37. Barometric Pressure Reduced
Chapter Editor: Walter Dümmer
Table of Contents
Figures and Tables
Ventilatory Acclimatization to High Altitude
John T. Reeves and John V. Weil
Physiological Effects of Reduced Barometric Pressure
Kenneth I. Berger and William N. Rom
Health Considerations for Managing Work at High Altitudes
John B. West
Prevention of Occupational Hazards at High Altitudes
Walter Dümmer
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.
Ventilatory Acclimatization to High Altitude
People are increasingly working at high altitudes. Mining operations, recreational facilities, modes of transportation, agricultural pursuits and military campaigns are often at high altitude, and all of these require human physical and mental activity. All such activity involves increased requirements for oxygen. A problem is that as one ascends higher and higher above sea level, both the total air pressure (the barometric pressure, PB) and the amount of oxygen in the ambient air (that portion of total pressure due to oxygen, PO2) progressively fall. As a result, the amount of work we can accomplish progressively decreases. These principles affect the workplace. For example, a tunnel in Colorado was found to require 25% more time to complete at an altitude of 11,000 ft than comparable work at sea level, and altitude effects were implicated in the delay. Not only is there increased muscular fatigue, but also, deterioration of mental function. Memory, computation, decision making and judgement all become impaired. Scientists doing calculations at the Mona Loa Observatory at an altitude above 4,000 m on the island of Hawaii have found they require more time to perform their calculations and they make more mistakes than at sea level. Because of the increasing scope, magnitude, variety and distribution of human activities on this planet, more people are working at high altitude, and effects of altitude become an occupational issue.
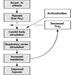
Fundamentally important to occupational performance at altitude is maintaining the oxygen supply to the tissues. We (and other animals) have defences against low oxygen states (hypoxia). Chief among these is an increase in breathing (ventilation), which begins when the oxygen pressure in the arterial blood (PaO2) decreases (hypoxemia), is present for all altitudes above sea level, is progressive with altitude and is our most effective defence against low oxygen in the environment. The process whereby breathing increases at high altitude is called ventilatory acclimatization. The importance of the process can be seen in figure 1, which shows that the oxygen pressure in the arterial blood is higher in acclimatized subjects than in unacclimatized subjects. Further, the importance of acclimatization in maintaining the arterial oxygen pressure increases progressively with increasing altitude. Indeed, the unacclimatized person is unlikely to survive above an altitude of 20,000 ft, whereas acclimatized persons have been able to climb to the summit of Mount Everest (29,029 ft, 8,848 m) without artificial sources of oxygen.
Figure 1. Ventilatory acclimatization
Mechanism
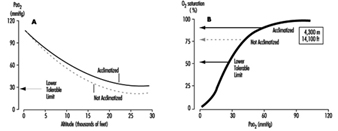
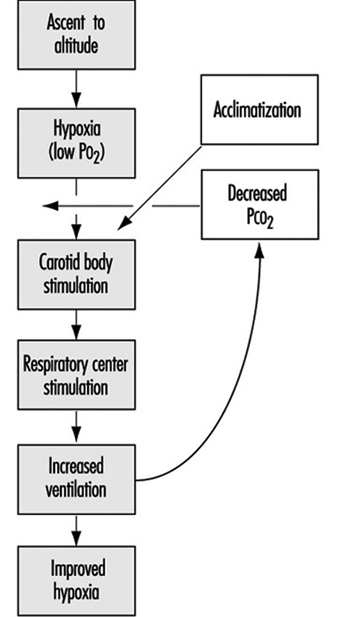
The stimulus for the increase in ventilation at high altitude largely and almost exclusively arises in a tissue which monitors the oxygen pressure in the arterial blood and is contained within an organ called the carotid body, about the size of a pinhead, located at a branch point in each of the two carotid arteries, at the level of the angle of the jaw. When the arterial oxygen pressure falls, nerve-like cells (chemoreceptor cells) in the carotid body sense this decrease and increase their firing rate along the 9th cranial nerve, which carries the impulses directly to the respiratory control centre in the brain stem. When the respiratory centre receives increased numbers of impulses, it stimulates an increase in the frequency and depth of breathing via complex nerve pathways, which activate the diaphragm and the muscles of the chest wall. The result is an increased amount of air ventilated by the lungs, figure 2, which in turn acts to restore the arterial oxygen pressure. If a subject breathes oxygen or air enriched with oxygen, the reverse happens. That is, the chemoreceptor cells decrease their firing rate, which decreases the nerve traffic to the respiratory centre, and breathing decreases. These small organs on each side of the neck are very sensitive to small changes in oxygen pressure in the blood. Also, they are almost entirely responsible for maintaining the body’s oxygen level, for when both of them are damaged or removed, ventilation no longer increases when blood oxygen levels fall. Thus an important factor controlling breathing is the arterial oxygen pressure; a decrease in oxygen level leads to an increase in breathing, and an increase in oxygen level leads to a decrease in breathing. In each case the result is, in effect, the body’s effort to maintain blood oxygen levels constant.
Figure 2. Sequence of events in acclimatization
Time course (factors opposing the increase in ventilation at altitude)
Oxygen is required for the sustained production of energy, and when oxygen supply to tissues is reduced (hypoxia), tissue function may become depressed. Of all organs, the brain is most sensitive to lack of oxygen, and, as noted above, centres within the central nervous system are important in the control of breathing. When we breathe a low-oxygen mixture, the initial response is an increase in ventilation, but after 10 minutes or so the increase is blunted to some extent. While the cause for this blunting is not known, its suggested cause is depression of some central neural function related to the ventilation pathway, and has been called hypoxic ventilatory depression. Such depression has been observed shortly after ascent to high altitude. The depression is transient, lasting only a few hours, possibly because there is some tissue adaptation within the central nervous system.
Nevertheless, some increase in ventilation usually begins immediately on going to high altitude, although time is required before maximum ventilation is achieved. On arrival at altitude, increased carotid body activity attempts to increase ventilation, and thereby to raise the arterial oxygen pressure back to the sea level value. However, this presents the body with a dilemma. An increase in breathing causes an increased excretion of carbon dioxide (CO2) in the exhaled air. When CO2 is in body tissues, it creates an acid aqueous solution, and when it is lost in exhaled air, the body fluids, including blood, become more alkaline, thus altering the acid-base balance in the body. The dilemma is that ventilation is regulated not only to keep oxygen pressure constant, but also for acid-base balance. CO2 regulates breathing in the opposite direction from oxygen. Thus when the CO2 pressure (i.e., the degree of acidity somewhere within the respiratory centre) rises, ventilation rises, and when it falls, ventilation falls. On arrival at high altitude, any increase in ventilation caused by the low oxygen environment will lead to a fall in CO2 pressure, which causes alkalosis and acts to oppose the increased ventilation (figure 2). Therefore, the dilemma on arrival is that the body cannot maintain constancy in both oxygen pressure and acid-base balance. Human beings require many hours and even days to regain proper balance.
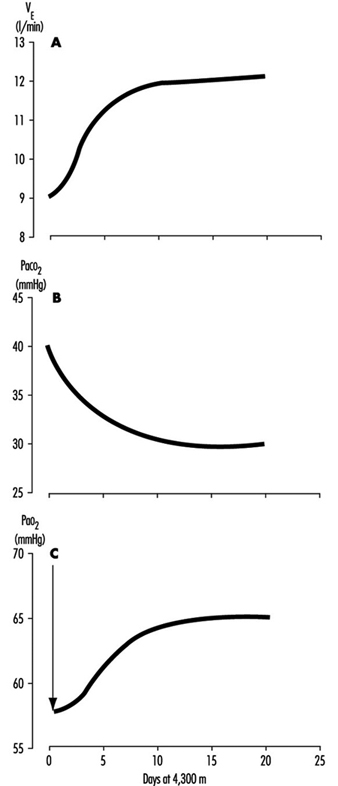
One method for rebalancing is for the kidneys to increase alkaline bicarbonate excretion in the urine, which compensates for the respiratory loss of acidity, thus helping to restore the body’s acid-base balance toward the sea-level values. The renal excretion of bicarbonate is a relatively slow process. For example, on going from sea level to 4,300 m (14,110 ft), acclimatization requires from seven to ten days (figure 3). This action of the kidneys, which reduces the alkaline inhibition of ventilation, was once thought to be the major reason for the slow increase in ventilation following ascent, but more recent research assigns a dominant role to a progressive increase in the sensitivity of the hypoxic sensing ability of the carotid bodies during the early hours to days following ascent to altitude. This is the interval of ventilatory acclimatization. The acclimatization process allows, in effect, ventilation to rise in response to low arterial oxygen pressure even though the CO2 pressure is falling. As the ventilation rises and CO2 pressure falls with acclimatization at altitude, there is a resultant and concomitant rise in oxygen pressure within the lung alveoli and the arterial blood.
Figure 3. Time course of ventilatory acclimatization for sea level subjects taken to 4,300 m altitude
Because of the possibility of transient hypoxic ventilatory depression at altitude, and because acclimatization is a process which begins only upon entering a low oxygen environment, the minimal arterial oxygen pressure occurs upon arrival at altitude. Thereafter, the arterial oxygen pressure rises relatively rapidly for the initial days and thereafter increases more slowly, as in figure 3. Because the hypoxia is worse soon after arrival, the lethargy and symptoms which accompany altitude exposure are also worse during the first hours and days. With acclimatization, a restored sense of well-being usually develops.
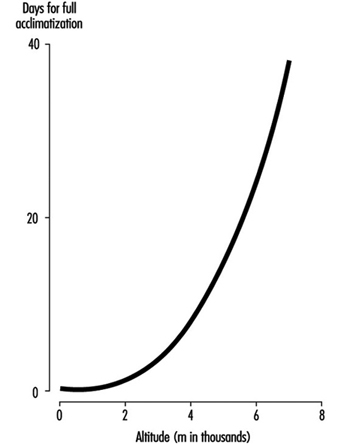
The time required for acclimatization increases with increasing altitude, consistent with the concept that greater increase in ventilation and acid-base adjustments require longer intervals for renal compensation to occur. Thus while acclimatization may require three to five days for a sea-level native to acclimatize at 3,000 m, for altitudes above 6,000 to 8,000 m, complete acclimatization, even if it is possible, may require six weeks or more (figure 4). When the altitude-acclimatized person returns to sea level, the process reverses. That is, the arterial oxygen pressure now rises to the sea-level value and ventilation falls. Now there is less CO2 exhaled, and CO2 pressure rises in the blood and in the respiratory centre. The acid-base balance is altered toward the acid side, and the kidneys must retain bicarbonate to restore balance. Although the time required for the loss of acclimatization is not as well understood, it seems to require approximately as long an interval as the acclimatization process itself. If so, then return from altitude, hypothetically, gives a mirror image of altitude ascent, with one important exception: arterial oxygen pressures immediately become normal on descent.
Figure 4. Effects of altitude on barometric pressure and inspired PO2
Variability among individuals
As might be expected, individuals vary with regard to time required for, and magnitude of, ventilatory acclimatization to a given altitude. One very important reason is the large variation between individuals in the ventilatory response to hypoxia. For example, at sea level, if one holds the CO2 pressure constant, so that it does not confound the ventilatory response to low oxygen, some normal persons show little or no increase in ventilation, while others show a very large (up to fivefold) increase. The ventilatory response to breathing low-oxygen mixtures seems to be an inherent characteristic of an individual, because family members behave more alike than do persons who are not related. Those persons who have poor ventilatory responses to low oxygen at sea level, as expected, also seem to have smaller ventilatory responses over time at high altitude. There may be other factors causing inter-individual variability in acclimatization, such as variability in the magnitude of ventilatory depression, in the function of the respiratory centre, in sensitivity to acid-base changes, and in renal handling of bicarbonate, but these have not been evaluated.
Sleep
Poor sleep quality, particularly before there is ventilatory acclimatization, is not only a common complaint, but also a factor that will impair occupational efficiency. Many things interfere with the act of breathing., including emotions, physical activity, eating and the degree of wakefulness. Ventilation decreases during sleep, and the capacity for breathing to be stimulated by low oxygen or high CO2 also decreases. Respiratory rate and depth of breathing both decrease. Further, at high altitude, where there are fewer oxygen molecules in the air, the amount of oxygen stored in the lung alveoli between breaths is less. Thus if breathing ceases for a few seconds (called apnoea, which is a common event at high altitude), the arterial oxygen pressure falls more rapidly than at sea level, where, in essence, the reservoir for oxygen is greater.
Periodic cessation of breathing is almost universal during the first few nights following ascent to high altitude. This is a reflection of the respiratory dilemma of altitude, described earlier, working in cyclic fashion: hypoxic stimulation increases ventilation, which in turn lowers carbon dioxide levels, inhibits breathing, and increases hypoxic stimulation, which again stimulates ventilation. Usually there is an apnoeic period of 15 to 30 seconds, followed by several very large breaths, which often briefly awakens the subject, after which there is another apnoea. The arterial oxygen pressure sometimes falls to alarming levels as a result of the apnoeic periods. There may be frequent awakenings, and even when total sleep time is normal its fragmentation impairs sleep quality such that there is the impression of having had a restless or sleepless night. Giving oxygen eliminates the cycling of hypoxic stimulation, and alkalotic inhibition abolishes the periodic breathing and restores normal sleep.
Middle-aged males in particular also are at risk for another cause of apnoea, namely intermittent obstruction of the upper airway, the common cause of snoring. While intermittent obstruction at the back of the nasal passages usually causes only annoying noise at sea level, at high altitude, where there is a smaller reservoir of oxygen in the lungs, such obstruction may lead to severely low levels of arterial oxygen pressure and poor sleep quality.
Intermittent Exposure
There are work situations, particularly in the Andes of South America, that require a worker to spend several days at altitudes above 3,000 to 4,000 m, and then to spend several days at home, at sea level. The particular work schedules (how many days are to be spent at altitude, say four to 14, and how many days, say three to seven, at sea level) are usually determined by the economics of the workplace more than by health considerations. However, a factor to be considered in the economics is the interval required both for acclimatization and loss of acclimatization to the altitude in question. Particular attention should be placed on the worker’s sense of well-being and performance on the job on arrival and the first day or two thereafter, regarding fatigue, time required to perform routine and non-routine functions, and errors made. Also strategies should be considered to minimize the time required for acclimatization at altitude, and to improve function during the waking hours.
Physiological Effects of Reduced Barometric Pressure
The major effects of high altitude on humans relate to the changes in barometric pressure (PB) and its consequential changes in the ambient pressure of oxygen (O2). Barometric pressure decreases with increasing altitude in a logarithmic fashion and can be estimated by the following equation:
![]()
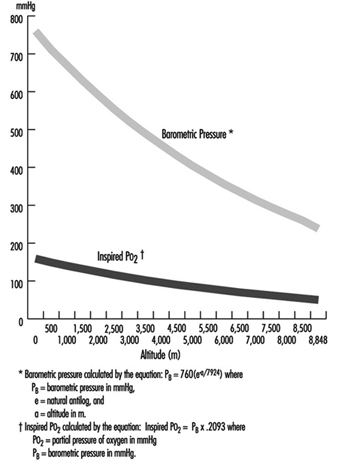
where a = altitude, expressed in metres. In addition, the relationship of barometric pressure to altitude is influenced by other factors such as distance from the equator and season. West and Lahiri (1984) found that direct measurements of barometric pressure near the equator and at the summit of Mt. Everest (8,848 m) were greater than predictions based on the International Civil Aviation Organization Standard Atmosphere. Weather and temperature also affect the relationship between barometric pressure and altitude to the extent that a low-pressure weather system can reduce pressure, making sojourners to high altitude “physiologically higher”. Since the inspired partial pressure of oxygen (PO2) remains constant at approximately 20.93% of barometric pressure, the most important determinant of inspired PO2 at any altitude is the barometric pressure. Thus, inspired oxygen decreases with increasing altitude due to decreased barometric pressure, as shown in figure 1.
Figure 1. Effects of altitude on barometric pressure and inspired PO2
Temperature and ultraviolet radiation also change at high altitudes. Temperature decreases with increasing altitude at a rate of approximately 6.5 °C per 1,000 m. Ultraviolet radiation increases approximately 4% per 300 m due to decreased cloudiness, dust, and water vapour. In addition, as much as 75% of ultraviolet radiation can be reflected back by snow, further increasing exposure at high altitude. Survival in high altitude environments is dependent on adaptation to and/or protection from each of these elements.
Acclimatization
While rapid ascent to high altitudes often results in death, slow ascent by mountaineers can be successful when accompanied by compensatory physiological adaptation measures. Acclimatization to high altitudes is geared towards maintaining an adequate supply of oxygen to meet metabolic demands despite the decreasing inspired PO2. In order to achieve this goal, changes occur in all organ systems involved with oxygen uptake into the body, distribution of O2 to the necessary organs, and O2 unloading to the tissues.
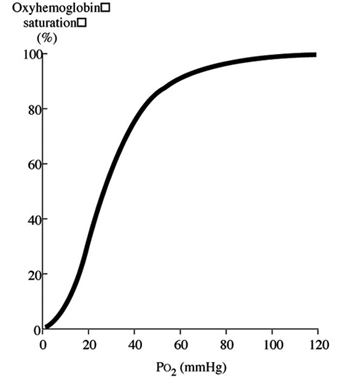
Discussion of oxygen uptake and distribution requires understanding the determinants of oxygen content in the blood. As air enters the alveolus, the inspired PO2 decreases to a new level (called the alveolar PO2) because of two factors: increased partial pressure of water vapour from humidification of inspired air, and increased partial pressure of carbon dioxide (PCO2) from CO2 excretion. From the alveolus, oxygen diffuses across the alveolar capillary membrane into the blood as a result of a gradient between alveolar PO2 and blood PO2. The majority of oxygen found in blood is bound to haemoglobin (oxyhaemoglobin). Thus, oxygen content is directly related to both the haemoglobin concentration in the blood and the percentage of O2 binding sites on haemoglobin that are saturated with oxygen (oxyhaemoglobin saturation). Therefore, understanding the relationship between arterial PO2 and oxyhaemoglobin saturation is essential for understanding the determinants of oxygen content in the blood. Figure 2 illustrates the oxyhaemoglobin dissociation curve. With increasing altitude, inspired PO2 decreases and, therefore, arterial PO2 and oxyhaemoglobin saturation decreases. In normal subjects, altitudes greater than 3,000 m are associated with sufficiently decreased arterial PO2 that oxyhaemoglobin saturation falls below 90%, on the steep portion of the oxyhaemoglobin dissociation curve. Further increases in altitude will predictably result in significant desaturation in the absence of compensatory mechanisms.
Figure 2. Oxyhaemoglobin dissociation curve
The ventilatory adaptations that occur in high-altitude environments protect the arterial partial pressure of oxygen against the effects of decreasing ambient oxygen levels, and can be divided into acute, subacute and chronic changes. Acute ascent to high altitude results in a fall in the inspired PO2 which in turn leads to a decrease in the arterial PO2 (hypoxia). In order to minimize the effects of decreased inspired PO2 on arterial oxyhaemoglobin saturation, the hypoxia that occurs at high altitude triggers an increase in ventilation, mediated through the carotid body (hypoxic ventilatory response–HVR). Hyperventilation increases carbon dioxide excretion and subsequently the arterial and then the alveolar partial pressure of carbon dioxide (PCO2) falls. The fall in alveolar PCO2 allows alveolar PO2 to rise, and consequently, arterial PO2 and arterial O2 content increases. However, the increased carbon dioxide excretion also causes a decrease in blood hydrogen ion concentration ([H+]) leading to the development of alkalosis. The ensuing alkalosis inhibits the hypoxic ventilatory response. Thus, on acute ascent to high altitude there is an abrupt increase in ventilation that is modulated by the development of an alkalosis in the blood.
Over the next several days at high altitude, further changes in ventilation occur, commonly referred to as ventilatory acclimatization. Ventilation continues to increase over the next several weeks. This further increase in ventilation occurs as the kidney compensates for the acute alkalosis by excretion of bicarbonate ions, with a resultant rise in blood [H+]. It was initially believed that renal compensation for the alkalosis removed the inhibitory influence of alkalosis on the hypoxic ventilatory response, thereby allowing the full potential of the HVR to be reached. However, measurements of blood pH revealed that the alkalosis persists despite the increase in ventilation. Other postulated mechanisms include: (1) cerebrospinal fluid (CSF) pH surrounding the respiratory control centre in the medulla may have returned to normal despite the persistent serum alkalosis; (2) increased sensitivity of the carotid body to hypoxia; (3) increased response of the respiratory controller to CO2. Once ventilatory acclimatization has occurred, both hyperventilation and the increased HVR persist for several days after return to lower altitudes, despite resolution of hypoxia.
Further ventilatory changes occur after several years of living at high altitude. Measurements in high-altitude natives have shown a decreased HVR when compared to values obtained in acclimatized individuals, although not to levels seen in subjects at sea level. The mechanism for the decreased HVR is unknown, but may be related to hypertrophy of the carotid body and/or development of other adaptive mechanisms for preserving tissue oxygenation such as: increased capillary density; increased gas exchange capacity of the tissues; increased number and density of mitochondria; or increased vital capacity.
In addition to its effect on ventilation, hypoxia also induces constriction of the vascular smooth muscle in the pulmonary arteries (hypoxic vasoconstriction). The ensuing increase in pulmonary vascular resistance and pulmonary artery pressure redirects blood flow away from poorly ventilated alveoli with low alveolar PO2 and towards better ventilated alveoli. In this manner, pulmonary arterial perfusion is matched to lung units that are well ventilated, providing another mechanism for preserving arterial PO2.
Oxygen delivery to the tissues is further enhanced by adaptations in the cardiovascular and haematological systems. On initial ascent to high altitude, heart rate increases, resulting in an increase in cardiac output. Over several days, cardiac output falls due to decreased plasma volume, caused by an increased water loss that occurs at high altitudes. With more time, increased erythropoietin production leads to increased haemoglobin concentration, providing the blood with increased oxygen-carrying capacity. In addition to increasing levels of haemoglobin, changes in the avidity of oxygen binding to haemoglobin may also help maintain tissue oxygenation. A shift of the oxyhaemoglobin dissociation curve to the right may be expected because it would favour release of oxygen to the tissues. However, data obtained from the summit of Mt. Everest and from hypobaric chamber experiments simulating the summit suggest that the curve is shifted to the left (West and Lahiri 1984; West and Wagner 1980; West et al. 1983). Although a left shift would make oxygen unloading to the tissues more difficult, it may be advantageous at extreme altitudes because it would facilitate oxygen uptake in the lungs despite markedly reduced inspired PO2 (43 mmHg on the summit of Mt. Everest versus 149 mmHg at sea level).
The last link in the chain of oxygen supply to the tissues is cellular uptake and utilization of O2. Theoretically, there are two potential adaptations that can occur. First, minimization of the distance that oxygen has to travel on diffusion out of the blood vessel and into the intracellular site responsible for oxidative metabolism, the mitochondria. Second, biochemical alterations can occur that improve mitochondrial function. Minimization of diffusion distance has been suggested by studies that show either increased capillary density or increased mitochondrial density in muscle tissue. It is unclear whether these changes reflect either recruitment or development of capillaries and mitochondria, or are an artefact due to muscle atrophy. In either case, the distance between the capillaries and the mitochondria would be decreased, thereby facilitating oxygen diffusion. Biochemical alterations that may improve mitochondrial function include increased myoglobin levels. Myoglobin is an intracellular protein that binds oxygen at low tissue PO2 levels and facilitates oxygen diffusion into the mitochondria. Myoglobin concentration increases with training and correlates with muscle cell aerobic capacity. Although these adaptations are theoretically beneficial, conclusive evidence is lacking.
Early accounts of high altitude explorers describe changes in cerebral function. Decreased motor, sensory and cognitive abilities, including decreased ability to learn new tasks and difficulty expressing information verbally, have all been described. These deficits may lead to poor judgement and to irritability, further compounding the problems encountered in high-altitude environments. On return to sea level, these deficits improve with a variable time course; reports have indicated impaired memory and concentration lasting from days to months, and decreased finger-tapping speed for one year (Hornbein et al. 1989). Individuals with greater HVR are more susceptible to long-lasting deficits, possibly because the benefit of hyperventilation on arterial oxyhaemoglobin saturation may be offset by hypocapnia (decreased PCO2 in the blood), which causes constriction of the cerebral blood vessels leading to decreased cerebral blood flow.
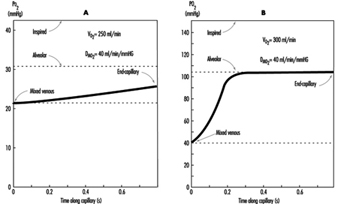
The preceding discussion has been limited to resting conditions; exercise provides an additional stress as oxygen demand and consumption increases. The fall in ambient oxygen at high altitude causes a fall in maximal oxygen uptake and, therefore, maximal exercise. In addition, the decreased inspired PO2 at high altitudes severely impairs oxygen diffusion into the blood. This is illustrated in figure 3, which plots the time course of oxygen diffusion into the alveolar capillaries. At sea level, there is excess time for equilibration of end-capillary PO2 to alveolar PO2, whereas at the summit of Mt. Everest, full equilibration is not realized. This difference is due to the decreased ambient oxygen level at high altitudes leading to a decreased diffusion gradient between alveolar and venous PO2. With exercise, cardiac output and blood flow increase, thereby reducing transit time of blood cells across the alveolar capillary, further exacerbating the problem. From this discussion, it becomes apparent that the left shift in the O2 and haemoglobin dissociation curve with altitude is necessary as compensation for the decreased diffusion gradient for oxygen in the alveolus.
Figure 3. The calculated time course of oxygen tension in the alveolar capillary
Disturbed sleep is common among sojourners at high altitude. Periodic (Cheyne-Stokes) breathing is universal and characterized by periods of rapid respiratory rate (hyperpnoea) alternating with periods of absent respirations (apnoea) leading to hypoxia. Periodic breathing tends to be more pronounced in individuals with the greatest hypoxic ventilatory sensitivity. Accordingly, sojourners with lower HVR have less severe periodic breathing. However, sustained periods of hypoventilation are then seen, corresponding with sustained decreases in oxyhaemoglobin saturation. The mechanism for periodic breathing probably relates to increased HVR causing increased ventilation in response to hypoxia. The increased ventilation leads to increased blood pH (alkalosis), which in turn suppresses ventilation. As acclimatization progresses, periodic breathing improves. Treatment with acetazolamide reduces periodic breathing and improves arterial oxyhaemoglobin saturation during sleep. Caution should be used with medications and alcohol that suppress ventilation, as they may exacerbate the hypoxia seen during sleep.
Pathophysiological Effects of Reduced Barometric Pressure
The complexity of human physiological adaptation to high altitude provides numerous potential maladaptive responses. Although each syndrome will be described separately, there is considerable overlap between them. Illnesses such as acute hypoxia, acute mountain sickness, high-altitude pulmonary oedema, and high-altitude cerebral oedema most likely represent a spectrum of abnormalities that share a similar pathophysiology.
Hypoxia
Hypoxia occurs with ascent to high altitudes because of the decreased barometric pressure and the resultant decrease in ambient oxygen. With rapid ascent, hypoxia occurs acutely, and the body does not have time to adjust. Mountaineers have generally been protected from the effects of acute hypoxia because of the time that elapses, and hence the acclimatization that occurs, during the climb. Acute hypoxia is problematic for both aviators and rescue personnel in high-altitude environments. Acute oxyhaemoglobin desaturation to values less than 40 to 60% leads to loss of consciousness. With less severe desaturation, individuals note headache, confusion, drowsiness and loss of coordination. Hypoxia also induces a state of euphoria which Tissandier, during his balloon flight in 1875, described as experiencing “inner joy”. With more severe desaturation, death occurs. Acute hypoxia responds rapidly and completely to either administration of oxygen or descent.
Acute mountain sickness
Acute mountain sickness (AMS) is the most common disorder in high-altitude environments and afflicts up to two-thirds of sojourners. The incidence of acute mountain sickness is dependent on multiple factors, including rate of ascent, length of exposure, degree of activity, and individual susceptibility. Identification of affected individuals is important in order to prevent progression to pulmonary or cerebral oedema. Identification of acute mountain sickness is made through recognition of characteristic signs and symptoms occurring in the appropriate setting. Most often, acute mountain sickness occurs within a few hours of a rapid ascent to altitudes greater than 2,500 m. The most common symptoms include headache that is more pronounced at night, loss of appetite that may be accompanied by nausea and vomiting, disturbed sleep, and fatigue. Individuals with AMS often complain of shortness of breath, cough and neurological symptoms such as memory deficits and auditory or visual disturbances. Findings on physical exam may be lacking, although fluid retention may be an early sign. The pathogenesis of acute mountain illness may relate to relative hypoventilation that would increase cerebral blood flow and intracranial pressure by increasing arterial PCO2 and decreasing arterial PO2. This mechanism may explain why persons with greater HVR are less likely to develop acute mountain sickness. The mechanism for fluid retention is not well understood, but may be related to abnormal plasma levels for proteins and/or hormones that regulate renal excretion of water; these regulators may respond to the increased activity of the sympathetic nervous system noted in patients with acute mountain sickness. The accumulation of water may in turn lead to the development of oedema or swelling of the interstitial spaces in the lungs. More severe cases may go on to develop pulmonary or cerebral oedema.
Prevention of acute mountain sickness can be accomplished through slow, graded ascent, allowing adequate time for acclimatization. This may be especially important for those individuals with greater susceptibility or a prior history of acute mountain sickness. In addition, administration of acetazolamide before or during ascent may help prevent and ameliorate symptoms of acute mountain sickness. Acetazolamide inhibits the action of carbonic anhydrase in the kidneys and leads to increased excretion of bicarbonate ions and water, producing an acidosis in the blood. The acidosis stimulates respiration, leading to increased arterial oxyhaemoglobin saturation and decreased periodic breathing during sleep. Through this mechanism, acetazolamide speeds the natural process of acclimatization.
Treatment of acute mountain sickness can be accomplished most effectively by descent. Further ascent to high altitudes is contra-indicated, as the disease may progress. When descent is not possible, oxygen may be administered. Alternatively, portable lightweight fabric hyperbaric chambers may be brought on expeditions to high-altitude environments. Hyperbaric bags are particularly valuable when oxygen is not available and descent is not possible. Several drugs are available that improve symptoms of acute mountain sickness, including acetazolamide and dexamethasone. The mechanism of action of dexamethasone is unclear, although it may act by decreasing oedema formation.
High-altitude pulmonary oedema
High-altitude pulmonary oedema affects approximately 0.5 to 2.0% of individuals who ascend to altitudes greater than 2,700 m and is the most common cause of death due to illnesses encountered at high altitudes. High-altitude pulmonary oedema develops from 6 to 96 hours after ascent. Risk factors for the development of high-altitude pulmonary oedema are similar to those for acute mountain sickness. Common early signs include symptoms of acute mountain sickness accompanied by decreased exercise tolerance, increased recovery time after exercise, shortness of breath on exertion, and persistent dry cough. As the condition worsens, the patient develops shortness of breath at rest, findings of audible congestion in the lungs, and cyanosis of the nail beds and lips. The pathogenesis of this disorder is uncertain but probably relates to increased microvascular pressure or increased permeability of the microvasculature leading to the development of pulmonary oedema. Although pulmonary hypertension may help explain the pathogenesis, elevation in the pulmonary artery pressure due to hypoxia has been observed in all individuals who ascend to high altitude, including those who do not develop pulmonary oedema. Nevertheless, susceptible individuals may possess uneven hypoxic constriction of the pul-monary arteries, leading to over-perfusion of the microvasculature in localized areas where hypoxic vasoconstriction was absent or diminished. The resulting increase in pressure and shear forces may damage the capillary membrane, leading to oedema formation. This mechanism explains the patchy nature of this disease and its appearance on x-ray examination of the lungs. As with acute mountain sickness, individuals with a lower HVR are more likely to develop high-altitude pulmonary oedema as they have lower oxyhaemoglobin saturations and, therefore, greater hypoxic pulmonary vasoconstriction.
Prevention of high-altitude pulmonary oedema is similar to prevention of acute mountain sickness and includes gradual ascent and use of acetazolamide. Recently, use of the smooth-muscle relaxing agent nifedipine has been shown to be of benefit in preventing disease in individuals with a prior history of high-altitude pulmonary oedema. Additionally, avoidance of exercise may have a preventive role, although it is probably limited to those individuals who already posses a subclinical degree of this disease.
Treatment of high-altitude pulmonary oedema is best accomplished by assisted evacuation to a lower altitude, keeping in mind that the victim needs to limit his or her exertion. After descent, improvement is rapid and additional treatment other than bed rest and oxygen are usually not necessary. When descent is not possible, oxygen therapy may be beneficial. Drug treatment has been attempted with multiple agents, most successfully with the diuretic furosemide and with morphine. Caution must be used with these drugs, as they can lead to dehydration, decreased blood pressure, and respiratory depression. Despite the effectiveness of descent as therapy, mortality remains at approximately 11%. This high mortality rate may reflect failure to diagnose the disease early in its course, or inability to descend coupled with lack of availability of other treatments.
High-altitude cerebral oedema
High-altitude cerebral oedema represents an extreme form of acute mountain sickness that has progressed to include generalized cerebral dysfunction. The incidence of cerebral oedema is unclear because it is difficult to differentiate a severe case of acute mountain sickness from a mild case of cerebral oedema. The pathogenesis of high-altitude cerebral oedema is an extension of the pathogenesis of acute mountain sickness; hypoventilation increases cerebral blood flow and intracranial pressure progressing to cerebral oedema. Early symptoms of cerebral oedema are identical to symptoms of acute mountain sickness. As the disease progresses, additional neurological symptoms are noted, including severe irritability and insomnia, ataxia, hallucinations, paralysis, seizures and eventually coma. Examination of the eyes commonly reveals swelling of the optic disc or papilloedema. Retinal haemorrhages are frequently noted. In addition, many cases of cerebral oedema have concurrent pulmonary oedema.
Treatment of high-altitude cerebral oedema is similar to treatment of other high-altitude disorders, with descent being the preferred therapy. Oxygen should be administered to maintain oxyhaemoglobin saturation greater that 90%. Oedema formation may be decreased with use of corticosteroids such as dexamethasone. Diuretic agents have also been utilized to decrease oedema, with uncertain efficacy. Comatose patients may require additional support with airway management. The response to treatment is variable, with neurological deficits and coma persisting for days to weeks after evacuation to lower altitudes. Preventative measures for cerebral oedema are identical to measures for other high-altitude syndromes.
Retinal haemorrhages
Retinal haemorrhages are extremely common, affecting up to 40% of individuals at 3,700 m and 56% at 5,350 m. Retinal haemorrhages are usually asymptomatic. They are most likely caused by increased retinal blood flow and vascular dilatation due to arterial hypoxia. Retinal haemorrhages are more common in individuals with headaches and can be precipitated by strenuous exercise. Unlike other high-altitude syndromes, retinal haemorrhages are not preventable by acetazolamide or furosemide therapy. Spontaneous resolution is usually seen within two weeks.
Chronic mountain sickness
Chronic mountain sickness (CMS) afflicts residents and long-term inhabitants of high altitude. The first description of chronic mountain sickness reflected Monge’s observations of Andean natives living at altitudes above 4,000 m. Chronic mountain sickness, or Monge’s disease, has since been described in most high-altitude dwellers except Sherpas. Males are more commonly affected than females. Chronic mountain sickness is characterized by plethora, cyanosis and elevated red blood cell mass leading to neurological symptoms that include headache, dizziness, lethargy and impaired memory. Victims of chronic mountain sickness may develop right heart failure, also called cor pulmonale, due to pulmonary hypertension and markedly reduced oxyhaemoglobin saturation. The pathogenesis of chronic mountain sickness is unclear. Measurements from affected individuals have revealed a decreased hypoxic ventilatory response, severe hypoxemia that is exacerbated during sleep, increased haemoglobin concentration and increased pulmonary artery pressure. Although a cause-and-effect relationship seems likely, evidence is lacking and often confusing.
Many symptoms of chronic mountain sickness can be ameliorated by descent to sea level. Relocation to sea level removes the hypoxic stimulus for red blood cell production and pulmonary vasoconstriction. Alternate treatments include: phlebotomy to reduce red blood cell mass, and low-flow oxygen during sleep to improve hypoxia. Therapy with medroxyprogesterone, a respiratory stimulant, has also been found to be effective. In one study, ten weeks of medroxyprogesterone therapy was followed by improved ventilation and hypoxia, and decreased red blood cell counts.
Other conditions
Patients with sickle cell disease are more likely to suffer from painful vaso-occlusive crisis at high altitude. Even moderate altitudes of 1,500 m have been known to precipitate crises, and altitudes of 1,925 m are associated with a 60% risk of crises. Patients with sickle cell disease residing at 3,050 m in Saudi Arabia have twice as many crises as patients residing at sea level. In addition, patients with sickle cell trait may develop splenic infarct syndrome on ascent to high altitude. Likely aetiologies for the increased risk of vaso-occlusive crisis include: dehydration, increased red blood cell count, and immobility. Treatment of vaso-occlusive crisis includes descent to sea level, oxygen and intravenous hydration.
Essentially no data exist describing the risk to pregnant patients on ascent to high altitudes. Although patients residing at high altitude have an increased risk of pregnancy-induced hypertension, no reports of increased foetal demise exist. Severe hypoxia may cause abnormalities in foetal heart rate; however, this occurs only at extreme altitudes or in the presence of high-altitude pulmonary oedema. Therefore, the greatest risk to the pregnant patient may relate to the remoteness of the area rather than to altitude-induced complications.
Health Considerations for Managing Work at High Altitudes
Large numbers of people work at high altitudes, particularly in the cities and villages of the South American Andes and the Tibetan plateau. The majority of these people are highlanders who have lived in the area for many years and perhaps several generations. Much of the work is agricultural in nature—for example, tending domesticated animals.
However, the focus of this article is different. Recently there has been a large increase in commercial activities at altitudes of 3,500 to 6,000 m. Examples include mines in Chile and Peru at altitudes of around 4,500 m. Some of these mines are very large, employing over 1,000 workers. Another example is the telescope facility at Mauna Kea, Hawaii, at an altitude of 4,200 m.
Traditionally, the high mines in the South American Andes, some of which date back to the Spanish colonial period, have been worked by indigenous people who have been at high altitude for generations. Recently however, increasing use is being made of workers from sea level. There are several reasons for this change. One is that there are not enough people in these remote areas to operate the mines. An equally important reason is that as the mines become increasingly automated, skilled people are required to operate large digging machines, loaders and trucks, and local people may not have the necessary skills. A third reason is the economics of developing these mines. Whereas previously whole towns were set up in the vicinity of the mine to accommodate the workers’ families, and necessary ancillary facilities such as schools and hospitals, it is now seen to be preferable to have the families live at sea level, and have the workers commute to the mines. This is not purely an economic issue. The quality of life at an altitude of 4,500 m is less than at lower altitudes (e.g., children grow more slowly). Therefore the decision to have the families remain at sea level while the workers commute to high altitude has a sound socio-economic basis.
The situation where a workforce moves from sea level to altitudes of approximately 4,500 m raises many medical issues, many of which are poorly understood at the present time. Certainly most people who travel from sea level to an altitude of 4,500 m develop some symptoms of acute mountain sickness initially. Tolerance to the altitude often improves after the first two or three days. However, the severe hypoxia of these altitudes has a number of deleterious effects on the body. Maximal work capacity is decreased, and people fatigue more rapidly. Mental efficiency is reduced and many people find it is much more difficult to concentrate. Sleep quality is often poor, with frequent arousals and periodic breathing (the breathing waxes and wanes three or four times every minute) with the result that that the arterial PO2 falls to low levels following the periods of apnoea or reduced breathing.
Tolerance to high altitude varies greatly between individuals, and it is often very difficult to predict who is going to be intolerant of high altitude. A substantial number of people who would like to work at an altitude of 4,500 m find that they are unable to do so, or that the quality life is so poor that they refuse to remain at that altitude. Topics such as the selection of workers who are likely to tolerate high altitude, and the scheduling of their work between high altitude and the period with their families at sea level, are relatively new and not well understood.
Pre-employment Examination
In addition to the usual type of pre-employment examination, special attention should be given to the cardio-pulmonary system, because working at high altitude makes great demands on the respiratory and cardiovascular systems. Medical conditions such as early chronic obstructive pulmonary disease and asthma will be much more disabling at high altitude because of the high levels of ventilation, and should be specifically looked for. A heavy cigarette smoker with symptoms of early bronchitis is likely to have difficulty tolerating high altitude. Forced spirometry should be measured in addition to the usual chest examination including chest radiograph. If possible, an exercise test should be carried out because any exercise intolerance will be exaggerated at high altitude.
The cardiovascular system should be carefully examined, including an exercise electrocardiogram if that is feasible. Blood counts should be made to exclude workers with unusual degrees of anaemia or polycythaemia.
Living at high altitude increases the psychological stress in many people, and a careful history should be taken to exclude prospective workers with previous behavioural problems. Many modern mines at high altitude are dry (no alcohol permitted). Gastro-intestinal symptoms are common in some people at high altitude, and workers who have a history of dyspepsia may do poorly.
Selection of Workers to Tolerate High Altitude
In addition to excluding workers with lung or heart disease who are likely to do poorly at high altitude, it would be very valuable if tests could be carried out to determine who is likely to tolerate altitude well. Unfortunately little is known at the present time about predictors of tolerance to high altitude, though considerable work is being done on this at the present time.
The best predictor of tolerance to high altitude is probably previous experience at high altitude. If someone has been able to work at an altitude of 4,500 m for several weeks without appreciable problems, it is very likely that he or she will be able to do this again. By the same token, somebody who tried to work at high altitude and found that he or she could not tolerate it, is very likely to have the same problem next time. Therefore in selecting workers, a great deal of emphasis should be placed on successful previous employment at high altitude. However, clearly this criterion cannot be used for all workers because otherwise no new people would enter the high-altitude working pool.
Another possible predictor is the magnitude of the ventilatory response to hypoxia. This can be measured at sea level by giving the prospective worker a low concentration of oxygen to breathe and measuring the increase in ventilation. There is some evidence that people who have a relatively weak hypoxic ventilatory response tolerate high altitude poorly. For example, Schoene (1982) showed that 14 high-altitude climbers had significantly higher hypoxic ventilatory responses than ten controls. Further measurements were made on the 1981 American Medical Research Expedition to Everest, where it was shown that the hypoxic ventilatory response measured before and on the Expedition correlated well with performance high on the mountain (Schoene, Lahiri and Hackett 1984). Masuyama, Kimura and Sugita (1986) reported that five climbers who reached 8,000 m in Kanchenjunga had a higher hypoxic ventilatory response than five climbers who did not.
However, this correlation is by no means universal. In a prospective study of 128 climbers going to high altitudes, a measure of hypoxic ventilatory response did not correlate with the height reached, whereas a measurement of maximal oxygen uptake at sea level did correlate (Richalet, Kerome and Bersch 1988). This study also suggested that the heart rate response to acute hypoxia might be a useful predictor of performance at high altitude. There have been other studies showing a poor correlation between hypoxic ventilatory response and performance at extreme altitude (Ward, Milledge and West 1995).
The problem with many of these studies is that the results are chiefly applicable to much higher altitudes than of interest here. Also there are many examples of climbers with moderate values of hypoxic ventilatory response who do well at high altitude. Nevertheless, an abnormally low hypoxic ventilatory response is probably a risk factor for tolerating even medium altitudes such as 4,500 m.
One way of measuring the hypoxic ventilatory response at sea level is to have the subject rebreathe into a bag which is initially filled with 24% oxygen, 7% carbon dioxide, and the balance nitrogen. During rebreathing the PCO2 is monitored and held constant by means of a variable bypass and carbon dioxide absorber. Rebreathing can be continued until the inspired PO2 falls to about 40 mmHg (5.3 kPa). The arterial oxygen saturation is measured continually with a pulse oximeter, and the ventilation plotted against the saturation (Rebuck and Campbell 1974). Another way of measuring the hypoxic ventilatory response is to determine the inspiratory pressure during a brief period of airway occlusion while the subject is breathing a low-oxygen mixture (Whitelaw, Derenne and Milic-Emili 1975).
Another possible predictor of tolerance to high altitude is work capacity during acute hypoxia at sea level. The rationale here is that someone who is not able to tolerate acute hypoxia is more likely to be intolerant of chronic hypoxia. There is little evidence for or against this hypothesis. Soviet physiologists used tolerance to acute hypoxia as one of the criteria for selection of climbers for their successful 1982 Everest expedition (Gazenko 1987). On the other hand, the changes that occur with acclimatization are so profound that it would not be surprising if exercise performance during acute hypoxia were poorly correlated with the ability to work during chronic hypoxia.
Another possible predictor is the increase in pulmonary artery pressure during acute hypoxia at sea level. This can be measured non-invasively in many people by Doppler ultrasound. The main rationale for this test is the known correlation between the development of high-altitude pulmonary oedema and the degree of hypoxic pulmonary vasoconstriction (Ward, Milledge and West 1995). However, since high-altitude pulmonary oedema is uncommon in people working at an altitude of 4,500 m, the practical value of this test is questionable.
The only way to determine whether these tests for the selection of workers have practical value is a prospective study where the results of the tests done at sea level are correlated with subsequent assessment of tolerance to high altitude. This raises the question of how high-altitude tolerance will be measured. The usual way of doing this is by questionnaires such as the Lake Louise questionnaire (Hackett and Oelz 1992). However, questionnaires may be unreliable in this population because workers perceive that if they admit to altitude intolerance, they might lose their jobs. It is true that there are objective measures of altitude intolerance such as quitting work, rales in the lungs as indications of subclinical pulmonary oedema, and mild ataxia as an indication of subclinical high-altitude cerebral oedema. However, these features will be seen only in people with severe altitude intolerance, and a prospective study based solely on such measurements would be very insensitive.
It should be emphasized that the value of these possible tests for determining tolerance to working at high altitude has not been established. However, the economic implications of taking on a substantial number of workers who are unable to perform satisfactorily at high altitude are such that it would be very valuable to have useful predictors. Studies are presently underway to determine whether some of these predictors are valuable and feasible. Measurements such as the hypoxic ventilatory response to hypoxia, and work capacity during acute hypoxia at sea level, are not particularly difficult. However, they need to be done by a professional laboratory, and the cost of these investigations can be justified only if the predictive value of the measurements is substantial.
Scheduling between High Altitude and Sea Level
Again, this article is addressed to the specific problems which occur when commercial activities such as mines at altitudes of about 4,500 m employ workers who commute from sea level where their families live. Scheduling is obviously not an issue where people live permanently at high altitude.
Designing the optimal schedule for moving between high altitude and sea level is a challenging problem, and as yet there is little scientific basis for the schedules that have been employed so far. These have been based mainly on social factors such as how long the workers are willing to spend at high altitude before seeing their families again.
The main medical rationale for spending several days at a time at high altitude is the advantage gained from acclimatization. Many people who develop symptoms of acute mountain sickness after going to high altitude feel much better after two to four days. Therefore rapid acclimatization is occurring over this period. In addition it is known that the ventilatory response to hypoxia takes seven to ten days to reach a steady state (Lahiri 1972; Dempsey and Forster 1982). This increase in ventilation is one of the most important features of the acclimatization process, and therefore it is reasonable to recommend that the working period at high altitude be at least ten days.
Other features of high-altitude acclimatization probably take much longer to develop. One example is polycythaemia, which takes several weeks to reach a steady state. However, it should be added that the physiological value of polycythaemia is much less certain than was thought at one time. Indeed, Winslow and Monge (1987) have shown that the severe degrees of polycythaemia which are sometimes seen in permanent dwellers at altitudes of about 4,500 m are counterproductive in that work capacity can sometimes be increased if the haematocrit is lowered by removing blood over several weeks.
Another important issue is the rate of deacclimatization. Ideally the workers should not lose all the acclimatization that they have developed at high altitude during their period with their families at sea level. Unfortunately, there has been little work on the rate of deacclimatization, although some measurements suggest that the rate of change of the ventilatory response during deacclimatization is slower than during acclimatization (Lahiri 1972).
Another practical issue is the time required to move workers from sea level to high altitude and back again. In a new mine at Collahuasi in north Chile, it takes only a few hours to reach the mine by bus from the coastal town of Iquique, where most of the families are expected to live. However, if the worker resides in Santiago, the trip could take over a day. Under these circumstances, a short working period of three or four days at high altitude would clearly be inefficient because of the time wasted in travelling.
Social factors also play a critical role in any scheduling that involves time away from the family. Even if there are medical and physiological reasons why an acclimatization period of 14 days is optimal, the fact that the workers are unwilling to leave their families for more than seven or ten days may be an overriding factor. Experience so far shows that a schedule of seven days at high altitude followed by seven days at sea level, or ten days at high altitude followed by the same period at sea level are probably the most acceptable schedules.
Note that with this type of schedule, the worker never fully acclimatizes to high altitude, nor fully deacclimatizes while at sea level. He therefore spends his time oscillating between the two extremes, never receiving the full benefit of either state. In addition, some workers complain of extreme tiredness when they return to sea level, and spend the first two or three days recovering. Possibly this is related to the poor quality of sleep which is often a feature of living at high altitude. These problems highlight our ignorance of the factors that determine the best schedules, and more work is clearly needed in this area.
Whatever schedule is used, it is highly advantageous if the workers can sleep at a lower altitude than the workplace. Naturally whether this is feasible depends on the topography of the region. A lower altitude for sleeping is not feasible if it takes several hours to reach it because this cuts too much off the working day. However, if there is a location several hundred metres lower which can be reached within, say, one hour, setting up sleeping quarters at this lower altitude will improve sleep quality, workers’ comfort and sense of well-being, and productivity.
Oxygen Enrichment of Room Air to Reduce the Hypoxia of High Altitude
The deleterious effects of high altitude are caused by the low partial pressure of oxygen in the air. In turn, this results from the fact that while the oxygen concentration is the same as at sea level, the barometric pressure is low. Unfortunately there is little that can be done at high altitude to counter this “climatic aggression”, as it was dubbed by Carlos Monge, the father of high-altitude medicine in Peru (Monge 1948).
One possibility is to increase the barometric pressure in a small area, and this is the principle of the Gamow bag, which is sometimes used for the emergency treatment of mountain sickness. However, pressurizing large spaces such as rooms is difficult from a technical point of view, and there are also medical problems associated with entering and leaving a room with increased pressure. An example is middle ear discomfort if the Eustachian tube is blocked.
The alternative is to raise the oxygen concentration in some parts of the work facility, and this is a relatively new development that shows great promise (West 1995). As pointed out earlier, even after a period of acclimatization of seven to ten days at an altitude of 4,500 m, severe hypoxia continues to reduce work capacity, mental efficiency and sleep quality. It would therefore be highly advantageous to reduce the degree of hypoxia in some parts of the work facility if that were feasible.
This can be done by adding oxygen to the normal air ventilation of some rooms. The value of relatively minor degrees of oxygen enrichment of the room air is remarkable. It has been shown that every 1% increase in oxygen concentration (for example from 21 to 22%) reduces the equivalent altitude by 300 m. The equivalent altitude is that which has the same inspired PO2 during air breathing as in the oxygen-enriched room. Thus at an altitude of 4,500 m, raising the oxygen concentration of a room from 21 to 26% would reduce the equivalent altitude by 1,500 m. The result would be an equivalent altitude of 3,000 m, which is easily tolerated. The oxygen would be added to the normal room ventilation and therefore would be part of the air conditioning. We all expect that a room will provide a comfortable temperature and humidity. Control of the oxygen concentration can be regarded as a further logical step in humanity’s control of our environment.
Oxygen enrichment has become feasible because of the introduction of relatively inexpensive equipment for providing large quantities of nearly pure oxygen. The most promising is the oxygen concentrator that uses a molecular sieve. Such a device preferentially adsorbs nitrogen and thus produces an oxygen-enriched gas from air. It is difficult to produce pure oxygen with this type of concentrator, but large amounts of 90% oxygen in nitrogen are readily available, and these are just as useful for this application. These devices can work continuously. In practice, two molecular sieves are used in an alternating fashion, and one is purged while the other is actively adsorbing nitrogen. The only requirement is electrical power, which is normally in abundant supply at a modern mine. As a rough indication of the cost of oxygen enrichment, a small commercial device can be bought off the shelf, and this produces 300 litres per hour of 90% oxygen. It was developed to produce oxygen for treating patients with lung disease in their homes. The device has a power requirement of 350 watts and the initial cost is about US$2,000. Such a machine is sufficient to raise the oxygen concentration in a room by 3% for one person at a minimal though acceptable level of room ventilation. Very large oxygen concentrators are also available, and they are used in the paper pulp industry. It is also possible that liquid oxygen might be economical under some circumstances.
There are several areas in a mine, for example, where oxygen enrichment might be considered. One would be the director’s office or conference room, where important decisions are being made. For example, if there is a crisis in the mine such as a serious accident, such a facility would probably result in clearer thinking than the normal hypoxic environment. There is good evidence that an altitude of 4,500 m impairs brain function (Ward, Milledge and West 1995). Another place where oxygen enrichment would be beneficial is a laboratory where quality control measurements are being carried out. A further possibility is oxygen enrichment of sleeping quarters to improve sleep quality. Double blind trials of the effectiveness of oxygen enrichment at altitudes of about 4,500 m would be easy to design and should be carried out as soon as possible.
Possible complications of oxygen enrichment should be considered. Increased fire hazard is one issue that has been raised. However, increasing the oxygen concentration by 5% at an altitude of 4,500 m produces an atmosphere which has a lower flammability than air at sea level (West 1996). It should be borne in mind that although oxygen enrichment increases the PO2, this is still much lower than the sea-level value. Flammability of an atmosphere depends on two variables (Roth 1964):
- the partial pressure of oxygen, which is much lower in the enriched air at high altitude than at sea level
- the quenching effect of the inert components (i.e., nitrogen) of the atmosphere.
This quenching is slightly reduced at high altitude, but the net effect is still a lower flammability. Pure or nearly pure oxygen is dangerous, of course, and the normal precautions should be taken in piping the oxygen from the oxygen concentrator to the ventilation ducting.
Loss of acclimatization to high altitude is sometimes cited as a disadvantage of oxygen enrichment. However, there is no basic difference between entering a room with an oxygen-enriched atmosphere, and descending to a lower altitude. Everybody would sleep at a lower altitude if they could, and therefore this is hardly an argument against using oxygen enrichment. It is true that frequent exposure to a lower altitude will result in less acclimatization to the higher altitude, other things being equal. However, the ultimate objective is effective working at the high altitude of the mine, and this can presumably be enhanced using oxygen enrichment.
It is sometimes suggested that altering the atmosphere in this way might increase the legal liability of the facility if some kind of hypoxia-related illness developed. Actually, the opposite view seems more reasonable. It is possible that a worker who develops, say, a myocardial infarction while working at high altitude could claim that the altitude was a contributing factor. Any procedure which reduces the hypoxic stress makes altitude-induced illnesses less likely.
Emergency Treatment
The various types of high-altitude sickness, including acute mountain sickness, high-altitude pulmonary oedema and high-altitude cerebral oedema, were discussed earlier in this chapter. Little needs to be added in the context of work at high altitude.
Anyone who develops a high-altitude illness should be allowed to rest. This may be sufficient for conditions such as acute mountain sickness. Oxygen should be given by mask if this is available. However, if the patient does not improve, or deteriorates, descent is by far the best treatment. Usually this is easily done in a large commercial facility, because transportation is always available. All the high-altitude-related illnesses usually respond rapidly to removal to lower altitude.
There may be a place in a commercial facility for a small pressurized container in which the patient can be placed, and the equivalent altitude reduced by pumping in air. In the field, this is commonly done using a strong bag. One design is known as the Gamow bag, after its inventor. However, the main advantage of the bag is its portability, and since this feature is not really essential in a commercial facility, it would probably be better to use a larger, rigid tank. This should be big enough for an attendant to be inside the facility with the patient. Of course adequate ventilation of such a container is essential. Interestingly, there is anecdotal evidence that raising the atmospheric pressure in this way is sometimes more efficacious in the treatment of high-altitude illness than giving the patient a high concentration of oxygen. It is not clear why this should be so.
Acute mountain sickness
This is usually self-limiting and the patient feels much better after a day or two. The incidence of acute mountain sickness can be reduced by taking acetazolamide (Diamox), one or two 250 mg tablets per day. These can be started before reaching high altitude or can be taken when symptoms develop. Even people with mild symptoms find that half a tablet at night often improves the quality of sleep. Aspirin or paracetamol is useful for headache. Severe acute mountain sickness can be treated with dexamethasone, 8 mg initially, followed by 4 mg every six hours. However, descent is by far the best treatment if the condition is severe.
High-altitude pulmonary oedema
This is a potentially serious complication of mountain sickness and must be treated. Again the best therapy is descent. While awaiting evacuation, or if evacuation is not possible, give oxygen or place in a high-pressure chamber. Nifedipine (a calcium channel blocker) should be given. The dose is 10 mg sublingually followed by 20 mg slow release. This results in a fall in pulmonary artery pressure and is often very effective. However, the patient should be taken down to a lower altitude.
High-altitude cerebral oedema
This is potentially a very serious complication and is an indication for immediate descent. While awaiting evacuation, or if evacuation is not possible, give oxygen or place in an increased pressure environment. Dexamethasone should be given, 8 mg initially, followed by 4 mg every six hours.
As indicated earlier, people who develop severe acute mountain sickness, high-altitude pulmonary oedema or high-altitude cerebral oedema are likely to have a recurrence if they return to high altitude. Therefore if a worker develops any of these conditions, attempts should be made to find employment at a lower altitude.
Prevention of Occupational Hazards at High Altitudes
Working at high altitudes induces a variety of biological responses, as described elsewhere in this chapter. The hyperventilatory response to altitude should cause a marked increase in the total dose of hazardous substances which may be inhaled by persons occupationally exposed, as compared to people working under similar conditions at sea level. This implies that 8-hour exposure limits used as the basis of exposure standards should be reduced. In Chile, for example, the observation that silicosis progresses faster in mines at high altitudes, led to the reduction of the permitted exposure level proportional to the barometric pressure at the workplace, when expressed in terms of mg/m3. While this may be overcorrecting at intermediate altitudes, the error will be in the favour the exposed worker. The threshold limit values (TLVs), expressed in terms of parts per million (ppm), require no adjustment, however, because both the proportion of millimoles of contaminant per mole of oxygen in air and the number of moles of oxygen required by a worker remain approximately constant at different altitudes, even though the air volume containing one mole of oxygen will vary.
In order to assure that this is true, however, the method of measurement used to determine the concentration in ppm must be truly volumetric, as is the case with Orsat’s apparatus or the Bacharach Fyrite instruments. Colourimetric tubes that are calibrated to read in ppm are not true volumetric measurements because the markings on the tube are actually caused by a chemical reaction between the air contaminant and some reagent. In all chemical reactions, substances combine in proportion to the number of moles present, not in proportion to volumes. The hand-operated air pump draws a constant volume of air through the tube at any altitude. This volume at a higher altitude will contain a smaller mass of contaminant, giving a reading lower than the actual volumetric concentration in ppm (Leichnitz 1977). Readings should be corrected by multiplying the reading by the barometric pressure at sea level and dividing the result by the barometric pressure at the sampling site, using the same units (such as torr or mbar) for both pressures.
Diffusional samplers: The laws of gas diffusion indicate that the collection efficiency of diffusional samplers is independent of barometric pressure changes. Experimental work by Lindenboom and Palmes (1983) shows that other, as yet undetermined factors influence the collection of NO2 at reduced pressures. The error is approximately 3.3% at 3,300 m and 8.5% at 5,400 m equivalent altitude. More research is needed on the causes of this variation and the effect of altitude on other gases and vapours.
No information is available on the effect of altitude on portable gas detectors calibrated in ppm, which are equipped with electrochemical diffusion sensors, but it could reasonably be expected that the same correction mentioned under colourimetric tubes would apply. Obviously the best procedure would be to calibrate them at altitude with a test gas of known concentration.
The principles of operation and measurement of electronic instruments should be examined carefully to determine whether they need recalibration when employed at high altitudes.
Sampling pumps: These pumps usually are volumetric—that is, they displace a fixed volume per revolution—but they usually are the last component of the sampling train, and the actual volume of air aspirated is affected by the resistance to flow opposed by the filters, hose, flow meters and orifices that are part of the sampling train. Rotameters will indicate a lower flow rate than that actually flowing through the sampling train.
The best solution of the problem of sampling at high altitudes is to calibrate the sampling system at the sampling site, obviating the problem of corrections. A briefcase sized bubble film calibration laboratory is available from sampling pump manufacturers. This is easily carried to location and permits rapid calibration under actual working conditions. It even includes a printer which provides a permanent record of calibrations made.
TLVs and Work Schedules
TLVs have been specified for a normal 8-hour workday and a 40-hour workweek. The present tendency in work at high altitudes is to work longer hours for a number of days and then commute to the nearest town for an extended rest period, keeping the average time at work within the legal limit, which in Chile is 48 hours per week.
Departures from the normal 8-hour working schedules make it necessary to examine the possible accumulation in the body of toxic substances due to the increase in exposure and reduction of detoxification times.
Chilean occupational health regulations have recently adopted the “Brief and Scala model’’ described by Paustenbach (1985) for reducing TLVs in the case of extended working hours. At altitude, the correction for barometric pressure should also be used. This usually results in very substantial reductions of permissible exposure limits.
In the case of cumulative hazards not subject to detoxifying mechanisms, such as silica, correction for extended working hours should be directly proportional to the actual hours worked in excess of the usual 2,000 hours per year.
Physical Hazards
Noise: The sound pressure level produced by noise of a given amplitude is in direct relation to air density, as is the amount of energy transmitted. This means that the reading obtained by a sound level meter and the effect on the inner ear are reduced in the same way, so no corrections would be required.
Accidents: Hypoxia has a pronounced influence on the central nervous system, reducing response time and disrupting vision. An increase in the incidence of accidents should be expected. Above 3,000 m, the performance of persons engaged in critical tasks will benefit from supplementary oxygen.
Precautionary Note: Air Sampling
Kenneth I. Berger and William N. Rom
The monitoring and maintenance of the occupational safety of workers requires special consideration for high altitude environments. High-altitude conditions can be expected to influence the accuracy of sampling and measuring instruments that have been calibrated for use at sea level. For example, active sampling devices rely on pumps to pull a volume of air onto a collection medium. Accurate measurement of the pump flow rate is essential in order to determine the exact volume of air drawn through the sampler and, therefore, the concentration of the contaminant. Flow calibrations are often performed at sea level. However, changes in air density with increasing altitude may alter the calibration, thereby invalidating subsequent measurements made in high altitude environments. Other factors that may influence the accuracy of sampling and measurement instruments at high altitude include changing temperature and relative humidity. An additional factor that should be considered when evaluating worker exposure to inhaled substances is the increased respiratory ventilation that occurs with acclimatization. Since ventilation is markedly increased after ascent to high altitude, workers may be exposed to excessive total doses of inhaled occupational contaminants, even though measured concentrations of the contaminant are below the threshold limit value.
" DISCLAIMER: The ILO does not take responsibility for content presented on this web portal that is presented in any language other than English, which is the language used for the initial production and peer-review of original content. Certain statistics have not been updated since the production of the 4th edition of the Encyclopaedia (1998)."