Most of the radiation that a human being will be exposed to during a lifetime comes from natural sources in outer space or from materials present in the earth’s crust. Radioactive materials may affect the organism from without or, if inhaled or ingested with food, from within. The dose received may be very variable because it depends, on the one hand, on the amount of radioactive minerals present in the area of the world where the person lives—which is related to the amount of radioactive nuclides in the air and the amount found both in food and especially in drinking water—and, on the other, on the use of certain construction materials and the use of gas or coal for fuel, as well as the type of construction employed and the traditional habits of people in the given locality.
Today, radon is considered the most prevalent source of natural radiation. Together with its “daughters," or radionuclides formed by its disintegration, radon constitutes approximately three fourths of the effective equivalent dose to which humans are exposed due to natural terrestrial sources. The presence of radon is associated with an increase in the occurrence of lung cancer due to the deposition of radioactive substances in the bronchial region.
Radon is a colourless, odourless and tasteless gas seven times as heavy as air. Two isotopes occur most frequently. One is radon-222, a radionuclide present in the radioactive series from the disintegration of uranium-238; its main source in the environment is the rocks and the soil in which its predecessor, radium-226, occurs. The other is radon-220 from the thorium radioactive series, which has a lower incidence than radon-222.
Uranium occurs extensively in the earth’s crust. The median concentration of radium in soil is in the order of 25 Bq/kg. A Becquerel (Bq) is the unit of the international system and it represents a unit of radionuclide activity equivalent to one disintegration per second. The average concentration of radon gas in the atmosphere at the surface of the earth is 3 Bq/m3, with a range of 0.1 (over the oceans) to 10 Bq/m3. The level depends on the porousness of the soil, the local concentration of radium-226 and the atmospheric pressure. Given that the half-life of radon-222 is 3.823 days, most of the dosage is not caused by the gas but by radon daughters.
Radon is found in existing materials and flows from the earth everywhere. Because of its characteristics it disperses easily outdoors, but it has a tendency to become concentrated in enclosed spaces, notably in caves and buildings, and especially in lower spaces where its elimination is difficult without proper ventilation. In temperate regions, the concentrations of radon indoors are estimated to be in the order of eight times higher than the concentrations outdoors.
Exposure to radon by most of the population, therefore, occurs for the most part within buildings. The median concentrations of radon depend, basically, on the geological characteristics of the soil, on the construction materials used for the building and on the amount of ventilation it receives.
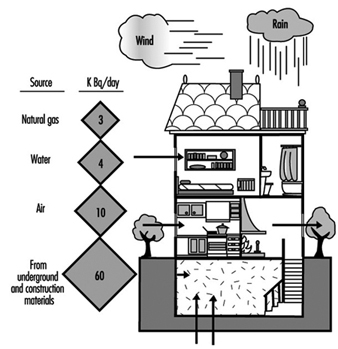
The main source of radon in indoor spaces is the radium present in the soil on which the building rests or the materials employed in its construction. Other significant sources—even though their relative influence is much less—are outside air, water and natural gas. Figure 1 shows the contribution that each source makes to the total.
Figure 1. Sources of radon in the indoor environment.
The most common construction materials, such as wood, bricks and cinder blocks, emit relatively little radon, in contrast to granite and pumice-stone. However, the main problems are caused by the use of natural materials such as alum slate in the production of construction materials. Another source of problems has been the use of by-products from the treatment of phosphate minerals, the use of by-products from the production of aluminium, the use of dross or slag from the treatment of iron ore in blast furnaces, and the use of ashes from the combustion of coal. In addition, in some instances, residues derived from uranium mining were also used in construction.
Radon can enter water and natural gas in the subsoil. The water used to supply a building, especially if it is from deep wells, may contain significant amounts of radon. If this water is used for cooking, boiling can free a large part of the radon it contains. If the water is consumed cold, the body eliminates the gas readily, so that drinking this water does not generally pose a significant risk. Burning natural gas in stoves without chimneys, in heaters and in other home appliances can also lead to an increase of radon in indoor spaces, especially dwellings. Sometimes the problem is more acute in bathrooms, because radon in water and in the natural gas used for the water heater accumulates if there is not enough ventilation.
Given that the possible effects of radon on the population at large were unknown just a few years ago, the data available on concentrations found in indoor spaces are limited to those countries which, because of their characteristics or special circumstances, are more sensitized to this problem. What is known for a fact is that it is possible to find concentrations in indoor spaces that are far above the concentrations found outdoors in the same region. In Helsinki (Finland), for instance, concentrations of radon in indoor air have been found that are five thousand times higher than the concentrations normally found outdoors. This may be due in large part to energy-saving measures that can noticeably favour the concentration of radon in indoor spaces, especially if they are heavily insulated. Buildings studied so far in different countries and regions show that the concentrations of radon found within them present a distribution that approximates the normal log. It is worth noting that a small number of the buildings in each region show concentrations ten times above the median. The reference values for radon in indoor spaces, and the remedial recommendations of various organizations are given in “Regulations, recommendations, guidelines and standards” in this chapter.
In conclusion, the main way to prevent exposures to radon is based on avoiding construction in areas that by their nature emit a greater amount of radon into the air. Where that is not possible, floors and walls should be properly sealed, and construction materials should not be used if they contain radioactive matter. Interior spaces, especially basements, should have an adequate amount of ventilation.