Cutaneous sensitivity shares the main elements of all the basic senses. Properties of the external world, such as colour, sound, or vibration, are received by specialized nerve cell endings called sensory receptors, which convert external data into nervous impulses. These signals are then conveyed to the central nervous system, where they become the basis for interpreting the world around us.
It is useful to recognize three essential points about these processes. First, energy, and changes in energy levels, can be perceived only by a sense organ capable of detecting the specific type of energy in question. (This is why microwaves, x rays, and ultraviolet light are all dangerous; we are not equipped to detect them, so that even at lethal levels they are not perceived.) Second, our perceptions are necessarily imperfect shadows of reality, as our central nervous system is limited to reconstructing an incomplete image from the signals conveyed by its sensory receptors. Third, our sensory systems provide us with more accurate information about changes in our environment than about static conditions. We are well-equipped with sensory receptors sensitive to flickering lights, for example, or to the tiny fluctuations of temperature provoked by a slight breeze; we are less well-equipped to receive information about a steady temperature, say, or a constant pressure on the skin.
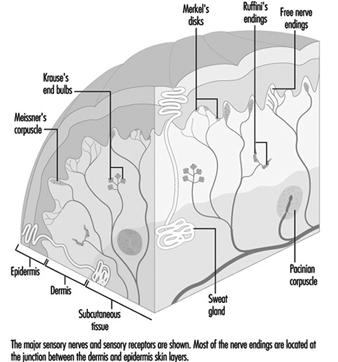
Traditionally the skin senses are divided into two categories: cutaneous and deep. While deep sensitivity relies on receptors located in muscle, tendons, joints, and the periosteum (membrane surrounding the bones), cutaneous sensitivity, with which we are concerned here, deals with information received by receptors in the skin: specifically, the various classes of cutaneous receptors that are located in or near the junction of the dermis and the epidermis.
All sensory nerves linking cutaneous receptors to the central nervous system have roughly the same structure. The cell’s large body resides in a cluster of other nerve cell bodies, called a ganglion, located near the spinal cord and connected to it by a narrow branch of the cell’s trunk, called its axon. Most nerve cells, or neurons, that originate at the spinal cord send axons to bones, muscle, joints, or, in the case of cutaneous sensitivity, to the skin. Just like an insulated wire, each axon is covered along its course and at its endings with protective layers of cells known as Schwann cells. These Schwann cells produce a substance known as myelin, which coats the axon like a sheath. At intervals along the way are tiny breaks in the myelin, known as nodes of Ranvier. Finally, at the end of the axon are found the components that specialize in receiving and retransmitting information about the external environment: the sensory receptors (Mountcastle 1974).
The different classes of cutaneous receptors, like all sensory receptors, are defined in two ways: by their anatomical structures, and by the type of electrical signals they send along their nerve fibres. Distinctly structured receptors are usually named after their discoverers. The relatively few classes of sensory receptors found in the skin can be divided into three main categories: mechanoreceptors, thermal receptors, and nociceptors.
All of these receptors can convey information about a particular stimulus only after they have first encoded it in a type of electrochemical neural language. These neural codes use varying frequencies and patterns of nerve impulses that scientists have only just begun to decipher. Indeed, an important branch of neurophysiological research is devoted entirely to the study of sensory receptors and the ways in which they translate energy states in the environment into neural codes. Once the codes are generated, they are conveyed centrally along afferent fibres, the nerve cells that serve receptors by conveying the signals to the central nervous system.
The messages produced by receptors can be subdivided on the basis of the response given to a continuous, unvarying stimulation: slowly adapting receptors send electrochemical impulses to the central nervous system for the duration of a constant stimulus, whereas rapidly adapting receptors gradually reduce their discharges in the presence of a steady stimulus until they reach a low baseline level or cease entirely, thereupon ceasing to inform the central nervous system about the continuing presence of the stimulus.
The distinctly different sensations of pain, warmth, cold, pressure, and vibration are thus produced by activity in distinct classes of sensory receptors and their associated nerve fibres. The terms “flutter” and “vibration,” for example, are used to distinguish two slightly different vibratory sensations encoded by two different classes of vibration-sensitive receptors (Mountcastle et al. 1967). The three important categories of pain sensation known as pricking pain, burning pain, and aching pain have each been associated with a distinct class of nociceptive afferent fibre. This is not to say, however, that a specific sensation necessarily involves only one class of receptor; more than one receptor class may contribute to a given sensation, and, in fact, sensations may differ depending on the relative contribution of different receptor classes (Sinclair 1981).
The preceding summary is based on the specificity hypothesis of cutaneous sensory function, first formulated by a German physician named Von Frey in 1906. Although at least two other theories of equal or perhaps greater popularity have been proposed during the past century, Von Frey’s hypothesis has now been strongly supported by factual evidence.
Receptors that Respond to Constant Skin Pressure
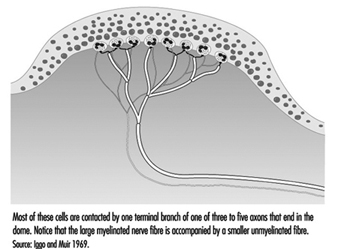
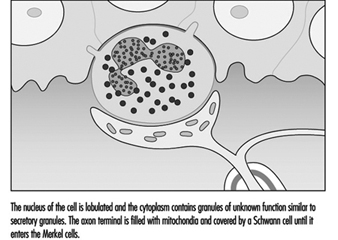
In the hand, relatively large myelinated fibres (5 to 15 mm in diameter) emerge from a subcutaneous nerve network called the subpapillary nerve plexus and end in a spray of nerve terminals at the junction of the dermis and the epidermis (figure 1). In hairy skin, these nerve endings culminate in visible surface structures known as touch domes; in glabrous, or hairless, skin, the nerve endings are found at the base of skin ridges (such as those forming the fingerprints). There, in the touch dome, each nerve fibre tip, or neurite, is enclosed by a specialized epithelial cell known as a Merkel cell (see figures 2 and 3).
Figure 1. A schematic illustration of a cross-section of the skin
Figure 2. The touch dome on each raised region of skin contains 30 to 70 Merkel cells.
Figure 3. At a higher magnification available with the electron microscope, the Merkel cell, a specialized epithelial cell, is seen to be attached to the basement membrane that separates the epidermis from the dermis.
The Merkel cell neurite complex transduces mechanical energy into nerve impulses. While little is known about the cell’s role or about its mechanism of transduction, it has been identified as a slowly adapting receptor. This means that pressure on a touch dome containing Merkel cells causes the receptors to produce nerve impulses for the duration of the stimulus. These impulses rise in frequency in proportion to the intensity of the stimulus, thereby informing the brain of the duration and magnitude of pressure on the skin.
Like the Merkel cell, a second slowly adapting receptor also serves the skin by signalling the magnitude and duration of steady skin pressures. Visible only through a microscope, this receptor, known as the Ruffini receptor, consists of a group of neurites emerging from a myelinated fibre and encapsulated by connective tissue cells. Within the capsule structure are fibres that apparently transmit local skin distortions to the neurites, which in turn produce the messages sent along the neural highway to the central nervous system. Pressure on the skin causes a sustained discharge of nerve impulses; as with the Merkel cell, the frequency of nerve impulses is proportional to the intensity of the stimulus.
Despite their similarities, there is one outstanding difference between Merkel cells and Ruffini receptors. Whereas sensation results when Ruffini receptors are stimulated, stimulation of touch domes housing Merkel cells produces no conscious sensation; the touch dome is thus a mystery receptor, for its actual role in neural function remains unknown. Ruffini receptors, then, are believed to be the only receptors capable of providing the neural signals necessary for the sensory experience of pressure, or constant touch. In addition, it has been shown that the slowly adapting Ruffini receptors account for the ability of humans to rate cutaneous pressure on a scale of intensity.
Receptors that Respond to Vibration and Skin Movement
In contrast with slowly adapting mechanoreceptors, rapidly adapting receptors remain silent during sustained skin indentation. They are, however, well-suited to signal vibration and skin movement. Two general categories are noted: those in hairy skin, which are associated with individual hairs; and those which form corpuscular endings in glabrous, or hairless, skin.
Receptors serving hairs
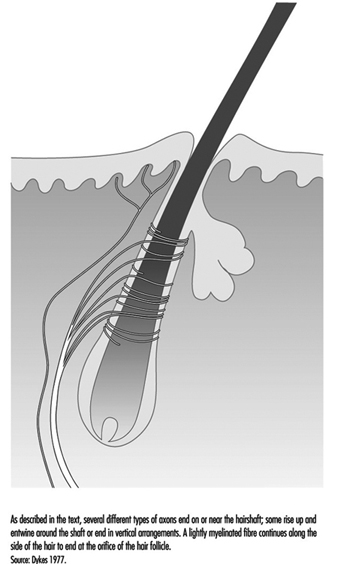
A typical hair is enveloped by a network of nerve terminals branching from five to nine large myelinated axons (figure 4). In primates, these terminals fall into three categories: lanceolate endings, spindle-like terminals, and papillary endings. All three are rapidly adapting, such that a steady deflection of the hair causes nerve impulses only while movement occurs. Thus, these receptors are exquisitely sensitive to moving or vibratory stimuli, but provide little or no information about pressure, or constant touch.
Figure 4. The shafts of hairs are a platform for nerve terminals that detect movements.
Lanceolate endings arise from a heavily myelinated fibre that forms a network around the hair. The terminal neurites lose their usual coverage of Schwann cells and work their way among the cells at the base of the hair.
Spindle-like terminals are formed by axon terminals surrounded by Schwann cells. The terminals ascend to the sloping hair shaft and end in a semicircular cluster just below a sebaceous, or oil-producing, gland. Papillary endings differ from spindle-like terminals because instead of ending on the hair shaft, they terminate as free nerve endings around the orifice of the hair.
There are, presumably, functional differences among the receptor types found on hairs. This can be inferred in part from structural differences in the way the nerves end on the hair shaft and in part from differences in the diameter of axons, as axons of different diameters connect to different central relay regions. Still, the functions of receptors in hairy skin remains an area for study.
Receptors in glabrous skin
The correlation of a receptor’s anatomical structure with the neural signals it generates is most pronounced in large and easily manipulable receptors with corpuscular, or encapsulated, endings. Particularly well understood are the pacininan and Meissner corpuscles, which, like the nerve endings in hairs discussed above, convey sensations of vibration.
The pacinian corpuscle is large enough to be seen with the naked eye, making it easy to link the receptor with a specific neural response. Located in the dermis, usually around tendons or joints, it is an onion-like structure, measuring 0.5 × 1.0 mm. It is served by one of the body’s largest afferent fibres, having a diameter of 8 to 13 μm and conducting at 50 to 80 metres per second. Its anatomy, well-studied by both light and electron microscopy, is well known.
The principal component of the corpuscle is an outer core formed of cellular material enclosing fluid-filled spaces. The outer core itself is then surrounded by a capsule that is penetrated by a central canal and a capillary network. Passing through the canal is a single myelinated nerve fibre 7 to 11 mm in diameter, which becomes a long, nonmyelinated nerve terminal that probes deep into the centre of the corpuscle. The terminal axon is elliptical, with branch-like processes.
The pacinian corpuscle is a rapidly adapting receptor. When subjected to sustained pressure, it thus produces an impulse only at the beginning and the end of the stimulus. It responds to high-frequency vibrations (80 to 400 Hz) and is most sensitive to vibrations around 250 Hz. Often, these receptors respond to vibrations transmitted along bones and tendons, and because of their extreme sensitivity, they may be activated by as little as a puff of air on the hand (Martin 1985).
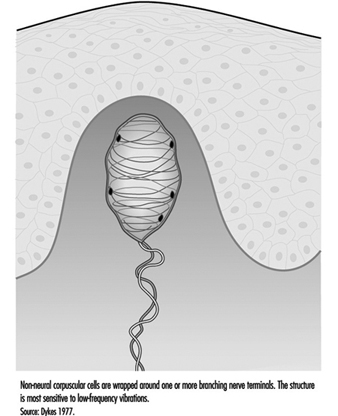
In addition to the pacinian corpuscle, there is another rapidly adapting receptor in glabrous skin. Most researchers believe it to be the Meissner corpuscle, located in the dermal papillae of the skin. Responsive to low-frequency vibrations of 2 to 40 Hz, this receptor consists of the terminal branches of a medium-sized myelinated nerve fibre enveloped in one or several layers of what appear to be modified Schwann cells, called laminar cells. The receptor’s neurites and laminar cells may connect to a basal cell in the epidermis (figure 5).
Figure 5. The Meissner corpuscle is a loosely encapsulated sensory receptor in the dermal papillae of glabrous skin.
If the Meissner corpuscle is selectively inactivated by the injection of a local anaesthetic through the skin, the sense of flutter or low-frequency vibration is lost. This suggests that it functionally complements the high frequency capacity of the pacinian corpuscles. Together, these two receptors provide neural signals sufficient to account for human sensibility to a full range of vibrations (Mountcastle et al. 1967).
Cutaneous Receptors Associated with Free Nerve Endings
Many still unidentifiable myelinated and unmyelinated fibres are found in the dermis. A large number are only passing through, on their way to skin, muscles, or periosteum, while others (both myelinated and unmyelinated) appear to end in the dermis. With a few exceptions, such as the pacinian corpuscle, most fibres in the dermis appear to end in poorly defined ways or simply as free nerve endings.
While more anatomical study is needed to differentiate these ill-defined endings, physiological research has clearly shown that these fibres encode a variety of environmental events. For example, free nerve endings found at the junction between the dermis and epidermis are responsible for encoding the environmental stimuli that will be interpreted as cold, warmth, heat, pain, itch, and tickle. It is not yet known which of these different classes of small fibres convey particular sensations.
The apparent anatomical similarity of these free nerve endings is probably due to the limitations of our investigative techniques, since structural differences among free nerve endings are slowly coming to light. For example, in glabrous skin, two different terminal modes of free nerve endings have been distinguished: a thick, short pattern and a long, thin one. Studies of human hairy skin have demonstrated histochemically recognizable nerve endings that terminate at the dermal-epidermal junction: the penicillate and papillary endings. The former arise from unmyelinated fibres and form a network of endings; in contrast, the latter arise from myelinated fibres and end around the hair orifices, as mentioned earlier. Presumably, these structural disparities correspond to functional differences.
Although it is not yet possible to assign specific functions to individual structural entities, it is clear from physiological experiments that there exist functionally different categories of free nerve endings. One small myelinated fibre has been found to respond to cold in humans. Another unmyelinated fibre serving free nerve endings responds to warmth. How one class of free nerve endings can respond selectively to a drop in temperature, while an increase of skin temperature can provoke another class to signal warmth is unknown. Studies show that activation of one small fibre with a free ending may be responsible for itching or tickling sensations, while there are believed to be two classes of small fibres specifically sensitive to noxious mechanical and noxious chemical or thermal stimuli, providing the neural basis for pricking and burning pain (Keele 1964).
The definitive correlation between anatomy and physiological response awaits the development of more advanced techniques. This is one of the major stumbling blocks in the management of disorders such as causalgia, paraesthesia, and hyperpathia, which continue to present a dilemma to the physician.
Peripheral Nerve Injury
Neural function can be divided into two categories: sensory and motor. Peripheral nerve injury, usually resulting from the crushing or severing of a nerve, can impair either function or both, depending on the types of fibres in the damaged nerve. Certain aspects of motor loss tend to be misinterpreted or overlooked, as these signals do not go to muscles but rather affect autonomic vascular control, temperature regulation, the nature and thickness of the epidermis, and the condition of cutaneous mechano-receptors. The loss of motor innervation will not be discussed here, nor will the loss of innervation affecting senses other than those responsible for cutaneous sensation.
The loss of sensory innervation to the skin creates a vulnerability to further injury, as it leaves an anaesthetic surface that is incapable of signalling potentially harmful stimuli. Once injured, anaesthetized skin surfaces are slow to heal, perhaps in part on account of the lack of autonomic innervation that normally regulates such key factors as temperature regulation and cellular nutrition.
Over a period of several weeks, denervated cutaneous sensory receptors begin to atrophy, a process which is easy to observe in large encapsulated receptors such as pacinian and Meissner corpuscles. If regeneration of the axons can occur, recovery of function may follow, but the quality of the recovered function will depend upon the nature of the original injury and upon the duration of denervation (McKinnon and Dellon 1988).
Recovery following a nerve crush is more rapid, much more complete and more functional than is recovery after a nerve is severed. Two factors explain the favourable prognosis for a nerve crush. First, more axons may again achieve contact with the skin than after a transection; second, the connections are guided back to their original site by Schwann cells and linings known as basement membranes, both of which remain intact in a crushed nerve, whereas after a nerve transection the nerves often travel to incorrect regions of the skin surface by following the wrong Schwann cell paths. The latter situation results in distorted spatial information being sent to the somatosensory cortex of the brain. In both cases, however, regenerating axons appear capable of finding their way back to the same class of sensory receptors that they previously served.
The reinnervation of a cutaneous receptor is a gradual process. As the growing axon reaches the skin surface, receptive fields are smaller than normal, while the threshold is higher. These receptive points expand with time and gradually coalesce into larger fields. Sensitivity to mechanical stimuli becomes greater and often approaches the sensitivity of normal sensory receptors of that class. Studies using the stimuli of constant touch, moving touch, and vibration have shown that the sensory modalities attributed to different types of receptors return to anaesthetic areas at different rates.
Viewed under a microscope, denervated glabrous skin is seen to be thinner than normal, having flattened epidermal ridges and fewer layers of cells. This confirms that nerves have a trophic, or nutritional, influence on skin. Soon after innervation returns, the dermal ridges become better developed, the epidermis becomes thicker, and axons can be found penetrating the basement membrane. As the axon comes back to the Meissner corpuscle, the corpuscle begins to increase in size, and the previously flattened, atrophic structure returns to its original form. If the denervation has been of long duration, a new corpuscle may form adjacent to the original atrophic skeleton, which remains denervated (Dellon 1981).
As can be seen, an understanding of the consequences of peripheral nerve injury requires knowledge of normal function as well as the degrees of functional recovery. While this information is available for certain nerve cells, others require further investigation, leaving a number of murky areas in our grasp of the role of cutaneous nerves in health and disease.