Historical Perspective
Asbestos is a term used to describe a group of naturally occurring fibrous minerals which are very widely distributed in rock outcrops and deposits throughout the world. Exploitation of the tensile and heat-resistant properties of asbestos for human use dates from ancient times. For instance, in the third century BC asbestos was used to strengthen clay pots in Finland. In classic times, shrouds woven from asbestos were used to preserve the ashes of the famous dead. Marco Polo returned from his travels in China with descriptions of a magic material which could be manufactured into a flame resistant cloth. By the early years of the nineteenth century, deposits were known to exist in several parts of the world, including the Ural Mountains, northern Italy and other Mediterranean areas, in South Africa and in Canada, but commercial exploitation only started in the latter half of the nineteenth century. By this time, the industrial revolution created not only the demand (such as that of insulating the steam engine) but also facilitated production, with mechanization replacing hand cobbing of fibre from the parent rock. The modern industry began in Italy and the United Kingdom after 1860 and was boosted by the development and exploitation of the extensive deposits of chrysotile (white) asbestos in Quebec (Canada) in the 1880s. Exploitation of the also extensive deposits of chrysotile in the Ural mountains was modest until the 1920s. The long thin fibres of chrysotile were particularly suitable for spinning into cloth and felts, one of the early commercial uses for the mineral. The exploitation of the deposits of crocidolite (blue) asbestos of the northwest Cape, South Africa, a fibre more water-resistant than chrysotile and better suited to marine use, and of the amosite (brown) asbestos deposits, also found in South Africa, started in the early years of this century. Exploitation of the Finnish deposits of anthophyllite asbestos, the only important commercial source of this fibre, took place between 1918 and 1966, while the deposits of crocidolite in Wittenoom, Western Australia, were mined from 1937 to 1966.
Fibre Types
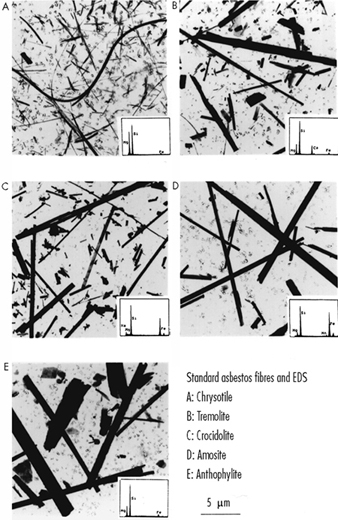
The asbestos minerals fall into two groups, the serpentine group which includes chrysotile, and the amphiboles, which include crocidolite, tremolite, amosite and anthophyllite (figure 1). Most ore deposits are heterogeneous mineralogically, as are most of the commercial forms of the mineral (Skinner, Roos and Frondel 1988). Chrysotile and the various amphibole asbestos minerals differ in crystalline structure, in chemical and surface characteristics and in the physical characteristics of their fibres, usually described in terms of the length-to-diameter (or aspect) ratio. They also differ in characteristics which distinguish commercial use and grade. Pertinent to the current discussion is the evidence that the different fibres differ in their biological potency (as considered below in the sections on various diseases).
Figure 1. Asbestos fibre types.
Seen on election microscopy together with energy dispersive x-ray spectra which enables identification of individual fibres. Courtesy of A. Dufresne and M. Harrigan, McGill University.
Commercial Production
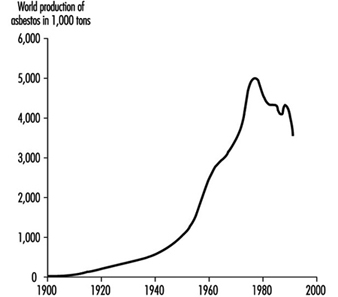
The growth of commercial production, illustrated in figure 2, was slow in the early years of this century. For instance, Canadian production exceeded 100,000 short tons per annum for the first time in 1911 and 200,000 tons in 1923. Growth between the two World Wars was steady, increased considerably to meet the demands of the Second World War and spectacularly to meet peacetime demands (including those of the cold war) to reach a peak in 1976 of 5,708,000 short tons (Selikoff and Lee 1978). After this, production faltered as the ill-health effects of exposure became a matter of increasing public concern in North America and Europe and remain at approximately 4,000,000 short tons per annum up to 1986, but decreased further in the 1990s. There was also a shift in the uses and sources of fibre in the 1980s; in Europe and North America demand declined as substitutes for many applications were introduced, while on the African, Asian and South American continents, demand for asbestos increased to meet the needs of a cheap durable material for use in construction and in water reticulation. By 1981, Russia had become the world’s major producer, with an increase in the commercial exploitation of large deposits in China and Brazil. In 1980, it was estimated that a total of over 100 million tons of asbestos had been mined worldwide, 90% of which was chrysotile, approximately 75% of which came from 4 chrysotile mining areas, located in Quebec (Canada), Southern Africa and the central and southern Ural Mountains. Two to three per cent of the world’s total production was crocidolite, from the Northern Cape, South Africa, and from Western Australia, and another 2 to 3% was amosite, from the Eastern Transvaal, South Africa (Skinner, Ross and Frondel 1988).
Figure 2. World production of asbestos in thousands of tons 1900-92
Asbestos-Related Diseases and Conditions
Like silica, asbestos has the capability of evoking scarring reactions in all biological tissue, human and animal. In addition, asbestos evokes malignant reactions, adding a further element to the concern for human health, as well as a challenge to science as to how asbestos exerts its ill effects. The first asbestos-related disease to be recognized, diffuse interstitial pulmonary fibrosis or scarring, later called asbestosis, was the subject of case reports in the United Kingdom in the early 1900s. Later, in the 1930s, case reports of lung cancer in association with asbestosis appeared in the medical literature though it was only over the next several decades that the scientific evidence was gathered establishing that asbestos was the carcinogenic factor. In 1960, the association between asbestos exposure and another much less common cancer, malignant mesothelioma, which involves the pleura (a membrane that covers the lung and lines the chest wall) was dramatically brought to attention by the report of a cluster of these tumours in 33 individuals, all of whom worked or lived in the asbestos mining area of the Northwest Cape (Wagner 1996). Asbestosis was the target of the dust control levels introduced and implemented with increasing rigour in the 1960s and 1970s, and in many industrialized countries, as the frequency of this disease decreased, asbestos-related pleural disease emerged as the most frequent manifestation of exposure and the condition which most frequently brought exposed subjects to medical attention. Table 1 lists diseases and conditions currently recognized as asbestos-related. The diseases in bold type are those most frequently encountered and for which a direct causal relationship is well established, while for the sake of completeness, certain other conditions, for which the relationship is less well established, are also listed (see footnote to Table 16) and the sections which follow in the text below that expand upon the various disease headings).
Table 1. Asbestos-related diseases and conditions
| Pathology | Organ(s) affected | Disease/condition1 |
| Non-malignant | Lungs Pleura Skin | Asbestosis (diffuse interstitial fibrosis) Small airway disease2 (fibrosis limited to the peri-bronchiolar region) Chronic airways disease3 Pleural plaques Viscero-parietal reactions, including benign pleural effusion, diffuse pleural fibrosis and rounded atelectasis Asbestos corns4 |
| Malignant | Lungs Pleura Other mesothelium-lined cavities Gastrointestinal tract5 Other5 | Lung cancer (all cell types) Cancer of larynx Mesothelioma of pleura Mesothelioma of the peritoneum, pericardium and scrotum (in decreasing frequency of occurrence) Cancer of stomach, oesophagus, colon, rectum Ovary, gall bladder, bile ducts, pancreas, kidney |
1 The diseases or conditions indicated in bold type are those most frequently encountered and the ones for which a causal relationship is well established and/or generally recognized.
2 Fibrosis in the walls of the small airways of the lung (including the membranous and respiratory bronchioles) is thought to represent the early lung parenchymal response to retained asbestos (Wright et al. 1992) which will progress to asbestosis if exposure continues and/or is heavy, but if exposure is limited or light, the lung response may be limited to these areas (Becklake in Liddell & Miller 1991).
3 Included are bronchitis, chronic obstructive pulmonary disease (COPD) and emphysema. All have been shown to be associated with work in dusty environments. The evidence for causality is reviewed in the section Chronic Airways Diseases and Becklake (1992).
4 Related to direct handling of asbestos and of historical rather than current interest.
5 Data not consistent from all studies (Doll and Peto 1987); some of the highest risks were reported in a cohort of over 17,000 American and Canadian asbestos insulation workers (Selikoff 1990), followed from January 1, 1967 to December 31, 1986 in whom exposure had been particularly heavy.
Sources: Becklake 1994; Liddell and Miller 1992; Selikoff 1990; Doll and Peto in Antman and Aisner 1987; Wright et al. 1992.
Uses
Table 2 lists the major sources, products and uses of the asbestos minerals.
Table 2. Main commercial sources, products and uses of asbestos
| Fibre type | Location of major deposits | Commercial products and/or uses |
| Chrysotile (white) |
Russia, Canada (Québec, also British Columbia, Newfoundland), China (Szechwan province); Mediterranean countries (Italy, Greece, Corsica, Cyprus); Southern Africa (South Africa, Zimbabwe, Swaziland); Brazil; smaller deposits in United States (Vermont, Arizona, California) and in Japan | Construction materials (tiles, shingles, gutters and cisterns; roofing, sheeting, and siding) Pressure and other pipes Fire proofing (marine and other) Insulation and sound proofing Reinforced plastic products (fan blades, switch gear) Friction materials usually in combination with resins in brakes, clutches, other Textiles (used in belts, clothing, casing, fire barriers, autoclaves, yarns and packing) Paper products (used in millboard, insulators, gaskets, roof felt, wall coverings, etc.) Floats in paints, coatings and welding rods |
| Crocidolite (blue) |
South Africa (Northwest Cape, Eastern Transvaal), Western Australia1 | Used mainly in combination in cement products (in particular pressure pipes) but also in many of the other products listed above |
| Amosite (brown) |
South Africa (Northern Transvaal)1 | Used mainly in cement, thermal insulation and roofing products particularly in the United States2 , but also in combination in many of the products listed under chrysotile |
| Anthophyllite | Finland1 | Filler in the rubber, plastics and chemical industries |
| Tremolite | Italy, Korea and some Pacific Islands; mined on a small scale in Turkey, China and elsewhere; contaminates the ore bearing rock in some asbestos, iron, talc and vermiculite mines; also found in agricultural soils in the Balkan Peninsula and in Turkey | Used as a filler in talc; may or may not be removed in processing the ore so it may appear in end products |
| Actinolite | Contaminates amosite, and less often, chrysotile, talc and vermiculite deposits | Not usually exploited commercially |
1 A list such as this is obviously not comprehensive and the readers should consult the sources cited and other chapters in this Encyclopedia for more complete information.
2 No longer in operation.
Sources: Asbestos Institute (1995); Browne (1994); Liddell and Miller (1991); Selikoff and Lee (1978); Skinner et al (1988).
Though necessarily incomplete, this table emphasizes that:
- Deposits are found in many parts of the world, most of which have been exploited non-commercially or commercially in the past, and some of which are currently commercially exploited.
- There are many manufactured products in current or past use which contain asbestos, particularly in the construction and transport industries.
- Disintegration of these products or their removal carries with it the risk of the resuspension of fibres and of renewed human exposure.
A figure of over 3,000 has been commonly quoted for the number of uses of asbestos and no doubt led to asbestos being dubbed the “magic mineral” in the 1960s. A 1953 industry list contains as many as 50 uses for raw asbestos, in addition to its use in the manufacture of the products listed in Table 17, each of which has many other industrial applications. In 1972, the consumption of asbestos in an industrialized country like the United States was attributed to the following categories of product: construction (42%); friction materials, felts, packings and gaskets (20%); floor tiles (11%); paper (9%); insulation and textiles (3%) and other uses (15%) (Selikoff and Lee 1978). By contrast, a 1995 industry list of the main product categories shows major redistribution on a worldwide basis as follows: asbestos cement (84%); friction materials (10%); textiles (3%); seals and gaskets (2%); and other uses (1%) (Asbestos Institute 1995).
Occupational Exposures, Past and Current
Occupational exposure, certainly in industrialized countries, has always been and is still the most likely source of human exposure (see Table 17 and the references cited in its footnote; other sections of this Encyclopaedia contain further information). There have, however, been major changes in industrial processes and procedures aimed at diminishing the release of dust into the working environment (Browne 1994; Selikoff and Lee 1978). In countries with mining operations, milling usually takes place at the minehead. Most chrysotile mines are open cast, while amphibole mines usually involve underground methods which generate more dust. Milling involves separating fibre from rock by means of mechanized crushing and screening, which were dusty processes until the introduction of wet methods and/or enclosure in most mills during the 1950s and 1960s. The handling of waste was also a source of human exposure, as was transporting bagged asbestos, whether it involved loading and unloading trucks and railcars or work on the dockside. These exposures have diminished since the introduction of leak-proof bags and the use of sealed containers.
Workers have had to use raw asbestos directly in packing and lagging, particularly in locomotives, and in spraying walls, ceilings and airducts, and in the marine industry, deckheads and bulkheads. Some of these uses have been phased out voluntarily or have been banned. In the manufacture of asbestos cement products, exposure occurs in receiving and opening bags containing raw asbestos, in preparing the fibre for mixing in the slurry, in machining end-products and in dealing with waste. In the manufacture of vinyl tiles and flooring, asbestos was used as a reinforcing and filler agent to blend with organic resins, but has now largely been replaced by organic fibre in Europe and North America. In the manufacture of yarns and textiles, exposure to fibre occurs in receiving, preparing, blending, carding, spinning, weaving and calendaring the fibre—processes which were until recently dry and potentially very dusty. Dust exposure has been considerably reduced in modern plants through use of a colloidal suspension of fibre extruded through a coagulant to form wet strands for the last-mentioned three processes. In the manufacture of asbestos paper products, human exposure to asbestos dust is also most likely to occur in the reception and preparation of the stock mix and in cutting the final products which in the 1970s contained from 30 to 90% asbestos. In the manufacture of asbestos friction products (dry mix-moulded, roll-formed, woven or endless wound) human exposure to asbestos dust is also most likely to occur during the initial handling and blending processes as well as in finishing the end product, which in the 1970s contained from 30 to 80% of asbestos. In the construction industry, prior to regular use of appropriate exhaust ventilation (which came in the 1960s), the high-speed power sawing, drilling and sanding of asbestos-containing boards or tiles led to the release of fibre-containing dust close to the operator’s breathing zone, particularly when such operations were conducted in closed spaces (for instance in high-rise buildings under construction). In the period after the Second World War, a major source of human exposure was in the use, removal or replacement of asbestos-containing materials in the demolition or refurbishing of buildings or ships. One of the chief reasons for this state of affairs was the lack of awareness, both of the composition of these materials (i.e., that they contained asbestos) and that exposure to asbestos could be harmful to health. Improved worker education, better work practices and personal protection have reduced the risk in the 1990s in some countries. In the transport industry, sources of exposure were the removal and replacement of lagging in locomotive engines and of braking material in trucks and cars in the automobile repair industry. Other sources of past exposure leading to, in particular, pleural disease, continue to attract notice, even in the 1990s, usually on the basis of case reports, for instance those describing workers using asbestos string in the manufacture of welding rods, in the formation of asbestos rope for grouting furnaces and maintaining underground mine haulage systems.
Other Sources of Exposure
Exposure of individuals engaged in trades which do not directly involve use or handling of asbestos but who work in the same area as those who do deal with it directly is called para-occupational (bystander) exposure. This has been an important source of exposure not only in the past but also for cases presenting for diagnosis in the 1990s. Workers involved include electricians, welders and carpenters in the construction and in the ship building or repair industries; maintenance personnel in asbestos factories; fitters, stokers and others in power stations and ships and boiler houses where asbestos lagging or other insulation is in place, and maintenance personnel in post-war high-rise buildings incorporating various asbestos-containing materials. In the past, domestic exposure occurred primarily from dust-laden workclothes being shaken or laundered at home, the dust so released becoming entrapped in carpets or furnishings and resuspended into the air with the activities of daily living. Not only could levels of airborne fibre reach levels as high as 10 fibre per millilitre (f/ml), that is, ten times the occupational exposure limit proposed by a WHO consultation (1989) of 1.0 f/ml but the fibres tended to remain airborne for several days. Since the 1970s, the practice of retaining all work clothes at the worksite for laundering has been widely but not universally adopted. In the past also, residential exposure occurred from contamination of air from industrial sources. For instance, increased levels of airborne asbestos have been documented in the neighbourhood of mines and asbestos plants and are determined by production levels, emission controls and weather. Given the long lag time for, in particular, asbestos-related pleural disease, such exposures are still likely to be responsible for some cases presenting for diagnosis in the 1990s. In the 1970s and 1980s, with the increase in public awareness of both the ill-health consequences of asbestos exposure and of the fact that asbestos containing materials are used extensively in modern construction (particularly in the friable form used for spray-on applications to walls, ceilings and ventilation ducts), a major cause of concern centred on whether, as such buildings age and are subject to daily wear and tear, asbestos fibres may be released into the air in sufficient numbers to become a threat to the health of those working in modern high-rise buildings (see below for risk estimates). Other sources of contamination of the air in urban areas include the release of fibre from brakes of vehicles and rescattering of fibres released by passing vehicles (Bignon, Peto and Saracci 1989).
Non-industrial sources of environmental exposure include naturally occurring fibres in soils, for instance in eastern Europe, and in rock outcrops in the Mediterranean region, including Corsica, Cyprus, Greece and Turkey (Bignon, Peto and Saracci 1989). An additional source of human exposure results from the use of tremolite for whitewash and stucco in Greece and Turkey, and according to more recent reports, in New Caledonia in the South Pacific (Luce et al. 1994). Furthermore, in several rural villages in Turkey, a zeolite fibre, erionite, has been found to be used both in stucco and in domestic construction and has been implicated in mesothelioma production (Bignon, Peto and Saracci 1991). Finally, human exposure may occur through drinking water, mainly from natural contamination, and given the widespread natural distribution of the fibre in outcrops, most water sources contain some fibre, levels being highest in mining areas (Skinner, Roos and Frondel 1988).
Aetiopathology of Asbestos-Related Disease
Fate of inhaled fibres
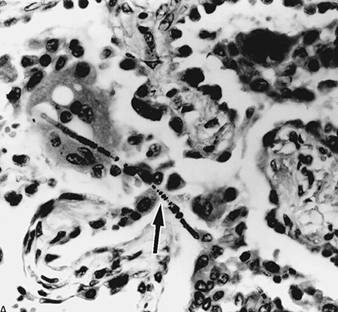
Inhaled fibres align themselves with the airstream and their capability of penetrating into the deeper lung spaces depends on their dimension, fibres of 5mm or less in aerodynamic diameter showing an over 80% penetration, but also less than 10 to 20% retention. Larger particles may impact in the nose and in major airways at bifurcations, where they tend to collect. Particles deposited in the major airways are cleared by the action of ciliated cells and are transported up the mucus escalator. Individual differences associated with what appears to be the same exposure are due, at least in part, to differences between individuals in the penetration and retention of inhaled fibres (Bégin, Cantin and Massé 1989). Small particles deposited beyond the major airways are phagocytosed by alveolar macrophages, scavenger cells which ingest foreign material. Longer fibres, that is, those over 10mm, often come under attack by more than one macrophage, are more likely to become coated and to form the nucleus of an asbestos body, a characteristic structure recognized since the early 1900s as a marker of exposure (see figure 3). Coating a fibre is considered to be part of the lungs’ defense to render it inert and non-immunogenic. Asbestos bodies are more likely to form on amphibole than on chrysotile fibres, and their density in biological material (sputum, bronchoalveolar lavage, lung tissue) is an indirect marker of lung burden. Coated fibres may persist in the lung for long periods, to be recovered from sputum or bronchoalveolar lavage fluid up to 30 years after last exposure. Clearance of non-coated fibres deposited in the lung’s parenchyma is towards the lung periphery and subpleural regions, and then to lymph nodes at the root of the lung.
Figure 3. Asbestos body
Magnification x 400, seen on microscopic section of the lung as a slightly curved elongated structure with a finely beaded iron protein coat. The asbestos fibre itself can be identified as the thin line near one end of the asbestos body (arrow). Source: Fraser et al. 1990
Theories to explain how fibres evoke the various pleural reactions associated with asbestos exposure include:
- direct penetration into the pleural space and drainage with the pleural fluid to pores in the pleura lining the chest wall
- release of mediators into the pleural space from subpleural lymphatic collections
- retrograde flow from lymph nodes at the root of the lung to the parietal pleura (Browne 1994)
There may also be retrograde flow via the thoracic duct to the abdominal lymph nodes to explain the occurrence of peritoneal mesothelioma.
Cellular effects of inhaled fibres
Animal studies indicate that the initial events which follow asbestos retention in the lung include:
- an inflammatory reaction, with accumulation of white blood cells followed by a macrophagic alveolitis with release of fibronectin, growth factor and various neutrophil chemotactic factors and, over time, the release of superoxide ion and
- proliferation of alveolar, epithelial, interstitial and endothelial cells (Bignon, Peto and Saracci 1989).
These events are reflected in the material recovered by bronchoalveolar lavage in animals and humans (Bégin, Cantin and Massé 1989). Both fibre dimensions and their chemical characteristics appear to determine biological potency for fibrogenesis, and these characteristics, in addition to surface properties, are also thought to be important for carcinogenesis. Long, thin fibres are more active than short ones, although the activity of the latter cannot be discounted, and amphiboles are more active than chrysotile, a property attributed to their greater biopersistence (Bégin, Cantin and Massé 1989). Asbestos fibres may also affect the human immune system and change the circulating population of blood lymphocytes. For instance, human cell mediated immunity to cell antigens (such as is exhibited in a tuberculin skin test) may be impaired (Browne 1994). In addition, since asbestos fibres appear to be capable of inducing chromosome abnormality, the view has been expressed that they can also be considered capable of inducing as well as promoting cancer (Jaurand in Bignon, Peto and Saracci 1989).
Dose versus exposure response relationships
In biological sciences such as pharmacology or toxicology in which dose-response relationships are used to estimate the probability of desired effects or the risk of undesired effects, a dose is conceptualized as the amount of agent delivered to and remaining in contact with the target organ for sufficient time to evoke a reaction. In occupational medicine, surrogates for dose, such as various measures of exposure, are usually the basis for risk estimates. However, exposure-response relationships can usually be demonstrated in workforce-based studies; the most appropriate exposure measure may, however, differ between diseases. Somewhat disconcerting is the fact that although exposure-response relationships will differ between workforces, these differences can be explained only in part by the fibre, particle size and industrial process. Nevertheless, such exposure-response relationships have formed the scientific basis for risk assessment and for setting permissible exposure levels, which were originally focused on controlling asbestosis (Selikoff and Lee 1978). As the prevalence and/or incidence of this condition has decreased, concern has switched to assure protection of human health against asbestos-related cancers. Over the last decade, techniques have been developed for the quantitative measurement of lung dust burden or biological dose directly in terms of fibres per gram of dry lung tissue. In addition, energy dispensive x-ray analysis (EDXA) permits precise characterization of each fibre by fibre type (Churg 1991). Though standardization of results between laboratories has not yet been achieved, comparisons of results obtained within a given laboratory are useful, and lung burden measurements have added a new tool for case evaluation. In addition, the application of these techniques in epidemiological studies has
- confirmed the biopersistence of amphibole fibres in the lung compared to chrysotile fibres
- identified fibre burden in the lungs of some individuals in whom exposure was forgotten, remote or thought to be unimportant
- demonstrated a gradient in lung burden associated with rural and urban residence and with occupational exposure and
- confirmed a fibre gradient in the lung dust burden associated with the major asbestos-related diseases (Becklake and Case 1994).
Asbestosis
Definition and history
Asbestosis is the name given to the pneumoconiosis consequent on exposure to asbestos dust. The term pneumoconiosis is used here as defined in the article “Pneumoconioses: Definitions”, of this Encyclopaedia as a condition in which there is “accumulation of dust in the lungs and tissue responses to the dust”. In the case of asbestosis, the tissue reaction is collagenous, and results in permanent alteration of the alveolar architecture with scarring. As early as 1898, the Annual Report of Her Majesty’s Chief Inspector of Factories contained reference to a lady factory inspector’s report on the adverse health consequences of asbestos exposure, and the 1899 Report contained details of one such case in a man who had worked for 12 years in one of the recently established textile factories in London, England. Autopsy revealed diffuse severe fibrosis of the lung and what subsequently came to be known as asbestos bodies were seen on subsequent histologic re-examination of the slides. Since fibrosis of the lung is an uncommon condition, the association was thought to be causal and the case was presented in evidence to a committee on compensation for industrial disease in 1907 (Browne 1994). Despite the appearance of reports of a similar nature filed by inspectors from the United Kingdom, Europe and Canada over the next decade, the role of exposure to asbestos in the genesis of the condition was not generally recognized until a case report was published in the British Medical Journal in 1927. In this report, the term pulmonary asbestosis was first used to describe this particular pneumoconiosis, and comment was made on the prominence of the associated pleural reactions, in contrast, for instance, to silicosis, the main pneumoconiosis recognized at the time (Selikoff and Lee 1978). In the 1930s, two major workforce-based studies carried out among textile workers, one in the United Kingdom and one in the United States, provided evidence of an exposure-response (and therefore likely causal) relationship between level and duration of exposure and radiographic changes indicative of asbestosis. These reports formed the basis of the first control regulations in the United Kingdom, promulgated in 1930, and the first threshold limit values for asbestos published by the American Conference of Government and Industrial Hygienists in 1938 (Selikoff and Lee 1978).
Pathology
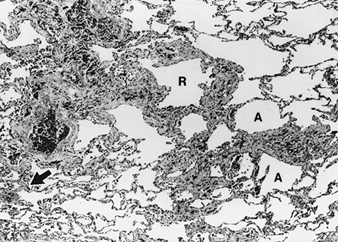
The fibrotic changes which characterize asbestosis are the consequence of an inflammatory process set up by fibres retained in the lung. The fibrosis of asbestosis is interstitial, diffuse, tends to involve the lower lobes and peripheral zones preferentially and, in the advanced case, is associated with obliteration of the normal lung architecture. Fibrosis of the adjacent pleura is common. Nothing in the histological features of asbestosis distinguish it from interstitial fibrosis due to other causes, except the presence of asbestos in the lung either in the form of asbestos bodies, visible to light microscopy, or as uncoated fibres, most of which are too fine to be seen except by means of electron microscopy. Thus, the absence of asbestos bodies in images derived from light microscopy does not rule out either exposure or the diagnosis of asbestosis. At the other end of the spectrum of disease severity, the fibrosis may be limited to relatively few zones and affect mainly the peribronchiolar regions (see figure 4), giving rise to what has been called asbestos-related small airways disease. Again, except perhaps for more extensive involvement of membranous small airways, nothing in the histologic changes of this condition distinguishes it from small airways disease due to other causes (such as cigarette smoking or exposure to other mineral dusts) other than the presence of asbestos in the lung. Small airways disease may be the only manifestation of asbestos-related lung fibrosis or it may coexist with varying degrees of interstitial fibrosis, that is, asbestosis (Wright et al. 1992). Carefully considered criteria have been published for the pathological grading of asbestosis (Craighead et al. 1982). In general, the extent and intensity of the lung fibrosis relates to the measured lung dust burden (Liddell and Miller 1991).
Figure 4. Asbestos-related small airways disease
Peribronchiolar fibrosis and infiltration by inflammatory cells is seen on a histologic section of a respiratory bronchiole (R) and its distal divisions or alveolar ducts (A). The surrounding lung is mostly normal but with focal thickening of the interstitial tissue (arrow), representing early asbestosis. Source: Fraser et al. 1990
Clinical features
Shortness of breath, the earliest, most consistently reported and most distressing complaint, has led to asbestosis being called a monosymptomatic disease (Selikoff and Lee 1978). Shortness of breath precedes other symptoms which include a dry, often distressing cough, and chest tightness—which is thought to be associated pleural reactions. Late inspiratory rales or crackles which persist after coughing are heard, first in the axilla and over the lung bases, before becoming more generalized as the condition advances, and are thought to be due to the explosive opening of airways which close on expiration. Coarse rales and rhonchi, if present, are thought to reflect bronchitis either in response to working in a dusty environment, or due to smoking.
Chest imaging
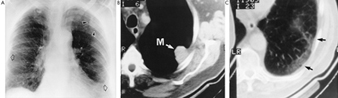
Traditionally, the chest radiograph has been the most important single diagnostic tool for establishing the presence of asbestosis. This has been facilitated by the use of the ILO (1980) radiological classification, which grades the small irregular opacities that are characteristic of asbestosis on a continuum from no disease to the most advanced disease, both for severity (described as profusion on a 12-point scale from –/0 to 3/+) and extent (described as the number of zones affected). Despite between reader differences, even among those who have completed training courses in reading, this classification has proved particularly useful in epidemiological studies, and has also been used clinically. However, pathological changes of asbestosis can be present on lung biopsy in up to 20% of subjects with a normal chest radiograph. In addition, small irregular opacities of low profusion (e.g., 1/0 on the ILO scale) are not specific for asbestosis but can be seen in relation to other exposures, for instance to cigarette smoking (Browne 1994). Computer tomography (CT) has revolutionized the imaging of interstitial lung disease, including asbestosis, with high resolution computer tomography (HRCT) adding increased sensitivity to the detection of interstitial and pleural disease (Fraser et al. 1990). Characteristics of asbestosis which can be identified by HRCT include thickened interlobular (septal) and intralobular core lines, parenchymal bands, curvilinear subpleural lines and subpleural dependent densities, the first two being the most distinctive for asbestosis (Fraser et al. 1990). HRCT can also identify these changes in cases with pulmonary function deficit in whom the chest radiograph is inconclusive. Based on postmortem HRCT, thickened intralobular lines have been shown to correlate with peribronchiolar fibrosis, and thickened interlobular lines with interstitial fibrosis (Fraser et al. 1990). As yet, no standardized reading method has been developed for the use of HRCT in asbestos-related disease. In addition to its cost, the fact that a CT device is a hospital installation makes it unlikely that it will replace the chest radiograph for surveillance and epidemiological studies; its role will likely remain limited to individual case investigation or to planned studies intended to address specific issues. Figure 21 illustrates the use of chest imaging in the diagnosis of asbestos-related lung disease; the case shown exhibits asbestosis, asbestos-related pleural disease and lung cancer. Large opacities, a complication of other pneumoconioses, in particular silicosis, are unusual in asbestosis and are usually due to other conditions such as lung cancer (see the case described in figure 5) or rounded atelectasis.
Figure 5. Chest imaging in asbestos-related lung disease.
A posteroanterior chest radiograph (A) shows asbestosis involving both lungs and assessed as ILO category 1/1, associated with bilateral pleural thickening (open arrows) and a vaguely defined opacity (arrow heads) in the left upper lobe. On HRCT scan (B), this was shown to be a dense mass (M) abutting onto the pleura and transthoracic needle biopsy revealed an adenocarcinoma of the lung. Also on CT scan (C), at high attenuation pleural plaques can be seen (arrow heads) as well as a thin curvilinear opacity in the parenchyma underlying the plaques with interstitial abnormality in the lung between the opacity and the pleura. Source: Fraser et al. 1990
Lung function tests
Established interstitial lung fibrosis due to asbestos exposure, like established lung fibrosis due to other causes, is usually but not invariably associated with a restrictive lung function profile (Becklake 1994). Its features include reduced lung volumes, in particular vital capacity (VC) with preservation of the ratio of forced expiratory volume in 1second to forced vital capacity (FEV1/FVC%), reduced lung compliance, and impaired gas exchange. Air flow limitation with reduced FEV1/FVC may, however, also be present as a response to a dusty work environment or to cigarette smoke. In the earlier stages of asbestosis, when the pathological changes are limited to peribronchiolar fibrosis and even before small irregular opacities are evident on the chest radiograph, impairment of tests reflecting small airway dysfunction such as the Maximum Mid-expiratory Flow Rate may be the only sign of respiratory dysfunction. Responses to the stress of exercise may also be impaired early in the disease, with increased ventilation in relation to the oxygen requirement of the exercise (due to an increased breathing frequency and shallow breathing) and impaired O2 exchange. As the disease progresses, less and less exercise is required to compromise O2 exchange. Given that the asbestos-exposed worker may exhibit features of both a restrictive and an obstructive lung function profile, the wise physician interprets the lung function profile in the asbestos worker for what it is, as a measure of impairment, rather than as an aid to diagnosis. Lung functions, in particular vital capacity, provide a useful tool for the follow-up of subjects individually, or in epidemiological studies, for instance after exposure has ceased, to monitor the natural history of asbestosis or asbestos-related pleural disease.
Other laboratory tests
Bronchoalveolar lavage is increasingly used as a clinical tool in the investigation of asbestos-related lung disease:
- to rule out other diagnoses
- to assess the activity of the pulmonary reactions under study such as fibrosis or
- to identify the agent in the form of asbestos bodies or fibres.
It is also used to study disease mechanisms in humans and animals (Bégin, Cantin and Massé 1989). The uptake of Gallium-67 is used as a measure of the activity of the pulmonary process, and serum antinuclear antibodies (ANA) and rheumatoid factors (RF), both of which reflect the immunological status of the individual, have also been investigated as factors influencing disease progression, and/or accounting for between individual differences in response to what appears to be the same level and dose of exposure.
Epidemiology including natural history
The prevalence of radiological asbestosis documented in workforce-based surveys varies considerably and, as might be expected, these differences relate to differences in exposure duration and intensity rather than differences between workplaces. However, even when these are taken into account by restricting comparison of exposure response relationships to those studies in which exposure estimates were individualized for each cohort member and based on job history and industrial hygiene measurements, marked fibre and process related gradients are evident (Liddell and Miller 1991). For instance, a 5% prevalence of small irregular opacities (1/0 or more on the ILO classification) resulted from a cumulative exposure to approximately 1,000 fibre years in Quebec chrysotile miners, to approximately 400 fibre years in Corsican chrysotile miners, and to under 10 fibre years in South African and Australian crocidolite miners. By contrast, for textile workers exposed to Quebec chrysotile, a 5% prevalence of irregular small opacities resulted from a cumulative exposure to under 20 fibre years. Lung dust burden studies are also consistent with a fibre gradient for evoking asbestosis: in 29 men in Pacific shipyard trades with asbestosis associated with mainly amosite exposure, the average lung burden found in autopsy material was 10 million amosite fibres per gram of dry lung tissue compared to an average chrysotile burden of 30 million fibres per gram of dry lung tissue in 23 Quebec chrysotile miners and millers (Becklake and Case 1994). Fibre size distribution contributes to but does not fully explain these differences, suggesting that other plant-specific factors, including other workplace contaminants, may play a role.
Asbestosis may remain stable or progress, but probably does not regress. Progression rates increase with age, with cumulative exposure, and with the extent of existing disease, and are more likely to occur if exposure was to crocidolite. Radiological asbestosis can both progress and appear long after exposure ceases. Deterioration of lung functions may also occur after exposure has ceased (Liddell and Miller 1991). An important issue (and one on which the epidemiological evidence is not consistent) is whether continued exposure increases the chance of progression once radiological changes have developed (Browne 1994; Liddell and Miller 1991). In some jurisdictions, for example in the United Kingdom, the number of cases of asbestosis presenting for worker’s compensation have decreased over the last decades, reflecting the workplace controls put in place in the 1970s (Meredith and McDonald 1994). In other countries, for instance in Germany (Gibbs, Valic and Browne 1994), rates of asbestosis continue to rise. In the United States, age-adjusted asbestos-related mortality rates (based on mention of asbestosis on the death certificate as either the cause of death or as playing a contributory role) for age 1+ increased from under 1 per million in 1960 to over 2.5 in 1986, and to 3 in 1990 (US Dept. of Health and Human Services, 1994).
Diagnosis and case management
Clinical diagnosis depends on:
- establishing the presence of disease
- establishing whether exposure occurred and
- evaluating whether the exposure was likely to have caused the disease.
The chest radiograph remains the key tool to establish the presence of disease, supplemented by HRCT if available in cases where there is doubt. Other objective features are the presence of basal crackles, while lung function level, including exercise challenge, is useful in establishing impairment, a step required for compensation evaluation. Since neither the pathology, radiological changes, nor the symptoms and lung function changes associated with asbestosis are different from those associated with interstitial lung fibrosis due to other causes, establishing exposure is key to diagnosis. In addition, the many uses of asbestos products whose content is often not known to the user makes an exposure history a much more daunting exercise in interrogation than was previously thought. If the exposure history appears inadequate, identification of the agent in biological specimens (sputum, bronchoalveolar lavage and when indicated, biopsy) can corroborate exposure; dose in the form of lung burden can be assessed quantitatively by autopsy or in surgically removed lungs. Evidence of disease activity (from a gallium-67 scan or bronchoalveolar lavage) may assist in estimating prognosis, a key issue in this irreversible condition. Even in the absence of consistent epidemiological evidence that progression is slowed once exposure ceases, such a course may be prudent and certainly desirable. It is not, however, a decision easy to take or recommend, particularly for older workers with little opportunity for job retraining. Certainly exposure should not continue in any workplace not in conformity with current permissible exposure levels. Criteria for the diagnosis of asbestosis for epidemiological purposes are less demanding, particularly for cross-sectional workforce-based studies which include those well enough to be at work. These usually address issues of causality and often use markers that indicate minimal disease, based either on lung function level or on changes in the chest radiograph. By contrast, criteria for diagnosis for medicolegal purposes are considerably more stringent and vary according to the legal administrative systems under which they operate, varying between states within countries as well as between countries.
Asbestos-Related Pleural Disease
Historical perspective
Early descriptions of asbestosis mention fibrosis of the visceral pleura as part of the disease process (see “Pathology”, page 10.55). In the 1930s there were also reports of circumscribed pleural plaques, often calcified, in the parietal pleura (which lines the chest wall and covers the surface of the diaphragm), and occurring in those with environmental, not occupational, exposure. A 1955 workforce-based study of a German factory reported a 5% prevalence of pleural changes on the chest radiograph, thereby drawing attention to the fact that pleural disease might be the primary if not the only manifestation of exposure. Visceroparietal pleural reactions, including diffuse pleural fibrosis, benign pleural effusion (reported first in the 1960s) and rounded atelectasis (first reported in the 1980s) are now all considered interrelated reactions which are usefully distinguished from pleural plaques on the basis of pathology and probably pathogenesis, as well as clinical features and presentation. In jurisdictions in which the prevalence and/or incidence rates of asbestosis are decreasing, pleural manifestations, increasingly common in surveys, are increasingly the basis of detection of past exposure, and increasingly the reason for an individual seeking medical attention.
Pleural plaques
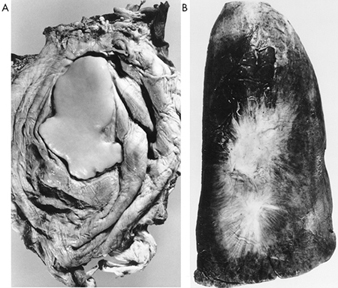
Pleural plaques are smooth, raised, white irregular lesions covered with mesothelium and found on the parietal pleura or diaphragm (figure 6). They vary in size, are often multiple, and tend to calcify with increasing age (Browne 1994). Only a small proportion of those detected at autopsy are seen on the chest radiograph, though most can be detected by HRCT. In the absence of pulmonary fibrosis, pleural plaques may cause no symptoms and may be detected only in screening surveys using chest radiography. Nevertheless, in workforce surveys, they are consistently associated with modest but measurable lung function impairment, mainly in VC and FVC (Ernst and Zejda 1991). In radiological surveys in the United States, rates of 1% are reported in men without known exposure, and 2.3% in men which include those in urban populations, with occupational exposure. Rates are also higher in communities with asbestos industries or high usage rates, while in some workforces, such as sheet metal workers, insulators, plumbers and railroad workers, rates may exceed 50%. In a 1994 Finnish autopsy survey of 288 men aged 35 to 69 years who died suddenly, pleural plaques were detected in 58%, and exhibited the tendency to increase with age, with the probability of exposure (based on history), with the concentration of asbestos fibres in lung tissue, and with smoking (Karjalainen et al. 1994). The aetiologic fraction of plaques attributable to a lung dust burden of 0.1 million fibres per gram of lung tissue was estimated at 24%, (this value is considered to be an underestimate). Lung dust burden studies are also consistent with fibre gradient in potency for evoking pleural reactions; in 103 men with amosite exposure in Pacific shipyard trades, all with pleural plaques, the average autopsy lung burden was 1.4 million fibres per gram of lung tissue, compared to 15.5 and 75 million fibres per gram of lung tissue for chrysotile and tremolite respectively in 63 Quebec chrysotile miners and millers examined in the same way (Becklake and Case 1994).
Figure 6. Asbestos-related pleural disease
A diaphragmatic pleural plaque (A) is seen in an autopsy specimen as a smooth well defined focus of fibrosis on the diaphragm of a construction worker with incidental exposure to asbestos and asbestos bodies in the lung. Visceral pleural fibrosis (B) is seen on an inflated autopsy lung specimen, and radiates from two central foci on the visceral pleura of the lung of a construction worker with asbestos exposure who also exhibited several parietal pleural plaques. Source: Fraser et al. 1990.
Visceroparietal pleural reactions
Though the pathology and pathogenesis of the different forms of visceroparietal reaction to asbestos exposure are almost certainly interrelated, their clinical manifestations and how they come to attention differs. Acute exudative pleural reactions may occur in the form of effusions in subjects whose lungs do not manifest other asbestos-related disease, or as an exacerbation in the severity and extent of existing pleural reactions. Such pleural effusions are called benign by way of distinguishing them from effusions associated with malignant mesothelioma. Benign pleural effusions occur typically 10 to 15 years after first exposure (or after limited past exposure) in individuals in their 20s and 30s. They are usually transient but may reoccur, may involve one or both sides of the chest simultaneously or sequentially, and may be either silent or associated with symptoms including chest tightness and/or pleural pain and dyspnoea. The pleural fluid contains leucocytes, often blood, and is albumin-rich; only rarely does it contain asbestos bodies or fibres which may, however, be found in biopsy material of the pleura or underlying lung. Most benign pleural effusions clear spontaneously, though in a small proportion of subjects (of the order of 10% in one series) these effusions may evolve into diffuse pleural fibrosis (see figure 6), with or without the development of lung fibrosis. Local pleural reactions may also fold in upon themselves, trapping lung tissue and causing well defined lesions called rounded atelectasis or pseudotumour because they may have the radiological appearance of lung cancer. In contrast to pleural plaques, which seldom cause symptoms, visceroparietal pleural reactions are usually associated with some shortness of breath as well as lung function impairment, particularly when there is obliteration of the costophrenic angle. In one study, for instance, average FVC deficit was 0.07 l when the chest wall was involved and 0.50 l when the costophrenic angle was involved (Ernst and Zejda in Liddell and Miller 1991). As already indicated, the distribution and determinants of pleural reactions vary considerably between workforces, with prevalence rates increasing with:
- estimated residence time of fibre in the lung (measured as time since first exposure)
- exposures primarily to or including amphibole and
- possibly intermittence of exposure, given the high rates of contamination in occupations in which use of asbestos materials is intermittent, but exposure probably heavy.
Lung Cancer
Historical perspective
The 1930s saw the publication of a number of clinical case reports from the United States, the United Kingdom and Germany of lung cancer (a condition much less common then than it is today) in asbestos workers, most of whom also had asbestosis of varying degrees of severity. Further evidence of the association between the two conditions was provided in the 1947 Annual Report of His Majesty’s Chief Inspector of Factories, which noted that lung cancer had been reported in 13.2% of male deaths attributed to asbestosis in the period 1924 to 1946 and in only 1.3% of male deaths attributed to silicosis. The first study to address the causal hypothesis was a cohort mortality study of a large United Kingdom asbestos textile plant (Doll 1955), one of the first such workforce-based studies, and by 1980, after at least eight such studies in as many workforces had confirmed an exposure-response relationship, the association was generally accepted as causal (McDonald and McDonald in Antman and Aisner 1987).
Clinical features and pathology
In the absence of other associated asbestos disease, the clinical features and criteria for the diagnosis of asbestos-associated lung cancer are no different from those for lung cancer not associated with asbestos exposure. Originally, asbestos-associated lung cancers were considered to be scar cancers, similar to lung cancer seen in other forms of diffuse lung fibrosis such as scleroderma. Features which favoured this view were their location in the lower lung lobes (where asbestosis is usually more marked), their sometimes multicentric origin and a preponderance of adenocarcinoma in some series. However, in most reported workforce-based studies, the distribution of cell types was no different from that seen in studies of non-asbestos-exposed populations, supporting the view that asbestos itself may be a human carcinogen, a conclusion reached by the International Agency for Research on Cancer (World Health Organization: International Agency for Research on Cancer 1982). Most but not all asbestos-related lung cancers occur in association with radiologic asbestosis (see below).
Epidemiology
Cohort studies confirm that lung cancer risk increases with exposure, though the fractional rate of increase for each fiber per milliliter per year exposed varies, and is related both to fibre type and to industrial process (Health Effects Institute—Asbestos Research 1991). For instance, for mainly chrysotile exposures in mining, milling and friction product manufacture, the increase ranged from approximately 0.01 to 0.17%, and in textile manufacture from 1.1 to 2.8%, while for exposure to amosite insulation products and some cement product exposures involving mixed fibre, rates of as high as 4.3 and 6.7% have been recorded (Nicholson 1991). Cohort studies in asbestos workers also confirm that cancer risk is demonstrable for non-smokers and that risk is increased (closer to multiplicative than additive) by cigarette smoking (McDonald and McDonald in Antman and Aisner 1987). The relative risk for lung cancer declines after exposure ceases, although the decline appears slower than that which occurs after quitting smoking. Lung dust burden studies are also consistent with a fibre gradient in lung cancer production; 32 men in Pacific shipyard trades with mainly amosite exposure had a lung dust burden of 1.1 million amosite fibres per gram of dry lung tissue compared to 36 Quebec chrysotile miners with an average lung dust burden of 13 million chrysotile fibres per gram of lung tissue (Becklake and Case 1994).
Relationship to asbestosis
In the 1955 autopsy study of causes of death in 102 workers employed in the United Kingdom asbestos textile factory referred to above (Doll 1955), lung cancer was found in 18 individuals, 15 of whom also had asbestosis. All subjects in whom both conditions were found had worked for at least 9 years before 1931, when national regulations for asbestos dust control were introduced. These observations suggested that as exposure levels decreased, the competing risk of death from asbestosis also decreased and workers lived long enough to exhibit the development of cancer. In most workforce-based studies, older workers with long service have some pathological evidence of asbestosis (or asbestos-related small airways disease) at autopsy even though this may be minimal and not detectable on the chest radiograph in life (McDonald and McDonald in Antman and Aisner 1987). Several but not all cohort studies are consistent with the view that not all excess lung cancers in populations exposed to asbestos are related to asbestosis. More than one pathogenetic mechanism may in fact be responsible for lung cancers in individuals exposed to asbestos depending on the site and deposition of the fibres. For instance, long thin fibres, which are deposited preferentially at airway bifurcations, are thought to become concentrated and to act as inducers of the process of cancerogenesis through chromosomal damage. Promoters of this process may include continued exposure to asbestos fibres or to tobacco smoke (Lippman 1995). Such cancers are more likely to be squamous cell in type. By contrast, in lungs which are the site of fibrosis, cancerogenesis may result from the fibrotic process: such cancers are more likely to be adenocarcinomas.
Implications and attributability
While determinants of excess cancer risk can be derived for exposed populations, attributability in the individual case cannot. Obviously, attributability to asbestos exposure is more likely and credible in an exposed individual with asbestosis who has never smoked than in an exposed individual without asbestosis who smokes. Nor can this probability be modelled reasonably. Lung dust burden measurements may supplement a careful clinical assessment but each case must be evaluated on its merits (Becklake 1994).
Malignant Mesothelioma
Pathology, diagnosis, ascertainment and clinical features
Malignant mesotheliomas arise from the serous cavities of the body. Approximately two-thirds arise in the pleura, about one-fifth in the peritoneum, while the pericardium and tunica vaginalis are much less frequently affected (McDonald and McDonald in Lidell and Miller 1991). Since mesothelial cells are pluripotential, the histological features of mesothelial tumours may vary; in most series, epithelial, sarcomatous and mixed forms account for approximately 50, 30 and 10% of cases respectively. Diagnosis of this rare tumour, even in the hands of experienced pathologists, is not easy, and mesothelioma panel pathologists often confirm only a small percentage, in some studies less than 50% of cases submitted for review. A variety of cytological and immunohistochemical techniques have been developed to assist in differentiating malignant mesothelioma from the main alternative clinical diagnoses, namely, secondary cancer or reactive mesothelial hyperplasia; this remains an active research field in which expectations are high but findings inconclusive (Jaurand, Bignon and Brochard 1993). For all these reasons, ascertainment of cases for epidemiological surveys is not straightforward, and even when based on cancer registries, may be incomplete. In addition, confirmation by expert panels using specified pathological criteria is necessary to assure comparability in criteria for registration.
Clinical features
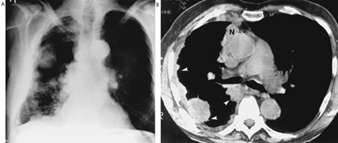
Pain is usually the presenting feature. For pleural tumours, this starts in the chest and/or shoulders, and may be severe. Breathlessness follows, associated with pleural effusion and/or progressive encasement of the lung by tumour, and weight loss. With peritoneal tumours, abdominal pain is usually accompanied by swelling. Imaging features are illustrated in figure 7. The clinical course is usually rapid and median survival times, six months in a 1973 report and eight months in a 1993 report, have changed little over the last two decades, despite the greater public and medical awareness which often leads to earlier diagnosis and despite advances in diagnostic techniques and an increase in the number of treatment options for cancer.
Figure 7. Malignant mesothelioma
Seen on an overpenetrated chest roetngenogram (A) as a large mass in the axillary region. Note the associated reduction in volume of the right haemothorax with marked irregular nodular thickening of the pleura of the whole right lung. CT scan (B) confirms the extensive pleural thickening involving parietal and mediastinal pleura (closed arrows) in and around the ribs. Source: Fraser et al. 1990
Epidemiology
In the 15 years which followed the 1960 report of the mesothelioma case series from the Northwest Cape, South Africa (Wagner 1996), international confirmation of the association came from reports of other case series from Europe (United Kingdom, France, Germany, Holland), the United States (Illinois, Pennsylvania and New Jersey) and Australia, and of case control studies from the United Kingdom (4 cities), Europe (Italy, Sweden, Holland) and from the United States and Canada. Odds ratios in these studies ranged from 2 to 9. In Europe in particular, the association with shipyard occupations was strong. In addition, proportional mortality studies in asbestos-exposed cohorts suggested that risk was associated both with fibre type and with industrial process, with rates attributable to mesothelioma ranging from 0.3% in chrysotile mining to 1% in chrysotile manufacturing, compared with 3.4% in amphibole mining and manufacturing and as high as 8.6% for exposure to mixed fibre in insulation (McDonald and McDonald in Liddell and Miller 1991). Similar fibre gradients are shown in cohort mortality studies which, given the short survival times of these tumours, are a reasonable reflection of incidence. These studies also show longer latent periods when exposure was to chrysotile compared to amphiboles. Geographical variation in incidence has been documented using Canadian age-and sex-specific rates for 1966 to 1972 to calculate expected rates (McDonald and McDonald in Liddell and Miller 1991); rate ratios (values actually observed over expected) were 0.8 for the United States (1972), 1.1 for Sweden (1958 to 1967), 1.3 for Finland (1965 to 1969), 1.7 for United Kingdom (1967 to 1968), and 2.1 for the Netherlands (1969 to 1971). While technical factors including ascertainment may obviously contribute to the variation recorded, the results do suggest higher rates in Europe than in North America.
Time trends and gender differences in mesothelioma incidence have been used as a measure of the health impact of asbestos exposure on populations. The best estimates for overall rates in industrialized countries before 1950 are under 1.0 per million for men and women (McDonald and McDonald in Jaurand and Bignon 1993). Subsequently, rates increased steadily in men and either not at all or less in women. For instance, overall rates in men and women per million were reported at 11.0 and under 2.0 in the United States in 1982, 14.7 and 7.0 in Denmark for 1975-80, 15.3 and 3.2 in the United Kingdom for 1980-83, and 20.9 and 3.6 in the Netherlands for 1978-87. Higher rates in men and women, but excluding younger subjects, were reported for crocidolite mining countries: 28.9 and 4.7 respectively in Australia (aged 2+) for 1986, and 32.9 and 8.9 respectively in South African Whites (aged 1+) for 1988 (Health Effects Institute—Asbestos Research 1991). The rising rates in men are likely to reflect occupational exposure, and if so, they should level off or decrease within the 20-to 30-year “incubation” period following the introduction of workplace controls and reduction of exposure levels in most workplaces in most industrialized countries in the 1970s. In countries in which the rates in women are rising, this increase may reflect their increasing engagement in occupations with risk exposure, or the increasing environmental or indoor contamination of urban air (McDonald 1985).
Aetiology
Environmental factors are clearly the main determinants of mesothelioma risk, exposure to asbestos being the most important, though the occurrence of family clusters maintains interest in the potential role of genetic factors. All asbestos fibre types have been implicated in mesothelioma production, including anthophyllite for the first time in a recent report from Finland (Meurman, Pukkala and Hakama 1994). However, there is a substantial body of evidence, from proportional and cohort mortality studies and lung burden studies, which suggests the role of a fibre gradient in mesothelioma production, risk being higher for exposures to mainly amphiboles or amphibole chrysotile mixtures, compared with mainly chrysotile exposures. In addition, there are rate differences between workforces for the same fibre at what appears to be the same exposure level; these remain to be explained, though fibre size distribution is a likely contributing factor.
The role of tremolite has been widely debated, a debate sparked by the evidence of its biopersistence in lung tissue, animal and human, compared to that of chrysotile. A plausible hypothesis is that the many short fibres which reach and are deposited in peripheral lung airways and alveoli are cleared to subpleural lymphatics where they collect; their potency in mesothelioma production depends on their biopersistence in contact with pleural surfaces (Lippmann 1995). In human studies, mesothelioma rates are lower for populations exposed at work to chrysotile relatively uncontaminated by tremolite (for instance, in Zimbabwean mines) compared to those exposed to chrysotile which is so contaminated (for instance, in Quebec mines), and these findings have been replicated in animal studies (Lippmann 1995). Also, in a multivariate analysis of lung fibre burden in material from a Canada-wide mesothelioma case control study (McDonald et al. 1989), the results suggested that most if not all mesotheliomas could be explained by tremolite lung fibre burden. Finally, a recent analysis of the mortality in the cohort of over 10,000 Quebec chrysotile miners and millers born between 1890 and 1920, and followed to 1988 (McDonald and McDonald 1995), supports this view: in almost 7,300 deaths, the 37 mesothelioma deaths were concentrated in certain mines from the Thetford area, yet the lung burden of 88 cohort members from the mines implicated did not differ from that of miners from other mines in terms of chrysotile fibre burden, only in terms of tremolite burden (McDonald et al. 1993).
What has been called the tremolite question is perhaps the most important of the currently debated scientific issues, and it also has public health implications. Note must also be made of the important fact that in all series and jurisdictions, a certain proportion of cases occur without reported asbestos exposure, and that only in some of these cases do lung dust burden studies point to previous environmental or occupational exposure. Other occupational exposures have been implicated in mesothelioma production, for instance in talc, vermiculite and possibly mica mining, but in these, the ore contained either tremolite or other fibres (Bignon, Peto and Saracci 1989). An open search for other exposures, occupational or non-occupational, to fibres, inorganic and organic, and to other agents which may be associated with mesothelioma production, should continue.
Other Asbestos-Related Diseases
Chronic airways disease
Usually included under this rubric are chronic bronchitis and chronic obstructive pulmonary disease (COPD), both of which can be diagnosed clinically, and emphysema, until recently diagnosed only by pathological examination of lungs removed at autopsy or otherwise (Becklake 1992). A major cause is smoking, and, over the past decades, mortality and morbidity due to chronic airways disease has increased in most industrialized countries. However, with the decline of pneumoconiosis in many workforces, evidence has emerged to implicate occupational exposures in the genesis of chronic airways disease, after taking into account the dominant role of smoking. All forms of chronic airways disease have been shown to be associated with work in a variety of dusty occupations, including those occupations in which an important component of the dust contaminating the workplace was asbestos (Ernst and Zejda in Liddell and Miller 1991). Total pollutant burden, rather than exposure to any of its particular components, in this case asbestos dust, is thought to be implicated, in much the same way as the effect of smoking exposure on chronic airways diseases is viewed, that is, in terms of total exposure burden (e.g., as pack-years), not exposure to any one of the over 4,000 constituents of tobacco smoke. (see elsewhere in this volume for a further discussion of the relationship between occupational exposures and chronic airways disease).
Other cancers
In several of the earlier cohort studies of asbestos exposed workers, mortality attributable to all cancers exceeded that expected, based on national or regional vital statistics. While lung cancer accounted for most of the excess, other cancers implicated were gastro-intestinal cancers, laryngeal cancer and cancer of the ovaries, in that order of frequency. For gastro-intestinal cancers, (including those affecting the oesophagus, the stomach, the colon and the rectum), the relevant exposure in occupational cohorts is presumed to be via swallowing asbestos-laden sputum raised from the major airways in the lung, and in earlier times, (before protection measures were taken against exposure at lunch sites) direct contamination of food in workplaces which had no lunch areas separate from working areas of plants and factories. Retrograde flow via the thoracic duct from lymph nodes draining the lung might also occur (see “Fate of inhaled fibres”, page 10.54). Because the association was inconsistent in the different cohorts studied, and because exposure response relationships were not always seen, there has been a reluctance to accept the evidence of the association between occupational exposure and asbestos exposure as causal (Doll and Peto 1987; Liddell and Miller 1991).
Cancer of the larynx is much less common than gastro-intestinal or lung cancer. As early as the 1970s, there were reports of an association between cancer of the larynx and asbestos exposure. Like lung cancer, a major risk factor and cause of laryngeal cancer is smoking. Laryngeal cancer is also strongly associated with alcohol consumption. Given the location of the larynx (an organ exposed to all the inhaled pollutants to which the lungs are exposed) and given the fact that it is lined by the same epithelium that lines the major bronchi, it is certainly biologically plausible that cancer of the larynx occurs as a result of asbestos exposure. However, the overall evidence available to date is inconsistent, even from large cohort studies such as the Quebec and Balangero (Italy) chrysotile miners, possibly because it is a rare cancer and there is still reluctance to regard the association as causal (Liddell and Miller 1991) despite its biological plausibility. Cancer of the ovaries has been recorded in excess of expected in three cohort studies (WHO 1989). Misdiagnosis, in particular as peritoneal mesothelioma, may explain most of the cases (Doll and Peto 1987).
Prevention, Surveillance and Assessment
Historical and current approaches
Prevention of any pneumoconiosis, including asbestosis, has traditionally been through:
- engineering and work practices to maintain airborne fibre levels as low as possible, or at least in conformity with permissible exposure levels usually set by law or regulation
- surveillance, conducted to record trends of markers of disease in exposed populations and monitor the results of control measures
- education and product labelling aimed at assisting workers as well as the general public in avoiding non-occupational exposure.
Permissible exposure levels were originally directed at controlling asbestosis and were based on industrial hygiene measurements in million particles per cubic foot, gathered using the same methods as were used for the control of silicosis. With the shift in biological focus to fibres, in particular long thin ones, as the cause of asbestosis, methods more appropriate to their identification and measurement in air were developed and, given these methods, the focus on the more abundant short fibres which contaminate most workplaces was minimized. Aspect (length to diameter) ratios for most particles of milled chrysotile asbestos fall within the range 5:1 to 20:1, going up to 50:1, in contrast to most particles of milled amphibole asbestos (including cleavage fragments) whose values fall below 3:1. The introduction of the membrane filter for fibre counting of air samples led to an arbitrary industrial hygiene and medical definition of a fibre as a particle at least 5μm long, 3μm or less thick, and with a length to width ratio of at least 3:1. This definition, used for many of the studies of exposure-response relationships, forms the scientific basis for setting environmental standards.
For instance, it was used in a meeting sponsored by the World Health Organization (1989) to propose occupational exposure limits and has been adopted by agencies such as the US Occupational Safety and Health Administration; it is retained mainly for reasons of comparability. The WHO meeting, chaired by Sir Richard Doll, while recognizing that the occupational exposure limit in any country can only be set by the appropriate national body, recommended that countries having high limits should take urgent steps to lower the occupational exposure for an individual worker to 2 f/ml (eight-hour time-weighted average) and that all countries should move as quickly as possible to 1 f/ml (eight-hour time-weighted average) if they had not already done so. With the decrease in asbestosis rates in some industrialized countries, and concern over asbestos-related cancers in all, attention has now shifted to determining whether the same fibre parameters—that is, at least 5mm long, 3mm or less thick, and with a length to width ratio of at least 3:1—are also appropriate for controlling carcinogenesis (Browne 1994). A current theory of asbestos carcinogenesis implicates short as well as long fibres (Lippmann 1995). In addition, given the evidence for a fibre gradient in mesothelioma and lung cancer production, and to a lesser extent, for asbestosis production, an argument could be made for permissible exposure levels taking fibre type into account. Some countries have addressed the issue by banning the use (and thus the import) of crocidolite, and setting more stringent exposure levels for amosite, namely 0.1 f/l (McDonald and McDonald 1987).
Exposure levels in the workplace
Permissible exposure levels embody the hypothesis, based on all available evidence, that human health will be preserved if exposure is maintained within those limits. Revision of permissible exposure levels, when it occurs, is invariably towards greater stringency (as described in the paragraph above). Nevertheless, despite good compliance with workplace controls, cases of disease continue to occur, for reasons of personal susceptibility (for instance, higher-than-average fibre retention rates) or because of failure of workplace controls for certain jobs or processes. Engineering controls, improved workplace practices and the use of substitutes, described elsewhere in the chapter, have been implemented internationally (Gibbs, Valic and Browne 1994) in larger establishments through industry, union and other initiatives. For instance, according to a 1986 worldwide industry review, compliance with the current recommended standard of 1 f/ml had been achieved at 83% of production sites (mines and mills) covering 13,499 workers in 6 countries; in 96% of 167 cement factories operating in 23 countries; in 71% of 40 textile factories covering over 2,000 workers operating in 7 countries; and in 97% of 64 factories manufacturing friction materials, covering 10,190 workers in 10 countries (Bouige 1990). However, a not unimportant proportion of such workplaces still do not comply with regulations, not all manufacturing countries participated in this survey, and the anticipated health benefits are evident only in some national statistics, not in others (“Diagnosis and case management”, page 10.57). Control in demolition processes and small enterprises using asbestos continues to be less than successful, even in many industrialized countries.
Surveillance
The chest radiograph is the main tool for asbestosis surveillance, cancer registries and national statistics for asbestos-related cancers. A commendable initiative in international surveillance of mining, tunnelling and quarrying, undertaken by the ILO through voluntary reporting from governmental sources, focuses on coal and hard-rock mining but could include asbestos. Unfortunately, follow-through has been poor, with the last report, which was based on data for 1973-77, being published in 1985 (ILO 1985). Several countries issue national mortality and morbidity data, an excellent example being the Work-related Lung Disease Surveillance Report for the United States, a report referred to above (USDHSS 1994). Such reports provide information to interpret trends and evaluate the impact of control levels at a national level. Larger industries should (and many do) keep their own surveillance statistics, as do some unions. Surveillance of smaller industries may require specific studies at appropriate intervals. Other sources of information include programmes such as the Surveillance of Work-related Respiratory Diseases (SWORD) in the United Kingdom, which gathers regular reports from a sample of the country’s chest and occupational physicians (Meredith and McDonald 1994), and reports from compensation boards (which often, however, do not provide information on workers at risk).
Product labelling, education and the information highway
Mandatory product labelling together with worker education and education of the general public are powerful tools in prevention. While in the past, this took place within the context of worker organizations, worker management committees, and union education programmes, future approaches could exploit electronic highways to make available databases on health and safety in toxicology and medicine.
Exposure in buildings and from water supplies
In 1988, a review of potential health risks associated with working in buildings constructed using asbestos-containing materials was mandated by the US Congress (Health Effects Institute—Asbestos Research 1991). The results of a large number of indoor sampling studies from Europe, the United States and Canada were used in risk estimates. The lifetime risk for premature cancer death was estimated to be 1 per million for those exposed for 15 years in schools (for estimated exposure levels ranging from .0005 to .005 f/ml) and 4 per million for those exposed for 20 years working in office buildings (for estimated exposure levels ranging from .0002 to .002 f/ml). For comparison, the risk for occupational exposure to 0.1 f/ml (i.e., in compliance with the permissible exposure limit proposed by the US Occupational Safety and Health Administration) for 20 years was estimated at 2,000 per million exposed. Measurements in drinking water in urban communities show considerable variation, from undetectable levels to high levels ranging from 0.7 million f/l in Connecticut, USA, to levels ranging from 1.1 million to 1.3 billion f/l in the mining areas of Quebec (Bignon, Peto and Saracci 1989). Some contamination may also occur from the asbestos cement pipes which must service most urban water reticulation services in the world. However, a working group which reviewed the evidence in 1987 did not discount the potential associated hazard, but did not regard the health risks associated with asbestos ingestion as “one of the most pressing public health hazards” (USDHHS 1987), a view concordant with the concluding remarks in an IARC (WHO) monograph on non-occupational exposure to mineral fibres (Bignon, Peto and Saracci 1989).
Asbestos and other fibres in the 21st century
The first half of the twentieth century was characterized by what could be described as gross neglect of asbestos-related ill health. Before the Second World War, the reasons for this are not clear; the scientific basis for control was there but perhaps not the will and not the worker militancy. During the war, there were other national and international priorities, and after the war, pressures of urbanization by a rapidly increasing world population took precedence, and perhaps fascination in an industrial age with the versatility of the “magic” mineral diverted attention from its dangers. Following the first International Conference on the Biological Effects of Asbestos in 1964 (Selikoff and Churg 1965), asbestos-related disease became a cause célèbre, not only on its own account, but also because it marked a period of labour-management confrontation concerning the rights of the worker to knowledge about workplace hazards, health protection and fair compensation for injury or illness. In countries with no-fault worker’s compensation, asbestos-related disease on the whole received fair recognition and handling. In countries where product liability and class action suits were more usual, large awards have been made to some affected workers (and their lawyers) while others have been left destitute and without support. While the need for fibres in modern societies is unlikely to diminish, the role of the mineral fibres vis-à-vis other fibres may change. There has already been a shift in uses both within and between countries (see “Other sources of exposure”, page 10.53). Though the technology exists to diminish workplace exposures, there remain workplaces in which it has not been applied. Given the current knowledge, given international communication and product labelling, and given worker education and industry commitment, it should be possible to use this mineral to provide cheap and durable products for use in construction and water reticulation on an international basis without risk to user, worker, manufacturer or miner, or to the general public at large.