Climate Change
The major greenhouse gases (GHGs) consist of carbon dioxide, methane, nitrous oxide, water vapour and chlorofluorocarbons (CFCs). These gases allow sunlight to penetrate to the earth’s surface, yet prevent infrared radiant heat from escaping. The Intergovernmental Panel on Climate Change (IPCC) of the United Nations has concluded that emissions, primarily from industry, and destruction of greenhouse gas sinks, via poor land use management, especially deforestation, have substantially increased the concentrations of GHGs beyond natural processes. Without major policy shifts, pre-industrial carbon dioxide levels are expected to increase, yielding a 1.0-3.5°C rise in average global temperature by the year 2100 (IPCC in press).
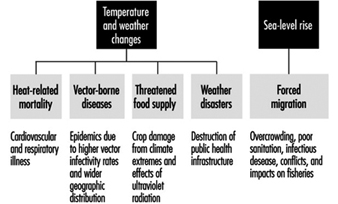
The two primary components of climate change include (1) temperature elevation with concomitant weather instability and extremes and (2) rising sea-level due to thermoexpansion. These changes may result in an increased frequency of heat waves and hazardous air pollution episodes, reduced soil moisture, higher incidence of disruptive weather events, and coastal inundation (IPCC 1992). Subsequent health effects may include an increase in (1) heat-related mortality and morbidity; (2) infectious diseases, particularly those that are insect borne; (3) malnutrition from food supply shortages; and (4) public health infrastructural crises from weather disasters and sea-level rise, coupled with climate-related human migration (see figure 1).
Figure 1. Public health effects from the major components of global climate change
 Humans have an enormous capacity to adapt to climatic and environmental conditions. However, the rate of predicted climatic and potential ecological change is of great concern to medical and earth scientists alike. Many of the health effects will be mediated through ecological responses to altered climate conditions. For example, spread of vector-borne diseases will depend on shifts in vegetation and availability of reservoir or intermediate hosts, in conjunction with the direct effects of temperature and humidity on parasites and their vectors (Patz et al. 1996). Understanding the hazards of climate change will, therefore, require an integrated ecological risk assessment which demands new and complex approaches compared to conventional single-agent cause-and-effect risk analysis from empirical data (McMichael 1993).
Humans have an enormous capacity to adapt to climatic and environmental conditions. However, the rate of predicted climatic and potential ecological change is of great concern to medical and earth scientists alike. Many of the health effects will be mediated through ecological responses to altered climate conditions. For example, spread of vector-borne diseases will depend on shifts in vegetation and availability of reservoir or intermediate hosts, in conjunction with the direct effects of temperature and humidity on parasites and their vectors (Patz et al. 1996). Understanding the hazards of climate change will, therefore, require an integrated ecological risk assessment which demands new and complex approaches compared to conventional single-agent cause-and-effect risk analysis from empirical data (McMichael 1993).
Stratospheric Ozone Depletion
Stratospheric ozone depletion is occurring primarily from reactions with halogen free radicals from chlorofluorocarbons (CFCs), along with other halocarbons and methyl bromide (Molina and Rowland 1974). Ozone specifically blocks the penetration of ultravioletB radiation (UVB), which contains the most biologically destructive wavelengths (290-320 nanometres). UVB levels are expected to rise disproportionately in temperate and arctic zones, since a clear relationship has been established between higher latitudes and the extent of ozone thinning (Stolarski et al. 1992).
For the period 1979-91, average ozone loss has been estimated at 2.7% per decade, correcting for solar cycle and other factors (Gleason et al. 1993). In 1993, researchers using a sensitive new spectroradiometer in Toronto, Canada, discovered that current ozone depletion has caused local increases in ambient UVB radiation of 35% in winter and 7% in summer, relative to 1989 levels (Kerr and McElroy 1993). Earlier estimates by the UN Environment Programme (UNEP) predicted a 1.4% rise in UVB per 1% drop in stratospheric ozone (UNEP 1991a).
The direct health impacts from stratospheric ozone depletion, which leads to increased ambient UVB radiation, include (1) skin cancer (2) ocular diseases and (3) immunosuppression. Indirect effects to health may occur from crop damage by ultraviolet radiation.
Health Effects of Temperature and Precipitation Change
Heat-related morbidity and mortality
Physiologically, humans have a great capacity for thermoregulation up to a threshold temperature. Weather conditions exceeding threshold temperatures and persisting for several consecutive days cause increased mortality in the population. In large cities, poor housing combined with the urban “heat island” effect further exacerbate conditions. In Shanghai, for instance, this effect can be as high as 6.5 °C on a windless evening during winter (IPCC 1990). Most heat-related fatalities occur in the elderly population and are attributed to cardiovascular and respiratory disorders (Kilbourne 1989). Key meteorological variables contribute to heat-related mortality, the most significant being high night-time readings; the greenhouse effect is predicted to especially elevate these minimum temperatures (Kalkstein and Smoyer 1993).
Temperate and polar regions are expected to warm disproportionately more than tropical and subtropical zones (IPCC 1990). Based on predictions by the US National Aeronautics and Space Administration (NASA), average summer temperatures in New York and St. Louis, for example, would rise by 3.1 and 3.9 °C, respectively, if ambient CO2 doubles. Even with adjustment for physiological acclimatization, annual summer mortality in temperate cities such as these could rise over fourfold (Kalkstein and Smoyer 1993).
Atmospheric chemistry is an important contributing factor in the formation of urban photochemical smog, whereby photodecomposition of NO2 in the presence of volatile organic compounds results in the production of tropospheric (ground-level) ozone. Both increased ambient UV radiation and warmer temperatures would further drive these reactions. Adverse health effects from air pollution are well known, and continued fossil fuel use will extend acute and chronic health impacts. (see “Air pollution” in this chapter).
Infectious diseases and climate/ecosystem change
Coupled atmosphere-ocean general circulation models predict that high latitudes in the northern hemisphere will experience the largest surface temperature elevation based on current IPCC scenarios (IPCC 1992). Minimum winter temperatures are expected to be disproportionately more affected, allowing for certain viruses and parasites to extend into regions where they previously could not live. In addition to direct climate effects on vectors, transformation of ecosystems could have marked implications for diseases whereby the geographic range of vector and/or reservoir host species is defined by these ecosystems.
Vector-borne diseases may spread to temperate regions in both hemispheres and intensify in endemic areas. Temperature determines vector infectivity by affecting pathogen replication, maturation and the period of infectivity (Longstreth and Wiseman 1989). Elevated temperature and humidity also intensify the biting behaviour of several mosquito species. Extreme heat, on the other hand, can shorten insect survival time.
Infectious diseases which incorporate a cold-blooded species (invertebrate) within their life cycles, are most susceptible to subtle climate variations (Sharp 1994). Diseases whose infectious agents, vectors or hosts are affected by climate change include malaria, schistosomiasis, filariasis, leishmaniasis, onchocerciasis (river blindness), trypanosomiasis (Chagas’ and African sleeping sickness), dengue, yellow fever and arboviral encephalitis. Current figures of the number of people at risk of these diseases are listed in table 1 (WHO 1990d).
Table 1. Global status of major vector-borne diseases
|
No.a |
Disease |
Population at risk |
Prevalence of infection |
Present distribution |
Possible change of distribution as a result of climatic change |
|
1. |
Malaria |
2,100 |
270 |
Tropics/subtropics |
++ |
|
2. |
Lymphatic filariases |
900 |
90.2 |
Tropics/subtropics |
+ |
|
3. |
Onchocerciasis |
90 |
17.8 |
Africa/L. America |
+ |
|
4. |
Schistosomiasis |
600 |
200 |
Tropics/subtropics |
++ |
|
5. |
African trypanosomiasis |
50 |
(25,000 new cases/year) |
Tropical Africa |
+ |
|
6. |
Leishmaniases |
350 |
12 million infected |
Asia/S.Europe/Africa/S. America |
? |
|
7. |
Dracunculiasis |
63 |
1 |
Tropics (Africa/Asia) |
0 |
|
Arboviral diseases |
|||||
|
8. |
Dengue |
1,500 |
Tropics/subtropics |
++ |
|
|
9. |
Yellow fever |
+++ |
Africa/L. America |
+ |
|
|
10. |
Japanese encephalitis |
+++ |
E/S.E. Asia |
+ |
|
|
11. |
Other arboviral diseases |
+++ |
+ |
||
a The numbers refer to explanations in the text. b Based on a world population estimated at 4.8 billion (1989).
0 = unlikely; + = likely; ++ = very likely; +++ = no estimate available; ? = not known.
Worldwide, malaria is the most prevalent vector-borne disease and causes one to two million deaths annually. An estimated one million additional annual fatalities may arise from climate change by the middle of the next century, according to Martens et al. (1995). The Anopheline mosquito which carries malaria can extend to the 16 °C winter isotherm, since parasite development does not occur below this temperature (Gilles and Warrell 1993). Epidemics occurring at higher altitudes generally coincide with above average temperatures (Loevinsohn 1994). Deforestation also affects malaria, since cleared areas provide an abundance of freshwater pools in which Anopheline larvae can develop (see “Species extinction, biodiversity loss and human health” in this chapter).
Over the past two decades, efforts to control malaria have made only marginal gains. Treatment has not improved as drug-resistance has become a major problem for the most virulent strain, Plasmodium falciparum, and antimalarial vaccines have shown only limited efficacy (Institute of Medicine 1991). Great capacity for antigenic variation of protozoans has thus far prevented acquisition of effective vaccines for malaria and sleeping sickness, leaving little optimism for readily available new pharmaceutical agents against these diseases. Diseases which involve intermediate reservoir hosts (e.g., deer and rodents in the case of Lyme disease) make human herd immunity from vaccination programmes essentially unattainable, representing another hurdle to preventive medical intervention.
As climate change alters habitat, causing a potential reduction of biodiversity, insect vectors will be forced to find new hosts (see “Species extinction, biodiversity loss and human health”). In Honduras, for example, blood-seeking insects such as the assassin beetle, which carries incurable Chagas’ disease (or American Trypanosomiasis), has been forced to seek human hosts as biodiversity decreases from deforestation. Of 10,601 Hondurans studied in endemic regions, 23.5% are now seropositive for Chagas’ disease (Sharp 1994). Zoonotic diseases are frequently the source of human infections, and generally affect man after an environmental change or alteration of human activity (Institute of Medicine l992). Many “newly emerging” diseases in humans are actually long-standing zoonoses of animal host species. For example, Hantavirus, recently found to be the cause of human fatalities in the southwest United States, has long been established in rodents and the recent outbreak was felt to be related to climatic/ecological conditions (Wenzel 1994).
Marine effects
Climate change may further impact public health through effects on harmful marine phytoplankton (or algae) blooms. Increases in phytoplankton globally has been a consequence of poor erosion control management, liberal agricultural application of fertilizers, and coastal sewage release, all resulting in effluents rich in nutrients which promote algae growth. Conditions that favour this growth could be augmented by warmer sea surface temperatures expected with global warming. Overharvesting of fish and shellfish (algae consumers) coupled with widespread pesticide use toxic to fish, further contribute to plankton overgrowth (Epstein 1995).
Red tides causing diarrhoeal and paralytic diseases and amnesic shellfish poisoning are prime examples of diseases stemming from algal overgrowth. Vibrio cholera has been found to be harboured by marine phytoplankton; thus blooms could represent an expanded reservoir from which cholera epidemics may initiate (Huq et al. 1990).
Food supply and human nutrition
Malnutrition is a major cause of infant mortality and childhood morbidity due to immunosuppression (see “Food and agriculture”). Climate change could adversely affect agriculture both by long-term changes, such as reducing soil moisture through evapotranspiration, and, more immediately, by extreme weather events such as droughts, flooding (and erosion) and tropical storms. Plants may initially benefit from “CO2 fertilization”, which can enhance photosynthesis (IPCC 1990). Even accounting for this, agriculture in developing countries will suffer most, and it is estimated that in these nations, 40-300 million additional people will be at risk from hunger due to climate change (Sharp 1994).
Indirect ecological changes affecting crops will need to be considered as well, since agricultural pests may change in distribution (IPCC 1992) (see “Food and agriculture”). Considering complex ecosystem dynamics, complete assessment will need to extend beyond the direct impacts of changing atmospheric and/or soil conditions.
Health Effects of Weather Disasters and Sea Level Rise
Thermal expansion of oceans may cause sea level to rise at a relatively rapid rate of two to four centimetres per decade, and projected extremes of the hydrologic cycle are expected to produce more severe weather patterns and storms. Such events would directly disrupt dwellings and public health infrastructures, such as sanitation systems and stormwater drainage (IPCC 1992). Vulnerable populations in low-lying coastal areas and small islands would be forced to migrate to safer locations. Resulting overcrowding and poor sanitation among these environmental refugees could amplify the spread of infectious diseases such as cholera, and vector-borne disease transmission rates would escalate due to crowding and potential influxes of infected individuals (WHO 1990d). Flooded drainage systems may further exacerbate the situation, and psychological impacts must also be considered from post-traumatic stress syndrome following major storms.
Fresh water supply would diminish due to saline intrusion of coastal aquifers and coastal farmland lost to salination or outright inundation. For example, a sea-level rise of one metre would destroy 15% and 20% of agriculture in Egypt and Bangladesh respectively (IPCC 1990). As for droughts, adaptive irrigation methods could affect arthropod and invertebrate breeding sites of vectors (e.g., similar to schistosomiasis in Egypt), but cost/benefit evaluation of such impacts will be difficult.
Health Effects of Stratospheric Ozone Depletion
Direct health effects of ultravioletB radiation
Ozone specifically blocks the penetration of ultravioletB radiation, which contains the most biologically destructive wavelengths of 290-320 nanometres. UVB induces the formation of pyrimidine dimers within DNA molecules, which if unrepaired can evolve to cancer (IARC 1992). Non-melanoma skin cancer (squamous and basal cell carcinoma) and superficial spreading melanoma are correlated with sunlight exposure. In Western populations, melanoma incidence has increased by 20 to 50% every five years over the past two decades (Coleman et al. 1993). While there is no direct relationship between cumulative ultraviolet exposure and melanoma, excessive UV exposure during childhood is associated with incidence. For a sustained 10% decline in the stratospheric ozone layer, non-melanoma skin cancer cases could rise by 26%, or 300,000 globally per year; melanoma could increase by 20%, or 4,500 more cases annually (UNEP 1991a).
Eye cataract formation causes half of the world’s blindness (17 million cases annually) and is associated with UVB radiation in a dose-response relationship (Taylor 1990). Amino acids and membrane transport systems in the lens of the eye are especially prone to photo oxidation by oxygen radicals generated by UVB irradiation (IARC 1992). A doubling of UVB exposure could cause a 60% increase in cortical cataracts over current levels (Taylor et al. 1988). UNEP estimates that a 10% sustained loss of stratospheric ozone would result in nearly 1.75 million extra cataracts annually (UNEP 1991a). Other ocular effects of UVB exposure include photokeratitis, photokerato-conjunctivitis, pinguecula and pterygium (or overgrowth of the conjunctival epithelium) and climatic droplet keratopathy (IARC 1992).
The ability of the immune system to function effectively depends on “local” antigen processing and presentation to T-cells, as well as augmentation of the “systemic” response via lymphokine (biochemical messenger) production and resultant T-helper/T-suppressor cell ratios. UVB causes immunosuppression at both levels. UVB in animal studies can affect the course of infectious skin diseases, such as onchocerciasis, leishmaniasis and dermatophytosis, and impair immunosurveillance of transformed, precancerous epidermal cells. Preliminary studies further show an influence on vaccine efficacy (Kripke and Morison 1986; IARC 1992).
Indirect public health effects of UVB
Historically, terrestrial plants became established only after the formation of the shielding ozone layer, since UVB inhibits photosynthesis (UNEP 1991a). Weakening of food crops susceptible to UVB damage could further extend the impacts on agriculture due to climate changes and sea-level rise.
Phytoplankton are at the foundation of the marine food chain and also serve as an important carbon dioxide “sink”. UV damage to these algae in polar regions, therefore, would detrimentally affect the marine food chain and exacerbate the greenhouse effect. UNEP estimates that a 10% loss of marine phytoplankton would limit the oceans’ annual CO2 uptake by five gigatonnes, which equals the yearly anthropogenic emissions from fossil fuel combustion (UNEP 1991a).
Occupational Hazards and Control Strategies
Occupational hazards
With regard to reduction in GHG emissions from fossil fuels, alternate renewable energy sources will need to be expanded. The public and occupational hazards of nuclear energy are well known, and safeguarding plants, workers and spent fuel will be necessary. Methanol may serve to replace much gasoline usage; however, formaldehyde emission from these sources will present a new environmental hazard. Superconducting materials for energy efficient electricity transfer are mostly ceramics comprised of calcium, strontium, barium, bismuth, thallium and yttrium (WHO in press).
Less is known about the occupational safety in the manufacturing units for solar energy capture. Silicon, gallium, indium, thallium, arsenic and antimony are the primary elements used to build photovoltaic cells (WHO in press). Silicon and arsenic adversely affect the lungs; gallium is concentrated in the kidney, liver, and bone; and ionic forms of indium are nephrotoxic.
The destructive effects of CFCs on the stratospheric ozone layer were recognized in the 1970s, and the US EPA banned these inert propellants in aerosols in 1978. By 1985, widespread concern erupted when an Antarctic-based British team discovered the “hole” in the ozone layer (Farman, Gardiner and Shanklin 1985). Subsequent passage of the Montreal Protocol in 1987, with amendments in 1990 and 1992, has already mandated sharp cuts in CFC production.
The replacement chemicals for CFCs are the hydrochlorofluorocarbons (HCFCs) and the hydrofluorocarbons (HFCs). The presence of the hydrogen atom may more readily subject these compounds to degradation by hydroxyl radicals (OH–) in the troposphere, thus reducing potential stratospheric ozone depletion. These CFC replacement chemicals are, however, more biologically reactive than CFCs. The nature of a C-H bond makes these chemicals prone to oxidation via the cytochrome P-450 system (WHO in press).
Mitigation and adaptation
Meeting the public health challenges presented by global climate change will require (1) an integrated ecological approach; (2) reduction of greenhouse gases through industrial emission control, land use policies to maximize the extent of CO2 “sinks” and population policies to achieve both; (3) monitoring of biological indicators on both regional and global scales; (4) adaptive public health strategies to minimize the impacts from unavoidable climate change; and (5) cooperation between developed and developing nations. In short, increased integration of environmental and public health policies must be promoted.
Climate change and ozone depletion present a vast number of health risks at multiple levels and underscore the important relationship between ecosystem dynamics and sustained human health. Preventive measures must therefore be systems based, and must anticipate significant ecological responses to climate change as well as the direct physical hazards predicted. Some key elements to consider in an ecological risk assessment will include spatial and temporal variations, feedback mechanisms and use of lower level organisms as early biological indicators.
Reduction of greenhouse gases by diverting from fossil fuels to renewable energy resources represents primary prevention of climate change. Similarly, strategic land use planning and stabilization of population stress on the environment will preserve important natural greenhouse gas sinks.
Because some climate change may be unavoidable, secondary prevention through early detection by monitoring of health parameters will require unprecedented coordination. For the first time in history, attempts are being made to monitor the earth system in its entirety. The Global Climate Observing System incorporates the World Weather Watch and Global Atmosphere Watch of the World Meteorological Organization (WMO) with parts of UNEP’s Global Environmental Monitoring System. The Global Ocean Observing System is a new joint endeavour by the Intergovernmental Oceanographic Commission of UN Educational, Scientific and Cultural Organization (UNESCO), WMO and the International Council of Scientific Unions (ICSU). Both satellite and underwater measurements will be utilized to monitor changes in marine systems. The Global Terrestrial Observing System is a new system sponsored by UNEP, UNESCO, WMO, ICSU and the Food and Agricultural Organization (FAO), and will provide the terrestrial component of the Global Climate Observing System (WMO 1992).
Adaptive options to reduce unavoidable health consequences include disaster preparedness programmes; urban planning to reduce the “heat island” effect and improve housing; land use planning to minimize erosion, flash flooding and unnecessary deforestation (e.g., halting the creation of rangeland for meat exportation); personal adaptive behaviours, such as avoiding sun exposure; and vector-control and expanded vaccination efforts. Unintended costs of adaptive control measures of, for example, increased pesticide use will require consideration. Over-dependence on pesticides not only leads to insect resistance but also eliminates natural, beneficial, predatory organisms. The adverse effect on public health and the environment due to current pesticide use is estimated to be between US$100 billion and US$200 billion annually (Institute of Medicine 1991).
Developing countries will suffer disproportionately more from the consequences of climate change, though industrialized nations are presently more responsible for GHGs in the atmosphere. In the future poorer countries will influence the course of global warming significantly more, both through the technologies they choose to adopt as their development accelerates, and by land use practices. Developed nations will need to embrace more environmentally sound energy policies and promptly transfer new (and affordable) technology to developing countries.
Case Study: Mosquito-borne viruses
Mosquito-borne encephalitis and dengue fever are prime examples of vector-borne diseases whose distributions are limited by climate. Epidemics of St. Louis encephalitis (SLE), the most common arboviral encephalitis in the United States, generally occur south of the 22°C June isotherm, but northerly outbreaks have occurred during unseasonably warm years. Human outbreaks are highly correlated with several-day periods when temperature exceeds 27°C (Shope 1990).
Field studies on SLE indicate that a 1°C increase in temperature significantly shortens the elapsed time between a mosquito blood-meal and viral replication to the point of infectivity within the vector, or the extrinsic incubation period. Adjusting for reduced adult mosquito survival at elevated temperatures, a 3 to 5 °C temperature increase is predicted to cause a significant northern shift of SLE outbreaks (Reeves et al. 1994).
The range of the primary mosquito vector of dengue (and yellow fever), Aedes aegypti, extends to 35° latitude because freezing temperatures kill both larvae and adults. Dengue is widespread in the Caribbean, tropical America, Oceania, Asia, Africa and Australia. Over the past 15 years, dengue epidemics have increased in both numbers and severity, especially in tropical urban centres. Dengue haemorrhagic fever now ranks as one of the leading causes for hospitalization and mortality of children in Southeast Asia (Institute of Medicine 1992). The same increasing pattern observed in Asia 20 years ago is now occurring in the Americas.
Climate change can potentially alter dengue transmission. In Mexico in 1986, the most important predictor of dengue transmission was found to be the median temperature during the rainy season, with an adjusted fourfold risk observed between 17 °C and 30 °C (Koopman et al. 1991). Lab studies support these field data. In vitro, the extrinsic incubation period for dengue type-2 virus was 12 days at 30 °C and only seven days at 32 to 35 °C (Watts et al. 1987). This temperature effect of shortening the incubation period by five days translates to a potentially threefold higher transmission rate of disease (Koopman et al. 1991). Finally, warmer temperatures result in the hatching of smaller adults, which must bite more frequently to develop an egg batch. In summary, increased temperatures can lead to more infectious mosquitoes that bite more frequently (Focks et al. 1995).
