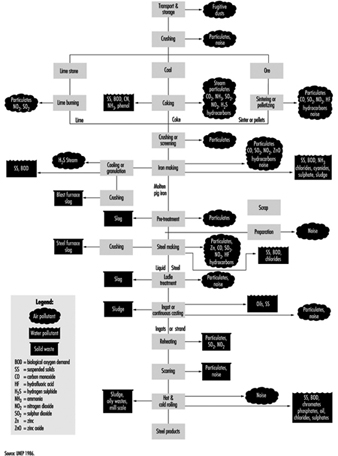
Adapted from UNEP and IISI 1997 and an unpublished article by Jerry Spiegel.
Because of the sheer volume and complexity of its operations and its extensive use of energy and raw materials, the iron and steel industry, like other “heavy” industries, has the potential of having a significant impact on the environment and the population of nearby communities. Figure 1 summarizes the pollutants and wastes generated by its major production processes. They comprise three primary categories: air pollutants, waste water contaminants and solid wastes.
Figure 1. Flow chart of pollutants & wastes generated by different processes
Historically, investigations of the public health impact of the iron and steel industry have concentrated on the localized effects in the densely populated local areas in which steel production has been concentrated and particularly in specific regions where acute air pollution episodes have been experienced, such as the Donora and Meuse valleys, and the triangle between Poland, the former Czechoslovakia and the former German Democratic Republic (WHO 1992).
Air Pollutants
Air pollutants from iron- and steel-making operations have historically been an environmental concern. These pollutants include gaseous substances such as oxides of sulphur, nitrogen dioxide and carbon monoxide. In addition, particulates such as soot and dust, which may contain iron oxides, have been the focus of controls. Emissions from coke ovens and from coke oven by-product plants have been a concern, but the continuous improvements in the technology of steel-making and of emissions control during the past two decades, coupled with more stringent government regulations, have significantly reduced such emissions in North America, Western Europe and Japan. Total pollution control costs, over half of which relate to air emissions, have been estimated to range from 1 to 3% of total production costs; air- pollution control installations have represented approximately 10 to 20% of total plant investments. Such costs create a barrier to the global application of state-of-the-art controls in developing countries and for older, economically marginal enterprises.
Air pollutants vary with the particular process, the engineering and construction of the plant, the raw materials employed, the sources and amounts of the energy required, the extent to which waste products are recycled into the process and the efficiency of the pollution controls. For example, the introduction of basic-oxygen steel making has permitted the collection and recycling of waste gases in a controlled manner, reducing the amounts to be exhausted, while the use of the continuous-casting process has reduced the consumption of energy, resulting in a reduction of emissions. This has increased product yield and improved quality.
Sulphur dioxide
The amount of sulphur dioxide, formed largely in the combustion processes, depends primarily on the sulphur content of the fossil fuel employed. Both coke and coke-oven gas used as fuels are major sources of sulphur dioxide. In the atmosphere, sulphur dioxide may react with oxygen radicals and water to form a sulphuric acid aerosol and, in combination with ammonia, may form an ammonium sulphate aerosol. The health effects attributed to sulphur oxides are not only due to the sulphur dioxide but also to its tendency to form such respirable aerosols. In addition, sulphur dioxide may be adsorbed onto particulates, many of which are in the respirable range. Such potential exposures may be reduced not only by use of fuels with low sulphur content but also by reduction of the concentration of the particulates. The increased use of electric furnaces has decreased the emission of sulphur oxides by eliminating the need for coke, but this has passed on this pollution control burden to the plants generating electricity. Desulphurization of coke-oven gas is achieved by the removal of reduced sulphur compounds, primarily hydrogen sulphide, prior to combustion.
Nitrogen oxides
Like the sulphur oxides, oxides of nitrogen, primarily nitrogen oxide and nitrogen dioxide, are formed in fuel combustion processes. They react with oxygen and volatile organic compounds (VOCs) in the presence of ultraviolet (UV) radiation to form ozone. They also combine with water to form nitric acid, which, in turn, combines with ammonia to form ammonium nitrate. These may also form respirable aerosols which can be removed from the atmosphere through wet or dry deposition.
Particulate matter
Particulate matter, the most visible form of pollution, is a varying, complex mixture of organic and inorganic materials. Dust may be blown from stockpiles of iron ore, coal, coke and limestone or it may enter the air during their loading and transport. Coarse materials generate dust when they are rubbed together or crushed under vehicles. Fine particles are generated in the sintering, smelting and melting processes, particularly when molten iron comes in contact with air to form iron oxide. Coke ovens produce fine coal coke and tar emissions. Potential health effects depend on the number of particles in the respirable range, the chemical composition of the dust and the duration and concentration of exposure.
Sharp reductions in the levels of particulate pollution have been achieved. For example, by using electrostatic precipitators to clean dry waste gases in oxygen steel making, one German steel works decreased the level of emitted dust from 9.3 kg/t of crude steel in 1960 to 5.3 kg/t in 1975 and to somewhat less than 1 kg/t by 1990. The cost, however, was a marked rise in energy consumption. Other methods of particulate pollution control include the use of wet scrubbers, bag houses and cyclones (which are effective only against large particles).
Heavy metals
Metals such as cadmium, lead, zinc, mercury, manganese, nickel and chromium can be emitted from a furnace as a dust, fume or vapour or they may be adsorbed by particulates. Health effects, which are described elsewhere in this Encyclopaedia, depend on the level and duration of exposure.
Organic emissions
Organic emissions from primary steel operations may include benzene, toluene, xylene, solvents, PAHs, dioxins and phenols. The scrap steel used as raw material may include a variety of these substances, depending on its source and the way it was used (e.g., paint and other coatings, other metals and lubricants). Not all of these organic pollutants are captured by the conventional gas cleaning systems.
Radioactivity
In recent years, there have been reports of instances in which radioactive materials have inadvertently been included in the scrap steel. The physicochemical properties of the nuclides (e.g., melting and boiling temperatures and affinity for oxygen) will determine what happens to them in the steel making process. There may be an amount sufficient to contaminate the steel products, the by-products and the various types of wastes and thus require a costly clean-up and disposal. There is also the potential contamination of the steel-making equipment, with resultant potential exposure of the steel workers. However, many steel operations have installed sensitive radiation detectors to screen all purchased steel scrap.
Carbon dioxide
Although it has no effect on human health or ecosystems at the usual atmospheric levels, carbon dioxide is important because of its contribution to the “greenhouse effect”, which is associated with global warming. The steel industry is a major generator of carbon dioxide, more from the use of carbon as a reducing agent in the production of iron from iron ore than from its use as a source of energy. By 1990, through a variety of measures for blast furnace coke rate reduction, waste-heat recovery and energy saving, carbon dioxide emissions by the iron and steel industry had been reduced to 47% of the levels in 1960.
Ozone
Ozone, a major constituent of atmospheric smog near the surface of the earth, is a secondary pollutant formed in air by the photochemical reaction of sunlight on nitrogen oxides, facilitated to a varying degree, depending on their structure and reactivity, by a range of VOCs. The major source of ozone precursors is motor vehicle exhausts, but some are also generated by iron and steel plants as well as by other industries. As a result of atmospheric and topographic conditions, the ozone reaction may take place at great distances from their source.
Waste Water Contaminants
Steel works discharge large volumes of water to lakes, rivers and streams, with additional volumes being vaporized while cooling coke or steel. Waste water retained in unsealed or leaking holding ponds can seep through and may contaminate the local water table and underground streams. These may also be contaminated by the leaching of rainwater through piles of raw materials or accumulations of solid wastes. Contaminants include suspended solids, heavy metals and oils and greases. Temperature changes in natural waters due to discharge of higher temperature process water (70% of steel-making process water is used for cooling) may affect the ecosystems of these waters. Consequently, cooling treatment prior to discharge is essential and can be achieved through application of available technology.
Suspended solids
Suspended solids (SS) are the main waterborne pollutants discharged during steel production. They comprise mainly iron oxides from scale formation during processing; coal, biological sludge, metallic hydroxides and other solids may also be present. These are largely non-toxic in aqueous environments at normal discharge levels. Their presence at higher levels may lead to discolouration of streams, de-oxygenation and silting.
Heavy metals
Steel-making process water may contain high levels of zinc and manganese, while discharges from cold-rolling and coatings areas may contain zinc, cadmium, aluminium, copper and chromium. These metals are naturally present in the aquatic environment; it is their presence at higher than usual concentrations that creates concern about potential effects on humans and the ecosystems. These concerns are increased by the fact that, unlike many organic pollutants, these heavy metals do not biodegrade to harmless end products and may become concentrated in sediments and in the tissues of fish and other aquatic life. Further, by being combined with other contaminants (e.g., ammonia, organic compounds, oils, cyanides, alkalis, solvents and acids), their potential toxicity may be increased.
Oils and greases
Oils and greases may be present in waste water in both soluble and insoluble forms. Most heavy oils and greases are insoluble and are relatively easily removed. They may become emulsified, however, by contact with detergents or alkalis or by being agitated. Emulsified oils are routinely used as part of the process in cold mills. Except for causing discolouration of the water surface, small quantities of most aliphatic oil compounds are innocuous. Monohydric aromatic oil compounds, however, may be toxic. Further, oil components may contain such toxicants as PCBs, lead and other heavy metals. In addition to the question of toxicity, the biological and chemical oxygen demand (BOD and COD) of oils and other organic compounds can decrease the oxygen content of the water, thus affecting the viability of aquatic life.
Solid Wastes
Much of the solid waste produced in steel making is reusable. The process of producing coke, for example, gives rise to coal derivatives which are important raw materials for the chemical industry. Many by-products (e.g., coke dust) can be fed back into the production processes. Slag produced when the impurities present in coal and iron ore melt and combine with the lime used as a flux in smelting can be used in a number of ways: land fill for reclamation projects, in road building and as raw material for sintering plants that supply blast furnaces. Steel, regardless of grade, size, use or length of time in service, is completely recyclable and can be recycled repeatedly without any degradation of its mechanical, physical or metallurgical properties. The recycling rate is estimated to be 90%. Table 1presents an overview of the degree to which the Japanese steel making industry has achieved the recycling of waste materials.
Table 1. Waste generated and recycled in steel production in Japan
|
Generation (A) |
Landfill (B) |
Re-use |
|
|
Slag Blast furnaces |
24,717 |
712 |
97.1 |
|
Dust |
4,763 |
238 |
95.0 |
|
Sludge |
519 |
204 |
60.7 |
|
Waste oil |
81 |
||
|
Total |
41,519 |
3,570 |
91.4 |
Source: IISI 1992.
Energy Conservation
Energy conservation is desirable not only for economic reasons but also for reducing pollution at energy-supply facilities such as electric utilities. The amount of energy consumed in steel production varies widely with the processes used and the mix of scrap metal and iron ore in the feed material. The energy intensity of United States scrap-based plants in 1988 averaged 21.1 gigajoules per tonne while the Japanese plants consumed about 25% less. A model International Iron and Steel Institute (IISI) scrap-based plant required only 10.1 gigajoules per tonne (IISI 1992).
Increases in the cost of energy have stimulated development of energy- and materials-saving technologies. Low-energy gases, such as by-product gases produced in the blast-furnace and coke-oven processes, are recovered, cleaned and used as a fuel. Consumption of coke and auxiliary fuel by the German steel industry, which averaged 830 kg/tonne in 1960, was reduced to 510 kg/tonne in 1990. The Japanese steel industry was able to reduce its share of total Japanese energy consumption from 20.5% in 1973 to about 7% in 1988. The United States steel industry has made major investments in energy conservation. The average mill has reduced energy consumption by 45% since 1975 through process modification, new technology and restructuring (carbon dioxide emissions have fallen proportionately).
Facing the Future
Traditionally, governments, trade associations and individual industries have approached environmental concerns on a media-specific basis, dealing separately, for example, with air, water and waste disposal problems. While useful, this has sometimes merely shifted the problem from one environmental area to another, as in the case of costly waste water treatment which leaves the subsequent problem of disposing of the treatment sludge, which can also cause serious ground water pollution.
In recent years, however, the international steel industry has addressed this problem through Integrated Pollution Control, which has further developed into Total Environmental Risk Management, a programme that looks at all impacts simultaneously and addresses the priority areas systematically. A second development of equal importance has been a focus on preventive rather than remedial action. This addresses such issues as plant siting, site preparation, plant layout and equipment, specification of day-to-day management responsibilities, and the assurance of adequate staff and resources to monitor compliance with environmental regulations and report the results to appropriate authorities.
The Industry and Environment Centre, established in 1975 by the United Nations Environment Programme (UNEP), aims to encourage cooperation between the industries and governments in order to promote environmentally sound industrial development. Its goals include:
- encouragement of the incorporation of environmental criteria in industrial development plans
- facilitation of the implementation of procedures and principles for the protection of the environment
- promotion of the use of safe and clean techniques
- stimulation of the exchange of information and experience throughout the world.
The UNEP works closely with the IISI, the first international industry association devoted to a single industry. The IISI’s members include publicly- and privately-owned steel-producing companies and national and regional steel industry associations, federations and research institutes in the 51 countries which, together, account for over 70% of the total world steel production. IISI, often in concert with UNEP, produces statements of environmental policy and principles and technical reports such as the one on which much of this article has been based (UNEP and IISI 1997). Together, they are working to address the economic, social, moral, personal, management and technological factors that influence compliance with environmental principles, policies and regulations.