Adapted from the 3rd edition, Encyclopaedia of Occupational Health and Safety.
There is a wide variety of techniques for finishing the surfaces of metal products so that they resist corrosion, fit better and look better (see table 1). Some products are treated by a sequence of several of these techniques. This article will briefly describe some of those most commonly used.
Table 1. Summary of the hazards associated with the different metal treatment methods
|
Metal treatment method |
Hazards |
Precautions |
|
Electrolytic polishing |
Burns and irritation from caustic and corrosive chemicals |
Use appropriate personal protective equipment. Install effective exhaust ventilation. |
|
Electroplating |
Exposure to potentially cancer causing chromium and nickel; exposure to cyanides; burns and irritation from caustic and corrosive chemicals; electric shock; the process can be wet, causing slip and fall hazards; potential explosive dust generation; ergonomic hazards |
Use appropriate personal protective equipment. Install effective exhaust ventilation, often slotted, push-pull system. Clean up spills immediately. Install non-skid flooring. Use effective design of work procedures and stations to avoid ergonomic stress. |
|
Enamels and glazing |
Physical hazards from grinders, conveyers, mills; burn hazard from high temperature liquids and equipment; exposure to dusts that may cause lung disease |
Install proper machine guards, including interlocks. Use appropriate personal protective equipment. Install effective exhaust ventilation to avoid dust exposure. HEPA-filtered equipment may be necessary. |
|
Etching |
Exposure to hydrofluoric acid; burns and irritation from caustic and corrosive chemicals; burn hazard from high temperature liquids and equipment |
Implement a programme to avoid exposure to hydrofluoric acid. Use appropriate personal protective equipment. Install effective exhaust ventilation. |
|
Galvanizing |
Burn hazard from high temperature liquids, metals, and equipment; burns and irritation from caustic and corrosive chemicals; metal fume fever; potential lead exposure |
Use appropriate personal protective equipment. Install effective exhaust ventilation. Implement a lead exposure reduction/monitoring programme. |
|
Heat treatment |
Burn hazard from high temperature liquids, metals and equipment; burns and irritation from caustic and corrosive chemicals; possible explosive atmospheres of hydrogen; potential exposure to carbon monoxide; potential exposure to cyanides; fire hazard from oil quenching |
Use appropriate personal protective equipment. Install effective exhaust ventilation. Display signs warning of high temperature equipment and surfaces. Install systems to monitor the concentration of carbon monoxide. Install adequate fire-suppression systems. |
|
Metallizing |
Burn hazard from high temperature metals and equipment; possible explosive atmospheres of dust, acetylene; zinc metal fume fever |
Install adequate fire suppression systems. Properly separate chemicals and gases. Use appropriate personal protective equipment. Install effective exhaust ventilation. |
|
Phosphating |
Burns and irritation from caustic and corrosive chemicals |
Use appropriate personal protective equipment. Install effective exhaust ventilation. |
|
Plastics coating |
Exposure to chemical sensitizers |
Seek alternatives to sensitizers. Use appropriate personal protective equipment. Install effective exhaust ventilation. |
|
Priming |
Exposure to various solvents which are potentially toxic and flammable, exposure to chemical sensitizers, exposure to potentially carcinogenic chromium |
Seek alternatives to sensitizers. Use appropriate personal protective equipment. Install effective exhaust ventilation. Properly separate chemicals/gases. |
Before any of these techniques can be applied, the products must be thoroughly cleaned. A number of methods of cleaning are used, individually or in sequence. They include mechanical grinding, brushing and polishing (which produce metallic or oxidic dust—aluminium dust may be explosive), vapour degreasing, washing with organic grease solvents, “pickling” in concentrated acid or alkaline solutions and electrolytic degreasing. The last involves immersion in baths containing cyanide and concentrated alkali in which electrolytically formed hydrogen or oxygen remove the grease, resulting in “blank” metal surfaces that are free from oxides and grease. The cleaning is followed by adequate rinsing and drying of the product.
Proper design of the equipment and effective LEV will reduce some of the risk. Workers exposed to the hazard of splashes must be provided with protective goggles or eye shields and protective gloves, aprons and clothing. Showers and eyewash fountains should be nearby and in good working order, and splashes and spills should be washed away promptly. With electrolytic equipment, the gloves and shoes must be non-conducting, and other standard electrical precautions, such as the installation of ground fault circuit interrupters and lockout/tagout procedures should be followed.
Treatment Processes
Electrolytic polishing
Electrolytic polishing is used to produce a surface of improved appearance and reflectivity, to remove excess metal to accurately fit the required dimensions and to prepare the surface for inspection for imperfections. The process involves preferential anodic dissolution of high spots on the surface after vapour degreasing and hot alkaline cleaning. Acids are frequently used as the electrolyte solutions; accordingly, adequate rinsing is required afterwards.
Electroplating
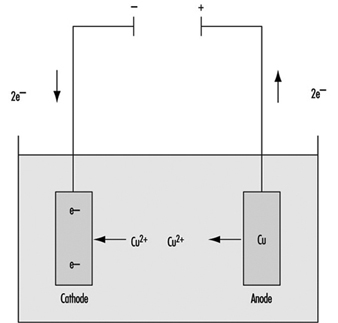
Electroplating is a chemical or electrochemical process for applying a metallic layer to the product—for example, nickel to protect against corrosion, hard chromium to improve the surface properties or silver and gold to beautify it. Occasionally, non-metallic materials are used. The product, wired as the cathode, and an anode of the metal to be deposited are immersed in an electrolyte solution (which can be acidic, alkaline or alkaline with cyanide salts and complexes) and connected externally to a source of direct current. The positively charged cations of the metallic anode migrate to the cathode, where they are reduced to the metal and deposited as a thin layer (see figure 1). The process is continued until the new coating reaches the desired thickness, and the product is then washed, dried and polished.
Figure 1. Electroplating: Schematic representation
Anode: Cu → Cu+2 + 2e- ; Cathode: Cu+2 + 2e- → Cu
In electroforming, a process closely related to electroplating, objects moulded of, for example, plaster or plastic are made conductive by the application of graphite and then are connected as the cathode so that the metal is deposited on them.
In anodization, a process that has become increasingly important in recent years, products of aluminium (titanium and other metals are also used) are connected as the anode and immersed in dilute sulphuric acid. However, instead of the formation of positive aluminium ions and migrating for deposition on the cathode, they are oxidized by the oxygen atoms arising at the anode and become bound to it as an oxide layer. This oxide layer is partially dissolved by the sulphuric acid solution, making the surface layer porous. Subsequently, coloured or light-sensitive materials can be deposited in these pores, as in the fabrication of nameplates, for example.
Enamels and glazes
Vitreous enamel or porcelain enamel is used to give a high heat-, stain- and corrosion-resistant covering to metals, usually iron or steel, in a wide range of fabricated products including bath tubs, gas and electric cookers, kitchen ware, storage tanks and containers, and electrical equipment. In addition, enamels are used in the decoration of ceramics, glass, jewellery and decorative ornaments. The specialized use of enamel powders in the production of such ornamental ware as Cloisonné and Limoges has been known for centuries. Glazes are applied to pottery ware of all kinds.
The materials used in the manufacture of vitreous enamels and glazes include:
- refractories, such as quartz, feldspar and clay
- fluxes, such as borax (sodium borate decahydrate), soda ash (anhydrous sodium carbonate), sodium nitrate, fluorspar, cryolite, barium carbonate, magnesium carbonate, lead monoxide, lead tetroxide and zinc oxide
- colours, such as oxides of antimony, cadmium, cobalt, iron, nickel, manganese, selenium, vanadium, uranium and titanium
- opacifiers, such as oxides of antimony, titanium, tin and zirconium, and sodium antimoninate
- electrolytes, such as borax, soda ash, magnesium carbonate and sulphate, sodium nitrite and sodium aluminate
- flocculating agents, such as clay, gums, ammonium alginate, bentonite and colloidal silica.
The first step in all types of vitreous enamelling or glazing is the making of the frit, the enamel powder. This involves preparation of the raw materials, smelting and frit handing.
After careful cleaning of the metal products (e.g., shot blasting, pickling, degreasing), the enamel may be applied by a number of procedures:
- In the wet process, the object is dipped into the aqueous enamel slip, withdrawn and allowed to drain or, in “slushing”, the enamel slip is thicker and must be shaken from the object.
- In the dry process, the ground-coated object is heated to the enamelling temperature and then dry enamel powder is dusted through sieves onto it. The enamel sinters into place and, when the object is returned to the furnace, it melts down to a smooth surface.
- Spray application is being used increasingly, usually in a mechanized operation. It requires a cabinet under exhaust ventilation.
- Decorative enamels are usually applied by hand, using brushes or similar tools.
- Glazes for porcelain and pottery articles are usually applied by dipping or spraying. Although some dipping operations are being mechanized, pieces are usually dipped by hand in the domestic porcelain industry. The object is held in the hand, dipped into a large tub of glaze, the glaze is removed by a flick of the wrist and the object is placed in a dryer. An enclosed hood or cabinet with efficient exhaust ventilation should be provided when the glaze is sprayed.
The prepared objects are then “fired” in a furnace or kiln, which usually is gas fuelled.
Etching
Chemical etching produces a satin or matte finish. Most frequently, it is used as a pre-treatment prior to anodizing, lacquering, conversion coating, buffing or chemical brightening. It is most frequently applied to aluminium and stainless steel, but is also used for many other metals.
Aluminium is usually etched in alkaline solutions containing various mixtures of sodium hydroxide, potassium hydroxide, trisodium phosphate and sodium carbonate, together with other ingredients to prevent sludge formation. One of the most common processes uses sodium hydroxide at a concentration of 10 to 40 g/l maintained at a temperature of 50 to 85°C with an immersion time as long as 10 minutes.
The alkaline etching is usually preceded and followed by treatment in various mixtures of hydrochloric, hydrofluoric, nitric, phosphoric, chromic or sulphuric acid. A typical acid treatment involves immersions of 15 to 60 seconds in a mixture of 3 parts by volume of nitric acid and 1 part by volume of hydrofluoric acid that is maintained at a temperature of 20°C.
Galvanizing
Galvanizing applies a zinc coating to a variety of steel products to protect against corrosion. The product must be clean and oxide-free for the coating to adhere properly. This usually involves a number of cleaning, rinsing, drying or annealing processes before the product enters the galvanizing bath. In “hot dip” galvanizing, the product is passed through a bath of molten zinc; “cold” galvanizing is essentially electroplating, as described above.
Manufactured products are usually galvanized in a batch process, while the continuous strip method is used for steel strip, sheet or wire. Flux may be employed to maintain satisfactory cleaning of both the product and the zinc bath and to facilitate drying. A prefluxing step may be followed by an ammonium chloride flux cover on the surface of the zinc bath, or the latter may be used alone. In galvanizing pipe, the pipe is immersed in a hot solution of zinc ammonium chloride after cleaning and before the pipe enters the molten zinc bath. The fluxes decompose to form irritating hydrogen chloride and ammonia gas, requiring LEV.
The various types of continuous hot-dip galvanizing differ essentially in how the product is cleaned and whether the cleaning is done on-line:
- cleaning by flame oxidation of the surface oils with subsequent reduction in the furnace and annealing done in-line
- electrolytic cleaning done prior to in-line annealing
- cleaning by acid pickling and alkali cleaning, using a flux prior to the preheat furnace and annealing in a furnace before galvanizing
- cleaning by acid pickling and alkali cleaning, eliminating the flux and preheating in a reducing gas (e.g., hydrogen) prior to galvanizing.
The continuous galvanizing line for light-gauge strip steel omits pickling and the use of flux; it uses alkaline cleaning and maintains the clean surface of the strip by heating it in a chamber or furnace with a reducing atmosphere of hydrogen until it passes below the surface of the molten zinc bath.
Continuous galvanizing of wire requires annealing steps, usually with a molten lead pan in front of the cleaning and galvanizing tanks; air or water cooling; pickling in hot, dilute hydrochloric acid; rinsing; application of a flux; drying; and then galvanizing in the molten zinc bath.
A dross, an alloy of iron and zinc, settles to the bottom of the molten zinc bath and must be removed periodically. Various types of materials are floated on the surface of the zinc bath to prevent oxidation of the molten zinc. Frequent skimming is needed at the points of entry and exit of the wire or strip being galvanized.
Heat treatment
Heat treatment, the heating and cooling of a metal which remains in the solid state, is usually an integral part of the processing of metal products. It almost always involves a change in the crystalline structure of the metal which results in a modification of its properties (e.g., annealing to make the metal more malleable, heating and slow cooling to reduce hardness, heating and quenching to increase hardness, low-temperature heating to minimize internal stresses).
Annealing
Annealing is a “softening” heat treatment widely used to allow further cold working of the metal, improve machinability, stress-relieve the product before it is used and so on. It involves heating the metal to a specific temperature, holding it at that temperature for a specific length of time and allowing it to cool at a particular rate. A number of annealing techniques are used:
- Blue annealing, in which a layer of blue oxide is produced on the surface of iron-based alloys
- Bright annealing, which is carried out in a controlled atmosphere to minimize surface oxidation
- Close annealing or box annealing, a method in which both ferrous and non-ferrous metals are heated in a sealed metal container with or without a packing material and then slowly cooled
- Full annealing, usually carried out in a protective atmosphere, aimed at obtaining the maximum softness economically feasible
- Malleablizing, a special kind of anneal given to iron castings to make them malleable by transforming the combined carbon in the iron to fine carbon (i.e., graphite)
- Partial annealing, a low-temperature process to remove internal stresses induced in the metal by cold working
- Sub-critical or spheroidizing annealing, which produces improved machinability by allowing the iron carbide in the crystalline structure to acquire a spheroid shape.
Age-hardening
Age-hardening is a heat treatment often used on aluminium-copper alloys in which the natural hardening that takes place in the alloy is accelerated by heating to about 180°C for about 1 hour.
Homogenizing
Homogenizing, usually applied to ingots or powdered metal compacts, is designed to remove or greatly reduce segregation. It is achieved by heating to a temperature about 20°C below the metal’s melting point for about 2 hours or more and then quenching.
Normalizing
A process similar to full annealing, ensures the uniformity of the mechanical properties to be obtained and also produces greater toughness and resistance to mechanical loading.
Patenting
Patenting is a special type of annealing process that is usually applied to materials of small cross-section which are intended to be drawn (e.g., 0.6% carbon steel wire). The metal is heated in an ordinary furnace to above the transformation range and then passes from the furnace directly into, for example, a lead bath held at a temperature of about 170°C.
Quench-hardening and tempering
An increase in hardness can be produced in an iron-based alloy by heating to above the transformation range and rapidly cooling to room temperature by quenching in oil, water or air. The article is often too highly stressed to be put into service and, in order to increase its toughness, it is tempered by reheating to a temperature below the transformation range and allowing it to cool at the desired rate.
Martempering and austempering are similar processes except that the article is quenched, for example, in a salt or lead bath held at a temperature of 400°C.
Surface- and case-hardening
This is another heat-treatment process applied most frequently to iron-based alloys, which allows the surface of the object to remain hard while its core remains relatively ductile. It has a number of variations:
- Flame hardening involves hardening the surfaces of the object (e.g., gear teeth, bearings, slideways) by heating with a high-temperature gas torch and then quenching in oil, water or another suitable medium.
- Electrical induction hardening is similar to flame hardening except that the heating is produced by eddy currents induced in the surface layers.
- Carburizing increases the carbon content of the surface of an iron-based alloy by heating the object in a solid, liquid or gaseous carbonaceous medium (e.g., solid charcoal and barium carbonate, liquid sodium cyanide and sodium carbonate, gaseous carbon monoxide, methane and so on) at a temperature of about 900°C.
- Nitriding increases the nitrogen content of the surface of a special low-alloy cast iron or steel object by heating it in a nitrogenous medium, usually ammonia gas, at about 500 to 600°C.
- Cyaniding is a method of case-hardening in which the surface of a low-carbon steel object is enriched in both carbon and nitrogen simultaneously. It usually involves heating the object for 1 hour in a bath of molten 30% sodium cyanide at 870°C, and then quenching in oil or water.
- Carbo-nitriding is a gaseous process for the simultaneous absorption of carbon and nitrogen into the surface layer of steel by heating it to 800 to 875°C in an atmosphere of a carburizing gas (see above) and a nitriding gas (e.g., 2 to 5% anhydrous ammonia).
Metallizing
Metallizing, or metal spraying, is a technique for applying a protective metallic coating to a mechanically roughened surface by spraying it with molten droplets of metal. It is also used to build up worn or corroded surfaces and for salvaging badly-machined component parts. The process is widely known as Schooping, after the Dr. Schoop who invented it.
It uses the Schooping gun, a hand-held, pistol-shaped spray gun through which the metal in wire form is fed into a fuel gas/oxygen blowpipe flame which melts it and, using compressed air, sprays it onto the object. The heat source is a mixture of oxygen and either acetylene, propane or compressed natural gas. The coiled wire is usually straightened before being fed into the gun. Any metal that can be made into a wire may be used; the gun can also accept the metal in powder form.
Vacuum metallizing is a process in which the object is placed in a vacuum jar into which the coating metal is sprayed.
Phosphating
Phosphating is used mainly on mild and galvanized steel and aluminium to augment the adhesion and corrosion resistance of paint, wax and oil finishes. It is also used to form a layer which acts as a parting film in the deep drawing of sheet metal and improves its wear resistance. It essentially consists of allowing the metal surface to react with a solution of one or more phosphates of iron, zinc, manganese, sodium or ammonium. Sodium and ammonium phosphate solutions are used for combined cleaning and phosphating. The need to phosphate multi-metal objects and the desire to increase line speeds in automated operations have led to reducing reaction times by the addition of accelerators such as fluorides, chlorates, molybdates and nickel compounds to the phosphating solutions.To reduce crystal size and, consequently, increase the flexibility of zinc phosphate coatings, crystal refining agents such as tertiary zinc phosphate or titanium phosphate are added to the pre-treatment rinse.
The phosphating sequence typically includes the following steps:
- hot caustic cleaning
- brushing and rinsing
- further hot caustic cleaning
- conditioning water rinse
- spraying or dipping in hot solutions of acid phosphates
- cold water rinse
- warm chromic acid rinse
- another cold water rinse
- drying.
Priming
Organic paint primers are applied to metal surfaces to promote the adhesion of subsequently applied paints and to retard corrosion at the paint-metal interface. The primers usually contain resins, pigments and solvents and may be applied to the prepared metal surfaces by brush, spray, immersion, roller coating or electrophoresis.
The solvents may be any combination of aliphatic and aromatic hydrocarbons, ketones, esters, alcohols and ethers. The most commonly used resins are polyvinyl butynol, phenolic resins, drying oil alkyds, epoxidized oils, epoxyesters, ethyl silicates and chlorinated rubbers. In complex primers, cross-linking agents such as tetraethylene pentamine, pentaethylene hexamine, isocyanates and urea formaldehyde are used. Inorganic pigments used in primer formulations include lead, barium, chromium, zinc and calcium compounds.
Plastic coating
Plastic coatings are applied to metals in liquid form, as powders which are subsequently cured or sintered by heating, or in the form of fabricated sheets which are laminated to the metal surface with an adhesive. The most commonly used plastics include polyethylene, polyamides (nylons) and PVC. The latter may include plasticizers based on monomeric and polymeric esters and stabilizers such as lead carbonate, fatty acid salts of barium and cadmium, dibutyltin dilaurate, alkyltin mercaptides and zinc phosphate. Although generally of low toxicity and non-irritating, some of the plasticizers are skin sensitizers.
Hazards and Their Prevention
As might be deduced from the complexity of the processes outlined above, there is a large variety of safety and health hazards associated with the surface treatment of metals. Many are regularly encountered in manufacturing operations; others are presented by the uniqueness of the techniques and materials employed. Some are potentially life threatening. By and large, however, they can be prevented or controlled.
Workplace design
The workplace should be designed to allow the delivery of raw materials and supplies and the removal of the finished products without interfering with the ongoing processing. Since many of the chemicals are flammable or prone to react when mixed, proper separation in storage and in transit is essential. Many of the metal finishing operations involve liquids, and when leaks, spills or splashes of acids or alkalis occur they must be washed away promptly. Accordingly, adequately drained, slip-resistant floors must be provided. Housekeeping must be diligent to keep the work areas and other spaces clean and free from accumulations of materials. Systems for disposal of solid and liquid wastes and effluents from furnaces and exhaust ventilation must be designed with environmental concerns in mind.
Work stations and work assignments should use ergonomic principles to minimize strains, sprains, excessive fatigue and RSIs. Machine guards must have automatic lockout so the machine is de-energized if the guard is removed. Splash guards are essential. Because of the danger of splashes of hot acid and alkali solutions, eyewash fountains and whole-body showers must be installed within easy reach. Signs should be posted to warn other production and maintenance personnel of such dangers as chemical baths and hot surfaces.
Chemical assessment
All chemicals should be evaluated for potential toxicity and physical hazards, and less hazardous materials should be substituted where possible. However, since the less toxic material may be more flammable, the hazard of fire and explosion must also be considered. In addition, the chemical compatibility of materials must be considered. For example, mixing of nitrate and cyanide salts by accident could cause an explosion due to the strong oxidizing properties of nitrates.
Ventilation
Most of the metal coating processes require LEV that is strategically placed to draw the vapours or other contaminants away from the worker. Some systems push fresh air across the tank to “push” airborne contaminants to the exhaust side of the system. Fresh air intakes must be located away from exhaust vents so that potentially toxic gases are not recirculated.
Personal protective equipment
Processes should be engineered to prevent potentially toxic exposures, but since they cannot always be totally avoided, employees will have to be provided with appropriate PPE (e.g., goggles with or without face shields as appropriate, gloves, aprons or coveralls and shoes). Because many of the exposures involve hot corrosive or caustic solutions, the protective items should be insulated and chemical-resistant. If there is possible exposure to electricity, PPE should be non-conductive. PPE must be available in adequate quantity to allow contaminated, wet items to be cleaned and dried before re-using them. Insulated gloves and other protective clothing should be available where there is the risk of thermal burns from hot metal, furnaces and so on.
An important adjunct is the availability of wash-up facilities and clean lockers and dressing rooms, so that workers’ clothing remains uncontaminated and workers do not carry toxic materials back into their homes.
Employee training and supervision
Employee education and training are essential both when new to the job or when there have been changes in the equipment or the process. MSDSs must be provided for each of the chemical products which explain the chemical and physical hazards, in languages and at educational levels that ensure they will be understood by the workers. Competence testing and periodic retraining will assure that workers have retained the needed information. Close supervision is advisable to make sure that the proper procedures are being followed.
Selected hazards
Certain hazards are unique to the metal coating industry and deserve special consideration.
Alkaline and acid solutions
The heated alkaline and acid solutions used in cleaning and treatment of metals are particularly corrosive and caustic. They are irritating to the skin and mucous membranes and are especially dangerous when splashed into the eye. Eyewash fountains and emergency showers are essential. Proper protective clothing and goggles will guard against the inevitable splashes; when a splash reaches the skin, the area should be immediately and copiously rinsed with cool, clean water for at least 15 minutes; medical attention may be necessary, particularly when the eye is involved.
Care should be exercised when utilizing chlorinated hydrocarbons as phosgene may result from a reaction of the chlorinated hydrocarbon, acids and metals. Nitric and hydrofluoric acid are particularly dangerous when their gases are inhaled, because it may take 4 hours or more before the effects on the lungs become apparent. Bronchitis, pneumonitis and even potentially fatal pulmonary oedema may appear belatedly in a worker who apparently had no initial effect from the exposure. Prompt prophylactic medical treatment and, often, hospitalization are advisable for workers who have been exposed. Skin contact with hydrofluoric acid can cause severe burns without pain for several hours. Prompt medical attention is essential.
Dust
Metallic and oxidic dusts are a particular problem in grinding and polishing operations, and are most effectively removed by LEV as they are created. Ductwork should be designed to be smooth and air velocity should be sufficient to keep the particulates from settling out of the air stream. Aluminium and magnesium dust may be explosive and should be collected in a wet trap. Lead has become less of a problem with the decline of its use in ceramics and porcelain glazes, but it remains the ubiquitous occupational hazard and must always be guarded against. Beryllium and its compounds have received interest recently due to the possibility of carcinogenicity and chronic beryllium disease.
Certain operations present a risk of silicosis and pneumoconiosis: the calcining, crushing and drying of flint, quartz or stone; the sieving, mixing and weighing out of these substances in the dry state; and the charging of furnaces with such materials. They also represent a danger when they are used in a wet process and are splashed about the workplace and on workers’ clothing, to become dusts again when they dry out. LEV and rigorous cleanliness and personal hygiene are important preventive measures.
Organic solvents
Solvents and other organic chemicals used in degreasing and in certain processes are dangerous when inhaled. In the acute phase, their narcotic effects may lead to respiratory paralysis and death. In chronic exposure, toxicity of the central nervous system and liver and kidney damage are most frequent. Protection is provided by LEV with a safety zone of at least 80 to 100 cm between the source and the breathing area of the worker. Bench ventilation must also be installed to remove residual vapours from the finished workpieces. Defatting of the skin by organic solvents may be a precursor of dermatitis. Many solvents are also flammable.
Cyanide
Baths containing cyanides are frequently used in electrolytic degreasing, electroplating and cyaniding. Reaction with acid will form the volatile, potentially lethal hydrogen cyanide (prussic acid). The lethal concentration in air is 300 to 500 ppm. Fatal exposures may also result from skin absorption or ingestion of cyanides. Optimum cleanliness is essential for workers using cyanide. Food should not be eaten before washing, and should never be in the work area. Hands and clothing must be carefully cleaned following a potential cyanide exposure.
First aid measures for cyanide poisoning include transport into the open air, removal of contaminated clothing, copious washing of the exposed areas with water, oxygen therapy and inhalation of amyl nitrite. LEV and skin protection are essential.
Chromium and nickel
Chromic and nickel compounds used in galvanic baths in electroplating may be hazardous. Chromium compounds can cause burns, ulceration and eczema of the skin and mucosa and a characteristic perforation of the nasal septum. Bronchial asthma may occur. Nickel salts can cause obstinate allergic or toxic-irritative skin injury. There is evidence that both chromium and nickel compounds may be carcinogenic. LEV and skin protection are essential.
Furnaces and ovens
Special precautions are needed when working with the furnaces employed, for example, in the heat treatment of metals where components are handled at high temperatures and the materials used in the process may either be toxic or explosive or both. The gaseous media (atmospheres) in the furnace may react with the metal charge (oxidizing or reducing atmospheres) or they may be neutral and protective. Most of the latter contain up to 50% hydrogen and 20% carbon monoxide, which, in addition to being combustible, form highly explosive mixtures with air at elevated temperatures. The ignition temperature varies from 450 to 750 °C, but a local spark may cause ignition even at lower temperatures. The danger of explosion is greater when the furnace is being started up or shut down. Since a cooling furnace tends to suck in air (a particular danger when the fuel or power supply is interrupted), a supply of inert gas (e.g., nitrogen or carbon dioxide) should be available for purging when the furnace is shut down as well as when a protective atmosphere is introduced into a hot furnace.
Carbon monoxide is perhaps the greatest hazard from furnaces and ovens. Since it is colourless and odourless, it frequently reaches toxic levels before the worker becomes aware of it. Headache is one of the earliest symptoms of toxicity, and, therefore, a worker developing a headache on the job should immediately be removed into fresh air. Danger zones include recessed pockets in which the carbon monoxide may collect; it should be remembered that brickwork is porous and may retain the gas during normal purging and emit it when the purging is completed.
Lead furnaces may be dangerous since lead tends to vaporize quite rapidly at temperatures above 870°C. Accordingly, an effective fume extraction system is required. A pot breakage or failure may also be hazardous; a sufficiently large well or pit should be provided to capture the molten metal if this occurs.
Fire and explosion
Many of the compounds used in metal coating are flammable and, under certain circumstances, explosive. For the most part, the furnaces and drying ovens are gas fired, and special precautions such as flame-failure devices at burners, low-pressure cut-off valves in the supply lines and explosion relief panels in the structure of the stoves should be installed. In electrolytic operations, hydrogen formed in the process may collect at the surface of the bath and, if not exhausted, may reach explosive concentrations. Furnaces should be properly ventilated and burners protected from being clogged by dripping material.
Oil quenching is also a fire hazard, especially if the metal charge is not completely immersed. Quenching oils should have a high flashpoint, and their temperature should not exceed 27°C.
Compressed oxygen and fuel gas cylinders used in metallizing are fire and explosion hazards if not stored and operated properly. See the article “Welding and thermal cutting” in this chapter for detailed precautions.
As required by local ordinances, firefighting equipment, including alarms, should be provided and maintained in working order, and the workers drilled in using it properly.
Heat
The use of furnaces, open flames, ovens, heated solutions and molten metals inevitably presents the risk of excessive heat exposure, which is compounded in hot, humid climates and, particularly, by occlusive protective garments and gear. Complete air conditioning of a plant may not be economically feasible, but supplying cooled air in local ventilation systems is helpful. Rest breaks in cool surroundings and adequate fluid intake (fluids taken at the work station should be free of toxic contaminants) will help to avert heat toxicity. Workers and supervisors should be trained in the recognition of heat stress symptoms.
Conclusion
Surface treatment of metals involves a multiplicity of processes entailing a broad range of potentially toxic exposures, most of which can be prevented or controlled by the diligent application of well-recognized preventive measures.