This article presents information on basic process equipment, storage, plant layout and operations considerations in chemical process industries, including major items and concepts that are broadly applicable throughout the chemical industry. However, much of the equipment required in chemical processing is highly specialized and cannot be broadly generalized. More detailed information on toxicity and hazardous materials and process safety are reviewed elsewhere in this Encyclopaedia.
There are two basic categories of layout in chemical processing industries: plant layout, which covers all process units, utilities, storage areas, loading/unloading areas, buildings, shops and warehousing, and unit or process layout, which covers only equipment placement for a specific process, also termed a process block.
Plant Layout
Siting
Locating or siting an overall plant is based upon a number of general factors, as shown in table 1 (CCPS 1993). These factors vary considerably with locations, governments and economic policies. Of these various factors, safety considerations are an extremely important concern, and in some locations they can be the major factor that governs plant siting.
Table 1. Some general site selection factors
- Population density around the site
- Natural disaster occurrence (earthquake, flood, etc.)
- Prevailing winds and meteorological data
- Availability of power, steam and water
- Safety considerations
- Air, water and waste regulations and their complexity
- Accessibility to raw materials and markets
- Transportation
- Siting permits and complexity of obtaining them
- Interaction requirements in industrial developments
- Labour availability and costs
- Investment incentives
One important aspect of plant safety in siting is defining a buffer zone between a plant with hazardous processes and nearby plants, dwellings, schools, hospitals, highways, waterways and airplane corridors. Some overall safety considerations are presented in table 2. The buffer zone is important because distance tends to reduce or mitigate potential exposures from various accidents. The distance necessary to reduce toxic concentrations to acceptable levels through atmospheric interaction and the dispersion of toxic materials from an accidental release can be defined. Moreover, the time lag between a toxic release and public exposure created by a buffer zone can be used to warn the population through pre-planned emergency response programmes. Since plants have various types of facilities containing toxic materials, dispersion analyses should be conducted on the potentially hazardous systems to ensure the buffer zone is adequate in each area surrounding the plant perimeter.
Table 2. Plant siting safety considerations
- Buffer zone
- Location of other hazardous installations in vicinity
- Inventory of toxic and hazardous materials
- Adequacy of firefighting water supply
- Emergency equipment access
- Availability of emergency response support from adjacent industries and the community
- Weather extremes and prevailing winds
- Location of highways, waterways, railroad and airplane corridors
- Environmental and waste disposal restrictions during emergencies
- Draining and grade slope
- Maintenance and inspection
Fire is a potential hazard in process plants and facilities. Large fires can be a source of thermal radiation which can also be mitigated by distance. Elevated flares can also be a source of thermal radiation during an emergency or startup/shutdown operation. A flare is a device that automatically burns exhaust gases or emergency vapour releases at elevated positions or special ground locations. These should be sited away from the plant perimeter (for community protection) and an area at the flare base should be prohibited to workers. If not operated properly, liquid carryover into the flare can result in burning liquid droplets. In addition to fire, there can be explosions within equipment or a vapour cloud that produces blast waves. Although distance will reduce the blast intensity somewhat over the buffer zone, the blast will still have an effect on the nearby community.
The potential of accidental releases or fires from existing facilities that may be near the proposed site should also be considered. Potential incidents should be modelled and evaluated to determine the possible effect on the proposed plant layout. Emergency responses to an external event should be evaluated and responses coordinated with other plants and affected communities.
Other considerations
Dow Chemical Company has developed another approach to plant layout based on an acceptable level of Maximum Probable Property Damage (MPPD) and Business Interruption Risk (B1) (Dow Chemical Company 1994a). These considerations are important for both new and existing plants. The Dow Fire and Explosion Index is useful in new plant layouts or in the addition of equipment to existing plants. If risks calculated from the Index are found to be unacceptable, the separation distances should be increased. Alternatively, layout changes may also reduce the risk potential.
Overall layout
In an overall plant layout, the prevailing winds are an important consideration. Ignition sources should be located upwind of potential leak sources. Fired heaters, boilers, incinerators and flares are in this category (CCPS 1993). The location of storage tanks downwind of process units and utilities is another recommendation (CCPS 1993). Environmental regulations have led to significantly reduced leakage from tankage (Lipton and Lynch 1994).
Minimum separation distances have been outlined in various publications for process units, equipment and different plant functions (CCPS 1993; Dow Chemical Company 1994a; IRI 1991). General facilities that normally have recommended distance separations in overall plant layouts are shown in table 3. Actual distance recommendations should be carefully defined. While fired heaters and process furnaces are not shown in table 3, they are an important item and recommended distance separations must be included in a unit process layout.
Table 3. Facilities generally separated in overall plant layouts
- Process units
- Tank farms
- Loading and unloading facilities
- Flares
- Power, boilers and incinerators
- Cooling towers
- Substations, large electrical switch yards
- Central control houses
- Warehouses
- Analytical laboratories
- Incoming utility metering and block systems
- Fire hoses, fixed monitors, reservoirs and emergency fire pumps
- Waste treatment areas
- Maintenance buildings and areas
- Administrative buildings
In addition, roads are necessary for emergency and maintenance vehicle or equipment access and require careful placement between process units and throughout the various sections of the plant. Acceptable clearances for overhead pipe racks and other overhead equipment should be established along with lateral clearances at cross-roads and entrances to all facilities.
The layout requirements can be based on recommended minimum separation distances (CCPS 1993; NFPA 1990; IRI 1991; Mecklenburgh 1985) or determined through a hazard analysis (Dow Chemical Company 1994a).
Process Unit Layout
Table 3 presents an overall plant separations layout summary. The process units are contained within the specific block shown in the general layout. The chemical process is generally shown in detail in process and implementation diagrams (P&IDs). A process layout requires considerations beyond specific equipment separation distances, some of which are shown in table 4.
Table 4. General considerations in a process unit layout
- Area definition for future expansion and unit accessibility
- Repair equipment accessibility for frequent maintenance
- Space requirements for individual equipment repair (e.g., area needed for pulling heat exchanger bundle or accessibility for control valve)
- Barriers for high pressure equipment or reactors with explosion potential
- Mechanical and space requirements for loading/unloading solids-filled reactors or towers
- Space for venting dust explosions
- Separation of frequently opened or maintained equipment from high temperature piping, vessels, etc.
- Special buildings or structures and necessary clearance (e.g., a compressor house with an internal bridge crane or external crane)
The assemblage of equipment in any particular process unit will vary considerably, depending on the process. The toxicity and hazardous characteristics of the streams and materials within the units also vary widely. Despite these differences, minimum distance standards have been developed for many equipment items (CCPS 1993; NFPA 1990; IRI 1991; Mecklenburgh 1985). Procedures for calculating potential leakage and toxic exposures from process equipment that can also affect separation distance are available (Dow Chemical Company 1994b). In addition, dispersion analysis can be applied when leakage estimates have been calculated.
Equipment and separation distance
A matrix technique can be used to calculate the space needed for separating equipment (CCPS 1993; IRI 1991). Calculations based upon specific processing conditions and an equipment hazard evaluation may result in separation distances that differ from a standard matrix guide.
Extensive lists for a matrix can be developed by refinement of individual categories and by the addition of equipment. For example, compressors may be split into several types, such as those handling inert gas, air and hazardous gases. Separation distances for engine-driven compressors may differ from motor- or steam-driven machines. Separation distances in storage facilities that house liquefied gases should be analysed on the basis of whether the gas is inert.
The process battery limits should be carefully defined. They are the boundary lines or plot limits for a process unit (the name derives from the early use of a battery of ovens in processing). Other units, roads, utilities, pipeways, runoff ditches and so on are plotted based upon battery limits. While unit equipment location does not extend to the battery limits, separation distances of equipment from battery limits should be defined.
Control rooms or control houses
In the past each process unit was designed with a control room that provided operational control of the process. With the advent of electronic instrumentation and computer-controlled processing, individual control rooms have been replaced by a central control room that controls a number of process units in many operations. The centralized control room is economically advantageous because of process optimization and increases in efficiency of personnel. Individual process units still exist and, in some specialized units, older control houses which have been supplanted by centralized control rooms may still be used for local process monitoring and for emergency control. Although control room functions and locations are generally determined by process economics, the design of the control room or control house is very important for maintaining emergency control and for worker protection. Some considerations for both central and local control houses include:
- pressurizing the control house to prevent the entrance of toxic and hazardous vapours
- designing the control house for blast and explosion resistance
- establishing a location that is at minimal risk (based upon separation distance and probability of gas releases)
- purifying all inlet air and installing an inlet stack location that minimizes the intake of toxic or hazardous vapours
- sealing all sewer outlets from the control house
- installing a fire suppression system.
Inventory reduction
An important consideration in process and plant layouts is the quantity of toxic and hazardous material in the overall inventory, including the equipment. The consequences of a leak are more severe as the volume of material increases. Consequently, the inventory should be minimized wherever possible. Improved processing that reduces the number and size of pieces of equipment reduces the inventory, lowers the risk and also results in lower investment and improved operating efficiencies.
Some potential inventory reduction considerations are shown in table 6. Where a new process facility will be installed, processing should be optimized by taking into consideration some of the objectives shown in table 5.
Table 5. Steps for limiting inventory
- Reducing storage tank inventory reduction through improved process control, operation and just-in-time inventory control
- Eliminating or minimizing onsite tank inventory through process integration
- Using reaction variable analysis and development for reactor volume reduction
- Replacing batch reactors with continuous reactors, which also reduces downstream holdup
- Lowering distillation column holdup through bottoms-volume reductions and tray holdup with either more advanced trays or packings
- Replacing kettle reboilers with thermosyphon reboilers
- Minimizing overhead drum and bottoms surge drum volumes
- Improving pipe layout and sizing to minimize holdup
- Where toxic materials are produced, minimizing the toxic section holdup
Storage Facilities
The storage facilities in a chemical processing plant can house liquid and solid feed, intermediate chemicals, by-products and process products. Products stored in many facilities serve as intermediates or precursors for other processes. Storage may also be required for diluents, solvents or other process materials. All of these materials are generally stored in above-ground storage tankage (AST). Underground tankage is still used in some locations, but use is generally limited due to access problems and limited capacity. In addition, potential leakage of such underground storage tanks (USTs) presents environmental problems when leaks contaminate ground water. General earth contamination can lead to potential atmospheric exposures with higher vapour-pressure materials leaks. Leaked materials can be a potential exposure problem during ground remediation efforts. UST leakage has resulted in stringent environmental regulations in many countries, such as the requirements for double-walled tanks and underground monitoring.
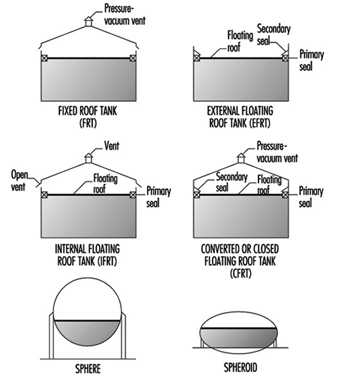
Typical above-ground storage tanks are shown in figure 1. Vertical ASTs are cone or domed roof tanks, floating roof tanks that are covered or non-covered floating roof or external floating roof tanks (EFRTs). Converted or closed roof tanks are EFRTs with covers installed on the tanks that are frequently geodesic type domes. Since EFRTs over time do not maintain a perfectly circular shape, sealing the floating roof is difficult and a covering is installed on the tank. A geodesic dome design eliminates roof trusses needed for cone roof tanks (FRTs). The geodesic dome is more economical than a cone roof and, in addition, the dome reduces losses of materials to the environment.
Figure 1. Typical above-ground storage tanks
Normally, the tanks are limited to liquid storage where the liquid vapour pressure does not exceed 77 kPa. Where the pressure exceeds this value, spheroids or spheres are used since both are designed for pressure operation. Spheroids can be quite large but are not installed where the pressure may exceed certain limits defined by the mechanical design. For most higher vapour-pressure storage applications, spheres are normally the storage container and are equipped with pressure relief valves to prevent over pressuring. A safety concern that has developed with spheres is rollover, which generates excessive vapour and results in relief valve discharges or in more extreme situations such as sphere wall rupture (CCPS 1993). In general, the liquid contents stratify and if warm (less dense) material is loaded into the sphere bottom, the warm material rises to the surface with the cooler, higher density surface material rolled over to the bottom. The warm surface material vaporizes, raising the pressure, which may result in relief valve discharge or sphere overpressuring.
Tank layout
Tankage layout requires careful planning. There are recommendations for tank separation distances and other considerations (CCPS 1988; 1993). In many locations, separation distances are not specified by code, but minimum distances (OSHA 1994) can be a result of various decisions applicable to separation distances and locations. Some of these considerations are presented in table 6. In addition, tank service is a factor in tank separation for pressurized, refrigerated and atmospheric tanks (CCPS 1993).
Table 6. Tank separation and location considerations
- Separation based on shell to shell distances can be based on references and subject to calculating the thermal radiation distance in the event of fire in an adjacent tank.
- Tanks should be separated from process units.
- A tank location, preferably downwind from other areas, minimizes ignition problems in the event of a tank releasing a significant vapour quantity.
- Storage tanks should have dykes, which are also required by law in most regions.
- Tanks can be grouped for utilization of common dykes and firefighting equipment.
- Dykes should have isolation capability in an emergency.
Dykes are required and are nominally sized volumetrically to hold the contents of a tank. Where multiple tanks are within a dyke, the minimum volumetric dyke capacity is equivalent to the capacity of the largest tank (OSHA 1994). The dyke walls can be constructed of earth, steel, concrete or solid masonry. However, the earth dykes should be impenetrable and have a flat top with a minimum width of 0.61 m. In addition, the soil within the dyked area should also have an impenetrable layer to prevent any chemical or oil leakage into the soil.
Tank leakage
A problem that has been developing through the years is tank leakage as a result of corrosion in the tank bottom. Frequently, tanks have water layers in the tank bottom that can contribute to corrosion, and electrolytic corrosion may occur due to contact with the earth. As a result, regulatory requirements have been instituted in various regions to control tank bottom leakage and underground soil and water contamination from contaminants in the water. A variety of design procedures have been developed to control and monitor leakage (Hagen and Rials 1994). In addition, double bottoms have also been installed. In some installations, cathodic protection has been installed to further control metal deterioration (Barletta, Bayle and Kennelley 1995).
Water draw off
Manually discharging water periodically from the tank bottom can result in exposure. Visual observation to determine the interface through open manual draining can result in worker exposure. A closed discharge can be installed with an interface sensor and control valve minimizing potential worker exposures (Lipton and Lynch 1994). A variety of sensors are commercially available for this service.
Overfilling tanks
Frequently, tanks are overfilled, creating potential safety and worker exposure hazards. This can be prevented with redundant or dual-level instruments controlling inlet block valves or feed pumps (Bahner 1996). For many years, overflow lines were installed on chemical tanks, but they terminated a short distance above a drain opening to permit visual observation of the overflow discharge. Moreover, the drain had to be sized for greater than the maximum fill rate to ensure proper drainage. However, such a system is a potential exposure source. This can be eliminated by connecting the overflow line directly to the drain with a flow indicator in the line to show the overflow. Although this will function satisfactorily, this results in overloading the drain system with a very large contaminant volume and potential health and safety problems.
Tank inspection and cleaning
Periodically, tanks are removed from service for inspection and/ or cleaning. These procedures must be carefully controlled to prevent worker exposure and minimize potential safety hazards. Following draining, tanks are frequently flushed with water to remove process liquid traces. Historically, the tanks have then been cleaned manually or mechanically where necessary. When tanks are drained, they are filled with vapour that may be toxic and can be within a combustible range. Water flushing may not significantly affect vapour toxicity, but it may reduce potential combustion problems. With floating roofs, the material below the floating roof can be flushed and drained, but some tanks may still have material in the sump. This bottom material must be removed manually and may present potential exposure concerns. Personnel may be required to wear personal protective equipment (PPE).
Normally, enclosed tanks and any volume below the floating roofs are purged with air until a specified oxygen concentration level is achieved before entry is permitted. However, concentration measurements should be continually obtained to ensure toxic concentration levels are satisfactory and do not change.
Vapour venting and emission control
For fixed roof or converted floating roof tanks (CFRTs), venting to the atmosphere may not be acceptable in many locations. The pressure-vacuum (PV) vent (shown in figure 2 these tanks are removed and the vapours flow through a closed duct to a control device where the contaminants are destroyed or recovered. For both tanks, an inert purge (e.g., nitrogen) can be injected to eliminate the diurnal vacuum effect and maintain a positive pressure for the recovery device. In the CFRT tank, the nitrogen eliminates the diurnal effect and reduces any vapours to the atmosphere through a PV vent. However, vapour emissions are not eliminated. A large number of control devices and techniques are available including combustion, absorbers, condensers and absorption (Moretti and Mukhopadhyay 1993; Carroll and Ruddy 1993; Basta 1994; Pennington 1996; Siegall 1996). Selection of a control system is a function of final emission targets and operating and investment costs.
In floating roof tanks, both external and internal, seals and auxiliary fitting controls effectively minimize vapour losses.
Safety hazards
Flammability is a major concern in tankage and fire-fighting systems are required to aid in control and prevention of expanded fire zones. Firewater systems and installation recommendations are available (CCPS 1993; Dow Chemical Company 1994a; NFPA 1990). Water can be sprayed directly on a fire under certain conditions and is essential in cooling adjacent tankage or equipment to prevent overheating. In addition, foam is an effective fire-fighting agent and permanent foam equipment can be installed on tanks. The installation of foam equipment on mobile fire-fighting equipment should be reviewed with a manufacturer. Environmentally acceptable and low toxicity foams are now available that are effective and comparable to other foams in quickly extinguishing fires.
Processing Equipment
A wide variety of process equipment is required in chemicals processing as a result of the numerous processes, specialized process requirements and variations in products. Consequently, all of the chemical equipment in use today cannot be reviewed; this section will concentrate on the more widely applied equipment found in processing sequences.
Reactors
There are a large number of reactor types in the chemical industry. The basis for reactor selection is a function of a number of variables, beginning with classifying whether the reaction is a batch or continuous reaction. Frequently, batch reactions are converted to continuous operations as experience with the reaction increases and some modifications, such as improved catalysts, become available. Continuous reaction processing is generally more efficient and produces a more consistent product, which is desirable in meeting product quality targets. However, there are still a large number of batch operations.
Reaction
In all reactions, the classifications of a reaction as exothermic or endothermic (producing heat or requiring heat) is necessary in order to define the heating or cooling requirements necessary to control the reaction. In addition, runaway reaction criteria must be established to install instrument sensors and controls that can prevent a reaction from becoming out of control. Prior to full-scale operation of a reactor, emergency procedures must be investigated and developed to ensure the runaway reaction is safely contained. Some of the various potential solutions are emergency control equipment that is automatically activated, injection of a chemical that stops the reaction and vent facilities that can accommodate and contain the reactor contents. Safety valve and vent operation are extremely important requiring well-maintained and functioning equipment at all times. Consequently, multiple interlocked safety valves are frequently installed to ensure that maintenance on one valve will not reduce the required relief capacity.
Should a safety valve or vent discharge due to malfunction, the discharge effluent must be contained in practically all circumstances to minimize potential safety and health hazards. As a result, the method of containing the emergency discharge through piping along with final disposition of the reactor discharge should be carefully analysed. In general, liquid and vapour should be separated with the vapour sent to a flare or recovery and liquid recycled where possible. Solids removal may require some study.
Batch
In reactors involving exothermic reactions, an important consideration is fouling on the walls or internal tubing by the cooling media used to maintain the temperature. Removal of fouled material varies considerably and the method of removal is a function of the fouled material characteristics. Fouled material can be removed with a solvent, a high-pressure jet nozzle stream or, in some cases, manually. In all these procedures, safety and exposure must be carefully controlled. Movement of material in and out of the reactor must not permit the entrance of air, which may result in a flammable vapour mixture. Vacuums should be broken with an inert gas (e.g., nitrogen). Vessel entry for inspection or work can be classified as entry into a confined space and the rules for this procedure should be observed. Vapour and dermal toxicity should be understood and technicians must be knowledgeable about health hazards.
Continuous
Flow-through reactors can be filled with liquid or a vapour and liquid. Some reactions produce slurries in the reactors. Also, there are reactors that contain solid catalysts. The reaction fluid may be liquid, vapour or a combination of vapour and liquid. Solid catalysts, which promote a reaction without participating in it, are normally contained within grids and are termed fixed beds. The fixed-bed reactors may have single or multiple beds and can have exotherinic or endothermic reactions, with most reactions requiring a constant temperature (isothermal) through each bed. This frequently requires the injection of feed streams or a diluent at various locations between beds to control the temperature. With these reaction systems, temperature indication and sensor location through the beds are extremely important to prevent a reaction runaway and product yield or quality changes.
Fixed beds generally lose their activity and must be regenerated or replaced. For regeneration, deposits on the bed may be burned off, dissolved in a solvent or, in some cases, regenerated through the injection of a chemical in an inert fluid into the bed, thereby restoring catalyst activity. Depending on the catalyst, one of these techniques may be applied. Where beds are burned, the reactor is emptied and purged of all process fluids then filled with an inert gas (usually nitrogen), which is heated and recirculated, raising the bed to a specified temperature level. At this point, a very small volume of oxygen is added to the inert stream to initiate a flame front that gradually moves through the bed and controls the temperature rise. Excessive oxygen quantities have a deleterious effect on the catalyst.
Fixed-bed catalyst removal
Removal of fixed-bed catalysts must be carefully controlled. The reactors are drained of process fluid and then the remaining fluid is displaced with a flushing fluid or purged with a vapour until all of the process fluid has been removed. Final purging may require other techniques before the vessel can be purged with an inert gas or air prior to opening the vessel or discharging the catalyst from the vessel under an inert blanket. Should water be used in this process, the water is drained through closed piping to a process sewer. Some catalysts are sensitive to air or oxygen, becoming pyrophoric or toxic. These require special procedures to eliminate air during filling or emptying the vessels. Personal protection along with handling procedures must be carefully defined to minimize potential exposures and protect personnel.
Spent catalyst disposal may require further treating before it is sent to a catalyst manufacturer for recycling or into an environmentally acceptable disposal procedure.
Other catalyst systems
Gas flowing through a loose solid catalyst bed expands the bed and forms a suspension that is similar to a liquid and termed a fluid bed. This type of reaction is used in various processes. Spent catalysts are removed as a gas-solids side stream for regeneration and then returned to the process through an enclosed system. In other reactions, catalyst activity may be very high and, although catalyst is discharged in the product, the concentration is extremely low and does not pose a problem. Where a high concentration of catalyst solids in the product vapour is undesirable, solids carryover must be removed before purification. However, traces of solids will remain. These are removed for disposal in one of the by-product streams, which in turn must be clarified.
In situations where spent catalyst is regenerated through burning, extensive solids recovery facilities are required in fluid-bed systems to meet environmental restrictions. Recovery may consist of various combinations of cyclones, electric precipitators, bag filters) and/ or scrubbers. Where burning occurs in fixed beds, the basic concern is temperature control.
Since fluid-bed catalysts are frequently within the respiratory range, care must be exercised during solids handling to ensure worker protection with either fresh or recovered catalysts.
In some instances a vacuum may be used to remove various components from a fixed bed. In these situations, a steam-driven vacuum jet is frequently the vacuum producer. This produces a steam discharge that frequently contains toxic materials although in very low concentration in the jet stream. However, the discharge of a steam jet should be carefully reviewed to determine contaminant quantities, toxicity and potential dispersion if it is discharged directly to the atmosphere. Should this be unsatisfactory, the jet discharge may require condensing in a sump where all vapours are controlled and the water is sent to the closed sewer system. A rotary vacuum pump will perform in this service. The discharge from a reciprocating vacuum pump may not be permitted to discharge directly to the atmosphere, but can in some instances discharge into a flare line, incinerator or process heater.
Safety
In all reactors, pressure increases are a major concern since the vessel pressure rating must not be exceeded. These pressure increases may be a result of poor process control, malfunction or a runaway reaction. Consequently, pressure relief systems are required to maintain vessel integrity by preventing reactor overpressuring. Relief valve discharges must be carefully designed to maintain adequate relief under all conditions, including relief-valve maintenance. Multiple valves may be required. Should a relief valve be designed to discharge into the atmosphere, the discharge point should be elevated above all nearby structures and a dispersion analysis should be conducted to ensure adequate protection for workers and nearby communities.
If a rupture disk is installed with a safety valve, the discharge should also be enclosed and the final discharge location designated as described above. Since a disk rupture will not reseat, a disk without a safety valve will probably release most of the reactor contents and air may enter the reactor at the end of the release. This requires a careful analysis to ensure that a flammable situation is not created and that highly undesirable reactions do not occur. Moreover, the discharge from a disk may release liquid and the vent system must be designed to contain all liquids with vapour discharged, as described above. Atmospheric emergency releases must be approved by regulatory authorities before installation.
Mixer agitators installed in reactors are sealed. Leaks may be hazardous and if they occur the seal must be repaired which requires a reactor shutdown. The reactor contents may require special handling or precautions and an emergency shutdown procedure should include reaction termination and disposition of the reactor contents. Flammability and exposure control must be carefully reviewed for each step including final disposition of the reactor mix. Since a shutdown can be expensive and involve production loss, magnetic driven mixers and newer seal systems have been introduced to reduce maintenance and reactor shutdowns.
Entrance to all reactors requires compliance with safe confined-space entry procedures.
Fractionation or distillation towers
Distillation is a process whereby chemical substances are separated through methods which take advantage of differences in boiling points. The familiar towers in chemical plants and refineries are distillation towers.
Distillation in various forms is a processing step found in the great majority of chemical processes. Fractionation or distillation can be found in purification, separation, stripping, azeotropic and extractive process steps. These applications now include reactive distillation, where a reaction occurs in a separate section of the distillation tower.
Distillation is conducted with a series of trays in a tower, or it can be conducted in a tower filled with packing. The packings have special configurations that readily permit the passage of vapour and liquid, but provide sufficient surface area for vapour-liquid contact and efficient fractionation.
Operation
Heat is normally supplied to a tower with a reboiler, although the heat content of specific streams may be sufficient to eliminate the reboiler. With reboiler heat, multiple step vapour-liquid separation occurs on the trays and lighter materials ascend through the tower. Vapours from the top tray are fully or partially condensed in the overhead condenser. The condensed liquid is collected in the distillate recovery drum, where part of the liquid is recycled to the tower and the other portion is withdrawn and sent to a specific location. Non-condensed vapours may be recovered elsewhere or sent to a control device which can be a combustor or recovery system.
Pressure
Towers typically operate at pressures higher than atmospheric pressure. However, towers are frequently operated under vacuum to minimize liquid temperatures that may affect product quality or in situations where tower materials become a mechanical and economic concern due to the temperature level that may be difficult to achieve. Also, high temperatures may affect the fluid. In heavy petroleum fractions, very high tower bottoms temperatures frequently result in coking problems.
Vacuums are typically obtained with ejectors or vacuum pumps. In process units, vacuum loadings consist of some light vapour materials, inerts that may have been in the tower feed stream and air from leakage. Normally the vacuum system is installed after a condenser to reduce the organic loading to the vacuum system. The vacuum system is sized based upon the estimated vapour loading, with ejectors handling larger vapour loadings. In certain systems a vacuum machine may be directly connected to a condenser outlet. A typical ejector system operation is a combination of ejectors and direct barometric condensers where the ejector vapours have direct contact with the cooling water. Barometric condensers are very large consumers of water and the steam-water mixture results in high water outlet temperatures that tend to vaporize any organic compound traces in the atmospheric barometric sump, potentially increasing workplace exposures. In addition, a large effluent load is added to the waste-water system.
A large water reduction is achieved along with a substantial reduction in steam consumption in modified vacuum systems. Since the vacuum pump will not handle a large vapour load, a steam ejector is used in the first stage in combination with a surface condenser to reduce the vacuum pump load. In addition, a sump drum is installed for above-ground operation. The simpler system reduces waste-water loading and maintains a closed system that eliminates potential vapour exposures.
Safety
All towers and drums must be protected from overpressure that may result from malfunction, fire (Mowrer 1995) or utility failure. A hazard review is necessary and is required by law in some countries. A general process safety management approach that is applicable to process and plant operation improves safety, minimizes losses and protects worker health (Auger 1995; Murphy 1994; Sutton 1995). Protection is provided by pressure relief valves (PRVs) that discharge to the atmosphere or to a closed system. The PRV is generally mounted at the tower top to relieve the large vapour load, although some installations locate the PRV in other tower locations. The PRV can also be located on the distillate overhead recovery drum as long as valves are not placed between the PRV and the tower top. If block valves are installed in the process lines to the condenser then the PRV must be installed on the tower.
When distillation tower overpressure is relieved, under certain emergency scenarios, the PRV discharge may be exceedingly large. Very high loading in a closed system discharge vent line may be the largest load in the system. Since a PRV discharge can be sudden and the overall relieving time may be quite short (less than 15 minutes), this extremely large vapour load must be carefully analysed (Bewanger and Krecter 1995; Boicourt 1995). Since this short, large peak load is difficult to process in control devices such as absorbers, adsorbers, furnaces and so on, the preferable control device in most situations is a flare for vapour destruction. Normally, a number of PRVs are connected to a flare line header that in turn is connected to a single flare. However, the flare and overall system must be carefully designed to cover a large group of potential contingencies (Boicourt 1995).
Health hazards
For direct relief to the atmosphere, a detailed dispersion analysis of the relief valve discharge vapours should be conducted to ensure that workers are not exposed and that community concentrations are well within allowable concentration guidelines. In controlling dispersion, atmospheric relief valve discharge lines may have to be raised to prevent excessive concentrations on nearby structures. A very tall flare-like stack may be necessary to control dispersion.
Another area of concern is entering a tower for maintenance or mechanical changes during a shutdown. This entails entering a confined space and exposes workers to the associated hazards. The flushing and purging method prior to opening must be carefully conducted to ensure minimal exposures by reducing any toxic concentrations below recommended levels. Before commencing with flushing and purging operations, the tower pressure must be reduced and all piping connections to the tower must be blinded (i.e., flat metal disks must be placed between the tower flanges and the connecting pipe flanges). This step should be carefully managed to ensure minimum exposures. In different processes, the methods of clearing the tower of toxic fluids vary. Frequently, the tower fluid is displaced with a fluid that has very low toxicity characteristics. This displacement fluid is then drained and pumped to a selected location. The remaining liquid film and droplets can be steamed to the atmosphere through a top flange that has a special stand-off blind with an opening between the blind and tower flange. Following steaming, air enters the tower through the special blind opening as the tower cools. A manhole at the tower bottom and one at the tower top are opened permitting the blowing of air through the tower. When the internal tower concentration reaches a predetermined level, the tower can be entered.
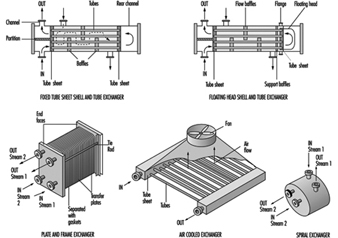
Heat exchangers
There are a wide variety of heat exchangers in the chemical process industry. Heat exchangers are mechanical devices for the transfer of heat to or from a process stream. They are selected in accordance with process conditions and exchanger designs. A few of the common exchanger types are shown in figure 2. Selection of the optimum exchanger for a process service is somewhat complicated and requires a detailed investigation (Woods 1995). In many situations, certain types are not suitable because of pressure, temperature, solids concentration, viscosity, flow quantity and other factors. Moreover, an individual heat exchanger design can vary considerably; several types of floating head tube and sheet exchangers are available (Green, Maloney and Perry 1984). The floating head is normally selected where the temperatures may cause excessive tube expansion that otherwise could not maintain integrity in a fixed tube sheet exchanger. In the simplified floating head exchanger in figure 2, the floating head is contained completely within the exchanger and does not have any connection with the shell cover. In other floating head designs, there may be packing around the floating tubesheet (Green, Maloney and Perry 1984).
Figure 2. Typical heat exchangers
Leakage
The packing on floating tubesheets is in contact with the atmosphere and may be a source of leakage and potential exposure. Other exchangers may also have potential leakage sources and should be examined carefully. As a result of their heat transfer characteristics, plate and frame exchangers are often installed in the chemical industry. The plates have various corrugations and configurations. Plates are separated by gaskets that prevent mixing of the streams and provide an external seal. However, the seals limit temperature applications to about 180 ºC, although seal improvements may overcome this limitation. Since there are a number of plates, the plates must be compressed properly to ensure proper sealing between them. Consequently, careful mechanical installation is necessary to prevent leakage and potential hazards. Since there are a large number of seals, careful seal monitoring is important to minimize potential exposures.
Air cooled exchangers are attractive economically and have been installed in a wide number of process applications and in various locations within process units. To save space, these exchangers are often installed over pipe runs and are frequently stacked. Since tube material selection is important, a variety of materials is used in the chemical industry. These tubes are connected to the tube sheet. This requires use of compatible materials. Leakage through a tube crack or at the tube sheet is a concern since the fan will circulate vapours from the leak and dispersion may result in potential exposures. Air dilution may significantly reduce the potential exposure hazard. However, fans are frequently shut down under some weather conditions and in these circumstances leak concentrations can increase thereby increasing potential exposures. Moreover, if leaking tubes are not repaired, the crack may worsen. With toxic liquids that do not readily vaporize, dripping can occur and result in potential dermal exposure.
Shell and tube heat exchangers may develop leaks through any of the various flanges (Green, Maloney and Perry 1984). Since shell and tube heat exchangers vary in size from small to very large surface areas, the diameter of outer flanges is generally much larger than typical pipe flanges. With these large flanges, the gaskets must not only withstand process conditions, but provide a seal under bolt load variations. Various gasket designs are used. Maintaining constant bolt load stresses on all of the flange bolts is difficult, resulting in leakage in many exchangers. The flange leakage can be controlled with flange sealing rings (Lipton and Lynch 1994).
Tube leakage may occur in any of the available exchanger types, with the exception of plate exchangers and a few other specialty exchangers. However, these latter exchangers have other potential problems. Where tubes leak into a cooling water system, the cooling water discharges the contaminant into a cooling tower which can be an exposure source to both workers and a nearby community. Consequently, the cooling water should be monitored.
The dispersion of cooling tower vapours can be widespread as a result of the fans in forced and induced draft cooling towers. In addition, natural convection towers discharge vapours to the atmosphere which then disperse. However, dispersion varies considerably based upon both weather conditions and the discharge elevation. Less volatile toxic materials remain in the cooling water and the cooling tower blowdown stream, which should have sufficient treatment capability to destroy contaminants. The cooling tower and tower basin must be cleaned periodically and contaminants add to the potential hazards in the basin and in the tower fill. Personal protection is necessary for much of this work.
Exchanger cleaning
A problem with tubes in cooling water service is the build-up of material in the tubes resulting from corrosion, biological organisms and solids deposition. As described above, tubes may also leak through cracks, or leakage may occur where tubes are rolled into striations in the tube sheet. When any of these conditions occur, exchanger repair is required and the process fluids must be removed from the exchanger. This requires a completely contained operation, which is necessary to meet environmental, safety and health exposure objectives.
Generally, the process fluid is drained to a receiver and the remaining material is flushed out of the exchanger with a solvent or inert material. The latter material is also sent to a receiver for the contaminated material by draining or pressuring with nitrogen. Where toxic material was in the exchanger, the exchanger should be monitored for any traces of toxic material. If testing results are unsatisfactory, the exchanger can be steamed to vaporize and remove all traces of material. However, the steam vent should be connected to a closed system to prevent vapour escape into the atmosphere. While the closed vent may not be absolutely necessary, at times there may be more contaminant material in the exchanger, requiring closed steam venting at all times to control potential hazards. Following steaming, a vent to the atmosphere admits air. This general procedure is applicable to the exchanger side or sides containing toxic material.
Chemicals then used for cleaning the tubes or the shell side should be circulated in a closed system. Normally, the cleaning solution is recirculated from a tank truck system and the contaminated solution in the system is drained to a truck for disposition.
Pumps
One of the most important process functions is the movement of liquids and in the chemical industry all types of liquid materials are moved with a wide variety of pumps. Canned and magnetic pumps are sealless centrifugal pumps. Magnetic pump drivers are available for installation on other pump types to prevent leakage. Types of pumps used in the chemical process industry are listed in table 7.
Table 7. Pumps in the chemicals process industry
- Centrifugal
- Reciprocating (plunger)
- Canned
- Magnetic
- Turbine
- Gear
- Diaphragm
- Axial flow
- Screw
- Moving cavity
- Lobe
- Vane
Sealing
From a health and safety standpoint, sealing and repairing centrifugal pumps are major concerns. Mechanical seals, which constitute the prevalent shaft sealing system, can leak and at times have blown out. However, there have been major advances in seal technology since the 1970s that have resulted in significant leakage reductions and extended pump service life. Some of these improvements are bellows seals, cartridge seals, improved face designs, better face materials and improvements in pump variable monitoring. Moreover, continuing research in seal technology should result in further technology improvements.
Where process fluids are highly toxic, leakless or sealless canned or magnetic pumps are frequently installed. Operating service periods or the mean time between maintenance (MTBM) has improved markedly and generally varies between three and five years. In these pumps, the process fluid is the lubricating fluid for the rotor bearings. Vaporization of the internal fluid adversely affects the bearings and often makes bearing replacement necessary. Liquid conditions in the pumps can be maintained by ensuring the internal pressure in the bearing system is always greater than the liquid vapour pressure at the operating temperature. When repairing a sealless pump, completely draining a relatively low volatility material is important and should be carefully reviewed with the supplier.
In typical centrifugal process pumps, packing has essentially been replaced with mechanical seals. These seals are generally classified as single or dual mechanical seals, with the latter term covering tandem or double mechanical seals. There are other dual seal combinations, but they are not as widely used. In general, tandem or double mechanical seals with liquid buffer fluids between the seals are installed to reduce seal leakage. Pump mechanical seal standards for both centrifugal and rotary pumps covering single and dual mechanical seal specification and installation were issued by the American Petroleum Institute (API 1994). A mechanical seal application guide is now available to aid in the evaluation of seal types (STLE 1994).
To prevent excessive leakage or blow-out from a failed seal, a gland plate is installed following the seal. It may have a gland flush fluid to move the leakage into a closed drain system (API 1994). Since the gland system is not a complete seal, auxiliary seal systems, such as throttle bushings are available.They are installed in the gland that controls excessive leakage to the atmosphere or seal blow-out (Lipton and Lynch 1994). These seals are not designed for continuous operation; after activation they will operate for up to two weeks before failure, thereby providing time for operations to switch pumps or make process adjustments.
A newer mechanical seal system is available that essentially reduces emissions to the nil level. This is a double mechanical seal system with a gas buffer system that replaces the liquid buffer in the standard dual mechanical seal system (Fone 1995; Netzel 1996; Adams, Dingman and Parker 1995). In the liquid buffer systems, the seal faces are separated by an extremely thin lubricating film of buffer fluid that also cools the seal faces. Although separated slightly, a certain amount of face contact exists which results in seal wear and seal face heating. The gas seals are called non-contact seals since one seal face with curved indentations pumps gas through the seal faces and builds a gas layer or dam that completely separates the seal faces. This lack of contact results in a very long seal life and also reduces the seal friction loss, thereby noticeably decreasing power consumption. Since the seal pumps gas there is a very small flow into the process and to the atmosphere.
Health hazards
A major concern with pumps is draining and flushing to prepare the pump for maintenance or repair. Draining and removal covers both process fluid and buffer fluids. Procedures should require discharge of all fluids into a closed connection drain system. In the pump stuffing box where a throat bushing separates the impeller from the stuffing box, the bushing acts as a weir in holding some liquid in the stuffing box. Weep holes in the bushing or a drain in the stuffing box will permit complete process liquid removal through draining and flushing. For buffer fluids, there should be a method of draining all fluid from the dual seal area. Maintenance requires seal removal and if the seal volume is not completely drained and flushed, the seals are a potential source of exposure during repair.
Dust and powders
Handling of dusts and powders in solids processing equipment is a concern due to the potential for fire or explosion. An explosion within equipment may burst through a wall or enclosure as a result of explosion-generated pressure sending a combined pressure and fire wave into the workplace area. Workers can be at risk, and adjacent equipment can be severely impacted with drastic effects. Dusts or powders suspended in air or in a gas with oxygen present and in a confined space are susceptible to explosion when a source of ignition with sufficient energy is present. Some typical explosive equipment environments are shown in table 8.
Table 8. Potential explosion sources in equipment
|
Conveying equipment |
Storage |
|
Pneumatic ducts |
Bins |
|
Mechanical conveyors |
Hoppers |
|
Rotary valves |
|
|
Processing equipment |
|
|
Filter dust collectors |
Grinders |
|
Fluid bed dryers |
Ball mills |
|
Transfer line dryers |
Powder mixing |
|
Screening |
Cyclones |
An explosion produces heat and rapid gas expansion (pressure increase) and generally results in deflagration, which is a flame front that moves rapidly but at less than the sound velocity for these conditions. When the flame front velocity is greater than the sound velocity or is at supersonic velocity the condition is termed detonation, which is more destructive than deflagration. Explosion and flame front expansion occur in milliseconds and do not provide sufficient time for standard process responses. Consequently, the potential fire and explosion characteristics of the powder must be defined to determine the potential hazards that may exist in the various processing steps (CCPS 1993; Ebadat 1994; Bartknecht 1989; Cesana and Siwek 1995). This information can then provide a basis for the installation of controls and the prevention of explosions.
Explosion hazard quantification
Since the explosions generally occur in enclosed equipment, various tests are conducted in specially-designed laboratory equipment. While powders may appear similar, published results should not be used since small differences in the powders can have very different explosion characteristics.
A variety of tests conducted on powder can define the explosion hazard and the test series should encompass the following.
The classification test determines whether a powder dust cloud can initiate and propagate flames (Ebadat 1994). Powders that have these characteristics are considered Class A powders. Those powders that do not ignite are termed Class B. The Class A powders then require a further series of tests to evaluate their explosion and hazard potential.
The minimum ignition energy test defines the minimum spark energy necessary for ignition of a powder cloud (Bartknecht 1989).
In explosion severity and analysis Group A powders are then tested as a dust cloud in a sphere where the pressure is measured during a test explosion based on minimum ignition energy. The maximum explosion pressure is defined along with the rate of change in pressure per unit time. From this information, the explosion specific characteristic value (Kst) in bar metres per second is determined and the explosion class is defined (Bartknecht 1989; Garzia and Senecal 1996):
Kst(bar·m/s) Dust explosion class Relative strength
1-200 St 1 Somewhat weaker
201-300 St 2 Strong
300+ St 3 Very strong
A large number of powders have been tested and the majority were in the St 1 class (Bartknecht 1989; Garzia and Senecal 1996).
In assessment of non-cloud powders, powders are tested to determine safe operating procedures and conditions.
Explosion prevention tests
Explosion prevention tests can be helpful where explosion suppression systems cannot be installed. They provide some information on desirable operating conditions (Ebadat 1994).
The minimum oxygen test defines the oxygen level below which the dust will not ignite (Fone 1995). Inert gas in the process will prevent ignition if the gas is acceptable.
The minimum dust concentration is determined in order to establish the operating level below which ignition will not occur.
Electrostatic hazard tests
Many explosions are a result of electrostatic ignitions and various tests indicate the potential hazards. Some of the tests cover the minimum ignition energy, powder electric charge characteristics and volume resistivity. From the test results, certain steps can be taken to prevent explosions. Steps include increasing humidity, modifying construction materials, proper grounding, controlling certain aspects of equipment design and preventing sparks (Bartknecht 1989; Cesana and Siwek 1995).
Explosion control
There are basically two methods of controlling explosions or fronts from propagating from one location and another or containing an explosion within a piece of equipment. These two methods are chemical suppressants and isolation valves (Bartknecht 1989; Cesana and Siwek 1995; Garzia and Senecal 1996). Based upon the explosion pressure data from the explosion severity tests, rapid response sensors are available that will trigger a chemical suppressant and/ or rapidly close isolation barrier valves. Suppressants are commercially available, but suppressant injector design is very important.
Explosion vents
In equipment where a potential explosion may occur, explosion vents that rupture at specific pressures are frequently installed. These must be carefully designed and the exhaust path from the equipment must be defined to prevent a worker presence in this path area. Moreover, impingement on equipment in the explosion path should be analysed to ensure equipment safety. A barrier may be required.
Loading and Unloading
Products, intermediates and by-products are loaded into tank trucks and railcars. (In some cases, depending on location of facilities and dockage requirements, tankers and barges are used.) Location of the loading and unloading facilities are important. While the materials loaded and unloaded usually are liquids and gases, solids are also loaded and unloaded at preferred locations based upon the type of solids moved, potential explosion hazard and the degree of transfer difficulty.
Open hatches
In loading tank trucks or railcars through top opening hatches, a very important consideration is minimizing splashing as the container is filled. If the fill pipe is located well above the bottom of the container, filling results in splashing and generation of vapour or mixed liquid-vapour evolvement. Splashing and vapour generation can be minimized by locating the fill pipe outlet well below the liquid level. The fill pipe is normally extended through the container a minimum distance above the container bottom. Since liquid filling also displaces vapour, toxic vapours can be a potential health hazard and also present safety concerns. Consequently, the vapours should be collected. Fill arms are commercially available that have deep fill pipes and extend through a special cover that closes the hatch opening (Lipton and Lynch 1994). In addition, a vapour collection pipe extends a short distance below the special hatch cover. At the upstream end of the arm, the vapour outlet is connected to a recovery device (e.g., an absorber or condenser), or the vapour can be returned to the storage tank as a vapour balance transfer (Lipton and Lynch 1994).
In the tank truck open hatch system, the arm is raised to permit draining into the tank truck and some of the liquid in the arm can be pressured with nitrogen as the arm is withdrawn, but the fill pipes during this operation should remain within the hatch opening. As the fill arm clears the hatch, a bucket should be placed over the outlet to catch arm drippings.
Railcars
Many railcars have closed hatches with deep fill legs very close to the bottom of the container and a separate vapour collection outlet. Through an arm that extends to the closed hatch, liquid is loaded and vapour collected in a fashion similar to the open hatch arm method. In railcar loading systems, following valve shut off at the arm inlet, nitrogen is injected into the container side of the arms to blow the liquid remaining in the arm into the railcar before the fill valve on the railcar is closed (Lipton and Lynch 1994).
Tank trucks
Many tank trucks are filled through the bottom to minimize vapour generation (Lipton and Lynch 1994). The fill lines can be special hoses or manoeuvrable arms. Dry break couplers are placed on the hose or arm ends and on the tank truck bottom connections. When the tank truck is filled and the line is automatically blocked, the arm or hose is disconnected at the drybreak coupling, which automatically closes as the couplings are separated. Newer couplings have been designed to disconnect with almost zero leakage.
In bottom loading, vapour is collected through a top vapour vent and the vapour is conducted through an external line that terminates near the bottom of the container (Lipton and Lynch 1994). This permits worker access to the vapour coupling connections. The collected vapour, which is at a pressure slightly above atmospheric, must be collected and sent to a recovery device (Lipton and Lynch 1994). These devices are selected based upon initial cost, effectiveness, maintenance and operability. Generally, the recovery system is preferable to a flare, which destroys the recovered vapours.
Loading control
In tank trucks, level sensors are permanently installed within the truck body to indicate when the fill level has been reached and signal a remote control block valve that stops flow to the truck. (Lipton and Lynch 1994). There may be more than one sensor in the tank truck as backup to ensure that the truck is not overfilled. Overfilling can result in serious safety and health exposure problems.
Railcars in dedicated chemical service may have level sensors mounted internally in the car. For non-dedicated cars, a flow totalizer controls the amount of liquid sent to the railcar and automatically shuts the remote control block valve at a predetermined setting (Lipton and Lynch 1994). Both container types should be investigated to determine whether liquid remains in the container prior to filling. Many railcars have manual level indicators that can be used for this service. However, where level is shown by opening a small level stick vent to the atmosphere, this procedure should only be performed under properly controlled and approved conditions due to the toxicity of some of the loaded chemicals.
Unloading
Where chemicals have a very high vapour pressure and the railcar or tank truck has a relatively high pressure, the chemical is unloaded under its own vapour pressure. Should the vapour pressure fall to a level that will interfere with the unloading procedure, nitrogen gas can be injected to maintain a satisfactory pressure. Vapour from a tank of the same chemical can also be compressed and injected to raise the pressure.
For toxic chemicals that have a relatively low vapour pressure, such as benzene, the liquid is unloaded under nitrogen pressure, which eliminates pumping and simplifies the system (Lipton and Lynch 1994). Tank trucks and railcars for this service have design pressures capable of handling the pressures and variations encountered. However, lower pressures after unloading a container are maintained until the tank truck or railcar is refilled; the pressure rebuilds during loading. Nitrogen can be added if sufficient pressure has not been attained during loading.
One of the problems in loading and unloading operations is draining and purging lines and equipment in the loading/unloading facilities. Closed drains and particularly low point drains are necessary with nitrogen purges to remove all traces of the toxic chemicals. These materials can be collected in a drum and returned to a receiving or recovery facility (Lipton and Lynch 1994).