Contents Page
CONTENTS
Chapter Editor Gunnar Nordberg
- General Profile
- Acknowledgements
- Aluminium
- Antimony
- Arsenic
- Barium
- Bismuth
- Cadmium
- Chromium
- Copper
- Iron
- Gallium
- Germanium
- Indium
- Iridium
- Lead
- Magnesium
- Manganese
- Metal Carbonyls (especially Nickel Carbonyl)
- Mercury
- Molybdenum
- Nickel
- Niobium
- Osmium
- Palladium
- Platinum
- Rhenium
- Rhodium
- Ruthenium
- Selenium
- Silver
- Tantalum
- Tellurium
- Thallium
- Tin
- Titanium
- Tungsten
- Vanadium
- Zinc
- Zirconium and Hafnium
CARDIOVASCULAR MORBIDITY AND MORTALITY IN THE WORKFORCE
In the following article, the term cardiovascular diseases (CVDs) refers to organic and functional disorders of the heart and circulatory system, including the resultant damage to other organ systems, which are classified under numbers 390 to 459 in the 9th revision of the International Classification of Diseases (ICD) (World Health Organization (WHO) 1975). Based essentially on international statistics assembled by the WHO and data collected in Germany, the article discusses the prevalence of CVDs, new disease rates, and frequency of deaths, morbidity and disability.
Definition and Prevalence in the Working-Age Population
Coronary artery disease (ICD 410-414) resulting in ischaemia of the myocardium is probably the most significant CVD in the working population, particularly in industrialized countries. This condition results from a constriction in the vascular system that supplies the heart muscle, a problem caused primarily by arteriosclerosis. It affects 0.9 to 1.5% of working-age men and 0.5 to 1.0% of women.
Inflammatory diseases (ICD 420-423) may involve the endocardium, the heart valves, the pericardium and/or the heart muscle (myocardium) itself. They are less common in industrialized countries, where their frequency is well below 0.01% of the adult population, but are seen more frequently in developing countries, perhaps reflecting the greater prevalence of nutritional disorders and infectious diseases.
Heart rhythm disorders (ICD 427) are relatively rare, although much media attention has been given to recent instances of disability and sudden death among prominent professional athletes. Although they can have a significant impact on the ability to work, they are often asymptomatic and transitory.
The myocardiopathies (ICD 424) are conditions which involve enlargement or thickening of the heart musculation, effectively narrowing the vessels and weakening the heart. They have attracted more attention in recent years, largely because of improved methods of diagnosis, although their pathogenesis is often obscure. They have been attributed to infections, metabolic diseases, immunologic disorders, inflammatory diseases involving the capillaries and, of particular importance in this volume, to toxic exposures in the workplace. They are divided into three types:
- dilative—the most common form (5 to 15 cases per 100,000 people), which is associated with the functional weakening of the heart
- hypertrophic—thickening and enlargement of the myocardium resulting in relative insufficiency of the coronary arteries
- restrictive—a rare type in which myocardial contractions are limited.
Hypertension (ICD 401-405) (increased systolic and/or diastolic blood pressure) is the most common circulatory disease, being found among 15 to 20% of working people in industrialized countries. It is discussed in greater detail below.
Atherosclerotic changes in the major blood vessels (ICD 440), often associated with hypertension, cause disease in the organs they serve. Foremost among these is cerebrovascular disease (ICD 430-438), which may result in a stroke due to infarction and/or haemorrhage. This occurs in 0.3 to 1.0% of working people, most commonly among those aged 40 and older.
Atherosclerotic diseases, including coronary artery disease, stroke and hypertension, by far the most common cardiovascular diseases in the working population, are multifactorial in origin and have their onset early in life. They are of importance in the workplace because:
- so large a proportion of the workforce has an asymptomatic or unrecognized form of cardiovascular disease
- the development of that disease may be aggravated or acute symptomatic events precipitated by working conditions and job demands
- the acute onset of a symptomatic phase of the cardiovascular disease is often attributed to the job and/or the workplace environment
- most individuals with an established cardiovascular disease are capable of working productively, albeit, sometimes, only after effective rehabilitation and job retraining
- the workplace is a uniquely propitious arena for primary and secondary preventive programmes.
Functional circulatory disorders in the extremities (ICD 443) include Raynaud’s disease, short-term pallor of the fingers, and are relatively rare. Some occupational conditions, such as frostbite, long-term exposure to vinyl chloride and hand-arm exposure to vibration can induce these disorders.
Varicosities in the leg veins (ICD 454), often improperly dismissed as a cosmetic problem, are frequent among women, especially during pregnancy. While a hereditary tendency to weakness of the vein walls may be a factor, they are usually associated with long periods of standing in one position without movement, during which the static pressure within the veins is increased. The resultant discomfort and leg oedema often dictate change or modification of the job.
Annual incidence rates
Among the CVDs, hypertension has the highest annual new case rate among working people aged 35 to 64. New cases develop in approximately 1% of that population every year. Next in frequency are coronary heart disease (8 to 92 new cases of acute heart attack per 10,000 men per year, and 3 to 16 new cases per 10,000 women per year) and stroke (12 to 30 cases per 10,000 men per year, and 6 to 30 cases per 10,000 women per year). As demonstrated by global data collected by the WHO-Monica project (WHO-MONICA 1994; WHO-MONICA 1988), the lowest new incidence rates for heart attack were found among men in China and women in Spain, while the highest rates were found among both men and women in Scotland. The significance of these data is that in the population of working age, 40 to 60% of heart attack victims and 30 to 40% of stroke victims do not survive their initial episodes.
Mortality
Within the primary working ages of 15 to 64, only 8 to 18% of deaths from CVDs occur prior to age 45. Most occur after age 45, with the annual rate increasing with age. The rates, which have been changing, vary considerably from country to country (WHO 1994b).
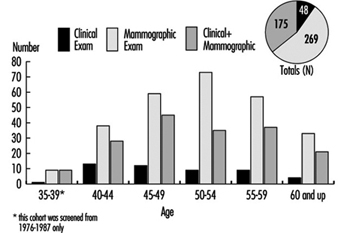
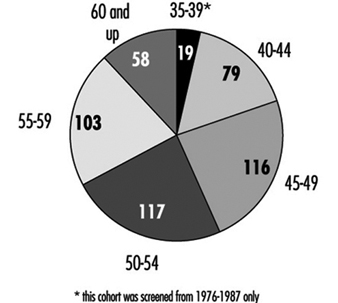
Table 3.1 [CAR01TE] shows the death rates for men and for women aged 45 to 54 and 55 to 64 for some countries. Note that the death rates for men are consistently higher than those for women of corresponding ages. Table 3.2 [CAR02TE] compares the death rates for various CVDs among people aged 55 to 64 in five countries.
Work Disability and Early Retirement
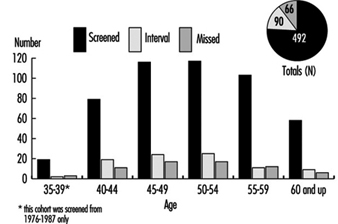
Diagnosis-related statistics on time lost from work represent an important perspective on the impact of morbidity on the working population, even though the diagnostic designations are usually less precise than in cases of early retirement because of disability. The case rates, usually expressed in cases per 10,000 employees, provide an index of the frequency of the disease categories, while the average number of days lost per case indicates the relative seriousness of particular diseases. Thus, according to statistics on 10 million workers in western Germany compiled by the Allgemeinen Ortskrankenkasse, CVDs accounted for 7.7% of the total disability in 1991-92, although the number of cases for that period was only 4.6% of the total (table 3.3 [CAR03TE]). In some countries, where early retirement is provided when work ability is reduced due to illness, the pattern of disability mirrors the rates for different categories of CVD.
Traumatic Head Injuries
Aetiological Factors
Head trauma consists of skull injury, focal brain injury and diffuse brain tissue injury (Gennarelli and Kotapa 1992). In work-related head trauma falls account for the majority of the causes (Kraus and Fife 1985). Other job-related causes include being struck by equipment, machinery or related items, and by on-road motor vehicles. The rates of work-related brain injury are markedly higher among young workers than older ones (Kraus and Fife 1985).
Occupations at Risk
Workers involved in mining, construction, driving motor vehicles and agriculture are at higher risk. Head trauma is common in sportsmen such as boxers and soccer players.
Neuropathophysiology
Skull fracture can occur with or without damage to the brain. All forms of brain injury, whether resulting from penetrating or closed head trauma, can lead to the development of swelling of the cerebral tissue. Vasogenic and cytogenic pathophysiologic processes active at the cellular level result in cerebral oedema, increased intracranial pressure and cerebral ischaemia.
Focal brain injuries (epidural, subdural or intracranial haematomas) may cause not only local brain damage, but a mass effect within the cranium, leading to midline shift, herniation and ultimately brain stem (mid-brain, pons and medulla oblongata) compression, causing, first a declining level of consciousness, then respiratory arrest and death (Gennarelli and Kotapa 1992).
Diffuse brain injuries represent shearing trauma to innumerable axons of the brain, and may be manifested as anything from subtle cognitive dysfunction to severe disability.
Epidemiological Data
There are few reliable statistics on the incidence of head injury from work-related activities.
In the United States, estimates of the incidence of head injury indicate that at least 2 million people incur such injuries each year, with nearly 500,000 resultant hospital admissions (Gennarelli and Kotapa 1992). Approximately half of these patients were involved in motor accidents.
A study of brain injury in residents of San Diego County, California in 1981 showed that the overall work-related injury rate for males was 19.8 per 100,000 workers (45.9 per 100 million work hours). The incidence rates of work-related brain injuries for male civilian and military personnel were 15.2 and 37.0 per 100,000 workers, respectively. In addition, the annual incidence of such injuries was 9.9 per 100 million work hours for males in the work force (18.5 per 100 million hours for military personnel and 7.6 per 100 million hours for civilians) (Kraus and Fife 1985). In the same study, about 54% of the civilian work-related brain injuries resulted from falls, and 8% involved on-road motor vehicle accidents (Kraus and Fife 1985).
Signs and Symptoms
The signs and symptoms vary among different forms of head trauma (table 1) (Gennarelli and Kotapa 1992) and different locations of traumatic brain lesion (Gennarelli and Kotapa 1992; Gorden 1991). On some occasions, multiple forms of head trauma may occur in the same patient.
Table 1. Classification of traumatic head injuries.
|
Skull injuries |
Brain tissue injuries |
|
|
Focal |
Diffuse |
|
|
Vault fracture |
Haematoma |
Concussion |
|
Linear |
Epidural |
Mild |
|
Depressed |
Subdural |
Classical |
|
Basilar fracture |
Contusion |
Prolonged coma (diffuse axonal injury) |
Skull injuries
Fractures of cerebral vault, either linear or depressed, can be detected by radiological examinations, in which the location and depth of the fracture are clinically most important.
Fractures of the skull base, in which the fractures are usually not visible on conventional skull radiographs, can best be found by computed tomography (CT scan). It can also be diagnosed by clinical findings such as the leakage of cerebropinal fluid from the nose (CSF rhinorrhea) or ear (CSF otorrhea), or subcutaneous bleeding at the periorbital or mastoid areas, though these may take 24 hours to appear.
Focal brain tissue injuries (Gennarelli and Kotapa 1992;Gorden 1991)
Haematoma:
Epidural haematoma is usually due to arterial bleeding and may be associated with a skull fracture. The bleeding is seen distinctly as a biconvex density on the CT scan. It is characterized clinically by transient loss of consciousness immediately after injury, followed by a lucid period. Consciousness may deteriorate rapidly due to increasing intracranial pressure.
Subdural haematoma is the result of venous bleeding beneath the dura. Subdural haemorrhage may be classified as acute, subacute or chronic, based on the time course of the development of symptoms. Symptoms result from direct pressure to the cortex under the bleed. The CT scan of the head often shows a crescent-shaped deficit.
Intracerebral haematoma results from bleeding within the parenchyma of the cerebral hemispheres. It may occur at the time of trauma or may appear a few days later (Cooper 1992). Symptoms are usually dramatic and include an acutely depressed level of consciousness and signs of increased intracranial pressure, such as headache, vomiting, convulsions and coma. Subarachnoid haemorrhage may occur spontaneously as the result of a ruptured berry aneurysm, or it may be caused by head trauma.
In patients with any form of haematoma, deterioration of consciousness, ipsilateral dilated pupil and contralateral haemiparesis suggests an expanding haematoma and the need for immediate neurosurgical evaluation. Brain stem compression accounts for approximately 66% of deaths from head injuries (Gennarelli and Kotapa 1992).
Cerebral contusion:
This presents as temporary loss of consciousness or neurologic deficits. Memory loss may be retrograde—loss of memory a time period before the injury, or antegrade—loss of current memory. CT scans shows multiple small isolated haemorrhages in the cerebral cortex. Patients are at higher risk of subsequent intracranial bleeding.
Diffuse brain tissue injuries (Gennarelli and Kotapa 1992;Gorden 1991)
Concussion:
Mild concussion is defined as a rapidly resolving (less than 24 hours) interruption of function (such as memory), secondary to trauma. This includes symptoms as subtle as memory loss and as obvious as unconsciousness.
Classic cerebral concussion manifests as slowly resolving, temporary, reversible neurologic dysfunction such as memory loss, often accompanied by a significant loss of consciousness (more than 5 minutes, less than 6 hours). The CT scan is normal.
Diffuse axonal injury:
This results in a prolonged comatose state (more than 6 hours). In the milder form, the coma is of 6 to 24 hours duration, and may be associated with long-standing or permanent neurologic or cognitive deficits. A coma of moderate form lasts for more than 24 hours and is associated with a mortality of 20%. The severe form shows brain stem dysfunction with the coma lasting for more than 24 hours or even months, because of the involvement of the reticular activating system.
Diagnosis and Differential Diagnosis
Apart from the history and serial neurologic examinations and a standard assessment tool such as the Glasgow Coma Scale (table 2), the radiological examinations are helpful in making a definitive diagnosis. A CT scan of the head is the most important diagnostic test to be performed in patients with neurologic findings after head trauma (Gennarelli and Kotapa 1992; Gorden 1991; Johnson and Lee 1992), and allows rapid and accurate assessment of surgical and nonsurgical lesions in the critically injured patients (Johnson and Lee 1992). Magnetic resonance (MR) imaging is complementary to the evaluation of cerebral head trauma. Many lesions are identified by MR imaging such as cortical contusions, small subdural haematomas and diffuse axonal injuries that may not be seen on CT examinations (Sklar et al. 1992).
Table 2. Glasgow Coma Scale.
|
Eyes |
Verbal |
Motor |
|
Does not open eyes |
Makes no noise |
(1) No motor response to pain |
Treatment and Prognosis
Patients with head trauma need to be referred to an emergency department, and a neurosurgical consultation is important. All patients known to be unconscious for more than 10 to 15 minutes, or with a skull fracture or a neurologic abnormality, require hospital admission and observation, because the possibility exists of delayed deterioration from expanding mass lesions (Gennarelli and Kotapa 1992).
Depending on the type and severity of head trauma, provision of supplemental oxygen, adequate ventilation, decrease of brain water by intravenous administration of faster-acting hyperosmolar agents (e.g., mannitol), corticosteroids or diuretics, and surgical decompression may be necessary. Appropriate rehabilitation is advisable at a later stage.
A multicentre study revealed that 26% of patients with severe head injury had good recovery, 16% were moderately disabled, and 17% were either severely disabled or vegetative (Gennarelli and Kotapa 1992). A follow-up study also found persistent headache in 79% of the milder cases of head injury, and memory difficulties in 59% (Gennarelli and Kotapa 1992).
Prevention
Safety and health education programmes for the prevention of work-related accidents should be instituted at the enterprise level for workers and management. Preventive measures should be applied to mitigate the occurrence and severity of head injuries due to work-related causes such as falls and transport accidents.
First Aid
First aid is the immediate care given to victims of accidents before trained medical workers arrive. Its goal is to stop and, if possible, reverse harm. It involves rapid and simple measures such as clearing the air passageway, applying pressure to bleeding wounds or dousing chemical burns to eyes or skin.
The critical factors which shape first aid facilities in a workplace are work-specific risk and availability of definitive medical care. The care of a high-powered saw injury is obviously radically different from that of a chemical inhalation.
From a first aid perspective, a severe thigh wound occurring near a surgical hospital requires little more than proper transport; for the same injury in a rural area eight hours from the nearest medical facility, first aid would include—among other things—debridement, tying off bleeding vessels and administration of tetanus immunoglobulin and antibiotics.
First aid is a fluid concept not only in what (how long, how complex) must be done, but in who can do it. Though a very careful attitude is required, every worker can be trained in the top five or ten do’s and don’ts of first aid. In some situations, immediate action can save life, limb or eyesight. Co-workers of victims should not remain paralyzed while waiting for trained personnel to arrive. Moreover, the “top-ten” list will vary with each workplace and must be taught accordingly.
Importance of First Aid
In cases of cardiac arrest, defibrillation administered within four minutes yields survival rates of 40 to 50%, versus less than 5% if given later. Five hundred thousand people die of cardiac arrest every year in the United States alone. For chemical eye injuries, immediate flushing with water can save eyesight. For spinal cord injuries, correct immobilization can make the difference between full recovery and paralysis. For haemorrhages, the simple application of a fingertip to a bleeding vessel can stop life-threatening blood loss.
Even the most sophisticated medical care in the world often cannot undo the effects of poor first aid.
First Aid in the Context of the GeneralOrganization of Health and Safety
The provision of first aid should always have a direct relationship to general health and safety organization, because first aid itself will not handle more than a small part of workers’ total care. First aid is a part of the total health care for workers. In practice, its application will depend to a large extent on persons present at the time of an accident, whether co-workers or formally trained medical personnel. This immediate intervention must be followed by specialized medical care whenever needed.
First aid and emergency treatment in cases of accident and indisposition of workers at the workplace are listed as an important part of the functions of the occupational health services in the ILO Occupational Health Services Convention (No. 161), Article 5, and the Recommendation of the same name. Both adopted in 1985, they provide for the progressive development of occupational health services for all workers.
Any comprehensive occupational safety and health programme should include first aid, which contributes to minimizing the consequences of accidents and is therefore one of the components of tertiary prevention. There is a continuum leading from the knowledge of the occupational hazards, their prevention, first aid, emergency treatment, further medical care and specialized treatment for reintegration into and readaptation to work. There are important roles that occupational health professionals can play along this continuum.
It is not infrequent that several small incidents or minor accidents take place before a severe accident occurs. Accidents requiring only first aid represent a signal which should be heard and used by the occupational health and safety professionals to guide and promote preventive action.
Relation to Other Health-Related Services
The institutions which may be involved in the organization of first aid and provide assistance following an accident or illness at work include the following:
- the occupational health service of the enterprise itself or other occupational health entities
- other institutions which may provide services, such as: ambulance services; public emergency and rescue services; hospitals, clinics and health centres, both public and private; private physicians; poison centres; civil defence; fire departments; and police.
Each of these institutions has a variety of functions and capabilities, but it must be understood that what applies to one type of institution—say a poison centre—in one country, may not necessarily apply to a poison centre in another country. The employer, in consultation with, for example, the factory physician or outside medical advisers, must ensure that the capabilities and facilities of neighbouring medical institutions are adequate to deal with the injuries expected in the event of serious accidents. This assessment is the basis for deciding which institutions will be entered into the referral plan.
The cooperation of these related services is very important in providing proper first aid, particularly for small enterprises. Many of them may provide advice on the organization of first aid and on planning for emergencies. There are good practices which are very simple and effective; for example, even a shop or a small enterprise may invite the fire brigade to visit its premises. The employer or owner will receive advice on fire prevention, fire control, emergency planning, extinguishers, the first aid box and so on. Conversely, the fire brigade will know the enterprise and will be ready to intervene more rapidly and efficiently.
There are many other institutions which may play a role, such as industrial and trade associations, safety associations, insurance companies, standards organizations, trade unions and other non-governmental organizations. Some of these organizations may be knowledgeable about occupational health and safety and can be a valuable resource in the planning and organization of first aid.
An Organized Approach to First Aid
Organization and planning
First aid cannot be planned in isolation. First aid requires an organized approach involving people, equipment and supplies, facilities, support and arrangements for the removal of victims and non-victims from the site of an accident. Organizing first aid should be a cooperative effort, involving employers, occupational health and public health services, the labour inspectorate, plant managers and relevant non-governmental organizations. Involving workers themselves is essential: they are often the best source on the likelihood of accidents in specific situations.
Whatever the degree of sophistication or the absence of facilities, the sequence of actions to be taken in the case of an unforeseen event must be determined in advance. This must be done taking due account of existing and potential occupational and non-occupational hazards or occurrences, as well as ways of obtaining immediate and appropriate assistance. Situations vary not only with the size of the enterprise but also with its location (in a town or a rural area) and with the development of the health system and of labour legislation at the national level.
As regards the organization of first aid, there are several key variables to be considered:
- type of work and associated level of risk
- potential hazards
- size and layout of the enterprise
- other enterprise characteristics (e.g., configuration)
- availability of other health services.
Type of work and associated level of risk
The risks of injury vary greatly from one enterprise and from one occupation to another. Even within a single enterprise, such as a metalworking firm, different risks exist depending on whether the worker is engaged in the handling and cutting of metal sheets (where cuts are frequent), welding (with the risk of burns and electrocution), the assembly of parts, or metal plating (which has the potential of poisoning and skin injury). The risks associated with one type of work vary according to many other factors, such as the design and age of the machinery used, the maintenance of the equipment, the safety measures applied and their regular control.
The ways in which the type of work or the associated risks influence the organization of first aid have been fully recognized in most legislation concerning first aid. The equipment and supplies required for first aid, or the number of first aid personnel and their training, may vary in accordance with the type of work and the associated risks. Countries use different models for classifying them for the purpose of planning first aid and deciding whether higher or lower requirements are to be set. A distinction is sometimes made between the type of work and the specific potential risks:
- low risk-for example, in offices or shops
- higher risk-for example, in warehouses, farms and in some factories and yards
- specific or unusual risks-for example, in steelmaking (especially when working on furnaces), coking, non-ferrous smelting and processing, forging, foundries; shipbuilding; quarrying, mining or other underground work; work in compressed air and diving operations; construction, lumbering and woodworking; abattoirs and rendering plants; transportation and shipping; most industries involving harmful or dangerous substances.
Potential hazards
Even in enterprises which seem clean and safe, many types of injury can occur. Serious injuries may result from falling, striking against objects or contact with sharp edges or moving vehicles. The specific requirements for first aid will vary depending on whether the following occur:
- falls
- serious cuts, severed limbs
- crushing injuries and entanglements
- high risks of spreading fire and explosions
- intoxication by chemicals at work
- other chemical exposure
- electrocution
- exposure to excessive heat or cold
- lack of oxygen
- exposure to infectious agents, animal bites and stings.
The above is only a general guide. The detailed assessment of the potential risks in the working environment helps greatly to identify the need for first aid.
Size and layout of the enterprise
First aid must be available in every enterprise, regardless of size, taking into account that the frequency rate of accidents is often inversely related to the size of the enterprise.
In larger enterprises, the planning and organization of first aid can be more systematic. This is because individual workshops have distinct functions and the workforce is more specifically deployed than in smaller enterprises. Therefore the equipment, supplies and facilities for first aid, and first aid personnel and their training, can normally be organized more precisely in response to the potential hazards in a large enterprise than in a smaller one. Nevertheless, first aid can also be effectively organized in smaller enterprises.
Countries use different criteria for the planning of first aid in accordance with the size and other characteristics of the enterprise. No general rule can be set. In the United Kingdom, enterprises with fewer than 150 workers and involving low risks, or enterprises with fewer than 50 workers with higher risks, are considered small, and different criteria for the planning of first aid are applied in comparison with enterprises where the number of workers present at work exceeds these limits. In Germany, the approach is different: whenever there are fewer than 20 workers expected at work one set of criteria would apply; if the number of workers exceeds 20, other criteria will be used. In Belgium, one set of criteria applies to industrial enterprises with 20 or fewer workers at work, a second to those with between 20 and 500 workers, and a third to those with 1,000 workers and more.
Other enterprise characteristics
The configuration of the enterprise (i.e., the site or sites where the workers are at work) is important to the planning and organization of first aid. An enterprise might be located at one site or spread over several sites either within a town or region, or even a country. Workers may be assigned to areas away from the enterprise’s central establishment, such as in agriculture, lumbering, construction or other trades. This will influence the provision of equipment and supplies, the number and distribution of first aid personnel, and the means for the rescue of injured workers and their transportation to more specialized medical care.
Some enterprises are temporary or seasonal in nature. This implies that some workplaces exist only temporarily or that in one and the same place of work some functions will be performed only at certain periods of time and may therefore involve different risks. First aid must be available whenever needed, irrespective of the changing situation, and planned accordingly.
In some situations employees of more than one employer work together in joint ventures or in an ad hoc manner such as in building and construction. In such cases the employers may make arrangements to pool their provision of first aid. A clear allocation of responsibilities is necessary, as well as a clear understanding by the workers of each employer as to how first aid is provided. The employers must ensure that the first aid organized for this particular situation is as simple as possible.
Availability of other health services
The level of training and the extent of organization for first aid is, in essence, dictated by the proximity of the enterprise to, and its integration with, readily available health services. With close, good backup, avoiding delay in transport or calling for help can be more crucial to a good outcome than is skilful application of medical manoeuvres. Each workplace’s first aid programme must mold itself to—and become an extension of—the medical facility which provides the definitive care for its injured workers.
Basic Requirements of a First Aid Programme
First aid must be considered part of sound management and making work safe. Experience in countries where first aid is strongly established suggests that the best way to ensure effective first aid provision is to make it mandatory by legislation. In countries which have chosen this approach, the main requirements are set out in specific legislation or, more commonly, in national labour codes or similar regulations. In these cases, subsidiary regulations contain more detailed provisions. In most cases, the overall responsibility of the employer for providing and organizing first aid is laid down in the basic enabling legislation. The basic elements of a first aid programme include the following:
Equipment, supplies and facilities
- equipment to rescue the victim at the site of the accident so as to prevent further harm (e.g., in the case of fire, gassing, electrocution)
- first aid boxes, first aid kits or similar containers, with a sufficient quantity of the materials and appliances required for the delivery of basic first aid
- specialized equipment and supplies which may be required in enterprises involving specific or unusual risks at work
- an adequately identified first aid room or a similar facility where first aid can be administered
- provision of means of evacuation and emergency transportation of the injured persons to the first aid facility or the sites where further medical care is available
- means of giving the alarm and communicating the alert
Human resources
- selection, training and retraining of suitable persons for administering first aid, their appointment and location at critical sites throughout the enterprise, and the assurance that they are permanently available and accessible
- retraining, including practical exercises simulating emergency situations, with due account given to specific occupational hazards in the enterprise
Other
- establishment of a plan, including links between the relevant health or public health services, with a view to the delivery of medical care following first aid
- education and information of all workers concerning the prevention of accidents and injuries, and the actions workers must themselves take following an injury (e.g., a shower immediately after a chemical burn)
- information on the arrangements for first aid, and the periodic updating of this information
- posting of information, visual guides (e.g., posters) and instruction about first aid, and plans with a view to the delivery of medical care after first aid
- record keeping (the first aid treatment record is an internal report which will provide information concerning the health of the victim, as well as contributing to safety at work; it should include information on: the accident (time, location, occurrence), the type and severity of the injury, the first aid delivered, the additional medical care requested, the name of the casualty and the names of witnesses and other workers involved, especially those transporting the casualty)
Although basic responsibility for implementing a first aid programme lies with the employer, without full participation of the workers, first aid cannot be effective. For example, workers may need to cooperate in rescue and first aid operations; they should thus be informed of first aid arrangements and should make suggestions, based on their knowledge of the workplace. Written instructions about first aid, preferably in the form of posters, should be displayed by the employer at strategic places within the enterprise. In addition, the employer should organize briefings for all workers. The following are essential parts of the briefing:
- the organization of first aid in the enterprise, including the procedure for access to additional care
- colleagues who have been appointed as first aid personnel
- ways in which information about an accident should be communicated, and to whom
- location of the first aid box
- location of the first aid room
- location of the rescue equipment
- what the workers must do in case of an accident
- location of the escape routes
- workers’ actions following an accident
- ways of supporting first aid personnel in their task.
First Aid Personnel
First aid personnel are persons on the spot, generally workers who are familiar with the specific conditions of work, and who might not be medically qualified but must be trained and prepared to perform very specific tasks. Not every worker is suitable to be trained for providing first aid. First aid personnel should be selected carefully, taking into account attributes such as reliability, motivation and the ability to cope with people in a crisis situation.
Type and number
National regulations for first aid vary with respect to both the type and number of first aid personnel required. In some countries the emphasis is on the number of persons employed in the workplace. In other countries, the overriding criteria are the potential risks at work. In yet others, both of these factors are taken into account. In countries with a long tradition of occupational safety and health practices and where the frequency of accidents is lower, more attention is usually given to the type of first aid personnel. In countries where first aid is not regulated, emphasis is normally placed on numbers of first aid personnel.
A distinction may be made in practice between two types of first aid personnel:
- the basic-level first-aider, who receives basic training as outlined below and who qualifies for appointment where the potential risk at work is low
- the advanced-level first-aider, who will receive the basic and advanced training and will qualify for appointment where the potential risk is higher, special or unusual.
The following four examples are indicative of the differences in approach used in determining the type and number of first aid personnel in different countries:
United Kingdom
- If the work involves relatively low hazards only, no first aid personnel are required unless there are 150 or more workers present at work; in this case a ratio of one first-aider per 150 workers is considered adequate. Even if fewer than 150 workers are at work, the employer should nevertheless designate an “appointed person” at all times when workers are present.
- Should the work involve higher risk, one first-aider will normally be required when the number of workers at work is between 50 and 150. If more than 150 workers are at work, one additional first-aider for every 150 will be required and, if the number of workers at work is less than 50, an “appointed person” should be designated.
- If the potential risk is unusual or special, there will be a need, in addition to the number of first aid personnel already required under the criteria set out above, for an additional person who will be trained specifically in first aid in case of accidents arising from these unusual or special hazards (the occupational first-aider).
Belgium
- One first-aider is usually required for every 20 workers present at work. However, a full-time occupational health staff member is required if there are special hazards and if the number of workers exceeds 500, or in the case of any enterprise where the number of workers on site is 1,000 or more.
- Some degree of flexibility is possible in accordance with particular situations.
Germany
- One first-aider is required if there are 20 or fewer workers present at work.
- If more than 20 workers are present, the number of first-aiders should be 5% of those at work in offices or general trade, or 10% in all other enterprises. Depending on other measures which may have been taken by the enterprise to deal with emergencies and accidents, these numbers may be revised.
- If work involves unusual or specific risks (for instance, if hazardous substances are involved), a special type of first aid personnel needs to be provided and trained; no specific number is stipulated for such personnel (i.e., the above-mentioned numbers apply).
- If more than 500 workers are present and if unusual or special hazards exist (burns, poisonings, electrocutions, impairment of vital functions such as respiratory or cardiac arrest), specially trained full-time personnel must be made available to deal with cases where a delay in arrival of no more than 10 minutes may be allowable. This provision will apply in most larger construction sites where a number of enterprises often employ a workforce of several hundred workers.
New Zealand
- If more than five workers are present, an employee is appointed and put in charge of the equipment, supplies and facilities for first aid.
- If more than 50 persons are present, the person appointed must be either a registered nurse or hold a certificate (issued by the St. John’s Ambulance Association or the New Zealand Red Cross Society).
Training
The training of first aid personnel is the single most important factor determining the effectiveness of organized first aid. Training programmes will depend on the circumstances within the enterprise, especially the type of work and the risks involved.
Basic Training
Basic training programmes are usually on the order of 10 hours. This is a minimum. Programmes can be divided into two parts, dealing with the general tasks to be performed and the actual delivery of first aid. They will cover the areas listed below.
General tasks
- how first aid is organized
- how to assess the situation, the magnitude and severity of the injuries and the need for additional medical help
- how to protect the casualty against further injury without creating a risk for oneself; the location and use of the rescue equipment
- how to observe and interpret the victim’s general condition (e.g., unconsciousness, respiratory and cardiovascular distress, bleeding)
- the location, use and maintenance of first aid equipment and facilities
- the plan for access to additional care.
Delivery of first aid
The objective is to provide basic knowledge and delivery of first aid. At the basic level, this includes, for example:
- wounds
- bleeding
- fractured bones or joints
- crushing injuries (e.g., to the thorax or abdomen)
- unconsciousness, especially if accompanied by respiratory difficulties or arrest
- eye injuries
- burns
- low blood pressure, or shock
- personal hygiene in dealing with wounds
- care of amputated digits.
Advanced Training
The aim of advanced training is specialization rather than comprehensiveness. It is of particular importance in relation to the following types of situation (though specific programmes normally deal only with some of these, in accordance with needs, and their duration varies considerably):
- cardiopulmonary resuscitation
- poisoning (intoxication)
- injuries caused by electric current
- severe burns
- severe eye injuries
- skin injuries
- contamination by radioactive material (internal, and skin or wound contamination)
- other hazard-specific procedures (e.g., heat and cold stress, diving emergencies).
Training Materials and Institutions
A wealth of literature is available on training programmes for first aid. The national Red Cross and Red Crescent Societies and various organizations in many countries have issued material which covers much of the basic training programme. This material should be consulted in the design of actual training programmes, though it may need adaptation to the specific requirements of first aid at work (in contrast with first aid after traffic accidents, for instance).
Training programmes should be approved by the competent authority or a technical body authorized to do so. In many cases, this may be the national Red Cross or Red Crescent Society or related institutions. Sometimes safety associations, industrial or trade associations, health institutions, certain non-governmental organizations and the labour inspectorate (or their subsidiary bodies) may contribute to the design and provision of the training programme to suit specific situations.
This authority should also be responsible for testing first aid personnel upon completion of their training. Examiners independent of the training programmes should be designated. Upon successfully completing the examination, the candidates should be awarded a certificate upon which the employer or enterprise will base their appointment. Certification should be made obligatory and should also follow refresher training, other instruction or participation in field work or demonstrations.
First Aid Equipment, Supplies and Facilities
The employer is responsible for providing first aid personnel with adequate equipment, supplies and facilities.
First aid boxes, first aid kits and similar containers
In some countries, only the principal requirements are set out in regulations (e.g., that adequate amounts of suitable materials and appliances are included, and that the employer must determine what precisely may be required, depending on the type of work, the associated risks and the configuration of the enterprise). In most countries, however, more specific requirements have been set out, with some distinction made as to the size of the enterprise and the type of work and potential risks involved.
Basic content
The contents of these containers must obviously match the skills of the first aid personnel, the availability of a factory physician or other health personnel and the proximity of an ambulance or emergency service. The more elaborate the tasks of the first aid personnel, the more complete must be the contents of the containers. A relatively simple first aid box will usually include the following items:
- individually wrapped sterile adhesive dressing
- bandages (and pressure dressings, where appropriate)
- a variety of dressings
- sterile sheets for burns
- sterile eye pads
- triangular bandages
- safety pins
- a pair of scissors
- antiseptic solution
- cotton wool balls
- a card with first aid instructions
- sterile plastic bags
- access to ice.
Location
First aid boxes should always be easily accessible, near areas where accidents could occur. They should be able to be reached within one to two minutes. They should be made of suitable materials, and should protect the contents from heat, humidity, dust and abuse. They need to be identified clearly as first aid material; in most countries, they are marked with a white cross or a white crescent, as applicable, on a green background with white borders.
If the enterprise is subdivided into departments or shops, at least one first aid box should be available in each unit. However, the actual number of boxes required will be determined on the basis of the needs assessment made by the employer. In some countries the number of containers required, as well as their contents, has been established by law.
Auxiliary kits
Small first aid kits should always be available where workers are away from the establishment in such sectors as lumbering, agricultural work or construction; where they work alone, in small groups or in isolated locations; where work involves travelling to remote areas; or where very dangerous tools or pieces of machinery are used. The contents of such kits, which should also be readily available to self-employed persons, will vary according to circumstances, but they should always include:
- a few medium-sized dressings
- a bandage
- a triangular bandage
- safety pins.
Specialized equipment and supplies
Further equipment may be needed for the provision of first aid where there are unusual or specific risks. For example, if poisonings are a possibility, antidotes must be immediately available in a separate container, though it must be made clear that their administration is subject to medical instruction. Long lists of antidotes exist, many for specific situations. Potential risks will determine which antidotes are needed.
Specialized equipment and material should always be located near the sites of potential accidents and in the first aid room. Transporting the equipment from a central location such as an occupational health service facility to the site of the accident may take too long.
Rescue equipment
In some emergency situations, specialized rescue equipment to remove or disentangle an accident victim may be necessary. Although it may not be easy to predict, certain work situations (such as working in confined spaces, at heights or above water) may have a high potential for this type of incident. Rescue equipment may include items such as protective clothing, blankets for fire-fighting, fire extinguishers, respirators, self-contained breathing apparatus, cutting devices and mechanical or hydraulic jacks, as well as equipment such as ropes, harnesses and specialized stretchers to move the victim. It must also include any other equipment required to protect the first aid personnel against becoming casualties themselves in the course of delivering first aid. Although initial first aid should be given before moving the patient, simple means should also be provided for transporting an injured or sick person from the scene of the accident to the first aid facility. Stretchers should always be accessible.
The first aid room
A room or a corner, prepared for administering first aid, should be available. Such facilities are required by regulations in many countries. Normally, first aid rooms are mandatory when there are more than 500 workers at work or when there is a potentially high or specific risk at work. In other cases, some facility must be available, even though this may not be a separate room—for example, a prepared corner with at least the minimum furnishings of a full-scale first aid room, or even a corner of an office with a seat, washing facilities and a first aid box in the case of a small enterprise. Ideally, a first aid room should:
- be accessible to stretchers and must have access to an ambulance or other means of transportation to a hospital
- be large enough to hold a couch, with space for people to work around it
- be kept clean, well ventilated, well lit and maintained in good order
- be reserved for the administration of first aid
- be clearly identified as a first aid facility, be appropriately marked and be under the responsibility of first aid personnel
- have clean running water, preferably both hot and cold, soap and a nail brush. If running water is not available, water should be kept in disposable containers near the first aid box for eye washing and irrigation
- include towels, pillows and blankets, clean clothing for use by the first aid personnel, and a refuse container.
Communication and Referral Systems
Means for communicating the alert
Following an accident or sudden illness, it is important that immediate contact be made with first aid personnel. This requires means of communication between work areas, the first aid personnel and the first aid room. Communications by telephone may be preferable, especially if distances are more than 200 metres, but this will not be possible in all establishments. Acoustic means of communication, such as a hooter or buzzer, may serve as a substitute as long as it can be assured that the first aid personnel arrive at the scene of the accident rapidly. Lines of communication should be pre-established. Requests for advanced or specialized medical care, or an ambulance or emergency service, are normally made by telephone. The employer should ensure that all relevant addresses, names and telephone numbers are clearly posted throughout the enterprise and in the first aid room, and that they are always available to the first aid personnel.
Access to additional care
The need for a referral of the victim to more advanced or specialized medical care must always be foreseen. The employer should have plans for such a referral, so that when the case arises everybody involved will know exactly what to do. In some cases the referral systems will be rather simple, but in others they may be elaborate, especially where unusual or special risks are involved at work. In the construction industry, for instance, referral may be required after serious falls or crushings, and the end point of referral will most probably be a general hospital, with adequate orthopaedic or surgical facilities. In the case of a chemical works, the end point of referral will be a poison centre or a hospital with adequate facilities for the treatment of poisoning. No uniform pattern exists. Each referral plan will be tailored to the needs of the enterprise under consideration, especially if higher, specific or unusual risks are involved. This referral plan is an important part of the enterprise’s emergency plan.
The referral plan must be supported by a system of communication and means for transporting the casualty. In some cases, this may involve communication and transport systems organized by the enterprise itself, especially in the case of larger or more complex enterprises. In smaller enterprises, transport of the casualty may need to rely on outside capacity such as public transport systems, public ambulance services, taxis and so on. Stand-by or alternative systems should be set up.
The procedures for emergency conditions must be communicated to everyone: workers (as part of their overall briefing on health and safety), first-aiders, safety officers, occupational health services, health facilities to which a casualty may be referred, and institutions which play a role in communications and the transport of the casualty (e.g., telephone services, ambulance services, taxi companies and so on).
Prevention
Occupational exposures account for only a minor proportion of the total number of cancers in the entire population. It has been estimated that 4% of all cancers can be attributed to occupational exposures, based on data from the United States, with a range of uncertainty from 2 to 8%. This implies that even total prevention of occupationally induced cancers would result in only a marginal reduction in national cancer rates.
However, for several reasons, this should not discourage efforts to prevent occupationally induced cancers. First, the estimate of 4% is an average figure for the entire population, including unexposed persons. Among people actually exposed to occupational carcinogens, the proportion of tumours attributable to occupation is much larger. Second, occupational exposures are avoidable hazards to which individuals are involuntarily exposed. An individual should not have to accept an increased risk of cancer in any occupation, especially if the cause is known. Third, occupationally induced cancers can be prevented by regulation, in contrast to cancers associated with lifestyle factors.
Prevention of occupationally induced cancer involves at least two stages: first, identification of a specific compound or occupational environment as carcinogenic; and second, imposing appropriate regulatory control. The principles and practice of regulatory control of known or suspected cancer hazards in the work environment vary considerably, not only among different parts of the developed and developing world, but also among countries of similar socio-economic development.
The International Agency for Research on Cancer (IARC) in Lyon, France, systematically compiles and evaluates epidemiological and experimental data on suspected or known carcinogens. The evaluations are presented in a series of monographs, which provide a basis for decisions on national regulations on the production and use of carcinogenic compounds (see “Occupational Carcinogens”, above.
Historical Background
The history of occupational cancer dates back to at least 1775, when Sir Percivall Pott published his classical report on scrotal cancer in chimney-sweeps, linking exposure to soot to the incidence of cancer. The finding had some immediate impact in that sweeps in some countries were granted the right to bathe at the end of the working day. Current studies of sweeps indicate that scrotal and skin cancer are now under control, although sweeps are still at increased risk for several other cancers.
In the 1890s, a cluster of bladder cancer was reported at a German dye factory by a surgeon at a nearby hospital. The causative compounds were later identified as aromatic amines, and these now appear in lists of carcinogenic substances in most countries. Later examples include skin cancer in radium-dial painters, nose and sinus cancer among woodworkers caused by inhalation of wood dust, and “mule-spinner’s disease”—that is, scrotal cancer among cotton industry workers caused by mineral oil mist. Leukaemia induced by exposure to benzene in the shoe repair and manufacturing industry also represents a hazard that has been reduced after the identification of carcinogens in the workplace.
In the case of linking asbestos exposure to cancer, this history illustrates a situation with a considerable time-lag between risk identification and regulatory action. Epidemiological results indicating that exposure to asbestos was associated with an increased risk of lung cancer were already starting to accumulate by the 1930s. More convincing evidence appeared around 1955, but it was not until the mid-1970s that effective steps for regulatory action began.
The identification of the hazards associated with vinyl chloride represents a different history, where prompt regulatory action followed identification of the carcinogen. In the 1960s, most countries had adopted an exposure limit value for vinyl chloride of 500 parts per million (ppm). In 1974, the first reports of an increased frequency of the rare tumour liver angiosarcoma among vinyl chloride workers were soon followed by positive animal experimental studies. After vinyl chloride was identified as carcinogenic, regulatory actions were taken for a prompt reduction of the exposure to the current limit of 1 to 5 ppm.
Methods Used for the Identificationof Occupational Carcinogens
The methods in the historical examples cited above range from observations of clusters of disease by astute clinicians to more formal epidemiological studies—that is, investigations of the disease rate (cancer rate) among human beings. Results from epidemiological studies are of high relevance for evaluations of the risk to humans. A major drawback of cancer epidemiological studies is that a long time period, usually at least 15 years, is necessary to demonstrate and evaluate the effects of an exposure to a potential carcinogen. This is unsatisfactory for surveillance purposes, and other methods must be applied for a quicker evaluation of recently introduced substances. Since the beginning of this century, animal carcinogenicity studies have been used for this purpose. However, the extrapolation from animals to humans introduces considerable uncertainty. The methods also have limitations in that a large number of animals must be followed for several years.
The need for methods with a more rapid response was partly met in 1971, when the short-term mutagenicity test (Ames test) was introduced. This test uses bacteria to measure the mutagenic activity of a substance (its ability to cause irreparable changes in the cellular genetic material, DNA). A problem in the interpretation of the results of bacterial tests is that not all substances causing human cancers are mutagenic, and not all bacterial mutagens are considered to be cancer hazards for human beings. However, the finding that a substance is mutagenic is usually taken as an indication that the substance might represent a cancer hazard for humans.
New genetic and molecular biology methods have been developed during the last 15 years, with the aim of detecting human cancer hazards. This discipline is termed “molecular epidemiology.” Genetic and molecular events are studied in order to clarify the process of cancer formation and thus develop methods for early detection of cancer, or indications of increased risk of the development of cancer. These methods include analysis of damage to the genetic material and the formation of chemical linkages (adducts) between pollutants and the genetic material. The presence of chromosomal aberrations clearly indicates effects on the genetic material which may be associated with cancer development. However, the role of molecular epidemiological findings in human cancer risk assessment remains to be settled, and research is under way to indicate more clearly exactly how results of these analyses should be interpreted.
Surveillance and Screening
The strategies for prevention of occupationally induced cancers differ from those applied for control of cancer associated with lifestyle or other environmental exposures. In the occupational field, the main strategy for cancer control has been reduction or total elimination of exposure to cancer-causing agents. Methods based on early detection by screening programmes, such as those applied for cervical cancer or breast cancer, have been of very limited importance in occupational health.
Surveillance
Information from population records on cancer rates and occupation may be used for surveillance of cancer frequencies in various occupations. Several methods to obtain such information have been applied, depending on the registries available. The limitations and possibilities depend largely on the quality of the information in the registries. Information on disease rate (cancer frequency) is typically obtained from local or national cancer registries (see below), or from death certificate data, while information on the age-composition and size of occupational groups is obtained from population registries.
The classical example of this type of information is the “Decennial supplements on occupational mortality,” published in the UK since the end of the nineteenth century. These publications use death certificate information on cause of death and on occupation, together with census data on frequencies of occupations in the entire population, to calculate cause-specific death rates in different occupations. This type of statistic is a useful tool to monitor the cancer frequency in occupations with known risks, but its ability to detect previously unknown risks is limited. This type of approach may also suffer from problems associated with systematic differences in the coding of occupations on the death certificates and in the census data.
The use of personal identification numbers in the Nordic countries has offered a special opportunity to link individual census data on occupations with cancer registration data, and to directly calculate cancer rates in different occupations. In Sweden, a permanent linkage of the censuses of 1960 and 1970 and the cancer incidence during subsequent years have been made available for researchers and have been used for a large number of studies. This Swedish Cancer-Environment Registry has been used for a general survey of certain cancers tabulated by occupation. The survey was initiated by a governmental committee investigating hazards in the work environment. Similar linkages have been performed in the other Nordic countries.
Generally, statistics based on routinely collected cancer incidence and census data have the advantage of ease in providing large amounts of information. The method gives information on the cancer frequencies regarding occupation only, not in relation to certain exposures. This introduces a considerable dilution of the associations, since exposure may differ considerably among individuals in the same occupation. Epidemiological studies of the cohort type (where the cancer experience among a group of exposed workers is compared with that in unexposed workers matched for age, sex and other factors) or the case-control type (where the exposure experience of a group of persons with cancer is compared to that in a sample of the general population) give better opportunities for detailed exposure description, and thus better opportunities for investigation of the consistency of any observed risk increase, for example by examining the data for any exposure-response trends.
The possibility of obtaining more refined exposure data together with routinely collected cancer notifications was investigated in a prospective Canadian case-control study. The study was set up in the Montreal metropolitan area in 1979. Occupational histories were obtained from males as they were added to the local cancer registry, and the histories were subsequently coded for exposure to a number of chemicals by occupational hygienists. Later, the cancer risks in relation to a number of substances were calculated and published (Siemiatycki 1991).
In conclusion, the continuous production of surveillance data based on recorded information provides an effective and comparatively easy way to monitor cancer frequency by occupation. While the main purpose achieved is surveillance of known risk factors, the possibilities for the identification of new risks are limited. Registry-based studies should not be used for conclusions regarding the absence of risk in an occupation unless the proportion of individuals significantly exposed is more precisely known. It is quite common that only a relatively small percentage of members of an occupation actually are exposed; for these individuals the substance may represent a substantial hazard, but this will not be observable (i.e., will be statistically diluted) when the entire occupational group is analysed as a single group.
Screening
Screening for occupational cancer in exposed populations for purposes of early diagnosis is rarely applied, but has been tested in some settings where exposure has been difficult to eliminate. For example, much interest has focused on methods for early detection of lung cancer among people exposed to asbestos. With asbestos exposures, an increased risk persists for a long time, even after cessation of exposure. Thus, continuous evaluation of the health status of exposed individuals is justified. Chest x rays and cytological investigation of sputum have been used. Unfortunately, when tested under comparable conditions neither of these methods reduces the mortality significantly, even if some cases may be detected earlier. One of the reasons for this negative result is that the prognosis of lung cancer is little affected by early diagnosis. Another problem is that the x rays themselves represent a cancer hazard which, while small for the individual, may be significant when applied to a large number of individuals (i.e., all those screened).
Screening also has been proposed for bladder cancer in certain occupations, such as the rubber industry. Investigations of cellular changes in, or mutagenicity of, workers’ urine have been reported. However, the value of following cytological changes for population screening has been questioned, and the value of the mutagenicity tests awaits further scientific evaluation, since the prognostic value of having increased mutagenic activity in the urine is not known.
Judgements on the value of screening also depend on the intensity of the exposure, and thus the size of the expected cancer risk. Screening might be more justified in small groups exposed to high levels of carcinogens than among large groups exposed to low levels.
To summarize, no routine screening methods for occupational cancers can be recommended on the basis of present knowledge. The development of new molecular epidemiological techniques may improve the prospects for early cancer detection, but more information is needed before conclusions can be drawn.
Cancer Registration
During this century, cancer registries have been set up at several locations throughout the world. The International Agency for Research on Cancer (IARC) (1992) has compiled data on cancer incidence in different parts of the world in a series of publications, “Cancer Incidence in Five Continents.” Volume 6 of this publication lists 131 cancer registries in 48 countries.
Two main features determine the potential usefulness of a cancer registry: a well-defined catchment area (defining the geographical area involved), and the quality and completeness of the recorded information. Many of those registries that were set up early do not cover a geographically well-defined area, but rather are confined to the catchment area of a hospital.
There are several potential uses of cancer registries in the prevention of occupational cancer. A complete registry with nationwide coverage and a high quality of recorded information can result in excellent opportunities for monitoring the cancer incidence in the population. This requires access to population data to calculate age-standardized cancer rates. Some registries also contain data on occupation, which therefore facilitates the monitoring of cancer risk in different occupations.
Registries also may serve as a source for the identification of cases for epidemiological studies of both the cohort and case-control types. In the cohort study, personal identification data of the cohort is matched to the registry to obtain information on the type of cancer (i.e., as in record linkage studies). This assumes that a reliable identifying system exists (for example, personal identification numbers in the Nordic countries) and that confidentiality laws do not prohibit use of the registry in this way. For case-control studies, the registry may be used as a source for cases, although some practical problems arise. First, the cancer registries cannot, for methodological reasons, be quite up to date regarding recently diagnosed cases. The reporting system, and necessary checks and corrections of the obtained information, results in some lag time. For concurrent or prospective case-control studies, where it is desirable to contact the individuals themselves soon after a cancer diagnosis, it usually is necessary to set up an alternative way of identifying cases, for example via hospital records. Second, in some countries, confidentiality laws prohibit the identification of potential study participants who are to be contacted personally.
Registries also provide an excellent source for calculating background cancer rates to use for comparison of the cancer frequency in cohort studies of certain occupations or industries.
In studying cancer, cancer registries have several advantages over mortality registries commonly found in many countries. The accuracy of the cancer diagnoses is often better in cancer registries than in mortality registries, which are usually based on death certificate data. Another advantage is that the cancer registry often holds information on histological tumour type, and also permits the study of living persons with cancer, and is not limited to deceased persons. Above all, registries hold cancer morbidity data, permitting the study of cancers that are not rapidly fatal and/or not fatal at all.
Environmental Control
There are three main strategies for reducing workplace exposures to known or suspected carcinogens: elimination of the substance, reduced exposure by reduced emission or improved ventilation, and personal protection of the workers.
It has long been debated whether a true threshold for carcinogen exposure exists, below which no risk is present. It is often assumed that the risk should be extrapolated linearly down to zero risk at zero exposure. If this is the case, then no exposure limit, no matter how low, would be considered entirely risk-free. Despite this, many countries have defined exposure limits for some carcinogenic substances, while, for others, no exposure limit value has been assigned.
Elimination of a compound may give rise to problems when replacement substances are introduced and when the toxicity of the replacement substance must be lower than that of the substance replaced.
Reducing the exposure at the source may be relatively easily accomplished for process chemicals by encapsulation of the process and ventilation. For example, when the carcinogenic properties of vinyl chloride were discovered, the exposure limit value for vinyl chloride was lowered by a factor of one hundred or more in several countries. Although this standard was at first considered impossible to achieve by industry, later techniques allowed compliance with the new limit. Reduction of exposure at the source may be difficult to apply to substances that are used under less controlled conditions, or are formed during the work operation (e.g., motor exhausts). The compliance with exposure limits requires regular monitoring of workroom air levels.
When exposure cannot be controlled either by elimination or by reduced emissions, the use of personal protection devices is the only remaining way to minimize the exposure. These devices range from filter masks to air-supplied helmets and protective clothing. The main route of exposure must be considered in deciding appropriate protection. However, many personal protection devices cause discomfort to the user, and filter masks introduce an increased respiratory resistance which may be very significant in physically demanding jobs. The protective effect of respirators is generally unpredictable and depends on several factors, including how well the mask is fitted to the face and how often filters are changed. Personal protection must be considered as a last resort, to be attempted only when more effective ways of reducing exposure fail.
Research Approaches
It is striking how little research has been done to evaluate the impact of programmes or strategies to reduce the risk to workers of known occupational cancer hazards. With the possible exception of asbestos, few such evaluations have been conducted. Developing better methods for control of occupational cancer should include an evaluation of how present knowledge is actually put to use.
Improved control of occupational carcinogens in the workplace requires the development of a number of different areas of occupational safety and health. The process of identification of risks is a basic prerequisite for reducing exposure to carcinogens in the workplace. Risk identification in the future must solve certain methodological problems. More refined epidemiological methods are required if smaller risks are to be detected. More precise data on exposure for both the substance under study and possible confounding exposures will be necessary. More refined methods for description of the exact dose of the carcinogen delivered to the specific target organ also will increase the power of exposure-response calculations. Today, it is not uncommon that very crude substitutes are used for the actual measurement of target organ dose, such as the number of years employed in the industry. It is quite clear that such estimates of dose are considerably misclassified when used as a surrogate for dose. The presence of an exposure-response relationship is usually taken as strong evidence of an aetiological relationship. However, the reverse, lack of demonstration of an exposure-response relationship, is not necessarily evidence that no risk is involved, especially when crude measures of target organ dose are used. If target organ dose could be determined, then actual dose-response trends would carry even more weight as evidence for causation.
Molecular epidemiology is a rapidly growing area of research. Further insight into the mechanisms of cancer development can be expected, and the possibility of the early detection of carcinogenic effects will lead to earlier treatment. In addition, indicators of carcinogenic exposure will lead to improved identification of new risks.
Development of methods for supervision and regulatory control of the work environment are as necessary as methods for the identification of risks. Methods for regulatory control differ considerably even among western countries. The systems for regulation used in each country depend largely on socio-political factors and the status of labour rights. The regulation of toxic exposures is obviously a political decision. However, objective research into the effects of different types of regulatory systems could serve as a guide for politicians and decision-makers.
A number of specific research questions also need to be addressed. Methods to describe the expected effect of withdrawal of a carcinogenic substance or reduction of exposure to the substance need to be developed (i.e., the impact of interventions must be assessed). The calculation of the preventive effect of risk reduction raises certain problems when interacting substances are studied (e.g., asbestos and tobacco smoke). The preventive effect of removing one of two interacting substances is comparatively greater than when the two have only a simple additive effect.
The implications of the multistage theory of carcinogenesis for the expected effect of withdrawal of a carcinogen also adds a further complication. This theory states that the development of cancer is a process involving several cellular events (stages). Carcinogenic substances may act either in early or late stages, or both. For example, ionizing radiation is believed to affect mainly early stages in inducing certain cancer types, while arsenic acts mainly at late stages in lung cancer development. Tobacco smoke affects both early and late stages in the carcinogenic process. The effect of withdrawing a substance involved in an early stage would not be reflected in a reduced cancer rate in the population for a long time, while the removal of a “late-acting” carcinogen would be reflected in a reduced cancer rate within a few years. This is an important consideration when evaluating the effects of risk-reduction intervention programmes.
Finally, the effects of new preventive factors have recently attracted considerable interest. During the last five years, a large number of reports have been published on the preventive effect on lung cancer of consuming fruits and vegetables. The effect seems to be very consistent and strong. For example, the risk of lung cancer has been reported as double among those with a low consumption of fruits and vegetables versus those with high intake. Thus, future studies of occupational lung cancer would have greater precision and validity if individual data on fruit and vegetable consumption can be included in the analysis.
In conclusion, improved prevention of occupational cancer involves both improved methods for risk identification and more research on the effects of regulatory control. For risk identification, developments in epidemiology should mainly be directed toward better exposure information, while in the experimental field, validation of the results of molecular epidemiological methods regarding cancer risk are needed.
Environmental Cancer
Cancer is a common disease in all countries of the world. The probability that a person will develop cancer by the age of 70 years, given survival to that age, varies between about 10 and 40% in both sexes. On average, in developed countries, about one person in five will die from cancer. This proportion is about one in 15 in developing countries. In this article, environmental cancer is defined as cancer caused (or prevented) by non-genetic factors, including human behaviour, habits, lifestyle and external factors over which the individual has no control. A stricter definition of environmental cancer is sometimes used, comprising only the effect of factors such as air and water pollution, and industrial waste.
Geographical Variation
Variation between geographical areas in the rates of particular types of cancer can be much greater than that for cancer as a whole. Known variation in the incidence of the more common cancers is summarized in table 1. The incidence of nasopharyngeal carcinoma, for example, varies some 500-fold between South East Asia and Europe. This wide variation in frequency of the various cancers has led to the view that much of human cancer is caused by factors in the environment. In particular, it has been argued that the lowest rate of a cancer observed in any population is indicative of the minimum, possibly spontaneous, rate occurring in the absence of causative factors. Thus the difference between the rate of a cancer in a given population and the minimum rate observed in any population is an estimate of the rate of the cancer in the first population which is attributable to environmental factors. On this basis it has been estimated, very approximately, that some 80 to 90% of all human cancers are environmentally determined (International Agency for Research on Cancer 1990).
Table 1. Variation between populations covered by cancer registration in the incidence of common cancers.1
|
Cancer (ICD9 code) |
High-incidence area |
CR2 |
Low-incidence area |
CR2 |
Range of variation |
|
Mouth (143-5) |
France, Bas Rhin |
2 |
Singapore (Malay) |
0.02 |
80 |
|
Nasopharynx (147) |
Hong Kong |
3 |
Poland, Warsaw (rural) |
0.01 |
300 |
|
Oesophagus (150) |
France, Calvados |
3 |
Israel (Israeli-born Jews) |
0.02 |
160 |
|
Stomach (151) |
Japan, Yamagata |
11 |
USA, Los Angeles (Filipinos) |
0.3 |
30 |
|
Colon (153) |
USA, Hawaii (Japanese) |
5 |
India, Madras |
0.2 |
30 |
|
Rectum (154) |
USA, Los Angeles (Japanese) |
3 |
Kuwait (non-Kuwaiti) |
0.1 |
20 |
|
Liver (155) |
Thailand, Khon Khaen |
11 |
Paraguay, Asuncion |
0.1 |
110 |
|
Pancreas (157) |
USA, Alameda County (Calif.) (Blacks) |
2 |
India, Ahmedabad |
0.1 |
20 |
|
Lung (162) |
New Zealand (Maori) |
16 |
Mali, Bamako |
0.5 |
30 |
|
Melanoma of skin (172) |
Australia, Capital Terr. |
3 |
USA, Bay Area (Calif.)(Blacks) |
0.01 |
300 |
|
Other skin cancers (173) |
Australia, Tasmania |
25 |
Spain, Basque Country |
0.05 |
500 |
|
Breast (174) |
USA, Hawaii (Hawaiian) |
12 |
China, Qidong |
1.0 |
10 |
|
Cervix uteri (180) |
Peru, Trujillo |
6 |
USA, Hawaii (Chinese) |
0.3 |
20 |
|
Corpus uteri (182) |
USA, Alameda County (Calif.) (Whites) |
3 |
China, Qidong |
0.05 |
60 |
|
Ovary (183) |
Iceland |
2 |
Mali, Bamako |
0.09 |
20 |
|
Prostate (185) |
USA, Atlanta (Blacks) |
12 |
China, Qidong |
0.09 |
140 |
|
Bladder (188) |
Italy, Florence |
4 |
India, Madras |
0.2 |
20 |
|
Kidney (189) |
France, Bas Rhin |
2 |
China, Qidong |
0.08 |
20 |
1 Data from cancer registries included in IARC 1992. Only cancer sites with cumulative rate larger or equal to 2% in the high-incidence area are included. Rates refer to males except for breast, cervix uteri, corpus uteri and ovary cancers.
2 Cumulative rate % between 0 and 74 years of age.
Source: International Agency for Research on Cancer 1992.
There are, of course, other explanations for geographical variation in cancer rates. Under-registration of cancer in some populations may exaggerate the range of variation, but certainly cannot explain differences of the size shown in table 1. Genetic factors also may be important. It has been observed, however, that when populations migrate along a gradient of cancer incidence they often acquire a rate of cancer which is intermediate between that of their home country and that of the host country. This suggests that a change in environment, without genetic change, has changed the cancer incidence. For example, when Japanese migrate to the United States their rates of colon and breast cancer, which are low in Japan, rise, and their rate of stomach cancer, which is high in Japan, falls, both tending more closely towards United States’ rates. These changes may be delayed until the first post-migration generation but they still occur without genetic change. For some cancers, change with migration does not occur. For example, the Southern Chinese retain their high rate of cancer of the nasopharynx wherever they live, thus suggesting that genetic factors, or some cultural habit which changes little with migration, are responsible for this disease.
Time Trends
Further evidence of the role of environmental factors in cancer incidence has come from the observation of time trends. The most dramatic and well-known change has been the rise in lung cancer rates in males and females in parallel with but occurring some 20 to 30 years after the adoption of cigarette use, which has been seen in many regions of the world; more recently in a few countries, such as the United States, there has been the suggestion of a fall in rates among males following a reduction in tobacco smoking. Less well understood are the substantial falls in incidence of cancers including those of the stomach, oesophagus and cervix which have paralleled economic development in many countries. It would be difficult to explain these falls, however, except in terms of reduction in exposure to causal factors in the environment or, perhaps, increasing exposure to protective factors—again environmental.
Main Environmental Carcinogenic Agents
The importance of environmental factors as causes of human cancer has been further demonstrated by epidemiological studies relating particular agents to particular cancers. The main agents which have been identified are summarized in table 10. This table does not contain the drugs for which a causal link with human cancer has been established (such as diethylstilboestrol and several alkylating agents) or suspected (such as cyclophosphamide) (see also Table 9). In the case of these agents, the risk of cancer has to be balanced with the benefits of the treatment. Similarly, Table 10 does not contain agents that occur primarily in the occupational setting, such as chromium, nickel and aromatic amines. For a detailed discussion of these agents see the previous article “Occupational Carcinogens.” The relative importance of the agents listed in table 8 varies widely, depending on the potency of the agent and the number of people involved. The evidence of carcinogenicity of several environmental agents has been evaluated within the IARC Monographs programme (International Agency for Research on Cancer 1995) (see again “Occupational Carcinogens” for a discussion of the Monographs programme); table 10 is based mainly on the IARC Monograph evaluations. The most important agents among those listed in table 10 are those to which a substantial proportion of the population is exposed in relatively large amounts. They include particularly: ultraviolet (solar) radiation; tobacco smoking; alcohol drinking; betel quid chewing; hepatitis B; hepatitis C and human papilloma viruses; aflatoxins; possibly dietary fat, and dietary fiber and vitamin A and C deficiency; reproductive delay; and asbestos.
Attempts have been made to estimate numerically the relative contributions of these factors to the 80 or 90% of cancers which might be attributed to environmental factors. The pattern varies, of course, from population to population according to differences in exposures and possibly in the genetic susceptibility to various cancers. In many industrialized countries, however, tobacco smoking and dietary factors are likely to be responsible each for roughly one-third of environmentally determined cancers (Doll and Peto 1981); while in developing countries the role of biological agents is likely to be large and that of tobacco relatively small (but increasing, following the recent increase in the consumption of tobacco in these populations).
Interactions between Carcinogens
An additional aspect to consider is the presence of interactions between carcinogens. Thus for example, in the case of alcohol and tobacco, and cancer of the oesophagus, it has been shown that an increasing consumption of alcohol multiplies manyfold the rate of cancer produced by a given level of tobacco consumption. Alcohol by itself may facilitate transport of tobacco carcinogens, or others, into the cells of susceptible tissues. Multiplicative interaction may also be seen between initiating carcinogens, as between radon and its decay products and tobacco smoking in miners of uranium. Some environmental agents may act by promoting cancers which have been initiated by another agent—this is the most likely mechanism for an effect of dietary fat on the development of breast cancer (probably through increased production of the hormones which stimulate the breast). The reverse may also occur, as, for example, in the case of vitamin A, which probably has an anti-promoting effect on lung and possibly other cancers initiated by tobacco. Similar interactions may also occur between environmental and constitutional factors. In particular, genetic polymorphism to enzymes implicated in the metabolism of carcinogenic agents or DNA repair is probably an important requirement of individual susceptibility to the effect of environmental carcinogens.
The significance of interactions between carcinogens, from the point of view of cancer control, is that withdrawal of exposure to one of two (or more) interacting factors may give rise to a greater reduction in cancer incidence than would be predicted from consideration of the effect of the agent when acting alone. Thus, for example, withdrawal of cigarettes may eliminate almost entirely the excess rate of lung cancer in asbestos workers (although rates of mesothelioma would be unaffected).
Implications for Prevention
The realization that environmental factors are responsible for a large proportion of human cancers has laid the foundation for primary prevention of cancer by modification of exposure to the factors identified. Such modification may comprise: removal of a single major carcinogen; reduction, as discussed above, in exposure to one of several interacting carcinogens; increasing exposure to protective agents; or combinations of these approaches. While some of this may be achieved by community-wide regulation of the environment through, for example, environmental legislation, the apparent importance of lifestyle factors suggests that much of primary prevention will remain the responsibility of individuals. Governments, however, may still create a climate in which individuals find it easier to take the right decision.
Occupational Carcinogens
The control of occupational carcinogens is based on the critical review of scientific investigations both in humans and in experimental systems. There are several review programmes being undertaken in different countries aimed at controlling occupational exposures which could be carcinogenic to humans. The criteria used in different programmes are not entirely consistent, leading occasionally to differences in the control of agents in different countries. For example, 4,4-methylene-bis-2-chloroaniline (MOCA) was classified as an occupational carcinogen in Denmark in 1976 and in the Netherlands in 1988, but only in 1992 has a notation “suspected human carcinogen” been introduced by the American Conference of Governmental Industrial Hygienists in the United States.
The International Agency for Research on Cancer (IARC) has established, within the framework of its Monographs programme, a set of criteria to evaluate the evidence of the carcinogenicity of specific agents. The IARC Monographs programme represents one of the most comprehensive efforts to review systematically and consistently cancer data, is highly regarded in the scientific community and serves as the basis for the information in this article. It also has an important impact on national and international occupational cancer control activities. The evaluation scheme is given in table 1.
Table 1. Evaluation of evidence of carcinogenicity in the IARC Monographs programme.
1. The evidence for the induction of cancer in humans, which obviously plays an important role in the identification of human carcinogens is considered. Three types of epidemiological studies contribute to an assessment of carcinogenicity in humans: cohort studies, case-control studies and correlation (or ecological) studies. Case reports of cancer in humans may also be reviewed. The evidence relevant to carcinogenicity from studies in humans is classified into one of the following categories:
- Sufficient evidence of carcinogenicity: A causal relationship has been established between exposure to the agent, mixture or exposure circumstance and human cancer. That is, a positive relationship has been observed between the exposure and cancer in studies in which chance, bias and confounding could be ruled out with reasonable confidence.
- Limited evidence of carcinogenicity: A positive association has been observed between exposure to the agent, mixture or exposure circumstance and cancer for which a causal interpretation is considered to be credible, but chance, bias or confounding could not be ruled out with reasonable confidence.
- Inadequate evidence of carcinogenicity: The available studies are of insufficient quality, consistency or statistical power to permit a conclusion regarding the presence or absence of a causal association, or no data on cancer in humans are available.
- Evidence suggesting lack of carcinogenicity: There are several adequate studies covering the full range of levels of exposure that human beings are known to encounter, which are mutually consistent in not showing a positive association between exposure to the agent and the studied cancer at any observed level of exposure.
2. Studies in which experimental animals (mainly rodents) are exposed chronically to potential carcinogens and examined for evidence of cancer are reviewed and the degree of evidence of carcinogenicity is then classified into categories similar to those used for human data.
3. Data on biological effects in humans and experimental animals that are of particular relevance are reviewed. These may include toxicological, kinetic and metabolic considerations and evidence of DNA binding, persistence of DNA lesions or genetic damage in exposed humans. Toxicological information, such as that on cytotoxicity and regeneration, receptor binding and hormonal and immunological effects, and data on structure-activity relationship are used when considered relevant to the possible mechanism of the carcinogenic action of the agent.
4. The body of evidence is considered as a whole, in order to reach an overall evaluation of the carcinogenicity to humans of an agent, mixture or circumstance of exposure (see table 2).
Agents, mixtures and exposure circumstances are evaluated within the IARC Monographs if there is evidence of human exposure and data on carcinogenicity (either in humans or in experimental animals) (for IARC classification groups, see table 2).
Table 2. IARC Monograph programme classification groups.
The agent, mixture or exposure circumstance is described according to the wording of one of the following categories:
| Group 1— | The agent (mixture) is carcinogenic to humans. The exposure circumstance entails exposures that are carcinogenic to humans. |
| Group 2A— | The agent (mixture) is probably carcinogenic to humans. The exposure circumstance entails exposures that are probably carcinogenic to humans. |
| Group 2B— | The agent (mixture) is possibly carcinogenic to humans. The exposure circumstance entails exposures that are possibly carcinogenic to humans. |
| Group 3— | The agent (mixture, exposure circumstance) is not classifiable as to its carcinogenicity to humans. |
| Group 4— | The agent (mixture, exposure circumstance) is probably not carcinogenic to humans. |
Known and Suspected Occupational Carcinogens
At present, there are 22 chemicals, groups of chemicals or mixtures for which exposures are mostly occupational, without considering pesticides and drugs, which are established human carcinogens (table 3). While some agents such as asbestos, benzene and heavy metals are currently widely used in many countries, other agents have mainly an historical interest (e.g., mustard gas and 2-naphthylamine).
Table 3. Chemicals, groups of chemicals or mixtures for which exposures are mostly occupational (excluding pesticides and drugs).
Group 1-Chemicals carcinogenic to humans1
| Exposure2 | Human target organ(s) | Main industry/use |
| 4-Aminobiphenyl (92-67-1) | Bladder | Rubber manufacture |
| Arsenic (7440-38-2) and arsenic compounds3 | Lung, skin | Glass, metals, pesticides |
| Asbestos (1332-21-4) | Lung, pleura, peritoneum | Insulation, filter material, textiles |
| Benzene (71-43-2) | Leukaemia | Solvent, fuel |
| Benzidine (92-87-5) | Bladder | Dye/pigment manufacture, laboratory agent |
| Beryllium (7440-41-7) and beryllium compounds | Lung | Aerospace industry/metals |
| Bis(chloromethyl)ether (542-88-11) | Lung | Chemical intermediate/by-product |
| Chloromethyl methylether (107-30-2) (technical grade) | Lung | Chemical intermediate/by-product |
| Cadmium (7440-43-9) and cadmium compounds | Lung | Dye/pigment manufacture |
| Chromium (VI) compounds | Nasal cavity, lung | Metal plating, dye/pigment manufacture |
| Coal-tar pitches (65996-93-2) | Skin, lung, bladder | Building material, electrodes |
| Coal-tars (8007-45-2) | Skin, lung | Fuel |
| Ethylene oxide (75-21-8) | Leukaemia | Chemical intermediate, sterilant |
| Mineral oils, untreated and mildly treated | Skin | Lubricants |
| Mustard gas (sulphur mustard) (505-60-2) |
Pharynx, lung | War gas |
| 2-Naphthylamine (91-59-8) | Bladder | Dye/pigment manufacture |
| Nickel compounds | Nasal cavity, lung | Metallurgy, alloys, catalyst |
| Shale-oils (68308-34-9) | Skin | Lubricants, fuels |
| Soots | Skin, lung | Pigments |
| Talc containing asbestiform fibers | Lung | Paper, paints |
| Vinyl chloride (75-01-4) | Liver, lung, blood vessels | Plastics, monomer |
| Wood dust | Nasal cavity | Wood industry |
1 Evaluated in the IARC Monographs, Volumes 1-63 (1972-1995) (excluding pesticides and drugs).
2 CAS Registry Nos. appear between parentheses.
3 This evaluation applies to the group of chemicals as a whole and not necessarily to all individual chemicals within the group.
An additional 20 agents are classified as probably carcinogenic to humans (Group 2A); they are listed in table 4, and include exposures that are currently prevalent in many countries, such as crystalline silica, formaldehyde and 1,3-butadiene. A large number of agents are classified as possible human carcinogens (Group 2B, table 5) - for example, acetaldehyde, dichloromethane and inorganic lead compounds. For the majority of these chemicals the evidence of carcinogenicity comes from studies in experimental animals.
Table 4. Chemicals, groups of chemicals or mixtures for which exposures are mostly occupational (excluding pesticides and drugs).
Group 2A—Probably carcinogenic to humans1
| Exposure2 | Suspected human target organ(s) | Main industry/use |
| Acrylonitrile (107-13-1) | Lung, prostate, lymphoma | Plastics, rubber, textiles, monomer |
| Benzidine-based dyes | – | Paper, leather, textile dyes |
| 1,3-Butadiene (106-99-0) | Leukaemia, lymphoma | Plastics, rubber, monomer |
| p-Chloro-o-toluidine (95-69-2) and its strong acid salts | Bladder | Dye/pigment manufacture, textiles |
| Creosotes (8001-58-9) | Skin | Wood preservation |
| Diethyl sulphate (64-67-5) | – | Chemical intermediate |
| Dimethylcarbamoyl chloride (79-44-7) | – | Chemical intermediate |
| Dimethyl sulphate (77-78-1) | – | Chemical intermediate |
| Epichlorohydrin (106-89-8) | – | Plastics/resins monomer |
| Ethylene dibromide (106-93-4) | – | Chemical intermediate, fumigant, fuels |
| Formaldehyde (50-0-0) | Nasopharynx | Plastics, textiles, laboratory agent |
| 4,4´-Methylene- bis-2-chloroaniline (MOCA) (101-14-4) |
Bladder | Rubber manufacture |
| Polychlorinated biphenyls (1336-36-3) | Liver, bile ducts, leukaemia, lymphoma | Electrical components |
| Silica (14808-60-7), crystalline | Lung | Stone cutting, mining, glass, paper |
| Styrene oxide (96-09-3) | – | Plastics, chemical intermediate |
| Tetrachloroethylene (127-18-4) |
Oesophagus, lymphoma | Solvent, dry cleaning |
| Trichloroethylene (79-01-6) | Liver, lymphoma | Solvent, dry cleaning, metal |
| Tris(2,3-dibromopropylphosphate (126-72-7) |
– | Plastics, textiles, flame retardant |
| Vinyl bromide (593-60-2) | – | Plastics, textiles, monomer |
| Vinyl fluoride (75-02-5) | – | Chemical intermediate |
1 Evaluated in the IARC Monographs, Volumes 1-63 (1972-1995) (excluding pesticides and drugs).
2 CAS Registry Nos. appear between parentheses.
Table 5. Chemicals, groups of chemicals or mixtures for which exposures are mostly occupational (excluding pesticides and drugs).
Group 2B—Possibly carcinogenic to humans1
| Exposure2 | Main industry/use |
| Acetaldehyde (75-07-0) | Plastics manufacture, flavors |
| Acetamide (60-35-5) | Solvent, chemical intermediate |
| Acrylamide (79-06-1) | Plastics, grouting agent |
| p-Aminoazotoluene (60-09-3) | Dye/pigment manufacture |
| o-Aminoazotoluene (97-56-3) | Dyes/pigments, textiles |
| o-Anisidine (90-04-0) | Dye/pigment manufacture |
| Antimony trioxide (1309-64-4) | Flame retardant, glass, pigments |
| Auramine (492-80-8) (technical-grade) | Dyes/pigments |
| Benzyl violet 4B (1694-09-3) | Dyes/pigments |
| Bitumens (8052-42-4), extracts of steam-refined and air-refined |
Building material |
| Bromodichloromethane (75-27-4) | Chemical intermediate |
| b-Butyrolactone (3068-88-0) | Chemical intermediate |
| Carbon-black extracts | Printing inks |
| Carbon tetrachloride (56-23-5) | Solvent |
| Ceramic fibers | Plastics, textiles, aerospace |
| Chlorendic acid (115-28-6) | Flame retardant |
| Chlorinated paraffins of average carbon chain length C12 and average degree of chlorination approximately 60% | Flame retardant |
| a-Chlorinated toluenes | Dye/pigment manufacture, chemical intermediate |
| p-Chloroaniline (106-47-8) | Dye/pigment manufacture |
| Chloroform (67-66-3) | Solvent |
| 4-Chloro-o-phenylenediamine (95-83-9) | Dyes/pigments, hair dyes |
| CI Acid Red 114 (6459-94-5) | Dyes/pigments, textiles, leather |
| CI Basic Red 9 (569-61-9) | Dyes/pigments, inks |
| CI Direct Blue 15 (2429-74-5) | Dyes/pigments, textiles, paper |
| Cobalt (7440-48-4)and cobalt compounds | Glass, paints, alloys |
| p-Cresidine (120-71-8) | Dye/pigment manufacture |
| N,N´-Diacetylbenzidine (613-35-4) | Dye/pigment manufacture |
| 2,4-Diaminoanisole (615-05-4) | Dye/pigment manufacture, hair dyes |
| 4,4´-Diaminodiphenyl ether (101-80-4) | Plastics manufacture |
| 2,4-Diaminotoluene (95-80-7) | Dye/pigment manufacture, hair dyes |
| p-Dichlorobenzene (106-46-7) | Chemical intermediate |
| 3,3´-Dichlorobenzidine (91-94-1) | Dye/pigment manufacture |
| 3,3´-Dichloro-4,4´-diaminodiphenyl ether (28434-86-8) | Not used |
| 1,2-Dichloroethane (107-06-2) | Solvent, fuels |
| Dichloromethane (75-09-2) | Solvent |
| Diepoxybutane (1464-53-5) | Plastics/resins |
| Diesel fuel, marine | Fuel |
| Di(2-ethylhexyl)phthalate (117-81-7) | Plastics, textiles |
| 1,2-Diethylhydrazine (1615-80-1) | Laboratory reagent |
| Diglycidyl resorcinol ether (101-90-6) | Plastics/resins |
| Diisopropyl sulphate (29973-10-6) | Contaminant |
| 3,3´-Dimethoxybenzidine (o-Dianisidine) (119-90-4) |
Dye/pigment manufacture |
| p-Dimethylaminoazobenzene (60-11-7) | Dyes/pigments |
| 2,6-Dimethylaniline (2,6-Xylidine)(87-62-7) | Chemical intermediate |
| 3,3´-Dimethylbenzidine (o-Tolidine)(119-93-7) | Dye/pigment manufacture |
| Dimethylformamide (68-12-2) | Solvent |
| 1,1-Dimethylhydrazine (57-14-7) | Rocket fuel |
| 1,2-Dimethylhydrazine (540-73-8) | Research chemical |
| 1,4-Dioxane (123-91-1) | Solvent |
| Disperse Blue 1 (2475-45-8) | Dyes/pigments, hair dyes |
| Ethyl acrylate (140-88-5) | Plastics, adhesives, monomer |
| Ethylene thiourea (96-45-7) | Rubber chemical |
| Fuel oils, residual (heavy) | Fuel |
| Furan (110-00-9) | Chemical intermediate |
| Gasoline | Fuel |
| Glasswool | Insulation |
| Glycidaldehyde (765-34-4) | Textile, leather manufacture |
| HC Blue No. 1 (2784-94-3) | Hair dyes |
| Hexamethylphosphoramide (680-31-9) | Solvent, plastics |
| Hydrazine (302-01-2) | Rocket fuel, chemical intermediate |
| Lead (7439-92-1) and lead compounds, inorganic | Paints, fuels |
| 2-Methylaziridine(75-55-8) | Dyes, paper, plastics manufacture |
| 4,4’-Methylene-bis-2-methylaniline (838-88-0) | Dye/pigment manufacture |
| 4,4’-Methylenedianiline(101-77-9) | Plastics/resins, dye/pigment manufacture |
| Methylmercury compounds | Pesticide manufacture |
| 2-Methyl-1-nitroanthraquinone (129-15-7) (uncertain purity) | Dye/pigment manufacture |
| Nickel, metallic (7440-02-0) | Catalyst |
| Nitrilotriacetic acid (139-13-9) and its salts | Chelating agent, detergent |
| 5-Nitroacenaphthene (602-87-9) | Dye/pigment manufacture |
| 2-Nitropropane (79-46-9) | Solvent |
| N-Nitrosodiethanolamine (1116-54-7) | Cutting fluids, impurity |
| Oil Orange SS (2646-17-5) | Dyes/pigments |
| Phenyl glycidyl ether (122-60-1) | Plastics/adhesives/resins |
| Polybrominated biphenyls (Firemaster BP-6) (59536-65-1) | Flame retardant |
| Ponceau MX (3761-53-3) | Dyes/pigments, textiles |
| Ponceau 3R (3564-09-8) | Dyes/pigments, textiles |
| 1,3-Propane sulphone (1120-71-4) | Dye/pigment manufacture |
| b-Propiolactone (57-57-8) | Chemical intermediate; plastics manufacture |
| Propylene oxide (75-56-9) | Chemical intermediate |
| Rockwool | Insulation |
| Slagwool | Insulation |
| Styrene (100-42-5) | Plastics |
| 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) (1746-01-6) | Contaminant |
| Thioacetamide (62-55-5) | Textile, paper, leather, rubber manufacture |
| 4,4’-Thiodianiline (139-65-1) | Dye/pigment manufacture |
| Thiourea (62-56-6) | Textile, rubber ingredient |
| Toluene diisocyanates (26471-62-5) | Plastics |
| o-Toluidine (95-53-4) | Dye/pigment manufacture |
| Trypan blue (72-57-1) | Dyes/pigments |
| Vinyl acetate (108-05-4) | Chemical intermediate |
| Welding fumes | Metallurgy |
1 Evaluated in the IARC Monographs, Volumes 1-63 (1972-1995) (excluding pesticides and drugs).
2 CAS Registry Nos. appear between parentheses.
Occupational exposures may also occur during the production and use of some pesticides and drugs. Table 6 presents an evaluation of the carcinogenicity of pesticides; two of them, captafol and ethylene dibromide, are classified as probable human carcinogens, while a total of 20 others, including DDT, atrazine and chlorophenols, are classified as possible human carcinogens.
Table 6. Pesticides evaluated in IARC Monographs, Volumes 1-63(1972-1995)
| IARC Group | Pesticide1 |
| 2A—Probably carcinogenic to humans | Captafol (2425-06-1) Ethylene dibromide (106-93-4) |
| 2B—Possibly carcinogenic to humans | Amitrole (61-82-5) Atrazine (1912-24-9) Chlordane (57-74-9) Chlordecone (Kepone) (143-50-0) Chlorophenols Chlorophenoxy herbicides DDT (50-29-3) 1,2-Dibromo-3-chloropropane (96-12-8) 1,3-Dichloropropene (542-75-6) (technical-grade) Dichlorvos (62-73-7) Heptachlor (76-44-8) Hexachlorobenzene (118-74-1) Hexachlorocyclohexanes (HCH) Mirex (2385-85-5) Nitrofen (1836-75-5), technical-grade Pentachlorophenol (87-86-5) Sodium o-phenylphenate (132-27-4) Sulphallate (95-06-7) Toxaphene (Polychlorinated camphenes) (8001-35-2) |
1 CAS Registry Nos. appear between parentheses.
Several drugs are human carcinogens (table 9): they are mainly alkylating agents and hormones; 12 more drugs, including chloramphenicol, cisplatine and phenacetin, are classified as probable human carcinogens (Group 2A). Occupational exposure to these known or suspected carcinogens, used mainly in chemotherapy, can occur in pharmacies and during their administration by nursing staff.
Table 7. Drugs evaluated in IARC Monographs, Volumes 1-63 (1972-1995).
| Drug1 | Target organ2 |
| IARC GROUP 1—Carcinogenic to humans | |
| Analgesic mixtures containing phenacetin | Kidney, bladder |
| Azathioprine (446-86-6) | Lymphoma, hepatobiliary system, skin |
| N,N-Bis(2-chloroethyl)- b-naphthylamine (Chlornaphazine) (494-03-1) | Bladder |
| 1,4-Butanediol dimethanesulphonate (Myleran) (55-98-1) |
Leukaemia |
| Chlorambucil (305-03-3) | Leukaemia |
| 1-(2-Chloroethyl)-3-(4-methylcyclohexyl)-1-nitrosourea (Methyl-CCNU) (13909-09-6) | Leukaemia |
| Cyclosporin (79217-60-0) | Lymphoma, skin |
| Cyclophosphamide (50-18-0) (6055-19-2) | Leukaemia, bladder |
| Diethylstilboestrol (56-53-1) | Cervix, vagina, breast |
| Melphalan (148-82-3) | Leukaemia |
| 8-Methoxypsoralen (Methoxsalen) (298-81-7) plus ultraviolet A radiation | Skin |
| MOPP and other combined chemotherapy including alkylating agents | Leukaemia |
| Oestrogen replacement therapy | Uterus |
| Oestrogens, nonsteroidal | Cervix, vagina, breast |
| Oestrogens, steroidal | Uterus |
| Oral contraceptives, combined | Liver |
| Oral contraceptives, sequential | Uterus |
| Thiotepa (52-24-4) | Leukaemia |
| Treosulfan (299-75-2) | Leukaemia |
| IARC GROUP 2A—Probably carcinogenic to humans | |
| Adriamycin (23214-92-8) | – |
| Androgenic (anabolic) steroids | (Liver) |
| Azacitidine (320-67-2) | – |
| Bischloroethyl nitrosourea (BCNU) (154-93-8) | (Leukaemia) |
| Chloramphenicol (56-75-7) | (Leukaemia) |
| 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) (13010-47-4) | – |
| Chlorozotocine (54749-90-5) | – |
| Cisplatin (15663-27-1) | – |
| 5-Methoxypsoralen (484-20-8) | – |
| Nitrogen mustard (51-75-2) | (Skin) |
| Phenacetin (62-44-2) | (Kidney, bladder) |
| Procarbazine hydrochloride (366-70-1) | – |
1 CAS Registry Nos. appear between parentheses.
2 Suspected target organs are given in parentheses.
Several environmental agents are known or suspected causes of cancer in humans and are listed in table 8; although exposure to such agents is not primarily occupational, there are groups of individuals exposed to them because of their work: examples are uranium miners exposed to radon decay products, hospital workers exposed to hepatitis B virus, food processors exposed to aflatoxins from contaminated foods, outdoor workers exposed to ultraviolet radiation or diesel engine exhaust, and bar staff or waiters exposed to environmental tobacco smoke.
The IARC Monograph programme has covered most of the known or suspected causes of cancer; there are, however, some important groups of agents that have not been evaluated by IARC—namely, ionizing radiation and electrical and magnetic fields.
Table 8. Environmental agents/exposures known or suspected to cause cancer in humans.1
| Agent/exposure | Target organ2 | Strength of evidence3 |
| Air pollutants | ||
| Erionite | Lung, pleura | 1 |
| Asbestos | Lung, pleura | 1 |
| Polycyclic aromatic hydrocarbons4 | (Lung, bladder) | S |
| Water pollutants | ||
| Arsenic | Skin | 1 |
| Chlorination by-products | (Bladder) | S |
| Nitrate and nitrite | (Oesophagus, stomach) | S |
| Radiation | ||
| Radon and its decay products | Lung | 1 |
| Radium, thorium | Bone | E |
| Other X-irradiation | Leukaemia, breast, thyroid, others | E |
| Solar radiation | Skin | 1 |
| Ultraviolet radiation A | (Skin) | 2A |
| Ultraviolet radiation B | (Skin) | 2A |
| Ultraviolet radiation C | (Skin) | 2A |
| Use of sunlamps and sunbeds | (Skin) | 2A |
| Electric and magnetic fields | (Leukaemia) | S |
| Biological agents | ||
| Chronic infection with hepatitis B virus | Liver | 1 |
| Chronic infection with hepatitis C virus | Liver | 1 |
| Infection with Helicobacter pylori | Stomach | 1 |
| Infection with Opistorchis viverrini | Bile ducts | 1 |
| Infection with Chlonorchis sinensis | (Liver) | 2A |
| Human Papilloma virus types 16 and18 | Cervix | 1 |
| Human Papilloma virus types 31 and 33 | (Cervix) | 2A |
| Human Papilloma virus types other than 16, 18, 31 and 33 | (Cervix) | 2B |
| Infection with Schistosoma haematobium | Bladder | 1 |
| Infection with Schistosoma japonicum | (Liver, colon) | 2B |
| Tobacco, alcohol and related substances | ||
| Alcoholic beverages | Mouth, pharynx, oesophagus, liver, larynx | 1 |
| Tobacco smoke | Lip, mouth, pharynx, oesophagus, pancreas, larynx, lung, kidney, bladder, (others) | 1 |
| Smokeless tobacco products | Mouth | 1 |
| Betel quid with tobacco | Mouth | 1 |
| Dietary factors | ||
| Aflatoxins | Liver | 1 |
| Aflatoxin M1 | (Liver) | 2B |
| Ochratoxin A | (Kidney) | 2B |
| Toxins derived from Fusarium moniliforme | (Oesophagus) | 2B |
| Chinese style salted fish | Nasopharynx | 1 |
| Pickled vegetables (traditional in Asia) | (Oesophagus, stomach) | 2B |
| Bracken fern | (Oesophagus) | 2B |
| Safrole | – | 2B |
| Coffee | (Bladder) | 2B |
| Caffeic acid | – | 2B |
| Hot mate | (Oesophagus) | 2A |
| Fresh fruits and vegetables (protective) | Mouth, oesophagus, stomach, colon, rectum, larynx, lung (others) | E |
| Fat | (Colon, breast, endometrium) | S |
| Fiber (protective) | (Colon, rectum) | S |
| Nitrate and nitrite | (Oesophagus, stomach) | S |
| Salt | (Stomach) | S |
| Vitamin A, b-carotene (protective) | (Mouth, oesophagus, lung, others) | S |
| Vitamin C (protective) | (Oesophagus, stomach) | S |
| IQ | (Stomach, colon, rectum) | 2A |
| MeIQ | – | 2B |
| MeIQx | – | 2B |
| PhIP | – | 2B |
| Reproductive and sexual behavior | ||
| Late age at first pregnancy | Breast | E |
| Low parity | Breast, ovary, corpus uteri | E |
| Early age at first intercourse | Cervix | E |
| Number of sexual partners | Cervix | E |
1 Agents and exposures, as well as medicines, occurring mainly in the occupational setting are excluded.
2 Suspected target organs are given in parentheses.
3 IARC Monograph evaluation reported wherever available (1: human carcinogen; 2A: probable human carcinogen; 2B: possible human carcinogen); otherwise E: established carcinogen; S: suspected carcinogen.
4 Human exposure to polycyclic aromatic hydrocarbons occurs in mixtures, such as engine emissions, combustion fumes and soots. Several mixtures and individual hydrocarbons have been evaluated by IARC.
Industries and Occupations
Current understanding of the relationship between occupational exposures and cancer is far from complete; in fact, only 22 individual agents are established occupational carcinogens (table 9), and for many more experimental carcinogens no definitive evidence is available based on exposed workers. In many cases, there is considerable evidence of increased risks associated with particular industries and occupations, although no specific agents can be identified as aetiological factors. Table 10 present lists of industries and occupations associated with excess carcinogenic risks, together with the relevant cancer sites and the known (or suspected) causative agent(s).
Table 9. Industries, occupations and exposures recognized as presenting a carcinogenic risk.
| Industry (ISIC code) | Occupation/process | Cancer site/type | Known or suspected causative agent |
| Agriculture, forestry and fishing (1) | Vineyard workers using arsenic insecticides Fishermen | Lung, skin Skin, lip | Arsenic compounds Ultraviolet radiation |
| Mining and quarrying (2) | Arsenic mining Iron ore (haematite) mining Asbestos mining Uranium mining Talc mining and milling | Lung, skin Lung Lung, pleural and peritoneal mesothelioma Lung Lung | Arsenic compounds Radon decay products Asbestos Radon decay products Talc containing asbestiform fibers |
| Chemical (35) | Bis(chloromethyl) ether (BCME) and chloromethyl-methyl ether (CMME) production workers and users Vinyl chloride production Isopropyl alcohol manufacture (strong-acid process) Pigment chromate production Dye manufacturers and users Auramine manufacture p-chloro-o-toluidine production | Lung (oat-cell carcinoma) Liver angiosarcoma Sinonasal Lung, sinonasal Bladder Bladder Bladder | BCME, CMME Vinyl chloride monomer Not identified Chromium (VI) compounds Benzidine, 2-naphthylamine, 4-aminobiphenyl Auramine and other aromatic amines used in the process p-chloro-o-toluidine and its strong acid salts |
| Leather (324) | Boot and shoe manufacture | Sinonasal, leukaemia | Leather dust, benzene |
| Wood and wood products (33) | Furniture and cabinet makers | Sinonasal | Wood dust |
| Pesticides and herbicides production (3512) | Arsenical insecticides production and packaging | Lung | Arsenic compounds |
| Rubber industry (355) | Rubber manufacture Calendering, tyre curing, tyre building Millers, mixers Synthetic latex production, tyre curing, calender operatives, reclaim, cable makers Rubber film production | Leukaemia Bladder Leukaemia Bladder Bladder Leukaemia | Benzene Aromatic amines Benzene Aromatic amines Aromatic amines Benzene |
| Asbestos production (3699) | Insulated material production (pipes, sheeting, textile, clothes, masks, asbestos cement products) | Lung, pleural and peritoneal mesothelioma | Asbestos |
| Metals (37) | Aluminum production Copper smelting Chromate production, chromium plating Iron and steel founding Nickel refining Pickling operations Cadmium production and refining; nickel-cadmium battery manufacture; cadmium pigment manufacture; cadmium alloy production; electroplating; zinc smelters; brazing and polyvinyl chloride compounding Beryllium refining and machining; production of beryllium-containing products | Lung, bladder Lung Lung, sinonasal Lung Sinonasal, lung Larynx, lung Lung Lung | Polycyclic aromatic hydrocarbons, tar Arsenic compounds Chromium (VI) compounds Not identified Nickel compounds Inorganic acid mists containing sulphuric acid Cadmium and cadmium compounds Beryllium and beryllium compounds |
| Shipbuilding, motor vehicle and railroad equipment manufacture (385) | Shipyard and dockyard, motor vehicle and railroad manufacture workers | Lung, pleural and peritoneal mesothelioma | Asbestos |
| Gas (4) | Coke plant workers Gas workers Gas-retort house workers | Lung Lung, bladder, scrotum Bladder | Benzo(a)pyrene Coal carbonization products, 2-naphthylamine Aromatic amines |
| Construction (5) | Insulators and pipe coverers Roofers, asphalt workers | Lung, pleural and peritoneal mesothelioma Lung | Asbestos Polycyclic aromatic hydrocarbons |
| Other | Medical personnel (9331) Painters (construction, automotive industry and other users) | Skin, leukaemia Lung | Ionizing radiation Not identified |
Table 10. Industries, occupations and exposures reported to present a cancer excess but for which the assessment of the carcinogenic risk is not definitive.
| Industry (ISIC code) | Occupation/process | Cancer site/type | Known (or suspected) causative agent |
| Agriculture, forestry and fishing (1) | Farmers, farm workers Herbicide application Insecticide application | Lymphatic and haematopoietic system (leukaemia, lymphoma) Malignant lymphomas, soft-tissue sarcomas Lung, lymphoma | Not identified Chlorophenoxy herbicides, chlorophenols (presumably contaminated with polychlorinated dibenzodioxins) Non-arsenical insecticides |
| Mining and quarrying (2) | Zinc-lead mining Coal Metal mining Asbestos mining | Lung Stomach Lung Gastrointestinal tract | Radon decay products Coal dust Crystalline silica Asbestos |
| Food industry (3111) | Butchers and meat workers | Lung | Viruses, PAH1 |
| Beverage industry (3131) | Beer brewers | Upper aero-digestive tract | Alcohol consumption |
| Textile manufacture (321) | Dyers Weavers | Bladder Bladder, sinonasal, mouth | Dyes Dusts from fibers and yarns |
| Leather (323) | Tanners and processors Boot and shoe manufacture and repair | Bladder, pancreas, lung Sinonasal, stomach, bladder | Leather dust, other chemicals, chromium Not identified |
| Wood and wood products (33), pulp and paper industry (341) | Lumbermen and sawmill workers Pulp and papermill workers Carpenters, joiners Woodworkers, unspecified Plywood production, particle-board production | Nasal cavity, Hodgkin lymphoma, skin Lymphopoietic tissue, lung Nasal cavity, Hodgkin lymphoma Lymphomas Nasopharynx, sinonasal | Wood dust, chlorophenols, creosotes Not identified Wood dust, solvents Not identified Formaldehyde |
| Printing (342) | Rotogravure workers, binders, printing pressmen, machine-room workers and other jobs | Lymphocytic and haemopoietic system, oral, lung, kidney | Oil mist, solvents |
| Chemical (35) | 1,3-Butadiene production Acrylonitrile production Vinylidene chloride production Isopropyl alcohol manufacture (strong-acid process) Polychloroprene production Dimethylsulphate production Epichlorohydrin production Ethylene oxide production Ethylene dibromide production Formaldehyde production Flame retardant and plasticizer use Benzoyl chloride production | Lymphocytic and haemopoietic system Lung, colon Lung Larynx Lung Lung Lung, lymphatic and haemopoietic system (leukaemia) Lymphatic and haemopoietic system (leukaemia), stomach Digestive system Nasopharynx, sinonasal Skin (melanoma) Lung | 1,3-Butadiene Acrylonitrile Vinylidene chloride (mixed exposure with acrylonitrile) Not identified Chloroprene Dimethylsulphate Epichlorohydrin Ethylene oxide Ethylene dibromide Formaldehyde Polychlorinated biphenyls Benzoyl chloride |
| Herbicides production (3512) | Chlorophenoxy herbicide production | Soft-tissue sarcoma | Chlorophenoxy herbicides, chlorophenols (contaminated with polychlorinated dibenzodioxins) |
| Petroleum (353) | Petroleum refining | Skin, leukaemia, brain | Benzene, PAH, untreated and mildly treated mineral oils |
| Rubber (355) | Various occupations in rubber manufacture Styrene-butadiene rubber production | Lymphoma, multiple myeloma, stomach, brain, lung Lymphatic and haematopoietic system | Benzene, MOCA,2 other not identified 1,3-Butadiene |
| Ceramic, glass and refractory brick (36) | Ceramic and pottery workers Glass workers (art glass, container and pressed ware) | Lung Lung | Crystalline silica Arsenic and other metal oxides, silica, PAH |
| Asbestos production (3699) | Insulation material production (pipes, sheeting, textiles, clothes, masks, asbestos cement products) | Larynx, gastrointestinal tract | Asbestos |
| Metals (37, 38) | Lead smelting Cadmium production and refining; nickel-cadmium battery manufacture; cadmium pigment manufacture; cadmium alloy production; electroplating; zinc smelting; brazing and polyvinyl chloride compounding Iron and steel founding | Respiratory and digestive systems Prostate Lung | Lead compounds Cadmium and cadmium compounds Crystalline silica |
| Shipbuilding (384) | Shipyard and dockyard workers | Larynx, digestive system | Asbestos |
| Motor vehicle manufacturing (3843, 9513) | Mechanics, welders, etc. | Lung | PAH, welding fumes, engine exhaust |
| Electricity (4101, 9512) | Generation, production, distribution, repair | Leukaemia, brain tumors Liver, bile ducts | Extremely low frequency magnetic fields PCBs3 |
| Construction (5) | Insulators and pipe coverers Roofers, asphalt workers | Larynx, gastrointestinal tract Mouth, pharynx, larynx, oesophagus, stomach | Asbestos PAH, coal tar, pitch |
| Transport (7) | Railroad workers, filling station attendants, bus and truck drivers, operators of excavating machines | Lung, bladder Leukaemia | Diesel engine exhaust Extremely low frequency magnetic fields |
| Other | Service station attendants (6200) Chemists and other laboratory workers (9331) Embalmers, medical personnel (9331) Health workers (9331) Laundry and dry cleaners (9520) Hairdressers (9591) Radium dial workers | Leukaemia and lymphoma Leukaemia and lymphoma, pancreas Sinonasal, nasopharynx Liver Lung, oesophagus, bladder Bladder, leukaemia and lymphoma Breast | Benzene Not identified (viruses, chemicals) Formaldehyde Hepatitis B virus Tri- and tetrachloroethylene and carbon tetrachloride Hair dyes, aromatic amines Radon |
1 PAH, polycyclic aromatic hydrocarbon.
2 MOCA, 4,4’-methylene-bis-2-chloroaniline.
3 PCBs, polychlorinated biphenyls.
Table 9 presents industries, occupations and exposures in which the presence of a carcinogenic risk is considered to be established, whereas Table 10 shows industrial processes, occupations and exposures for which an excess cancer risk has been reported but evidence is not considered to be definitive. Also included in table 10 are some occupations and industries already listed in table 9, for which there is inconclusive evidence of association with cancers other than those mentioned in table 9. For example, the asbestos production industry is included in table 9 in relation to lung cancer and pleural and peritoneal mesothelioma, whereas the same industry is included in table 10 in relation to gastrointestinal neoplasms. A number of industries and occupations listed intables 9 and 10 have also been evaluated under the IARC Monographs programme. For example, “occupational exposure to strong inorganic acid mist containing sulphuric acid” was classified in Group 1 (carcinogenic to humans).
Constructing and interpreting such lists of chemical or physical carcinogenic agents and associating them with specific occupations and industries is complicated by a number of factors: (1) information on industrial processes and exposures is frequently poor, not allowing a complete evaluation of the importance of specific carcinogenic exposures in different occupations or industries; (2) exposures to well-known carcinogenic exposures, such as vinyl chloride and benzene, occur at different intensities in different occupational situations; (3) changes in exposure occur over time in a given occupational situation, either because identified carcinogenic agents are substituted by other agents or (more frequently) because new industrial processes or materials are introduced; (4) any list of occupational exposures can refer only to the relatively small number of chemical exposures which have been investigated with respect to the presence of a carcinogenic risk.
All of the above issues emphasize the most critical limitation of a classification of this type, and in particular its generalization to all areas of the world: the presence of a carcinogen in an occupational situation does not necessarily mean that workers are exposed to it and, in contrast, the absence of identified carcinogens does not exclude the presence of yet unidentified causes of cancer.
A particular problem in developing countries is that much of the industrial activity is fragmented and takes place in local settings. These small industries are often characterized by old machinery, unsafe buildings, employees with limited training and education, and employers with limited financial resources. Protective clothing, respirators, gloves and other safety equipment are seldom available or used. The small companies tend to be geographically scattered and inaccessible to inspections by health and safety enforcement agencies.
Introduction
Magnitude of the Problem
The first clear-cut evidence of cancer causation involved an occupational carcinogen (Checkoway, Pearce and Crawford-Brown 1989). Pott (1775) identified soot as the cause of scrotal cancer in London chimney-sweeps, and graphically described the abysmal working conditions, which involved children climbing up narrow chimneys that were still hot. Despite this evidence, reports of the need to prevent fires in chimneys were used to delay legislation on child labour in this industry until 1840 (Waldron 1983). An experimental model of soot carcinogenesis was first demonstrated in the 1920s (Decoufle 1982), 150 years after the original epidemiological observation.
In subsequent years, a number of other occupational causes of cancer have been demonstrated through epidemiological studies (although the association with cancer has usually first been noted by occupational physicians or by workers). These include arsenic, asbestos, benzene, cadmium, chromium, nickel and vinyl chloride. Such occupational carcinogens are very important in public health terms because of the potential for prevention through regulation and improvements in industrial hygiene practices (Pearce and Matos 1994). In most instances, these are hazards which markedly increase the relative risk of a particular type or types of cancer. It is possible that other occupational carcinogens remain undetected because they involve only a small increase in risk or because they simply have not been studied (Doll and Peto 1981). Some key facts about occupational cancer are given in table 1.
Table 1. Occupational cancer: Key facts.
- Some 20 agents and mixtures are established occupational carcinogens; a similar number of chemicals are highly suspected occupational carcinogens.
- In industrialized countries, occupation is causally linked to 2 to 8% of all cancers; among exposed workers, however, this proportion is higher.
- No reliable estimates are available on either the burden of occupational cancer or the extent of workplace exposure to carcinogens in developing countries.
- The relatively low overall burden of occupational cancer in industrialized countries is the result of strict regulations on several known carcinogens; exposure to other known or highly suspected agents, however, is still allowed.
- Although several occupational cancers are listed as occupational diseases in many countries, a very small fraction of cases is actually recognized and compensated.
- Occupational cancer is-to a very large extent-a preventable disease.
Occupational causes of cancer have received considerable emphasis in epidemiological studies in the past. However, there has been much controversy regarding the proportion of cancers which are attributable to occupational exposures, with estimates ranging from 4 to 40% (Higginson 1969; Higginson and Muir 1976; Wynder and Gori 1977; Higginson and Muir 1979; Doll and Peto 1981; Hogan and Hoel 1981; Vineis and Simonato 1991; Aitio and Kauppinen 1991). The attributable cancer risk is the total cancer experience in a population that would not have occurred if the effects associated with the occupational exposures of concern were absent. It may be estimated for the exposed population, as well as for a broader population. A summary of existing estimates is shown in table 2. Universal application of the International Classification of Diseases is what makes such tabulations possible (see box).
Table 2. Estimated proportions of cancer (PAR) attributable to occupations in selected studies.
| Study | Population | PAR and cancer site | Comments |
| Higginson 1969 | Not stated | 1% Oral cancer 1-2% Lung cancer 10% Bladder cancer 2% Skin cancer |
No detailed presentation of exposure levels and other assumptions |
| Higginson and Muir 1976 | Not stated | 1-3% Total cancer | No detailed presentation of assumptions |
| Wynder and Gori 1977 | Not stated | 4% Total cancer in men, 2% for women |
Based on one PAR for bladder cancer and two personal communications |
| Higginson and Muir 1979 | West Midland, United Kingdom | 6% Total cancer in men, 2% total cancer |
Based on 10% of non-tobacco related lung cancer, mesothelioma, bladder cancer (30%), and leukaemia in women (30%) |
| Doll and Peto 1981 | United States early 1980 | 4% (range 2-8%) Total cancer |
Based on all studied cancer sites; reported as ‘tentative’ estimate |
| Hogan and Hoel 1981 | United States | 3% (range 1.4-4%) Total cancer |
Risk associated with occupational asbestos exposure |
| Vineis and Simonato 1991 | Various | 1-5% Lung cancer, 16-24% bladder cancer |
Calculations on the basis of data from case-control studies. Percentage for lung cancer considers only exposure to asbestos. In a study with a high proportion of subjects exposed to ionising radiation, a 40% PAR was estimated. Estimates of PAR in a few studies on bladder cancer were between 0 and 3%. |
The International Classification of Diseases
Human diseases are classified according to the International Classification of Diseases (ICD), a system that was started in 1893 and is regularly updated under the coordination of the World Health Organization. The ICD is used in almost all countries for tasks such as death certification, cancer registration and hospital discharge diagnosis. The Tenth Revision (ICD-10), which was approved in 1989 (World Health Organization 1992), differs considerably from the previous three revisions, which are similar to each other and have been in use since the 1950s. It is therefore likely that the Ninth Revision (ICD-9, World Health Organization 1978), or even earlier revisions, will still be used in many countries during the coming years.
The large variability in the estimates arises from the differences in the data sets used and the assumptions applied. Most of the published estimates on the fraction of cancers attributed to occupational risk factors are based on rather simplified assumptions. Furthermore, although cancer is relatively less common in developing countries due to the younger age structure (Pisani and Parkin 1994), the proportion of cancers due to occupation may be higher in developing countries due to the relatively high exposures which are encountered (Kogevinas, Boffetta and Pearce 1994).
The most generally accepted estimates of cancers attributable to occupations are those presented in a detailed review on the causes of cancer in the population of the United States in 1980 (Doll and Peto 1981). Doll and Peto concluded that about 4% of all the deaths due to cancer may be caused by occupational carcinogens within “acceptable limits” (i.e., still plausible in view of all the evidence at hand) of 2 and 8%. These estimates being proportions, they are dependent on how causes other than occupational exposures contribute to produce cancers. For example, the proportion would be higher in a population of lifetime non-smokers (such as the Seventh-Day Adventists) and lower in a population in which, say, 90% are smokers. Also the estimates do not apply uniformly to both sexes or to different social classes. Furthermore, if one considers not the whole population (to which the estimates refer), but the segments of the adult population in which exposure to occupational carcinogens almost exclusively occurs (manual workers in mining, agriculture and industry, broadly taken, who in the United States numbered 31 million out of a population, aged 20 and over, of 158 million in the late 1980s), the proportion of 4% in the overall population would increase to about 20% among those exposed.
Vineis and Simonato (1991) provided estimates on the number of cases of lung and bladder cancer attributable to occupation. Their estimates were derived from a detailed review of case-control studies, and demonstrate that in specific populations located in industrial areas, the proportion of lung cancer or bladder cancer from occupational exposures may be as high as 40% (these estimates being dependent not only on the local prevailing exposures, but also to some extent on the method of defining and assessing exposure).
Mechanisms and Theories of Carcinogenesis
Studies of occupational cancer are complicated because there are no “complete” carcinogens; that is, occupational exposures increase the risk of developing cancer, but this future development of cancer is by no means certain. Furthermore, it may take 20 to 30 years (and at least five years) between an occupational exposure and the subsequent induction of cancer; it may also take several more years for cancer to become clinically detectable and for death to occur (Moolgavkar et al. 1993). This situation, which also applies to non-occupational carcinogens, is consistent with current theories of cancer causation.
Several mathematical models of cancer causation have been proposed (e.g., Armitage and Doll 1961), but the model which is simplest and most consistent with current biological knowledge is that of Moolgavkar (1978). This assumes that a healthy stem cell occasionally mutates (initiation); if a particular exposure encourages the proliferation of intermediate cells (promotion) then it becomes more likely that at least one cell will undergo one or more further mutations producing a malignant cancer (progression) (Ennever 1993).
Thus, occupational exposures can increase the risk of developing cancer either by causing mutations in DNA or by various “epigenetic” mechanisms of promotion (those not involving damage to DNA), including increased cell proliferation. Most occupational carcinogens which have been discovered to date are mutagens, and therefore appear to be cancer initiators. This explains the long “latency” period which is required for further mutations to occur; in many instances the necessary further mutations may never occur, and cancer may never develop.
In recent years, there has been increasing interest in occupational exposures (e.g., benzene, arsenic, phenoxy herbicides) which do not appear to be mutagens, but which may act as promoters. Promotion may occur relatively late in the carcinogenic process, and the latency period for promoters may therefore be shorter than for initiators. However, the epidemiological evidence for cancer promotion remains very limited at this time (Frumkin and Levy 1988).
Transfer of Hazards
A major concern in recent decades has been the problem of the transfer of hazardous industries to the developing world (Jeyaratnam 1994). Such transfers have occurred in part due to the stringent regulation of carcinogens and increasing labour costs in the industrialized world, and in part from low wages, unemployment and the push for industrialization in the developing world. For example, Canada now exports about half of its asbestos to the developing world, and a number of asbestos-based industries have been transferred to developing countries such as Brazil, India, Pakistan, Indonesia and South Korea (Jeyaratnam 1994). These problems are further compounded by the magnitude of the informal sector, the large numbers of workers who have little support from unions and other worker organizations, the insecure status of workers, the lack of legislative protection and/or the poor enforcement of such protection, the decreasing national control over resources, and the impact of the third world debt and associated structural adjustment programmes (Pearce et al. 1994).
As a result, it cannot be said that the problem of occupational cancer has been reduced in recent years, since in many instances the exposure has simply been transferred from the industrialized to the developing world. In some instances, the total occupational exposure has increased. Nevertheless, the recent history of occupational cancer prevention in industrialized countries has shown that it is possible to use substitutes for carcinogenic compounds in industrial processes without leading industry to ruin, and similar successes would be possible in developing countries if adequate regulation and control of occupational carcinogens were in place.
Prevention of Occupational Cancer
Swerdlow (1990) outlined a series of options for the prevention of exposure to occupational causes of cancer. The most successful form of prevention is to avoid the use of recognized human carcinogens in the workplace. This has rarely been an option in industrialized countries, since most occupational carcinogens have been identified by epidemiological studies of populations that were already occupationally exposed. However, at least in theory, developing countries could learn from the experience of industrialized countries and prevent the introduction of chemicals and production processes that have been found to be hazardous to the health of workers.
The next best option for avoiding exposure to established carcinogens is their removal once their carcinogenicity has been established or suspected. Examples include the closure of plants making the bladder carcinogens 2-naphthylamine and benzidine in the United Kingdom (Anon 1965), termination of British gas manufacture involving coal carbonization, closure of Japanese and British mustard gas factories after the end of the Second World War (Swerdlow 1990) and gradual elimination of the use of benzene in the shoe industry in Istanbul (Aksoy 1985).
In many instances, however, complete removal of a carcinogen (without closing down the industry) is either not possible (because alternative agents are not available) or is judged politically or economically unacceptable. Exposure levels must therefore be reduced by changing production processes and through industrial hygiene practices. For example, exposures to recognized carcinogens such as asbestos, nickel, arsenic, benzene, pesticides and ionizing radiation have been progressively reduced in industrialized countries in recent years (Pearce and Matos 1994).
A related approach is to reduce or eliminate the activities that involve the heaviest exposures. For example, after an 1840 act was passed in England and Wales prohibiting chimney-sweeps from being sent up chimneys, the number of cases of scrotal cancer decreased (Waldron 1983). Exposure also can be minimized through the use of protective equipment, such as masks and protective clothing, or by imposing more stringent industrial hygiene measures.
An effective overall strategy in the control and prevention of exposure to occupational carcinogens generally involves a combination of approaches. One successful example is a Finnish registry which has as its objectives to increase awareness about carcinogens, to evaluate exposure at individual workplaces and to stimulate preventive measures (Kerva and Partanen 1981). It contains information on both workplaces and exposed workers, and all employers are required to maintain and update their files and to supply information to the registry. The system appears to have been at least partially successful in decreasing carcinogenic exposures in the workplace (Ahlo, Kauppinen and Sundquist 1988).
Worksite Strategies to Improve Maternal and Infant Health: Experiences of US Employers
There is a growing awareness among public and private sector employers in the United States that healthy birth outcomes, productivity and the organization’s economic status are connected. Concurrently, there is heightened concern about occupational reproductive health hazards. Never before have employers had better reasons to improve maternal and infant health among employees and their families. Rising health care costs, changing workforce demographics, and increasing evidence that healthy employees lead to productivity gains, are compelling reasons to make maternal and infant health an addition to their health education and promotion programs.
A maternal and infant health strategy is a term broadly used to define any thoughtfully planned employer-sponsored or union-sponsored initiative that promotes the health and well-being of women, before, during, and after pregnancy, and supports the health of infants during the first year of life as well. There is no single solution or approach to improving maternal and infant health. Rather, for most employers, the effort is a combination of the following activities, custom-fit to meet the environment that makes their workplace unique.
Health Care Benefits
It is helpful to view maternal and infant health care benefits as a continuum of care that provides reproductive health awareness and family planning counseling and services throughout the reproductive life span. The benefits listed in table 1 represent those a health insurance plan should cover because of their significance in improving maternal and infant health.
Table 1. Health insurance benefits.
|
Pre-pregnancy |
Pregnancy |
Post-pregnancy |
Infancy |
|
Annual preconception or interconception care visit (includes family planning services) Genetic counseling and testing Prescription drug plan Substance abuse treatment |
Genetic counseling and testing Prenatal care–should be offered with no deductibles or copayments Labor and delivery at a hospital or birthing centre should be offered with no deductibles or copayments
|
Postpartum care Prescription drug plan Home health care services Substance abuse treatment |
Normal newborn nursery care Neonatal intensive care–no pre-existing conditions exclusions for newborns Prescription drug plan Home health care services |
Source: March of Dimes Birth Defects Foundation 1994.
Benefits design
While many American health care plans provide coverage for preconception and prenatal care, there are a number of reasons why it may be difficult for some women to obtain high quality, affordable care. For example, some providers require payment in advance for prenatal care and delivery services, yet most insurers will not make payment until after delivery. Other barriers to accessing proper care include high deductible fees or copayments, inconvenient office hours, lack of coverage for dependants, and geographic inaccessibility. Employers cannot eliminate all of these barriers, but it would represent an excellent beginning to help remove the burdens of upfront payments and high deductible fees and to offer assistance to the employee in finding acceptance by a suitable provider of prenatal care.
At Texas Instruments (TI), the goal is to make prenatal care affordable regardless of an employee’s income level or health care provider. Mothers seeking prenatal care inside the TI network pay only 10% of an upfront negotiated fee, a single charge that covers prenatal care services and both uncomplicated deliveries and Caesarean sections.
The Haggar Apparel Company pays 100% of the cost of prenatal care upfront if an employee or dependant accesses prenatal care in the first trimester of pregnancy. The Home Depot (a retailer of builder’s wares and related merchandise) waives the expectant mother’s hospital deductible fee if prenatal care visits begin in the first trimester.
While many plans provide for adequate care for a newborn’s first few days of life, coverage for the infant’s ongoing preventive care after leaving the hospital, frequently referred to as well-baby care, is often inadequate or nonexistent.
At the First National Bank of Chicago, expectant mothers who are enrolled in the indemnity plan and who complete a prenatal education program by the end of their fourth month of pregnancy have the $400 deductible charge waived from their newborn’s first year health insurance coverage. The Monfort Company, a beef packing plant in Greeley, Colorado, totally covers well-baby care up to age three.
Benefits-related Services and Employee Programs
Table 2 lists benefits-related services and programs that are considered important supportive features to a maternal and infant health strategy. These services and programs may be provided directly by the employer, either in the workplace or a nearby location, or under a contract with an outside agency or vendor, depending on the structure, location and size of the organization and may be administered by the benefits, employee health, health promotion or employee assistance department, for example.
Few companies can offer all of these components; however, the more complete and comprehensive the strategy, the better the chance of improving the health of mothers and babies.
Table 2. Other benefits-related services provided by the employer.
|
SERVICES |
|||
|
Pre-pregnancy |
Pregnancy |
Post-pregnancy |
Infancy |
|
|
||
|
PROGRAMMES |
|||
|
Pre-pregnancy |
Pregnancy |
Post-pregnancy |
Infancy |
|
|
|
|
Source: March of Dimes Birth Defects Foundation 1994.
Pre-pregnancy and pregnancy period
Maternity management programs are gaining popularity because they offer attractive features to both the expectant parents and the employer. While not designed to replace prenatal care delivered by a health care professional, maternity management is a benefit-related service that provides personalized advice and support customized to a mother’s needs and risk levels.
Levi Strauss & Company, one of the nation’s largest clothing and apparel producers, offers a maternity management program administered by an insurance company. Employees are encouraged to access the program as soon as they are pregnant and they will receive $100 cash for calling the toll-free maternity management number. In 1992, costs for newborns whose mothers participated in the program were nearly 50% lower than for those whose mothers who did not.
The First National Bank of Chicago offers the March of Dimes Babies and You prenatal health promotion program as part of its maternal and infant health strategy. This program is described below and in the case study on p. 15.23 above.
Babies and You: A prenatal health promotion program
The March of Dimes’ Babies and You prenatal health promotion program was developed in 1982 in partnership with maternal and infant health care specialists throughout the country. Extensively field-tested by March of Dimes chapters and worksites, the program is continuously updated and enhanced.
Babies and You educates adults about how to practice healthy lifestyle behaviors before and during pregnancy, motivates women to get early and regular prenatal care, and influences employers to implement strategies that support healthy pregnancy outcomes.
Prenatal health promotion activities should be reaching male as well as female employees, partners, other family members and friends. Babies and You is adaptable to the unique needs of any given workforce. Consideration is given to the educational level, culture and language of prospective participants, as well as to any worksite restrictions and available community resources.
Because employers are at different stages in their health promotion activities, Babies and You offers three levels of implementation: an information campaign, educational seminars, and training of health professionals (see box). The most popular topics for informational materials and educational seminars are preconception and prenatal care, fetal development, genetics, the male role in pregnancy, nutrition during pregnancy, and parenting. The topics covered in the prenatal programs of 31 companies surveyed by the New York Business Group on Health found the dominant themes to be understanding what goes on during pregnancy and delivery; timely care by qualified health professionals; practicing healthy behaviors related to pregnancy and avoidance of hazards that might affect mother and/or fetus; care of the newborn; and maintaining satisfactory family and work relationships (Duncan, Barr and Warshaw 1992).
BABIES AND YOU: Levels of Implementation
Level I Informational Campaign is designed to create awareness at the worksite about the importance of early and regular prenatal care. To sustain this level of implementation, a variety of print and audiovisual materials is available from the March of Dimes.
Level II Educational Seminars are delivered at the worksite by March of Dimes volunteer health professionals. Fourteen different seminar topics are available to choose from, including: preconception care, prenatal care, nutrition, exercise and pregnancy, pregnancy after 35, stress and pregnancy, pregnancy complications, well-baby care, male role in pregnancy, and breastfeeding.
Level III Training of Health Professionals allows a worksite to establish Babies and You as an on-going component of its wellness activities. The March of Dimes provides a one-day training on program delivery and implementation to on-site health professionals such as occupational health nurses, benefits managers, medical directors and health promotion specialists.
But no matter what level of Babies and You a worksite chooses to implement, there are eight goals of a successful prenatal health promotion effort that this program strives to achieve:
- Management commitment
- Inter-departmental program planning
- Employee input
- The offering of incentives
- Supportive benefits and policies
- Establishment of communications channels
- Access to community resources
- Evaluation
Post-pregnancy and infancy period
In addition to implementing health promotion programs and other services that focus on a mother’s health before and during pregnancy, many employers also offer programs that support parents and infants after pregnancy, during the critical first twelve months and beyond. Maternity disability benefits, lactation programs, dependant care reimbursement accounts (e.g., pre-tax set-asides of earnings that employees may draw on to pay for dependant care expenses), parenting classes and onsite child care are just a few of the benefits and programs now offered.
For example, to maintain goodwill with its employees, Lancaster Laboratories, based in Lancaster, Pennsylvania, and providing contract laboratory research and consulting to the environmental, food and pharmaceutical industries, continues to provide health care insurance benefits during both maternity disability leave and unpaid parental leave whether or not the employee plans to return to work after having given birth. This family-supportive management approach has gotten results: in an industry where a 27% turnover rate is the norm, the rate at Lancaster is only 8% (March of Dimes 1994).
Lactation programs also are easy and beneficial for employers to implement. The health benefits of breastfeeding extend beyond the child’s own. A recent study shows that improving an infant’s health through breastfeeding has a direct effect on employee productivity. Healthier infants mean mothers and fathers miss significantly fewer days of work to care for a sick child (Ryan and Martinez 1989). Offering a lactation program simply requires providing onsite space and equipment for pumping and storing breast milk.
The Los Angeles Department of Water and Power was able to quantify some benefits of its lactation program: for example, 86% of participants state that the program eased their transition back to work; 71% report taking less time off since participating; and program participants have a 2% turnover rate (March of Dimes 1994).
Employer Policies
There are many workplace policies that employers can initiate to create a maternal and infant-health supportive culture. Instituting new policies and changing old ones can send an important message to employees about the company’s corporate culture.
Some policies affect the health of all workers, like creating a smoke-free environment. Others focus on selected groups, such as those that address occupational reproductive health hazards and which are targeted to meet the needs of men and women who are planning to have a child. Still more, including flexible work policies, support pregnant women in scheduling prenatal visits and ease the burden of parents with infants and small children. Finally, policies relating to modifying work assignments when needed during pregnancy and resolving questions of disability and its duration help to protect the health of the pregnant worker while minimizing interference with her work assignments.
When the Warner-Lambert Company, a leader in the pharmaceutical, consumer health care and confectionary products industries, initiated its maternity management and prenatal education programs, the company also introduced comprehensive guidelines for managing reproductive health. The guidelines encourage employees to complete questionnaires assessing the potential of reproductive health hazards in their jobs or worksites. If necessary, a Warner-Lambert safety engineer will conduct an assessment to determine what, if any, control of workplace hazards or job restrictions may be necessary.
In addition to reproductive health hazards policies, a number of employers offer flexible family leave policies. For example, at AT&T, the communications giant, employees can take up to 12 months of unpaid leave to care for a newborn or adopted child. More than 50% of the employees who have taken advantage of this leave policy since 1990 returned to work within three months. Within six months, 82% of the employees were back at work (March of Dimes 1994).
And at PepsiCo Inc., the large beverage and food conglomerate based in Purchase, New York, fathers of newborns can take up to eight weeks of paid leave and an additional eight weeks of unpaid leave with a guarantee of the same or a comparable job when they return (March of Dimes 1994).
Designing a Maternal and Infant Health Strategy to Meet Business Needs
Any sustainable employer-based maternal and infant health strategy, in addition to being acceptable to employees, must meet sound business objectives. Depending on a company’s objectives, different benefits, employee programs, or policies may take priority. The following steps are useful in developing a preliminary strategy:
- Document existing benefits, programmes, and policies that support maternal and infant health in order to create the foundation of a formal strategy.
- Find out about community resources available to assist the company’s efforts.
- Prepare a prioritized list of preliminary maternal and infant health initiatives which includes changes or introductions in benefits, programmes, or policies.
- Gain preliminary support from top management before taking the next step.
- Assess perceived needs and test proposed strategies with employees to validate preliminary recommendations.
- Develop a formal maternal and infant health strategy by articulating a mission, outlining objectives, allocating the resources needed, identifying potential obstacles and key players, preparing an implementation timetable and gaining necessary support at all levels of the company.
Implementing maternal and infant health initiatives
The next step is to implement the benefits, programs and policies that are part of the strategy. The implementation process typically includes the following steps:
- Assign responsibility for implementation.
- Select quality measurements by which to manage the programme.
- Evaluate and select vendors.
- Review incentives and other methods to increase employee participation.
- Communicate initiatives to employees and family members.
Managing the success of a maternal and infant health strategy
After implementation, an employer’s maternal and infant health strategy should be reviewed for effectiveness in meeting original objectives and business needs. Evaluation and feedback are essential and help to ensure that the maternal and infant health initiatives are meeting both the employer’s and employees’ needs.
Mother and Child Health in France
Shortly after World War II, France instituted Protection maternelle et infantile (PMI), a nationwide system through which public and private health professionals, in collaboration with social services, provide basic preventive health, medical, social and educational services to pregnant women, infants and children through to the age of six.
For the most part, families and private physicians arrange individually for preconception counseling, family planning, early and regular prenatal care and preventive health examinations and vaccinations for children up to the age of six. Participation in the program is encouraged through 100% reimbursement by national health insurance (in order to qualify for this coverage, women must register their pregnancies by the 15th week of gestation), monthly (family) allowance payments from a woman’s fourth month of gestation through to the child’s third month of life as an incentive for compliance with the national guidelines for preventive care, and a continuing program of information and education.
Women not able to participate in care via the private sector are covered by 96 locally controlled PMI centers, one in each French département. In addition to providing free neighborhood health clinics, these centers identify and target for intervention pregnant women and children at risk, conduct home visits and monitor the progress of all women and infants to ensure that the preventive services called for in the national guidelines are received.
The employers’ role in this system is regulated by law. They provide pregnant women with:
- Job changes; flexible hours to ease commuting burdens and rest periods in order to reduce the stress and fatigue that may lead to premature delivery
- Maternity leave with job security for mothers who bear or adopt children to promote bonding and healthy child development (a maternity benefit amounting to 84% of the salary, is paid by social security up to a ceiling)
- Part-time work arrangements and unpaid parental leave with job security to enable parents to balance child care and work responsibilities (a national parental allowance helps to offset the cost of the unpaid leave) (Richardson 1994)
Conclusion
The need to address maternal and infant health in the American workplace will increase as more and more women enter the labor force and as family and workplace issues become inseparable. Forward-thinking companies have already recognized this and are developing innovative approaches. Employers are in a unique and powerful position to influence change and become leaders in promoting healthy mothers and babies.
Mammography Programme at Marks and Spencer: A Case Study
This case study describes the mammography program at Marks and Spencer, the first to be offered by an employer on a nationwide scale. Marks and Spencer is an international retail operation with 612 stores worldwide, the majority being in the United Kingdom, Europe and Canada. In addition to a number of international franchise operations, the company owns Brooks Brothers and Kings Super Markets in the United States and D’Allaird’s in Canada and pursues extensive financial activities.
The company employs 62,000 people, the majority of whom work in 285 stores in the United Kingdom and the Republic of Ireland. The company’s reputation as a good employer is legendary and its policy of good human relations with staff has included the provision of comprehensive, high-quality health and welfare programs.
Although a treatment service is provided at some work locations, this need is largely met by community-based primary care physicians. The company health policy emphasizes the early detection and prevention of disease. A number of innovative screening programs have consequently been developed over the past 20 years, many of which have predated similar projects in the National Health Service (NHS). Over 80% of the workforce is female, a fact that has influenced the choice of screening programs, which include cervical cytology, ovarian cancer screening and mammography.
Breast Cancer Screening
In the mid-1970s the New York HIP study (Shapiro 1977) proved that mammography was capable of detecting impalpable breast cancers with the expectation that earlier detection would reduce mortality. To an employer of large numbers of middle-aged women, the appeal of mammography was obvious and a screening program was introduced in 1976 (Hutchinson and Tucker 1984; Haslehurst 1986). At that time there was virtually no access to reliable high-quality mammography in the public sector and that available in private health care organizations was of variable quality and expensive. The first task therefore was to ensure access to a uniformly high quality and this challenge was met by using mobile screening units, each equipped with a waiting area, examination cubicle and mammography equipment.
Centralized administration and film processing allowed continuous checks on all aspects of quality and allowed film interpretation to be undertaken by an experienced group of mammographers. There was, however, a disadvantage in that the radiographer was not able to immediately examine the developed film to verify that there were no technical errors so that if there had been any, the employee could be recalled or other arrangements made for the necessary repeat examination.
Compliance has always been exceptionally high and has remained over 80% for all age groups. Doubtless this is due peer group pressure, the easy availability of the service at or near the worksite and, until recently, a lack of mammography facilities in the NHS.
Women are invited to join the screening program and attendance is entirely voluntary. Prior to screening, short educational sessions are carried out by the company doctor or nurse, both of whom are available to answer queries and give explanations. Common anxieties include concern about radiation dosage and worry that the compression of the breast may cause pain. Women who are recalled for further tests are seen during working hours and fully recompensed for travel expenses for themselves and a companion.
Three modalities were used for the first five years of the program: clinical examination by a highly trained nurse-practitioner, thermography and mammography. Thermography was a time-consuming examination with a high rate of false positives and made no contribution to the cancer detection rate; accordingly it was discontinued in 1981. Although of limited value in cancer detection, clinical examination, which includes a detailed review of personal and family history, provides invaluable information to the radiologist and allows the client time to discuss her fears and other health issues with a sympathetic health professional. Mammography is the most sensitive of the three tests. Cranio-caudal and lateral oblique views are taken at the initial examination with single views only at the interval check. Single reading of films is the norm, though double reading is used for difficult cases and as a random quality check. Figure 1 shows the contribution of clinical examination and mammography to the total cancer detection rate. Of the 492 cases of cancer found, 10% were detected by clinical examination alone, 54% by mammography alone, and 36% were noted by clinical examination and mammography.
Figure 1. Screening for breast cancer. Contribution of clinical examination and mammography to cancer detection, by age group.
Women aged 35 to 70 were offered screening when the program was first introduced but the low cancer detection rate and high incidence of benign breast disease among those in the 35 to 39 age group led to withdrawal of the service in 1987 from these women. Figure 19 shows the numbers of screen-detected cancers by age group.
Figure 2. Age distribution of screen-detected cancers.
Similarly, the screening interval has changed from a yearly interval (reflecting initial enthusiasm) to a two-year gap. Figure 3 shows the number of screen-detected cancers by age group with the corresponding numbers of interval tumors and missed tumors. Interval cases are defined as those occurring after a truly negative screen during the time between routine tests. Missed cases are defined as those cancers which can be seen retrospectively on the films but were not identified at the time of the screening test.
Figure 3. Number of screen-detected cancers, interval cancers and missed cancers, by age group.
Among the screened population, 76% of breast cancers were detected at screening with a further 14% of cases occurring during the interval between examinations. The interval cancer rate will be carefully monitored to ensure that it does not rise to an unacceptably high level.
The survival benefit of screening women under the age of 50 remains unproven although it is agreed that smaller cancers are detected and this allows some women to choose between mastectomy or breast conservation therapy—a choice valued highly by many. Figure 4 shows the sizes of screen-detected cancers, the majority being under two centimeters in size and node negative.
Figure 4. Sizes of screen-detected cancers.
Impact of the Forrest Report
In the late 1980s, Professor Sir Patrick Forrest recommended that regular breast screening be made available to women over the age of 50 via the NHS (i.e., with no charge at the point of delivery of the service) (Forrest 1987). His most important recommendation was that the service should not start until specialist staff had been fully trained in the multidisciplinary approach to breast care diagnosis. Such staff was to include radiologists, nurse counselors and breast physicians. Since 1990, the United Kingdom has had an outstanding breast screening and assessment service for women over 50.
Coincidentally with this national development, Marks and Spencer reviewed its data and a major flaw in the program became apparent. The recall rate following routine screening was in excess of 8% for women over fifty and 12% for younger women. Analysis of the data showed that common reasons for recall were technical problems, such as malpositioning, processing errors, difficulties with grid lines or a need for further views. Additionally, it was clear that the use of ultrasonography, specialized mammography and fine needle aspiration cytology could cut the recall and referral rate even further. An initial study confirmed these impressions, and it was decided to redefine the screening protocol so that clients who needed further tests were not referred back to their family practitioners, but were retained within the screening program until a definitive diagnosis was made. Most of these women were returned to a schedule of routine recall after the further investigations and this reduced the formal surgical referral rate to a minimum.
Instead of duplicating the service provided by the National Health Service, a policy of partnership was developed which allowed Marks and Spencer to draw upon the expertise of the public sector while company funding is used to improve service for all. The breast screening program is now delivered by a number of providers: about half the requirement is met by the original mobile service but employees at the larger city stores now receive routine screening at specialist centers, which may either be in the private or public sectors. This cooperation with the National Health Service has been an exciting and challenging development and has helped to improve the overall standards of breast diagnosis and care for the entire population. By marrying together both private worksite and public sector programs it is possible to deliver an exceptionally high quality service to a widely distributed population.
" DISCLAIMER: The ILO does not take responsibility for content presented on this web portal that is presented in any language other than English, which is the language used for the initial production and peer-review of original content. Certain statistics have not been updated since the production of the 4th edition of the Encyclopaedia (1998)."