Background
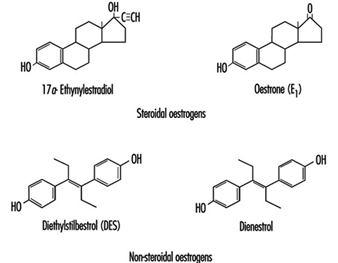
Oestrogens used in the pharmaceutical industry can generally be classified as natural or synthetic and as steroidal or non-steroidal. All steroidal oestrogens, both natural (e.g., oestrone) and synthetic (e.g., ethynyloestradiol and moestranol) have a typical multi-ring structure, as depicted in figure 6 Diethylstilboestrol (DES) and dienoestrol are examples of the non-steroidal oestrogens. The principal uses of oestrogenic compounds are in oral contraceptive tablets and tablets intended for oestrogen replacement therapy. The pure compounds (naturally derived or synthesized) are no longer manufactured in the United States, but are imported.
Figure 1. Examples of steroidal and non-steroidal oestrogen structure
Manufacturing processes
The following description is a generalized, and composite, description of the manufacturing process used in many US pharmaceutical companies. Specific product processes may not follow the flow exactly as described below; some steps may be absent in some processes, and, in other cases, additional steps may be present that are not described here.
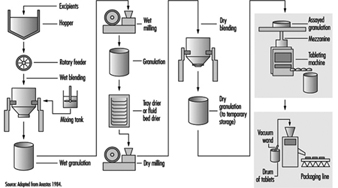
As with most dry-product drugs, pharmaceutical products made from oestrogenic compounds are manufactured in a step-wise batch operation (figure 2). The manufacturing steps begin with the assembly and pre-weighing of both active ingredients and excipients (inactive ingredients) in an isolated room under local exhaust ventilation. When needed, the ingredients are moved to a blending room equipped with mechanical blenders. Excipients are usually loaded dry from a hopper above the blender. The active ingredients are almost always dissolved first in an alcohol, and are added manually or are fed through tubing through the side of the blender. The initial blending of the ingredients is done in a wet state. At the end of the wet blending process, the granulation is typically moved to a wet mill, where particles in the mix are reduced to a specific size. The milled granulation is then dried using a fluid bed drier or is tray-dried in ovens designed for the purpose. The dried granulation may or may not undergo the addition of a lubricant before dry-blending and/or dry-milling, depending on the specific product and process. The final granulation, ready to be made into tablets, is then stored in sealed containers. The raw materials and granulation, and sometimes the intermediate products, are typically sampled and assayed by quality-control personnel prior to being moved to the next process step.
Figure 2. Typical oral contraceptive tablet manufacturing process flow
When needed, the granulation is moved to a compression room, where it is made into tablets by means of a tablet press. The granulation is typically fed from the storage container (typically a plastic-lined fibre drum or a lined stainless steel container) into the tablet press hopper by gravity or pneumatically by means of a vacuum wand. Formed tablets exit from the machine through tubing at the side, and drop into plastic-lined drums. When filled, the drums are sampled and inspected. After assay by quality-control personnel, the drums are sealed, stored and staged for packaging operations. Some tablets also undergo a coating process, in which layers of edible wax and sometimes sugars are used to seal the tablet.
The tablets are packaged by sealing them in blister packs or bottled, depending on the nature of the product. In this process, the containers of tablets are moved to the packaging area. The tablets may be manually scooped into the packaging machine hopper or fed by means of a vacuum wand. The tablets are then either immediately sealed between layers of aluminium foil and plastic film (blister-packaging) or they are bottled. The blister packs or bottles are then conveyed along a line on which they are inspected and placed in pouches or boxed with appropriate inserts.
Health effects on male and female pharmaceutical workers
Reports of occupational exposures and the effects on males have been relatively few, compared with the considerable literature that exists regarding acute and chronic effects of oestrogens in women as a result of non-occupational exposures. The non-occupational literature is primarily a result of widespread contraceptive and other medical uses of oestrogenic pharmaceuticals (but also environmental pollutants with oestrogenic properties, such as the organochlorines) and focuses particularly on the relationships between that exposure and a variety of human cancers, such as that of the endometrium, cervix and breast in women (Hoover 1980; Houghton and Ritter 1995). In the occupational literature, the hyperoestrogenic syndrome in both male and female workers has been associated with exposures to DES and its derivatives, natural or conjugated oestrogens, hexoestrol and its derivatives and steroidal synthetics such as ethynyloestradiol and moestranol. Shortly after the initiation of commercial production of oestrogens, reports began to surface of their effects, such as gynaecomastia (abnormal enlargement of the breasts in a male) and decreased libido among male workers, and menstrual disorders (increased flow or inter-menstrual spotting) among female workers (Scarff and Smith 1942; Fitzsimons 1944; Klavis 1953; Pagani 1953; Watrous 1947; Watrous and Olsen 1959; Pacynski et al. 1971; Burton and Shumnes 1973; Meyer, Peteet and Harrington 1978; Katzenellenbogen 1956; Dunn 1940; Stoppleman and van Valkenburg 1955; Goldzieher and Goldzieher 1949; Fisk 1950). There have also been a few reports of toxicity syndrome associated with some progoestogenic compounds, including acetoxyprogoesterone (Suciu et al. 1973), and vinyloestrenolone in combination with ethynyloestradiol (Gambini, Farine and Arbosti 1976).
A total of 181 cases of hyperoestrogenism in both males and females (occurring over the period 1940–1978) were recorded and reported by company physicians in 10 pharmaceutical companies (13 plant sites) in the United States (Zaebst, Tanaka and Haring 1980). The 13 plant sites included 9 sites manufacturing primarily oral contraceptives containing various synthetic oestrogens and progoestogens, one firm manufacturing oestrogen replacement pharmaceuticals from natural conjugated oestrogens and one firm manufacturing pharmaceuticals from DES (which had in earlier years also synthesized DES).
Investigators from the US National Institute for Occupational Safety and Health (NIOSH) conducted a pilot industrial hygiene and medical study in 1984 of male and female workers in two plants (Tanaka and Zaebst 1984). Measurable exposures were documented to both moestranol and natural conjugated oestrogens, both inside and outside the respiratory protective equipment used. However, no statistically significant changes in oestrogen-stimulated neurophysins (ESN), corticosteroid-binding globulins (CBG), testosterone, thyroid function, blood-clotting factors, liver function, glucose, blood lipids or gonadotropic hormones were noted in these workers. On physical examination, no adverse physical changes were noted in either male or female workers. However, in the plant using moestranol and norethindrone to manufacture oral contraceptive tablets, serum ethynyloestradiol levels appeared to show possible oestrogen exposure and absorption despite the use of respirators. Inside-respirator air samples obtained at this plant suggested less effective workplace protection factors than expected.
Hyperoestrogenic symptoms in males reported in these studies have included nipple sensitivity (manifested as tingling or tenderness of the nipple) or a feeling of pressure in the breast area and, in some cases, breast hyperplasia and gynaecomastia. Additional subjective symptoms reported by some of the male workers also included decreased libido and/or sexual potency. Findings in females included irregular menstruation, nausea, headaches, breast pain, leucorrhoea (thick, whitish discharge from the vagina or cervical canal) and ankle oedema. There have been no long-term follow-up studies in persons occupationally exposed to oestrogens or progoestogens.
Hazards and control of exposure
One of the most serious hazards in the manufacture of oestrogenic pharmaceuticals is inhalation (and to some extent oral ingestion) of the pure active oestrogenic compound during weighing, assembly and quality-assurance testing. However, substantial inhalation of the dry, blended dust (which contains a low percentage of active ingredient) may also occur to workers during granulation, compression and packaging operations. Skin absorption may also occur, particularly during the wet phases of granulation, since alcohol solutions are used. Quality-control and laboratory personnel are also at risk of exposure while sampling, assaying or otherwise handling pure oestrogenic substances, granulation or tablets. Maintenance personnel can be exposed while cleaning, repairing or inspecting mixers, hoppers, mills, vacuum lines and ventilation systems, or changing filters. NIOSH investigators have conducted an in-depth evaluation of engineering controls which have been used during the manufacturing of oral contraceptive tablets (Anastas 1984). This report provides a detailed review of controls and an evaluation of their effectiveness for granulation, milling, material transfers, powder and tablet feed equipment, and general and local exhaust ventilation systems.
The four main elements of hazard control employed in plants using oestrogenic pharmaceuticals are:
- Engineering controls. These include isolation of processing equipment rooms, control of air flow within a facility from least contaminated areas to most contaminated, local exhaust ventilation at any open transfer points, enclosure of machines, sealed process streams and enclosed powder feed systems. Frequently, implementation of engineering controls, such as general or local exhaust ventilation, is complicated by the fact that good manufacturing regulations (such as those required by the US Food and Drug Administration), which are designed to ensure a safe and effective product, conflict with the best health and safety practices. For example, pressure differentials achieved by general ventilation systems, designed to protect workers outside the hazardous process, conflict with the regulatory requirement to prevent contamination of the product by dust or contaminants external to the process. Because it eliminates direct contact between people and the hazardous contaminants, process or equipment containment is often the best option.
- Good work practices. These include separate clean and contaminated locker rooms separated by showers, changes of clothing, washing or showering before exiting contaminated areas and, where it is feasible and appropriate, systematic rotations of all workers between exposed and non-exposed areas. Appropriate training and education regarding the hazards of oestrogens, and good work practices, are an integral part of an effective worker protection programme. The best engineering controls and personal protective equipment can be defeated if the operators are not knowledgeable about the hazards and controls, and if they are not properly trained to take advantage of the controls and to use the personal protective equipment provided.
- Aggressive environmental and medical monitoring of exposed workers. In addition to normally administered physicals, routine screening should, at a minimum, include review for symptoms (breast tenderness, libido change and so on), examinations of the breast and axillary nodes and measurement of areolae. The screening frequency will vary, depending on the severity of the exposure hazard. Of course, medical screening and monitoring (e.g., physical exams, health questionnaires or testing of body fluids) should be implemented with the utmost sensitivity to workers’ overall welfare, their health and their privacy, since their cooperation and assistance in such a programme are critical to its success. Monitoring of worker exposures to the active oestrogenic or progoestogenic substances should be done regularly and should include not only breathing-zone sampling for air contaminants, but also evaluations of skin contamination and the effectiveness of personal protective equipment.
- Use of appropriate personal protective equipment: Personal protective equipment typically includes disposable or launderable coveralls; separate steroid-area shoes, socks, underclothing and rubber gloves; and effective respirators tailored to the degree of hazard. In the most hazardous areas, air-supplied respiratory protective equipment and impervious (to dusts and/or organic solvents) suits may be required.
Because of the potency of the oestrogenic substances, particularly the synthetic ones such as moestranol and ethynyloestradiol, all of these measures are needed to control exposures adequately. The use of personal protective equipment alone may not provide complete protection. Primary reliance should be placed on controlling exposures at the source, by process containment and by isolation.
Monitoring methods
Both high-performance liquid chromatography and radio-immunoassay procedures have been used to determine oestrogens or progoestogens in environmental samples. Serum samples have been analysed for the exogenous active compound, its metabolite (e.g., ethynyloestradiol is the main metabolite of moestranol), oestrogen-stimulated neurophysins or any of a number of other hormones (e.g., gonadotropic hormones and CBGs) considered appropriate for the specific process and hazard. Airborne monitoring usually includes breathing-zone personal monitoring, but area sampling can be useful in detecting departures from expected values over time. Personal monitoring has the advantages of detecting breakdowns or problems with processing equipment, personal protective equipment or ventilation systems and can provide an earlier warning of exposure. Biological monitoring, on the other hand, can detect exposures which may be missed by environmental monitoring (e.g., skin absorption or ingestion). In general, good practice combines both environmental and biological sampling to protect workers.