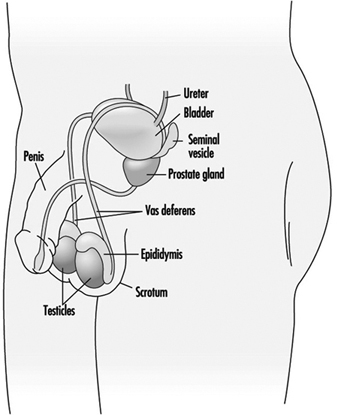
Spermatogenesis and spermiogenesis are the cellular processes that produce mature male sex cells. These processes take place within the seminiferous tubules of the testes of the sexually mature male, as shown in Figure 1. The human seminiferous tubules are 30 to 70 cm long and 150 to 300 mm in diameter (Zaneveld 1978). The spermatogonia (stem cells) are ppositioned along the basement membrane of the seminiferous tubules and are the basic cells for the production of sperm.
Figure 1. The male reproductive system
Sperm mature through a series of cellular divisions in which the spermatogonia proliferate and become primary spermatocytes. The resting primary spermatocytes migrate through tight junctions formed by the Sertoli cells to the luminal side of this testis barrier. By the time the spermatocytes reach the membrane barrier in the testis, the synthesis of DNA, the genetic material in the nucleus of the cell, is essentially complete. When the primary spermatocytes actually encounter the lumen of the seminiferous tubule, these undergo a special type of cell division which occurs only in germ cells and is known as meiosis. Meiotic cellular divison results in the splitting up of the chromosomes pairs in the nucleus, so that each resulting germ cell contains only a single copy of each chromosome strand rather than a matched pair.
During meiosis the chromosomes change shape by condensing and becoming filamentous. At a certain point, the nuclear membrane which surrounds them breaks down and microtubular spindles attach to the chromosomal pairs, causing them to separate. This completes the first meiotic division and two haploid secondary spermatocytes are formed. The secondary spermatocytes then undergo a second meiotic division to form equal numbers of X- and Y-chromosome bearing spermatids.
The morphological transformation of spermatids to spermatozoa is called spermiogenesis. When spermiogenesis is complete, each sperm cell is released by the Sertoli cell into the seminiferous tubule lumen by a process referred to as spermiation. The sperm migrate along the tubule to the rete testis and into the head of the epididymis. Sperm leaving the seminiferous tubules are immature: unable to fertilize an ovum and unable to swim. Spermatozoa released into the lumen of the seminiferous tubule are suspended in fluid pproduced primarily by the Sertoli cells. Concentrated sperm suspended within this fluid flow continuously from the seminiferous tubules, through slight changes in the ionic milieu within the rete testis, through the vasa efferentia, and into the epididymis. The epididymis is a single highly coiled tube (five to six metres long) in which sperm spend 12 to 21 days.
Within the epididymis, sperm progressively acquire motility and fertilizing capacity. This may be due to the changing nature of the suspension fluid in the epididymis. That is, as the cells mature the epididymis absorbs components from the fluid including secretions from the Sertoli cells (e.g., androgen binding protein), thereby increasing the concentration of spermatozoa. The epididymis also contributes its own secretions to the suspension fluid, including the chemicals glycerylphosphorylcholine (GPC) and carnitine.
Sperm morphology continues to transform in the epididymis. The cytoplasmic droplet is shed and the sperm nucleus condenses further. While the epididymis is the principal storage reservoir for sperm until ejaculation, about 30% of the sperm in an ejaculate have been stored in the vas deferens. Frequent ejaculation accelerates passage of sperm through the epididymis and may increase the number of immature (infertile) sperm in the ejaculate (Zaneveld 1978).
Ejaculation
Once within the vas deferens, the sperm are transported by the muscular contractions of ejaculation rather than by the flow of fluid. During ejaculation, fluids are forcibly expelled from the accessory sex glands giving rise to the seminal plasma. These glands do not expel their secretions at the same time. Rather, the bulbourethral (Cowper’s) gland first extrudes a clear fluid, followed by the prostatic secretions, the sperm-concentrated fluids from the epididymides and ampulla of the vas deferens, and finally the largest fraction primarily from the seminal vesicles. Thus, seminal plasma is not a homogeneous fluid.
Toxic Actions on Spermatogenesisand Spermiogenesis
Toxicants may disrupt spermatogenesis at several points. The most damaging, because of irreversibility, are toxicants that kill or genetically alter (beyond repair mechanisms) spermatogonia or Sertoli cells. Animal studies have been useful to determine the stage at which a toxicant attacks the spermatogenic process. These studies employ short term exposure to a toxicant before sampling to determine the effect. By knowing the duration for each spermatogenic stage, one can extrapolate to estimate the affected stage.
Biochemical analysis of seminal plasma pprovides insights into the function of the accessory sex glands. Chemicals that are secreted primarily by each of the accessory sex glands are typically selected to serve as a marker for each respective gland. For example, the epididymis is represented by GPC, the seminal vesicles by fructose, and the prostate gland by zinc. Note that this type of analysis pprovides only gross information on glandular function and little or no information on the other secretory constituents. Measuring semen pH and osmolality provide additional general information on the nature of seminal plasma.
Seminal plasma may be analysed for the presence of a toxicant or its metabolite. Heavy metals have been detected in seminal plasma using atomic absorption spectrophotometry, while halogenated hydrocarbons have been measured in seminal fluid by gas chromatography after extraction or protein-limiting filtration (Stachel et al. 1989; Zikarge 1986).
The viability and motility of spermatozoa in seminal plasma is typically a reflection of seminal plasma quality. Alterations in sperm viability, as measured by stain exclusion or by hypoosmotic swelling, or alterations in sperm motility parameters would suggest post-testicular toxicant effects.
Semen analyses also can indicate whether production of sperm cells has been affected by a toxicant. Sperm count and sperm morphology provide indices of the integrity of spermatogenesis and spermiogenesis. Thus, the number of sperm in the ejaculate is directly correlated with the number of germ cells per gram of testis (Zukerman et al. 1978), while abnormal morphology is probably a result of abnormal spermiogenesis. Dead sperm or immotile sperm often reflect the effects of post-testicular events. Thus, the type or timing of a toxic effect may indicate the target of the toxicant. For example, exposure of male rats to 2-methoxyethanol resulted in reduced fertility after four weeks (Chapin et al. 1985). This evidence, corroborated by histological examination, indicates that the target of toxicity is the spermatocyte (Chapin et al. 1984). While it is not ethical to intentionally expose humans to suspected reproductive toxicants, semen analyses of serial ejaculates of men inadvertently exposed for a short time to potential toxicants may provide similar useful information.
Occupational exposure to 1,2-dibromochloropropane (DBCP) reduced sperm concentration in ejaculates from a median of 79 million cells/ml in unexposed men to 46 million cells/ml in exposed workers (Whorton et al. 1979). Upon removing the workers from the exposure, those with reduced sperm counts experienced a partial recovery, while men who had been azoospermic remained sterile. Testicular biopsy revealed that the target of DBCP was the spermatogonia. This substantiates the severity of the effect when stem cells are the target of toxicants. There were no indications that DBCP exposure of men was associated with adverse pregnancy outcome (Potashnik and Abeliovich 1985). Another example of a toxicant targeting spermatogenesis/spermiogenesis was the study of workers exposed to ethylene dibromide (EDB). They had more sperm with tapered heads and fewer sperm per ejaculate than did controls (Ratcliffe et al. 1987).
Genetic damage is difficult to detect in human sperm. Several animal studies using the dominant lethal assay (Ehling et al. 1978) indicate that paternal exposure can produce an adverse pregnancy outcome. Epidemiological studies of large populations have demonstrated increased frequency of spontaneous abortions in women whose husbands were working as motor vehicle mechanics (McDonald et al. 1989). Such studies indicate a need for methods to detect genetic damage in human sperm. Such methods are being developed by several laboratories. These methods include DNA probes to discern genetic mutations (Hecht 1987), sperm chromosome karyotyping (Martin 1983), and DNA stability assessment by flow cytometry (Evenson 1986).
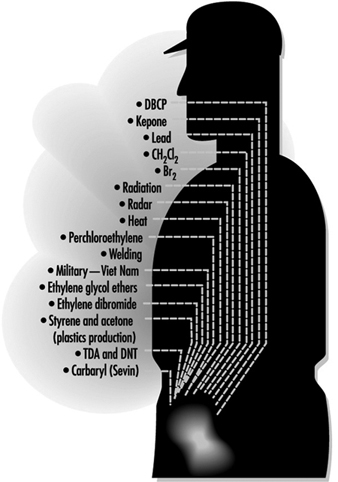
Figure 2. Exposures positively associated with adversely affecting semen quality
Figure 2 lists exposures known to affect sperm quality and table 1 provides a summary of the results of epidemiological studies of paternal effects on reproductive outcomes.
Table 1. Epidemiological studies of paternal effects on pregnancy outcome
| Reference | Type of exposure or occupation | Association with exposure1 | Effect |
| Record-based population studies | |||
| Lindbohm et al. 1984 | Solvents | – | Spontaneous abortion |
| Lindbohm et al. 1984 | Service station | + | Spontaneous abortion |
| Daniell and Vaughan 1988 | Organic solvents | – | Spontaneous abortion |
| McDonald et al. 1989 | Mechanics | + | Spontaneous abortion |
| McDonald et al. 1989 | Food processing | + | Developmental defects |
| Lindbohm et al. 1991a | Ethylene oxide | + | Spontaneous abortion |
| Lindbohm et al. 1991a | Petroleum refinery | + | Spontaneous abortion |
| Lindbohm et al. 1991a | Impregnates of wood | + | Spontaneous abortion |
| Lindbohm et al. 1991a | Rubber chemicals | + | Spontaneous abortion |
| Olsen et al. 1991 | Metals | + | Child cancer risk |
| Olsen et al. 1991 | Machinists | + | Child cancer risk |
| Olsen et al. 1991 | Smiths | + | Child cancer risk |
| Kristensen et al. 1993 | Solvents | + | Preterm birth |
| Kristensen et al. 1993 | Lead and solvents | + | Preterm birth |
| Kristensen et al. 1993 | Lead | + | Perinatal death |
| Kristensen et al. 1993 | Lead | + | Male child morbidity |
| Case-control studies | |||
| Kucera 1968 | Printing industry | (+) | Cleft lip |
| Kucera 1968 | Paint | (+) | Cleft palate |
| Olsen 1983 | Paint | + | Damage to central nervous system |
| Olsen 1983 | Solvents | (+) | Damage to central nervous system |
| Sever et al. 1988 | Low-level radiation | + | Neural tube defects |
| Taskinen et al. 1989 | Organic solvents | + | Spontaneous abortion |
| Taskinen et al. 1989 | Aromatic hydrocarbons | + | Spontaneous abortion |
| Taskinen et al. 1989 | Dust | + | Spontaneous abortion |
| Gardner et al. 1990 | Radiation | + | Childhood leukaemia |
| Bonde 1992 | Welding | + | Time to conception |
| Wilkins and Sinks 1990 | Agriculture | (+) | Child brain tumour |
| Wilkins and Sinks 1990 | Construction | (+) | Child brain tumour |
| Wilkins and Sinks 1990 | Food/tobacco processing | (+) | Child brain tumour |
| Wilkins and Sinks 1990 | Metal | + | Child brain tumour |
| Lindbohmn et al. 1991b | Lead | (+) | Spontaneous abortion |
| Sallmen et al. 1992 | Lead | (+) | Congenital defects |
| Veulemans et al. 1993 | Ethylene glycol ether | + | Abnormal spermiogram |
| Chia et al. 1992 | Metals | + | Cadmium in semen |
1 – no significant association; (+) marginally significant association; + significant association.
Source: Adapted from Taskinen 1993.
Neuroendocrine System
The overall functioning of the reproductive system is controlled by the nervous system and the hormones pproduced by the glands (the endocrine system). The reproductive neuroendocrine axis of the male involves principally the central nervous systems (CNS), the anterior pituitary gland and the testes. Inputs from the CNS and from the periphery are integrated by the hypothalamus, which directly regulates gonadotrophin secretion by the anterior pituitary gland. The gonadotrophins, in turn, act principally upon the Leydig cells within the interstitium and Sertoli and germ cells within the seminiferous tubules to regulate spermatogenesis and hormone production by the testes.
Hypothalamic–Pituitary Axis
The hypothalamus secretes the neurohormone gonadotrophin releasing hormone (GnRH) into the hypophysial portal vasculature for transport to the anterior pituitary gland. The pulsatile secretion of this decapeptide causes the concomitant release of luteinizing hormone (LH), and with lesser synchrony and one-fifth the potency, the release of follicle stimulating hormone (FSH) (Bardin 1986). Substantial evidence exists to support the presence of a separate FSH releasing hormone, although none has yet been isolated (Savy-Moore and Schwartz 1980; Culler and Negro-Vilar 1986). These hormones are secreted by the anterior pituitary gland. LH acts directly upon the Leydig cells to stimulate synthesis and release of testosterone, whereas FSH stimulates aromatization of testosterone to estradiol by the Sertoli cell. Gonadotropic stimulation causes the release of these steroid hormones into the spermatic vein.
Gonadotrophin secretion is, in turn, checked by testosterone and estradiol through negative feedback mechanisms. Testosterone acts principally upon the hypothalamus to regulate GnRH secretion and thereby reduces the pulse frequency, primarily, of LH release. Estradiol, on the other hand, acts upon the pituitary gland to reduce the magnitude of gonadotrophin release. Through these endocrine feedback loops, testicular function in general and testosterone secretion specifically are maintained at a relatively steady state.
Pituitary–Testicular Axis
LH and FSH are generally viewed as necessary for normal spermatogenesis. Presumably the effect of LH is secondary to inducing high intratesticular concentrations of testosterone. Therefore, FSH from the pituitary gland and testosterone from the Leydig cells act upon the Sertoli cells within the seminiferous tubule epithelium to initiate spermatogenesis. Sperm production persists, although quantitatively reduced, after removing either LH (and presumably the high intratesticular testosterone concentrations) or FSH. FSH is required for initiating spermatogenesis at puberty and, to a lesser extent, to reinitiate spermatogenesis that has been arrested (Matsumoto 1989; Sharpe 1989).
The hormonal synergism that serves to maintain spermatogenesis may entail recruitment by FSH of differentiated spermatogonia to enter meiosis, while testosterone may control specific, subsequent stages of spermatogenesis. FSH and testosterone may also act upon the Sertoli cell to stimulate production of one or more paracrine factors which may affect the number of Leydig cells and testosterone production by these cells (Sharpe 1989). FSH and testosterone stimulate protein synthesis by Sertoli cells including synthesis of androgen binding protein (ABP), while FSH alone stimulates synthesis of aromatase and inhibin. ABP is secreted primarily into the seminiferous tubular fluid and is transported to the proximal portion of the caput epididymis, possibly serving as a local carrier of androgens (Bardin 1986). Aromatase catalyses the conversion of testosterone to estradiol in the Sertoli cells and in other peripheral tissues.
Inhibin is a glycoprotein consisting of two dissimilar, disulphide-linked subunits, a and b. Although inhibin preferentially inhibits FSH release, it may also attenuate LH release in the presence of GnRH stimulation (Kotsugi et al. 1988). FSH and LH stimulate inhibin release with approximately equal potency (McLachlan et al. 1988). Interestingly, inhibin is secreted into the spermatic vein blood as pulses which are synchronous to those of testosterone (Winters 1990). This probably does not reflect direct actions of LH or testosterone on Sertoli cell activity, but rather the effects of other Leydig cell products secreted either into the interstitial spaces or the circulation.
Prolactin, which is also secreted by the anterior pituitary gland, acts synergistically with LH and testosterone to promote male reproductive function. Prolactin binds to specific receptors on the Leydig cell and increases the amount of androgen receptor complex within the nucleus of androgen responsive tissues (Baker et al. 1977). Hyperprolactinaemia is associated with reductions of testicular and prostate size, semen volume and circulating concentrations of LH and testosterone (Segal et al. 1979). Hyperprolactinaemia has also been associated with impotency, apparently independent of altering testosterone secretion (Thorner et al. 1977).
If measuring steroid hormone metabolites in urine, consideration must be given to the potential that the exposure being studied may alter the metabolism of excreted metabolites. This is especially pertinent since most metabolites are formed by the liver, a target of many toxicants. Lead, for example, reduced the amount of sulphated steroids that were excreted into the urine (Apostoli et al. 1989). Blood levels for both gonadotrophins become elevated during sleep as the male enters puberty, while testosterone levels maintain this diurnal pattern through adulthood in men (Plant 1988). Thus blood, urine or saliva samples should be collected at approximately the same time of day to avoid variations due to diurnal secretory patterns.
The overt effects of toxic exposure targeting the reproductive neuroendocrine system are most likely to be revealed through altered biological manifestations of the androgens. Manifestations significantly regulated by androgens in the adult man that may be detected during a basic physical examination include: (1) nitrogen retention and muscular development; (2) maintenance of the external genitalia and accessory sexual organs; (3) maintenance of the enlarged larynx and thickened vocal cords causing the male voice; (4) beard, axillary and pubic hair growth and temporal hair recession and balding; (5) libido and sexual performance; (6) organ specific proteins in tissues (e.g., liver, kidneys, salivary glands); and (7) aggressive behaviour (Bardin 1986). Modifications in any of these traits may indicate that androgen production has been affected.
Examples of Toxicant Effects
Lead is a classic example of a toxicant that directly affects the neuroendocrine system. Serum LH concentrations were elevated in men exposed to lead for less than one year. This effect did not progress in men exposed for more than five years. Serum FSH levels were not affected. On the other hand, serum levels of ABP were elevated and those of total testosterone were reduced in men exposed to lead for more than five years. Serum levels of free testosterone were significantly reduced after exposure to lead for three to five years (Rodamilans et al. 1988). In contrast, serum concentrations of LH, FSH, total testosterone, prolactin, and total neutral 17-ketosteroids were not altered in workers with lower circulating levels of lead, even though the distribution frequency of sperm count was altered (Assennato et al. 1986).
Exposure of shipyard painters to 2-ethoxyethanol also reduced sperm count without a concurrent change in serum LH, FSH, or testosterone concentrations (Welch et al. 1988). Thus toxicants may affect hormone production and sperm measures independently.
Male workers involved in the manufacture of the nematocide DBCP experienced elevated serum levels of LH and FSH and reduced sperm count and fertility. These effects are apparently sequelae to DBCP actions upon the Leydig cells to alter androgen production or action (Mattison et al. 1990).
Several compounds may exert toxicity by virtue of structural similarity to reproductive steroid hormones. Thus, by binding to the respective endocrine receptor, toxicants may act as agonists or antagonists to disrupt biological responses. Chlordecone (Kepone), an insecticide that binds to oestrogen receptors, reduced sperm count and motility, arrested sperm maturation and reduced libido. While it is tempting to suggest that these effects result from chlordecone interfering with oestrogen actions at the neuroendocrine or testicular level, serum levels of testosterone, LH and FSH were not shown to be altered in these studies in a manner similar to the effects of oestradiol therapy. DDT and its metabolites also exhibit steroidal properties and might be expected to alter male reproductive function by interfering with steroidal hormone functions. Xenobiotics such as polychlorinated biphenyls, polybrominated biphenyls, and organochlorine pesticides may also interfere with male reproductive functions by exerting oestrogenic agonist/antagonist activity (Mattison et al. 1990).
Sexual Function
Human sexual function refers to the integrated activities of the testes and secondary sex glands, the endocrine control systems, and the central nervous system-based behavioural and psychological components of reproduction (libido). Erection, ejaculation and orgasm are three distinct, independent, physiological and psychodynamic events which normally occur concurrently in men.
Little reliable data are available on occupational exposure effects on sexual function due to the problems described above. Drugs have been shown to affect each of the three stages xof male sexual function (Fabro 1985), indicating the potential for occupational exposures to exert similar effects. Antidepressants, testosterone antagonists and stimulants of prolactin release effectively reduce libido in men. Antihypertensive drugs which act on the sympathetic nervous system induce impotence in some men, but surprisingly, priapism in others. Phenoxybenzamine, an adrenoceptive antagonist, has been used clinically to block seminal emission but not orgasm (Shilon, Paz and Homonnai 1984). Anticholinergic antidepressant drugs permit seminal emission while blocking seminal ejection and orgasm which results in seminal plasma seeping from the urethra rather than being ejected.
Recreational drugs also affect sexual function (Fabro 1985). Ethanol may reduce impotence while enhancing libido. Cocaine, heroin and high doses of cannabinoids reduce libido. Opiates also delay or impair ejaculation.
The vast and varied array of pharmaceuticals that has been shown to affect the male reproductive system pprovides support for the notion that chemicals found in the workplace may also be reproductive toxicants. Research methods that are reliable and practical for field study conditions are needed to assess this important area of reproductive toxicology.