Infrared radiation is that part of the non-ionizing radiation spectrum located between microwaves and visible light. It is a natural part of the human environment and thus people are exposed to it in small amounts in all areas of daily life—for example, at home or during recreational activities in the sun. Very intense exposure, however, may result from certain technical processes at the workplace.
Many industrial processes involve thermal curing of various kinds of materials. The heat sources used or the heated material itself will usually emit such high levels of infrared radiation that a large number of workers are potentially at risk of being exposed.
Concepts and Quantities
Infrared radiation (IR) has wavelengths ranging from 780 nm to 1 mm. Following the classification by the International Commission on Illumination (CIE), this band is subdivided into IRA (from 780 nm to 1.4 μm), IRB (from 1.4 μm to 3 μm) and IRC (from 3 μm to 1 mm). This subdivision approximately follows the wavelength-dependent absorption characteristics of IR in tissue and the resulting different biological effects.
The amount and the temporal and spatial distribution of infrared radiation are described by different radiometric quantities and units. Due to optical and physiological properties, especially of the eye, a distinction is usually made between small “point” sources and “extended” sources. The criterion for this distinction is the value in radians of the angle (α) measured at the eye that is subtended by the source. This angle can be calculated as a quotient, the light source dimension DL divided by the viewing distance r. Extended sources are those which subtend a viewing angle at the eye greater than αmin, which normally is 11 milliradians. For all extended sources there is a viewing distance where α equals αmin; at greater viewing distances, the source can be treated like a point source. In optical radiation protection the most important quantities concerning extended sources are the radiance (L, expressed in Wm–2sr–1) and the time-integrated radiance (Lp in Jm–2sr–1), which describe the “brightness” of the source. For health risk assessment, the most relevant quantities concerning point sources or exposures at such distances from the source where α< αmin, are the irradiance (E, expressed in Wm–2), which is equivalent to the concept of exposure dose rate, and the radiant exposure (H, in Jm–2), equivalent to the exposure dose concept.
In some bands of the spectrum, the biological effects due to exposure are strongly dependent on wavelength. Therefore, additional spectroradiometric quantities must be used (e.g., the spectral radiance, Ll, expressed in Wm–2 sr–1 nm–1) to weigh the physical emission values of the source against the applicable action spectrum related to the biological effect.
Sources and Occupational Exposure
Exposure to IR results from various natural and artificial sources. The spectral emission from these sources may be limited to a single wavelength (laser) or may be distributed over a broad wavelength band.
The different mechanisms for the generation of optical radiation in general are:
- thermal excitation (black-body radiation)
- gas discharge
- light amplification by stimulated emission of radiation (laser), with the mechanism of gas discharge being of lesser importance in the IR band.
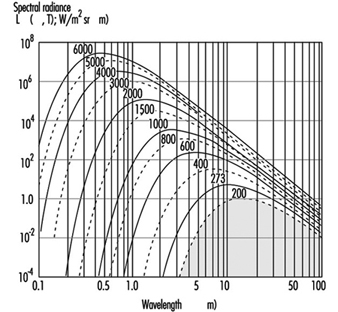
The emission from the most important sources used in many industrial processes results from thermal excitation, and can be approximated using the physical laws of black-body radiation if the absolute temperature of the source is known. The total emission (M, in Wm–2) of a black-body radiator (figure 1) is described by the Stefan-Boltzmann law:
M(T) = 5.67 x 10-8T4
and depends on the 4th power of the temperature (T, in K) of the radiating body. The spectral distribution of the radiance is described by Planck’s radiation law:

and the wavelength of maximum emission (λmax) is described according to Wien’s law by:
λmax = (2.898 x 10-8) / T
Figure 1. Spectral radiance λmaxof a black body radiator at the absolute temperature shown in degrees Kelvin on each curve
Many lasers used in industrial and medical processes will emit very high levels of IR. In general, compared with other radiation sources, laser radiation has some unusual features that may influence the risk following an exposure, such as very short pulse duration or extremely high irradiance. Therefore, laser radiation is discussed in detail elsewhere in this chapter.
Many industrial processes require the use of sources emitting high levels of visible and infrared radiation, and thus a large number of workers like bakers, glass blowers, kiln workers, foundry workers, blacksmiths, smelters and fire-fighters are potentially at risk of exposure. In addition to lamps, such sources as flames, gas torches, acetylene torches, pools of molten metal and incandescent metal bars must be considered. These are encountered in foundries, steel mills and in many other heavy industrial plants. Table 1 summarizes some examples of IR sources and their applications.
Table 1. Different sources of IR, population exposed and approximate exposure levels
|
Source |
Application or exposed population |
Exposure |
|
Sunlight |
Outdoor workers, farmers, construction workers, seafarers, general public |
500 Wm–2 |
|
Tungsten filament lamps |
General population and workers |
105–106 Wm–2sr–1 |
|
Tungsten halogen filament lamps |
(See tungsten filament lamps) |
50–200 Wm–2 (at 50 cm) |
|
Light emitting diodes (e.g. GaAs diode) |
Toys, consumer electronics, data transmission technology, etc. |
105 Wm–2sr–1 |
|
Xenon arc lamps |
Projectors, solar simulators, search lights |
107 Wm–2sr–1 |
|
Iron melt |
Steel furnace, steel mill workers |
105 Wm–2sr–1 |
|
Infrared lamp arrays |
Industrial heating and drying |
103 to 8.103 Wm–2 |
|
Infrared lamps in hospitals |
Incubators |
100–300 Wm–2 |
Biological Effects
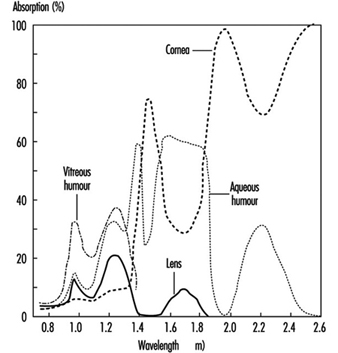
Optical radiation in general does not penetrate very deeply into biological tissue. Therefore, the primary targets of an IR exposure are the skin and the eye. Under most exposure conditions the main interaction mechanism of IR is thermal. Only the very short pulses that lasers may produce, but which are not considered here, can also lead to mechanothermal effects. Effects from ionization or from the breakage of chemical bonds are not expected to appear with IR radiation because the particle energy, being less than approximately 1.6 eV, is too low to cause such effects. For the same reason, photochemical reactions become significant only at shorter wavelengths in the visual and in the ultraviolet region. The different wavelength-dependent health effects of IR arise mainly from the wavelength-dependent optical properties of tissue—for example, the spectral absorption of the ocular media (figure 2).
Figure 2. Spectral absorption of the ocular media
Effects on the eye
In general, the eye is well adapted to protect itself against optical radiation from the natural environment. In addition, the eye is physiologically protected against injury from bright light sources, such as the sun or high intensity lamps, by an aversion response that limits the duration of exposure to a fraction of a second (approximately 0.25 seconds).
IRA affects primarily the retina, because of the transparency of the ocular media. When directly viewing a point source or laser beam, the focusing properties in the IRA region additionally render the retina much more susceptible to damage than any other part of the body. For short exposure periods, heating of the iris from the absorption of visible or near IR is considered to play a role in the development of opacities in the lens.
With increasing wavelength, above approximately 1 μm, the absorption by ocular media increases. Therefore, absorption of IRA radiation by both the lens and the pigmented iris is considered to play a role in the formation of lenticular opacities. Damage to the lens is attributed to wavelengths below 3 μm (IRA and IRB). For infrared radiation of wavelengths longer than 1.4 μm, the aqueous humour and the lens are particularly strongly absorbent.
In the IRB and IRC region of the spectrum, the ocular media become opaque as a result of the strong absorption by their constituent water. Absorption in this region is primarily in the cornea and in the aqueous humour. Beyond 1.9 μm, the cornea is effectively the sole absorber. The absorption of long wavelength infrared radiation by the cornea may lead to increased temperatures in the eye due to thermal conduction. Because of a quick turnover rate of the surface corneal cells, any damage limited to the outer corneal layer can be expected to be temporary. In the IRC band the exposure can cause a burn on the cornea similar to that on the skin. Corneal burns are not very likely to occur, however, because of the aversion reaction triggered by the painful sensation caused by strong exposure.
Effects on the skin
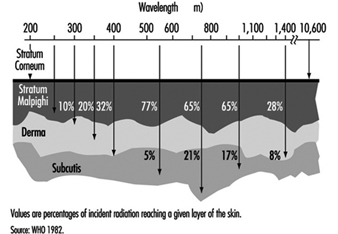
Infrared radiation will not penetrate the skin very deeply. Therefore, exposure of the skin to very strong IR may lead to local thermal effects of different severity, and even serious burns. The effects on the skin depend on the optical properties of the skin, such as wavelength-dependent depth of penetration (figure 3 ). Especially at longer wavelengths, an extensive exposure may cause a high local temperature rise and burns. The threshold values for these effects are time dependent, because of the physical properties of the thermal transport processes in the skin. An irradiation of 10 kWm–2, for example, may cause a painful sensation within 5 seconds, whereas an exposure of 2 kWm–2 will not cause the same reaction within periods shorter than approximately 50 seconds.
Figure 3. Depth of penetration into the skin for different wavelengths
If the exposure is extended over very long periods, even at values well below the pain threshold, the burden of heat to the human body may be great. Especially if the exposure covers the whole body as, for example, in front of a steel melt. The result may be an imbalance of the otherwise physiologically well balanced thermoregulation system. The threshold for tolerating such an exposure will depend on different individual and environmental conditions, such as the individual capacity of the thermoregulation system, the actual body metabolism during exposure or the environmental temperature, humidity and air movement (wind speed). Without any physical work, a maximum exposure of 300 Wm–2 may be tolerated over eight hours under certain environmental conditions, but this value decreases to approximately 140 Wm–2 during heavy physical work.
Exposure Standards
The biological effects of IR exposure which are dependent on wavelength and on the duration of exposure, are intolerable only if certain threshold intensity or dose values are exceeded. To protect against such intolerable exposure conditions, international organizations such as the World Health Organization (WHO), the International Labour Office (ILO), the International Committee for Non-Ionizing Radiation of the International Radiation Protection Association (INIRC/IRPA), and its successor, the International Commission on Non-Ionizing Radiation Protection (ICNIRP) and the American Conference of Governmental Industrial Hygienists (ACGIH) have suggested exposure limits for infrared radiation from both coherent and incoherent optical sources. Most of the national and international suggestions on guidelines for limiting human exposure to infrared radiation are either based on or even identical with the suggested threshold limit values (TLVs) published by the ACGIH (1993/1994). These limits are widely recognized and are frequently used in occupational situations. They are based on current scientific knowledge and are intended to prevent thermal injury of the retina and cornea and to avoid possible delayed effects on the lens of the eye.
The 1994 revision of the ACGIH exposure limits is as follows:
1. For the protection of the retina from thermal injury in case of exposure to visible light, (for example, in the case of powerful light sources), the spectral radiance Lλ in W/(m² sr nm) weighted against the retinal thermal hazard function Rλ (see table 2) over the wavelength interval Δλ and summed over the range of wavelength 400 to 1400 nm, should not exceed:
![]()
where t is the viewing duration limited to intervals from 10-3 to 10 seconds (that is, for accidental viewing conditions, not fixated viewing), and α is the angular subtense of the source in radians calculated by α = maximum extension of the source/distance to the source Rλ (table 2 ).
2. To protect the retina from the exposure hazards of infrared heat lamps or any near IR source where a strong visual stimulus is absent, the infrared radiance over the wavelength range 770 to 1400 nm as viewed by the eye (based on a 7 mm pupil diameter) for extended duration of viewing conditions should be limited to:
![]()
This limit is based on a pupil diameter of 7 mm since, in this case, the aversion response (closing the eye, for example) may not exist due to the absence of visible light.
3. To avoid possible delayed effects on the lens of the eye, such as delayed cataract, and to protect the cornea from overexposure, the infrared radiation at wavelengths greater than 770 nm should be limited to 100 W/m² for periods greater than 1,000 s and to:
![]()
or for shorter periods.
4. For aphakic patients, separate weighting functions and resulting TLVs are given for the wavelength range of ultraviolet and visible light (305–700 nm).
Table 2. Retinal thermal hazard function
|
Wavelength (nm) |
Rλ |
Wavelength (nm) |
Rλ |
|
400 |
1.0 |
460 |
8.0 |
|
405 |
2.0 |
465 |
7.0 |
|
410 |
4.0 |
470 |
6.2 |
|
415 |
8.0 |
475 |
5.5 |
|
420 |
9.0 |
480 |
4.5 |
|
425 |
9.5 |
485 |
4.0 |
|
430 |
9.8 |
490 |
2.2 |
|
435 |
10.0 |
495 |
1.6 |
|
440 |
10.0 |
500–700 |
1.0 |
|
445 |
9.7 |
700–1,050 |
10((700 - λ )/500) |
|
450 |
9.4 |
1,050–1,400 |
0.2 |
|
455 |
9.0 |
Source: ACGIH 1996.
Measurement
Reliable radiometric techniques and instruments are available that make it possible to analyse the risk to the skin and the eye from exposure to sources of optical radiation. For characterizing a conventional light source, it is generally very useful to measure the radiance. For defining hazardous exposure conditions from optical sources, the irradiance and the radiant exposure are of greater importance. The evaluation of broad-band sources is more complex than the evaluation of sources that emit at single wavelengths or very narrow bands, since spectral characteristics and source size must be considered. The spectrum of certain lamps consists of both a continuum emission over a wide wavelength band and emission on certain single wavelengths (lines). Significant errors may be introduced into the representation of those spectra if the fraction of energy in each line is not properly added to the continuum.
For health-hazard assessment the exposure values must be measured over a limiting aperture for which the exposure standards are specified. Typically a 1 mm aperture has been considered to be the smallest practical aperture size. Wavelengths greater than 0.1 mm present difficulties because of significant diffraction effects created by a 1 mm aperture. For this wavelength band an aperture of 1 cm² (11 mm diameter) was accepted, because hot spots in this band are larger than at shorter wavelengths. For the evaluation of retinal hazards, the size of the aperture was determined by an average pupil size and therefore an aperture of 7 mm was chosen.
In general, measurements in the optical region are very complex. Measurements taken by untrained personnel may lead to invalid conclusions. A detailed summary of measurement procedures is to be found in Sliney and Wolbarsht (1980).
Protective Measures
The most effective standard protection from exposure to optical radiation is the total enclosure of the source and all of the radiation pathways that may exit from the source. By such measures, compliance with the exposure limits should be easy to achieve in the majority of cases. Where this is not the case, personal protection is applicable. For example, available eye protection in the form of suitable goggles or visors or protective clothing should be used. If the work conditions will not allow for such measures to be applied, administrative control and restricted access to very intense sources may be necessary. In some cases a reduction of either the power of the source or the working time (work pauses to recover from heat stress), or both, might be a possible measure to protect the worker.
Conclusion
In general, infrared radiation from the most common sources such as lamps, or from most industrial applications, will not cause any risk to workers. At some workplaces, however, IR can cause a health risk for the worker. In addition, there is a rapid increase in the application and use of special-purpose lamps and in high temperature processes in industry, science and medicine. If the exposure from those applications is sufficiently high, detrimental effects (mainly in the eye but also on the skin) cannot be excluded. The importance of internationally recognized optical radiation exposure standards is expected to increase. To protect the worker from excessive exposure, protective measures like shielding (eye shields) or protective clothing should be mandatory.
The principal adverse biological effects attributed to infrared radiation are cataracts, known as glass blower’s or furnaceman’s cataracts. Long-term exposure even at relatively low levels causes heat stress to the human body. At such exposure conditions additional factors such as body temperature and evaporative heat loss as well as environmental factors must be considered.
In order to inform and instruct workers some practical guides were developed in industrial countries. A comprehensive summary can be found in Sliney and Wolbarsht (1980).