86. Woodworking
Chapter Editor: Jon Parish
Table of Contents
Tables and Figures
General Profile
Debra Osinsky
Woodworking Processes
Jon K. Parish
Routing Machines
Beat Wegmüller
Wood Planing Machines
Beat Wegmüller
Health Effects and Disease Patterns
Leon J. Warshaw
Tables
Click a link below to view table in article context.
1. Poisonous, allergenic & biologically active wood varieties
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.
General Profile
Traditionally, furniture factories have been located in Europe and North America. With the increased cost of labour in industrialized countries, more furniture production, which is labour intensive, has shifted to Far Eastern countries. It is likely that this movement will continue unless more automated equipment can be developed.
Most furniture manufacturers are small enterprises. For example, in the United States, approximately 86% of the factories in the wood furniture industry have fewer than 50 employees (EPA 1995); this is representative of the situation internationally.
The woodworking industry in the United States is responsible for manufacturing household, office, store, public building and restaurant furniture and fixtures. The woodworking industry falls under the US Bureau of the Census Standard Industrial Classification (SIC) Code 25 (equivalent to International SIC Code 33) and includes: wood household furniture, such as beds, tables, chairs and bookshelves; wood television and radio cabinets; wood office furniture, such as cabinets, chairs and desks; and wood office and store fixtures and partitions, such as bar fixtures, counters, lockers and shelves.
Because production lines for assembling furniture are costly, most manufacturers do not supply an exceptionally large range of items. Manufacturers may specialize in the product manufactured, the product group or the production process (EPA 1995).
Woodworking Processes
For the purposes of this article, the processes of the woodworking industry will be considered to start with the reception of converted timber from the sawmill and continue until the shipping of a finished wood article or product. Earlier stages in the handling of wood are dealt with in the chapters Forestry and Lumber industry.
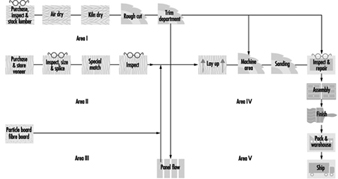
The woodworking industry produces furniture and a variety of building materials, ranging from plywood floors to shingles. This article covers the main stages in the processing of wood for the production of wooden products, which are machine working of natural wood or manufactured panels, assembly of machined parts and surface finishing (e.g., painting, staining, lacquering, veneering and so on). Figure 1 is a flow diagram for wood furniture manufacturing, which covers nearly the whole range of these processes.
Figure 1. Flow diagram for wood furniture manufacturing
Drying. Some furniture manufacturing facilities may purchase dried lumber, but others perform drying onsite using a drying kiln or oven, fired by a boiler. Usually wood waste is the fuel.
Machining. Once the lumber is dried, it is sawed and otherwise machined into the shape of the final furniture part, such as a table leg. In a normal plant, the wood stock moves from rough planer, to cutoff saw, to rip saw, to finish planer, to moulder, to lathe, to table saw, to band saw, to router, to shaper, to drill and mortiser, to carver and then to a variety of sanders.
Wood can be hand carved/worked with a variety of hand tools, including chisels, rasps, files, hand saws, sandpaper and the like.
In many instances, the design of furniture pieces requires bending of certain wooden parts. This occurs after the planing process, and usually involves the application of pressure in conjunction with a softening agent, such as water, and increased atmospheric pressure. After bending into the desired shape, the piece is dried to remove excess moisture.
Assembly. Wood furniture can either be finished and then assembled, or the reverse. Furniture made of irregularly shaped components is usually assembled and then finished.
The assembly process usually involves the use of adhesives (either synthetic or natural) in conjunction with other joining methods, such as nailing, followed by the application of veneers. Purchased veneers are trimmed to correct size and patterns, and bonded to purchased chipboard.
After assembly, the furniture part is examined to ensure a smooth surface for finishing.
Pre-finishing. After initial sanding, an even smoother surface is attained by spraying, sponging or dipping the furniture part with water to cause the wood fibres to swell and “raise”. After the surface has dried, a solution of glue or resin is applied and allowed to dry. The raised fibres are then sanded down to form a smooth surface.
If the wood contains rosin, which can interfere with the effectiveness of certain finishes, it may be derosinated by applying a mixture of acetone and ammonia. The wood is then bleached by spraying, sponging or dipping the wood into a bleaching agent such as hydrogen peroxide.
Surface finishing. Surface finishing may involve the use of a large variety of coatings. These coatings are applied after the product is assembled or in a flat line operation before assembly. Coatings could normally include fillers, stains, glazes, sealers, lacquers, paints, varnishes and other finishes. The coatings may be applied by spray, brush, pad, dip, roller or flow-coating machine.
Coatings can be either solvent based or water based. Paints may contain a wide variety of pigments, depending on the desired colour.
Hazards and Precautions
Machining safety
Woodworking manufacturing has many of the hazards to safety and health that are common to general industry, with a much larger proportion of extremely hazardous equipment and operations than most. Consequently, safety requires constant attention to safe work habits by employees, vigilant supervision, and maintenance of a safe work environment by employers.
Although in many instances woodworking machinery and equipment may be purchased without the necessary guards and other safety devices, it is management’s responsibility to provide adequate safeguards before such machinery and equipment is used. See also the articles “Routing machines” and “Wood planing machines”.
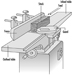
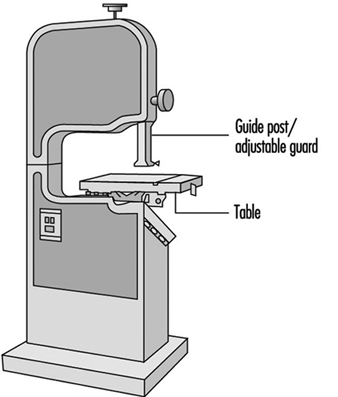
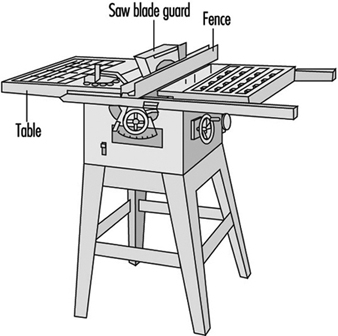
Sawing machines. Employees should be made aware of the safe operating practices necessary for the proper use of various woodworking saws (see figure 2 and figure 3).
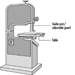
Figure 2. Band saw
Specific guidelines are as follows:
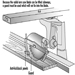
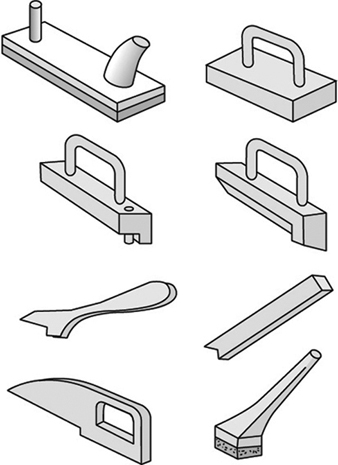
1. When feeding a table saw, hands must be kept out of the line of the cut. No guard can prevent a person’s hand from following the stock into the saw. When ripping with the fence gauge near the saw, a push stick or suitable jig must be used to complete the cut. See figure 4.
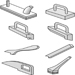
Figure 4. Push sticks
2. The saw blade must be positioned so as to minimize its protrusion above the stock; the lower the blade, the less chance for kickbacks. It is good practice to stand out of the line of the stock being ripped. A heavy leather apron or other guard for the abdomen is recommended.
3. Freehand sawing is always dangerous. The stock must always be held against a gauge or fence. See figure 3.
4. The saw must be appropriate for the job. For instance, it is an unsafe practice to rip with a table saw not equipped with a non-kickback device. Kickback aprons are recommended.
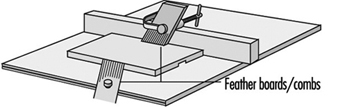
5. The dangerous practice of removing a hood guard because of narrow clearance on the gauge side can be avoided by clamping a filler board to the table between the gauge and the saw and using it to guide the stock. Employees must never be permitted to bypass guards. Combs, featherboards (see figure 5) or suitable jigs must be provided where standard guards cannot be used.
Figure 5. Featherboards & combs
6. Crosscutting long boards on a table saw should be avoided because the operator is required to use considerable hand pressure near the saw blade. Also, boards extending beyond the table may be struck by people or trucks. Long stock should be crosscut on a swing pull saw or radial arm saw with adequate supporting bench.
7. Work that should be done on special power-feed machines should not be done on general-purpose hand-fed machines.
8.To set a gauge of a table saw without taking off the guards, a permanent mark should designate the line of cut on the table top.
9. It is considered safe practice to bring equipment to a complete stop before adjusting blades or fences, and to disconnect the power source when changing blades.
10. A brush or stick should be used to clean sawdust and scrap from a saw.
A table saw is also called a variety saw because it can perform a wide variety of sawing functions. For this reason the operator should have a variety of guards, because no one guard can protect from every function. See figure 3.
Cutting machines. Cutting machines can also be hazardous if not adequately guarded and always used with respect and alertness. Cutting tools should be kept well sharpened and correctly balanced on their spindles.
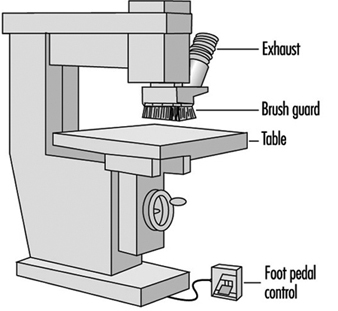
The router shown in figure 6 has a brush guard. Other routers may have a ring guard, a round guard that encircles the router bit. The purpose of guards is to keep the hands away from the cutting bit. Computer numerical controlled (CNC) routers may have several bits and are high production machines. On CNC machines the operator’s hands are kept further from the bit area. However, another problem is the high amount of wood dust. See also the article “Routing machines”.
Figure 6. Router
Guarding on a jointer or surface planing machine is mainly to keep the operator’s hands away from the revolving knives. The “mutton chop”-type guard allows only the portion of the knives which are cutting the stock to be exposed (see figure 7). The exposed portion of the knives behind the fence should also be guarded.
Figure 7. Jointer
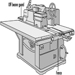
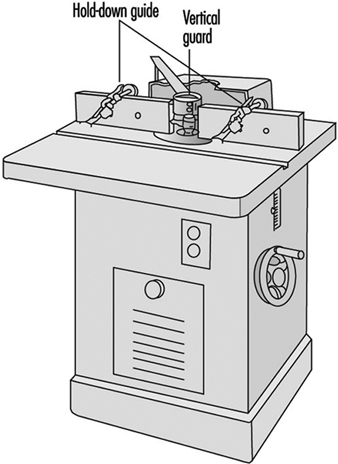
The shaper is a potentially very dangerous machine (see figure 8). If the shaper knives become separated from the above and below collars on the arbor, they can be thrown with great force. Also, stock must often be held close to the knives. This holding must be done with a fixture instead of by the operator’s hands. Featherboards can be used to hold the stock down against the table. Ring or saucer guards should be used whenever possible. A saucer guard is a round, flat, plastic disk that is mounted horizontally on the arbor above the shaper knives.
Figure 8. Shaper
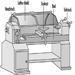
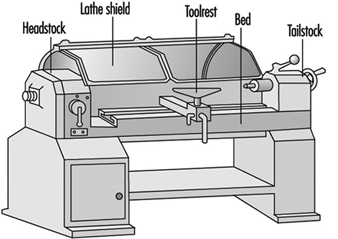
A lathe should be guarded by a hood guard because there is a danger of the stock being thrown from the machine. See figure 9. It is good practice for the hood to be interlocked with the motor so the lathe cannot be run unless the hood guard is in place.
Figure 9. Lathe
A ripsaw should have anti-kickback fingers installed to prevent the stock from reversing its direction and striking the operator. See figure 10. Also, the operator should wear a padded apron to lessen the impact if a kickback does occur.
Figure 10. Ripsaw
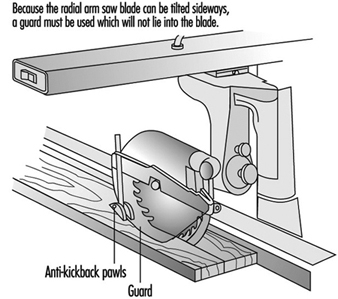
Because the radial arm saw blade can be tilted sideways, a guard must be used which will not lie into the blade. See figure 11.
Figure 11. Radial arm saw
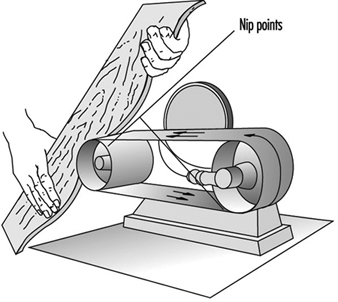
Sanding machines. Machined stock pieces are sanded down using belt, jitterbug, disc, drum or orbital sanders. Nip points are created in sanding belts. See figure 12. Often these nip points can be guarded with a hood which will also be part of a dust exhaust system.
Figure 12. Sander
Machine guarding. Figure 13 illustrates that the opening between a guard and the point of contact must be decreased as the distance decreases.
Figure 13. Distance between guard & point of operation
Miscellaneous machine safety concerns. Care must be taken that the use of stock-clamping/holding devices do not create additional hazards.
Most woodworking machines create the necessity of the operator (and helper) wearing eye protection.
It is common practice for employees to blow dust off of themselves with compressed air. They should be cautioned to keep air pressure below 30 psi and to avoid blowing into eyes or open cuts.
Wood dust hazards
Machines that produce wood dust should be equipped with dust-collecting systems. If the exhaust system is inadequate to dispose of the wood dust, the operator may need to wear a dust respirator. The International Agency for Research on Cancer (IARC) has now determined that “there is sufficient evidence in humans for the carcinogenicity of wood dust”, and that “Wood dust is carcinogenic to humans (Group 1)”. Other studies indicate that wood dust may prove an irritant to the mucous membranes of the eyes, nose and throat. Some toxic woods are more actively pathogenic and may produce allergic reactions and occasionally pulmonary disorders and systemic poisoning. See table 1.
Table 1. Poisonous, allergenic and biologically active wood varieties
|
Scientific names |
Selected commercial names |
Family |
Health Impairment |
|
Abies alba Mill (A. pectinata D.C.) |
Silver fir |
Pinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Acacia spp. |
Australian blackwood |
Mimosaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Acer spp. |
Maple |
Aceraceae |
Dermatitis |
|
Afrormosia elata Harms. |
Afrormosia, kokrodua, asamala, obang, oleo pardo, bohele, mohole |
Papilionaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Afzelia africana Smith |
Doussié, afzelia, aligua, apa, chanfuta, lingue merbau, intsia, hintsy |
Caesalpinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Agonandra brasiliensis Miers |
Pao, marfim, granadillo |
Olacaceae |
Dermatitis |
|
Ailanthus altissima Mill |
Chinese sumac |
Simaroubaceae |
Dermatitis |
|
Albizzia falcata Backer |
Iatandza |
Mimosaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; |
|
Alnus spp. |
Common alder |
Betulaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Amyris spp. |
Venezuelan or West Indian sandalwood |
Rutaceae |
Dermatitis; toxic effects |
|
Anacardium occidentale L. |
Cashew |
Anacardiaceae |
Dermatitis |
|
Andira araroba Aguiar. (Vataireopsis araroba Ducke) |
Red cabbage tree |
Papilionaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Aningeria spp. |
Aningeria |
Sapotaceae |
Conjunctivitis-rhinitis; asthma |
|
Apuleia molaris spruce (A. leiocarpa MacBride) |
Redwood |
Caesalpinaceae |
Dermatitis; toxic effects |
|
Araucaria angustifolia O. Ktze |
Parana pine, araucaria |
Araucariaceae |
Toxic effects |
|
Aspidosperma spp. |
Red peroba |
Apocynaceae |
Dermatitis; conjunctivitis- |
|
Astrocaryum spp. |
Palm |
Palmaceae |
Dermatitis; toxic effects |
|
Aucoumea klaineana Pierre |
Gabon mahogany |
Burseraceae |
Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis |
|
Autranella congolensis |
Mukulungu, autracon, elang, bouanga, kulungu |
Sapotaceae |
Dermatitis |
|
Bactris spp. (Astrocaryum spp.) |
Palm |
Palmaceae |
Dermatitis; toxic effects |
|
Balfourodendron riedelianum Engl. |
Guatambu, gutambu blanco |
Rutaceae |
Dermatitis |
|
Batesia floribunda Benth. |
Acapu rana |
Caesalpinaceae |
Toxic effects |
|
Berberis vulgaris L. |
Barberry |
Berberidaceae |
Toxic effects |
|
Betula spp. |
Birch |
Betulaceae |
Dermatitis |
|
Blepharocarva involucrigera F. Muell. |
Rosebutternut |
Anacardiaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Bombax brevicuspe Sprague |
Kondroti, alone |
Bombacaceae |
Dermatitis |
|
Bowdichia spp. |
Black sucupira |
Papilionaceae |
Dermatitis |
|
Brachylaena hutchinsii Hutch. |
Muhuhu |
Compositae |
Dermatitis |
|
Breonia spp. |
Molompangady |
Rubiaceae |
Dermatitis |
|
Brosimum spp. |
Snakewood, letterwood, tigerwood |
Moraceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Brya ebenus DC. (Amerimnum ebenus Sw.) |
Brown ebony, green ebony, Jamaican ebony, tropical American ebony |
Papilionaceae |
Dermatitis |
|
Buxus sempervirens L. |
European boxwood, East London b., Cape b. |
Buxaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Caesalpinia echinata Lam. (Guilandina echinata Spreng.) |
Brasilwood |
Caesalpinaceae |
Dermatitis; toxic effects |
|
Callitris columellaris F. Muell. |
White cypress pine |
Cupressaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Calophyllum spp. |
Santa maria, jacareuba, kurahura, galba |
Guttiferae |
Dermatitis; toxic effects |
|
Campsiandra laurifolia Benth. |
Acapu rana |
Caesalpinaceae |
Toxic effects |
|
Carpinus betulus |
Hornbeam |
Betulaceae |
Dermatitis |
|
Cassia siamea Lamk. |
Tagayasan, muong ten, djohar |
Caesalpinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Castanea dentata Borkh |
Chestnut, sweet chestnut |
Fagaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Castanospermum australe A. Cunn. |
Black bean, Australian or Moreton Bay chestnut |
Papilionaceae |
Dermatitis |
|
Cedrela spp. (Toona spp.) |
Red cedar, Australian cedar |
Meliaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Cedrus deodara (Roxb. ex. Lamb.) G. Don |
Deodar |
Pinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Celtis brieyi De Wild. |
Diania |
Ulmaceae |
Dermatitis |
|
Chlorophora excelsa Benth. and Hook I. |
Iroko, gelbholz, yellowood, kambala, mvule, odum, moule, African teak, abang, tatajuba, fustic, mora |
Moraceae |
Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis |
|
Chloroxylon spp. |
Ceylon satinwood |
Rutaceae |
Dermatitis; toxic effects |
|
Chrysophyllum spp. |
Najara |
Sapotaceae |
Dermatitis |
|
Cinnamomum camphora Nees and Ebeim |
Asian camphorwood, cinnamon |
Lauraceae |
Toxic effects |
|
Cryptocarya pleurosperma White and Francis |
Poison walnut |
Lauraceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Dacrycarpus dacryoides (A. Rich.) de Laub. |
New Zealand white pine |
Podocarpaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Dacrydium cupressinum Soland |
Sempilor, rimu |
Podocarpaceae |
conjunctivitis-rhinitis; asthma |
|
Dactylocladus stenostachys Oliv. |
Jong kong, merebong, medang tabak |
Melastomaceae |
Toxic effects |
|
Dalbergia spp. |
Ebony |
Papilionaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; |
|
Dialium spp. |
Eyoum, eyum |
Caesalpinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Diospyros spp. |
Ebony, African ebony |
Ebenaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Dipterocarpus spp. |
Keruing, gurjum, yang, keruing |
Dipterocarpaceae |
Dermatitis |
|
Distemonanthus benthamianus Baill. |
Movingui, ayan, anyaran, Nigerian satinwood |
Caesalpinaceae |
Dermatitis |
|
Dysoxylum spp. |
Mahogany, stavewood, red bean |
Meliaceae |
dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
D. muelleri Benth. |
Rose mahogany |
||
|
Echirospermum balthazarii Fr. All. (Plathymenia reticulata Benth.) |
Vinhatico |
Mimosaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Entandophragma spp. |
Tiama |
Meliaceae |
Dermatitis; |
|
Erythrophloeum guineense G. Don |
Tali, missanda, eloun, massanda, sasswood, erun, redwater tree |
Caesalpinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Esenbeckia leiocarpa Engl. |
Guaranta |
Rutaceae |
Dermatitis |
|
Eucalyptus spp. |
|
Myrtaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Euxylophora paraensis Hub. |
Boxwood |
Rutaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Excoecaria africana M. Arg. (Spirostachys africana Sand) |
African sandalwood, tabootie, geor, aloewood, blind-your-eye |
Euphorbiaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Fagara spp. |
Yellow sanders, West Indian satinwood, atlaswood, olon, bongo, mbanza |
Rutaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Fagus spp. (Nothofagus spp.) |
Beech |
Fagaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Fitzroya cupressoides (Molina) Johnston |
Alerce |
Cupressaceae |
Dermatitis |
|
Flindersia australis R. Br. |
Australian teak, Queensland maple, maple |
Rutaceae |
Dermatitis |
|
Fraxinus spp. |
Ash |
Oleaceae |
Dermatitis |
|
Gluta spp. |
Rengas, gluta |
Anacardiaceae |
Dermatitis; toxic effects |
|
Gonioma kamassi E. Mey. |
Knysna boxwood, kamassi |
Apocynaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Gonystylus bancanus Baill. |
Ramin, melawis, akenia |
Gonystylaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis |
|
Gossweilerodendron balsamiferum (Verm.) Harms. |
Nigerian cedar |
Caesalpinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Grevillea robusta A. Cunn. |
Silky oak |
Proteaceae |
Dermatitis |
|
Guaiacum officinale L. |
Gaiac, lignum vitae |
Zygophyllaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Guarea spp. |
Bossé |
Meliaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Halfordia scleroxyla F. Muell. |
Saffron-heart |
Polygonaceae |
Dermatitis; allergic extrinsic alveolitis |
|
Hernandia spp. |
Mirobolan, topolite |
Hernandiaceae |
Dermatitis |
|
Hippomane mancinella L. |
Beach apple |
Euphorbiaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Illipe latifolia F. Muell. |
Moak, edel teak |
Sapotaceae |
Dermatitis |
|
Jacaranda spp. |
Jacaranda |
Bignoniaceae |
Dermatitis |
|
Juglans spp. |
Walnut |
Juglandaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Juniperus sabina L. |
|
Cupressaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Khaya antotheca C. DC. |
Ogwango, African mahogany, krala |
Meliaceae |
Dermatitis; allergic extrinsic alveolitis |
|
Laburnum anagyroides Medic. (Cytisus laburnum L.) |
Laburnum |
Papilionaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Larix spp. |
Larch |
Pinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Liquidambar styracifolia L. |
Amberbaum, satin-nussbaum |
Hamamelidaceae |
Dermatitis |
|
Liriodendron tulipifera L. |
American whitewood, tulip tree |
Magnoliaceae |
Dermatitis |
|
Lovoa trichilioides Harms. (L. klaineana Pierre) |
Dibetou, African walnut, apopo, tigerwood, side |
Meliaceae |
dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Lucuma spp. (Pouteria spp.) |
Guapeva, abiurana |
Sapotaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Maba ebenus Wight. |
Makassar-ebenholz |
Ebenaceae |
Dermatitis |
|
Machaerium pedicellatum Vog. |
Kingswood |
Papilionaceae |
Dermatitis |
|
Mansonia altissima A. Chev. |
Nigerian walnut |
Sterculiaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Melanoxylon brauna Schott |
Brauna, grauna |
Caesalpinaceae |
Dermatitis |
|
Microberlinia brazzavillensis A. Chev. |
African zebrawood |
Caesalpinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Millettia laurentii De Wild. |
Wenge |
Papilionaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; |
|
Mimusops spp. (Manilkara spp.) |
Muirapiranga |
Sapotaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; |
|
Mitragyna ciliata Aubr. and Pell. |
Vuku, African poplar |
Rubiaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; |
|
Nauclea diderrichii Merrill (Sarcocephalus diderrichii De Wild.) |
Bilinga, opepe, kussia, badi, West African boxwood |
Rubiaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Nesogordonia papaverifera R. Capuron |
Kotibé, danta, epro, otutu, ovové, aborbora |
Tiliaceae |
Toxic effects |
|
Ocotea spp. |
Stinkwood |
Lauraceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Paratecoma spp. |
|
Bignoniaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Parinarium spp. |
|
Rosaceae |
Dermatitis |
|
Peltogyne spp. |
Blue wood, purpleheart |
Caesalpinaceae |
Toxic effects |
|
Phyllanthus ferdinandi F.v.M. |
Lignum vitae, chow way, tow war |
Euphorbiaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Picea spp. |
European spruce, whitewood |
Pinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis |
|
Pinus spp. |
Pine |
Pinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Piptadenia africana Hook f. |
Dabema, dahoma, ekhimi |
Mimosaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Platanus spp. |
Plane |
Platanaceae |
Dermatitis |
|
Pometia spp. |
Taun |
Sapindaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Populus spp. |
Poplar |
Salicaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Prosopis juliflora D.C. |
Cashaw |
Mimosaceae |
Dermatitis |
|
Prunus spp. |
Cherry |
Rosaceae |
dermatitis; conjunctivitis-rhinitis; asthma |
|
Pseudomorus brunoniana Bureau |
White handlewood |
Moraceae |
Dermatitis; toxic effects |
|
Pseudotsuga douglasii Carr. (P. menziesii Franco) |
Douglas fir, red fir, Douglas spruce |
Pinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Pterocarpus spp. |
African padauk, New Guinea rosewood, red sandalwood, red sanders, quassia wood |
Papilionaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Pycnanthus angolensis Warb. (P. kombo Warb.) |
Ilomba |
Myristicaceae |
Toxic effects |
|
Quercus spp. |
Oak |
Fagaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Raputia alba Engl. |
Arapoca branca, arapoca |
Rutaceae |
Dermatitis |
|
Rauwolfia pentaphylla Stapf. O. |
Peroba |
Apocynaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Sandoricum spp. |
Sentul, katon, kra-ton, ketjapi, thitto |
Meliaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Schinopsis lorentzii Engl. |
Quebracho colorado, red q., San Juan, pau mulato |
Anacardiaceae |
Dermatitis; toxic effects |
|
Semercarpus australiensis Engl. |
Marking nut |
Anacardiaceae |
Dermatitis; toxic effects |
|
Sequoia sempervirens Endl. |
Sequoia, California |
Taxodiaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Shorea spp. |
Alan, almon, red balau |
Dipterocarpaceae |
Dermatitis |
|
S. assamica Dyer |
Yellow lauan, white meranti |
||
|
Staudtia stipitata Warb. (S. gabonensis Warb.) |
Niové |
Myristicaceae |
Dermatitis |
|
Swietenia spp. |
Mahogany, Honduras mahogany, Tabasco m., baywood, American mahogany, |
Meliaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis; toxic effects |
|
Swintonia spicifera Hook. |
Merpauh |
Anacardiaceae |
Dermatitis |
|
Tabebuia spp. |
Araguan, ipé preto, lapacho |
Bignoniaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Taxus baccata L. |
Yew |
Taxaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis; toxic effects |
|
Tecoma spp. |
Green heart |
Bignoniaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Tectona grandis L. |
Teak, djati, kyun, teck |
Verbenaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis |
|
Terminalia alata Roth. |
Indian laurel |
Combretaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Thuja occidentalis L. |
White cedar |
Cupressaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Tieghemella africana A. Chev. (Dumoria spp.) |
Makoré, douka, okola, ukola, makoré, abacu, baku, African cherry |
Sapotaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Triplochiton scleroxylon K. Schum |
Obeche, samba, wawa, abachi, African whitewood, arere |
Sterculiaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Tsuga heterophylla Sarg. |
Tsuga, Western hemlock |
Pinaceae |
Dermatitis |
|
Turraeanthus africana Pell. |
Avodiré |
Meliaceae |
Dermatitis; allergic extrinsic alveolitis |
|
Ulmus spp. |
Elm |
Ulmaceae |
Dermatitis |
|
Vitex ciliata Pell. |
Verbenaceae |
Dermatitis |
|
|
V. congolensis De Wild. and Th. Dur |
Difundu |
||
|
V. pachyphylla Bak. |
Evino |
||
|
Xylia dolabriformis Benth. |
Mimosaceae |
Conjunctivitis-rhinitis; |
|
|
X. xylocarpa Taub. |
Pyinkado |
asthma |
|
|
Zollernia paraensis Huber |
Santo wood |
Caesalpinaceae |
Dermatitis; toxic effects |
Source: Istituto del Legno, Florence, Italy.
Increased use of high-production CNC machinery such as routers, tenoners and lathes creates more wood dust and will require new dust-collection technology.
Dust control. Most dust in a woodworking production shop is removed by local exhaust systems. However, often there is a considerable accumulation of very fine dust that has settled on rafters and other structural members, especially in areas where sanding is done. This is a hazardous situation, with great potential for fire and explosion. A flash fire over dust-covered surfaces may be followed by explosions of increasing force. In order to minimize this probability, it would be wise to use a checklist. See sample checklist in box.
Assembly hazards
A wide range of adhesives is used in the bonding of veneers to manufactured panels, depending on the characteristics required of the final product. Apart from casein glue, natural adhesives are less widely employed and the greatest use is made of synthetic adhesives such as urea-formaldehyde. Synthetic adhesives may pose a hazard of skin disease or systemic intoxication, especially those which release free formaldehyde or organic solvents into the atmosphere. Adhesives should be handled in well ventilated premises and sources of vapour emission should be equipped with exhaust ventilation. Employees should be provided with gloves, protective creams, respirators and eye protection when necessary.
The moving parts, especially blades, of veneer slicing, jointing and clipping machines should be fully guarded. Two-hand controls may be necessary.
Finishing hazards
Surface finishing. Solvents used for carrying the sprayed pigments or for thinning can include a wide variety of volatile organic compounds which may reach toxic and explosive concentrations in the air. In addition, many pigments are toxic by inhalation of spray mist (e.g., lead, manganese and cadmium pigments). Wherever dangerous concentrations of vapour or mist can occur, use exhaust ventilation (e.g., spray painting in a booth) or use water sprays. All sources of ignition, including fires, electrical equipment and static electricity, should be eliminated before any operations begin.
An active hazardous material communication programme should be in place to alert employees to all hazards created by toxic, reactive, corrosive and/or ignitable finish, glue and solvent chemicals and the protective measures that should be taken. Eating in the presence of these chemicals should be prohibited. Proper storage of flammables and proper disposal of soiled rags and steel wool which could cause spontaneous ignition are imperative.
Fire prevention. In view of the highly flammable nature of wood (especially in the form of dust and shavings) and of the other items found in a woodworking plant (such as solvents, glues and coatings), the importance of fire prevention measures cannot be overemphasized. Measures include:
- installing automatic wood-dust and shaving collection equipment on saws, planers, moulders and so on, which transport the waste to storage silos pending disposal or recovery
- prohibiting smoking at the workplace and eliminating all sources of ignition (e.g., open flames)
- ensuring regular clean-up procedures of deposited dust and shavings
- adequate maintenance of machines to prevent occurrences such as the overheating of bearings
- installation of fire barriers, sprinkler systems, fire extinguishers, fire hoses and a crew trained to use this equipment
- proper storage of flammables
- explosion-proof electrical equipment where needed.
Environmental and Public Health Concerns
The production of finished products from wood can be done without long-range environmental damage. The harvesting of trees can be done in such a manner that new growth can replace what is cut. Major deforestation such as has been the case in rain forests can be discouraged. Waste products from the machining of wood (i.e., sawdust, wood chips) can be used in chipcore or as fuel.
While there are solid waste and process wastewater implications for the woodworking industry, the major concerns are the air emissions resulting from the use of waste wood as fuel and from solvent-intensive finishing operations. Wood-fired boilers are commonly used in drying operations, while many of the finishing materials are applied by spray. In both instances, engineering controls are required to reduce air-borne particulates and recover and/or incinerate the volatile compounds.
Controls should result in operators being exposed to less toxic chemicals as less hazardous substitutes are found. Use of water-based finishes instead of solvent-based will decrease fire hazards.
Routing Machines
Stationary routing machines are used in general for the manufacture of wood articles and furniture elements, but sometimes also for machining plastics and light alloys. Important types of routing machines are copy routers, pattern millers, machines with mobile router heads and automatic copying machines. The automatic copying machines are generally used for machining several workpieces simultaneously.
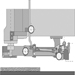
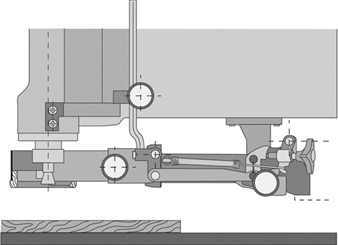
A common feature of all routing machines is that the tool is located above the workpiece support, which is normally a table. The tool-spindle axis is nearly always vertical, but on some machines the router head, and thus also the tool-spindle axis, may be tilted. The machining head is lowered for machining and returns automatically to its initial (rest) position. On older machines the machining head is lowered manually by operating a mechanical foot-pedal or hand lever. On modern machines the head is generally lowered by a pneumatic or hydraulic system. Figure 1 shows various accessories (hold-down shoes, guides and so on) and the Swiss National Accident Insurance Organization (SUVA) safety guard.
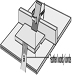
Figure 1. SUVA safety device with routing tool in working position
The tool-spindle is driven either by a belt drive or directly by a high-frequency motor, which is often of the two-speed type. The tool-spindle speeds generally range from 6,000 to 24,000 rpm. They are lower in pattern millers, where the lowest speed may be 250 rpm. Pattern millers are often equipped with a gearbox for the selection of different speeds.
The cutting diameter of the routing tool varies from 3 to 50 mm. However, on special pattern millers the cutting diameter of the tool may be as large as 300 mm.
Tooling
On routing machines single-edged spoon bits, double-edged panel cutters or solid-shaped cutters mainly are used. Like any tool they must be designed and made of such materials that will withstand the forces and loads to be expected during operation. Machines should be used and maintained in compliance with the manufacturer’s instructions.
The routing tools should be:
- clearly and permanently marked with the permissible speed, e.g., less than 20,000 rpm
- of a type tested by an approved body
- of round form design with a minimal radial cutting edge projection to reduce the danger of kickback.
Guarding of the tool
On routing machines where the tool is moving and the workpiece remains fixed, access to the rotating tool should be prevented by an adjustable guard (hand protector). It should be supplemented by a movable guard which can be lowered on to the workpiece surface. The lower end of this movable guard may be a brush.
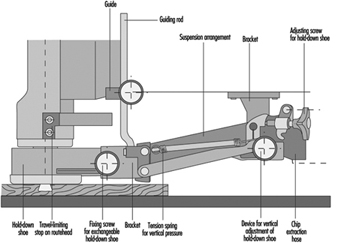
On routing machines where the workpiece is held and/or fed by hand, it is highly recommended to use a safety device exerting vertical pressure on the workpiece. The SUVA has designed such a guard. This safety device has been successfully used since the end of the 1940s and is still the most complete guard of its kind. Its main features are:
- prevention of unintentional contact with the rotating tool both in its rest position and in its working position
- fast and easy changeover from one size of hold-down shoe to another. Several sizes of hold-down shoes are available to fit tools with different cutting diameters
- manually adjustable amount of pressure exerted on the workpiece by the hold-down shoe
- automatic return of the hold-down shoe to its upper position when the router head rises to its rest position. The guarding device can be adjusted so that the hold-down shoe may be lifted only when the lower edge of the tool is above the level of the lower edge of the hold-down shoe; this is to prevent any unintentional contact with the tool from below (see figure 2. Serious injuries to the back of the hand, where the tendons are protected only by the skin, are thus avoided.
Figure 2. Safety device with routing tool in initial position
- possibility of connection of a chip extraction system to the guarding device.
This guarding device also enables workpieces to be routed along a guide with the aid of a horizontal pressure pad.
Hazards
Routing machines have been found to be less dangerous than vertical spindle moulding machines. One reason for this is the smaller diameter of most routing tools. However, the tools on routing machines are easily accessible and thus present a constant hazard for the hands and arms of the operator. Therefore, copy routers, where the workpiece is generally fed by hand, are by far the most dangerous routing machines.
Causes of accidents
The main causes of router accidents are:
- unintentional contact of the hand or arm with the rotating tool in its rest position (1) when removing chips and dust from the table by hand rather than using a wooden stick, (2) when the workpiece or the jig is not correctly handled or (3) when the sleeve of the operator’s clothing becomes entangled in the rotating tool
- unintentional contact of the hand with the routing tool as a result of kickback of the workpiece held by hand.
Kickback may happen because of:
- an unsafe working practice
- faults in the workpiece (knots, etc.)
- workpieces being fed into the tool too abruptly or from the wrong direction
- blunt cutter edges
- inadequate cutting speed
- incorrect fixing of the workpiece to the jig
- workpiece breakage
- ejection of the tool or parts of the tool due to bad tool design, excessive hardness of the tool material, faults in the material of the tool, overspeed of the tool or poor clamping of the tool in the tool holder.
In the event of ejection of a tool or workpiece, not only the operator but also other persons working in the area may be injured by ejected parts.
Measures to prevent accidents
Measures to prevent accidents should be directed at:
- the design and construction of the machine
- the tooling
- guarding the tool in its rest position (figure 15) and as far as possible in the working position (figure 14), in particular when the workpiece is held and fed by hand.
Design and Construction of the Machine
Routing machines must be designed to be safe to operate. It should be ensured that:
- the machine is sufficiently rigid
- the electrical equipment conforms to safety regulations
- actuators used to initiate a start function or movement of a machine element are constructed and mounted so as to minimize inadvertent operation
- access to moving machine parts, such as belt drives, hydraulic or pneumatically moved router heads or travelling tables on machines with automatic feeds, are prevented by adequate guarding
- the actual revolutions per minute of the tool are clearly visible to the operator
- the safety devices and chip extraction systems are easy to install
- the noise level of the machine is reduced as far as possible.
Furthermore, it is advisable to equip the tool drive of the routing machine with an automatic brake that activates when the machine is stopped. The braking time should not exceed 10 seconds.
Sample checklist
Housekeeping
1. A daily housekeeping programme is essential.
2. Dust accumulations of 1/8” depth in any area indicate a need for cleaning. It should be noted that any accumulation of dust may lead to a fire. The finer the dust, the greater the hazards.
3. Clean wood dust frequently.
a. Wipe down daily around hot surfaces.
b. Major blow down or vacuum when possible of all areas, including rafters, at least twice per year.
c. When concentrations are high, work small areas at a time.
d. Low humidity increases the potential for hazards and should be taken in consideration during blow downs.
4. Schedule blow downs or clean ups while equipment is down, such as Friday afternoons and weekends.
Electrical maintenance
1. Inspect/clean all motors regularly to avoid dust build-up.
2. Ensure all electrical boxes and panels meet the National Electrical Code requirements for their classified location.
3. Listen for unusual sounds, note unusual smells and watch for visual dust accumulations on machines and motors. Check motors and other electricals often to detect overheating.
4. Ensure that maintenance or operating personnel are lubricating bearings to motors, conveyors, chains and sprockets on a timely basis.
5. Ensure that electrical panels and boxes are kept closed and maintained to prevent dust accumulations, including keeping all knockout holes plugged.
Fire prevention
1. Actively prohibit smoking in unauthorized locations.
2. Adopt procedures for hot-work permits and ensure that procedures are followed.
3. Do not allow operator-controlled machines to operate unattended.
4. Install a device at the mouth of the dust collecting system to prevent sanding belts and other spark producing items from entering the system and causing a fire.
5. Trap metal in wood hogs by installing magnets in the conveyor system and metal detectors in the hog. Policies and procedures should be implemented to prevent metal and other foreign objects from reaching the hogs.
6. Conduct weekly and monthly inspections of fire protective systems including fire extinguishers, fire hoses, alarms and sprinkler control valves.
7. Ensure that boiler rooms and heating equipment are free of dust accumulations, that written boiler start-up procedures are being followed and that properly classified equipment is used.
8. Recognize the correct procedure in fighting dust fires.
9. Request a detailed inspection by the local fire marshal or insurance carrier.
10. Encourage mock drills/visits by the local fire department.
11. Install spark detection and extinguishing systems in dust collection systems and check periodically to ensure that they are working.
12. Review evacuation plans, emergency lighting, fire drills periodically for each work shift.
Miscellaneous
1. Contact insurance carrier for assistance in hazard identifications associated with safety, health and fire prevention.
2. Contact appropriate government safety agencies for additional assistance.
3. Employees should enter dust silos only when confined space procedures are followed.
4. All operators should ensure that dust collecting systems are working properly and report any malfunctions to management immediately.
5. Check for objects obstructing the ducts to the dust system.
6. It is recommended that all supervisors, safety committee members and other employees be made aware of the contents of this voluntary checklist to achieve maximum implementation.
Wood Planning Machines
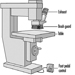
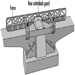
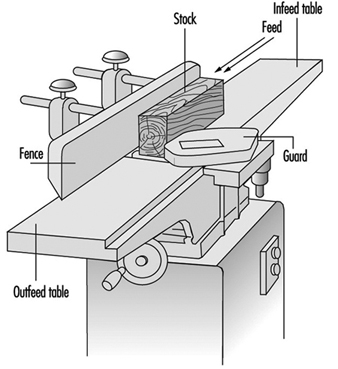
The development of stationary planing machines can be traced back to the beginning of the 19th century. On the first machines of this type, the workpiece was clamped to a carriage and fed below a horizontal shaft fitted with blades extending over the full working width. In 1850 a planing machine was built in Germany on which the workpiece was fed over a cutterblock located between two tables used to position and to support the workpiece. Apart from technical improvements this basic design has been maintained to this day. Such a machine is called a surface planing machine or a jointer (see figure 1).
Figure 1. Jointer
More recently, machines were designed to plane the upper surface of a workpiece to a predetermined thickness by means of a horizontally rotating cutterblock. The distance between the cutting circle diameter and the surface of the table supporting the workpiece is adjustable. Such machines are called one-side-thickness planing machines.
These two basic machine types were eventually combined into a machine which could be used for both surface and thickness planing. This development ended in planing machines for two-, three- and four-sided working in one pass.
From the point of view of occupational safety and health, it is strongly recommended that measures be taken for the extraction of wood dust and chips from the planing machine (e.g., by connecting the planing machine to a dust extraction system). Dust originating from hardwood (oak, beech) and tropical wood is considered a particular health hazard and must be extracted. Measures to reduce the noise level of planing machines should also be taken. An automatic brake for the cutterblock is compulsory in many countries.
Surface Planing Machines
A surface planing machine has rigid main frame that supports the infeed and the outfeed table. The cutterblock is located between the two tables and mounted on ball bearings. The main frame should be ergonomically designed (i.e., it should enable the operator to work comfortably).
Hand-operated control devices should be installed in such a way that the operator is not placed in a hazardous situation when operating them, and the possibility of inadvertent operation should be minimized.
The side of the main frame facing the operator’s position must be free of projecting parts such as handwheels, levers and so on. The table to the left of the cutterblock (outfeed table) is normally set at the same height as the cutting circle of the cutterblock. The table to the right of the cutterblock (infeed table) is set lower than the outfeed table to obtain the desired depth of cut. Contact between the table lips and the cutterblock should not be possible over the full setting range of the tables. However, the clearance between the table lips and the cutting circle of the cutterblock shall be as small as possible to provide for good support of the workpiece to be planed.
The major operations on a surface planing machine are flatting and edging. The position of the hands on the workpiece is important from an operational and safety viewpoint. When flatting, the workpiece should be fed with one hand, with the other hand holding it down initially on the infeed table. As soon as there is a sufficient portion of timber on the outfeed table, the latter hand can pass safely over the bridge-guard to apply pressure on the outfeed table and will be followed by the feeding hand to complete the feeding operation. When edging, the hands should not pass over the cutterblock while in contact with the timber. Their prime function is to exert horizontal pressure on the workpiece to maintain it square to the fence.
The noise produced by the rotating cutterblock often may exceed the level considered harmful to the ear. Measures to reduce the noise level are therefore necessary. Some of the noise reduction measures which have proved successful on surface planers are the following:
- use of a “quiet” cutterblock (e.g., a round form with minimum blade projection, helical blade instead of straight blade, segmental rotating tools with offset cutting)
- slotted or drilled table lips (the configuration and the dimensions of the apertures in the table lips must be selected so that no accident hazards arise; e.g., slots shall be no more than 6 mm wide and the diameter of the holes shall not exceed 6 mm)
- aerodynamic design of the chip deflectors below the table lips
- reduction of the cutterblock speed to below 1,000 rpm, provided the surface quality of the workpiece is still satisfactory.
Noise reduction up to 12 dBA when idling and 10 dBA under load can be achieved.
Cutterblocks should have a circular cross-section, and the chip clearance grooves and slots should be as small as possible. The blades and inserts shall be properly secured, preferably by form lock fixing.
The cutterblock rotates generally at speeds between 4,500 and 6,000 rpm. The diameters of conventional cutterblocks vary from 56 to 160 mm, and their lengths (working widths) from 200 to 900 mm. By analogy with the kinematics of conventional milling, the surface of the workpiece planed with a cutterblock is composed of cycloid arcs. The surface quality of the work therefore depends on the speed and diameter of the cutterblock, the number of cutting blades and the feed rate of the workpiece.
Equipping surface planing machines with an automatic brake for the cutterblock is recommended. The brake should be activated when the machine is stopped, and the braking time should not exceed 10 seconds.
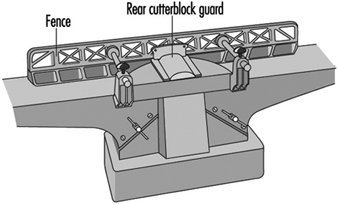
Access to the cutterblock at the rear of the fence should be prevented by a guard attached either to the fence or the fence support. The cutterblock in front of the fence should be guarded by an adjustable bridge-type guard fixed to the machine (e.g., to the main frame on the outfeed table side) (see figure 2). Access to the transmission elements should be prevented by a fixed guard.
Figure 2. Fence & rear cutterblock guard
Hazards
As the cutterblock rotates opposite to the direction in which the workpiece is fed, the hazard of kickback exists. If the workpiece is ejected, the operator’s hand or fingers may come in contact with the rotating cutterblock unless adequate guarding has been provided. It also frequently happens that the hand comes in contact with the cutterblock while feeding the workpiece with stretched fingers instead of pushing it forward with closed fist. Cutting blades not properly secured may be ejected by centrifugal force and may cause severe injury and/or material damage.
Guarding systems for surface planing machines
In many countries legislation covering the use of surface planing machines requires that the cutterblock be covered by an adjustable guarding system in order to prevent accidental contact of the operator’s hand with the rotating cutterblock.
In 1938, the SUVA introduced a planer guard which efficiently met all practical requirements. Over the years this guard has proved useful not only as a guarding system but also as an aid for most operations. It is well accepted by the woodworking trade in Switzerland, and almost all industrial surface planing machines are equipped with it. The design features of this guard have been introduced into the draft European standard for surface planing machines. The main features of this guard are the following:
- strong and rigid
- not easily deflected to expose cutterblock
- always stays parallel to the cutterblock axis regardless of its horizontal or vertical adjustment
- easily adjustable horizontally and vertically without the use of a tool.
However, accidents still happen. These accidents are mainly caused by failure to adjust the guard properly. Therefore, SUVA engineers have developed a bridge-type guard which covers the cutterblock in front of the fence automatically, and constantly exerts a defined pressure against the workpiece or the fence. This guard has been available since 1992.
The main design features of this new guard, called “Suvamatic”, are the following:
- complete guarding of the cutterblock. The full planing width is safeguarded by one single bridge-type guard. It can be folded down using a hinged locking system. This prevents the guard from projecting too far over the face of the machine.
- practical workpiece guiding system. The workpiece guiding system consists of a pressure pad and a guide for the workpiece. Both are fitted to the tip of the guard. The latter can be tilted to guide the workpiece both for flatting and edging.
- pressure application to assist work. For edging, the guard exerts pressure in direction of the fence. After edging, it automatically covers the full length of the cutterblock in front of the fence.
- automatic lifting and lowering of the guard. For flatting, the guard is lifted by the workpiece guide. After flatting, it lowers itself automatically to cover the cutterblock.
- guard can be locked in position for batch jobs. For batch jobs, the guard can be locked in vertical position to just accommodate the thickness of the workpiece. The guard will return automatically to this preset position after being pressed down.
- will fit all machines. The guard can be fitted to all surface planing machines and combined surface and thickness planing machines.
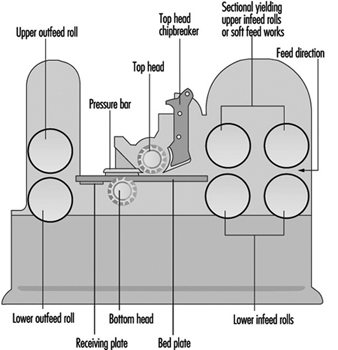
One-Side Thickness Planing Machines
The main frame of a one-side thickness planing machine houses the cutterblock, thickness planing table and feed elements.
Once the workpiece has been flattened and edged on a surface planing machine, it is planed to the desired thickness on the thickness planing machine. Unlike that of a surface planing machine, the cutterblock of a thickness planing machine is located above the planing table and the workpiece is no longer fed by hand but mechanically by feed rollers. The feed rollers are driven either by a separate motor (approximately 1 kW) or via a speed-reduction gearbox receiving its power from the cutterblock motor. With a separate drive the feed rate remains constant, but if the power is transmitted from the cutterblock motor the feed rate varies according to the cutterblock speed. Feed rates between 4 and 35 m/min are common.
Two spring-mounted feed rollers rest on the upper surface of the workpiece. The feed roller in front of the cutterblock is grooved for better grip on the workpiece; the feed roller at the outfeed end of the cutterblock is smooth. An infeed and an outfeed pressure bar located next to the cutterblock press the workpiece down onto the table, thereby ensuring a clean and even cut. The design and arrangement of the feed rollers and pressure bars should be such that contact with the rotating cutterblock is impossible.
Sectional feed rollers and pressure bars allow for the simultaneous working of two or more workpieces of slightly different thickness. From the point of view of accident prevention, sectional feed rollers and pressure bars are essential. The width of the individual feed roller or pressure bar section should not exceed 50 mm.
Two idle rollers are arranged in the table. They are designed to facilitate the passage of the workpiece over the table.
The surface of the table must be a plane free from slots or holes. Accidents involving an operator’s fingers being squeezed between openings and the workpiece have occurred. Vertical adjustment of the table may be manual or power assisted. A mechanical end-stop should prevent any contact of the table with the cutterblock or feed rollers. It must be ensured that the vertical adjustment mechanism hold the table in a stable position.
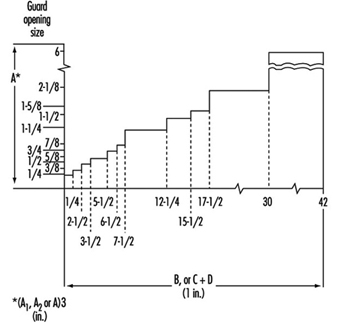
In order to prevent the feeding of oversize workpieces, a device (e.g., a fixed rod or fixed bar) is located on the infeed side of the machine, thereby limiting the maximum workpiece height. A maximum height of 250 mm between the surface of the table in its lowest position and the above-mentioned safety device is rarely exceeded. The usual working width varies between 315 and 800 mm (for special machines this width might go up to 1,300 mm).
The cutterblock diameter generally varies from 80 to 160 mm. Normally four blades are fitted to the cutterblock. The cutterblock rotates at speeds between 4,000 and 6,000 rpm, and its input power varies from 4 to 20 kW. The maximum depth of cut is 10 to 12 mm.
To minimize the danger of kickback, one-side thickness planing machines should be fitted with an anti-kickback device covering the full working width of the machine. This anti-kickback device generally consists of several grooved elements arranged on a rod. The individual element is between 8 and 15 mm wide, and it falls under its own weight to the rest position. The lowest point of the individual grooved element in its rest position should be 3 mm below the cutting circle of the cutterblock. The grooved elements should be made of a material (preferably steel) with a resilience strength of 15 J/cm2 and a surface hardness of 100 HB.
The following noise-reduction measures have proved to be successful on one-side thickness planing machines:
- use of a “quiet” cutterblock (like that suggested for surface planing machines)
- aerodynamic design of the pressure bars and the chip extraction hood
- reduction of the cutterblock speed
- partial or complete enclosure of the machine (tunnel-like design of the infeed and outfeed opening with sound-absorbing material on the surface facing the source of noise)
Noise reduction of up to 20 dBA may be achieved by a well designed complete enclosure.
Hazards
The major cause of accidents on one-side thickness planing machines is kickback of the workpiece. Kickback may happen because of:
- poor maintenance of the anti-kickback device (the individual elements may not fall free under their own weight but stick together because of dust accumulation; the grooves in the elements may be covered with resin, be blunt or be incorrectly reground)
- poor maintenance of the sectional feed rollers and pressure bars (e.g., resin-covered or rusty sections)
- insufficient spring load on feed rollers and pressure bars when several pieces of non-uniform thickness are fed at the same time.
Typical causes of other accidents are:
- contact of the hand with the rotating cutterblock when removing chips and dust from the table by hand rather than with a wooden stick or rake
- ejection of cutterblock blades due to incorrect fixing.
Combined Surface Planing and Thicknessing Machines
The design and operation of combined machines (see figure 3) are similar to those of the individual machines described above. The same can be said in regards to the feed rates, motor power, table and roller adjustments. For thickness planing the surface planing tables are either pulled away, folded down or lifted up sideways, exposing the cutterblock, which is covered by a chip extraction hood to prevent access Combined machines are mainly used in small workshops with few workers, or where space is limited (i.e., in cases where the installation of two individual machines is impossible or unprofitable).
Figure 3. Combined surface & thickness planer
The changeover from one operation to the other is often time-consuming and may be annoying if only a few pieces have to be machined. Moreover, usually only one person at a time can use the machine. However, since 1992 machines have been introduced to the market where simultaneous operation (surface and thickness planing at the same time) is possible.
The hazards of combined machines are to a large extent identical to the hazards listed for the individual machines.
Health Effects and Disease Patterns
The health and safety problems in the forestry and lumber industries are covered elsewhere in this Encyclopaedia. This article will deal with wood as it arrives from the mill and is used in carpentry and the making of furniture and other articles. These activities are predominantly performed in small enterprises. Many workers in these industries are individual contractors and, therefore, not listed as employees, and large numbers of individuals are exposed in do-it-yourself projects and in-home workshops. This means that many of the workers involved are inadequately trained and are supervised poorly or not at all, while proper safeguards and protective equipment are often lacking.
Ahman and colleagues (1995a, 1995b, 1996) call attention to the exposure of teachers of industrial arts and woodworking in Sweden. In contrast to unexposed controls, these teachers had notable (but mainly reversible) nasal effects and complaints that increased with the number of classes from the beginning of the week and receded over the weekends, even though the dust concentrations were below the Swedish threshold limit of 2 mg/m3. In several establishments in the Netherlands, dust levels regularly exceeded that limit, and during sanding operations in a furniture factory, almost all exposures were over the local threshold limit of 5 mg/m3 (Scheeper, Kromhout and Boleij 1995).
Accidental Injuries
The most common health problem in the wood and woodworking industries is accidental injuries. These are more frequent among younger, inexperienced workers, and, for the most part, they are relatively minor. On occasion, however, they may involve long-term impairment or loss of an extremity. They include: splinters, which may become infected, and lacerations, gouges and amputations resulting from improperly used or inadequately guarded woodworking machinery (Ma, Wang and Chou 1991); sprains and strains from injudicious lifting or working in an awkward position (Nestor, Bobick and Pizatella 1990); repetitive motion injuries involving the hand or shoulder; and eye injuries. Many if not most of these can be prevented by proper training, the judicious application of machine guards and restraints and the use of personal protective equipment such as gloves and safety glasses. When they occur, prompt removal of splinters and prevention of infection by expeditious cleansing and first aid treatment of wounds will minimize disability.
Wood Dust
Wood dust exposure occurs whenever wood is sawed, chipped, planed, routed or sanded. The effects vary with the intensity and duration of the exposure and the size of the particles. Particles in the eyes may cause irritation, and wood dust gathering in skin folds may be aggravated by perspiration and chemicals and lead to irritation and infection. These effects may be reduced by vacuum removal of the dust, protective masks and clothing and good personal hygiene practices.
Nasopharyngeal and respiratory passages
Wood dust in the nasal passages may diminish mucociliary clearance and impair olfactory sensitivity (Andersen, Solgaard and Andersen 1976; Ahman et al. 1996). These may lead to irritation, frequent sneezing, nosebleeds and infection of the sinuses (Imbus 1994).
Exposures in a furniture factory (Whitehead, Ashikaga and Vacek 1981) and in sawmill workers (Hessel et al. 1995) were shown to be accompanied by decreases in both 1-second forced expiratory volume (FEV1) and forced vital capacity (FVC), adjusted for age, height and smoking. These were accompanied by significant increases in shortness of breath and wheeze with chest tightness and the occurrence of bronchitis and asthma. There is, however, no convincing evidence of other lung disease due to wood dust exposures (Imbus 1994). In a 6-year prospective follow-up study of approximately 350,000 males in the United States, the 11,541 individuals who reported having been employed in wood-related occupations had a lower relative risk of mortality due to non-malignant respiratory disease than those who did not report exposure to wood dust (Demers et al. 1996).
Allergy and Asthma
Some woods, notably teak, mansonia and radiata pine, contain chemicals that are irritants (see table 1 for an extended list of wood species, their geographic origins, and their health effects). Some species may cause allergic contact dermatitis (e.g., Douglas fir, western red cedar, poplar, rosewood, teak, African mahogany and other “exotic” woods). Western red cedar, rosewood, mahogany and other exotic woods have been shown to cause asthma (Imbus 1994).
Cancer
An unusually high incidence of nasal cancer has been described among woodworkers in Australia, Canada, Denmark, Finland, France, Italy, the Netherlands, the United Kingdom and the United States (Imbus 1994). A recent pooled re-analysis of 12 case-control cohort studies conducted in seven countries confirmed a high risk of nasopharyngeal cancer among woodworkers (Demers et al. 1995). The cause of these excesses of nasal cancer is not known, but, according to recent reports from the United Kingdom and the United States, the risk of nasal cancer among furniture workers has declined since the Second World War, presumably reflecting changes in the manufacturing process (Imbus 1994). No excess risk of sino-nasal cancer was found among the 45,399 men exposed to wood dust included among the 362,823 men enrolled in the American Cancer Society’s 6-year Cancer Prevention Study, but, the researchers note, the number of cases was small. They did, however, find an especially high increase in lung cancer mortality among woodworkers who also reported exposure to asbestos or formaldehyde, and suggested that exposure to these known carcinogens was responsible for the observed increased risk (Stellman et al., in press).
Chemical Exposures
Wood may contain biological contaminants. Moulds and fungi, which often grow on the bark of trees, may cause allergic reactions. Inhalation of fungal spores found on maple, redwood and cork trees has been shown to cause maple bark disease, sequoiosis and suberosis (Imbus 1994).
Wood often contains exogenous chemicals applied in the course of its processing. These include adhesives, solvents, resin binders, insecticides and fungicides, waterproofing compounds, paints and pigments, lacquers and varnishes. Many of these are volatile and may be emitted when the wood is treated, heated or incinerated; they are also conveyed as elements in wood dust. The most important of these include: toluene, methanol, xylene, methyl ethyl ketone, n-butyl alcohol, 1, 1,1-trichlorethane and dichloromethane (EPA 1995).
Conclusion
Health hazards of the wood and woodworking industries may be controlled by installation of engineering controls (e.g., proper placement and guarding of power machinery, ventilation systems to control wood dust and chemical emissions) and personal protective equipment (e.g., gloves, safety glasses, respirators), coupled with periodic inspections to ensure that these are properly maintained and used. Perhaps most important is appropriate education and training of the workers and their supervisors.
Table 1. Poisonous, allergenic and biologically active wood varieties
| Scientific names | Selected commercial names | Family | Health Impairment |
| Abies alba Mill (A. pectinata D.C.) | Silver fir | Pinaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Acacia spp. A. harpophylla F. Muell. A. melanoxylon R. Br. A. seyal Del. A. shirley Maiden |
Australian blackwood | Mimosaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Acer spp. A. platanoides L. |
Maple | Aceraceae | Dermatitis |
| Afrormosia elata Harms. (Pericopsis elata Van Meeuwen) |
Afrormosia, kokrodua, asamala, obang, oleo pardo, bohele, mohole | Papilionaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Afzelia africana Smith A. bijuga A. Chev. (Intsia bijuga A. Cunn.) A. palembanica Bak. (Intsia palembanica Bak.) |
Doussié, afzelia, aligua, apa, chanfuta, lingue merbau, intsia, hintsy | Caesalpinaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Agonandra brasiliensis Miers | Pao, marfim, granadillo | Olacaceae | Dermatitis |
| Ailanthus altissima Mill | Chinese sumac | Simaroubaceae | Dermatitis |
| Albizzia falcata Backer A. ferruginea Benth. A. lebbek Benth A. toona F.M. Bail |
Iatandza Kokko, siris |
Mimosaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Alnus spp. A. glutinosa Gaertn. |
Common alder Black alder |
Betulaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Amyris spp. A. balsamifera L. A. toxifera Willd. |
Venezuelan or West Indian sandalwood | Rutaceae | Dermatitis; toxic effects |
| Anacardium occidentale L. A. excelsum Skels. |
Cashew | Anacardiaceae | Dermatitis |
| Andira araroba Aguiar. (Vataireopsis araroba Ducke) A. coriacea Pulle A. inermis H.B.K. |
Red cabbage tree Partridge wood | Papilionaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Aningeria spp. A. robusta Aubr. and Pell. A. altissima Aubr. and Pell. Antiaris africana Engl. A. welwitschi Engl. |
Aningeria Antiaris, ako, chen chen | Sapotaceae Moraceae | Conjunctivitis-rhinitis; asthma Toxic effects |
| Apuleia molaris spruce (A. leiocarpa MacBride) (A. ferrea Mart.) |
Redwood | Caesalpinaceae | Dermatitis; toxic effects |
| Araucaria angustifolia O. Ktze A. brasiliana A. Rich. |
Parana pine, araucaria | Araucariaceae | Toxic effects |
| Aspidosperma spp. A. peroba Fr. All. A. vargasii A. DC. |
Red peroba Pau marfim, pau amarello, pequia marfim, guatambu, amarilla, pequia | Apocynaceae | Dermatitis; conjunctivitis- rhinitis; asthma; toxic effects |
| Astrocaryum spp. | Palm | Palmaceae | Dermatitis; toxic effects |
| Aucoumea klaineana Pierre | Gabon mahogany | Burseraceae | Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis |
| Autranella congolensis A. Chev. (Mimusops congolensis De Wild.) |
Mukulungu, autracon, elang, bouanga, kulungu | Sapotaceae | Dermatitis |
| Bactris spp. (Astrocaryum spp.) | Palm | Palmaceae | Dermatitis; toxic effects |
| Balfourodendron riedelianum Engl. | Guatambu, gutambu blanco | Rutaceae | Dermatitis |
| Batesia floribunda Benth. | Acapu rana | Caesalpinaceae | Toxic effects |
| Berberis vulgaris L. | Barberry | Berberidaceae | Toxic effects |
| Betula spp. B. alba L. (B. pendula Roth.) |
Birch | Betulaceae | Dermatitis |
| Blepharocarva involucrigera F. Muell. | Rosebutternut | Anacardiaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Bombax brevicuspe Sprague B. chevalieri Pell |
Kondroti, alone | Bombacaceae | Dermatitis |
| Bowdichia spp. B. nitida Benth. B. guianensis Ducke (Diplotropis guianensis Benth.) (Diplotropis purpurea Amsh.) |
Black sucupira | Papilionaceae | Dermatitis |
| Brachylaena hutchinsii Hutch. | Muhuhu | Compositae | Dermatitis |
| Breonia spp. | Molompangady | Rubiaceae | Dermatitis |
| Brosimum spp. B. guianense Hub. (Piratinera guianensis Aubl.) |
Snakewood, letterwood, tigerwood | Moraceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Brya ebenus DC. (Amerimnum ebenus Sw.) Brya buxifolia Urb. |
Brown ebony, green ebony, Jamaican ebony, tropical American ebony | Papilionaceae | Dermatitis |
| Buxus sempervirens L. B. macowani Oliv. |
European boxwood, East London b., Cape b. | Buxaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Caesalpinia echinata Lam. (Guilandina echinata Spreng.) | Brasilwood | Caesalpinaceae | Dermatitis; toxic effects |
| Callitris columellaris F. Muell. | White cypress pine | Cupressaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Calophyllum spp. C. brasiliense Camb. |
Santa maria, jacareuba, kurahura, galba | Guttiferae | Dermatitis; toxic effects |
| Campsiandra laurifolia Benth. | Acapu rana | Caesalpinaceae | Toxic effects |
| Carpinus betulus | Hornbeam | Betulaceae | Dermatitis |
| Cassia siamea Lamk. | Tagayasan, muong ten, djohar | Caesalpinaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Castanea dentata Borkh C. sativa Mill. C. pumila Mill. |
Chestnut, sweet chestnut | Fagaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Castanospermum australe A. Cunn. | Black bean, Australian or Moreton Bay chestnut | Papilionaceae | Dermatitis |
| Cedrela spp. (Toona spp.) | Red cedar, Australian cedar | Meliaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Cedrus deodara (Roxb. ex. Lamb.) G. Don (C. libani Barrel. lc) |
Deodar | Pinaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Celtis brieyi De Wild. C. cinnamomea Ldl. |
Diania Gurenda |
Ulmaceae | Dermatitis |
| Chlorophora excelsa Benth. and Hook I. C. regia A. Chev. C. tinctoria (L.) Daub. |
Iroko, gelbholz, yellowood, kambala, mvule, odum, moule, African teak, abang, tatajuba, fustic, mora | Moraceae | Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis |
| Chloroxylon spp. C. swietenia A.DC. |
Ceylon satinwood | Rutaceae | Dermatitis; toxic effects |
| Chrysophyllum spp. | Najara | Sapotaceae | Dermatitis |
| Cinnamomum camphora Nees and Ebeim | Asian camphorwood, cinnamon | Lauraceae | Toxic effects |
| Cryptocarya pleurosperma White and Francis | Poison walnut | Lauraceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Dacrycarpus dacryoides (A. Rich.) de Laub. | New Zealand white pine | Podocarpaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Dacrydium cupressinum Soland | Sempilor, rimu | Podocarpaceae | conjunctivitis-rhinitis; asthma |
| Dactylocladus stenostachys Oliv. | Jong kong, merebong, medang tabak | Melastomaceae | Toxic effects |
| Dalbergia spp. D. amerimnon Benth. D. granadillo Pitt. D. hypoleuca Standl. D. latifolia Roxb. D. melanoxylon Guill. and Perr. D. nigra Fr. All. D. oliveri Gamble D. retusa Hemsl. D. sissoo Roxb. D. stevensonii Standl. |
Ebony Red foxwood Indian rosewood, Bombay blackwood, African blackwood, pallisander, riopalissandro, Brasilian rosewood, jacaranda Burma rosewood Red foxwood Nagaed wood, Honduras rosewood |
Papilionaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Dialium spp. D. dinklangeri Harms. |
Eyoum, eyum | Caesalpinaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Diospyros spp. D. celebica Bakh. D. crassiflora Hiern D. ebenum Koenig |
Ebony, African ebony Macassar ebony, African ebony, Ceylon ebony | Ebenaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Dipterocarpus spp. D. alatus Roxb. |
Keruing, gurjum, yang, keruing | Dipterocarpaceae | Dermatitis |
| Distemonanthus benthamianus Baill. | Movingui, ayan, anyaran, Nigerian satinwood | Caesalpinaceae | Dermatitis |
| Dysoxylum spp. D. fraseranum Benth. |
Mahogany, stavewood, red bean | Meliaceae | dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| D. muelleri Benth. | Rose mahogany | ||
| Echirospermum balthazarii Fr. All. (Plathymenia reticulataBenth.) | Vinhatico | Mimosaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Entandophragma spp. E. angolense C.D.C. E. candollei Harms. E. cylindricum Sprague E. utile Sprague |
Tiama Kosipo, omo Sapelli, sapele, aboudikro Sipo, utile, assié, kalungi, mufumbi |
Meliaceae | Dermatitis; allergic extrinsic alveolitis |
| Erythrophloeum guineense G. Don E. ivorense A. Chev. |
Tali, missanda, eloun, massanda, sasswood, erun, redwater tree | Caesalpinaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Esenbeckia leiocarpa Engl. | Guaranta | Rutaceae | Dermatitis |
| Eucalyptus spp. E. delegatensis R.T. Back E. hemiphloia F. Muell. E. leucoxylon Maiden E. maculata Hook. E. marginata Donn ex Sm. E. microtheca F. Muell. E. obliqua L. Herit. E. regnans F. Muell. E. saligna Sm. |
Alpine ash Grey box Yellow gum Spotted gum Mountain ash |
Myrtaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Euxylophora paraensis Hub. | Boxwood | Rutaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Excoecaria africana M. Arg. (Spirostachys africana Sand) E. agallocha L. |
African sandalwood, tabootie, geor, aloewood, blind-your-eye | Euphorbiaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Fagara spp. F. flava Krug and Urb. (Zanthoxylum flavum Vahl.) F. heitzii Aubr. and Pell. F. macrophylla Engl. |
Yellow sanders, West Indian satinwood, atlaswood, olon, bongo, mbanza | Rutaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Fagus spp. (Nothofagus spp.) F. sylvatica L. |
Beech | Fagaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Fitzroya cupressoides (Molina) Johnston (F. patagonica Hook. f.) |
Alerce | Cupressaceae | Dermatitis |
| Flindersia australis R. Br. F. brayleyana F. Muell. F. pimenteliana F. Muell. |
Australian teak, Queensland maple, maple Silkwood, Australian maple |
Rutaceae | Dermatitis |
| Fraxinus spp. F. excelsior L. |
Ash | Oleaceae | Dermatitis |
| Gluta spp. G. rhengas L. (Melanorrhoea spp.) M. curtisii Pierre M. laccifera wallichii Hook. |
Rengas, gluta Renga wood Rhengas |
Anacardiaceae | Dermatitis; toxic effects |
| Gonioma kamassi E. Mey. | Knysna boxwood, kamassi | Apocynaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Gonystylus bancanus Baill. | Ramin, melawis, akenia | Gonystylaceae | Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis |
| Gossweilerodendron balsamiferum (Verm.) Harms. | Nigerian cedar | Caesalpinaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Grevillea robusta A. Cunn. | Silky oak | Proteaceae | Dermatitis |
| Guaiacum officinale L. | Gaiac, lignum vitae | Zygophyllaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Guarea spp. G. cedrata Pell. G. laurentii De Wild. G. thompsonii Sprague |
Bossé Nigerian pearwood Cedar mahogany Scented guarea Black guarea |
Meliaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Halfordia scleroxyla F. Muell. H. papuana Lauterb. |
Saffron-heart | Polygonaceae | Dermatitis; allergic extrinsic alveolitis |
| Hernandia spp. H. sonora L. (H. guianensis Aubl.) |
Mirobolan, topolite | Hernandiaceae | Dermatitis |
| Hippomane mancinella L. | Beach apple | Euphorbiaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Illipe latifolia F. Muell. I. longifolia F. Muell. (Bassia latifolia Roxb.) (B. longifoliaRoxb.) |
Moak, edel teak | Sapotaceae | Dermatitis |
| Jacaranda spp. J. brasiliana Pers. Syn. (Bignonia brasiliana Lam.) J. coerulea (I.) Gris. |
Jacaranda Caroba, boxwood | Bignoniaceae | Dermatitis |
| Juglans spp. J. nigra L. J. regia L. |
Walnut | Juglandaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Juniperus sabina L. J. phoenicea L. J. virginiana L. |
Virginian pencil cedar, Eastern red cedar | Cupressaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Khaya antotheca C. DC. K. ivorensis A. Chev. K. senegalensis A. Juss. |
Ogwango, African mahogany, krala Dry-zone mahogany | Meliaceae | Dermatitis; allergic extrinsic alveolitis |
| Laburnum anagyroides Medic. (Cytisus laburnum L.) L. vulgare Gris |
Laburnum | Papilionaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Larix spp. L. decidua Mill. L. europea D.C. |
Larch European larch |
Pinaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Liquidambar styracifolia L. | Amberbaum, satin-nussbaum | Hamamelidaceae | Dermatitis |
| Liriodendron tulipifera L. | American whitewood, tulip tree | Magnoliaceae | Dermatitis |
| Lovoa trichilioides Harms. (L. klaineana Pierre) | Dibetou, African walnut, apopo, tigerwood, side | Meliaceae | dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Lucuma spp. (Pouteria spp.) L. procera |
Guapeva, abiurana Massaranduba |
Sapotaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Maba ebenus Wight. | Makassar-ebenholz | Ebenaceae | Dermatitis |
| Machaerium pedicellatum Vog. M. scleroxylon Tul. M. violaceum Vog. |
Kingswood | Papilionaceae | Dermatitis |
| Mansonia altissima A. Chev. | Nigerian walnut | Sterculiaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Melanoxylon brauna Schott | Brauna, grauna | Caesalpinaceae | Dermatitis |
| Microberlinia brazzavillensis A. Chev. M. bisulcata A. Chev. |
African zebrawood | Caesalpinaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Millettia laurentii De Wild. M. stuhlmannii Taub. |
Wenge Panga-panga |
Papilionaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Mimusops spp. (Manilkara spp.) Mimusops spp. (Dumoria spp.) (Tieghemella spp.) M. congolensis De Wild. (Autranella congolensis A. Chev.) M. djave Engl. (Baillonella toxisperma Pierre) M. heckelii Hutch. et Dalz. (Tieghemella heckelii Pierre) (Dumoria heckelii A. Chev.) |
Muirapiranga Makoré Mukulungu, autracon Moabi Cherry mahogany |
Sapotaceae | Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis; toxic effects |
| Mitragyna ciliata Aubr. and Pell. M. stipulosa O. Ktze |
Vuku, African poplar Abura |
Rubiaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Nauclea diderrichii Merrill (Sarcocephalus diderrichii De Wild.) Nauclea trillessi Merrill |
Bilinga, opepe, kussia, badi, West African boxwood | Rubiaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Nesogordonia papaverifera R. Capuron | Kotibé, danta, epro, otutu, ovové, aborbora | Tiliaceae | Toxic effects |
| Ocotea spp. O. bullata E. Mey O. porosa L. Barr. (Phoebe porosa Mez.) O. rodiaei Mez. (Nectandra rodiaei Schomb.) O. rubra Mez. O. usambarensis Engl. |
Stinkwood Laurel Brazilian walnut Greenheart Louro vermelho East African camphorwood |
Lauraceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Paratecoma spp. P. alba P. peroba Kuhlm. |
Brazilian white peroba Peroba white. p. |
Bignoniaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Parinarium spp. P. guianense (Parinari spp.) (Brosimum spp.) P. variegatum |
Guyana-satinholz Antillen-satinholz |
Rosaceae | Dermatitis |
| Peltogyne spp. P. densiflora Spruce |
Blue wood, purpleheart | Caesalpinaceae | Toxic effects |
| Phyllanthus ferdinandi F.v.M. | Lignum vitae, chow way, tow war | Euphorbiaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Picea spp. P. abies Karst. P. excelsa Link. P. mariana B.S.P. P. polita Carr. |
European spruce, whitewood Black spruce |
Pinaceae | Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis |
| Pinus spp. P. radiata D. Don |
Pine | Pinaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Piptadenia africana Hook f. Piptadeniastrum africanum Brenan |
Dabema, dahoma, ekhimi agobin, mpewere, bukundu |
Mimosaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Platanus spp. | Plane | Platanaceae | Dermatitis |
| Pometia spp. P. pinnata Forst. |
Taun Kasai |
Sapindaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Populus spp. | Poplar | Salicaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Prosopis juliflora D.C. | Cashaw | Mimosaceae | Dermatitis |
| Prunus spp. P. serotina Ehrl. |
Cherry Blackcherry |
Rosaceae | dermatitis; conjunctivitis-rhinitis; asthma |
| Pseudomorus brunoniana Bureau | White handlewood | Moraceae | Dermatitis; toxic effects |
| Pseudotsuga douglasii Carr. (P. menziesii Franco) | Douglas fir, red fir, Douglas spruce | Pinaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Pterocarpus spp. P. angolensis D.C. P. indicus Willd. P. santalinus L.f. (Vatairea guianensis Aubl.) |
African padauk, New Guinea rosewood, red sandalwood, red sanders, quassia wood | Papilionaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Pycnanthus angolensis Warb. (P. kombo Warb.) | Ilomba | Myristicaceae | Toxic effects |
| Quercus spp. | Oak | Fagaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Raputia alba Engl. R. magnifica Engl. |
Arapoca branca, arapoca | Rutaceae | Dermatitis |
| Rauwolfia pentaphylla Stapf. O. | Peroba | Apocynaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Sandoricum spp. S. indicum Cav. |
Sentul, katon, kra-ton, ketjapi, thitto | Meliaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Schinopsis lorentzii Engl. S. balansae Engl. |
Quebracho colorado, red q., San Juan, pau mulato | Anacardiaceae | Dermatitis; toxic effects |
| Semercarpus australiensis Engl. S. anacardium L. |
Marking nut | Anacardiaceae | Dermatitis; toxic effects |
| Sequoia sempervirens Endl. | Sequoia, California redwood |
Taxodiaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Shorea spp. | Alan, almon, red balau White heavy, red lauan, white L., yellow L., mayapis, meranti bakau, dark red M., light red M., red M., white M., yellow M., red seraya, white seraya |
Dipterocarpaceae | Dermatitis |
| S. assamica Dyer | Yellow lauan, white meranti | ||
| Staudtia stipitata Warb. (S. gabonensis Warb.) | Niové | Myristicaceae | Dermatitis |
| Swietenia spp. S. macrophylla King S. mahogany Jacq. |
Mahogany, Honduras mahogany, Tabasco m., baywood, American mahogany, Cuban mahogany |
Meliaceae | Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis; toxic effects |
| Swintonia spicifera Hook. S. floribunda Griff. |
Merpauh | Anacardiaceae | Dermatitis |
| Tabebuia spp. T. ipe Standl. (T. avellanedae Lor. ex Gris.) T. guayacan Hensl. (T. lapacho K. Schum) |
Araguan, ipé preto, lapacho | Bignoniaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Taxus baccata L. | Yew | Taxaceae | Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis; toxic effects |
| Tecoma spp. T. araliacea D.C. T. lapacho |
Green heart Lapacho |
Bignoniaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Tectona grandis L. | Teak, djati, kyun, teck | Verbenaceae | Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis |
| Terminalia alata Roth. T. superba Engl. and Diels. |
Indian laurel limba, afara, ofram, fraké, korina, akom |
Combretaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Thuja occidentalis L. T. plicata D. Don T. standishii Carr. |
White cedar Western red cedar |
Cupressaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Tieghemella africana A. Chev. (Dumoria spp.) T. heckelii Pierre |
Makoré, douka, okola, ukola, makoré, abacu, baku, African cherry | Sapotaceae | Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
| Triplochiton scleroxylon K. Schum | Obeche, samba, wawa, abachi, African whitewood, arere | Sterculiaceae | Dermatitis; conjunctivitis-rhinitis; asthma |
| Tsuga heterophylla Sarg. | Tsuga, Western hemlock | Pinaceae | Dermatitis |
| Turraeanthus africana Pell. | Avodiré Lusamba |
Meliaceae | Dermatitis; allergic extrinsic alveolitis |
| Ulmus spp. | Elm | Ulmaceae | Dermatitis |
| Vitex ciliata Pell. | Verbenaceae | Dermatitis | |
| V. congolensis De Wild. and Th. Dur | Difundu | ||
| V. pachyphylla Bak. | Evino | ||
| Xylia dolabriformis Benth. | Mimosaceae | Conjunctivitis-rhinitis; | |
| X. xylocarpa Taub. | Pyinkado | asthma | |
| Zollernia paraensis Huber | Santo wood | Caesalpinaceae | Dermatitis; toxic effects |
Source: Istituto del Legno, Florence, Italy.
" DISCLAIMER: The ILO does not take responsibility for content presented on this web portal that is presented in any language other than English, which is the language used for the initial production and peer-review of original content. Certain statistics have not been updated since the production of the 4th edition of the Encyclopaedia (1998)."