Toxicology in Health and Safety Regulation
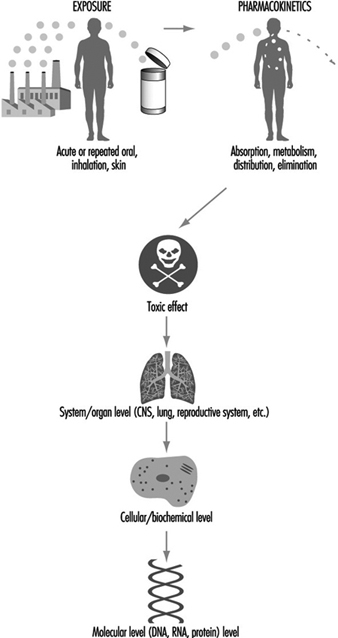
Toxicology plays a major role in the development of regulations and other occupational health policies. In order to prevent occupational injury and illness, decisions are increasingly based upon information obtainable prior to or in the absence of the types of human exposures that would yield definitive information on risk such as epidemiology studies. In addition, toxicological studies, as described in this chapter, can provide precise information on dose and response under the controlled conditions of laboratory research; this information is often difficult to obtain in the uncontrolled setting of occupational exposures. However, this information must be carefully evaluated in order to estimate the likelihood of adverse effects in humans, the nature of these adverse effects, and the quantitative relationship between exposures and effects.
Considerable attention has been given in many countries, since the 1980s, to developing objective methods for utilizing toxicological information in regulatory decision-making. Formal methods, frequently referred to as risk assessment, have been proposed and utilized in these countries by both governmental and non-governmental entities. Risk assessment has been varyingly defined; fundamentally it is an evaluative process that incorporates toxicology, epidemiology and exposure information to identify and estimate the probability of adverse effects associated with exposures to hazardous substances or conditions. Risk assessment may be qualitative in nature, indicating the nature of an adverse effect and a general estimate of likelihood, or it may be quantitative, with estimates of numbers of affected persons at specific levels of exposure. In many regulatory systems, risk assessment is undertaken in four stages: hazard identification, the description of the nature of the toxic effect; dose-response evaluation, a semi-quantitative or quantitative analysis of the relationship between exposure (or dose) and severity or likelihood of toxic effect; exposure assessment, the evaluation of information on the range of exposures likely to occur for populations in general or for subgroups within populations; risk characterization, the compilation of all the above information into an expression of the magnitude of risk expected to occur under specified exposure conditions (see NRC 1983 for a statement of these principles).
In this section, three approaches to risk assessment are presented as illustrative. It is impossible to provide a comprehensive compendium of risk assessment methods used throughout the world, and these selections should not be taken as prescriptive. It should be noted that there are trends towards harmonization of risk assessment methods, partly in response to provisions in the recent GATT accords. Two processes of international harmonization of risk assessment methods are currently underway, through the International Programme on Chemical Safety (IPCS) and the Organization for Economic Cooperation and Development (OECD). These organizations also maintain current information on national approaches to risk assessment.
Structure Activity Relationships
Structure activity relationships (SAR) analysis is the utilization of information on the molecular structure of chemicals to predict important characteristics related to persistence, distribution, uptake and absorption, and toxicity. SAR is an alternative method of identifying potential hazardous chemicals, which holds promise of assisting industries and governments in prioritizing substances for further evaluation or for early-stage decision making for new chemicals. Toxicology is an increasingly expensive and resource-intensive undertaking. Increased concerns over the potential for chemicals to cause adverse effects in exposed human populations have prompted regulatory and health agencies to expand the range and sensitivity of tests to detect toxicological hazards. At the same time, the real and perceived burdens of regulation upon industry have provoked concerns for the practicality of toxicity testing methods and data analysis. At present, the determination of chemical carcinogenicity depends upon lifetime testing of at least two species, both sexes, at several doses, with careful histopathological analysis of multiple organs, as well as detection of preneoplastic changes in cells and target organs. In the United States, the cancer bioassay is estimated to cost in excess of $3 million (1995 dollars).
Even with unlimited financial resources, the burden of testing the approximately 70,000 existing chemicals produced in the world today would exceed the available resources of trained toxicologists. Centuries would be required to complete even a first tier evaluation of these chemicals (NRC 1984). In many countries ethical concerns over the use of animals in toxicity testing have increased, bringing additional pressures upon the uses of standard methods of toxicity testing. SAR has been widely used in the pharmaceutical industry to identify molecules with potential for beneficial use in treatment (Hansch and Zhang 1993). In environmental and occupational health policy, SAR is used to predict the dispersion of compounds in the physical-chemical environment and to screen new chemicals for further evaluation of potential toxicity. Under the US Toxic Substances Control Act (TSCA), the EPA has used since 1979 an SAR approach as a “first screen” of new chemicals in the premanufacture notification (PMN) process; Australia uses a similar approach as part of its new chemicals notification (NICNAS) procedure. In the US SAR analysis is an important basis for determining that there is a reasonable basis to conclude that manufacture, processing, distribution, use or disposal of the substance will present an unreasonable risk of injury to human health or the environment, as required by Section 5(f) of TSCA. On the basis of this finding, EPA can then require actual tests of the substance under Section 6 of TSCA.
Rationale for SAR
The scientific rationale for SAR is based upon the assumption that the molecular structure of a chemical will predict important aspects of its behaviour in physical-chemical and biological systems (Hansch and Leo 1979).
SAR Process
The SAR review process includes identification of the chemical structure, including empirical formulations as well as the pure compound; identification of structurally analogous substances; searching databases and literature for information on structural analogs; and analysis of toxicity and other data on structural analogs. In some rare cases, information on the structure of the compound alone can be sufficient to support some SAR analysis, based upon well-understood mechanisms of toxicity. Several databases on SAR have been compiled, as well as computer-based methods for molecular structure prediction.
With this information, the following endpoints can be estimated with SAR:
- physical-chemical parameters: boiling point, vapour pressure, water solubility, octanol/water partition coefficient
- biological/environmental fate parameters: biodegradation, soil sorption, photodegradation, pharmacokinetics
- toxicity parameters: aquatic organism toxicity, absorption, acute mammalian toxicity (limit test or LD50), dermal, lung and eye irritation, sensitization, subchronic toxicity, mutagenicity.
It should be noted that SAR methods do not exist for such important health endpoints as carcinogenicity, developmental toxicity, reproductive toxicity, neurotoxicity, immunotoxicity or other target organ effects. This is due to three factors: the lack of a large database upon which to test SAR hypotheses, lack of knowledge of structural determinants of toxic action, and the multiplicity of target cells and mechanisms that are involved in these endpoints (see “The United States approach to risk assessment of reproductive toxicants and neurotoxic agents”). Some limited attempts to utilize SAR for predicting pharmacokinetics using information on partition coefficients and solubility (Johanson and Naslund 1988). More extensive quantitative SAR has been done to predict P450-dependent metabolism of a range of compounds and binding of dioxin- and PCB-like molecules to the cytosolic “dioxin” receptor (Hansch and Zhang 1993).
SAR has been shown to have varying predictability for some of the endpoints listed above, as shown in table 1. This table presents data from two comparisons of predicted activity with actual results obtained by empirical measurement or toxicity testing. SAR as conducted by US EPA experts performed more poorly for predicting physical-chemical properties than for predicting biological activity, including biodegradation. For toxicity endpoints, SAR performed best for predicting mutagenicity. Ashby and Tennant (1991) in a more extended study also found good predictability of short-term genotoxicity in their analysis of NTP chemicals. These findings are not surprising, given current understanding of molecular mechanisms of genotoxicity (see “Genetic toxicology”) and the role of electrophilicity in DNA binding. In contrast, SAR tended to underpredict systemic and subchronic toxicity in mammals and to overpredict acute toxicity to aquatic organisms.
Table 1. Comparison of SAR and test data: OECD/NTP analyses
| Endpoint | Agreement (%) | Disagreement (%) | Number |
| Boiling point | 50 | 50 | 30 |
| Vapour pressure | 63 | 37 | 113 |
| Water solubility | 68 | 32 | 133 |
| Partition coefficient | 61 | 39 | 82 |
| Biodegradation | 93 | 7 | 107 |
| Fish toxicity | 77 | 22 | 130 |
| Daphnia toxicity | 67 | 33 | 127 |
| Acute mammalian toxicity (LD50 ) | 80 | 201 | 142 |
| Skin irritation | 82 | 18 | 144 |
| Eye irritation | 78 | 22 | 144 |
| Skin sensitization | 84 | 16 | 144 |
| Subchronic toxicity | 57 | 32 | 143 |
| Mutagenicity2 | 88 | 12 | 139 |
| Mutagenicity3 | 82–944 | 1–10 | 301 |
| Carcinogenicity3 : Two year bioassay | 72–954 | — | 301 |
Source: Data from OECD, personal communication C. Auer ,US EPA. Only those endpoints for which comparable SAR predictions and actual test data were available were used in this analysis. NTP data are from Ashby and Tennant 1991.
1 Of concern was the failure by SAR to predict acute toxicity in 12% of the chemicals tested.
2 OECD data, based on Ames test concordance with SAR
3 NTP data, based on genetox assays compared to SAR predictions for several classes of “structurally alerting chemicals”.
4 Concordance varies with class; highest concordance was with aromatic amino/nitro compounds; lowest with “miscellaneous” structures.
For other toxic endpoints, as noted above, SAR has less demonstrable utility. Mammalian toxicity predictions are complicated by the lack of SAR for toxicokinetics of complex molecules. Nevertheless, some attempts have been made to propose SAR principles for complex mammalian toxicity endpoints (for instance, see Bernstein (1984) for an SAR analysis of potential male reproductive toxicants). In most cases, the database is too small to permit rigorous testing of structure-based predictions.
At this point it may be concluded that SAR may be useful mainly for prioritizing the investment of toxicity testing resources or for raising early concerns about potential hazard. Only in the case of mutagenicity is it likely that SAR analysis by itself can be utilized with reliability to inform other decisions. For no endpoint is it likely that SAR can provide the type of quantitative information required for risk assessment purposes as discussed elsewhere in this chapter and Encyclopaedia.
In Vitro Toxicity Testing
The emergence of sophisticated technologies in molecular and cellular biology has spurred a relatively rapid evolution in the life sciences, including toxicology. In effect, the focus of toxicology is shifting from whole animals and populations of whole animals to the cells and molecules of individual animals and humans. Since the mid-1980s, toxicologists have begun to employ these new methodologies in assessing the effects of chemicals on living systems. As a logical progression, such methods are being adapted for the purposes of toxicity testing. These scientific advances have worked together with social and economic factors to effect change in the evaluation of product safety and potential risk.
Economic factors are specifically related to the volume of materials that must be tested. A plethora of new cosmetics, pharmaceuticals, pesticides, chemicals and household products is introduced into the market every year. All of these products must be evaluated for their potential toxicity. In addition, there is a backlog of chemicals already in use that have not been adequately tested. The enormous task of obtaining detailed safety information on all of these chemicals using traditional whole animal testing methods would be costly in terms of both money and time, if it could even be accomplished.
There are also societal issues that relate to public health and safety, as well as increasing public concern about the use of animals for product safety testing. With regard to human safety, public interest and environmental advocacy groups have placed significant pressure on government agencies to apply more stringent regulations on chemicals. A recent example of this has been a movement by some environmental groups to ban chlorine and chlorine-containing compounds in the United States. One of the motivations for such an extreme action lies in the fact that most of these compounds have never been adequately tested. From a toxicological perspective, the concept of banning a whole class of diverse chemicals based simply on the presence of chlorine is both scientifically unsound and irresponsible. Yet, it is understandable that from the public’s perspective, there must be some assurance that chemicals released into the environment do not pose a significant health risk. Such a situation underscores the need for more efficient and rapid methods to assess toxicity.
The other societal concern that has impacted the area of toxicity testing is animal welfare. The growing number of animal protection groups throughout the world have voiced considerable opposition to the use of whole animals for product safety testing. Active campaigns have been waged against manufacturers of cosmetics, household and personal care products and pharmaceuticals in attempts to stop animal testing. Such efforts in Europe have resulted in the passage of the Sixth Amendment to Directive 76/768/EEC (the Cosmetics Directive). The consequence of this Directive is that cosmetic products or cosmetic ingredients that have been tested in animals after January 1, 1998 cannot be marketed in the European Union, unless alternative methods are insufficiently validated. While this Directive has no jurisdiction over the sale of such products in the United States or other countries, it will significantly affect those companies that have international markets that include Europe.
The concept of alternatives, which forms the basis for the development of tests other than those on whole animals, is defined by the three Rs: reduction in the numbers of animals used; refinement of protocols so that animals experience less stress or discomfort; and replacement of current animal tests with in vitro tests (i.e., tests done outside of the living animal), computer models or test on lower vertebrate or invertebrate species. The three Rs were introduced in a book published in 1959 by two British scientists, W.M.S. Russell and Rex Burch, The Principles of Humane Experimental Technique. Russell and Burch maintained that the only way in which valid scientific results could be obtained is through the humane treatment of animals, and believed that methods should be developed to reduce animal use and ultimately replace it. Interestingly, the principles outlined by Russell and Burch received little attention until the resurgence of the animal welfare movement in the mid-1970s. Today the concept of the three Rs is very much in the forefront with regard to research, testing and education.
In summary, the development of in vitro test methodologies has been influenced by a variety of factors that have converged over the last ten to 20 years. It is difficult to ascertain if any of these factors alone would have had such a profound effect on toxicity testing strategies.
Concept of In Vitro Toxicity Tests
This section will focus solely on in vitro methods for evaluating toxicity, as one of the alternatives to whole-animal testing. Additional non-animal alternatives such as computer modelling and quantitative structure-activity relationships are discussed in other articles of this chapter.
In vitro studies are generally conducted in animal or human cells or tissues outside of the body. In vitro literally means “in glass”, and refers to procedures carried out on living material or components of living material cultured in petri dishes or in test tubes under defined conditions. These may be contrasted with in vivo studies, or those carried out “in the living animal”. While it is difficult, if not impossible, to project the effects of a chemical on a complex organism when the observations are confined to a single type of cells in a dish, in vitro studies do provide a significant amount of information about intrinsic toxicity as well as cellular and molecular mechanisms of toxicity. In addition, they offer many advantages over in vivo studies in that they are generally less expensive and they may be conducted under more controlled conditions. Furthermore, despite the fact that small numbers of animals are still needed to obtain cells for in vitro cultures, these methods may be considered reduction alternatives (since many fewer animals are used compared to in vivo studies) and refinement alternatives (because they eliminate the need to subject the animals to the adverse toxic consequences imposed by in vivo experiments).
In order to interpret the results of in vitro toxicity tests, determine their potential usefulness in assessing toxicity and relate them to the overall toxicological process in vivo, it is necessary to understand which part of the toxicological process is being examined. The entire toxicological process consists of events that begin with the organism’s exposure to a physical or chemical agent, progress through cellular and molecular interactions and ultimately manifest themselves in the response of the whole organism. In vitro tests are generally limited to the part of the toxicological process that takes place at the cellular and molecular level. The types of information that may be obtained from in vitro studies include pathways of metabolism, interaction of active metabolites with cellular and molecular targets and potentially measurable toxic endpoints that can serve as molecular biomarkers for exposure. In an ideal situation, the mechanism of toxicity of each chemical from exposure to organismal manifestation would be known, such that the information obtained from in vitro tests could be fully interpreted and related to the response of the whole organism. However, this is virtually impossible, since relatively few complete toxicological mechanisms have been elucidated. Thus, toxicologists are faced with a situation in which the results of an in vitro test cannot be used as an entirely accurate prediction of in vivo toxicity because the mechanism is unknown. However, frequently during the process of developing an in vitro test, components of the cellular and molecular mechanism(s) of toxicity are elucidated.
One of the key unresolved issues surrounding the development and implementation of in vitro tests is related to the following consideration: should they be mechanistically based or is it sufficient for them to be descriptive? It is inarguably better from a scientific perspective to utilize only mechanistically based tests as replacements for in vivo tests. However in the absence of complete mechanistic knowledge, the prospect of developing in vitro tests to completely replace whole animal tests in the near future is almost nil. This does not, however, rule out the use of more descriptive types of assays as early screening tools, which is the case presently. These screens have resulted in a significant reduction in animal use. Therefore, until such time as more mechanistic information is generated, it may be necessary to employ to a more limited extent, tests whose results simply correlate well with those obtained in vivo.
In Vitro Tests for Cytotoxicity
In this section, several in vitro tests that have been developed to assess a chemical’s cytotoxic potential will be described. For the most part, these tests are easy to perform and analysis can be automated. One commonly used in vitro test for cytotoxicity is the neutral red assay. This assay is done on cells in culture, and for most applications, the cells can be maintained in culture dishes that contain 96 small wells, each 6.4mm in diameter. Since each well can be used for a single determination, this arrangement can accommodate multiple concentrations of the test chemical as well as positive and negative controls with a sufficient number of replicates for each. Following treatment of the cells with various concentrations of the test chemical ranging over at least two orders of magnitude (e.g., from 0.01mM to 1mM), as well as positive and negative control chemicals, the cells are rinsed and treated with neutral red, a dye that can be taken up and retained only by live cells. The dye may be added upon removal of the test chemical to determine immediate effects, or it may be added at various times after the test chemical is removed to determine cumulative or delayed effects. The intensity of the colour in each well corresponds to the number of live cells in that well. The colour intensity is measured by a spectrophotometer which may be equipped with a plate reader. The plate reader is programmed to provide individual measurements for each of the 96 wells of the culture dish. This automated methodology permits the investigator to rapidly perform a concentration-response experiment and to obtain statistically useful data.
Another relatively simple assay for cytotoxicity is the MTT test. MTT (3[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) is a tetrazolium dye that is reduced by mitochondrial enzymes to a blue colour. Only cells with viable mitochondria will retain the ability to carry out this reaction; therefore the colour intensity is directly related to the degree of mitochondrial integrity. This is a useful test to detect general cytotoxic compounds as well as those agents that specifically target mitochondria.
The measurement of lactate dehydrogenase (LDH) activity is also used as a broad-based assay for cytotoxicity. This enzyme is normally present in the cytoplasm of living cells and is released into the cell culture medium through leaky cell membranes of dead or dying cells that have been adversely affected by a toxic agent. Small amounts of culture medium may be removed at various times after chemical treatment of the cells to measure the amount of LDH released and determine a time course of toxicity. While the LDH release assay is a very general assessment of cytotoxicity, it is useful because it is easy to perform and it may be done in real time.
There are many new methods being developed to detect cellular damage. More sophisticated methods employ fluorescent probes to measure a variety of intracellular parameters, such as calcium release and changes in pH and membrane potential. In general, these probes are very sensitive and may detect more subtle cellular changes, thus reducing the need to use cell death as an endpoint. In addition, many of these fluorescent assays may be automated by the use of 96-well plates and fluorescent plate readers.
Once data have been collected on a series of chemicals using one of these tests, the relative toxicities may be determined. The relative toxicity of a chemical, as determined in an in vitro test, may be expressed as the concentration that exerts a 50% effect on the endpoint response of untreated cells. This determination is referred to as the EC50 (Effective Concentration for 50% of the cells) and may be used to compare toxicities of different chemicals in vitro. (A similar term used in evaluating relative toxicity is IC50, indicating the concentration of a chemical that causes a 50% inhibition of a cellular process, e.g., the ability to take up neutral red.) It is not easy to assess whether the relative in vitro toxicity of the chemicals is comparable to their relative in vivo toxicities, since there are so many confounding factors in the in vivo system, such as toxicokinetics, metabolism, repair and defence mechanisms. In addition, since most of these assays measure general cytotoxicity endpoints, they are not mechanistically based. Therefore, agreement between in vitro and in vivo relative toxicities is simply correlative. Despite the numerous complexities and difficulties in extrapolating from in vitro to in vivo, these in vitro tests are proving to be very valuable because they are simple and inexpensive to perform and may be used as screens to flag highly toxic drugs or chemicals at early stages of development.
Target Organ Toxicity
In vitro tests can also be used to assess specific target organ toxicity. There are a number of difficulties associated with designing such tests, the most notable being the inability of in vitro systems to maintain many of the features of the organ in vivo. Frequently, when cells are taken from animals and placed into culture, they tend either to degenerate quickly and/or to dedifferentiate, that is, lose their organ-like functions and become more generic. This presents a problem in that within a short period of time, usually a few days, the cultures are no longer useful for assessing organ-specific effects of a toxin.
Many of these problems are being overcome because of recent advances in molecular and cellular biology. Information that is obtained about the cellular environment in vivo may be utilized in modulating culture conditions in vitro. Since the mid-1980s, new growth factors and cytokines have been discovered, and many of these are now available commercially. Addition of these factors to cells in culture helps to preserve their integrity and may also help to retain more differentiated functions for longer periods of time. Other basic studies have increased the knowledge of the nutritional and hormonal requirements of cells in culture, so that new media may be formulated. Recent advances have also been made in identifying both naturally occurring and artificial extracellular matrices on which cells may be cultured. Culture of cells on these different matrices can have profound effects on both their structure and function. A major advantage derived from this knowledge is the ability to intricately control the environment of cells in culture and individually examine the effects of these factors on basic cell processes and on their responses to different chemical agents. In short, these systems can provide great insight into organ-specific mechanisms of toxicity.
Many target organ toxicity studies are conducted in primary cells, which by definition are freshly isolated from an organ, and usually exhibit a finite lifetime in culture. There are many advantages to having primary cultures of a single cell type from an organ for toxicity assessment. From a mechanistic perspective, such cultures are useful for studying specific cellular targets of a chemical. In some instances, two or more cell types from an organ may be cultured together, and this provides an added advantage of being able to look at cell-cell interactions in response to a toxin. Some co-culture systems for skin have been engineered so that they form a three dimensional structure resembling skin in vivo. It is also possible to co-culture cells from different organs—for example, liver and kidney. This type of culture would be useful in assessing the effects specific to kidney cells, of a chemical that must be bioactivated in the liver.
Molecular biological tools have also played an important role in the development of continuous cell lines that can be useful for target organ toxicity testing. These cell lines are generated by transfecting DNA into primary cells. In the transfection procedure, the cells and the DNA are treated such that the DNA can be taken up by the cells. The DNA is usually from a virus and contains a gene or genes that, when expressed, allow the cells to become immortalized (i.e., able to live and grow for extended periods of time in culture). The DNA can also be engineered so that the immortalizing gene is controlled by an inducible promoter. The advantage of this type of construct is that the cells will divide only when they receive the appropriate chemical stimulus to allow expression of the immortalizing gene. An example of such a construct is the large T antigen gene from Simian Virus 40 (SV40) (the immortalizing gene), preceded by the promoter region of the metallothionein gene, which is induced by the presence of a metal in the culture medium. Thus, after the gene is transfected into the cells, the cells may be treated with low concentrations of zinc to stimulate the MT promoter and turn on the expression of the T antigen gene. Under these conditions, the cells proliferate. When zinc is removed from the medium, the cells stop dividing and under ideal conditions return to a state where they express their tissue-specific functions.
The ability to generate immortalized cells combined with the advances in cell culture technology have greatly contributed to the creation of cell lines from many different organs, including brain, kidney and liver. However, before these cell lines may be used as a surrogate for the bona fide cell types, they must be carefully characterized to determine how “normal” they really are.
Other in vitro systems for studying target organ toxicity involve increasing complexity. As in vitro systems progress in complexity from single cell to whole organ culture, they become more comparable to the in vivo milieu, but at the same time they become much more difficult to control given the increased number of variables. Therefore, what may be gained in moving to a higher level of organization can be lost in the inability of the researcher to control the experimental environment. Table 1 compares some of the characteristics of various in vitro systems that have been used to study hepatotoxicity.
Table 1. Comparison of in vitro systems for hepatotoxicity studies
| System | Complexity (level of interaction) |
Ability to retain liver-specific functions | Potential duration of culture | Ability to control environment |
| Immortalized cell lines | some cell to cell (varies with cell line) | poor to good (varies with cell line) | indefinite | excellent |
| Primary hepatocyte cultures | cell to cell | fair to excellent (varies with culture conditions) | days to weeks | excellent |
| Liver cell co-cultures | cell to cell (between the same and different cell types) | good to excellent | weeks | excellent |
| Liver slices | cell to cell (among all cell types) | good to excellent | hours to days | good |
| Isolated, perfused liver | cell to cell (among all cell types), and intra-organ | excellent | hours | fair |
Precision-cut tissue slices are being used more extensively for toxicological studies. There are new instruments available that enable the researcher to cut uniform tissue slices in a sterile environment. Tissue slices offer some advantage over cell culture systems in that all of the cell types of the organ are present and they maintain their in vivo architecture and intercellular communication. Thus, in vitro studies may be conducted to determine the target cell type within an organ as well as to investigate specific target organ toxicity. A disadvantage of the slices is that they degenerate rapidly after the first 24 hours of culture, mainly due to poor diffusion of oxygen to the cells on the interior of the slices. However, recent studies have indicated that more efficient aeration may be achieved by gentle rotation. This, together with the use of a more complex medium, allows the slices to survive for up to 96 hours.
Tissue explants are similar in concept to tissue slices and may also be used to determine the toxicity of chemicals in specific target organs. Tissue explants are established by removing a small piece of tissue (for teratogenicity studies, an intact embryo) and placing it into culture for further study. Explant cultures have been useful for short-term toxicity studies including irritation and corrosivity in skin, asbestos studies in trachea and neurotoxicity studies in brain tissue.
Isolated perfused organs may also be used to assess target organ toxicity. These systems offer an advantage similar to that of tissue slices and explants in that all cell types are present, but without the stress to the tissue introduced by the manipulations involved in preparing slices. In addition, they allow for the maintenance of intra-organ interactions. A major disadvantage is their short-term viability, which limits their use for in vitro toxicity testing. In terms of serving as an alternative, these cultures may be considered a refinement since the animals do not experience the adverse consequences of in vivo treatment with toxicants. However, their use does not significantly decrease the numbers of animals required.
In summary, there are several types of in vitro systems available for assessing target organ toxicity. It is possible to acquire much information about mechanisms of toxicity using one or more of these techniques. The difficulty remains in knowing how to extrapolate from an in vitro system, which represents a relatively small part of the toxicological process, to the whole process occurring in vivo.
In Vitro Tests for Ocular Irritation
Perhaps the most contentious whole-animal toxicity test from an animal welfare perspective is the Draize test for eye irritation, which is conducted in rabbits. In this test, a small fixed dose of a chemical is placed in one of the rabbit’s eyes while the other eye is used as a control. The degree of irritation and inflammation is scored at various times after exposure. A major effort is being made to develop methodologies to replace this test, which has been criticized not only for humane reasons, but also because of the subjectivity of the observations and variability of the results. It is interesting to note that despite the harsh criticism the Draize test has received, it has proven to be remarkably successful in predicting human eye irritants, particularly slightly to moderately irritating substances, that are difficult to identify by other methods. Thus, the demands on in vitro alternatives are great.
The quest for alternatives to the Draize test is a complicated one, albeit one that is predicted to be successful. Numerous in vitro and other alternatives have been developed and in some cases they have been implemented. Refinement alternatives to the Draize test, which by definition, are less painful or distressful to the animals, include the Low Volume Eye Test, in which smaller amounts of test materials are placed in the rabbits’ eyes, not only for humane reasons, but to more closely mimic the amounts to which people may actually be accidentally exposed. Another refinement is that substances which have a pH less than 2 or greater than 11.5 are no longer tested in animals since they are known to be severely irritating to the eye.
Between 1980 and 1989, there has been an estimated 87% decline in the number of rabbits used for eye irritation testing of cosmetics. In vitro tests have been incorporated as part of a tier-testing approach to bring about this vast reduction in whole-animal tests. This approach is a multi-step process that begins with a thorough examination of the historical eye irritation data and physical and chemical analysis of the chemical to be evaluated. If these two processes do not yield enough information, then a battery of in vitro tests is performed. The additional data obtained from the in vitro tests might then be sufficient to assess the safety of the substance. If not, then the final step would be to perform limited in vivo tests. It is easy to see how this approach can eliminate or at least drastically reduce the numbers of animals needed to predict the safety of a test substance.
The battery of in vitro tests that is used as part of this tier-testing strategy depends upon the needs of the particular industry. Eye irritation testing is done by a wide variety of industries from cosmetics to pharmaceuticals to industrial chemicals. The type of information required by each industry varies and therefore it is not possible to define a single battery of in vitro tests. A test battery is generally designed to assess five parameters: cytotoxicity, changes in tissue physiology and biochemistry, quantitative structure-activity relationships, inflammation mediators, and recovery and repair. An example of a test for cytotoxicity, which is one possible cause for irritation, is the neutral red assay using cultured cells (see above). Changes in cellular physiology and biochemistry resulting from exposure to a chemical may be assayed in cultures of human corneal epithelial cells. Alternatively, investigators have also used intact or dissected bovine or chicken eyeballs obtained from slaughterhouses. Many of the endpoints measured in these whole organ cultures are the same as those measured in vivo, such as corneal opacity and corneal swelling.
Inflammation is frequently a component of chemical-induced eye injury, and there are a number of assays available to examine this parameter. Various biochemical assays detect the presence of mediators released during the inflammatory process such as arachidonic acid and cytokines. The chorioallantoic membrane (CAM) of the hen’s egg may also be used as an indicator of inflammation. In the CAM assay, a small piece of the shell of a ten-to-14-day chick embryo is removed to expose the CAM. The chemical is then applied to the CAM and signs of inflammation, such as vascular hemorrhaging, are scored at various times thereafter.
One of the most difficult in vivo processes to assess in vitro is recovery and repair of ocular injury. A newly developed instrument, the silicon microphysiometer, measures small changes in extracellular pH and can been used to monitor cultured cells in real time. This analysis has been shown to correlate fairly well with in vivo recovery and has been used as an in vitro test for this process. This has been a brief overview of the types of tests being employed as alternatives to the Draize test for ocular irritation. It is likely that within the next several years a complete series of in vitro test batteries will be defined and each will be validated for its specific purpose.
Validation
The key to regulatory acceptance and implementation of in vitro test methodologies is validation, the process by which the credibility of a candidate test is established for a specific purpose. Efforts to define and coordinate the validation process have been made both in the United States and in Europe. The European Union established the European Centre for the Validation of Alternative Methods (ECVAM) in 1993 to coordinate efforts there and to interact with American organizations such as the Johns Hopkins Centre for Alternatives to Animal Testing (CAAT), an academic centre in the United States, and the Interagency Coordinating Committee for the Validation of Alternative Methods (ICCVAM), composed of representatives from the National Institutes of Health, the US Environmental Protection Agency, the US Food and Drug Administration and the Consumer Products Safety Commission.
Validation of in vitro tests requires substantial organization and planning. There must be consensus among government regulators and industrial and academic scientists on acceptable procedures, and sufficient oversight by a scientific advisory board to ensure that the protocols meet set standards. The validation studies should be performed in a series of reference laboratories using calibrated sets of chemicals from a chemical bank and cells or tissues from a single source. Both intralaboratory repeatability and interlaboratory reproducibility of a candidate test must be demonstrated and the results subjected to appropriate statistical analysis. Once the results from the different components of the validation studies have been compiled, the scientific advisory board can make recommendations on the validity of the candidate test(s) for a specific purpose. In addition, results of the studies should be published in peer-reviewed journals and placed in a database.
The definition of the validation process is currently a work in progress. Each new validation study will provide information useful to the design of the next study. International communication and cooperation are essential for the expeditious development of a widely acceptable series of protocols, particularly given the increased urgency imposed by the passage of the EC Cosmetics Directive. This legislation may indeed provide the needed impetus for a serious validation effort to be undertaken. It is only through completion of this process that the acceptance of in vitro methods by the various regulatory communities can commence.
Conclusion
This article has provided a broad overview of the current status of in vitro toxicity testing. The science of in vitro toxicology is relatively young, but it is growing exponentially. The challenge for the years ahead is to incorporate the mechanistic knowledge generated by cellular and molecular studies into the vast inventory of in vivo data to provide a more complete description of toxicological mechanisms as well as to establish a paradigm by which in vitro data may be used to predict toxicity in vivo. It will only be through the concerted efforts of toxicologists and government representatives that the inherent value of these in vitro methods can be realized.
Genetic Toxicity Assessment
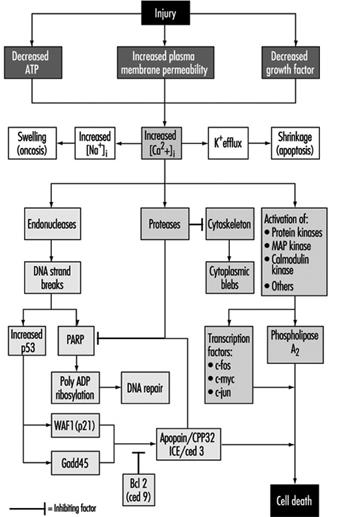
Genetic toxicity assessment is the evaluation of agents for their ability to induce any of three general types of changes (mutations) in the genetic material (DNA): gene, chromosomal and genomic. In organisms such as humans, the genes are composed of DNA, which consists of individual units called nucleotide bases. The genes are arranged in discrete physical structures called chromosomes. Genotoxicity can result in significant and irreversible effects upon human health. Genotoxic damage is a critical step in the induction of cancer and it can also be involved in the induction of birth defects and foetal death. The three classes of mutations mentioned above can occur within either of the two types of tissues possessed by organisms such as humans: sperm or eggs (germ cells) and the remaining tissue (somatic cells).
Assays that measure gene mutation are those that detect the substitution, addition or deletion of nucleotides within a gene. Assays that measure chromosomal mutation are those that detect breaks or chromosomal rearrangements involving one or more chromosomes. Assays that measure genomic mutation are those that detect changes in the number of chromosomes, a condition called aneuploidy. Genetic toxicity assessment has changed considerably since the development by Herman Muller in 1927 of the first assay to detect genotoxic (mutagenic) agents. Since then, more than 200 assays have been developed that measure mutations in DNA; however, fewer than ten assays are used commonly today for genetic toxicity assessment. This article reviews these assays, describes what they measure, and explores the role of these assays in toxicity assessment.
Identification of Cancer HazardsPrior to the Development of the Fieldof Genetic Toxicology
Genetic toxicology has become an integral part of the overall risk assessment process and has gained in stature in recent times as a reliable predictor for carcinogenic activity. However, prior to the development of genetic toxicology (before 1970), other methods were and are still being used to identify potential cancer hazards to humans. There are six major categories of methods currently used for identifying human cancer risks: epidemiological studies, long-term in vivo bioassays, mid-term in vivo bioassays, short-term in vivo and in vitro bioassays, artificial intelligence (structure-activity), and mechanism-based inference.
Table 1 gives advantages and disadvantages for these methods.
Table 1. Advantages and disadvantages of current methods for identifying human cancer risks
| Advantages | Disadvantages | |
| Epidemiological studies | (1) humans are ultimate indicators of disease; (2) evaluate sensitive or susceptible populations; (3) occupational exposure cohorts; (4) environmental sentinel alerts |
(1) generally retrospective (death certificates, recall biases, etc.); (2) insensitive, costly, lengthy; (3) reliable exposure data sometimes unavailable or difficult to obtain; (4) combined, multiple and complex exposures; lack of appropriate control cohorts; (5) experiments on humans not done; (6) cancer detection, not prevention |
| Long-term in vivo bioassays | (1) prospective and retrospective (validation) evaluations; (2) excellent correlation with identified human carcinogens; (3) exposure levels and conditions known; (4) identifies chemical toxicity and carcinogenicity effects; (5) results obtained relatively quickly; (6) qualitative comparisons among chemical classes; (7) integrative and interactive biologic systems related closely to humans | (1) rarely replicated, resource intensive; (3) limited facilities suitable for such experiments; (4) species extrapolation debate; (5) exposures used are often at levels far in excess of those experienced by humans; (6) single-chemical exposure does not mimic human exposures, which are generally to multiple chemicals simultaneously |
| Mid- and short-term in vivo and in vitro bioassays | (1) more rapid and less expensive than other assays; (2) large samples that are easily replicated; (3) biologically meaningful end points are measured (mutation, etc.); (4) can be used as screening assays to select chemicals for long-term bioassays |
(1) in vitro not fully predictive of in vivo; (2) usually organism or organ specific; (3) potencies not comparable to whole animals or humans |
| Chemical structure–biological activity associations | (1) relatively easy, rapid, and inexpensive; (2) reliable for certain chemical classes (e.g., nitrosamines and benzidine dyes); (3) developed from biological data but not dependent on additional biological experimentation | (1) not “biological”; (2) many exceptions to formulated rules; (3) retrospective and rarely (but becoming) prospective |
| Mechanism-based inferences | (1) reasonably accurate for certain classes of chemicals; (2) permits refinements of hypotheses; (3) can orient risk assessments to sensitive populations | (1) mechanisms of chemical carcinogenesis undefined, multiple, and likely chemical or class specific; (2) may fail to highlight exceptions to general mechanisms |
Rationale and Conceptual Basisfor Genetic Toxicology Assays
Although the exact types and numbers of assays used for genetic toxicity assessment are constantly evolving and vary from country to country, the most common ones include assays for (1) gene mutation in bacteria and/or cultured mammalian cells and (2) chromosomal mutation in cultured mammalian cells and/or bone marrow within living mice. Some of the assays within this second category can also detect aneuploidy. Although these assays do not detect mutations in germ cells, they are used primarily because of the extra cost and complexity of performing germ-cell assays. Nonetheless, germ-cell assays in mice are used when information about germ-cell effects is desired.
Systematic studies over a 25-year period (1970-1995), especially at the US National Toxicology Program in North Carolina, have resulted in the use of a discrete number of assays for detecting the mutagenic activity of agents. The rationale for evaluating the usefulness of the assays was based on their ability to detect agents that cause cancer in rodents and that are suspected of causing cancer in humans (i.e., carcinogens). This is because studies during the past several decades have indicated that cancer cells contain mutations in certain genes and that many carcinogens are also mutagens. Thus, cancer cells are viewed as containing somatic-cell mutations, and carcinogenesis is viewed as a type of somatic-cell mutagenesis.
The genetic toxicity assays used most commonly today have been selected not only because of their large database, relatively low cost, and ease of performance, but because they have been shown to detect many rodent and, presumptively, human carcinogens. Consequently, genetic toxicity assays are used to predict the potential carcinogenicity of agents.
An important conceptual and practical development in the field of genetic toxicology was the recognition that many carcinogens were modified by enzymes within the body, creating altered forms (metabolites) that were frequently the ultimate carcinogenic and mutagenic form of the parent chemical. To duplicate this metabolism in a petri dish, Heinrich Malling showed that the inclusion of a preparation from rodent liver contained many of the enzymes necessary to perform this metabolic conversion or activation. Thus, many genetic toxicity assays performed in dishes or tubes (in vitro) employ the addition of similar enzyme preparations. Simple preparations are called S9 mix, and purified preparations are called microsomes. Some bacterial and mammalian cells have now been genetically engineered to contain some of the genes from rodents or humans that produce these enzymes, reducing the need to add S9 mix or microsomes.
Genetic Toxicology Assays and Techniques
The primary bacterial systems used for genetic toxicity screening are the Salmonella (Ames) mutagenicity assay and, to a much lesser extent, strain WP2 of Escherichia coli. Studies in the mid-1980s indicated that the use of only two strains of the Salmonella system (TA98 and TA100) were sufficient to detect approximately 90% of the known Salmonella mutagens. Thus, these two strains are used for most screening purposes; however, various other strains are available for more extensive testing.
These assays are performed in a variety of ways, but two general procedures are the plate-incorporation and liquid-suspension assays. In the plate-incorporation assay, the cells, the test chemical and (when desired) the S9 are added together into a liquefied agar and poured onto the surface of an agar petri plate. The top agar hardens within a few minutes, and the plates are incubated for two to three days, after which time mutant cells have grown to form visually detectable clusters of cells called colonies, which are then counted. The agar medium contains selective agents or is composed of ingredients such that only the newly mutated cells will grow. The liquid-incubation assay is similar, except the cells, test agent, and S9 are incubated together in liquid that does not contain liquefied agar, and then the cells are washed free of the test agent and S9 and seeded onto the agar.
Mutations in cultured mammalian cells are detected primarily in one of two genes: hprt and tk. Similar to the bacterial assays, mammalian cell lines (developed from rodent or human cells) are exposed to the test agent in plastic culture dishes or tubes and then are seeded into culture dishes that contain medium with a selective agent that permits only mutant cells to grow. The assays used for this purpose include the CHO/HPRT, the TK6, and the mouse lymphoma L5178Y/TK+/- assays. Other cell lines containing various DNA repair mutations as well as containing some human genes involved in metabolism are also used. These systems permit the recovery of mutations within the gene (gene mutation) as well as mutations involving regions of the chromosome flanking the gene (chromosomal mutation). However, this latter type of mutation is recovered to a much greater extent by the tk gene systems than by the hprt gene systems due to the location of the tk gene.
Similar to the liquid-incubation assay for bacterial mutagenicity, mammalian cell mutagenicity assays generally involve the exposure of the cells in culture dishes or tubes in the presence of the test agent and S9 for several hours. The cells are then washed, cultured for several more days to allow the normal (wild-type) gene products to be degraded and the newly mutant gene products to be expressed and accumulate, and then they are seeded into medium containing a selective agent that permits only the mutant cells to grow. Like the bacterial assays, the mutant cells grow into visually detectable colonies that are then counted.
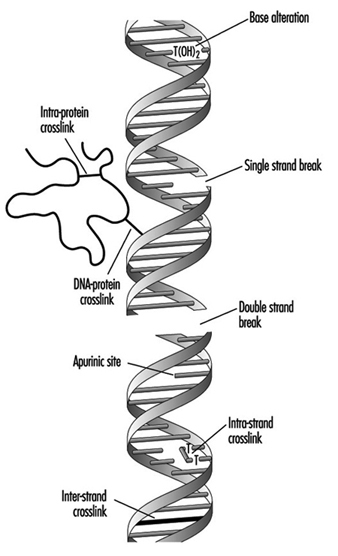
Chromosomal mutation is identified primarily by cytogenetic assays, which involve exposing rodents and/or rodent or human cells in culture dishes to a test chemical, allowing one or more cell divisions to occur, staining the chromosomes, and then visually examining the chromosomes through a microscope to detect alterations in the structure or number of chromosomes. Although a variety of endpoints can be examined, the two that are currently accepted by regulatory agencies as being the most meaningful are chromosomal aberrations and a subcategory called micronuclei.
Considerable training and expertise are required to score cells for the presence of chromosomal aberrations, making this a costly procedure in terms of time and money. In contrast, micronuclei require little training, and their detection can be automated. Micronuclei appear as small dots within the cell that are distinct from the nucleus, which contains the chromosomes. Micronuclei result from either chromosome breakage or from aneuploidy. Because of the ease of scoring micronuclei compared to chromosomal aberrations, and because recent studies indicate that agents that induce chromosomal aberrations in the bone marrow of living mice generally induce micronuclei in this tissue, micronuclei are now commonly measured as an indication of the ability of an agent to induce chromosomal mutation.
Although germ-cell assays are used far less frequently than the other assays described above, they are indispensable in determining whether an agent poses a risk to the germ cells, mutations in which can lead to health effects in succeeding generations. The most commonly used germ-cell assays are in mice, and involve systems that detect (1) heritable translocations (exchanges) among chromosomes (heritable translocation assay), (2) gene or chromosomal mutations involving specific genes (visible or biochemical specific-locus assays), and (3) mutations that affect viability (dominant lethal assay). As with the somatic-cell assays, the working assumption with the germ-cell assays is that agents positive in these assays are presumed to be potential human germ-cell mutagens.
Current Status and Future Prospects
Recent studies have indicated that only three pieces of information were necessary to detect approximately 90% of a set of 41 rodent carcinogens (i.e., presumptive human carcinogens and somatic-cell mutagens). These included (1) knowledge of the chemical structure of agent, especially if it contains electrophilic moieties (see section on structure-activity relationships); (2) Salmonella mutagenicity data; and (3) data from a 90-day chronic toxicity assay in rodents (mice and rats). Indeed, essentially all of the IARC-declared human carcinogens are detectable as mutagens using just the Salmonella assay and the mouse-bone marrow micronucleus assay. The use of these mutagenicity assays for detecting potential human carcinogens is supported further by the finding that most human carcinogens are carcinogenic in both rats and mice (trans-species carcinogens) and that most trans- species carcinogens are mutagenic in Salmonella and/or induce micronuclei in mouse bone marrow.
With advances in DNA technology, the human genome project, and an improved understanding of the role of mutation in cancer, new genotoxicity assays are being developed that will likely be incorporated into standard screening procedures. Among these are the use of transgenic cells and rodents. Transgenic systems are those in which a gene from another species has been introduced into a cell or organism. For example, transgenic mice are now in experimental use that permit the detection of mutation in any organ or tissue of the animal, based on the introduction of a bacterial gene into the mouse. Bacterial cells, such as Salmonella, and mammalian cells (including human cell lines) are now available that contain genes involved in the metabolism of carcinogenic/mutagenic agents, such as the P450 genes. Molecular analysis of the actual mutations induced in the trans-gene within transgenic rodents, or within native genes such as hprt, or the target genes within Salmonella can now be performed, so that the exact nature of the mutations induced by the chemicals can be determined, providing insights into the mechanism of action of the chemical and allowing comparisons to mutations in humans presumptively exposed to the agent.
Molecular advances in cytogenetics now permit more detailed evaluation of chromosomal mutations. These include the use of probes (small pieces of DNA) that attach (hybridize) to specific genes. Rearrangements of genes on the chromosome can then be revealed by the altered location of the probes, which are fluorescent and easily visualized as colored sectors on the chromosomes. The single-cell gel electrophoresis assay for DNA breakage (commonly called the “comet” assay) permits the detection of DNA breaks within single cells and may become an extremely useful tool in combination with cytogenetic techniques for detecting chromosomal damage.
After many years of use and the generation of a large and systematically developed database, genetic toxicity assessment can now be done with just a few assays for relatively small cost in a short period of time (a few weeks). The data produced can be used to predict the ability of an agent to be a rodent and, presumptively, human carcinogen/somatic-cell mutagen. Such an ability makes it possible to limit the introduction into the environment of mutagenic and carcinogenic agents and to develop alternative, nonmutagenic agents. Future studies should lead to even better methods with greater predictivity than the current assays.
Biomarkers
The word biomarker is short for biological marker, a term that refers to a measurable event occurring in a biological system, such as the human body. This event is then interpreted as a reflection, or marker, of a more general state of the organism or of life expectancy. In occupational health, a biomarker is generally used as an indicator of health status or disease risk.
Biomarkers are used for in vitro as well as in vivo studies that may include humans. Usually, three specific types of biological markers are identified. Although a few biomarkers may be difficult to classify, usually they are separated into biomarkers of exposure, biomarkers of effect or biomarkers of susceptibility (see table 1).
Table 1. Examples of biomarkers of exposure or biomarkers of effect that are used in toxicological studies in occupational health
| Sample | Measurement | Purpose |
| Exposure biomarkers | ||
| Adipose tissue | Dioxin | Dioxin exposure |
| Blood | Lead | Lead exposure |
| Bone | Aluminium | Aluminium exposure |
| Exhaled breath | Toluene | Toluene exposure |
| Hair | Mercury | Methylmercury exposure |
| Serum | Benzene | Benzene exposure |
| Urine | Phenol | Benzene exposure |
| Effect biomarkers | ||
| Blood | Carboxyhaemoglobin | Carbon monoxide exposure |
| Red blood cells | Zinc-protoporphyrin | Lead exposure |
| Serum | Cholinesterase | Organophosphate exposure |
| Urine | Microglobulins | Nephrotoxic exposure |
| White blood cells | DNA adducts | Mutagen exposure |
Given an acceptable degree of validity, biomarkers may be employed for several purposes. On an individual basis, a biomarker may be used to support or refute a diagnosis of a particular type of poisoning or other chemically-induced adverse effect. In a healthy subject, a biomarker may also reflect individual hypersusceptibility to specific chemical exposures and may therefore serve as a basis for risk prediction and counselling. In groups of exposed workers, some exposure biomarkers can be applied to assess the extent of compliance with pollution abatement regulations or the effectiveness of preventive efforts in general.
Biomarkers of Exposure
An exposure biomarker may be an exogenous compound (or a metabolite) within the body, an interactive product between the compound (or metabolite) and an endogenous component, or another event related to the exposure. Most commonly, biomarkers of exposures to stable compounds, such as metals, comprise measurements of the metal concentrations in appropriate samples, such as blood, serum or urine. With volatile chemicals, their concentration in exhaled breath (after inhalation of contamination-free air) may be assessed. If the compound is metabolized in the body, one or more metabolites may be chosen as a biomarker of the exposure; metabolites are often determined in urine samples.
Modern methods of analysis may allow separation of isomers or congeners of organic compounds, and determination of the speciation of metal compounds or isotopic ratios of certain elements. Sophisticated analyses allow determination of changes in the structure of DNA or other macromolecules caused by binding with reactive chemicals. Such advanced techniques will no doubt gain considerably in importance for applications in biomarker studies, and lower detection limits and better analytical validity are likely to make these biomarkers even more useful.
Particularly promising developments have occurred with biomarkers of exposure to mutagenic chemicals. These compounds are reactive and may form adducts with macromolecules, such as proteins or DNA. DNA adducts may be detected in white blood cells or tissue biopsies, and specific DNA fragments may be excreted in the urine. For example, exposure to ethylene oxide results in reactions with DNA bases, and, after excision of the damaged base, N-7-(2-hydroxyethyl)guanine will be eliminated in the urine. Some adducts may not refer directly to a particular exposure. For example, 8-hydroxy-2´-deoxyguanosine reflects oxidative damage to DNA, and this reaction may be triggered by several chemical compounds, most of which also induce lipid peroxidation.
Other macromolecules may also be changed by adduct formation or oxidation. Of special interest, such reactive compounds may generate haemoglobin adducts that can be determined as biomarkers of exposure to the compounds. The advantage is that ample amounts of haemoglobin can be obtained from a blood sample, and, given the four-month lifetime of red blood cells, the adducts formed with the amino acids of the protein will indicate the total exposure during this period.
Adducts may be determined by sensitive techniques such as high-performance lipid chromatography, and some immunological methods are also available. In general, the analytical methods are new, expensive and need further development and validation. Better sensitivity can be obtained by using the 32P post labelling assay, which is a nonspecific indication that DNA damage has taken place. All of these techniques are potentially useful for biological monitoring and have been applied in a growing number of studies. However, simpler and more sensitive analytical methods are needed. Given the limited specificity of some methods at low-level exposures, tobacco smoking or other factors may impact significantly on the measurement results, thus causing difficulties in interpretation.
Exposure to mutagenic compounds, or to compounds which are metabolized into mutagens, may also be determined by assessing the mutagenicity of the urine from an exposed individual. The urine sample is incubated with a strain of bacteria in which a specific point mutation is expressed in a way that can be easily measured. If mutagenic chemicals are present in the urine sample, then an increased rate of mutations will occur in the bacteria.
Exposure biomarkers must be evaluated with regard to temporal variation in exposure and the relation to different compartments. Thus, the time frame(s) represented by the biomarker, that is, the extent to which the biomarker measurement reflects past exposure(s) and/or accumulated body burden, must be determined from toxicokinetic data in order to interpret the result. In particular, the degree to which the biomarker indicates retention in specific target organs should be considered. Although blood samples are often used for biomarker studies, peripheral blood is generally not regarded as a compartment as such, although it acts as a transport medium between compartments. The degree to which the concentration in the blood reflects levels in different organs varies widely between different chemicals, and usually also depends upon the length of the exposure as well as time since exposure.
Sometimes this type of evidence is used to classify a biomarker as an indicator of (total) absorbed dose or an indicator of effective dose (i.e., the amount that has reached the target tissue). For example, exposure to a particular solvent may be evaluated from data on the actual concentration of the solvent in the blood at a particular time following the exposure. This measurement will reflect the amount of the solvent that has been absorbed into the body. Some of the absorbed amount will be exhaled due to the vapour pressure of the solvent. While circulating in the blood, the solvent will interact with various components of the body, and it will eventually become subject to breakdown by enzymes. The outcome of the metabolic processes can be assessed by determining specific mercapturic acids produced by conjugation with glutathione. The cumulative excretion of mercapturic acids may better reflect the effective dose than will the blood concentration.
Life events, such as reproduction and senescence, may affect the distribution of a chemical. The distribution of chemicals within the body is significantly affected by pregnancy, and many chemicals may pass the placental barrier, thus causing exposure of the foetus. Lactation may result in excretion of lipid-soluble chemicals, thus leading to a decreased retention in the mother along with an increased uptake by the infant. During weight loss or development of osteoporosis, stored chemicals may be released, which can then result in a renewed and protracted “endogenous” exposure of target organs. Other factors may affect individual absorption, metabolism, retention and distribution of chemical compounds, and some biomarkers of susceptibility are available (see below).
Biomarkers of Effect
A marker of effect may be an endogenous component, or a measure of the functional capacity, or some other indicator of the state or balance of the body or organ system, as affected by the exposure. Such effect markers are generally preclinical indicators of abnormalities.
These biomarkers may be specific or non-specific. The specific biomarkers are useful because they indicate a biological effect of a particular exposure, thus providing evidence that can potentially be used for preventive purposes. The non-specific biomarkers do not point to an individual cause of the effect, but they may reflect the total, integrated effect due to a mixed exposure. Both types of biomarkers may therefore be of considerable use in occupational health.
There is not a clear distinction between exposure biomarkers and effect biomarkers. For example, adduct formation could be said to reflect an effect rather than the exposure. However, effect biomarkers usually indicate changes in the functions of cells, tissues or the total body. Some researchers include gross changes, such as an increase in liver weight of exposed laboratory animals or decreased growth in children, as biomarkers of effect. For the purpose of occupational health, effect biomarkers should be restricted to those that indicate subclinical or reversible biochemical changes, such as inhibition of enzymes. The most frequently used effect biomarker is probably inhibition of cholinesterase caused by certain insecticides, that is, organophosphates and carbamates. In most cases, this effect is entirely reversible, and the enzyme inhibition reflects the total exposure to this particular group of insecticides.
Some exposures do not result in enzyme inhibition but rather in increased activity of an enzyme. This is the case with several enzymes that belong to the P450 family (see “Genetic determinants of toxic response”). They may be induced by exposures to certain solvents and polyaromatic hydrocarbons (PAHs). Since these enzymes are mainly expressed in tissues from which a biopsy may be difficult to obtain, the enzyme activity is determined indirectly in vivo by administering a compound that is metabolized by that particular enzyme, and then the breakdown product is measured in urine or plasma.
Other exposures may induce the synthesis of a protective protein in the body. The best example is probably metallothionein, which binds cadmium and promotes the excretion of this metal; cadmium exposure is one of the factors that result in increased expression of the metallothionein gene. Similar protective proteins may exist but have not yet been explored sufficiently to become accepted as biomarkers. Among the candidates for possible use as biomarkers are the so-called stress proteins, originally referred to as heat shock proteins. These proteins are generated by a range of different organisms in response to a variety of adverse exposures.
Oxidative damage may be assessed by determining the concentration of malondialdehyde in serum or the exhalation of ethane. Similarly, the urinary excretion of proteins with a small molecular weight, such as albumin, may be used as a biomarker of early kidney damage. Several parameters routinely used in clinical practice (for example, serum hormone or enzyme levels) may also be useful as biomarkers. However, many of these parameters may not be sufficiently sensitive to detect early impairment.
Another group of effect parameters relate to genotoxic effects (changes in the structure of chromosomes). Such effects may be detected by microscopy of white blood cells that undergo cell division. Serious damage to the chromosomes—chromosomal aberrations or formation of micronuclei—can be seen in a microscope. Damage may also be revealed by adding a dye to the cells during cell division. Exposure to a genotoxic agent can then be visualized as an increased exchange of the dye between the two chromatids of each chromosome (sister chromatid exchange). Chromosomal aberrations are related to an increased risk of developing cancer, but the significance of an increased rate of sister chromatid exchange is less clear.
More sophisticated assessment of genotoxicity is based on particular point mutations in somatic cells, that is, white blood cells or epithelial cells obtained from the oral mucosa. A mutation at a specific locus may make the cells capable of growing in a culture that contains a chemical that is otherwise toxic (such as 6-thioguanine). Alternatively, a specific gene product can be assessed (e.g., serum or tissue concentrations of oncoproteins encoded by particular oncogenes). Obviously, these mutations reflect the total genotoxic damage incurred and do not necessarily indicate anything about the causative exposure. These methods are not yet ready for practical use in occupational health, but rapid progress in this line of research would suggest that such methods will become available within a few years.
Biomarkers of Susceptibility
A marker of susceptibility, whether inherited or induced, is an indicator that the individual is particularly sensitive to the effect of a xenobiotic or to the effects of a group of such compounds. Most attention has been focused on genetic susceptibility, although other factors may be at least as important. Hypersusceptibility may be due to an inherited trait, the constitution of the individual, or environmental factors.
The ability to metabolize certain chemicals is variable and is genetically determined (see “Genetic determinants of toxic response”). Several relevant enzymes appear to be controlled by a single gene. For example, oxidation of foreign chemicals is mainly carried out be a family of enzymes belonging to the P450 family. Other enzymes make the metabolites more water soluble by conjugation (e.g., N-acetyltransferase and μ-glutathion-S-transferase). The activity of these enzymes is genetically controlled and varies considerably. As mentioned above, the activity can be determined by administering a small dose of a drug and then determining the amount of the metabolite in the urine. Some of the genes have now been characterized, and techniques are available to determine the genotype. Important studies suggest that a risk of developing certain cancer forms is related to the capability of metabolizing foreign compounds. Many questions still remain unanswered, thus at this time limiting the use of these potential susceptibility biomarkers in occupational health.
Other inherited traits, such as alpha1-antitrypsin deficiency or glucose-6-phosphate dehydrogenase deficiency, also result in deficient defence mechanisms in the body, thereby causing hypersusceptibility to certain exposures.
Most research related to susceptibility has dealt with genetic predisposition. Other factors play a role as well and have been partly neglected. For example, individuals with a chronic disease may be more sensitive to an occupational exposure. Also, if a disease process or previous exposure to toxic chemicals has caused some subclinical organ damage, then the capacity to withstand a new toxic exposure is likely to be less. Biochemical indicators of organ function may in this case be used as susceptibility biomarkers. Perhaps the best example regarding hypersusceptibility relates to allergic responses. If an individual has become sensitized to a particular exposure, then specific antibodies can be detected in serum. Even if the individual has not become sensitized, other current or past exposures may add to the risk of developing an adverse effect related to an occupational exposure.
A major problem is to determine the joint effect of mixed exposures at work. In addition, personal habits and drug use may result in an increased susceptibility. For example, tobacco smoke usually contains a considerable amount of cadmium. Thus, with occupational exposure to cadmium, a heavy smoker who has accumulated substantial amounts of this metal in the body will be at increased risk of developing cadmium-related kidney disease.
Application in Occupational Health
Biomarkers are extremely useful in toxicological research, and many may be applicable in biological monitoring. Nonetheless, the limitations must also be recognized. Many biomarkers have so far been studied only in laboratory animals. Toxicokinetic patterns in other species may not necessarily reflect the situation in human beings, and extrapolation may require confirmatory studies in human volunteers. Also, account must be taken of individual variations due to genetic or constitutional factors.
In some cases, exposure biomarkers may not at all be feasible (e.g., for chemicals which are short-lived in vivo). Other chemicals may be stored in, or may affect, organs which cannot be accessed by routine procedures, such as the nervous system. The route of exposure may also affect the distribution pattern and therefore also the biomarker measurement and its interpretation. For example, direct exposure of the brain via the olfactory nerve is likely to escape detection by measurement of exposure biomarkers. As to effect biomarkers, many of them are not at all specific, and the change can be due to a variety of causes, including lifestyle factors. Perhaps in particular with the susceptibility biomarkers, interpretation must be very cautious at the moment, as many uncertainties remain about the overall health significance of individual genotypes.
In occupational health, the ideal biomarker should satisfy several requirements. First of all, sample collection and analysis must be simple and reliable. For optimal analytical quality, standardization is needed, but the specific requirements vary considerably. Major areas of concern include: preparation of the in- dividual, sampling procedure and sample handling, and measurement procedure; the latter encompasses technical factors, such as calibration and quality assurance procedures, and individual- related factors, such as education and training of operators.
For documentation of analytical validity and traceability, reference materials should be based on relevant matrices and with appropriate concentrations of toxic substances or relevant metabolites at appropriate levels. For biomarkers to be used for biological monitoring or for diagnostic purposes, the responsible laboratories must have well-documented analytical procedures with defined performance characteristics, and accessible records to allow verification of the results. At the same time, nonetheless, the economics of characterizing and using reference materials to supplement quality assurance procedures in general must be considered. Thus, the achievable quality of results, and the uses to which they are put, have to be balanced against the added costs of quality assurance, including reference materials, manpower and instrumentation.
Another requirement is that the biomarker should be specific, at least under the circumstances of the study, for a particular type of exposure, with a clear-cut relationship to the degree of exposure. Otherwise, the result of the biomarker measurement may be too difficult to interpret. For proper interpretation of the measurement result of an exposure biomarker, the diagnostic validity must be known (i.e., the translation of the biomarker value into the magnitude of possible health risks). In this area, metals serve as a paradigm for biomarker research. Recent research has demonstrated the complexity and subtlety of dose-response relationships, with considerable difficulty in identifying no-effect levels and therefore also in defining tolerable exposures. However, this kind of research has also illustrated the types of investigation and the refinement that are necessary to uncover the relevant information. For most organic compounds, quantitative associations between exposures and the corresponding adverse health effects are not yet available; in many cases, even the primary target organs are not known for sure. In addition, evaluation of toxicity data and biomarker concentrations is often complicated by exposure to mixtures of substances, rather than exposure to a single compound at the time.
Before the biomarker is applied for occupational health purposes, some additional considerations are necessary. First, the biomarker must reflect a subclinical and reversible change only. Second, given that the biomarker results can be interpreted with regard to health risks, then preventive efforts should be available and should be considered realistic in case the biomarker data suggests a need to reduce the exposure. Third, the practical use of the biomarker must be generally regarded as ethically acceptable.
Industrial hygiene measurements may be compared with applicable exposure limits. Likewise, results on exposure biomarkers or effect biomarkers may be compared to biological action limits, sometimes referred to as biological exposure indices. Such limits should be based on the best advice of clinicians and scientists from appropriate disciplines, and responsible administrators as “risk managers” should then take into account relevant ethical, social, cultural and economic factors. The scientific basis should, if possible, include dose-response relationships supplemented by information on variations in susceptibility within the population at risk. In some countries, workers and members of the general public are involved in the standard-setting process and provide important input, particularly when scientific uncertainty is considerable. One of the major uncertainties is how to define an adverse health effect that should be prevented—for example, whether adduct formation as an exposure biomarker by itself represents an adverse effect (i.e., effect biomarker) that should be prevented. Difficult questions are likely to arise when deciding whether it is ethically defensible, for the same compound, to have different limits for adventitious exposure, on the one hand, and occupational exposure, on the other.
The information generated by the use of biomarkers should generally be conveyed to the individuals examined within the physician-patient relationship. Ethical concerns must in particular be considered in connection with highly experimental biomarker analyses that cannot currently be interpreted in detail in terms of actual health risks. For the general population, for example, limited guidance exists at present with regard to interpretation of exposure biomarkers other than the blood-lead concentration. Also of importance is the confidence in the data generated (i.e., whether appropriate sampling has been done, and whether sound quality assurance procedures have been utilized in the laboratory involved). An additional area of special worry relates to individual hypersusceptibility. These issues must be taken into account when providing the feedback from the study.
All sectors of society affected by, or concerned with carrying out, a biomarker study need to be involved in the decision-making process on how to handle the information generated by the study. Specific procedures to prevent or overcome inevitable ethical conflicts should be developed within the legal and social frameworks of the region or country. However, each situation represents a different set of questions and pitfalls, and no single procedure for public involvement can be developed to cover all applications of exposure biomarkers.
Target Organ Toxicology
The study and characterization of chemicals and other agents for toxic properties is often undertaken on the basis of specific organs and organ systems. In this chapter, two targets have been selected for in-depth discussion: the immune system and the gene. These examples were chosen to represent a complex target organ system and a molecular target within cells. For more comprehensive discussion of the toxicology of target organs, the reader is referred to standard toxicology texts such as Casarett and Doull, and Hayes. The International Programme on Chemical Safety (IPCS) has also published several criteria documents on target organ toxicology, by organ system.
Target organ toxicology studies are usually undertaken on the basis of information indicating the potential for specific toxic effects of a substance, either from epidemiological data or from general acute or chronic toxicity studies, or on the basis of special concerns to protect certain organ functions, such as reproduction or foetal development. In some cases, specific target organ toxicity tests are expressly mandated by statutory authorities, such as neurotoxicity testing under the US pesticides law (see “The United States approach to risk assessment of reproductive toxicants and neurotoxic agents,” and mutagenicity testing under the Japanese Chemical Substance Control Law (see “Principles of hazard identification: The Japanese approach”).
As discussed in “Target organ and critical effects,” the identification of a critical organ is based upon the detection of the organ or organ system which first responds adversely or to the lowest doses or exposures. This information is then used to design specific toxicology investigations or more defined toxicity tests that are designed to elicit more sensitive indications of intoxication in the target organ. Target organ toxicology studies may also be used to determine mechanisms of action, of use in risk assessment (see “The United States approach to risk assessment of reproductive toxicants and neurotoxic agents”).
Methods of Target Organ Toxicity Studies
Target organs may be studied by exposure of intact organisms and detailed analysis of function and histopathology in the target organ, or by in vitro exposure of cells, tissue slices, or whole organs maintained for short or long term periods in culture (see “Mechanisms of toxicology: Introduction and concepts”). In some cases, tissues from human subjects may also be available for target organ toxicity studies, and these may provide opportunities to validate assumptions of cross-species extrapolation. However, it must be kept in mind that such studies do not provide information on relative toxicokinetics.
In general, target organ toxicity studies share the following common characteristics: detailed histopathological examination of the target organ, including post mortem examination, tissue weight, and examination of fixed tissues; biochemical studies of critical pathways in the target organ, such as important enzyme systems; functional studies of the ability of the organ and cellular constituents to perform expected metabolic and other functions; and analysis of biomarkers of exposure and early effects in target organ cells.
Detailed knowledge of target organ physiology, biochemistry and molecular biology may be incorporated in target organ studies. For instance, because the synthesis and secretion of small-molecular-weight proteins is an important aspect of renal function, nephrotoxicity studies often include special attention to these parameters (IPCS 1991). Because cell-to-cell communication is a fundamental process of nervous system function, target organ studies in neurotoxicity may include detailed neurochemical and biophysical measurements of neurotransmitter synthesis, uptake, storage, release and receptor binding, as well as electrophysiological measurement of changes in membrane potential associated with these events.
A high degree of emphasis is being placed upon the development of in vitro methods for target organ toxicity, to replace or reduce the use of whole animals. Substantial advances in these methods have been achieved for reproductive toxicants (Heindel and Chapin 1993).
In summary, target organ toxicity studies are generally undertaken as a higher order test for determining toxicity. The selection of specific target organs for further evaluation depends upon the results of screening level tests, such as the acute or subchronic tests used by OECD and the European Union; some target organs and organ systems may be a priori candidates for special investigation because of concerns to prevent certain types of adverse health effects.
Immunotoxicology
The functions of the immune system are to protect the body from invading infectious agents and to provide immune surveillance against arising tumour cells. It has a first line of defence that is non-specific and that can initiate effector reactions itself, and an acquired specific branch, in which lymphocytes and antibodies carry the specificity of recognition and subsequent reactivity towards the antigen.
Immunotoxicology has been defined as “the discipline concerned with the study of the events that can lead to undesired effects as a result of interaction of xenobiotics with the immune system. These undesired events may result as a consequence of (1) a direct and/or indirect effect of the xenobiotic (and/or its biotransformation product) on the immune system, or (2) an immunologically based host response to the compound and/or its metabolite(s), or host antigens modified by the compound or its metabolites” (Berlin et al. 1987).
When the immune system acts as a passive target of chemical insults, the result can be decreased resistance to infection and certain forms of neoplasia, or immune disregulation/stimulation that can exacerbate allergy or auto-immunity. In the case that the immune system responds to the antigenic specificity of the xenobiotic or host antigen modified by the compound, toxicity can become manifest as allergies or autoimmune diseases.
Animal models to investigate chemical-induced immune suppression have been developed, and a number of these methods are validated (Burleson, Munson, and Dean 1995; IPCS 1996). For testing purposes, a tiered approach is followed to make an adequate selection from the overwhelming number of assays available. Generally, the objective of the first tier is to identify potential immunotoxicants. If potential immunotoxicity is identified, a second tier of testing is performed to confirm and characterize further the changes observed. Third-tier investigations include special studies on the mechanism of action of the compound. Several xenobiotics have been identified as immunotoxicants causing immunosuppression in such studies with laboratory animals.
The database on immune function disturbances in humans by environmental chemicals is limited (Descotes 1986; NRC Subcommittee on Immunotoxicology 1992). The use of markers of immunotoxicity has received little attention in clinical and epidemiological studies to investigate the effect of these chemicals on human health. Such studies have not been performed frequently, and their interpretation often does not permit unequivocal conclusions to be drawn, due for instance to the uncontrolled nature of exposure. Therefore, at present, immunotoxicity assessment in rodents, with subsequent extrapolation to man, forms the basis of decisions regarding hazard and risk.
Hypersensitivity reactions, notably allergic asthma and contact dermatitis, are important occupational health problems in industrialized countries (Vos, Younes and Smith 1995). The phenomenon of contact sensitization was investigated first in the guinea pig (Andersen and Maibach 1985). Until recently this has been the species of choice for predictive testing. Many guinea pig test methods are available, the most frequently employed being the guinea pig maximization test and the occluded patch test of Buehler. Guinea pig tests and newer approaches developed in mice, such as ear swelling tests and the local lymph node assay, provide the toxicologist with the tools to assess skin sensitization hazard. The situation with respect to sensitization of the respiratory tract is very different. There are, as yet, no well-validated or widely accepted methods available for the identification of chemical respiratory allergens although progress in the development of animal models for the investigation of chemical respiratory allergy has been achieved in the guinea pig and mouse.
Human data show that chemical agents, in particular drugs, can cause autoimmune diseases (Kammüller, Bloksma and Seinen 1989). There are a number of experimental animal models of human autoimmune diseases. Such comprise both spontaneous pathology (for example systemic lupus erythematosus in New Zealand Black mice) and autoimmune phenomena induced by experimental immunization with a cross-reactive autoantigen (for example the H37Ra adjuvant induced arthritis in Lewis strain rats). These models are applied in the preclinical evaluation of immunosuppressive drugs. Very few studies have addressed the potential of these models for assessment of whether a xenobiotic exacerbates induced or congenital autoimmunity. Animal models that are suitable to investigate the ability of chemicals to induce autoimmune diseases are virtually lacking. One model that is used to a limited extent is the popliteal lymph node assay in mice. Like the situation in humans, genetic factors play a crucial role in the development of autoimmune disease (AD) in laboratory animals, which will limit the predictive value of such tests.
The Immune System
The major function of the immune system is defence against bacteria, viruses, parasites, fungi and neoplastic cells. This is achieved by the actions of various cell types and their soluble mediators in a finely tuned concert. The host defence can be roughly divided into non-specific or innate resistance and specific or acquired immunity mediated by lymphocytes (Roitt, Brostoff and Male 1989).
Components of the immune system are present throughout the body (Jones et al. 1990). The lymphocyte compartment is found within lymphoid organs (figure 1). The bone marrow and thymus are classified as primary or central lymphoid organs; the secondary or peripheral lymphoid organs include lymph nodes, spleen and lymphoid tissue along secretory surfaces such as the gastrointestinal and respiratory tracts, the so-called mucosa-associated lymphoid tissue (MALT). About half of the body’s lymphocytes are located at any one time in MALT. In addition the skin is an important organ for the induction of immune responses to antigens present on the skin. Important in this process are epidermal Langerhans cells that have an antigen-presenting function.
Figure 1. Primary and secondary lymphoid organs and tissues
Phagocytic cells of the monocyte/macrophage lineage, called the mononuclear phagocyte system (MPS), occur in lymphoid organs and also at extranodal sites; the extranodal phagocytes include Kupffer cells in the liver, alveolar macrophages in the lung, mesangial macrophages in the kidney and glial cells in the brain. Polymorphonuclear leukocytes (PMNs) are present mainly in blood and bone marrow, but accumulate at sites of inflammation.
Non-specific defence
A first line of defence to micro-organisms is executed by a physical and chemical barrier, such as at the skin, the respiratory tract and the alimentary tract. This barrier is helped by non-specific protective mechanisms including phagocytic cells, such as macrophages and polymorphonuclear leukocytes, which are able to kill pathogens, and natural killer cells, which can lyse tumour cells and virus-infected cells. The complement system and certain microbial inhibitors (e.g., lysozyme) also take part in the non-specific response.
Specific immunity
After initial contact of the host with the pathogen, specific immune responses are induced. The hallmark of this second line of defence is specific recognition of determinants, so-called antigens or epitopes, of the pathogens by receptors on the cell surface of B- and T-lymphocytes. Following interaction with the specific antigen, the receptor-bearing cell is stimulated to undergo proliferation and differentiation, producing a clone of progeny cells that are specific for the eliciting antigen. The specific immune responses help the non-specific defence presented to the pathogens by stimulating the efficacy of the non-specific responses. A fundamental characteristic of specific immunity is that memory develops. Secondary contact with the same antigen provokes a faster and more vigorous but well-regulated response.
The genome does not have the capacity to carry the codes of an array of antigen receptors sufficient to recognize the number of antigens that can be encountered. The repertoire of specificity develops by a process of gene rearrangements. This is a random process, during which various specificities are brought about. This includes specificities for self components, which are undesirable. A selection process that takes place in the thymus (T cells), or bone marrow (B cells) operates to delete these undesirable specificities.
Normal immune effector function and homeostatic regulation of the immune response is dependent upon a variety of soluble products, known collectively as cytokines, which are synthesized and secreted by lymphocytes and by other cell types. Cytokines have pleiotropic effects on immune and inflammatory responses. Cooperation between different cell populations is required for the immune response—the regulation of antibody responses, the accumulation of immune cells and molecules at inflammatory sites, the initiation of acute phase responses, the control of macrophage cytotoxic function and many other processes central to host resistance. These are influenced by, and in many cases are dependent upon, cytokines acting individually or in concert.
Two arms of specific immunity are recognized—humoral immunity and cell-mediated or cellular immunity:
Humoral immunity. In the humoral arm B-lymphocytes are stimulated following recognition of antigen by cell-surface receptors. Antigen receptors on B-lymphocytes are immunoglobulins (Ig). Mature B cells (plasma cells) start the production of antigen-specific immunoglobulins that act as antibodies in serum or along mucosal surfaces. There are five major classes of immunoglobulins: (1) IgM, pentameric Ig with optimal agglutinating capacity, which is first produced after antigenic stimulation; (2) IgG, the main Ig in circulation, which can pass the placenta; (3) IgA, secretory Ig for the protection of mucosal surfaces; (4) IgE, Ig fixing to mast cells or basophilic granulocytes involved in immediate hypersensitivity reactions and (5) IgD, whose major function is as a receptor on B-lymphocytes.
Cell-mediated immunity. The cellular arm of the specific immune system is mediated by T-lymphocytes. These cells also have antigen receptors on their membranes. They recognize antigen if presented by antigen presenting cells in the context of histocompatibility antigens. Hence, these cells have a restriction in addition to the antigen specificity. T cells function as helper cells for various (including humoral) immune responses, mediate recruitment of inflammatory cells, and can, as cytotoxic T cells, kill target cells after antigen-specific recognition.
Mechanisms of Immunotoxicity
Immunosuppression
Effective host resistance is dependent upon the functional integrity of the immune system, which in turn requires that the component cells and molecules which orchestrate immune responses are available in sufficient numbers and in an operational form. Congenital immunodeficiencies in humans are often characterized by defects in certain stem cell lines, resulting in impaired or absent production of immune cells. By analogy with congenital and acquired human immunodeficiency diseases, chemical-induced immunosuppression may result simply from a reduced number of functional cells (IPCS 1996). The absence, or reduced numbers, of lymphocytes may have more or less profound effects on immune status. Some immunodeficiency states and severe immunosuppression, as can occur in transplantation or cytostatic therapy, have been associated in particular with increased incidences of opportunistic infections and of certain neoplastic diseases. The infections can be bacterial, viral, fungal or protozoan, and the predominant type of infection depends on the associated immunodeficiency. Exposure to immunosuppressive environmental chemicals may be expected to result in more subtle forms of immunosuppression, which may be difficult to detect. These may lead, for example, to an increased incidence of infections such as influenza or the common cold.
In view of the complexity of the immune system, with the wide variety of cells, mediators and functions that form a complicated and interactive network, immunotoxic compounds have numerous opportunities to exert an effect. Although the nature of the initial lesions induced by many immunotoxic chemicals have not yet been elucidated, there is increasing information available, mostly derived from studies in laboratory animals, regarding the immunobiological changes which result in depression of immune function (Dean et al. 1994). Toxic effects might occur at the following critical functions (and some examples are given of immunotoxic compounds affecting these functions):
- development and expansion of different stem cell populations (benzene exerts immunotoxic effects at the stem cell level, causing lymphocytopenia)
- proliferation of various lymphoid and myeloid cells as well as supportive tissues in which these cells mature and function (immunotoxic organotin compounds suppress the proliferative activity of lymphocytes in the thymic cortex through direct cytotoxicity; the thymotoxic action of 2,3,7,8-tetrachloro-dibenzo-p-dioxin (TCDD) and related compounds is likely due to an impaired function of thymic epithelial cells, rather than to direct toxicity for thymocytes)
- antigen uptake, processing and presentation by macrophages and other antigen-presenting cells (one of the targets of 7,12-dimethylbenz(a)anthracene (DMBA) and of lead is antigen presentation by macrophages; a target of ultraviolet radiation is the antigen-presenting Langerhans cell)
- regulatory function of T-helper and T-suppressor cells (T-helper cell function is impaired by organotins, aldicarb, polychlorinated biphenyls (PCBs), TCDD and DMBA; T-suppressor cell function is reduced by low-dose cyclophosphamide treatment)
- production of various cytokines or interleukins (benzo(a)pyrene (BP) suppresses interleukin-1 production; ultraviolet radiation alters production of cytokines by keratinocytes)
- synthesis of various classes of immunoglobulins IgM and IgG is suppressed following PCB and tributyltin oxide (TBT) treatment, and increased after hexachlorobenzene (HCB) exposure).
- complement regulation and activation (affected by TCDD)
- cytotoxic T cell function (3-methylcholanthrene (3-MC), DMBA, and TCDD suppress cytotoxic T cell activity)
- natural killer (NK) cell function (pulmonary NK activity is suppressed by ozone; splenic NK activity is impaired by nickel)
- macrophage and polymorphonuclear leukocyte chemotaxis and cytotoxic functions (ozone and nitrogen dioxide impair the phagocytic activity of alveolar macrophages).
Allergy
Allergy may be defined as the adverse health effects which result from the induction and elicitation of specific immune responses. When hypersensitivity reactions occur without involvement of the immune system the term pseudo-allergy is used. In the context of immunotoxicology, allergy results from a specific immune response to chemicals and drugs that are of interest. The ability of a chemical to sensitize individuals is generally related to its ability to bind covalently to body proteins. Allergic reactions may take a variety of forms and these differ with respect to both the underlying immunological mechanisms and the speed of the reaction. Four major types of allergic reactions have been recognized: Type I hypersensitivity reactions, which are effectuated by IgE antibody and where symptoms are manifest within minutes of exposure of the sensitized individual. Type II hypersensitivity reactions result from the damage or destruction of host cells by antibody. In this case symptoms become apparent within hours. Type III hypersensitivity, or Arthus, reactions are also antibody mediated, but against soluble antigen, and result from the local or systemic action of immune complexes. Type IV, or delayed-type hypersensitivity, reactions are effected by T-lymphocytes and normally symptoms develop 24to 48hours following exposure of the sensitized individual.
The two types of chemical allergy of greatest relevance to occupational health are contact sensitivity or skin allergy and allergy of the respiratory tract.
Contact hypersensitivity. A large number of chemicals are able to cause skin sensitization. Following topical exposure of a susceptible individual to a chemical allergen, a T-lymphocyte response is induced in the draining lymph nodes. In the skin the allergen interacts directly or indirectly with epidermal Langerhans cells, which transport the chemical to the lymph nodes and present it in an immunogenic form to responsive T-lymphocytes. Allergen- activated T-lymphocytes proliferate, resulting in clonal expansion. The individual is now sensitized and will respond to a second dermal exposure to the same chemical with a more aggressive immune response, resulting in allergic contact dermatitis. The cutaneous inflammatory reaction which characterizes allergic contact dermatitis is secondary to the recognition of the allergen in the skin by specific T-lymphocytes. These lymphocytes become activated, release cytokines and cause the local accumulation of other mononuclear leukocytes. Symptoms develop some 24 to 48 hours following exposure of the sensitized individual, and allergic contact dermatitis therefore represents a form of delayed-type hypersensitivity. Common causes of allergic contact dermatitis include organic chemicals (such as 2,4-dinitrochlorobenzene), metals (such as nickel and chromium) and plant products (such as urushiol from poison ivy).
Respiratory hypersensitivity. Respiratory hypersensitivity is usually considered to be a Type I hypersensitivity reaction. However, late phase reactions and the more chronic symptoms associated with asthma may involve cell-mediated (Type IV) immune processes. The acute symptoms associated with respiratory allergy are effected by IgE antibody, the production of which is provoked following exposure of the susceptible individual to the inducing chemical allergen. The IgE antibody distributes systemically and binds, via membrane receptors, to mast cells which are found in vascularized tissues, including the respiratory tract. Following inhalation of the same chemical a respiratory hypersensitivity reaction will be elicited. Allergen associates with protein and binds to, and cross-links, IgE antibody bound to mast cells. This in turn causes the degranulation of mast cells and the release of inflammatory mediators such as histamine and leukotrienes. Such mediators cause bronchoconstriction and vasodilation, resulting in the symptoms of respiratory allergy; asthma and/or rhinitis. Chemicals known to cause respiratory hypersensitivity in man include acid anhydrides (such as trimellitic anhydride), some diisocyanates (such as toluene diisocyanate), platinum salts and some reactive dyes. Also, chronic exposure to beryllium is known to cause hypersensitivity lung disease.
Autoimmunity
Autoimmunity can be defined as the stimulation of specific immune responses directed against endogenous “self” antigens. Induced autoimmunity can result either from alterations in the balance of regulatory T-lymphocytes or from the association of a xenobiotic with normal tissue components such as to render them immunogenic (“altered self”). Drugs and chemicals known to incidentally induce or exacerbate effects like those of autoimmune disease (AD) in susceptible individuals are low molecular weight compounds (molecular weight 100 to 500) that are generally considered to be not immunogenic themselves. The mechanism of AD by chemical exposure is mostly unknown. Disease can be produced directly by means of circulating antibody, indirectly through the formation of immune complexes, or as a consequence of cell-mediated immunity, but likely occurs through a combination of mechanisms. The pathogenesis is best known in immune haemolytic disorders induced by drugs:
- The drug can attach to the red-cell membrane and interact with a drug-specific antibody.
- The drug can alter the red-cell membrane so that the immune system regards the cell as foreign.
- The drug and its specific antibody form immune complexes that adhere to the red-cell membrane to produce injury.
- Red-cell sensitization occurs due to the production of red-cell autoantibody.
A variety of chemicals and drugs, in particular the latter, have been found to induce autoimmune-like responses (Kamüller, Bloksma and Seinen 1989). Occupational exposure to chemicals may incidentally lead to AD-like syndromes. Exposure to monomeric vinyl chloride, trichloroethylene, perchloroethylene, epoxy resins and silica dust may induce scleroderma-like syndromes. A syndrome similar to systemic lupus erythematosus (SLE) has been described after exposure to hydrazine. Exposure to toluene diisocyanate has been associated with the induction of thrombocytopenic purpura. Heavy metals such as mercury have been implicated in some cases of immune complex glomerulonephritis.
Human Risk Assessment
The assessment of human immune status is performed mainly using peripheral blood for analysis of humoral substances like immunoglobulins and complement, and of blood leukocytes for subset composition and functionality of subpopulations. These methods are usually the same as those used to investigate humoral and cell-mediated immunity as well as nonspecific resistance of patients with suspected congenital immunodeficiency disease. For epidemiological studies (e.g., of occupationally exposed populations) parameters should be selected on the basis of their predictive value in human populations, validated animal models, and the underlying biology of the markers (see table 1). The strategy in screening for immunotoxic effects after (accidental) exposure to environmental pollutants or other toxicants is much dependent on circumstances, such as type of immunodeficiency to be expected, time between exposure and immune status assessment, degree of exposure and number of exposed individuals. The process of assessing the immunotoxic risk of a particular xenobiotic in humans is extremely difficult and often impossible, due largely to the presence of various confounding factors of endogenous or exogenous origin that influence the response of individuals to toxic damage. This is particularly true for studies which investigate the role of chemical exposure in autoimmune diseases, where genetic factors play a crucial role.
Table 1. Classification of tests for immune markers
| Test category | Characteristics | Specific tests |
| Basic-general Should be included with general panels |
Indicators of general health and organ system status | Blood urea nitrogen, blood glucose, etc. |
| Basic-immune Should be included with general panels |
General indicators of immune status Relatively low cost Assay methods are standardized among laboratories Results outside reference ranges are clinically interpretable |
Complete blood counts Serum IgG, IgA, IgM levels Surface marker phenotypes for major lymphocyte subsets |
| Focused/reflex Should be included when indicated by clinical findings, suspected exposures, or prior test results |
Indicators of specific immune functions/events Cost varies Assay methods are standardized among laboratories Results outside reference ranges are clinically interpretable |
Histocompatibility genotype Antibodies to infectious agents Total serum IgE Allergen-specific IgE Autoantibodies Skin tests for hypersensitivity Granulocyte oxidative burst Histopathology (tissue biopsy) |
| Research Should be included only with control populations and careful study design |
Indicators of general or specific immune functions/events Cost varies; often expensive Assay methods are usually not standardized among laboratories Results outside reference ranges are often not clinically interpretable |
In vitro stimulation assays Cell activation surface markers Cytokine serum concentrations Clonality assays (antibody, cellular, genetic) Cytotoxicity tests |
As adequate human data are seldom available, the assessment of risk for chemical-induced immunosuppression in humans is in the majority of cases based upon animal studies. The identification of potential immunotoxic xenobiotics is undertaken primarily in controlled studies in rodents. In vivo exposure studies present, in this regard, the optimal approach to estimate the immunotoxic potential of a compound. This is due to the multifactoral and complex nature of the immune system and of immune responses. In vitro studies are of increasing value in the elucidation of mechanisms of immunotoxicity. In addition, by investigating the effects of the compound using cells of animal and human origin, data can be generated for species comparison, which can be used in the “parallelogram” approach to improve the risk assessment process. If data are available for three cornerstones of the parallelogram (in vivo animal, and in vitro animal and human) it may be easier to predict the outcome at the remaining cornerstone, that is, the risk in humans.
When assessment of risk for chemical-induced immunosuppression has to rely solely upon data from animal studies, an approach can be followed in the extrapolation to man by the application of uncertainty factors to the no observed adverse effect level (NOAEL). This level can be based on parameters determined in relevant models, such as host resistance assays and in vivo assessment of hypersensitivity reactions and antibody production. Ideally, the relevance of this approach to risk assessment requires confirmation by studies in humans. Such studies should combine the identification and measurement of the toxicant, epidemiological data and immune status assessments.
To predict contact hypersensitivity, guinea pig models are available and have been used in risk assessment since the 1970s. Although sensitive and reproducible, these tests have limitations as they depend on subjective evaluation; this can be overcome by newer and more quantitative methods developed in the mouse. Regarding chemical-induced hypersensitivity induced by inhalation or ingestion of allergens, tests should be developed and evaluated in terms of their predictive value in man. When it comes to setting safe occupational exposure levels of potential allergens, consideration has to be given to the biphasic nature of allergy: the sensitization phase and the elicitation phase. The concentration required to elicit an allergic reaction in a previously sensitized individual is considerably lower than the concentration necessary to induce sensitization in the immunologically naïve but susceptible individual.
As animal models to predict chemical-induced autoimmunity are virtually lacking, emphasis should be given to the development of such models. For the development of such models, our knowledge of chemical-induced autoimmunity in humans should be advanced, including the study of genetic and immune system markers to identify susceptible individuals. Humans that are exposed to drugs that induce autoimmunity offer such an opportunity.
Genetic Toxicology
Genetic toxicology, by definition, is the study of how chemical or physical agents affect the intricate process of heredity. Genotoxic chemicals are defined as compounds that are capable of modifying the hereditary material of living cells. The probability that a particular chemical will cause genetic damage inevitably depends on several variables, including the organism’s level of exposure to the chemical, the distribution and retention of the chemical once it enters the body, the efficiency of metabolic activation and/or detoxification systems in target tissues, and the reactivity of the chemical or its metabolites with critical macromolecules within cells. The probability that genetic damage will cause disease ultimately depends on the nature of the damage, the cell’s ability to repair or amplify genetic damage, the opportunity for expressing whatever alteration has been induced, and the ability of the body to recognize and suppress the multiplication of aberrant cells.
In higher organisms, hereditary information is organized in chromosomes. Chromosomes consist of tightly condensed strands of protein-associated DNA. Within a single chromosome, each DNA molecule exists as a pair of long, unbranched chains of nucleotide subunits linked together by phosphodiester bonds that join the 5 carbon of one deoxyribose moiety to the 3 carbon of the next (figure 1). In addition, one of four different nucleotide bases (adenine, cytosine, guanine or thymine) is attached to each deoxyribose subunit like beads on a string. Three-dimensionally, each pair of DNA strands forms a double helix with all of the bases oriented toward the inside of the spiral. Within the helix, each base is associated with its complementary base on the opposite DNA strand; hydrogen bonding dictates strong, noncovalent pairing of adenine with thymine and guanine with cytosine (figure 1). Since the sequence of nucleotide bases is complementary throughout the entire length of the duplex DNA molecule, both strands carry essentially the same genetic information. In fact, during DNA replication each strand serves as a template for the production of a new partner strand.
Figure 1. The (a) primary, (b) secondary and (c) tertiary organization of human hereditary information
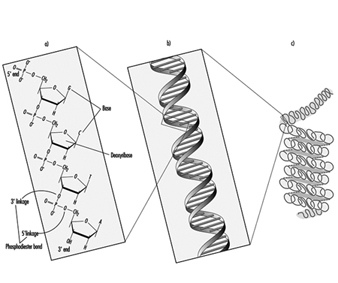 Using RNA and an array of different proteins, the cell ultimately deciphers the information encoded by the linear sequence of bases within specific regions of DNA (genes) and produces proteins that are essential for basic cell survival as well as normal growth and differentiation. In essence, the nucleotides function like a biological alphabet which is used to code for amino acids, the building blocks of proteins.
Using RNA and an array of different proteins, the cell ultimately deciphers the information encoded by the linear sequence of bases within specific regions of DNA (genes) and produces proteins that are essential for basic cell survival as well as normal growth and differentiation. In essence, the nucleotides function like a biological alphabet which is used to code for amino acids, the building blocks of proteins.
When incorrect nucleotides are inserted or nucleotides are lost, or when unnecessary nucleotides are added during DNA synthesis, the mistake is called a mutation. It has been estimated that less than one mutation occurs for every 109 nucleotides incorporated during the normal replication of cells. Although mutations are not necessarily harmful, alterations causing inactivation or overexpression of important genes can result in a variety of disorders, including cancer, hereditary disease, developmental abnormalities, infertility and embryonic or perinatal death. Very rarely, a mutation can lead to enhanced survival; such occurrences are the basis of natural selection.
Although some chemicals react directly with DNA, most require metabolic activation. In the latter case, electrophilic intermediates such as epoxides or carbonium ions are ultimately responsible for inducing lesions at a variety of nucleophilic sites within the genetic material (figure 2). In other instances, genotoxicity is mediated by by-products of compound interaction with intracellular lipids, proteins, or oxygen.
Figure 2. Bioactivation of: a) benzo(a)pyrene; and b) N-nitrosodimethylamine
Because of their relative abundance in cells, proteins are the most frequent target of toxicant interaction. However, modification of DNA is of greater concern due to the central role of this molecule in regulating growth and differentiation through multiple generations of cells.
At the molecular level, electrophilic compounds tend to attack oxygen and nitrogen in DNA. The sites that are most prone to modification are illustrated in figure 3. Although oxygens within phosphate groups in the DNA backbone are also targets for chemical modification, damage to bases is thought to be biologically more relevant since these groups are considered to be the primary informational elements in the DNA molecule.
Figure 3. Primary sites of chemically-induced DNA damage
Compounds that contain one electrophilic moiety typically exert genotoxicity by producing mono-adducts in DNA. Similarly, compounds that contain two or more reactive moieties can react with two different nucleophilic centres and thereby produce intra- or inter-molecular crosslinks in genetic material (figure 4). Interstrand DNA-DNA and DNA-protein crosslinks can be particularly cytotoxic since they can form complete blocks to DNA replication. For obvious reasons, the death of a cell eliminates the possibility that it will be mutated or neoplastically transformed. Genotoxic agents can also act by inducing breaks in the phosphodiester backbone, or between bases and sugars (producing abasic sites) in DNA. Such breaks may be a direct result of chemical reactivity at the damage site, or may occur during the repair of one of the aforementioned types of DNA lesion.
Figure 4. Various types of damage to the protein-DNA complex
Over the past thirty to forty years, a variety of techniques have been developed to monitor the type of genetic damage induced by various chemicals. Such assays are described in detail elsewhere in this chapter and Encyclopaedia.
Misreplication of “microlesions” such as mono-adducts, abasic sites or single-strand breaks may ultimately result in nucleotide base-pair substitutions, or the insertion or deletion of short polynucleotide fragments in chromosomal DNA. In contrast, “macrolesions,” such as bulky adducts, crosslinks, or double-strand breaks may trigger the gain, loss or rearrangement of relatively large pieces of chromosomes. In any case, the consequences can be devastating to the organism since any one of these events can lead to cell death, loss of function or malignant transformation of cells. Exactly how DNA damage causes cancer is largely unknown. It is currently believed the process may involve inappropriate activation of proto-oncogenes such as myc and ras, and/or inactivation of recently identified tumour suppressor genes such as p53. Abnormal expression of either type of gene abrogates normal cellular mechanisms for controlling cell proliferation and/or differentiation.
The preponderance of experimental evidence indicates that the development of cancer following exposure to electrophilic compounds is a relatively rare event. This can be explained, in part, by the cell’s intrinsic ability to recognize and repair damaged DNA or the failure of cells with damaged DNA to survive. During repair, the damaged base, nucleotide or short stretch of nucleotides surrounding the damage site is removed and (using the opposite strand as a template) a new piece of DNA is synthesized and spliced into place. To be effective, DNA repair must occur with great accuracy prior to cell division, before opportunities for the propagation of mutation.
Clinical studies have shown that people with inherited defects in the ability to repair damaged DNA frequently develop cancer and/or developmental abnormalities at an early age (table 1). Such examples provide strong evidence linking accumulation of DNA damage to human disease. Similarly, agents that promote cell proliferation (such as tetradecanoylphorbol acetate) often enhance carcinogenesis. For these compounds, the increased likelihood of neoplastic transformation may be a direct consequence of a decrease in the time available for the cell to carry out adequate DNA repair.
Table 1. Hereditary, cancer-prone disorders that appear to involve defects in DNA repair
| Syndrome | Symptoms | Cellular phenotype |
| Ataxia telangiectasia | Neurological deterioration Immunodeficiency High incidence of lymphoma |
Hypersensitivity to ionizing radiation and certain alkylating agents. Dysregulated replication of damaged DNA (may indicate shortened time for DNA repair) |
| Bloom’s syndrome | Developmental abnormalities Lesions on exposed skin High incidence of tumours of the immune system and gastrointestinal tract |
High frequency of chromosomal aberrations Defective ligation of breaks associated with DNA repair |
| Fanconi’s anaemia | Growth retardation High incidence of leukaemia |
Hypersensitivity to crosslinking agents High frequency of chromosomal aberrations Defective repair of crosslinks in DNA |
| Hereditary nonpolyposis colon cancer | High incidence of colon cancer | Defect in DNA mismatch repair (when insertion of wrong nucleotide occurs during replication) |
| Xeroderma pigmentosum | High incidence of epithelioma on exposed areas of skin Neurological impairment (in many cases) |
Hypersensitivity to UV light and many chemical carcinogens Defects in excision repair and/or replication of damaged DNA |
The earliest theories on how chemicals interact with DNA can be traced back to studies conducted during the development of mustard gas for use in warfare. Further understanding grew out of efforts to identify anticancer agents that would selectively arrest the replication of rapidly dividing tumour cells. Increased public concern over hazards in our environment has prompted additional research into the mechanisms and consequences of chemical interaction with the genetic material. Examples of various types of chemicals which exert genotoxicity are presented in table 2.
Table 2. Examples of chemicals that exhibit genotoxicity in human cells
| Class of chemical | Example | Source of exposure | Probable genotoxic lesion |
| Aflatoxins | Aflatoxin B1 | Contaminated food | Bulky DNA adducts |
| Aromatic amines | 2-Acetylaminofluorene | Environmental | Bulky DNA adducts |
| Aziridine quinones | Mitomycin C | Cancer chemotherapy | Mono-adducts, interstrand crosslinks and single-strand breaks in DNA. |
| Chlorinated hydrocarbons | Vinyl chloride | Environmental | Mono-adducts in DNA |
| Metals and metal compounds | Cisplatin | Cancer chemotherapy | Both intra- and inter-strand crosslinks in DNA |
| Nickel compounds | Environmental | Mono-adducts and single-strand breaks in DNA | |
| Nitrogen mustards | Cyclophosphamide | Cancer chemotherapy | Mono-adducts and interstrand crosslinks in DNA |
| Nitrosamines | N-Nitrosodimethylamine | Contaminated food | Mono-adducts in DNA |
| Polycyclic aromatic hydrocarbons | Benzo(a)pyrene | Environmental | Bulky DNA adducts |
Cellular Injury and Cellular Death
Virtually all of medicine is devoted to either preventing cell death, in diseases such as myocardial infarction, stroke, trauma and shock, or causing it, as in the case of infectious diseases and cancer. It is, therefore, essential to understand the nature and mechanisms involved. Cell death has been classified as “accidental”, that is, caused by toxic agents, ischaemia and so on, or “programmed”, as occurs during embryological development, including formation of digits, and resorption of the tadpole tail.
Cell injury and cell death are, therefore, important both in physiology and in pathophysiology. Physiological cell death is extremely important during embryogenesis and embryonic development. The study of cell death during development has led to important and new information on the molecular genetics involved, especially through the study of development in invertebrate animals. In these animals, the precise location and the significance of cells that are destined to undergo cell death have been carefully studied and, with the use of classic mutagenesis techniques, several involved genes have now been identified. In adult organs, the balance between cell death and cell proliferation controls organ size. In some organs, such as the skin and the intestine, there is a continual turnover of cells. In the skin, for example, cells differentiate as they reach the surface, and finally undergo terminal differentiation and cell death as keratinization proceeds with the formation of crosslinked envelopes.
Many classes of toxic chemicals are capable of inducing acute cell injury followed by death. These include anoxia and ischaemia and their chemical analogues such as potassium cyanide; chemical carcinogens, which form electrophiles that covalently bind to proteins in nucleic acids; oxidant chemicals, resulting in free radical formation and oxidant injury; activation of complement; and a variety of calcium ionophores. Cell death is also an important component of chemical carcinogenesis; many complete chemical carcinogens, at carcinogenic doses, produce acute necrosis and inflammation followed by regeneration and preneoplasia.
Definitions
Cell injury
Cell injury is defined as an event or stimulus, such as a toxic chemical, that perturbs the normal homeostasis of the cell, thus causing a number of events to occur (figure 1). The principal targets of lethal injury illustrated are inhibition of ATP synthesis, disruption of plasma membrane integrity or withdrawal of essential growth factors.
Lethal injuries result in the death of a cell after a variable period of time, depending on temperature, cell type and the stimulus; or they can be sublethal or chronic—that is, the injury results in an altered homeostatic state which, though abnormal, does not result in cell death (Trump and Arstila 1971; Trump and Berezesky 1992; Trump and Berezesky 1995; Trump, Berezesky and Osornio-Vargas 1981). In the case of a lethal injury, there is a phase prior to the time of cell death
during this time, the cell will recover; however, after a particular point in time (the “point of no return” or point of cell death), the removal of the injury does not result in recovery but instead the cell undergoes degradation and hydrolysis, ultimately reaching physical-chemical equilibrium with the environment. This is the phase known as necrosis. During the prelethal phase, several principal types of change occur, depending on the cell and the type of injury. These are known as apoptosis and oncosis.
Apoptosis
Apoptosis is derived from the Greek words apo, meaning away from, and ptosis, meaning to fall. The term falling away from is derived from the fact that, during this type of prelethal change, the cells shrink and undergo marked blebbing at the periphery. The blebs then detach and float away. Apoptosis occurs in a variety of cell types following various types of toxic injury (Wyllie, Kerr and Currie 1980). It is especially prominent in lymphocytes, where it is the predominant mechanism for turnover of lymphocyte clones. The resulting fragments result in the basophilic bodies seen within macrophages in lymph nodes. In other organs, apoptosis typically occurs in single cells which are rapidly cleared away before and following death by phagocytosis of the fragments by adjacent parenchymal cells or by macrophages. Apoptosis occurring in single cells with subsequent phagocytosis typically does not result in inflammation. Prior to death, apoptotic cells show a very dense cytosol with normal or condensed mitochondria. The endoplasmic reticulum (ER) is normal or only slightly dilated. The nuclear chromatin is markedly clumped along the nuclear envelope and around the nucleolus. The nuclear contour is also irregular and nuclear fragmentation occurs. The chromatin conden- sation is associated with DNA fragmentation which, in many instances, occurs between nucleosomes, giving a characteristic ladder appearance on electrophoresis.
In apoptosis, increased [Ca2+]i may stimulate K+ efflux resulting in cell shrinkage, which probably requires ATP. Injuries that totally inhibit ATP synthesis, therefore, are more likely to result in apoptosis. A sustained increase of [Ca2+]i has a number of deleterious effects including activation of proteases, endonucleases, and phospholipases. Endonuclease activation results in single and double DNA strand breaks which, in turn, stimulate increased levels of p53 and in poly-ADP ribosylation, and of nuclear proteins which are essential in DNA repair. Activation of proteases modifies a number of substrates including actin and related proteins leading to bleb formation. Another important substrate is poly(ADP-ribose) polymerase (PARP), which inhibits DNA repair. Increased [Ca2+]i is also associated with activation of a number of protein kinases, such as MAP kinase, calmodulin kinase and others. Such kinases are involved in activation of transcription factors which initiate transcription of immediate-early genes, for example, c-fos, c-jun and c-myc, and in activation of phospholipase A2 which results in permeabilization of the plasma membrane and of intracellular membranes such as the inner membrane of mitochondria.
Oncosis
Oncosis, derived from the Greek word onkos, to swell, is so named because in this type of prelethal change the cell begins to swell almost immediately following the injury (Majno and Joris 1995). The reason for the swelling is an increase in cations in the water within the cell. The principal cation responsible is sodium, which is normally regulated to maintain cell volume. However, in the absence of ATP or if Na-ATPase of the plasmalemma is inhibited, volume control is lost because of intracellular protein, and sodium in the water continuing to increase. Among the early events in oncosis are, therefore, increased [Na+]i which leads to cellular swelling and increased [Ca2+]i resulting either from influx from the extracellular space or release from intracellular stores. This results in swelling of the cytosol, swelling of the endoplasmic reticulum and Golgi apparatus, and the formation of watery blebs around the cell surface. The mitochondria initially undergo condensation, but later they too show high-amplitude swelling because of damage to the inner mitochondrial membrane. In this type of prelethal change, the chromatin undergoes condensation and ultimately degradation; however, the characteristic ladder pattern of apoptosis is not seen.
Necrosis
Necrosis refers to the series of changes that occur following cell death when the cell is converted to debris which is typically removed by the inflammatory response. Two types can be distinguished: oncotic necrosis and apoptotic necrosis. Oncotic necrosis typically occurs in large zones, for example, in a myocardial infarct or regionally in an organ after chemical toxicity, such as the renal proximal tubule following administration of HgCl2. Broad zones of an organ are involved and the necrotic cells rapidly incite an inflammatory reaction, first acute and then chronic. In the event that the organism survives, in many organs necrosis is followed by clearing away of the dead cells and regeneration, for example, in the liver or kidney following chemical toxicity. In contrast, apoptotic necrosis typically occurs on a single cell basis and the necrotic debris is formed within the phagocytes of macrophages or adjacent parenchymal cells. The earliest characteristics of necrotic cells include interruptions in plasma membrane continuity and the appearance of flocculent densities, representing denatured proteins within the mitochondrial matrix. In some forms of injury that do not initially interfere with mitochondrial calcium accumulation, calcium phosphate deposits can be seen within the mitochondria. Other membrane systems are similarly fragmenting, such as the ER, the lysosomes and the Golgi apparatus. Ultimately, the nuclear chromatin undergoes lysis, resulting from attack by lysosomal hydrolases. Following cell death, lysosomal hydrolases play an important part in clearing away debris with cathepsins, nucleolases and lipases since these have an acid pH optimum and can survive the low pH of necrotic cells while other cellular enzymes are denatured and inactivated.
Mechanisms
Initial stimulus
In the case of lethal injuries, the most common initial interactions resulting in injury leading to cell death are interference with energy metabolism, such as anoxia, ischaemia or inhibitors of respiration, and glycolysis such as potassium cyanide, carbon monoxide, iodo-acetate, and so on. As mentioned above, high doses of compounds that inhibit energy metabolism typically result in oncosis. The other common type of initial injury resulting in acute cell death is modification of the function of the plasma membrane (Trump and Arstila 1971; Trump, Berezesky and Osornio-Vargas 1981). This can either be direct damage and permeabilization, as in the case of trauma or activation of the C5b-C9 complex of complement, mechanical damage to the cell membrane or inhibition of the sodium-potassium (Na+-K+) pump with glycosides such as ouabain. Calcium ionophores such as ionomycin or A23187, which rapidly carry [Ca2+] down the gradient into the cell, also cause acute lethal injury. In some cases, the pattern in the prelethal change is apoptosis; in others, it is oncosis.
Signalling pathways
With many types of injury, mitochondrial respiration and oxidative phosphorylation are rapidly affected. In some cells, this stimulates anaerobic glycolysis, which is capable of maintaining ATP, but with many injuries this is inhibited. The lack of ATP results in failure to energize a number of important homeostatic processes, in particular, control of intracellular ion homeostasis (Trump and Berezesky 1992; Trump, Berezesky and Osornio-Vargas 1981). This results in rapid increases of [Ca2+]i, and increased [Na+] and [Cl-] results in cell swelling. Increases in [Ca2+]i result in the activation of a number of other signalling mechanisms discussed below, including a series of kinases, which can result in increased immediate early gene transcription. Increased [Ca2+]i also modifies cytoskeletal function, in part resulting in bleb formation and in the activation of endonucleases, proteases and phospholipases. These seem to trigger many of the important effects discussed above, such as membrane damage through protease and lipase activation, direct degradation of DNA from endonuclease activation, and activation of kinases such as MAP kinase and calmodulin kinase, which act as transcription factors.
Through extensive work on development in the invertebrate C. elegans and Drosophila, as well as human and animal cells, a series of pro-death genes have been identified. Some of these invertebrate genes have been found to have mammalian counterparts. For example, the ced-3 gene, which is essential for programmed cell death in C. elegans, has protease activity and a strong homology with the mammalian interleukin converting enzyme (ICE). A closely related gene called apopain or prICE has recently been identified with even closer homology (Nicholson et al. 1995). In Drosophila, the reaper gene seems to be involved in a signal that leads to programmed cell death. Other pro-death genes include the Fas membrane protein and the important tumour-suppressor gene, p53, which is widely conserved. p53 is induced at the protein level following DNA damage and when phosphorylated acts as a transcription factor for other genes such as gadd45 and waf-1, which are involved in cell death signalling. Other immediate early genes such as c-fos, c-jun, and c-myc also seem to be involved in some systems.
At the same time, there are anti-death genes which appear to counteract the pro-death genes. The first of these to be identified was ced-9 from C. elegans, which is homologous to bcl-2 in humans. These genes act in an as yet unknown way to prevent cell killing by either genetic or chemical toxins. Some recent evidence indicates that bcl-2 may act as an antioxidant. Currently, there is much effort underway to develop an understanding of the genes involved and to develop ways to activate or inhibit these genes, depending on the situation.
Introduction and Concepts
Mechanistic toxicology is the study of how chemical or physical agents interact with living organisms to cause toxicity. Knowledge of the mechanism of toxicity of a substance enhances the ability to prevent toxicity and design more desirable chemicals; it constitutes the basis for therapy upon overexposure, and frequently enables a further understanding of fundamental biological processes. For purposes of this Encyclopaedia the emphasis will be placed on animals to predict human toxicity. Different areas of toxicology include mechanistic, descriptive, regulatory, forensic and environmental toxicology (Klaassen, Amdur and Doull 1991). All of these benefit from understanding the fundamental mechanisms of toxicity.
Why Understand Mechanisms of Toxicity?
Understanding the mechanism by which a substance causes toxicity enhances different areas of toxicology in different ways. Mechanistic understanding helps the governmental regulator to establish legally binding safe limits for human exposure. It helps toxicologists in recommending courses of action regarding clean-up or remediation of contaminated sites and, along with physical and chemical properties of the substance or mixture, can be used to select the degree of protective equipment required. Mechanistic knowledge is also useful in forming the basis for therapy and the design of new drugs for treatment of human disease. For the forensic toxicologist the mechanism of toxicity often provides insight as to how a chemical or physical agent can cause death or incapacitation.
If the mechanism of toxicity is understood, descriptive toxicology becomes useful in predicting the toxic effects of related chemicals. It is important to understand, however, that a lack of mechanistic information does not deter health professionals from protecting human health. Prudent decisions based on animal studies and human experience are used to establish safe exposure levels. Traditionally, a margin of safety was established by using the “no adverse effect level” or a “lowest adverse effect level” from animal studies (using repeated-exposure designs) and dividing that level by a factor of 100 for occupational exposure or 1,000 for other human environmental exposure. The success of this process is evident from the few incidents of adverse health effects attributed to chemical exposure in workers where appropriate exposure limits had been set and adhered to in the past. In addition, the human lifespan continues to increase, as does the quality of life. Overall the use of toxicity data has led to effective regulatory and voluntary control. Detailed knowledge of toxic mechanisms will enhance the predictability of newer risk models currently being developed and will result in continuous improvement.
Understanding environmental mechanisms is complex and presumes a knowledge of ecosystem disruption and homeostasis (balance). While not discussed in this article, an enhanced understanding of toxic mechanisms and their ultimate consequences in an ecosystem would help scientists to make prudent decisions regarding the handling of municipal and industrial waste material. Waste management is a growing area of research and will continue to be very important in the future.
Techniques for Studying Mechanisms of Toxicity
The majority of mechanistic studies start with a descriptive toxicological study in animals or clinical observations in humans. Ideally, animal studies include careful behavioural and clinical observations, careful biochemical examination of elements of the blood and urine for signs of adverse function of major biological systems in the body, and a post-mortem evaluation of all organ systems by microscopic examination to check for injury (see OECD test guidelines; EC directives on chemical evaluation; US EPA test rules; Japan chemicals regulations). This is analogous to a thorough human physical examination that would take place in a hospital over a two- to three-day time period except for the post-mortem examination.
Understanding mechanisms of toxicity is the art and science of observation, creativity in the selection of techniques to test various hypotheses, and innovative integration of signs and symptoms into a causal relationship. Mechanistic studies start with exposure, follow the time-related distribution and fate in the body (pharmacokinetics), and measure the resulting toxic effect at some level of the system and at some dose level. Different substances can act at different levels of the biological system in causing toxicity.
Exposure
The route of exposure in mechanistic studies is usually the same as for human exposure. Route is important because there can be effects that occur locally at the site of exposure in addition to systemic effects after the chemical has been absorbed into the blood and distributed throughout the body. A simple yet cogent example of a local effect would be irritation and eventual corrosion of the skin following application of strong acid or alkaline solutions designed for cleaning hard surfaces. Similarly, irritation and cellular death can occur in cells lining the nose and/or lungs following exposure to irritant vapours or gases such as oxides of nitrogen or ozone. (Both are constituents of air pollution, or smog). Following absorption of a chemical into blood through the skin, lungs or gastrointestinal tract, the concentration in any organ or tissue is controlled by many factors which determine the pharmacokinetics of the chemical in the body. The body has the ability to activate as well as detoxify various chemicals as noted below.
Role of Pharmacokinetics in Toxicity
Pharmacokinetics describes the time relationships for chemical absorption, distribution, metabolism (biochemical alterations in the body) and elimination or excretion from the body. Relative to mechanisms of toxicity, these pharmacokinetic variables can be very important and in some instances determine whether toxicity will or will not occur. For instance, if a material is not absorbed in a sufficient amount, systemic toxicity (inside the body) will not occur. Conversely, a highly reactive chemical that is detoxified quickly (seconds or minutes) by digestive or liver enzymes may not have the time to cause toxicity. Some polycyclic halogenated substances and mixtures as well as certain metals like lead would not cause significant toxicity if excretion were rapid; but accumulation to sufficiently high levels determines their toxicity since excretion is not rapid (sometimes measured in years). Fortunately, most chemicals do not have such long retention in the body. Accumulation of an innocuous material still would not induce toxicity. The rate of elimination from the body and detoxication is frequently referred to as the half-life of the chemical, which is the time for 50% of the chemical to be excreted or altered to a non-toxic form.
However, if a chemical accumulates in a particular cell or organ, that may signal a reason to further examine its potential toxicity in that organ. More recently, mathematical models have been developed to extrapolate pharmacokinetic variables from animals to humans. These pharmacokinetic models are extremely useful in generating hypotheses and testing whether the experimental animal may be a good representation for humans. Numerous chapters and texts have been written on this subject (Gehring et al. 1976; Reitz et al. 1987; Nolan et al. 1995). A simplified example of a physiological model is depicted in figure 1.
Figure 1. A simplified pharmacokinetic model
Different Levels and Systems Can Be Adversely Affected
Toxicity can be described at different biological levels. Injury can be evaluated in the whole person (or animal), the organ system, the cell or the molecule. Organ systems include the immune, respiratory, cardiovascular, renal, endocrine, digestive, muscolo-skeletal, blood, reproductive and central nervous systems. Some key organs include the liver, kidney, lung, brain, skin, eyes, heart, testes or ovaries, and other major organs. At the cellular/biochemical level, adverse effects include interference with normal protein function, endocrine receptor function, metabolic energy inhibition, or xenobiotic (foreign substance) enzyme inhibition or induction. Adverse effects at the molecular level include alteration of the normal function of DNA-RNA transcription, of specific cytoplasmic and nuclear receptor binding, and of genes or gene products. Ultimately, dysfunction in a major organ system is likely caused by a molecular alteration in a particular target cell within that organ. However, it is not always possible to trace a mechanism back to a molecular origin of causation, nor is it necessary. Intervention and therapy can be designed without a complete understanding of the molecular target. However, knowledge about the specific mechanism of toxicity increases the predictive value and accuracy of extrapolation to other chemicals. Figure 2 is a diagrammatic representation of the various levels where interference of normal physiological processes can be detected. The arrows indicate that the consequences to an individual can be determined from top down (exposure, pharmaco- kinetics to system/organ toxicity) or from bottom up (molecular change, cellular/biochemical effect to system/organ toxicity).
Figure 2. Reresentation of mechanisms of toxicity
Examples of Mechanisms of Toxicity
Mechanisms of toxicity can be straightforward or very complex. Frequently, there is a difference among the type of toxicity, the mechanism of toxicity, and the level of effect, related to whether the adverse effects are due to a single, acute high dose (like an accidental poisoning), or a lower-dose repeated exposure (from occupational or environmental exposure). Classically, for testing purposes, an acute, single high dose is given by direct intubation into the stomach of a rodent or exposure to an atmosphere of a gas or vapour for two to four hours, whichever best resembles the human exposure. The animals are observed over a two-week period following exposure and then the major external and internal organs are examined for injury. Repeated-dose testing ranges from months to years. For rodent species, two years is considered a chronic (lifetime) study sufficient to evaluate toxicity and carcinogenicity, whereas for non-human primates, two years would be considered a subchronic (less than lifetime) study to evaluate repeated dose toxicity. Following exposure a complete examination of all tissues, organs and fluids is conducted to determine any adverse effects.
Acute Toxicity Mechanisms
The following examples are specific to high-dose, acute effects which can lead to death or severe incapacitation. However, in some cases, intervention will result in transient and fully reversible effects. The dose or severity of exposure will determine the result.
Simple asphyxiants. The mechanism of toxicity for inert gases and some other non-reactive substances is lack of oxygen (anoxia). These chemicals, which cause deprivation of oxygen to the central nervous system (CNS), are termed simple asphyxiants. If a person enters a closed space that contains nitrogen without sufficient oxygen, immediate oxygen depletion occurs in the brain and leads to unconsciousness and eventual death if the person is not rapidly removed. In extreme cases (near zero oxygen) unconsciousness can occur in a few seconds. Rescue depends on rapid removal to an oxygenated environment. Survival with irreversible brain damage can occur from delayed rescue, due to the death of neurons, which cannot regenerate.
Chemical asphyxiants. Carbon monoxide (CO) competes with oxygen for binding to haemoglobin (in red blood cells) and therefore deprives tissues of oxygen for energy metabolism; cellular death can result. Intervention includes removal from the source of CO and treatment with oxygen. The direct use of oxygen is based on the toxic action of CO. Another potent chemical asphyxiant is cyanide. The cyanide ion interferes with cellular metabolism and utilization of oxygen for energy. Treatment with sodium nitrite causes a change in haemoglobin in red blood cells to methaemoglobin. Methaemoglobin has a greater binding affinity to the cyanide ion than does the cellular target of cyanide. Consequently, the methaemoglobin binds the cyanide and keeps the cyanide away from the target cells. This forms the basis for antidotal therapy.
Central nervous system (CNS) depressants. Acute toxicity is characterized by sedation or unconsciousness for a number of materials like solvents which are not reactive or which are transformed to reactive intermediates. It is hypothesized that sedation/anaesthesia is due to an interaction of the solvent with the membranes of cells in the CNS, which impairs their ability to transmit electrical and chemical signals. While sedation may seem a mild form of toxicity and was the basis for development of the early anaesthetics, “the dose still makes the poison”. If sufficient dose is administered by ingestion or inhalation the animal can die due to respiratory arrest. If anaesthetic death does not occur, this type of toxicity is usually readily reversible when the subject is removed from the environment or the chemical is redistributed or eliminated from the body.
Skin effects. Adverse effects to the skin can range from irritation to corrosion, depending on the substance encountered. Strong acids and alkaline solutions are incompatible with living tissue and are corrosive, causing chemical burns and possible scarring. Scarring is due to death of the dermal, deep skin cells responsible for regeneration. Lower concentrations may just cause irritation of the first layer of skin.
Another specific toxic mechanism of skin is that of chemical sensitization. As an example, sensitization occurs when 2,4-dinitrochlorobenzene binds with natural proteins in the skin and the immune system recognizes the altered protein-bound complex as a foreign material. In responding to this foreign material, the immune system activates special cells to eliminate the foreign substance by release of mediators (cytokines) which cause a rash or dermatitis (see “Immunotoxicology”). This is the same reaction of the immune system when exposure to poison ivy occurs. Immune sensitization is very specific to the particular chemical and takes at least two exposures before a response is elicited. The first exposure sensitizes (sets up the cells to recognize the chemical), and subsequent exposures trigger the immune system response. Removal from contact and symptomatic therapy with steroid-containing anti-inflammatory creams are usually effective in treating sensitized individuals. In serious or refractory cases a systemic acting immunosuppresant like prednisone is used in conjunction with topical treatment.
Lung sensitization. An immune sensitization response is elicited by toluene diisocyanate (TDI), but the target site is the lungs. TDI over-exposure in susceptible individuals causes lung oedema (fluid build-up), bronchial constriction and impaired breathing. This is a serious condition and requires removing the individual from potential subsequent exposures. Treatment is primarily symptomatic. Skin and lung sensitization follow a dose response. Exceeding the level set for occupational exposure can cause adverse effects.
Eye effects. Injury to the eye ranges from reddening of the outer layer (swimming-pool redness) to cataract formation of the cornea to damage to the iris (coloured part of the eye). Eye irritation tests are conducted when it is believed serious injury will not occur. Many of the mechanisms causing skin corrosion can also cause injury to the eyes. Materials corrosive to the skin, like strong acids (pH less than 2) and alkali (pH greater than 11.5), are not tested in the eyes of animals because most will cause corrosion and blindness due to a mechanism similar to that which causes skin corrosion. In addition, surface active agents like detergents and surfactants can cause eye injury ranging from irritation to corrosion. A group of materials that requires caution is the positively charged (cationic) surfactants, which can cause burns, permanent opacity of the cornea and vascularization (formation of blood vessels). Another chemical, dinitrophenol, has a specific effect of cataract formation. This appears to be related to concentration of this chemical in the eye, which is an example of pharmacokinetic distributional specificity.
While the listing above is far from exhaustive, it is designed to give the reader an appreciation for various acute toxicity mechanisms.
Subchronic and Chronic Toxicity Mechanisms
When given as a single high dose, some chemicals do not have the same mechanism of toxicity as when given repeatedly as a lower but still toxic dose. When a single high dose is given, there is always the possibility of exceeding the person’s ability to detoxify or excrete the chemical, and this can lead to a different toxic response than when lower repetitive doses are given. Alcohol is a good example. High doses of alcohol lead to primary central nervous system effects, while lower repetitive doses result in liver injury.
Anticholinesterase inhibition. Most organophosphate pesticides, for example, have little mammalian toxicity until they are metabolically activated, primarily in the liver. The primary mechanism of action of organophosphates is the inhibition of acetylcholinesterase (AChE) in the brain and peripheral nervous system. AChE is the normal enzyme that terminates the stimulation of the neurotransmitter acetylcholine. Slight inhibition of AChE over an extended period has not been associated with adverse effects. At high levels of exposure, inability to terminate this neuronal stimulation results in overstimulation of the cholinergic nervous system. Cholinergic overstimulation ultimately results in a host of symptoms, including respiratory arrest, followed by death if not treated. The primary treatment is the administration of atropine, which blocks the effects of acetylcholine, and the administration of pralidoxime chloride, which reactivates the inhibited AChE. Therefore, both the cause and the treatment of organophosphate toxicity are addressed by understanding the biochemical basis of toxicity.
Metabolic activation. Many chemicals, including carbon tetrachloride, chloroform, acetylaminofluorene, nitrosamines, and paraquat are metabolically activated to free radicals or other reactive intermediates which inhibit and interfere with normal cellular function. At high levels of exposure this results in cell death (see “Cellular injury and cellular death”). While the specific interactions and cellular targets remain unknown, the organ systems which have the capability to activate these chemicals, like the liver, kidney and lung, are all potential targets for injury. Specifically, particular cells within an organ have a greater or lesser capacity to activate or detoxify these intermediates, and this capacity determines the intracellular susceptibility within an organ. Metabolism is one reason why an understanding of pharmacokinetics, which describes these types of transformations and the distribution and elimination of these intermediates, is important in recognizing the mechanism of action of these chemicals.
Cancer mechanisms. Cancer is a multiplicity of diseases, and while the understanding of certain types of cancer is increasing rapidly due to the many molecular biological techniques that have been developed since 1980, there is still much to learn. However, it is clear that cancer development is a multi-stage process, and critical genes are key to different types of cancer. Alterations in DNA (somatic mutations) in a number of these critical genes can cause increased susceptibility or cancerous lesions (see “Genetic toxic- ology”). Exposure to natural chemicals (in cooked foods like beef and fish) or synthetic chemicals (like benzidine, used as a dye) or physical agents (ultraviolet light from the sun, radon from soil, gamma radiation from medical procedures or industrial activity) are all contributors to somatic gene mutations. However, there are natural and synthetic substances (such as anti-oxidants) and DNA repair processes which are protective and maintain homeostasis. It is clear that genetics is an important factor in cancer, since genetic disease syndromes such as xeroderma pigmentosum, where there is a lack of normal DNA repair, dramatically increase susceptibility to skin cancer from exposure to ultraviolet light from the sun.
Reproductive mechanisms. Similar to cancer, many mechanisms of reproductive and/or developmental toxicity are known, but much is to be learned. It is known that certain viruses (such as rubella), bacterial infections and drugs (such as thalidomide and vitamin A) will adversely affect development. Recently, work by Khera (1991), reviewed by Carney (1994), show good evidence that the abnormal developmental effects in animal tests with ethylene glycol are attributable to maternal metabolic acidic metabolites. This occurs when ethylene glycol is metabolized to acid metabolites including glycolic and oxalic acid. The subsequent effects on the placenta and foetus appear to be due to this metabolic toxication process.
Conclusion
The intent of this article is to give a perspective on several known mechanisms of toxicity and the need for future study. It is important to understand that mechanistic knowledge is not absolutely necessary to protect human or environmental health. This knowledge will enhance the professional’s ability to better predict and manage toxicity. The actual techniques used in elucidating any particular mechanism depend upon the collective knowledge of the scientists and the thinking of those who make decisions regarding human health.
" DISCLAIMER: The ILO does not take responsibility for content presented on this web portal that is presented in any language other than English, which is the language used for the initial production and peer-review of original content. Certain statistics have not been updated since the production of the 4th edition of the Encyclopaedia (1998)."