From the standpoint of pollution, indoor air in non-industrial situations displays several characteristics that differentiate it from outside, or atmospheric, air and from the air in industrial workplaces. Besides contaminants found in atmospheric air, indoor air also includes contaminants generated by building materials and by the activities that take place within the building. The concentrations of contaminants in indoor air tend to be the same or less than concentrations found in outside air, depending on ventilation; contaminants generated by building materials are usually different from those found in outside air and can be found in high concentrations, while those generated by activities inside the building depend on the nature of such activities and may be the same as those found in outside air, as in the case of CO and CO2.
For this reason, the number of contaminants found in non-industrial inside air is large and varied and the levels of concentration are low (except for instances where there is an important generating source); they vary according to atmospheric/climatologic conditions, the type or characteristics of the building, its ventilation and the activities carried out within it.
Analysis
Much of the methodology used to gauge the quality of indoor air stems from industrial hygiene and from measurements of immission of outdoor air. There are few analytic methods validated specifically for this type of testing, although some organizations, such as the World Health Organization and the Environmental Protection Agency in the United States are conducting research in this field. An additional obstacle is the paucity of information on the exposure-effect relationship when dealing with long-term exposures to low concentrations of pollutants.
The analytical methods used for industrial hygiene are designed to measure high concentrations and have not been defined for many pollutants, while the number of contaminants in indoor air can be large and varied and the levels of concentration can be low, except in certain cases. Most methods used in industrial hygiene are based on the taking of samples and their analysis; many of these methods can be applied to indoor air if several factors are taken into account: adjusting the methods to the typical concentrations; increasing their sensitivity without detriment to precision (for example, increasing the volume of air tested); and validating their specificity.
The analytical methods used to measure concentrations of pollutants in outdoor air are similar to those used for indoor air, and therefore some can be used directly for indoor air while others can be easily adapted. However, it is important to keep in mind that some methods are designed for a direct reading of one sample, while others require bulky and sometimes noisy instrumentation and use large volumes of sampled air which can distort the reading.
Planning the Readings
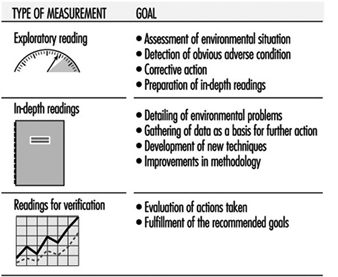
The traditional procedure in the field of workplace environmental control can be used to improve the quality of indoor air. It consists of identifying and quantifying a problem, proposing corrective measures, making sure that these measures are implemented, and then assessing their effectiveness after a period of time. This common procedure is not always the most adequate because often such an exhaustive evaluation, including the taking of many samples, is not necessary. Exploratory measures, which can range from a visual inspection to assaying of ambient air by direct reading methods, and which can provide an approximate concentration of pollutants, are sufficient for solving many of the existing problems. Once corrective measures have been taken, the results can be evaluated with a second measurement, and only when there is no clear evidence of an improvement a more thorough inspection (with in-depth measurements) or a complete analytical study can be undertaken (Swedish Work Environment Fund 1988).
The main advantages of such an exploratory procedure over the more traditional one are economy, speed and effectiveness. It requires competent and experienced personnel and the use of suitable equipment. Figure 1 summarizes the goals of the different stages of this procedure.
Figure 1. Planning the readings for exploratory evaluation.
Sampling Strategy
Analytical control of the quality of indoor air should be considered as a last resort only after the exploratory measurement has not given positive results, or if further evaluation or control of the initial tests is needed.
Assuming some previous knowledge of the sources of pollution and of the types of contaminants, the samples, even when limited in number, should be representative of the various spaces studied. Sampling should be planned to answer the questions What? How? Where? and When?
What
The pollutants in question must be identified in advance and, keeping in mind the different types of information that can be obtained, one should decide whether to make emission or immission measurements.
Emission measurements for indoor air quality can determine the influence of different sources of pollution, of climatic conditions, of the building’s characteristics, and of human intervention, which allow us to control or reduce the sources of emissions and improve the quality of indoor air. There are different techniques for taking this type of measurement: placing a collection system adjacent to the source of the emission, defining a limited work area and studying emissions as if they represented general working conditions, or working in simulated conditions applying monitoring systems that rely on head space measures.
Immission measurements allow us to determine the level of indoor air pollution in the different compartmentalized areas of the building, making it possible to produce a map of pollution for the entire structure. Using these measurements and identifying the different areas where people have carried out their activities and calculating the time they have spent at each task, it will be possible to determine the levels of exposure. Another way of doing this is by having individual workers wear monitoring devices while working.
It may be more practical, if the number of pollutants is large and varied, to select a few representative substances so that the reading is representative and not too expensive.
How
Selecting the type of reading to be made will depend on the available method (direct reading or sample-taking and analysis) and on the measuring technique: emission or immission.
Where
The location selected should be the most appropriate and representative for obtaining samples. This requires knowledge of the building being studied: its orientation relative to the sun, the number of hours it receives direct sunlight, the number of floors, the type of compartmentalization, if ventilation is natural or forced air, if its windows can be opened, and so on. Knowing the source of the complaints and the problems is also necessary, for example, whether they occur in the upper or the lower floors, or in the areas close to or far from the windows, or in the areas that have poor ventilation or illumination, among other locations. Selecting the best sites to draw the samples will be based on all of the available information regarding the above-mentioned criteria.
When
Deciding when to take the readings will depend on how concentrations of air pollutants change relative to time. Pollution may be detected first thing in the morning, during the workday or at the end of the day; it may be detected at the beginning or the end of the week; during the winter or the summer; when air-conditioning is on or off; as well as at other times.
To address these questions properly, the dynamics of the given indoor environment must be known. It is also necessary to know the goals of the measurements taken, which will be based on the types of pollutant that are being investigated. The dynamics of indoor environments are influenced by the diversity of the sources of pollution, the physical differences in the spaces involved, the type of compartmentalization, the type of ventilation and climate control used, outside atmospheric conditions (wind, temperature, season, etc.), and the building’s characteristics (number of windows, their orientation, etc.).
The goals of the measurements will determine if sampling will be carried out for short or long intervals. If the health effects of the given contaminants are thought to be long-term, it follows that average concentrations should be measured over long periods of time. For substances that have acute but not cumulative effects, measurements over short periods are sufficient. If intense emissions of short duration are suspected, frequent sampling over short periods is called for in order to detect the time of the emission. Not to be overlooked, however, is the fact that in many cases the possible choices in the type of sampling methods used may be determined by the analytical methods available or required.
If after considering all these questions it is not sufficiently clear what the source of the problem is, or when the problem occurs with greatest frequency, the decision as to where and when to take samples must be made at random, calculating the number of samples as a function of the expected reliability and cost.
Measuring techniques
The methods available for taking samples of indoor air and for their analysis can be grouped into two types: methods that involve a direct reading and those that involve taking samples for later analysis.
Methods based on a direct reading are those by which taking the sample and measuring the concentration of pollutants is done simultaneously; they are fast and the measurement is instantaneous, allowing for precise data at a relatively low cost. This group includes colorimetric tubes and specific monitors.
The use of colorimetric tubes is based on the change in the colour of a specific reactant when it comes in contact with a given pollutant. The most commonly used are tubes that contain a solid reactant and air is drawn through them using a manual pump. Assessing the quality of indoor air with colorimetric tubes is useful only for exploratory measurements and for measuring sporadic emissions since their sensitivity is generally low, except for some pollutants such as CO and CO2 that can be found at high concentrations in indoor air. It is important to keep in mind that the precision of this method is low and interference from unlooked-for contaminants is often a factor.
In the case of specific monitors, detection of pollutants is based on physical, electric, thermal, electromagnetic and chemoelectromagnetic principles. Most monitors of this type can be used to make measurements of short or long duration and gain a profile of contamination at a given site. Their precision is determined by their respective manufacturers and proper use demands periodic calibrations by means of controlled atmospheres or certified gas mixtures. Monitors are becoming increasingly precise and their sensitivity more refined. Many have built-in memory to store the readings, which can then be downloaded to computers for the creation of databases and the easy organization and retrieval of the results.
Sampling methods and analyses can be classified into active (or dynamic) and passive, depending on the technique.
With active systems, this pollution can be collected by forcing air through collecting devices in which the pollutant is captured, concentrating the sample. This is accomplished with filters, adsorbent solids, and absorbent or reactive solutions which are placed in bubblers or are impregnated onto porous material. Air is then forced through and the contaminant, or the products of its reaction, are analysed. For the analysis of air sampled with active systems the requirements are a fixative, a pump to move the air and a system to measure the volume of sampled air, either directly or by using flow and duration data.
The flow and the volume of sampled air are specified in the reference manuals or should be determined by previous tests and will depend on the quantity and type of absorbent or adsorbent used, the pollutants that are being measured, the type of measurement (emission or immission) and the condition of the ambient air during the taking of the sample (humidity, temperature, pressure). The efficacy of the collection increases by reducing the rate of intake or by increasing the amount of fixative used, directly or in tandem.
Another type of active sampling is the direct capture of air in a bag or any other inert and impermeable container. This type of sample gathering is used for some gases (CO, CO2, H2S, O2) and is useful as an exploratory measure when the type of pollutant is unknown. The drawback is that without concentrating the sample there may be insufficient sensitivity and further laboratory processing may be necessary to increase the concentration.
Passive systems capture pollutants by diffusion or permeation onto a base that may be a solid adsorbent, either alone or impregnated with a specific reactant. These systems are more convenient and easy to use than active systems. They do not require pumps to capture the sample nor highly trained personnel. But capturing the sample may take a long time and the results tend to furnish only medium concentration levels. This method cannot be used to measure peak concentrations; in those instances active systems should be used instead. To use passive systems correctly it is important to know the speed at which each pollutant is captured, which will depend on the diffusion coefficient of the gas or vapor and the design of the monitor.
Table 1 shows the salient characteristics of each sampling method and table 2 outlines the various methods used to gather and analyse the samples for the most significant indoor air pollutants.
Table 1. Methodology for taking samples
|
Characteristics |
Active |
Passive |
Direct reading |
|
Timed interval measurements |
+ |
+ |
|
|
Long-term measurements |
+ |
+ |
|
|
Monitoring |
+ |
||
|
Concentration of sample |
+ |
+ |
|
|
Immission measurement |
+ |
+ |
+ |
|
Emission measurement |
+ |
+ |
+ |
|
Immediate response |
+ |
+ Means that the given method is suitable to the method of measurement or desired measurement criteria.
Table 2. Detection methods for gases in indoor air
|
Pollutant |
Direct reading |
Methods |
Analysis |
||
|
Capture by diffusion |
Capture by concentration |
Direct capture |
|||
|
Carbon monoxide |
Electrochemical cell |
Bag or inert container |
GCa |
||
|
Ozone |
Chemiluminescence |
Bubbler |
UV-Visb |
||
|
Sulphur dioxide |
Electrochemical cell |
Bubbler |
UV-Vis |
||
|
Nitrogen dioxide |
Chemiluminescence |
Filter impregnated with a |
Bubbler |
UV-Vis |
|
|
Carbon dioxide |
Infrared spectroscopy |
Bag or inert container |
GC |
||
|
Formaldehyde |
— |
Filter impregnated with a |
Bubbler |
HPLCc |
|
|
VOCs |
Portable GC |
Adsorbent solids |
Adsorbent solids |
Bag or inert container |
GC (ECDd-FIDe-NPDf-PIDg) |
|
Pesticides |
— |
Adsorbent solids |
GC (ECD-FPD-NPD) |
||
|
Particulate matter |
— |
Optical sensor |
Filter |
Impactor |
Gravimetry |
— = Method unsuitable for pollutant.
a GC = gas chromatography.
b UV-Vis = visible ultraviolet spectrophotometry.
c HPLC = high precision liquid chromatography.
d CD = electron capture detector.
e FID = flame, ionization detector.
f NPD = nitrogen/phosphorous detector.
g PID = photoionization detector.
h MS = mass spectrometry.
Selecting the method
To select the best sampling method, one should first determine that validated methods for the pollutants being studied exist and see to it that the proper instruments and materials are available to gather and analyse the pollutant. One usually needs to know what their cost will be, and the sensitivity required for the job, as well as things that can interfere with the measurement, given the method chosen.
An estimate of the minimum concentrations of what one hopes to measure is very useful when evaluating the method used to analyse the sample. The minimum concentration required is directly related to the amount of pollutant that can be gathered given the conditions specified by the method used (i.e., the type of system used to capture the pollutant or the duration of sample taking and volume of air sampled). This minimum amount is what determines the sensitivity required of the method used for analysis; it can be calculated from reference data found in the literature for a particular pollutant or group of pollutants, if they were arrived at by a similar method to the one that will be used. For example, if it is found that hydrocarbon concentrations of 30 (mg/m3) are commonly found in the area under study, the analytical method used should allow the measurement of those concentrations easily. If the sample is obtained with a tube of active carbon in four hours and with a flow of 0.5 litres per minute, the amount of hydrocarbons gathered in the sample is calculated by multiplying the flow rate of the substance by the period of time monitored. In the given example this equals:
![]() of hydrocarbons
of hydrocarbons ![]()
Any method for detecting hydrocarbons that requires the amount in the sample to be under 3.6 μg can be used for this application.
Another estimate could be calculated from the maximum limit established as the allowable limit for indoor air for the pollutant being measured. If these figures don’t exist and the usual concentrations found in indoor air are not known, nor the rate at which the pollutant is being discharged into the space, approximations can be used based on the potential levels of the pollutant that can negatively affect health. The method chosen should be capable of measuring 10% of the established limit or of the minimal concentration that could affect health. Even if the method of analysis chosen has an acceptable degree of sensitivity, it is possible to find concentrations of pollutants that are below the lower limit of detection of the chosen method. This should be kept in mind when calculating average concentrations. For example, if out of ten readings taken three are below the detection limit, two averages should be calculated, one assigning these three readings the value of zero and another giving them the lowest detection limit, which renders a minimum average and a maximum average. The true measured average will be found between the two.
Analytical Procedures
The number of indoor air pollutants is great and they are found in small concentrations. The methodology that has been available is based on adapting methods used to monitor the quality of outdoor, atmospheric, air and air found in industrial situations. Adapting these methods for the analysis of indoor air implies changing the range of the concentration sought, when the method allows, using longer sampling times and greater amounts of absorbents or adsorbents. All these changes are appropriate when they do not lead to a loss in reliability or precision. Measuring a mixture of contaminants is usually expensive and the results obtained imprecise. In many cases all that will be ascertained will be a pollution profile that will indicate the level of contamination during sampling intervals, compared to clean air, to outside air, or to other indoor spaces. Direct reading monitors are used to monitor the pollution profile and may not be suitable if they are too noisy or too large. Ever smaller and quieter monitors, that afford greater precision and sensitivity, are being designed. Table 3 shows in outline the current state of the methods used to measure the different types of contaminants.
Table 3. Methods used for the analysis of chemical pollutants
|
Pollutant |
Direct-reading monitora |
Sampling and analysis |
|
Carbon monoxide |
+ |
+ |
|
Carbon dioxide |
+ |
+ |
|
Nitrogen dioxide |
+ |
+ |
|
Formaldehyde |
– |
+ |
|
Sulphur dioxide |
+ |
+ |
|
Ozone |
+ |
+ |
|
VOCs |
+ |
+ |
|
Pesticides |
– |
+ |
|
Particulates |
+ |
+ |
a ++ = most commonly used; + = less commonly used; – = not applicable.
Analysis of gases
Active methods are the most common for the analysis of gases, and are carried out using absorbent solutions or adsorbent solids, or by directly taking a sample of air with a bag or some other inert and airtight container. In order to prevent loss of part of the sample and increase the accuracy of the reading, the volume of the sample must be lower and the amount of absorbent or adsorbent used should be more than for other types of pollution. Care should also be taken in transporting and storing the sample (keeping it at low temperature) and minimizing the time before the sample is tested. Direct reading methods are widely used for measuring gases because of the considerable improvement in the capabilities of modern monitors, which are more sensitive and more precise than before. Because of their ease of use and the level and type of information that they furnish, they are increasingly replacing traditional methods of analysis. Table 4 shows the minimum detection levels for the various gases studied given the method of sampling and analysis used.
Table 4. Lower detection limits for some gases by monitors used to assess indoor air quality
|
Pollutant |
Direct-reading monitora |
Sample-taking and |
|
Carbon monoxide |
1.0 ppm |
0.05 ppm |
|
Nitrogen dioxide |
2 ppb |
1.5 ppb (1 week)b |
|
Ozone |
4 ppb |
5.0 ppb |
|
Formaldehyde |
5.0 ppb (1 week)b |
a Carbon dioxide monitors that use infrared spectroscopy are always sensitive enough.
b Passive monitors (length of exposure).
These gases are common pollutants in indoor air. They are measured by using monitors that detect them directly by electrochemical or infrared means, even though infrared detectors are not very sensitive. They can also be measured by taking air samples directly with inert bags and analysing the sample by gas chromatography with a flame ionization detector, transforming the gases into methane first by means of a catalytic reaction. Thermal conduction detectors are usually sensitive enough to measure normal concentrations of CO2.
Nitrogen dioxide
Methods have been developed to detect nitrogen dioxide, NO2, in indoor air by using passive monitors and taking samples for later analysis, but these methods have presented sensitivity problems that will hopefully be overcome in the future. The best known method is the Palmes tube, which has a detection limit of 300 ppb. For non-industrial situations, sampling should be for a minimum of five days in order to obtain a detection limit of 1.5 ppb, which is three times the value of the blank for a one-week exposure. Portable monitors that measure in real time have also been developed based on the chemiluminescence reaction between NO2 and the reactant luminol, but the results obtained by this method can be affected by temperature and their linearity and sensitivity depend on the characteristics of the solution of luminol used. Monitors that have electrochemical sensors have improved sensitivity but are subject to interference from compounds that contain sulphur (Freixa 1993).
Sulphur dioxide
A spectrophotometric method is used to measure sulphur dioxide, SO2, in an indoor environment. The air sample is bubbled through a solution of potassium tetrachloromercuriate to form a stable complex which is in turn measured spectrophotometrically after reacting with pararosaniline. Other methods are based on flame photometry and pulsating ultraviolet fluorescence, and there are also methods based on deriving the measurement before the spectroscopic analysis. This type of detection, which has been used for outside air monitors, is not suited for indoor air analysis because of a lack of specificity and because many of these monitors require a venting system to eliminate the gases that they generate. Because emissions of SO2 have been greatly reduced and it is not considered an important pollutant of indoor air, the development of monitors for its detection have not advanced very much. However, there are portable instruments available on the market that can detect SO2 based on the detection of pararosaniline (Freixa 1993).
Ozone
Ozone, O3, can only be found in indoor environments in special situations in which it is generated continuously, since it decays rapidly. It is measured by direct reading methods, by colorimetric tubes and by chemiluminescence methods. It can also be detected by methods used in industrial hygiene that can be easily adapted for indoor air. The sample is obtained with an absorbent solution of potassium iodide in a neutral medium and then subjected to spectrophotometric analysis.
Formaldehyde
Formaldehyde is an important pollutant of indoor air, and because of its chemical and toxic characteristics an individualized evaluation is recommended. There are different methods for detecting formaldehyde in air, all of them based on taking samples for later analysis, with active fixing or by diffusion. The most appropriate capturing method will be determined by the type of sample (emission or immission) used and the sensitivity of the analytical method. The traditional methods are based on obtaining a sample by bubbling air through distilled water or a solution of 1% sodium bisulphate at 5°C, and then analysing it with spectrofluorometric methods. While the sample is stored, it should also be kept at 5°C. SO2 and the components of tobacco smoke can create interference. Active systems or methods that capture pollutants by diffusion with solid adsorbents are used more and more frequently in indoor air analysis; they all consist of a base that can be a filter or a solid saturated with a reactant, such as sodium bisulphate or 2,4-diphenylhydrazine. Methods that capture the pollutant by diffusion, in addition to general advantages of that method, are more sensitive than active methods because the time required to obtain the sample is longer (Freixa 1993).
Detection of volatile organic compounds (VOCs)
The methods used to measure or monitor organic vapors in indoor air must meet a series of criteria: they should have a sensitivity in the order of parts per billion (ppb) to parts per trillion (ppt), the instruments used to take the sample or make a direct reading must be portable and easy to handle in the field, and the results obtained must be precise and capable of being duplicated. There are a great many methods that meet these criteria, but the ones most frequently used to analyse indoor air are based on sample taking and analysis. Direct detection methods exist that consist of portable gas chromatographs with different detection methods. These instruments are expensive, their handling is sophisticated and they can be operated only by trained personnel. For polar and nonpolar organic compounds that have a boiling point between 0°C and 300°C, the most widely used adsorbent both for active and passive sampling systems has been activated carbon. Porous polymers and polymer resins, such as Tenax GC, XAD-2 and Ambersorb are also used. The most widely used of these is Tenax. The samples obtained with activated carbon are extracted with carbon disulphide and they are analysed by gas chromatography with flame ionization, electron-capture, or mass spectrometry detectors, followed by qualitative and quantitative analysis. Samples obtained with Tenax are usually extracted by thermal desorption with helium and are condensed in a nitrogen cold trap before being fed to the chromatograph. Another common method consists in obtaining samples directly, using bags or inert containers, feeding the air directly to the gas chromatograph, or concentrating the sample first with an adsorbent and a cold trap. The detection limits of these methods depend on the compound analysed, the volume of the sample taken, the background pollution and the detection limits of the instrument used. Because quantifying each and every one of the compounds present is impossible, quantification is normally done by families, by using as a reference compounds that are characteristic of each family of compounds. In detecting VOCs in indoor air, the purity of the solvents used is very important. If thermal desorption is used, the purity of the gases is also important.
Detection of pesticides
To detect pesticides in indoor air, the methods commonly employed consist of taking samples with solid adsorbents, although the use of bubblers and mixed systems is not ruled out. The solid adsorbent most commonly used has been porous polymer Chromosorb 102, although polyurethane foams (PUFs) that can capture a wider number of pesticides are being used more and more. The methods of analysis vary according to the sampling method and the pesticide. Usually they are analysed by using gas chromatography with different specific detectors, from electron capture to mass spectrometry. The potential of the latter for identifying compounds is considerable. The analysis of these compounds presents certain problems, which include the contamination of glass parts in the sample-taking systems with traces of polychlorinated biphenyls (PCBs), phthalates or pesticides.
Detection of environmental dust or particles
For the capture and analysis of particles and fibres in air a great variety of techniques and equipment are available and suited for assessing indoor air quality. Monitors that permit a direct reading of the concentration of particles in the air use diffuse light detectors, and methods that employ sample taking and analysis use weighting and analysis with a microscope. This type of analysis requires a separator, such as a cyclone or an impactor, to sift out larger particles before a filter can be used. Methods that employ a cyclone can handle small volumes, which results in long sessions of sample taking. Passive monitors offer excellent precision, but they are affected by ambient temperature and tend to give readings with higher values when the particles are small.