The Chemistry and Physics of Fire
Fire is a manifestation of uncontrolled combustion. It involves combustible materials which are found around us in the buildings in which we live, work and play, as well as a wide range of gases, liquids and solids which are encountered in industry and commerce. They are commonly carbon-based, and may be referred to collectively as fuels in the context of this discussion. Despite the wide variety of these fuels in both their chemical and physical states, in fire they share features that are common to them all. Differences are encountered in the ease with which fire can be initiated (ignition), the rate with which fire can develop (flame spread), and the power that can be generated (rate of heat release), but as our understanding of the science of fire improves, we become better able to quantify and predict fire behaviour and apply our knowledge to fire safety in general. The purpose of this section is to review some of the underlying principles and provide guidance to an understanding of fire processes.
Basic Concepts
Combustible materials are all around us. Given the appropriate circumstances, they can be made to burn by subjecting them to an ignition source which is capable of initiating a self-sustaining reaction. In this process, the “fuel” reacts with oxygen from the air to release energy (heat), while being converted to products of combustion, some of which may be harmful. The mechanisms of ignition and burning need to be clearly understood.
Most everyday fires involve solid materials (e.g., wood, wood products and synthetic polymers), although gaseous and liquid fuels are not uncommon. A brief review of the combustion of gases and liquids is desirable before some of the basic concepts are discussed.
Diffusion and premixed flames
A flammable gas (e.g., propane, C3H8) can be burned in two ways: a stream or jet of gas from a pipe (cf. the simple Bunsen burner with the air inlet closed) can be ignited and will burn as a diffusion flame in which burning occurs in those regions where gaseous fuel and air mix by diffusive processes. Such a flame has a characteristic yellow luminosity, indicating the presence of minute soot particles formed as a result of incomplete combustion. Some of these will burn in the flame, but others will emerge from the flame tip to form smoke.
If the gas and air are intimately mixed before ignition, then premixed combustion will occur, provided that the gas/air mixture lies within a range of concentrations bounded by the lower and upper flammability limits (see table 1). Outside these limits, the mixture is non-flammable. (Note that a premixed flame is stabilized at the mouth of a Bunsen burner when the air inlet is open.) If a mixture is flammable, then it can be ignited by a small ignition source, such as an electrical spark. The stoichiometric mixture is the most readily ignited, in which the amount of oxygen present is in the correct proportion to burn all the fuel to carbon dioxide and water (see accompanying equation, below, in which nitrogen can be seen to be present in the same proportion as in air but does not take part in the reaction). Propane (C3H8) is the combustible material in this reaction:
C3H8 + 5O2 + 18.8N2 = 3CO2 + 4H2O + 18.8N2
An electrical discharge as small as 0.3 mJ is sufficient to ignite a stoichiometric propane/air mixture in the reaction illustrated. This represents a barely perceptible static spark, as experienced by someone who has walked across a synthetic carpet and touched a grounded object. Even smaller amounts of energy are required for certain reactive gases such as hydrogen, ethylene and ethyne. In pure oxygen (as in the reaction above, but with no nitrogen present as a diluent), even lower energies are sufficient.
Table 1. Lower and upper flammability limits in air
|
Lower flammability |
Upper flammability |
|
|
Carbon monoxide |
12.5 |
74 |
|
Methane |
5.0 |
15 |
|
Propane |
2.1 |
9.5 |
|
n-Hexane |
1.2 |
7.4 |
|
n-Decane |
0.75 |
5.6 |
|
Methanol |
6.7 |
36 |
|
Ethanol |
3.3 |
19 |
|
Acetone |
2.6 |
13 |
|
Benzene |
1.3 |
7.9 |
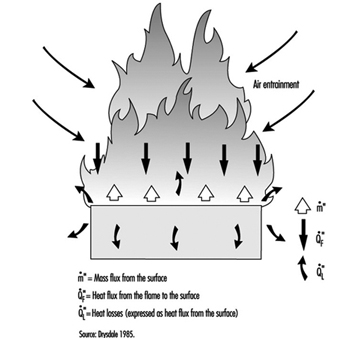
The diffusion flame associated with a flow of gaseous fuel exemplifies the mode of burning that is observed when a liquid or solid fuel is undergoing flaming combustion. However, in this case, the flame is fed by fuel vapours generated at the surface of the condensed phase. The rate of supply of these vapours is coupled to their rate of burning in the diffusion flame. Energy is transferred from the flame to the surface, thus providing the energy necessary to produce the vapours. This is a simple evaporative process for liquid fuels, but for solids, enough energy must be provided to cause chemical decomposition of the fuel, breaking large polymeric molecules into smaller fragments which can vaporize and escape from the surface. This thermal feedback is essential to maintain the flow of vapours, and hence support the diffusion flame (figure 1). Flames can be extinguished by interfering with this process in a number of ways (see below).
Figure 1. Schematic representation of a burning surface showing the heat and mass transfer processes.
Heat transfer
An understanding of heat (or energy) transfer is the key to an understanding of fire behaviour and fire processes. The subject deserves careful study. There are many excellent texts to which one may turn (Welty, Wilson and Wicks 1976; DiNenno 1988), but for the present purposes it is necessary only to draw attention to the three mechanisms: conduction, convection and radiation. The basic equations for steady-state heat transfer () are:
Conduction: ![]()
Convection: ![]()
Radiation: ![]()
Conduction is relevant to heat transfer through solids; (k is a material property known as thermal conductivity (kW/mK ) and l is the distance (m) over which the temperature falls from T1 to T2 (in degrees Kelvin). Convection in this context refers to the transfer of heat from a fluid (in this case, air, flames or fire products) to a surface (solid or liquid); h is the convective heat transfer coefficient kW/m2K) and depends on the configuration of the surface and nature of the flow of fluid past that surface. Radiation is similar to visible light (but with a longer wavelength) and requires no intervening medium (it can traverse a vacuum); e is the emissivity (efficiency by which a surface can radiate), s is the Stefan-Boltzman constant (). Thermal radiation travels at the speed of light (3 x 108 m/s) and an intervening solid object will cast a shadow.
Rate of burning and rate of heat release
Heat transfer from flames to the surface of condensed fuels (liquids and solids) involves a mixture of convection and radiation, although the latter dominates when the effective diameter of the fire exceeds 1 m. The rate of burning (, (g/s)) can be expressed by the formula:
![]()
![]() is the heat flux from the flame to the surface (kW/m2);
is the heat flux from the flame to the surface (kW/m2); ![]() is the heat loss from the surface (e.g., by radiation, and by conduction through the solid) expressed as a flux (kW/m2); Afuel is the surface area of the fuel (m2); and Lv is the heat of gasification (equivalent to the latent heat of evaporation for a liquid) (kJ/g). If a fire develops in a confined space, the hot smoky gases rising from the fire (driven by buoyancy) are deflected beneath the ceiling, heating the upper surfaces. The resulting smoke layer and the hot surfaces radiate down to the lower part of the enclosure, in particular to the fuel surface, thus increasing the rate of burning:
is the heat loss from the surface (e.g., by radiation, and by conduction through the solid) expressed as a flux (kW/m2); Afuel is the surface area of the fuel (m2); and Lv is the heat of gasification (equivalent to the latent heat of evaporation for a liquid) (kJ/g). If a fire develops in a confined space, the hot smoky gases rising from the fire (driven by buoyancy) are deflected beneath the ceiling, heating the upper surfaces. The resulting smoke layer and the hot surfaces radiate down to the lower part of the enclosure, in particular to the fuel surface, thus increasing the rate of burning:
![]()
where ![]() is the extra heat supplied by radiation from the upper part of the enclosure (kW/m2). This additional feedback leads to greatly enhanced rates of burning and to the phenomenon of flashover in enclosed spaces where there is an adequate supply of air and sufficient fuel to sustain the fire (Drysdale 1985).
is the extra heat supplied by radiation from the upper part of the enclosure (kW/m2). This additional feedback leads to greatly enhanced rates of burning and to the phenomenon of flashover in enclosed spaces where there is an adequate supply of air and sufficient fuel to sustain the fire (Drysdale 1985).
The rate of burning is moderated by the magnitude of the value of Lv, the heat of gasification. This tends to be low for liquids and relatively high for solids. Consequently, solids tend to burn much more slowly than liquids.
It has been argued that the most important single parameter which determines the fire behaviour of a material (or assembly of materials) is the rate of heat release (RHR) which is coupled to the rate of burning through the equation:
![]()
whereis the effective heat of combustion of the fuel (kJ/g). New techniques are now available for measuring the RHR at different heat fluxes (e.g., the Cone Calorimeter), and it is now possible to measure the RHR of large items, such as upholstered furniture and wall linings in large-scale calorimeters which use oxygen consumption measurements to determine the rate of heat release (Babrauskas and Grayson 1992).
It should be noted that as a fire grows in size, not only does the rate of heat release increase, but the rate of production of “fire products” also increases. These contain toxic and noxious species as well as particulate smoke, the yields of which will increase when a fire developing in a building enclosure becomes underventilated.
Ignition
Ignition of a liquid or solid involves raising the surface temperature until vapours are being evolved at a rate sufficient to support a flame after the vapours have been ignited. Liquid fuels can be classified according to their flashpoints, the lowest temperature at which there is a flammable vapour/air mixture at the surface (i.e., the vapour pressure corresponds to the lower flammability limit). These can be measured using a standard apparatus, and typical examples are given in table 2. A slightly higher temperature is required to produce a sufficient flow of vapours to support a diffusion flame. This is known as the firepoint. For combustible solids, the same concepts are valid, but higher temperatures are required as chemical decomposition is involved. The firepoint is typically in excess of 300 °C, depending on the fuel. In general, flame-retarded materials have significantly higher firepoints (see Table 2).
Table 2. Flashpoints and firepoints of liquid and solid fuels
|
Closed cup flashpoint1 (°C) |
Firepoint2 (°C) |
|
|
Gasoline (100 Octane) (l) |
–38 |
– |
|
n-Decane (l) |
46 |
61.5 |
|
n-Dodecane (l) |
74 |
103 |
|
Polymethylmethacrylate (s) |
– |
310 |
|
FR polymethylmethacrylate (s) |
– |
377 |
|
Polypropylene (s) |
– |
330 |
|
FR polypropylene (s) |
– |
397 |
|
Polystyrene (s) |
– |
367 |
|
FR polystyrene (s) |
– |
445 |
l = liquid; s = solid.
1 By Pensky-Martens closed cup apparatus.
2 Liquids: by Cleveland open cup apparatus. Solids: Drysdale and Thomson (1994).
(Note that the results for the flame-retarded species refer to a heat flux of 37 kW/m2).
Ease of ignition of a solid material is therefore dependent on the ease with which its surface temperature can be raised to the firepoint, e.g., by exposure to radiant heat or to a flow of hot gases. This is less dependent on the chemistry of the decomposition process than on the thickness and physical properties of the solid, namely, its thermal conductivity (k), density (r) and heat capacity (c). Thin solids, such as wood shavings (and all thin sections), can be ignited very easily because they have a low thermal mass, that is, relatively little heat is required to raise the temperature to the firepoint. However, when heat is transferred to the surface of a thick solid, some will be conducted from the surface into the body of the solid, thus moderating the temperature rise of the surface. It can be shown theoretically that the rate of rise of the surface temperature is determined by the thermal inertia of the material, that is, the product krc. This is borne out in practice, since thick materials with a high thermal inertia (e.g., oak, solid polyurethane) will take a long time to ignite under a given heat flux, whereas under identical conditions thick materials with a low thermal inertia (e.g., fibre insulating board, polyurethane foam) will ignite quickly (Drysdale 1985).
Ignition sources
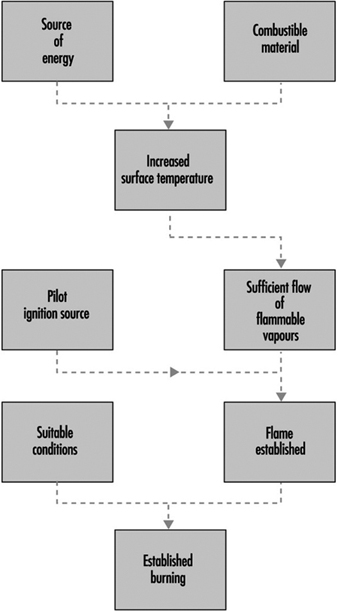
Ignition is illustrated schematically in figure 2 (piloted ignition). For successful ignition, an ignition source must be capable not only of raising the surface temperature to the firepoint, or above, but it must also cause the vapours to ignite. An impinging flame will act in both capacities, but an imposed radiative flux from a remote source may lead to the evolution of vapours at a temperature above the firepoint, without the vapours igniting. However, if the evolved vapours are hot enough (which requires the surface temperature to be much higher than the firepoint), they may ignite spontaneously as they mix with air. This process is known as spontaneous ignition.
Figure 2. The scenario for piloted ignition.
A large number of ignition sources can be identified, but they have one thing in common, which is that they are the result of some form of carelessness or inaction. A typical list would include naked flames, “smokers’ materials”, frictional heating, electrical devices (heaters, irons, cookers, etc.) and so on. An excellent survey may be found in Cote (1991). Some of these are summarized in table 3.
Table 3. Ignition sources
|
|
Examples
|
|
Electrically powered equipment |
Electric heaters, hair dryers, electric blankets, etc. |
|
Open flame source |
Match, cigarette lighter, blow torch, etc. |
|
Gas-fuelled equipment |
Gas fire, space heater, cooker, etc. |
|
Other fuelled equipment |
Wood stove, etc. |
|
Lighted tobacco product |
Cigar, pipe, etc. |
|
Hot object |
Hot pipes, mechanical sparks, etc. |
|
Exposure to heating |
Adjacent fire, etc. |
|
Spontaneous heating |
Linseed oil-soaked rags, coal piles, etc. |
|
Chemical reaction |
Rare-e.g., potassium permanganate with glycerol |
It should be noted that smouldering cigarettes cannot initiate flaming combustion directly (even in common gaseous fuels), but can cause smouldering in materials which have the propensity to undergo this type of combustion. This is observed only with materials which char on heating. Smouldering involves the surface oxidation of the char, which generates enough heat locally to produce fresh char from adjacent unburnt fuel. It is a very slow process, but may eventually undergo a transition to flaming. Thereafter, the fire will develop very rapidly.
Materials which have the propensity to smoulder can also exhibit the phenomenon of self-heating (Bowes 1984). This arises when such a material is stored in large quantities and in such a way that heat generated by slow surface oxidation cannot escape, leading to a rise in temperature within the mass. If the conditions are right, this can lead to a runaway process ultimately developing into a smouldering reaction at depth within the material.
Flame spread
A major component in the growth of any fire is the rate at which flame will spread over adjacent combustible surfaces. Flame spread can be modelled as an advancing ignition front in which the leading edge of the flame acts as an ignition source for the fuel that is not yet burning. The rate of spread is determined partly by the same material properties that control the ease of ignition and partly by the interaction between the existing flame and the surface ahead of the front. Upward, vertical spread is the most rapid as buoyancy ensures that the flames flow upwards, exposing the surface above the burning area to direct heat transfer from the flames. This should be contrasted with spread over a horizontal surface when the flames from the burning area rise vertically, away from the surface. Indeed, it is common experience that vertical spread is the most hazardous (e.g., flame spread on curtains and drapes and on loose clothing such as dresses and nightgowns).
The rate of spread is also affected by an imposed radiant heat flux. In the development of a fire in a room, the area of the fire will grow more rapidly under the increasing level of radiation that builds up as the fire progresses. This will contribute to the acceleration of fire growth that is characteristic of flashover.
Theory of Fire Extinguishment
Fire extinction and suppression can be examined in terms of the above outline of the theory of fire. The gas phase combustion processes (i.e., the flame reactions) are very sensitive to chemical inhibitors. Some of the flame retardants used to improve the “fire properties” of materials rely on the fact that small amounts of inhibitor released with the fuel vapours will suppress the establishment of flame. The presence of a flame retardant cannot render a combustible material non-combustible, but it can make ignition more difficult—perhaps preventing ignition altogether provided that the ignition source is small. However, if a flame-retarded material becomes involved in an existing fire, it will burn as the high heat fluxes overwhelm the effect of the retardant.
Extinction of a fire may be achieved in a number of ways:
1. stopping the supply of fuel vapours
2. quenching the flame by chemical extinguishers (inhibiting)
3. removing the supply of air (oxygen) to the fire (smothering)
4. “blow-out”.
Controlling the flow of fuel vapours
The first method, stopping the supply of fuel vapours, is clearly applicable to a gas-jet fire in which the supply of the fuel can simply be turned off. However, it is also the most common and safest method of extinguishing a fire involving condensed fuels. In the case of a fire involving a solid, this requires the fuel surface to be cooled below the firepoint, when the flow of vapours becomes too small to support a flame. This is achieved most effectively by the application of water, either manually or by means of an automatic system (sprinklers, water spray, etc.). In general, liquid fires cannot be dealt with in this manner: liquid fuels with low firepoints simply cannot be cooled sufficiently, while in the case of a high-firepoint fuel, vigorous vaporization of water when it comes into contact with the hot liquid at the surface can lead to burning fuel being ejected from the container. This can have very serious consequences for those fighting the fire. (There are some special cases in which an automatic high-pressure water-spray system may be designed to deal with the latter type of fire, but this is not common.)
Liquid fires are commonly extinguished by the use of fire-fighting foams (Cote 1991). This is produced by aspirating a foam concentrate into a stream of water which is then directed at the fire through a special nozzle which permits air to be entrained into the flow. This produces a foam which floats on top of the liquid, reducing the rate of supply of fuel vapours by a blockage effect and by shielding the surface from heat transfer from the flames. The foam has to be applied carefully to form a “raft” which gradually increases in size to cover the liquid surface. The flames will decrease in size as the raft grows, and at the same time the foam will gradually break down, releasing water which will aid the cooling of the surface. The mechanism is in fact complex, although the net result is to control the flow of vapours.
There are a number of foam concentrates available, and it is important to choose one that is compatible with the liquids that are to be protected. The original “protein foams” were developed for hydrocarbon liquid fires, but break down rapidly if brought into contact with liquid fuels that are water soluble. A range of “synthetic foams” have been developed to tackle the entire range of liquid fires that may be encountered. One of these, aqueous film-forming foam (AFFF), is an all-purpose foam which also produces a film of water on the surface of the liquid fuel, thus increasing its effectiveness.
Quenching the flame
This method makes use of chemical suppressants to extinguish the flame. The reactions which occur in the flame involve free radicals, a highly reactive species which have only a fleeting existence but are continuously regenerated by a branched chain process that maintains high enough concentrations to allow the overall reaction (e.g., an R1 type reaction) to proceed at a fast rate. Chemical suppressants applied in sufficient quantity will cause a dramatic fall in the concentration of these radicals, effectively quenching the flame. The most common agents that operate in this way are the halons and dry powders.
Halons react in the flame to generate other intermediate species with which the flame radicals react preferentially. Relatively small amounts of the halons are required to extinguish a fire, and for this reason they were traditionally considered highly desirable; extinguishing concentrations are “breathable” (although the products generated while passing through the flame are noxious). Dry powders act in a similar fashion, but under certain circumstances are much more effective. Fine particles are dispersed into the flame and cause termination of the radical chains. It is important that the particles are small and numerous. This is achieved by the manufacturers of many proprietary brands of dry powders by selecting a powder that “decrepitates”, that is, the particles fragment into smaller particles when they are exposed to the high temperatures of the flame.
For a person whose clothing has caught fire, a dry powder extinguisher is recognized as the best method to control flames and to protect that individual. Rapid intervention gives rapid “knockdown”, thus minimizing injury. However, the flame must be completely extinguished because the particles quickly fall to the ground and any residual flaming will quickly regain hold. Similarly, halons will only remain effective if the local concentrations are maintained. If it is applied out of doors, the halon vapour rapidly disperses, and once again the fire will rapidly re-establish itself if there is any residual flame. More significantly, the loss of the suppressant will be followed by re-ignition of the fuel if the surface temperatures are high enough. Neither halons nor dry powders have any significant cooling effect on the fuel surface.
Removing the supply of air
The following description is an oversimplification of the process. While “removing the supply of air” will certainly cause the fire to extinguish, to do this it is only necessary to reduce the oxygen concentration below a critical level. The well-known “oxygen index test” classifies combustible materials according to the minimum oxygen concentration in an oxygen/nitrogen mixture that will just support flaming. Many common materials will burn at oxygen concentrations down to approximately 14% at ambient temperatures (ca. 20°C) and in the absence of any imposed heat transfer. The critical concentration is temperature dependent, decreasing as the temperature is increased. Thus, a fire that has been burning for some time will be capable of supporting flames at concentrations perhaps as low as 7%. A fire in a room may be held in check and may even self-extinguish if the supply of oxygen is limited by keeping doors and windows closed. Flaming may cease, but smouldering will continue at very much lower oxygen concentrations. Admission of air by opening a door or breaking a window before the room has cooled sufficiently can lead to a vigorous eruption of the fire, known as backdraught, or backdraft.
“Removal of air” is difficult to achieve. However, an atmosphere may be rendered “inert” by total flooding by means of a gas which will not support combustion, such as nitrogen, carbon dioxide or gases from a combustion process (e.g., a ship’s engines) which are low in oxygen and high in carbon dioxide. This technique can only be used in enclosed spaces as it is necessary to maintain the required concentration of the “inert gas” until either the fire has extinguished completely or fire-fighting operations can begin. Total flooding has special applications, such as for ships’ holds and rare book collections in libraries. The required minimum concentrations of the inert gases are shown in Table 4. These are based on the assumption that the fire is detected at an early stage and that the flooding is carried out before too much heat has accumulated in the space.
Table 4: Comparison of concentrations of different gases required for inerting
|
Agent |
Minimum concentration (% volume) |
|
Halon 1301 |
8.0 |
|
Halon 1211 |
8.1 |
|
Nitrogen |
|
|
Carbon dioxide |
|
“Removal of air” can be effected in the immediate vicinity of a small fire by local application of a suppressant from an extinguisher. Carbon dioxide is the only gas that is used in this way. However, as this gas quickly disperses, it is essential to extinguish all flaming during the attack on the fire; otherwise, flaming will re-establish itself. Re-ignition is also possible because carbon dioxide has little if any cooling effect. It is worth noting that a fine water spray entrained into a flame can cause extinction as the combined result of evaporation of the droplets (which cools the burning zone) and reduction of the oxygen concentration by dilution by water vapour (which acts in the same way as carbon dioxide). Fine water sprays and mists are being considered as possible replacements for halons.
It is appropriate to mention here that it is inadvisable to extinguish a gas flame unless the gas flow can be stopped immediately thereafter. Otherwise, a substantial volume of flammable gas may build up and subsequently ignite, with potentially serious consequences.
Blow-out
This method is included here for completeness. A match flame can easily be blown out by increasing the air velocity above a critical value in the vicinity of the flame. The mechanism operates by destabilizing the flame in the vicinity of the fuel. In principle, larger fires can be controlled in the same way, but explosive charges are normally required to generate sufficient velocities. Oil well fires can be extinguished in this manner.
Finally, a common feature that needs to be emphasized is that the ease with which a fire can be extinguished decreases rapidly as the fire increases in size. Early detection permits extinction with minimal quantities of suppressant, with reduced losses. In choosing a suppressant system, one should take into account the potential rate of fire development and what type of detection system is available.
Explosions
An explosion is characterized by the sudden release of energy, producing a shock wave, or blast wave, that may be capable of causing remote damage. There are two distinct types of sources, namely, the high explosive and the pressure burst. The high explosive is typified by compounds such as trinitrotoluene (TNT) and cyclotrimethylenetrinitramine (RDX). These compounds are highly exothermic species, decomposing to release substantial quantities of energy. Although thermally stable (although some are less so and require desensitization to make them safe to handle), they can be induced to detonate, with decomposition, propagating at the velocity of sound through the solid. If the amount of energy released is high enough, a blast wave will propagate from the source with the potential to do significant damage at a distance.
By assessing remote damage, one can estimate the size of the explosion in terms of “TNT equivalent” (normally in metric tons). This technique relies on the large amount of data that has been gathered on the damage potential of TNT (much of it during wartime), and uses empirical scaling laws which have been developed from studies of the damage caused by known quantities of TNT.
In peacetime, high explosives are used in a variety of activities, including mining, quarrying and major civil engineering works. Their presence on a site represents a particular hazard that requires specific management. However, the other source of “explosions” can be equally devastating, particularly if the hazard has not been recognized. Overpressures leading to pressure bursts can be the result of chemical processes within plants or from purely physical effects, as will occur if a vessel is heated externally, leading to overpressurization. The term BLEVE (boiling liquid expanding vapour explosion) has its origins here, referring originally to the failure of steam boilers. It is now also commonly used to describe the event in which a pressure vessel containing a liquefied gas such as LPG (liquefied petroleum gas) fails in a fire, releasing the flammable contents, which then ignite to produce a “fireball”.
On the other hand, the overpressure may be caused internally by a chemical process. In the process industries, self-heating can lead to a runaway reaction, generating high temperatures and pressures capable of causing a pressure burst. However, the most common type of explosion is caused by the ignition of a flammable gas/air mixture which is confined within an item of a plant or indeed within any confining structure or enclosure. The prerequisite is the formation of a flammable mixture, an occurrence which should be avoided by good design and management. In the event of an accidental release, a flammable atmosphere will exist wherever the concentration of the gas (or vapour) lies between the lower and upper flammability limits (Table 1). If an ignition source is introduced to one of these regions, a premixed flame will propagate rapidly from the source, converting the fuel/air mixture into combustion products at an elevated temperature. This can be as high as 2,100 K, indicating that in a completely closed system initially at 300 K, an overpressure as high as 7 bars is possible. Only specially designed pressure vessels are capable of containing such overpressures. Ordinary buildings will fall unless protected by pressure relief panels or bursting discs or by an explosion suppression system. Should a flammable mixture form within a building, the subsequent explosion can cause significant structural damage—perhaps total destruction—unless the explosion can vent to the outside through openings (e.g., the failure of windows) created during the early stages of the explosion.
Explosions of this type are also associated with the ignition of dust suspensions in air (Palmer 1973). These are encountered when there is a substantial accumulation of “explosible” dust which is dislodged from shelves, rafters and ledges within a building to form a cloud, which is then exposed to an ignition source (e.g., in flour mills, grain elevators, etc.). The dust must (obviously) be combustible, but not all combustible dusts are explosible at ambient temperatures. Standard tests have been designed to determine whether a dust is explosible. These can also be used to illustrate that explosible dusts exhibit “explosibility limits”, similar in concept to the “flammability limits” of gases and vapours. In general, a dust explosion has the potential to do a great deal of damage because the initial event may cause more dust to be dislodged, forming an even greater dust cloud which will inevitably ignite, to produce an even greater explosion.
Explosion venting, or explosion relief, will only operate successfully if the rate of development of the explosion is relatively slow, such as associated with the propagation of a premixed flame through a stationary flammable mixture or an explosible dust cloud. Explosion venting is of no use if detonation is involved. The reason for this is that the pressure relief openings have to be created at an early stage of the event when the pressure is still relatively low. If a detonation occurs, the pressure rises too rapidly for relief to be effective, and the enclosing vessel or item of a plant experiences very high internal pressures which will lead to massive destruction. Detonation of a flammable gas mixture can occur if the mixture is contained within a long pipe or duct. Under certain conditions, propagation of the premixed flame will push the unburnt gas ahead of the flame front at a rate that will increase turbulence, which in turn will increase the rate of propagation. This provides a feedback loop which will cause the flame to accelerate until a shock wave is formed. This, combined with the combustion process, is a detonation wave which can propagate at velocities well in excess of 1,000 m/s. This may be compared with the fundamental burning velocity of a stoichiometric propane/air mixture of 0.45 m/s. (This is the rate at which a flame will propagate through a quiescent (i.e., non-turbulent) propane/air mixture.)
The importance of turbulence on the development of this type of explosion cannot be underestimated. The successful operation of an explosion protection system relies on early venting or early suppression. If the rate of development of the explosion is too fast, then the protection system will not be effective, and unacceptable overpressures can be produced.
An alternative to explosion relief is explosion suppression. This type of protection requires that the explosion is detected at a very early stage, as close to ignition as possible. The detector is used to initiate the rapid release of a suppressant into the path of the propagating flame, effectively arresting the explosion before the pressure has increased to an extent at which the integrity of the enclosing boundaries is threatened. The halons have been commonly used for this purpose, but as these are being phased out, attention is now being paid to the use of high-pressure water-spray systems. This type of protection is very expensive and has limited application as it can only be used in relatively small volumes within which the suppressant can be distributed quickly and uniformly (e.g., ducts carrying flammable vapour or explosible dusts).
Information Analysis for Fire Protection
In general terms, fire science has only recently been developed to a stage at which it is capable of providing the knowledge base on which rational decisions regarding engineering design, including safety issues, can be based. Traditionally, fire safety has developed on an ad hoc basis, effectively responding to incidents by imposing regulations or other restrictions to ensure that there will be no re-occurrence. Many examples could be quoted. For example, the Great Fire of London in 1666 led in due course to the establishment of the first building regulations (or codes) and the development of fire insurance. More recent incidents, such as the high-rise office block fires in São Paulo, Brazil, in 1972 and 1974, initiated changes to the building codes, framed in such a way as to prevent similar multiple-fatality fires in the future. Other problems have been addressed in a similar fashion. In California in the United States, the hazard associated with certain types of modern upholstered furniture (particularly those containing standard polyurethane foam) was recognized, and eventually strict regulations were introduced to control its availability.
These are simple cases in which observations of the consequences of fire have led to the imposition of a set of rules intended to improve the safety of the individual and the community in the event of fire. The decision for action on any issue has to be justified on the basis of an analysis of our knowledge of fire incidents. It is necessary to show that the problem is real. In some cases—such as the São Paulo fires—this exercise is academic, but in others, such as “proving” that modern furnishings are a problem, it is necessary to ensure that the associated costs are wisely spent. This requires a reliable database on fire incidents which over a number of years is capable of showing trends in the number of fires, the number of fatalities, the incidence of a particular type of ignition, etc. Statistical techniques can then be used to examine whether a trend, or a change, is significant, and appropriate measures taken.
In a number of countries, the fire brigade is required to submit a report on each fire attended. In the United Kingdom and the United States, the officer in charge completes a report form which is then submitted to a central organization (the Home Office in the United Kingdom, the National Fire Protection Association, NFPA, in the United States) which then codes and processes the data in a prescribed fashion. The data are then available for inspection by government bodies and other interested parties. These databases are invaluable in highlighting (for example) the principal sources of ignition and the items first ignited. An examination of the incidence of fatalities and their relationship to sources of ignition, etc. has shown that the number of people who die in fires started by smokers’ materials is significantly out of proportion with the number of fires which originate in this way.
The reliability of these databases depends on the skill with which the fire officers carry out the fire investigation. Fire investigation is not an easy task, and requires considerable ability and knowledge—in particular, a knowledge of fire science. The Fire Service in the United Kingdom has a statutory duty to submit a fire report form for every fire attended, which places a considerable responsibility on the officer in charge. The construction of the form is crucial, as it must elicit the required information in sufficient detail. The “Basic Incident Report Form” recommended by the NFPA is shown in the Fire Protection Handbook (Cote 1991).
The data can be used in two ways, either to identify a fire problem or to provide the rational argument necessary to justify a particular course of action that may require public or private expenditure. A long-established database can be used to show the effects of actions taken. The following ten points have been gleaned from NFPA statistics over the period 1980 to 1989 (Cote 1991):
1. Home smoke detectors are widely used and very effective (but significant gaps in the detector strategy remain).
2. Automatic sprinklers produce large reductions in loss of life and property. Increased use of portable and area heating equipment sharply increased home fires involving heating equipment.
3. Incendiary and suspicious fires continued to decline from the 1970’s peak, but associated property damage stopped declining.
4. A large share of fire-fighter fatalities are attributed to heart attacks and activities away from the fireground.
5. Rural areas have the highest fire death rates.
6. Smoking materials igniting upholstered furniture, mattresses or bedding produce the most deadly residential fire scenarios.
7. US and Canadian fire death rates are amongst the highest of all the developed countries.
8. The states of the Old South in the United States have the highest fire death rates.
9. Older adults are at particularly high risk of death in fire.
Such conclusions are, of course, country-specific, although there are some common trends. Careful use of such data can provide the means of formulating sound policies regarding fire safety in the community. However, it must be remembered that these are inevitably “reactive”, rather than “proactive”. Proactive measures can only be introduced following a detailed fire hazard assessment. Such a course of action has been introduced progressively, starting in the nuclear industry and moving into the chemical, petrochemical and offshore industries where the risks are much more easily defined than in other industries. Their application to hotels and public buildings generally is much more difficult and requires the application of fire modelling techniques to predict the course of a fire and how the fire products will spread through the building to affect the occupants. Major advances have been made in this type of modelling, although it must be said that there is a long way to go before these techniques can be used with confidence. Fire safety engineering is still in need of much basic research in fire safety science before reliable fire hazard assessment tools can be made widely available.