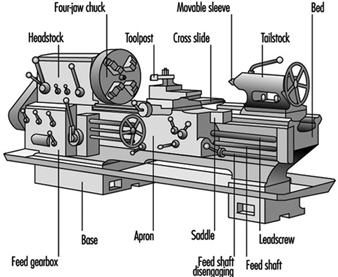
Metal Processing and Metal Working
Foundries
Founding, or metal casting, involves the pouring of molten metal into the hollow inside of a heat-resistant mould which is the outside or negative shape of the pattern of the desired metal object. The mould may contain a core to determine the dimensions of any internal cavity in the final casting. Foundry work comprises:
- making a pattern of the desired article
- making the mould and cores and assembling the mould
- melting and refining the metal
- pouring the metal into the mould
- cooling the metal casting
- removing the mould and core from the metal casting
- removing extra metal from the finished casting.
The basic principles of foundry technology have changed little in thousands of years. However, processes have become more mechanized and automatic. Wooden patterns have been replaced by metal and plastic, new substances have been developed for producing cores and moulds, and a wide range of alloys are used. The most prominent foundry process is sand moulding of iron.
Iron, steel, brass and bronze are traditional cast metals. The largest sector of the foundry industry produces grey and ductile iron castings. Gray iron foundries use iron or pig iron (new ingots) to make standard iron castings. Ductile iron foundries add magnesium, cerium or other additives (often called ladle additives) to the ladles of molten metal before pouring to make nodular or malleable iron castings. The different additives have little impact on workplace exposures. Steel and malleable iron make up the balance of the ferrous foundry industrial sector. The major customers of the largest ferrous foundries are the auto, construction and agricultural implement industries. Iron foundry employment has decreased as engine blocks become smaller and can be poured in a single mould, and as aluminium is substituted for cast iron. Non-ferrous foundries, especially aluminium foundry and die-cast operations, have heavy employment. Brass foundries, both free standing and those producing for the plumbing equipment industry, are a shrinking sector which, however, remains important from an occupational health perspective. In recent years, titanium, chromium, nickel and magnesium, and even more toxic metals such as beryllium, cadmium and thorium, are used in foundry products.
Although the metal founding industry may be assumed to start by remelting solid material in the form of metal ingots or pigs, the iron and steel industry in the large units may be so integrated that the division is less obvious. For instance, the merchant blast furnace may turn all its output into pig iron, but in an integrated plant some iron may be used to produce castings, thus taking part in the foundry process, and the blast furnace iron may be taken molten to be turned into steel, where the same thing can occur. There is in fact a separate section of the steel trade known for this reason as ingot moulding. In the normal iron foundry, the remelting of pig iron is also a refining process. In the non-ferrous foundries the process of melting may require the addition of metals and other substances, and thus constitutes an alloying process.
Moulds made from silica sand bound with clay predominate in the iron foundry sector. Cores traditionally produced by baking silica sand bound with vegetable oils or natural sugars have been substantially replaced. Modern founding technology has developed new techniques to produce moulds and cores.
In general, the health and safety hazards of foundries can be classified by type of metal cast, moulding process, size of casting and degree of mechanization.
Process Overview
On the basis of the designer’s drawings, a pattern conforming to the external shape of the finished metal casting is constructed. In the same way, a corebox is made that will produce suitable cores to dictate the internal configuration of the final article. Sand casting is the most widely used method, but other techniques are available. These include: permanent mould casting, using moulds of iron or steel; die casting, in which the molten metal, often a light alloy, is forced into a metal mould under pressures of 70 to 7,000 kgf/cm2; and investment casting, where a wax pattern is made of each casting to be produced and is covered with refractory which will form the mould into which the metal is poured. The “lost foam” process uses polystyrene foam patterns in sand to make aluminium castings.
Metals or alloys are melted and prepared in a furnace which may be of the cupola, rotary, reverberatory, crucible, electric arc, channel or coreless induction type (see table 1). Relevant metallurgical or chemical analyses are performed. Molten metal is poured into the assembled mould either via a ladle or directly from the furnace. When the metal has cooled, the mould and core material are removed (shakeout, stripping or knockout) and the casting is cleaned and dressed (despruing, shot-blasting or hydro-blasting and other abrasive techniques). Certain castings may require welding, heat treatment or painting before the finished article will meet the specifications of the buyer.
Table 1. Types of foundry furnaces
|
Furnace |
Description |
|
Cupola furnace |
A cupola furnace is a tall, vertical furnace, open at the top with hinged doors at the bottom. It is charged from the top with alternate layers of coke, limestone and metal; the molten metal is removed at the bottom. Special hazards include carbon monoxide and heat. |
|
Electric arc furnace |
The furnace is charged with ingots, scrap, alloy metals and fluxing agents. An arc is produced between three electrodes and the metal charge, melting the metal. A slag with fluxes covers the surface of the molten metal to prevent oxidation, to refine the metal and protect the furnace roof from excessive heat. When ready, the electrodes are raised and the furnace tilted to pour the molten metal into the receiving ladle. Special hazards include metal fumes and noise. |
|
Induction furnace |
An induction furnace melts the metal by passing a high electric current through copper coils on the outside of the furnace, inducing an electric current in the outer edge of the metal charge that heats the metal because of the high electrical resistance of the metal charge. Melting progresses from the outside of the charge to the inside. Special hazards include metal fumes. |
|
Crucible furnace |
The crucible or container holding the metal charge is heated by a gas or oil burner. When ready, the crucible is lifted out of the furnace and tilted for pouring into moulds. Special hazards include carbon monoxide, metal fumes, noise and heat. |
|
Rotary furnace |
A long, inclined rotating cylindrical furnace that is charged from the top and fired from the lower end. |
|
Channel furnace |
A type of induction furnace. |
|
Reverberatory furnace |
This horizontal furnace consists of a fireplace at one end, separated from the metal charge by a low partition wall called the fire-bridge, and a stack or chimney at the other end. The metal is kept from contact with the solid fuel. Both the fireplace and metal charge are covered by an arched roof. The flame in its path from the fireplace to the stack is reflected downwards or reverberated on the metal beneath, melting it. |
Hazards such as the danger arising from the presence of hot metal are common to most foundries, irrespective of the particular casting process employed. Hazards may also be specific to a particular foundry process. For example, the use of magnesium presents flare risks not encountered in other metal founding industries. This article emphasizes iron foundries, which contain most of the typical foundry hazards.
The mechanized or production foundry employs the same basic methods as the conventional iron foundry. When moulding is done, for example, by machine and castings are cleaned by shot blasting or hydroblasting, the machine usually has built-in dust control devices, and the dust hazard is reduced. However, sand is frequently moved from place to place on an open-belt conveyor, and transfer points and sand spillage may be sources of considerable quantities of airborne dust; in view of the high production rates, the airborne dust burden may be even higher than in the conventional foundry. A review of air sampling data in the middle 1970s showed higher dust levels in large American production foundries than in small foundries sampled during the same period. Installation of exhaust hoods over transfer points on belt conveyors, combined with scrupulous housekeeping, should be normal practice. Conveying by pneumatic systems is sometimes economically possible and results in a virtually dust-free conveying system.
Iron Foundries
For simplicity, an iron foundry can be presumed to comprise the following six sections:
- metal melting and pouring
- pattern-making
- moulding
- coremaking
- shakeout/knockout
- casting cleaning.
In many foundries, almost any of these processes may be carried out simultaneously or consecutively in the same workshop area.
In a typical production foundry, iron moves from melting to pouring, cooling, shakeout, cleaning and shipping as a finished casting. Sand is cycled from sand mix, moulding, shakeout and back to sand mixing. Sand is added to the system from core making, which starts with new sand.
Melting and pouring
The iron founding industry relies heavily on the cupola furnace for metal melting and refining. The cupola is a tall, vertical furnace, open at the top with hinged doors at the bottom, lined with refractory and charged with coke, scrap iron and limestone. Air is blown through the charge from openings (tuyers) at the bottom; combustion of coke heats, melts and purifies the iron. Charge materials are fed into the top of the cupola by crane during operation and must be stored close at hand, usually in compounds or bins in the yard adjacent to the charging machinery. Tidiness and efficient supervision of the stacks of raw materials are essential to minimize the risk of injury from slippages of heavy objects. Cranes with large electromagnets or heavy weights are often used to reduce the scrap metal to manageable sizes for charging into the cupola and for filling the charging hoppers themselves. The crane cab should be well protected and the operators properly trained.
Employees handling raw materials should wear hand leathers and protective boots. Careless charging can overfill the hopper and can cause dangerous spillage. If the charging process is found to be too noisy, the noise of metal-on-metal impact can be reduced by fitting rubber noise-dampening liners to storage skips and bins. The charging platform is necessarily above ground level and can present a hazard unless it is level and has a non-slip surface and strong rails around it and any floor openings.
Cupolas generate large quantities of carbon monoxide, which may leak from the charging doors and be blown back by local eddy currents. Carbon monoxide is invisible, odourless and can quickly produce toxic ambient levels. Employees working on the charging platform or surrounding catwalks should be well trained in order to recognize the symptoms of carbon monoxide poisoning. Both continuous and spot monitoring of exposure levels are needed. Self-contained breathing apparatus and resuscitation equipment should be maintained in readiness, and operators should be instructed in their use. When emergency work is carried out, a confined-space entry system of contaminant monitoring should be developed and enforced. All work should be supervised.
Cupolas are usually sited in pairs or groups, so that while one is being repaired the others operate. The period of use must be based on experience with durability of refractories and on engineering recommendations. Procedures must be worked out in advance for tapping out iron and for shutting down when hot spots develop or if the water cooling system is disabled. Cupola repair necessarily involves the presence of employees inside the cupola shell itself to mend or renew refractory linings. These assignments should be considered confined-space entries and appropriate precautions taken. Precautions should also be taken to prevent the discharge of material through the charging doors at such times. To protect the workers from falling objects, they should wear safety helmets and, if working at a height, safety harnesses.
Workers tapping cupolas (transferring molten metal from the cupola well to a holding furnace or ladle) must observe rigorous personal protection measures. Goggles and protective clothing are essential. The eye protectors should resist both high velocity impact and molten metal. Extreme caution should be exercised in order to prevent remaining molten slag (the unwanted debris removed from the melt with the aid of the limestone additives) and metal from coming into contact with water, which will cause a steam explosion. Tappers and supervisors must ensure that any person not involved in the operation of the cupola remains outside the danger area, which is delineated by a radius of about 4 m from the cupola spout. Delineation of a non-authorized no-entry zone is a statutory requirement under the British Iron and Steel Foundries Regulations of 1953.
When the cupola run is at an end, the cupola bottom is dropped to remove the unwanted slag and other material still inside the shell before employees can carry out the routine refractory maintenance. Dropping the cupola bottom is a skilled and dangerous operation requiring trained supervision. A refractory floor or layer of dry sand on which to drop the debris is essential. If a problem occurs, such as jammed cupola bottom doors, great caution must be exercised to avoid risks of burns to workers from the hot metal and slag.
Visible white-hot metal is a danger to workers’ eyes due to the emission of infrared and ultraviolet radiation, extensive exposure to which can cause cataracts.
The ladle must be dried before filling with molten metal, to prevent steam explosions; a satisfactory period of flame heating must be established.
Employees in metal and pouring sections of the foundry should be provided with hard hats, tinted eye protection and face shields, aluminized clothing such as aprons, gaiters or spats (lower-leg and foot coverings) and boots. Use of protective equipment should be mandatory, and there should be adequate instruction in its use and maintenance. High standards of housekeeping and exclusion of water to the highest degree possible are needed in all areas where molten metal is being manipulated.
Where large ladles are slung from cranes or overhead conveyors, positive ladle-control devices should be employed to ensure that spillage of metal cannot occur if the operator releases his or her hold. Hooks holding molten metal ladles must be periodically tested for metal fatigue to prevent failure.
In production foundries, the assembled mould moves along a mechanical conveyor to a ventilated pouring station. Pouring may be from a manually controlled ladle with mechanical assist, an indexing ladle controlled from a cab, or it can be automatic. Typically, the pouring station is provided with a compensating hood with a direct air supply. The poured mould proceeds along the conveyor through an exhausted cooling tunnel until shakeout. In smaller, job shop foundries, moulds may be poured on a foundry floor and allowed to burn off there. In this situation, the ladle should be equipped with a mobile exhaust hood.
Tapping and transport of molten iron and charging of electric furnaces creates exposure to iron oxide and other metal oxide fumes. Pouring into the mould ignites and pyrolyses organic materials, generating large amounts of carbon monoxide, smoke, carcinogenic polynuclear aromatic hydrocarbons (PAHs) and pyrolysis products from core materials which may be carcinogenic and also respiratory sensitizers. Moulds containing large polyurethane bound cold box cores release a dense, irritating smoke containing isocyanates and amines. The primary hazard control for mould burn off is a locally exhausted pouring station and cooling tunnel.
In foundries with roof fans for exhausting pouring operations, high metal fume concentrations may be found in the upper regions where crane cabs are located. If the cabs have an operator, the cabs should be enclosed and provided with filtered, conditioned air.
Pattern making
Pattern making is a highly skilled trade translating the two-dimensional design plans to a three-dimensional object. Traditional wooden patterns are made in standard workshops containing hand tools and electric cutting and planing equipment. Here, all reasonably practicable measures should be taken to reduce the noise to the greatest extent possible, and suitable ear protectors must be provided. It is important that the employees are aware of the advantages of using such protection.
Power-driven wood cutting and finishing machines are obvious sources of danger, and often suitable guards cannot be fitted without preventing the machine from functioning at all. Employees must be well versed in normal operating procedure and should also be instructed in the hazards inherent in the work.
Wood sawing can create dust exposure. Efficient ventilation systems should be fitted to eliminate wood dust from the pattern shop atmosphere. In certain industries using hard woods, nasal cancer has been observed. This has not been studied in the founding industry.
Casting in permanent metal moulds, as in die-casting, has been an important development in the foundry industry. In this case, pattern making is largely replaced by engineering methods and is really a die manufacture operation. Most of the pattern-making hazards and the risks from sand are eliminated, but are replaced by the risk inherent in the use of some sort of refractory material to coat the die or mould. In modern die-foundry work, increasing use is made of sand cores, in which case the dust hazards of the sand foundry are still present.
Moulding
The most common moulding process in the iron founding industry uses the traditional “green sand” mould made from silica sand, coal dust, clay and organic binders. Other methods of mould production are adapted from coremaking: thermosetting, cold self-setting and gas-hardened. These methods and their hazards will be discussed under coremaking. Permanent moulds or the lost foam process may also be used, especially in the aluminium foundry industry.
In production foundries, sand mix, moulding, mould assembly, pouring and shakeout are integrated and mechanized. Sand from shakeout is recycled back to the sand mix operation, where water and other additives are added and the sand is mixed in mullers to maintain the desired physical properties.
For ease of assembly, patterns (and their moulds) are made in two parts. In manual mould-making, the moulds are enclosed in metal or wooden frames called flasks. The bottom half of the pattern is placed in the bottom flask (the drag), and first fine sand and then heavy sand are poured around the pattern. The sand is compacted in the mould by a jolt-squeeze, sand slinger or pressure process. The top flask (the cope) is prepared similarly. Wooden spacers are placed in the cope to form the sprue and riser channels, which are the pathway for the molten metal to flow into the mould cavity. The patterns are removed, the core inserted, and then the two halves of the mould assembled and fastened together, ready for pouring. In production foundries, the cope and drag flasks are prepared on a mechanical conveyor, cores are placed in the drag flask, and the mould assembled by mechanical means.
Silica dust is a potential problem wherever sand is handled. Moulding sand is usually either damp or mixed with liquid resin, and is therefore less likely to be a significant source of respirable dust. A parting agent such as talc is sometimes added to promote the ready removal of the pattern from the mould. Respirable talc causes talcosis, a type of pneumoconiosis. Parting agents are more widespread where hand moulding is employed; in the larger, more automatic processes they are rarely seen. Chemicals are sometimes sprayed onto the mould surface, suspended or dissolved in isopropyl alcohol, which is then burned off to leave the compound, usually a type of graphite, coating the mould in order to achieve a casting with a finer surface finish. This involves an immediate fire risk, and all employees involved in applying these coatings should be provided with fire-retardant protective clothing and hand protection, as organic solvents can also cause dermatitis. Coatings should be applied in a ventilated booth to prevent the organic vapours from escaping into the workplace. Strict precautions should also be observed to ensure that the isopropyl alcohol is stored and used with safety. It should be transferred to a small vessel for immediate use, and the larger storage vessels should be kept well away from the burning-off process.
Manual mould making can involve the manipulation of large and cumbersome objects. The moulds themselves are heavy, as are the moulding boxes or flasks. They are often lifted, moved and stacked by hand. Back injuries are common, and power assists are needed so employees do not need to lift objects too heavy to be carried safely.
Standardized designs are available for enclosures of mixers, conveyors and pouring and shakeout stations with appropriate exhaust volumes and capture and transport velocities. Adherence to such designs and strict preventive maintenance of control systems will attain compliance with international recognized limits for dust exposure.
Coremaking
Cores inserted into the mould determine the internal configuration of a hollow casting, such as the water jacket of an engine block. The core must withstand the casting process but at the same time must not be so strong as to resist removal from the casting during the knocking-out stage.
Prior to the 1960s, core mixtures comprised sand and binders, such as linseed oil, molasses or dextrin (oil sand). The sand was packed in a core box with a cavity in the shape of the core, and then dried in an oven. Core ovens evolve harmful pyrolysis products and require a suitable, well maintained chimney system. Normally, convection currents within the oven will be sufficient to ensure satisfactory removal of fumes from the workplace, although they contribute enormously to air pollution After removal from the oven, the finished oil sand cores can still give rise to a small amount of smoke, but the hazard is minor; in some cases, however, small amounts of acrolein in the fumes may be a considerable nuisance. Cores may be treated with a “flare-off coating” to improve the surface finish of the casting, which calls for the same precautions as in the case of moulds.
Hot box or shell moulding and coremaking are thermosetting processes used in iron foundries. New sand may be mixed with resin at the foundry, or resin-coated sand may be shipped in bags for addition to the coremaking machine. Resin sand is injected into a metal pattern (the core box). The pattern is then heated—by direct natural gas fires in the hot box process or by other means for shell cores and moulding. Hot boxes typically use a furfuryl alcohol (furan), urea- or phenol-formaldehyde thermosetting resin. Shell moulding uses a urea- or phenol-formaldehyde resin. After a short curing time, the core hardens considerably and can be pushed clear of the pattern plate by ejector pins. Hot box and shell coremaking generate substantial exposure to formaldehyde, which is a probable carcinogen, and other contaminants, depending on the system. Control measures for formaldehyde include direct air supply at the operator station, local exhaust at the corebox, enclosure and local exhaust at the core storage station and low-formaldehyde-emission resins. Satisfactory control is difficult to achieve. Medical surveillance for respiratory conditions should be provided to coremaking workers. Phenol- or urea-formaldehyde resin contact with the skin or eyes must be prevented because the resins are irritants or sensitizers and can cause dermatitis. Copious washing with water will help to avoid the problem.
Cold-setting (no-bake) hardening systems presently in use include: acid-catalyzed urea- and phenol-formaldehyde resins with and without furfuryl alcohol; alkyd and phenolic isocyanates; Fascold; self-set silicates; Inoset; cement sand and fluid or castable sand. Cold-setting hardeners do not require external heating to set. The isocyanates employed in binders are normally based on methylene diphenyl isocyanate (MDI), which, if inhaled, can act as a respiratory irritant or sensitizer, causing asthma. Gloves and protective goggles are advisable when handling or using these compounds. The isocyanates themselves should be carefully stored in sealed containers in dry conditions at a temperature between 10 and 30°C. Empty storage vessels should be filled and soaked for 24 hours with a 5% sodium carbonate solution in order to neutralize any residual chemical left in the drum. Most general housekeeping principles should be strictly applied to resin moulding processes, but the greatest caution of all should be exercised when handling the catalysts used as setting agents. The catalysts for the phenol and oil isocyanate resins are usually aromatic amines based on pyridine compounds, which are liquids with a pungent smell. They can cause severe skin irritation and renal and hepatic damage and can also affect the central nervous system. These compounds are supplied either as separate additives (three-part binder) or are ready mixed with the oil materials, and LEV should be provided at the mixing, moulding, casting and knockout stages. For certain other no-bake processes the catalysts used are phosphoric or various sulphonic acids, which are also toxic; accidents during transport or use should be adequately guarded against.
Gas-hardened coremaking comprises the carbon dioxide (CO2)-silicate and the Isocure (or “Ashland”) processes. Many variations of the CO2-silicate process have been developed since the 1950s. This process has generally been used for the production of medium to large moulds and cores. The core sand is a mixture of sodium silicate and silica sand, usually modified by adding such substances as molasses as breakdown agents. After the core box is filled, the core is cured by passing carbon dioxide through the core mixture. This forms sodium carbonate and silica gel, which acts as a binder.
Sodium silicate is an alkaline substance, and can be harmful if it comes into contact with the skin or eyes or is ingested. It is advisable to provide an emergency shower close to areas where large quantities of sodium silicate are handled and gloves should always be worn. A readily available eye-wash fountain should be located in any foundry area where sodium silicate is used. The CO2 can be supplied as a solid, liquid or gas. Where it is supplied in cylinders or pressure tanks, a great many housekeeping precautions should be taken, such as cylinder storage, valve maintenance, handling and so on. There is also the risk from the gas itself, since it can lower the oxygen concentration in the air in enclosed spaces.
The Isocure process is used for cores and moulds. This is a gas-setting system in which a resin, frequently phenol-formaldehyde, is mixed with a di-isocyanate (e.g., MDI) and sand. This is injected into the core box and then gassed with an amine, usually either triethylamine or dimethylethylamine, to cause the crosslinking, setting reaction. The amines, often sold in drums, are highly volatile liquids with a strong smell of ammonia. There is a very real risk of fire or explosion, and extreme care should be taken, especially where the material is stored in bulk. The characteristic effect of these amines is to cause halo vision and corneal swelling, although they also affect the central nervous system, where they can cause convulsions, paralysis and, occasionally, death. Should some of the amine come into contact with the eyes or skin, first-aid measures should include washing with copious quantities of water for at least 15 minutes and immediate medical attention. In the Isocure process, the amine is applied as a vapour in a nitrogen carrier, with excess amine scrubbed through an acid tower. Leakage from the corebox is the principle cause of high exposure, although offgassing of amine from manufactured cores is also significant. Great care should be taken at all times when handling this material, and suitable exhaust ventilation equipment should be installed to remove vapours from the working areas.
Shakeout, casting extraction and core knockout
After the molten metal has cooled, the rough casting must be removed from the mould. This is a noisy process, typically exposing operators well above 90 dBA over an 8 hour working day. Hearing protectors should be provided if it is not practicable to reduce the noise output. The main bulk of the mould is separated from the casting usually by jarring impact. Frequently the moulding box, mould and casting are dropped onto a vibrating grid to dislodge the sand (shakeout). The sand then drops through the grid into a hopper or onto a conveyor where it can be subjected to magnetic separators and recycled for milling, treatment and re-use, or merely dumped. Sometimes hydroblasting can be used instead of a grid, creating less dust. The core is removed here, also sometimes using high-pressure water streams.
The casting is then removed and transferred to the next stage of the knockout operation. Often small castings can be removed from the flask by a “punch-out” process before shakeout, which produces less dust. The sand gives rise to hazardous silica dust levels because it has been in contact with molten metal and is therefore very dry. The metal and sand remain very hot. Eye protection is needed. Walking and working surfaces must be kept free of scrap, which is a tripping hazard, and of dust, which can be resuspended to pose an inhalation hazard.
Relatively few studies have been carried out to determine what effect, if any, the new core binders have on the health of the de-coring operator in particular. The furanes, furfuryl alcohol and phosphoric acid, urea- and phenol-formaldehyde resins, sodium silicate and carbon dioxide, no-bakes, modified linseed oil and MDI, all undergo some type of thermal decomposition when exposed to the temperatures of the molten metals.
No studies have yet been conducted on the effect of the resin-coated silica particle on the development of pneumoconiosis. It is not known whether these coatings will have an inhibiting or accelerating effect on lung-tissue lesions. It is feared that the reaction products of phosphoric acid may liberate phosphine. Animal experiments and some selected studies have shown that the effect of the silica dust on lung tissue is greatly accelerated when silica has been treated with a mineral acid. Urea- and phenol-formaldehyde resins can release free phenols, aldehydes and carbon monoxide. The sugars added to increase collapsibility produce significant amounts of carbon monoxide. No-bakes will release isocyanates (e.g., MDI) and carbon monoxide.
Fettling (cleaning)
Casting cleaning, or fettling, is carried out following shakeout and core knockout. The various processes involved are variously designated in different places but can be broadly classified as follows:
- Dressing covers stripping, roughing or mucking-off, removal of adherent moulding sand, core sand, runners, risers, flash and other readily disposable matter with hand tools or portable pneumatic tools.
- Fettling covers removal of burnt-on moulding sand, rough edges, surplus metal, such as blisters, stumps of gates, scabs or other unwanted blemishes, and the hand cleaning of the casting using hand chisels, pneumatic tools and wire brushes. Welding techniques, such as oxyacetylene-flame cutting, electric arc, arc-air, powder washing and the plasma torch, may be employed for burning off headers, for casting repair and for cutting and washing.
Sprue removal is the first dressing operation. As much as half of the metal cast in the mould is not part of the final casting. The mould must include reservoirs, cavities, feeders and sprue in order that it be filled with metal to complete the cast object. The sprue usually can be removed during the knockout stage, but sometimes this must be carried out as a separate stage of the fettling or dressing operation. Sprue removal is done by hand, usually by knocking the casting with a hammer. To reduce noise, the metal hammers can be replaced by rubber-covered ones and the conveyors lined with the same noise-damping rubber. Hot metal fragments are thrown off and pose an eye hazard. Eye protection must be used. Detached sprues should normally be returned to the charging region of the melting plant and should not be permitted to accumulate at the despruing section of the foundry. After despruing (but sometimes before) most castings are shot blasted or tumbled to remove mould materials and perhaps to improve the surface finish. Tumbling barrels generate high noise levels. Enclosures may be necessary, which can also require LEV.
Dressing methods in steel, iron and non-ferrous foundries are very similar, but special difficulties exist in the dressing and fettling of steel castings owing to greater amounts of burnt-on fused sand compared to iron and non-ferrous castings. Fused sand on large steel castings may contain cristobalite, which is more toxic than the quartz found in virgin sand.
Airless shot blasting or tumbling of castings before chipping and grinding is needed to prevent overexposure to silica dust. The casting must be free of visible dust, although a silica hazard may still be generated by grinding if silica is burnt into the apparently clean metal surface of the casting. The shot is centrifugally propelled at the casting, and no operator is required inside the unit. The blast cabinet must be exhausted so no visible dust escapes. Only when there is a breakdown or deterioration of the shot-blast cabinet and/or the fan and collector is there a dust problem.
Water or water and sand or pressure shot blasting may be used to remove adherent sand by subjecting the casting to a high-pressure stream of either water or iron or steel shot. Sand blasting has been banned in several countries (e.g., the United Kingdom) because of the silicosis risk as the sand particles become finer and finer and the respirable fraction thus continually increases. The water or shot is discharged through a gun and can clearly present a risk to personnel if not handled correctly. Blasting should always be carried out in an isolated, enclosed space. All blasting enclosures should be inspected at regular intervals to ensure that the dust extraction system is functioning and that there are no leaks through which shot or water could escape into the foundry. Blasters’ helmets should be approved and carefully maintained. It is advisable to post a notice on the door to the booth, warning employees that blasting is under way and that unauthorized entry is prohibited. In certain circumstances delay bolts linked to the blast drive motor can be fitted to the doors, making it impossible to open the doors until blasting has ceased.
A variety of grinding tools are used to smooth the rough casting. Abrasive wheels may be mounted on floor-standing or pedestal machines or in portable or swing-frame grinders. Pedestal grinders are used for smaller castings that can be easily handled; portable grinders, surface disc wheels, cup wheels and cone wheels are used for a number of purposes, including smoothing of internal surfaces of castings; swing-frame grinders are used primarily on large castings that require a great deal of metal removal.
Other Foundries
Steel founding
Production in the steel foundry (as distinct from a basic steel mill) is similar to that in the iron foundry; however, the metal temperatures are much higher. This means that eye protection with coloured lenses is essential and that the silica in the mould is converted by heat to tridymite or crystobalite, two forms of crystalline silica which are particularly dangerous to the lungs. Sand often becomes burnt on to the casting and has to be removed by mechanical means, which give rise to dangerous dust; consequently, effective dust exhaust systems and respiratory protection are essential.
Light-alloy founding
The light-alloy foundry uses mainly aluminium and magnesium alloys. These often contain small amounts of metals which may give off toxic fumes under certain circumstances. The fumes should be analysed to determine their constituents where the alloy might contain such components.
In aluminium and magnesium foundries, melting is commonly done in crucible furnaces. Exhaust vents around the top of the pot for removing fumes are advisable. In oil-fired furnaces, incomplete combustion due to faulty burners may result in products such as carbon monoxide being released into the air. Furnace fumes may contain complex hydrocarbons, some of which may be carcinogenic. During furnace and flue cleaning there is the hazard of exposure to vanadium pentoxide concentrated in furnace soot from oil deposits.
Fluorspar is commonly used as a flux in aluminium melting, and significant quantities of fluoride dust may be released to the environment. In certain cases barium chloride has been used as a flux for magnesium alloys; this is a significantly toxic substance and, consequently, considerable care is required in its use. Light alloys may occasionally be degassed by passing sulphur dioxide or chlorine (or proprietary compounds that decompose to produce chlorine) through the molten metal; exhaust ventilation and respiratory protective equipment are required for this operation. In order to reduce the cooling rate of the hot metal in the mould, a mixture of substances (usually aluminium and iron oxide) which react highly exothermically is placed on the mould riser. This “thermite” mixture gives off dense fumes which have been found to be innocuous in practice. When the fumes are brown in colour, alarm may be caused due to suspicion of the presence of nitrogen oxides; however, this suspicion is unfounded. The finely divided aluminium produced during the dressing of aluminium and magnesium castings constitutes a severe fire hazard, and wet methods should be used for dust collection.
Magnesium casting entails considerable potential fire and explosion hazard. Molten magnesium will ignite unless a protective barrier is maintained between it and the atmosphere; molten sulphur is widely employed for this purpose. Foundry workers applying the sulphur powder to the melting pot by hand may develop dermatitis and should be provided with gloves made of fireproof fabric. The sulphur in contact with the metal is constantly burning, so considerable quantities of sulphur dioxide are given off. Exhaust ventilation should be installed. Workers should be informed of the danger of a pot or ladle of molten magnesium catching fire, which may give rise to a dense cloud of finely divided magnesium oxide. Protective clothing of fireproof materials should be worn by all magnesium foundry workers. Clothing coated with magnesium dust should not be stored in lockers without humidity control, since spontaneous combustion may occur. The magnesium dust should be removed from the clothing.French chalk is used extensively in mould dressing in magnesium foundries; the dust should be controlled to prevent talcosis. Penetrating oils and dusting powders are employed in the inspection of light-alloy castings for the detection of cracks.
Dyes have been introduced to improve the effectiveness of these techniques. Certain red dyes have been found to be absorbed and excreted in sweat, thus causing soiling of personal clothing; although this condition is a nuisance, no effects on health have been observed.
Brass and bronze foundries
Toxic metal fumes and dust from typical alloys are a special hazard of brass and bronze foundries. Exposures to lead above safe limits in both melting, pouring and finishing operations are common, especially where alloys have a high lead composition. The lead hazard in furnace cleaning and dross disposal is particularly acute. Overexposure to lead is frequent in melting and pouring and can also occur in grinding. Zinc and copper fumes (the constituents of bronze) are the most common causes of metal fume fever, although the condition has also been observed in foundry workers using magnesium, aluminium, antimony and so on. Some high-duty alloys contain cadmium, which can cause chemical pneumonia from acute exposure and kidney damage and lung cancer from chronic exposure.
Permanent-mould process
Casting in permanent metal moulds, as in die-casting, has been an important development in the foundry. In this case, pattern making is largely replaced by engineering methods and is really a die-sinking operation. Most of the pattern making hazards are thereby removed and the risks from sand are also eliminated but are replaced by a degree of risk inherent in the use of some sort of refractory material to coat the die or mould. In modern die-foundry work, increasing use is made of sand cores, in which case the dust hazards of the sand foundry are still present.
Die casting
Aluminium is a common metal in die casting. Automotive hardware such as chrome trim is typically zinc die cast, followed by copper, nickel and chrome plating. The hazard of metal fume fever from zinc fumes should be constantly controlled, as must be chromic acid mist.
Pressure die-casting machines present all the hazards common to hydraulic power presses. In addition, the worker may be exposed to the mist of oils used as die lubricants and must be protected against the inhalation of these mists and the danger of oil-saturated clothing. The fire-resistant hydraulic fluids used in the presses may contain toxic organophosphorus compounds, and particular care should be taken during maintenance work on hydraulic systems.
Precision founding
Precision foundries rely on the investment or lost-wax casting process, in which patterns are made by injection moulding wax into a die; these patterns are coated with a fine refractory powder which serves as a mould-facing material, and the wax is then melted out prior to casting or by the introduction of the casting metal itself.
Wax removal presents a definite fire hazard, and decomposition of the wax produces acrolein and other hazardous decomposition products. Wax-burnout kilns must be adequately ventilated. Trichloroethylene has been used to remove the last traces of wax; this solvent may collect in pockets in the mould or be absorbed by the refractory material and vaporize or decompose during pouring. The inclusion of asbestos investment casting refractory materials should be eliminated due to the hazards of asbestos.
Health Problems and Disease Patterns
Foundries stand out among industrial processes because of a higher fatality rate arising from molten metal spills and explosions, cupola maintenance including bottom drop and carbon monoxide hazards during relining. Foundries report a higher incidence of foreign body, contusion and burn injuries and a lower proportion of musculoskeletal injuries than other facilities. They also have the highest noise exposure levels.
A study of several dozen fatal injuries in foundries revealed the following causes: crushing between mould conveyor cars and building structures during maintenance and trouble-shooting, crushing while cleaning mullers which were remotely activated, molten metal burns after crane failure, mould cracking, overflowing transfer ladle, steam eruption in undried ladle, falls from cranes and work platforms, electrocution from welding equipment, crushing from material-handling vehicles, burns from cupola bottom drop, high-oxygen atmosphere during cupola repair and carbon monoxide overexposure during cupola repair.
Abrasive wheels
The bursting or breaking of abrasive wheels may cause fatal or very serious injuries: gaps between the wheel and the rest at pedestal grinders may catch and crush the hand or forearm. Unprotected eyes are at risk at all stages. Slips and falls, especially when carrying heavy loads, may be caused by badly maintained or obstructed floors. Injuries to the feet may be caused by falling objects or dropped loads. Sprains and strains may result from overexertion in lifting and carrying. Badly maintained hoisting appliances may fail and cause materials to fall on workers. Electric shock may result from badly maintained or unearthed (ungrounded) electrical equipment, especially portable tools.
All dangerous parts of machinery, especially abrasive wheels, should have adequate guarding, with automatic lockout if the guard is removed during processing. Dangerous gaps between the wheel and the rest at pedestal grinders should be eliminated, and close attention should be paid to all precautions in the care and maintenance of abrasive wheels and in regulation of their speed (particular care is required with portable wheels). Strict maintenance of all electrical equipment and proper grounding arrangements should be enforced. Workers should be instructed in correct lifting and carrying techniques and should know how to attach loads to crane hooks and other hoisting appliances. Suitable PPE, such as eye and face shields and foot and leg protection, should also be provided. Provision should be made for prompt first aid, even for minor injuries, and for competent medical care when needed.
Dust
Dust diseases are prominent among foundry workers. Silica exposures are often close to or exceed prescribed exposure limits, even in well-controlled cleaning operations in modern production foundries and where castings are free of visible dust. Exposures many times above the limit occur where castings are dusty or cabinets leak. Overexposures are likely where visible dust escapes venting in shakeout, sand preparation or refractory repair.
Silicosis is the predominant health hazard in the steel fettling shop; a mixed pneumoconiosis is more prevalent in iron fettling (Landrigan et al. 1986). In the foundry, the prevalence increases with length of exposure and higher dust levels. There is some evidence that conditions in steel foundries are more likely to cause silicosis than those in iron foundries because of the higher levels of free silica present. Attempts to set an exposure level at which silicosis will not occur have been inconclusive; the threshold is probably less than 100 micrograms/m3 and perhaps as low as half that amount.
In most countries, the occurrence of new cases of silicosis is declining, in part because of changes in technology, a move away from silica sand in foundries and a shift away from silica brick and towards basic furnace linings in steel melting. A major reason is the fact that automation has resulted in the employment of fewer workers in steel production and foundries. Exposure to respirable silica dust remains stubbornly high in many foundries, however, and in countries where processes are labour intensive, silicosis remains a major problem.
Silico-tuberculosis has long been reported in foundry workers. Where the prevalence of silicosis has declined, there has been a parallel falling off in reported cases of tuberculosis, although that disease has not been completely eradicated. In countries where dust levels have remained high, dusty processes are labour intensive and the prevalence of tuberculosis in the general population is elevated, tuberculosis remains an important cause of death amongst foundry workers.
Many workers suffering from pneumoconiosis also have chronic bronchitis, often associated with emphysema; it has long been thought by many investigators that, in some cases at least, occupational exposures may have played a part. Cancer of the lung, lobar pneumonia, bronchopneumonia and coronary thrombosis have also been reported to be associated with pneumoconiosis in foundry workers.
A recent review of mortality studies of foundry workers, including the American auto industry, showed increased deaths from lung cancer in 14 of 15 studies. Because high lung cancer rates are found among cleaning room workers where the primary hazard is silica, it is likely that mixed exposures are also found.
Studies of the carcinogens in the foundry environment have concentrated on polycyclic aromatic hydrocarbons formed in the thermal breakdown of sand additives and binders. It has been suggested that metals such as chromium and nickel, and dusts such as silica and asbestos, may also be responsible for some of the excess mortality. Differences in moulding and core-making chemistry, sand type and the composition of iron and steel alloys may be responsible for different levels of risk in different foundries (IARC 1984).
Increased mortality from non-malignant respiratory disease was found in 8 of 11 studies. Silicosis deaths were recorded as well. Clinical studies found x-ray changes characteristic of pneumoconiosis, lung function deficits characteristic of obstruction, and increased respiratory symptoms among workers in modern “clean” production foundries. These resulted from exposures after the l960s and strongly suggest that the health risks prevalent in the older foundries have not yet been eliminated.
Prevention of lung disorders is essentially a matter of dust and fume control; the generally applicable solution is providing good general ventilation coupled with efficient LEV. Low-volume, high-velocity systems are most suitable for some operations, particularly portable grinding wheels and pneumatic tools.
Hand or pneumatic chisels used to remove burnt-on sand produce much finely divided dust. Brushing off excess materials with revolving wire brushes or hand brushes also produces much dust; LEV is required.
Dust control measures are readily adaptable to floor-standing and swing-frame grinders. Portable grinding on small castings can be carried out on exhaust-ventilated benches, or ventilation may be applied to the tools themselves. Brushing can also be carried out on a ventilated bench. Dust control on large castings presents a problem, but considerable progress has been made with low-volume, high-velocity ventilation systems. Instruction and training in their use is needed to overcome the objections of workers who find these systems cumbersome and complain that their view of the working area is impaired.
Dressing and fettling of very large castings where local ventilation is impracticable should be done in a separate, isolated area and at a time when few other workers are present. Suitable PPE that is regularly cleaned and repaired, should be provided for each worker, along with instruction in its proper use.
Since the 1950s, a variety of synthetic resin systems have been introduced into foundries to bind sand in cores and moulds. These generally comprise a base material and a catalyst or hardener which starts the polymerization. Many of these reactive chemicals are sensitizers (e.g., isocyanates, furfuryl alcohol, amines and formaldehyde) and have now been implicated in cases of occupational asthma among foundry workers. In one study, 12 out of 78 foundry workers exposed to Pepset (cold-box) resins had asthmatic symptoms, and of these, six had a marked decline in airflow rates in a challenge test using methyl di-isocyanate (Johnson et al. 1985).
Welding
Welding in fettling shops exposes workers to metal fumes with the consequent hazard of toxicity and metal fever, depending on the composition of the metals involved. Welding on cast iron requires a nickel rod and creates exposure to nickel fumes. The plasma torch produces a considerable amount of metal fumes, ozone, nitrogen oxide and ultraviolet radiation, and generates high levels of noise.
An exhaust-ventilated bench can be provided for welding small castings. Controlling exposures during welding or burning operations on large castings is difficult. A successful approach involves creating a central station for these operations and providing LEV through a flexible duct positioned at the point of welding. This requires training the worker to move the duct from one location to another. Good general ventilation and, when necessary, the use of PPE will aid in reducing the overall dust and fume exposures.
Noise and vibration
The highest levels of noise in the foundry are usually found in knockout and cleaning operations; they are higher in mechanized than in manual foundries. The ventilation system itself may generate exposures close to 90 dBA.
Noise levels in the fettling of steel castings may be in the range of 115 to 120 dBA, while those actually encountered in the fettling of cast iron are in the 105 to 115 dBA range. The British Steel Casting Research Association established that the sources of noise during fettling include:
- the fettling tool exhaust
- the impact of the hammer or wheel on the casting
- resonance of the casting and vibration against its support
- transmission of vibration from the casting support to surrounding structures
- reflection of direct noise by the hood controlling air flow through the ventilation system.
Noise control strategies vary with the size of the casting, the type of metal, the work area available, the use of portable tools and other related factors. Certain basic measures are available to reduce noise exposure of individuals and co-workers, including isolation in time and space, complete enclosures, partial sound-absorbing partitions, execution of work on sound-absorbing surfaces, baffles, panels and hoods made from sound-absorbing or other acoustical materials. The guidelines for safe daily exposure limits should be observed and, as a last resort, personal protective devices may be used.
A fettling bench developed by the British Steel Casting Research Association reduces the noise in chipping by about 4 to 5 dBA. This bench incorporates an exhaust system to remove dust. This improvement is encouraging and leads to hope that, with further development, even greater noise reductions will become possible.
Hand-arm vibration syndrome
Portable vibrating tools may cause Raynaud’s phenomenon (hand-arm vibration syndrome—HAVS). This is more prevalent in steel fettlers than in iron fettlers and more frequent among those using rotating tools. The critical vibratory rate for the onset of this phenomenon is between 2,000 and 3,000 revolutions per minute and in the range of 40 to 125 Hz.
HAVS is now thought to involve effects on a number of other tissues in the forearm apart from peripheral nerves and blood vessels. It is associated with carpal tunnel syndrome and degenerative changes in the joints. A recent study of steelworks chippers and grinders showed they were twice as likely to develop Dupuytren’s contracture than a comparison group (Thomas and Clarke 1992).
Vibration transmitted to the hands of the worker can be considerably reduced by: selection of tools designed to reduce the harmful ranges of frequency and amplitude; direction of the exhaust port away from the hand; use of multiple layers of gloves or an insulating glove; and shortening of exposure time by changes in work operations, tools and rest periods.
Eye problems
Some of the dusts and chemicals encountered in foundries (e.g., isocyanates, formaldehyde and tertiary amines, such as dimethlyethylamine, triethylamine and so on) are irritants and have been responsible for visual symptoms among exposed workers. These include itchy, watery eyes, hazy or blurred vision or so called “blue-grey vision”. On the basis of the occurrence of these effects, reducing time-weighted average exposures below 3 ppm has been recommended.
Other problems
Formaldehyde exposures at or above the US exposure limit are found in well-controlled hot-box core-making operations. Exposures many times above the limit may be found where hazard control is poor.
Asbestos has been used widely in the foundry industry and, until recently, it was often used in protective clothing for heat-exposed workers. Its effects have been found in x-ray surveys of foundry workers, both among production workers and maintenance workers who have been exposed to asbestos; a cross-sectional survey found the characteristic pleural involvement in 20 out of 900 steel workers (Kronenberg et al. 1991).
Periodic examinations
Preplacement and periodic medical examinations, including a survey of symptoms, chest x rays, pulmonary function tests and audiograms, should be provided for all foundry workers with appropriate follow-up if questionable or abnormal findings are detected. The compounding effects of tobacco smoke on the risk of respiratory problems among foundry workers mandate inclusion of advice on smoking cessation in a programme of health education and promotion.
Conclusion
Foundries have been an essential industrial operation for centuries. Despite continuing advances in technology, they present workers with a panoply of hazards to safety and health. Because hazards continue to exist even in the most modern plants with exemplary prevention and control programmes, protecting the health and well-being of workers remains an ongoing challenge to management and to the workers and their representatives. This remains difficult both in industry downturns (when concerns for worker health and safety tend to give way to economic stringencies) and in boom times (when the demand for increased output may lead to potentially dangerous short cuts in the processes). Education and training in hazard control, therefore, remain a constant necessity.
Forging and Stamping
Process Overview
Forming metal parts by application of high compressive and tensile forces is common throughout industrial manufacturing. In stamping operations, metal, most often in the form of sheets, strips or coils, is formed into specific shapes at ambient temperatures by shearing, pressing and stretching between dies, usually in a series of one or more discrete impact steps. Cold-rolled steel is the starting material in many stamping operations creating sheet metal parts in the automotive and appliance and other industries. Approximately 15% of workers in the automotive industry work in stamping operations or plants.
In forging, compressive force is applied to pre-formed blocks (blanks) of metal, usually heated to high temperatures, also in one or more discrete pressing steps. The shape of the final piece is determined by the shape of the cavities in the metal die or dies used. With open impression dies, as in drop hammer forging, the blank is compressed between one die attached to the bottom anvil and the vertical ram. With closed impression dies, as in press forging, the blank is compressed between the bottom die and an upper die attached to the ram.
Drop hammer forges use a steam or air cylinder to raise the hammer, which is then dropped by gravity or is driven by steam or air. The number and force of the hammer blows are manually controlled by the operator. The operator often holds the cold end of the stock while operating the drop hammer. Drop hammer forging once comprised about two-thirds of all forging done in the United States, but is less common today.
Press forges use a mechanical or hydraulic ram to shape the piece with a single, slow, controlled stroke (see figure 1). Press forging is usually controlled automatically. It can be done hot or at normal temperatures (cold-forging, extruding). A variation on normal forging is rolling, where continuous applications of force are used and the operator turns the part.
Figure 1. Press forging
Die lubricants are sprayed or otherwise applied to die faces and blank surfaces before and between hammer or press strokes.
High-strength machine parts such as shafts, ring gears, bolts and vehicle suspension components are common steel forging products. High-strength aircraft components such as wing spars, turbine disks and landing gear are forged from aluminium, titanium or nickel and steel alloys. Approximately 3% of automotive workers are in forging operations or plants.
Working Conditions
Many hazards common in heavy industry are present in stamping and forging operations. These include repetitive strain injuries (RSIs) from repeated handling and processing of parts and operation of machine controls such as palm buttons. Heavy parts place workers at risk for back and shoulder problems as well as upper extremity musculoskeletal disorders. Press operators in automotive stamping plants have rates of RSIs that are comparable to those of assembly plant workers in high-risk jobs. High-impulse vibration and noise are present in most stamping and some forging (e.g., steam or air hammer) operations, causing hearing loss and possible cardiovascular illness; these are among the highest-noise industrial environments (over 100 dBA). As in other forms of automation-driven systems, worker energy loads can be high, depending on the parts handled and machine cycling rates.
Catastrophic injuries resulting from unanticipated machine movements are common in stamping and forging. These can be due to: (1) mechanical failure of machine control systems, such as clutch mechanisms in situations where workers are routinely expected to be within the machine operating envelope (an unacceptable process design); (2) deficiencies in machine design or performance that invite unprogrammed worker interventions such as moving jammed or misaligned parts; or (3) improper, high-risk maintenance procedures performed without adequate lockout of the entire machine network involved, including parts transfer automation and the functions of other connected machines. Most automated machine networks are not configured for quick, efficient and effective lockout or safe trouble-shooting.
Mists from machine lubricating oils generated during normal operation are another generic health hazard in stamping and forging press operations powered by compressed air, potentially putting workers at risk for respiratory, dermatological and digestive diseases.
Health and Safety Problems
Stamping
Stamping operations have high risk of severe laceration due to the required handling of parts with sharp edges. Possibly worse is the handling of the scrap resulting from cut-off perimeters and punched out sections of parts. Scrap is typically collected by gravity-fed chutes and conveyors. Clearing occasional jams is a high-risk activity.
Chemical hazards specific to stamping typically arise from two main sources: drawing compounds (i.e., die lubricants) in actual press operations and welding emissions from assembly of the stamped parts. Drawing compounds (DCs) are required for most stamping. The material is sprayed or rolled onto sheet metal and further mists are generated by the stamping event itself. Like other metalworking fluids, drawing compounds may be straight oils or oil emulsions (soluble oils). Components include petroleum oil fractions, special lubricity agents (e.g., animal and vegetable fatty acid derivatives, chlorinated oils and waxes), alkanolamines, petroleum sulphonates, borates, cellulose-derived thickeners, corrosion inhibitors and biocides. Air concentrations of mist in stamping operations may reach those of typical machining operations, although these levels tend to be lower on average (0.05 to 2.0 mg/m3). However, visible fog and accumulated oil film on building surfaces are often present, and skin contact may be higher due to extensive handling of parts. Exposures most likely to present hazards are chlorinated oils (possible cancer, liver disease, skin disorders), rosin or tall oil fatty acid derivatives (sensitizers), petroleum fractions (digestive cancers) and, possibly, formaldehyde (from biocides) and nitrosamines (from alkanolamines and sodium nitrite, either as DC ingredients or in surface coatings on incoming steel). Elevated digestive cancer has been observed in two automotive stamping plants. Microbiological blooms in systems that apply DCs by rolling it onto sheet metal from an open reservoir can pose risks to workers for respiratory and dermatological problems analogous to those in machining operations.
Welding of stamped parts is often performed in stamping plants, usually without intermediate washing. This produces emissions that include metal fumes and pyrolysis and combustion products from drawing compound and other surface residues. Typical (primarily resistance) welding operations in stamping plants generate total particulate air concentrations in the range 0.05 to 4.0 mg/m3. Metal content (as fumes and oxides) usually makes up less than half of that particulate matter, indicating that up to 2.0 mg/m3 is poorly characterized chemical debris. The result is haze visible in many stamping plant welding areas. The presence of chlorinated derivatives and other organic ingredients raises serious concerns over the composition of welding smoke in these settings and strongly argues for ventilation controls. Application of other materials prior to welding (such as primer, paint and epoxy-like adhesives), some of which are then welded over, adds further concern. Welding production repair activities, usually done manually, often pose higher exposures to these same air contaminants. Excess rates of lung cancer have been observed among welders in an automotive stamping plant.
Forging
Like stamping, forging operations can pose high laceration risks when workers handle forged parts or trim the flash or unwanted edges off parts. High impact forging can also eject fragments, scale or tools, causing injury. In some forging activities, the worker grasps the working piece with tongs during the pressing or impact steps, increasing the risk for musculoskeletal injuries. In forging, unlike stamping, furnaces for heating parts (for forging and annealing) as well as bins of hot forgings are usually nearby. These create potential for high heat stress conditions. Additional factors in heat stress are the worker’s metabolic load during manual handling of materials and, in some cases, heat from combustion products of oil-based die lubricants.
Die lubrication is required in most forging and has the added feature that the lubricant comes in contact with high-temperature parts. This causes immediate pyrolysis and aerosolization not only in the dies but also subsequently from smoking parts in cooling bins. Forging die lubricant ingredients can include graphite slurries, polymeric thickeners, sulphonate emulsifiers, petroleum fractions, sodium nitrate, sodium nitrite, sodium carbonate, sodium silicate, silicone oils and biocides. These are applied as sprays or, in some applications, by swab. Furnaces used for heating metal to be forged are usually fired by oil or gas, or they are induction furnaces. Emissions can result from fuel-fired furnaces with inadequate draft and from non-ventilated induction furnaces when incoming metal stock has surface contaminants, such as oil or corrosion inhibitors, or if, prior to forging, it was lubricated for shearing or sawing (as in the case of bar stock). In the US, total particulate air concentrations in forging operations typically range from 0.1 to 5.0 mg/m3 and vary widely within forging operations due to thermal convection currents. An elevated lung cancer rate was observed among forging and heat treatment workers from two ball-bearing manufacturing plants.
Health and Safety Practices
Few studies have evaluated actual health effects in workers with stamping or forging exposures. Comprehensive characterization of the toxicity potential of most routine operations, including identification and measurement of priority toxic agents, has not been done. Evaluating the long-term health effects of die lubrication technology developed in the 1960s and 1970s has only recently become feasible. As a result, regulation of these exposures defaults to generic dust or total particulate standards such as 5.0 mg/m3 in the US. While probably adequate in some circumstances, this standard is not demonstrably adequate for many stamping and forging applications.
Some reduction in die lubricant mist concentrations is possible with careful management of the application procedure in both stamping and forging. Roll application in stamping is preferred when feasible, and using minimal air pressure in sprays is beneficial. Possible elimination of priority hazardous ingredients should be investigated. Enclosures with negative pressure and mist collectors can be highly effective but may be incompatible with parts handling. Filtering air released from high-pressure air systems in presses would reduce press oil mist (and noise). Skin contact in stamping operations can be reduced with automation and good personal protective wear, providing protection against both laceration and liquid saturation. For stamping plant welding, washing parts prior to welding is highly desirable, and partial enclosures with LEV would reduce smoke levels substantially.
Controls to reduce heat stress in stamping and hot forging include minimizing the amount of manual material handling in high-heat areas, shielding of furnaces to reduce radiation of heat, minimizing the height of furnace doors and slots and using cooling fans. The location of cooling fans should be an integral part of the design of air movement to control mist exposures and heat stress; otherwise, cooling may be obtained only at the expense of higher exposures.
Mechanization of material handling, switching from hammer to press forging when possible and adjusting the work rate to ergonomically practical levels can reduce the number of musculoskeletal injuries.
Noise levels can be reduced through a combination of switching from hammer to press forges when possible, well-designed enclosures and quieting of furnace blowers, air clutches, air leads and parts handling. A hearing conservation programme should be instituted.
PPE needed includes head protection, foot protection, goggles, hearing protectors (around are as with excessive noise), heat- and oil-proof aprons and leggings (with heavy use of oil-based die lubricants) and infrared eye and face protection (around furnaces).
Environmental Health Hazards
The environmental hazards arising from stamping plants, relatively minor compared to those from some other types of plants, include disposal of waste drawing compound and washing solutions and the exhausting of welding smoke without adequate cleaning. Some forging plants historically have caused acute degradation of local air quality with forging smoke and scale dust. However, with appropriate air cleaning capacity, this need not occur. Disposition of stamping scrap and forging scale containing die lubricants is another potential issue.
Welding and Thermal Cutting
This article is a revision of the 3rd edition of the Encyclopaedia of Occupational Health and Safety article “Welding and thermal cutting” by G.S. Lyndon.
Process Overview
Welding is a generic term referring to the union of pieces of metal at joint faces rendered plastic or liquid by heat or pressure, or both. The three common direct sources of heat are:
- flame produced by the combustion of fuel gas with air or oxygen
- electrical arc, struck between an electrode and a workpiece or between two electrodes
- electrical resistance offered to passage of current between two or more workpieces.
Other sources of heat for welding are discussed below (see table 1).
Table 1. Process materials inputs and pollution outputs for lead smelting and refining
|
Process |
Material input |
Air emissions |
Process wastes |
Other wastes |
|
Lead sintering |
Lead ore, iron, silica, limestone flux, coke, soda, ash, pyrite, zinc, caustic, baghouse dust |
Sulphur dioxide, particulate matter contain-ing cadmium and lead |
||
|
Lead smelting |
Lead sinter, coke |
Sulphur dioxide, particulate matter contain-ing cadmium and lead |
Plant washdown wastewater, slag granulation water |
Slag containing impurities such as zinc, iron, silica and lime, surface impoundment solids |
|
Lead drossing |
Lead bullion, soda ash, sulphur, baghouse dust, coke |
Slag containing such impurities as copper, surface impoundment solids |
||
|
Lead refining |
Lead drossing bullion |
In gas welding and cutting, oxygen or air and a fuel gas are fed to a blowpipe (torch) in which they are mixed prior to combustion at the nozzle. The blowpipe is usually hand held (see figure 1). The heat melts the metal faces of the parts to be joined, causing them to flow together. A filler metal or alloy is frequently added. The alloy often has a lower melting point than the parts to be joined. In this case, the two pieces are generally not brought to fusion temperature (brazing, soldering). Chemical fluxes may be used to prevent oxidation and facilitate the joining.
Figure 1. Gas welding with a torch & rod of filter metal. The welder is protected by a leather apron, gauntlets and goggles
In arc welding, the arc is struck between an electrode and the workpieces. The electrode can be connected to either an alternating current (AC) or direct current (DC) electric supply. The temperature of this operation is about 4,000°C when the workpieces fuse together. Usually it is necessary to add molten metal to the joint either by melting the electrode itself (consumable electrode processes) or by melting a separate filler rod which is not carrying current (non-consumable electrode processes).
Most conventional arc welding is done manually by means of a covered (coated) consumable electrode in a hand-held electrode holder. Welding is also accomplished by many semi or fully automatic electric welding processes such as resistance welding or continuous electrode feed.
During the welding process, the welding area must be shielded from the atmosphere in order to prevent oxidation and contamination. There are two types of protection: flux coatings and inert gas shielding. In flux-shielded arc welding, the consumable electrode consists of a metal core surrounded by a flux coating material, which is usually a complex mixture of mineral and other components. The flux melts as welding progresses, covering the molten metal with slag and enveloping the welding area with a protective atmosphere of gases (e.g., carbon dioxide) generated by the heated flux. After welding, the slag must be removed, often by chipping.
In gas-shielded arc welding, a blanket of inert gas seals off the atmosphere and prevents oxidation and contamination during the welding process. Argon, helium, nitrogen or carbon dioxide are commonly used as the inert gases. The gas selected depends upon the nature of the materials to be welded. The two most popular types of gas-shielded arc welding are metal- and tungsten inert gas (MIG and TIG).
Resistance welding involves using the electrical resistance to the passage of a high current at low voltage through components to be welded to generate heat for melting the metal. The heat generated at the interface between the components brings them to welding temperatures.
Hazards and Their Prevention
All welding involves hazards of fire, burns, radiant heat (infrared radiation) and inhalation of metal fumes and other contaminants. Other hazards associated with specific welding processes include electrical hazards, noise, ultraviolet radiation, ozone, nitrogen dioxide, carbon monoxide, fluorides, compressed gas cylinders and explosions. See table 2 for additional detail.
Table 2. Description and hazards of welding processes
|
Welding Process |
Description |
Hazards |
|
Gas welding and cutting |
||
|
Welding |
The torch melts the metal surface and filler rod, causing a joint to be formed. |
Metal fumes, nitrogen dioxide, carbon monoxide, noise, burns, infrared radiation, fire, explosions |
|
Brazing |
The two metal surfaces are bonded without melting the metal. The melting temperature of the filler metal is above 450 °C. Heating is done by flame heating, resistance heating and induction heating. |
Metal fumes (especially cadmium), fluorides, fire, explosion, burns |
|
Soldering |
Similar to brazing, except the melting temperature of the filler metal is below 450 °C. Heating is also done using a soldering iron. |
Fluxes, lead fumes, burns |
|
Metal cutting and flame gouging |
In one variation, the metal is heated by a flame, and a jet of pure oxygen is directed onto the point of cutting and moved along the line to be cut. In flame gouging, a strip of surface metal is removed but the metal is not cut through. |
Metal fumes, nitrogen dioxide, carbon monoxide, noise, burns, infrared radiation, fire, explosions |
|
Gas pressure welding |
The parts are heated by gas jets while under pressure, and become forged together. |
Metal fumes, nitrogen dioxide, carbon monoxide, noise, burns, infrared radiation, fire, explosions |
|
Flux-shielded arc welding |
||
|
Shielded metal arc welding (SMAC); “stick” arc welding; manual metal arc welding (MMA); open arc welding |
Uses a consumable electrode consisting of a metal core surrounded by a flux coating |
Metal fumes, fluorides (especially with low-hydrogen electrodes), infrared and ultraviolet radiation, burns, electrical, fire; also noise, ozone, nitrogen dioxide |
|
Submerged arc welding (SAW) |
A blanket of granulated flux is deposited on the workpiece, followed by a consumable bare metal wire electrode. The arc melts the flux to produce a protective molten shield in the welding zone. |
Fluorides, fire, burns, infrared radiation, electrical; also metal fumes, noise, ultraviolet radiation, ozone, and nitrogen dioxide |
|
Gas-shielded arc welding |
||
|
Metal inert gas (MIG); gas metal arc welding (GMAC) |
The electrode is normally a bare consumable wire of similar composition to the weld metal and is fed continuously to the arc. |
Ultraviolet radiation, metal fumes, ozone, carbon monoxide (with CO2 gas), nitrogen dioxide, fire, burns, infrared radiation, electrical, fluorides, noise |
|
Tungsten inert gas (TIG); gas tungsten arc welding (GTAW); heliarc |
The tungsten electrode is non-consumable, and filler metal is introduced as a consumable into the arc manually. |
Ultraviolet radiation, metal fumes, ozone, nitrogen dioxide, fire, burns, infrared radiation, electrical, noise, fluorides, carbon monoxide |
Plasma arc welding (PAW) and plasma arc spraying; tungsten arc cutting |
Similar to TIG welding, except that the arc and stream of inert gases pass through a small orifice before reaching the workpiece, creating a “plasma” of highly ionized gas which can achieve temperatures of over 33,400°C.This is also used for metallizing. |
Metal fumes, ozone, nitrogen dioxide, ultraviolet and infrared radiation, noise; fire, burns, electrical, fluorides, carbon monoxide, possible x rays |
|
Flux core arc welding (FCAW); metal active gas welding (MAG) |
Uses a flux-cored consumable electrode; may have carbon dioxide shield (MAG) |
Ultraviolet radiation, metal fumes, ozone, carbon monoxide (with CO2 gas), nitrogen dioxide, fire, burns, infrared radiation, electrical, fluorides, noise |
|
Electric resistance welding |
||
|
Resistance welding (spot, seam, projection or butt welding) |
A high current at low voltage flows through the two components from electrodes. The heat generated at the interface between the components brings them to welding temperatures. During the passage of the current, pressure by the electrodes produces a forge weld. No flux or filler metal is used. |
Ozone, noise (sometimes), machinery hazards, fire, burns, electrical, metal fumes |
|
Electro-slag welding |
Used for vertical butt welding. The workpieces are set vertically, with a gap between them, and copper plates or shoes are placed on one or both sides of the joint to form a bath. An arc is established under a flux layer between one or more continuously fed electrode wires and a metal plate. A pool of molten metal is formed, protected by molten flux or slag, which is kept molten by resistance to the current passing between the electrode and the workpieces. This resistance-generated heat melts the sides of the joint and the electrode wire, filling the joint and making a weld. As welding progresses, the molten metal and slag are retained in position by shifting the copper plates. |
Burns, fire, infrared radiation, electrical, metal fumes |
|
Flash welding |
The two metal parts to be welded are connected to a low-voltage, high-current source. When the ends of the components are brought into contact, a large current flows, causing “flashing” to occur and bringing the ends of the components to welding temperatures. A forge weld is obtained by pressure. |
Electrical, burns, fire, metal fumes |
Other welding processes |
||
|
Electron beam welding |
A workpiece in an vacuum chamber is bombarded by a beam of electrons from an electron gun at high voltages. The energy of the electrons is transformed into heat upon striking the workpiece, thus melting the metal and fusing the workpiece. |
X rays at high voltages, electrical, burns, metal dusts, confined spaces |
|
Arcair cutting |
An arc is struck between the end of a carbon electrode (in a manual electrode holder with its own supply of compressed air) and the workpiece. The molten metal produced is blown away by jets of compressed air. |
Metal fumes, carbon monoxide, nitrogen dioxide, ozone, fire, burns, infrared radiation, electrical |
|
Friction welding |
A purely mechanical welding technique in which one component remains stationary while the other is rotated against it under pressure. Heat is generated by friction, and at forging temperature the rotation ceases. A forging pressure then effects the weld. |
Heat, burns, machinery hazards |
|
Laser welding and drilling |
Laser beams can be used in industrial applications requiring exceptionally high precision, such as miniature assemblies and micro techniques in the electronics industry or spinnerets for the artificial fibre industry. The laser beam melts and joins the workpieces. |
Electrical, laser radiation, ultraviolet radiation, fire, burns, metal fumes, decomposition products of workpiece coatings |
|
Stud welding |
An arc is struck between a metal stud (acting as the electrode) held in a stud welding gun and the metal plate to be joined, and raises the temperature of the ends of the components to melting point. The gun forces the stud against the plate and welds it. Shielding is provided by a ceramic ferrule surrounding the stud. |
Metal fumes, infrared and ultraviolet radiation, burns, electrical, fire, noise, ozone, nitrogen dioxide |
|
Thermite welding |
A mixture of aluminium powder and a metal oxide powder (iron, copper, etc.) is ignited in a crucible, producing molten metal with the evolution of intense heat. The crucible is tapped and the molten metal flows into the cavity to be welded (which is surrounded by a sand mould). This is often used to repair castings or forgings. |
Fire, explosion, infrared radiation, burns |
Much welding is not done in shops where conditions can generally be controlled, but in the field in the construction or repair of large structures and machinery (e.g., frameworks of buildings, bridges and towers, ships, railroad engines and cars, heavy equipment and so on). The welder may have to carry all his or her equipment to the site, set it up and work in confined spaces or on scaffolds. Physical strain, inordinate fatigue and musculoskeletal injuries may follow being required to reach, kneel or work in other uncomfortable and awkward positions. Heat stress may result from working in warm weather and the occlusive effects of the personal protective equipment, even without the heat generated by the welding process.
Compressed gas cylinders
In high-pressure gas welding installations, oxygen and the fuel gas (acetylene, hydrogen, town gas, propane) are supplied to the torch from cylinders. The gases are stored in these cylinders at high pressure. The special fire and explosion hazards and precautions for the safe use and storage of the fuel gases are also discussed elsewhere in this Encyclopaedia. The following precautions should be observed:
- Only pressure regulators designed for the gas in use should be fitted to cylinders. For example, an acetylene regulator should not be used with coal gas or hydrogen (although it may be used with propane).
- Blowpipes must be kept in good order and cleaned at regular intervals. A hardwood stick or soft brass wire should be used for cleaning the tips. They should be connected to regulators with special canvas-reinforced hoses placed in such a way that they are unlikely to be damaged.
- Oxygen and acetylene cylinders must be stored separately and only on fire-resistant premises devoid of flammable material and must be so located that they may be readily removed in case of fire. Local building and fire protection codes must be consulted.
- The colour coding in force or recommended for identification of cylinders and accessories should be scrupulously observed. In many countries, the internationally accepted colour codes used for the transport of dangerous materials are applied in this field. The case for enforcement of uniform international standards in this respect is strengthened by safety considerations bound up with the increasing international migration of industrial workers.
Acetylene generators
In the low-pressure gas welding process, acetylene is generally produced in generators by reaction of calcium carbide and water. The gas is then piped to the welding or cutting torch into which oxygen is fed.
Stationary generating plants should be installed either in the open air or in a well-ventilated building away from the main workshops. The ventilation of the generator house should be such as to prevent the formation of an explosive or toxic atmosphere. Adequate lighting should be provided; switches, other electrical gear and electrical lamps should either be located outside the building or be explosion-proof. Smoking, flames, torches, welding plant or flammable materials must be excluded from the house or from the vicinity of an open-air generator. Many of these precautions also apply to portable generators. Portable generators should be used, cleaned and recharged only in the open air or in a well-ventilated shop, away from any flammable material.
Calcium carbide is supplied in sealed drums. The material should be stored and kept dry, on a platform raised above the floor level. Stores must be situated under cover, and if they adjoin another building the party wall must be fireproof. The storeroom should be suitably ventilated through the roof. Drums should be opened only immediately before the generator is charged. A special opener should be provided and used; a hammer and chisel should never be used to open drums. It is dangerous to leave calcium carbide drums exposed to any source of water.
Before a generator is dismantled, all calcium carbide must be removed and the plant filled with water. The water should remain in the plant for at least half an hour to ensure that every part is free from gas. The dismantling and servicing should be carried out only by the manufacturer of the equipment or by a specialist. When a generator is being recharged or cleaned, none of the old charge must be used again.
Pieces of calcium carbide wedged in the feed mechanism or adhering to parts of the plant should be carefully removed, using non-sparking tools made of bronze or another suitable non-ferrous alloy.
All concerned should be fully conversant with the manufacturer’s instructions, which should be conspicuously displayed. The following precautions should also be observed:
- A properly designed back-pressure valve must be fitted between the generator and each blowpipe to prevent backfire or reverse flow of gas. The valve should be regularly inspected after backfire, and the water level checked daily.
- Only blowpipes of the injector type designed for low-pressure operation should be used. For heating and cutting, town gas or hydrogen at low pressure are sometimes employed. In these cases, a non-return valve should be placed between each blowpipe and the supply main or pipeline.
- An explosion may be caused by “flash-back”, which results from dipping the nozzle-tip into the molten metal pool, mud or paint, or from any other stoppage. Particles of slag or metal that become attached to the tip should be removed. The tip should also be cooled frequently.
- Local building and fire codes should be consulted.
Fire and explosion prevention
In locating welding operations, consideration should be given to surrounding walls, floors, nearby objects and waste material. The following procedures should be followed:
- All combustible material must be removed or adequately protected by sheet metal or other suitable materials; tarpaulins should never be used.
- Wood structures should be discouraged or similarly protected. Wood floors should be avoided.
- Precautionary measures should be taken in the case of openings or cracks in walls and floors; flammable material in adjoining rooms or on the floor below should be removed to a safe position. Local building and fire codes should be consulted.
- Suitable fire-extinguishing apparatus should always be at hand. In the case of low-pressure plant using an acetylene generator, buckets of dry sand should also be kept available; fire extinguishers of dry powder or carbon dioxide types are satisfactory. Water must never be used.
- Fire brigades may be necessary. A responsible person should be assigned to keep the site under observation for at least half an hour after completion of the work, in order to deal with any outbreak of fire.
- Since explosions can occur when acetylene gas is present in air in any proportion between 2 and 80%, adequate ventilation and monitoring are required to ensure freedom from gas leaks. Only soapy water should be used to search for gas leaks.
- Oxygen must be carefully controlled. For example, it should never be released into the air in a confined space; many metals, clothing and other materials become actively combustible in the presence of oxygen. In gas cutting, any oxygen which may not be consumed will be released into the atmosphere; gas cutting should never be undertaken in a confined space without proper ventilation arrangements.
- Alloys rich in magnesium or other combustible metals should be kept away from welding flames or arcs.
- Welding of containers can be extremely hazardous. If the previous contents are unknown, a vessel should always be treated as if it had contained a flammable substance. Explosions may be prevented either by removing any flammable material or by making it non-explosive and non-flammable.
- The mixture of aluminium and iron oxide used in thermite welding is stable under normal conditions. However, in view of the ease with which aluminium powder will ignite, and the quasi-explosive nature of the reaction, appropriate precautions should be taken in handling and storage (avoidance of exposure to high heat and possible ignition sources).
- A written hot-work permit programme is required for welding in some jurisdictions. This programme outlines the precautions and procedures to be followed during welding, cutting, burning and so on. This programme should include the specific operations conducted along with the safety precautions to be implemented. It must be plant specific and may include an internal permit system that must be completed with each individual operation.
Protection from heat and burn hazards
Burns of the eyes and exposed parts of the body may occur due to contact with hot metal and spattering of incandescent metal particles or molten metal. In arc welding, a high-frequency spark used to initiate the arc can cause small, deep burns if concentrated at a point on the skin. Intense infrared and visible radiation from a gas welding or cutting flame and incandescent metal in the weld pool can cause discomfort to the operator and persons in the vicinity of the operation. Each operation should be considered in advance, and necessary precautions designed and implemented. Goggles made specifically for gas welding and cutting should be worn to protect the eyes from heat and light radiated from the work. Protective covers over filter glass should be cleaned as required and replaced when scratched or damaged. Where molten metal or hot particles are emitted, the protective clothing being worn should deflect spatter. The type and thickness of fire-resistant clothing worn should be chosen according to the degree of hazard. In cutting and arc welding operations, leather shoe coverings or other suitable spats should be worn to prevent hot particles from falling into boots or shoes. For protecting the hands and forearms against heat, spatter, slag and so on, the leather gauntlet type of glove with canvas or leather cuffs is sufficient. Other types of protective clothing include leather aprons, jackets, sleeves, leggings and head covering. In overhead welding, a protective cape and cap are necessary. All protective clothing should be free from oil or grease, and seams should be inside, so as not to trap globules of molten metal. Clothing should not have pockets or cuffs that could trap sparks, and it should be worn so sleeves overlap gloves, leggings overlap shoes and so on. Protective clothing should be inspected for burst seams or holes through which molten metal or slag may enter. Heavy articles left hot on completion of welding should always be marked “hot” as a warning to other workers. With resistance welding, the heat produced may not be visible, and burns can result from handling of hot assemblies. Particles of hot or molten metal should not fly out of spot, seam or projection welds if conditions are correct, but non-flammable screens should be used and precautions taken. Screens also protect passers-by from eye burns. Loose parts should not be left in the throat of the machine because they are liable to be projected with some velocity.
Electrical safety
Although no-load voltages in manual arc welding are relatively low (about 80 V or less), welding currents are high, and transformer primary circuits present the usual hazards of equipment operated at power supply line voltage. The risk of electric shock should therefore not be ignored, especially in cramped spaces or in insecure positions.
Before welding commences, the grounding installation on arc welding equipment should always be checked. Cables and connections should be sound and of adequate capacity. A proper grounding clamp or bolted terminal should always be used. Where two or more welding machines are grounded to the same structure, or where other portable electric tools are also in use, grounding should be supervised by a competent person. The working position should be dry, secure and free from dangerous obstructions. A well-arranged, well-lighted, properly ventilated and tidy workplace is important. For work in confined spaces or dangerous positions, additional electrical protection (no-load, low-voltage devices) can be installed in the welding circuit, ensuring that only extremely low-voltage current is available at the electrode holder when welding is not taking place. (See discussion of confined spaces below.) Electrode holders in which the electrodes are held by a spring grip or screw thread are recommended. Discomfort due to heating can be reduced by effective heat insulation on that part of the electrode holder which is held in the hand. Jaws and connections of electrode holders should be cleaned and tightened periodically to prevent overheating. Provision should be made to accommodate the electrode holder safely when not in use by means of an insulated hook or a fully insulated holder. The cable connection should be designed so that continued flexing of the cable will not cause wear and failure of the insulation. Dragging of cables and plastic gas supply tubes (gas-shielded processes) across hot plates or welds must be avoided. The electrode lead should not come in contact with the job or any other earthed object (ground). Rubber tubes and rubber-covered cables must not be used anywhere near the high-frequency discharge, because the ozone produced will rot the rubber. Plastic tubes and polyvinyl chloride (PVC) covered cables should be used for all supplies from the transformer to the electrode holder. Vulcanized or tough rubber-sheathed cables are satisfactory on the primary side. Dirt and metallic or other conducting dust can cause a breakdown in the high-frequency discharge unit. To avoid this condition, the unit should be cleaned regularly by blowing-out with compressed air. Hearing protection should be worn when using compressed air for more than a few seconds. For electron-beam welding, the safety of the equipment used must be checked prior to each operation. To protect against electric shock, a system of interlocks must be fitted to the various cabinets. A reliable system of grounding of all units and control cabinets is necessary. For plasma welding equipment used for cutting heavy thicknesses, the voltages may be as high as 400 V and danger should be anticipated. The technique of firing the arc by a high-frequency pulse exposes the operator to the dangers of an unpleasant shock and a painful, penetrating high-frequency burn.
Ultraviolet radiation
The brilliant light emitted by an electric arc contains a high proportion of ultraviolet radiation. Even momentary exposure to bursts of arc flash, including stray flashes from other workers’ arcs, may produce a painful conjunctivitis (photo-ophthalmia) known as “arc eye” or “eye flash”. If any person is exposed to arc flash, immediate medical attention must be sought. Excessive exposure to ultraviolet radiation may also cause overheating and burning of the skin (sunburn effect). Precautions include:
- A shield or helmet fitted with correct grade of filter should be used (see the article “Eye and face protection” elsewhere in this Encyclopaedia). For the gas-shielded arc welding processes and carbon-arc cutting, flat handshields provide insufficient protection from reflected radiation; helmets should be used. Filtered goggles or eyeglasses with sideshields should be worn under the helmet to avoid exposure when the helmet is lifted up for inspection of the work. Helmets will also provide protection from spatter and hot slag. Helmets and handshields are provided with a filter glass and a protective cover glass on the outside. This should be regularly inspected, cleaned and replaced when scratched or damaged.
- The face, nape of the neck and other exposed parts of the body should be properly protected, especially when working close to other welders.
- Assistants should wear suitable goggles at a minimum and other PPE as the risk requires.
- All arc welding operations should be screened to protect other persons working nearby. Where the work is carried out at fixed benches or in welding shops, permanent screens should be erected where possible; otherwise, temporary screens should be used. All screens should be opaque, of sturdy construction and of a flame-resistant material.
- The use of black paints for the inside of welding booths has become an accepted practice, but the paint should produce a matte finish. Adequate ambient lighting should be provided to prevent eye strain leading to headaches and accidents.
- Welding booths and portable screens should be checked regularly to ensure that there is no damage which might result in the arc affecting persons working nearby.
Chemical hazards
Airborne contaminants from welding and flame cutting, including fumes and gases, arise from a variety of sources:
- the metal being welded, the metal in the filler rod or constituents of various types of steel such as nickel or chromium)
- any metallic coating on the article being welded or on the filler rod (e.g., zinc and cadmium from plating, zinc from galvanizing and copper as a thin coating on continuous mild steel filler rods)
- any paint, grease, debris and the like on the article being welded (e.g., carbon monoxide, carbon dioxide, smoke and other irritant breakdown products)
- flux coating on the filler rod (e.g., inorganic fluoride)
- the action of heat or ultraviolet light on the surrounding air (e.g., nitrogen dioxide, ozone) or on chlorinated hydrocarbons (e.g., phosgene)
- inert gas used as a shield (e.g., carbon dioxide, helium, argon).
Fumes and gases should be removed at the source by LEV. This can be provided by partial enclosure of the process or by the installation of hoods which supply sufficiently high air velocity across the weld position so as to ensure capture of the fumes.
Special attention should be paid to ventilation in the welding of non-ferrous metals and certain alloy steels, as well as to protection from the hazard of ozone, carbon monoxide and nitrogen dioxide which may be formed. Portable as well as fixed ventilation systems are readily available. In general, the exhausted air should not be recirculated. It should be recirculated only if there are not hazardous levels of ozone or other toxic gases and the exhaust air is filtered through a high-efficiency filter.
With electron-beam welding and if materials being welded are of a toxic nature (e.g., beryllium, plutonium and so on), care must be taken to protect the operator from any dust cloud when opening the chamber.
When there is a risk to health from toxic fumes (e.g., lead) and LEV is not practicable—for example, when lead-painted structures are being demolished by flame cutting—the use of respiratory protective equipment is necessary. In such circumstances, an approved, high-efficiency full-facepiece respirator or ahigh-efficiency positive pressure powered air-purified respirator (PAPR) should be worn. A high standard of maintenance of the motor and the battery is necessary, especially with the original high-efficiency positive pressure power respirator. The use of positive pressure compressed air line respirators should be encouraged where a suitable supply of breathing-quality compressed air is available. Whenever respiratory protective equipment is to be worn, the safety of the workplace should be reviewed to determine whether extra precautions are necessary, bearing in mind the restricted vision, entanglement possibilities and so on of persons wearing respiratory protective equipment.
Metal fume fever
Metal fume fever is commonly seen in workers exposed to the fumes of zinc in the galvanizing or tinning process, in brass founding, in the welding of galvanized metal and in metallizing or metal spraying, as well as from exposure to other metals such as copper, manganese and iron. It occurs in new workers and those returning to work after a weekend or holiday hiatus. It is an acute condition that occurs several hours after the initial inhalation of particles of a metal or its oxides. It starts with a bad taste in the mouth followed by dryness and irritation of the respiratory mucosa resulting in cough and occasionally dyspnoea and “tightness” of the chest. These may be accompanied by nausea and headache and, some 10 to 12 hours after the exposure, chills and fever which may be quite severe. These last several hours and are followed by sweating, sleep and often by polyuria and diarrhoea. There is no particular treatment, and recovery is usually complete in about 24 hours with no residua. It can be prevented by keeping exposure to the offending metallic fumes well within the recommended levels through the use of efficient LEV.
Confined spaces
For entry into confined spaces, there may be a risk of the atmosphere being explosive, toxic, oxygen deficient or combinations of the above. Any such confined space must be certified by a responsible person as safe for entry and for work with an arc or flame. A confined-space entry programme, including an entry permit system, may be required and is highly recommended for work that must be carried out in spaces that are typically not constructed for continuous occupancy. Examples include, but are not limited to, manholes, vaults, ship holds and the like. Ventilation of confined spaces is crucial, since gas welding not only produces airborne contaminants but also uses up oxygen. Gas-shielded arc welding processes can decrease the oxygen content of the air. (See figure 2.)
Figure 2. Welding in an enclosed space
S. F. Gilman
Noise
Noise is a hazard in several welding processes, including plasma welding, some types of resistance welding machines and gas welding. In plasma welding, the plasma jet is ejected at very high speeds, producing intense noise (up to 90 dBA), particularly in the higher frequency bands. The use of compressed air to blow off dust also creates high noise levels. To prevent hearing damage, ear plugs or muffs must be worn and a hearing conservation programme should be instituted, including audiometric (hearing capacity) examinations and employee training.
Ionizing radiation
In welding shops where welds are inspected radiographically with x-ray or gamma-ray equipment, the customary warning notices and instructions must be strictly observed. Workers must be kept at a safe distance from such equipment. Radioactive sources must be handled only with the required special tools and subject to special precautions.
Local and governmental regulations must be followed. See the chapter Radiation, ionizing elsewhere in this Encyclopaedia.
Sufficient shielding must be provided with electron-beam welding to prevent x rays from penetrating the walls and windows of the chamber. Any parts of the machine providing shields against x-ray radiation should be interlocked so that the machine cannot be energized unless they are in position. Machines should be checked at the time of installation for leaks of x-ray radiation, and regularly thereafter.
Other hazards
Resistance welding machines have at least one electrode, which moves with considerable force. If a machine is operated while a finger or hand is lying between the electrodes, severe crushing will result. Where possible, a suitable means of guarding must be devised to safeguard the operator. Cuts and lacerations can be minimized by first deburring components and by wearing protective gloves or gauntlets.
Lockout/tagout procedures should be used when machinery with electrical, mechanical or other energy sources is being maintained or repaired.
When slag is being removed from welds by chipping and so on, the eyes should be protected by goggles or other means.
Lathes
Adapted from the 3rd edition, Encyclopaedia of Occupational Health and Safety.
The important part lathes play in metalworking shops is best illustrated by the fact that 90 to 95% of the swarf (metal shavings) produced in the valves and fittings industry originates from lathes. About one-tenth of the accidents reported in this industry are due to lathes; this corresponds to one-third of all machine accidents. According to a study of the relative accident frequency per machine unit carried out in a plant manufacturing small precision parts and electrical equipment, lathes rank fifth after woodworking machines, metal-cutting saws, power presses and drilling machines. The need for protective measures on lathes is therefore beyond doubt.
Turning is a machine process in which the diameter of material is reduced by a tool with a special cutting edge. The cutting movement is produced by rotating the workpiece, and the feed and traverse movements are produced by the tool. By varying these three basic movements, and also by choosing the appropriate tool cutting-edge geometry and material, it is possible to influence the rate of stock removal, surface quality, shape of the chip formed and tool wear.
Structure of Lathes
A typical lathe consists of:
- a bed or base with machined slideways for the saddle and tailstock
- a headstock mounted on the bed, with the spindle and chuck
- a feed gearbox attached to the front of the bed for transmitting the feed movement as a function of the cutting speed through the leadscrew or feed shaft and apron to the saddle
- a saddle (or carriage) carrying the cross slide which performs the traverse movement
- a toolpost mounted on the cross slide (see figure 1).
Figure 1. Lathes and similar machines
This basic model of a lathe can be infinitely varied, from the universal machine to the special automatic lathe designed for one type of work only.
The most important types of lathe are as follows:
- Centre lathe. This is the most frequently used turning machine. It corresponds to the basic model with horizontal turning axis. The work is held between centres, by a faceplate or in a chuck.
- Multiple-tool lathe. This enables several tools to be engaged at the same time.
- Turret lathe, capstan lathe. Machines of this type enable a workpiece to be machined by several tools which are engaged one after the other. The tools are held in the turret, which rotates for bringing them into cutting position. The turrets are generally of the disc or crown type, but there are also drum-type turret lathes.
- Copy-turning lathes. The desired shape is transmitted by tracer control from a template to the work.
- Automatic lathe. The various operations, including the change of the work, are automated. There are bar automatics and chucking automatics.
- Vertical lathe (boring and turning mill). The work turns about a vertical axis; it is clamped to a horizontal revolving table. This type of machine is generally used for machining large castings and forgings.
- NC and CNC lathes. All the aforementioned machines can be equipped with a numerical control (NC) or computer-assisted numerical control (CNC) system. The result is a semi-automated or fully automated machine which can be used rather universally, thanks to the great versatility and easy programmability of the control system.
The future development of the lathe will probably concentrate on control systems. Contact controls will be increasingly replaced by electronic control systems. As regards the latter, there is a trend in evolution from interpolation-programmed to memory-programmed controls. It is foreseeable in the long run that the use of increasingly efficient process computers will tend to optimize the machining process.
Accidents
Lathe accidents are generally caused by:
- disregard for safety regulations when the machines are installed in workshops (e.g., not enough space between machines, no power disconnect switch for each machine)
- missing guards or the absence of auxiliary devices (severe injuries have been caused to workers who tried to brake the spindle of their lathes by pressing one of their hands against unguarded belt pulleys and to operators who inadvertently engaged unguarded clutch levers or pedals; injuries due to flying chips because of the absence of hinged or sliding covers have also occurred)
- inadequately located control elements (e.g., a turner’s hand can be pierced by the tailstock centre if the pedal controlling the chuck is mistaken for the one controlling the hydraulic circuit of the tailstock centre movement)
- adverse conditions of work (i.e., shortcomings from the point of view of occupational physiology)
- lack of PPE or wearing unsuitable work clothing (severe and even fatal injuries have been caused to lathe operators who wore loose clothes or had long, free-hanging hair)
- insufficient instruction of personnel (an apprentice was fatally injured when he filed a short shaft which was fixed between centres and rotated by a cranked carrier on the spindle nose and a straight one on the shaft; the lathe carrier seized his left-hand sleeve, which was wrapped around the workpiece, dragging the apprentice violently into the lathe)
- poor work organization leading to the use of unsuitable equipment (e.g., a long bar was machined on a conventional production lathe; it was too long for this lathe, and it projected more than 1 m beyond the headstock; moreover, the chuck aperture was too large for the bar and was made up by inserting wooden wedges; when the lathe spindle started rotating, the free bar end bent by 45° and struck the operator’s head; the operator died during the following night)
- defective machine elements (e.g., a loose carrier pin in a clutch may cause the lathe spindle to start rotating while the operator is adjusting a workpiece in the chuck).
Accident Prevention
The prevention of lathe accidents starts at the design stage. Designers should give special attention to control and transmission elements.
Control elements
Each lathe must be equipped with a power disconnect (or isolating) switch so that maintenance and repair work may be carried out safely. This switch must disconnect the current on all poles, reliably cut the pneumatic and hydraulic power and vent the circuits. On large machines, the disconnect switch should be so designed that it can be padlocked in its out position—a safety measure against accidental reconnection.
The layout of the machine controls should be such that the operator can easily distinguish and reach them, and that their manipulation presents no hazard. This means that controls must never be arranged at points which can be reached only by passing the hand over the working zone of the machine or where they may be hit by flying chips.
Switches which monitor guards and interlock them with the machine drive should be chosen and installed in such a way that they positively open the circuit as soon as the guard is shifted from its protecting position.
Emergency stop devices must cause the immediate standstill of the dangerous movement. They must be designed and located in such a way that they can be easily operated by the threatened worker. Emergency stop buttons must be easily reached and should be in red.
The actuating elements of control gear which may trip a dangerous machine movement must be guarded so as to exclude any inadvertent operation. For instance, the clutch engaging levers on the headstock and apron should be provided with safety locking devices or screens. A push-button can be made safe by lodging it in a recess or by shrouding it with a protective collar.
Hand-operated controls should be designed and located in such a way that the hand movement corresponds to the controlled machine movement.
Controls should be identified with easily readable and understandable markings. To avoid misunderstandings and linguistic difficulties, it is advisable to use symbols.
Transmission elements
All moving transmission elements (belts, pulleys, gears) must be covered with guards. An important contribution to the prevention of lathe accidents can be made by the persons responsible for the installation of the machine. Lathes should be so installed that the operators tending them do not hinder or endanger each other. The operators should not turn their backs towards passageways. Protective screens should be installed where neighbouring workplaces or passageways are within the range of flying chips.
Passageways must be clearly marked. Enough space should be left for materials-handling equipment, for stacking workpieces and for tool boxes. Bar-stock guides must not protrude into the passageways.
The floor on which the operator stands must be insulated against cold. Care should be taken that the insulation forms no stumbling obstacle, and the flooring should not become slippery even when covered with a film of oil.
Conduit and pipework should be installed in such a way that they do not become obstacles. Temporary installations should be avoided.
Safety engineering measures on the shop floor should be directed in particular at the following points:
- work-holding fixtures (faceplates, chucks, collets) should be dynamically balanced before use
- the maximum permissible speed of a chuck should be indicated on the chuck by the manufacturer and respected by the lathe operator
- when scroll chucks are used, it should be ensured that the jaws cannot be slung out when the lathe is started
- chucks of this type should be designed in such a manner that the key cannot be taken off before the jaws have been secured. The chuck keys in general should be so designed that it is impossible to leave them in the chuck.
It is important to provide for auxiliary lifting equipment to facilitate mounting and removing of heavy chucks and faceplates. To prevent chucks from running off the spindle when the lathe is suddenly braked, they must be securely fixed. This can be achieved by putting a retaining nut with left-hand thread on the spindle nose, by using a “Camlock” quick-action coupling, by fitting the chuck with a locking key or by securing it with a two-part locking ring.
When powered work-holding fixtures are used, such as hydraulically operated chucks, collets and tailstock centres, measures must be taken which make it impossible for the hands to be introduced into the danger zone of closing fixtures. This can be achieved by limiting the travel of the clamping element to 6 mm, by choosing the location of deadman’s controls so as to exclude the introduction of the hands into the danger zone or by providing a moving guard which has to be closed before the clamping movement can be started.
If starting the lathe while the chuck jaws are open presents a danger, the machine should be equipped with a device which prevents the spindle rotation being started before the jaws are closed. The absence of power must not cause the opening or closure of a powered work-holding fixture.
If the gripping force of a power chuck diminishes, the spindle rotation must be stopped, and it must be impossible to start the spindle. Reversing the gripping direction from inside to outside (or vice versa) while the spindle rotates must not cause the chuck to be dislodged from the spindle. Removal of holding fixtures from the spindle should be possible only when the spindle has ceased rotating.
When machining bar stock, the portion projecting beyond the lathe must be enclosed by bar-stock guides. Bar feed weights must be guarded by hinged covers extending to the floor.
Carriers
To prevent serious accidents—in particular, when filing work in a lathe—unprotected carriers must not be used. A centring safety carrier should be used, or a protective collar should be fitted to a conventional carrier. It is also possible to use self-locking carriers or to provide the carrier disc with a protective cover.
Working zone of the lathe
Universal-lathe chucks should be guarded by hinged covers. If possible, protective covers should be interlocked with spindle drive circuits. Vertical boring and turning mills should be fenced with bars or plates to prevent injury from revolving parts. To enable the operator to watch the machining process safely, platforms with railings must be provided. In certain cases, TV cameras can be installed so that the operator may monitor the tool edge and tool in-feed.
The working zones of automatic lathes, NC and CNC lathes should be completely enclosed. Enclosures of fully automatic machines should only have openings through which the stock to be machined is introduced, the turned part ejected and the swarf removed from the working zone. These openings must not constitute a hazard when work passes through them, and it must be impossible to reach through them into the danger zone.
The working zones of semi-automatic, NC and CNC lathes must be enclosed during the machining process. The enclosures are generally sliding covers with limit switches and interlocking circuit.
Operations requiring access to the working zone, such as change of work or tools, gauging and so on, must not be carried out before the lathe has been safely stopped. Zeroing a variable-speed drive is not considered a safe standstill. Machines with such drives must have locked protective covers that cannot be unlocked before the machine is safely stopped (e.g., by cutting the spindle-motor power supply).
If special tool-setting operations are required, an inching control is to be provided which enables certain machine movements to be tripped while the protective cover is open. In such cases, the operator can be protected by special circuit designs (e.g., by permitting only one movement to be tripped at a time). This can be achieved by using two-hand controls.
Turning swarf
Long turning chips are dangerous because they may get entangled with arms and legs and cause serious injury. Continuous and ravelled chips can be avoided by choosing appropriate cutting speeds, feeds and chip thicknesses or by using lathe tools with chip breakers of the gullet or step type. Swarf hooks with handle and buckle should be used for removing chips.
Ergonomics
Every machine should be so designed that it enables a maximal output to be obtained with a minimum of stress on the operator. This can be achieved by adapting the machine to the worker.
Ergonomic factors must be taken into account when designing the human-machine interface of a lathe. Rational workplace design also includes providing for auxiliary handling equipment, such as loading and unloading attachments.
All controls must be located within the physiological sphere or reach of both hands. The controls must be clearly laid out and should be logical to operate. Pedal-operated controls should be avoided in machines tended by standing operators.
Experience has shown that good work is performed when the workplace is designed for both standing and sitting postures. If the operator has to work standing up, he or she should be given the possibility of changing posture. Flexible seats are in many cases a welcome relief for strained feet and legs.
Measures should be taken to create optimal thermal comfort, taking into account the air temperature, relative humidity, air movement and radiant heat. The workshop should be adequately ventilated. There should be local exhaust devices to eliminate gaseous emanations. When machining bar stock, sound-absorbent-lined guide tubes should be used.
The workplace should preferably be provided with uniform lighting, affording an adequate level of illumination.
Work Clothing and Personal Protection
Overalls should be close fitting and buttoned or zipped to the neck. They should be without breast pockets, and the sleeves must be tightly buttoned at the wrists. Belts should not be worn. No finger rings and bracelets should be worn when working on lathes. Wearing of safety spectacles should be obligatory. When heavy workpieces are machined, safety shoes with steel toe caps must be worn. Protective gloves must be worn whenever swarf is being collected.
Training
The lathe operator’s safety depends to a large extent on working methods. It is therefore important that he or she should receive thorough theoretical and practical training to acquire skills and develop a behaviour affording the best possible safeguards. Correct posture, correct movements, correct choice and handling of tools should become routine to such an extent that the operator works correctly even if his or her concentration is temporarily relaxed.
Important points in a training programme are an upright posture, the proper mounting and removal of the chuck and the accurate and secure fixing of workpieces. Correct holding of files and scrapers and safe working with abrasive cloth must be intensively practised.
Workers must be well informed about the hazards of injury which may be caused when gauging work, checking adjustments and cleaning lathes.
Maintenance
Lathes must be regularly maintained and lubricated. Faults must be corrected immediately. If safety is at stake in the event of a fault, the machine should be put out of operation until corrective action has been taken.
Repair and maintenance work must be carried out only after the machine has been isolated from the power supply
.
Grinding and Polishing
Adapted from the 3rd edition, Encyclopaedia of Occupational Health and Safety.
Grinding generally involves the use of a bonded abrasive to wear away parts of a workpiece. The aim is to give the work a certain shape, correct its dimensions, increase the smoothness of a surface or improve the sharpness of cutting edges. Examples include removal of sprues and rough edges from a foundry casting, removal of surface scale from metals before forging or welding and deburring of parts in sheet metal and machine shops. Polishing is used to remove surface imperfections such as tool marks. Buffing does not remove metal, but uses a soft abrasive blended in a wax or grease base to produce a high-lustre surface.
Grinding is the most comprehensive and diversified of all machining methods and is employed on many materials—predominantly iron and steel but also other metals, wood, plastics, stone, glass, pottery and so on. The term covers other methods of producing very smooth and glossy surfaces, such as polishing, honing, whetting and lapping.
The tools used are wheels of varying dimensions, grinding segments, grinding points, sharpening stones, files, polishing wheels, belts, discs and so on. In grinding wheels and the like, the abrasive material is held together by bonding agents to form a rigid, generally porous body. In the case of abrasive belts, the bonding agent holds the abrasive secured to a flexible base material. Buffing wheels are made from cotton or other textile disks sewn together.
The natural abrasives—natural corundum or emery (aluminium oxides), diamond, sandstone, flint and garnet—have been largely superseded by artificial abrasives including aluminium oxide (fused alumina), silicon carbide (carborundum) and synthetic diamonds. A number of fine-grained materials such as chalk, pumice, tripoli, tin putty and iron oxide are also used, especially for polishing and buffing.
Aluminium oxide is most widely used in grinding wheels, followed by silicon carbide. Natural and artificial diamonds are used for important special applications. Aluminium oxide, silicon carbide, emery, garnet and flint are used in grinding and polishing belts.
Both organic and inorganic bonding agents are used in grinding wheels. The main type of inorganic bonds are vitrified silicate and magnesite. Notable among organic bonding agents are phenol- or urea- formaldehyde resin, rubber and shellac. The vitrified bonding agents and phenolic resin are completely dominating within their respective groups. Diamond grinding wheels can also be metal bonded. The various bonding agents give the wheels different grinding properties, as well as different properties with regard to safety.
Abrasive and polishing belts and discs are composed of a flexible base of paper or fabric to which the abrasive is bonded by means of a natural or synthetic adhesive.
Different machines are used for different types of operations, such as surface grinding, cylindrical (including centreless) grinding, internal grinding, rough grinding and cutting. The two main types are: those where either the grinder or the work is moved by hand and machines with mechanical feeds and chucks. Common equipment types include: surface-type grinders; pedestal-type grinders, polishers and buffers; disk grinders and polishers; internal grinders; abrasive cut-off machines; belt polishers; portable grinders, polishers and buffers; and multiple polishers and buffers.
Hazards and Their Prevention
Bursting
The major injury risk in the use of grinding wheels is that the wheel may burst during grinding. Normally, grinding wheels operate at high speeds. There is a trend towards ever-increasing speeds. Most industrialized nations have regulations limiting the maximum speeds at which the various types of grinding wheels may be run.
The fundamental protective measure is to make the grinding wheel as strong as possible; the nature of the bonding agent is most important. Wheels with organic bonds, in particular phenolic resin, are tougher than those with inorganic bonds and more resistant to impacts. High peripheral speeds may be permissible for wheels with organic bonds.
Very high-speed wheels, in particular, often incorporate various types of reinforcement. For example, certain cup wheels are fitted with steel hubs to increase their strength. During rotation the major stress develops around the centre hole. To strengthen the wheel, the section around the centre hole, which takes no part in the grinding, can thus be made of an especially strong material which is not suitable for grinding. Large wheels with a centre section reinforced in this way are used particularly by the steel works for grinding slabs, billets and the like at speeds up to 80 m/s.
The most common method of reinforcing grinding wheels, however, is to include glass fibre fabric in their construction. Thin wheels, such as those used for cutting, may incorporate glass fibre fabric at the centre or at each side, while thicker wheels have a number of fabric layers depending on the thickness of the wheel.
With the exception of some grinding wheels of small dimensions, either all wheels or a statistical sampling of them must be given speed tests by the manufacturer. In tests the wheels are run over a certain period at a speed exceeding that permitted in grinding. Test regulations vary from country to country, but usually the wheel has to be tested at a speed 50% above the working speed. In some countries, regulations require special testing of wheels that are to operate at higher speeds than normal at a central testing institute. The institute may also cut specimens from the wheel and investigate their physical properties. Cutting wheels are subjected to certain impact tests, bending tests and so on. The manufacturer is also obliged to ensure that the grinding wheel is well balanced prior to delivery.
The bursting of a grinding wheel may cause fatal or very serious injuries to anyone in the vicinity and heavy damage to plant or premises. In spite of all precautions taken by the manufacturers, occasional wheel bursts or breaks may still occur unless proper care is exercised in their use. Precautionary measures include:
- Handling and storing. A wheel may become damaged or cracked during transit or handling. Moisture may attack the bonding agent in phenolic resin wheels, ultimately reducing their strength. Vitrified wheels may be sensitive to repeated temperature variations. Irregularly absorbed moisture may throw the wheel out of balance. Consequently, it is most important that wheels are carefully handled at all stages and kept in an orderly manner in a dry and protected place.
- Checking for cracks. A new wheel should be checked to ensure that it is undamaged and dry, most simply by tapping with a wooden mallet. A faultless vitrified wheel will give a clear ring, an organic bonded wheel a less ringing tone; but either can be differentiated from the cracked sound of a defective wheel. In case of doubt, the wheel should not be used and the supplier should be consulted.
- Testing. Before the new wheel is put into service, it should be tested at full speed with due precautions being observed. After wet grinding, the wheel should be run idle to eject the water; otherwise the water may collect at the bottom of the wheel and cause imbalance, which may result in bursting when the wheel is next used.
- Mounting. Accidents and breakages occur when grinding wheels are mounted on unsuitable apparatus—for example, on spindle ends of buffing machines. The spindle should be of adequate diameter but not so large as to expand the centre hole of the wheel; flanges should be not less than one-third the diameter of the wheel and made of mild steel or of similar material.
- Speed. In no circumstances should the maximum permissible operating speed specified by the makers be exceeded. A notice indicating the spindle speed should be fitted to all grinding machines, and the wheel should be marked with the maximum permissible peripheral speed and the corresponding number of revolutions for a new wheel. Special precautions are necessary with variable speed grinding machines and to ensure the fitting of wheels of appropriate permissible speeds in portable grinders.
- Work rest. Wherever practicable, rigidly mounted work rests of adequate dimensions should be provided. They should be adjustable and kept as close as possible to the wheel to prevent a trap in which the work might be forced against the wheel and break it or, more probable, catch and injure the operator’s hand.
- Guarding. Abrasive wheels should be provided with guards strong enough to contain the parts of a bursting wheel (see figure 1). Some countries have detailed regulations regarding the design of the guards and the materials to be used. In general, cast iron and cast aluminium are to be avoided. The grinding opening should be as small as possible, and an adjustable nose piece may be necessary. Exceptionally, where the nature of the work precludes the use of a guard, special protective flanges or safety chucks may be used. The spindles and tapered ends of double-ended polishing machines can cause entanglement accidents unless they are effectively guarded.
Figure 1. A well guarded, vitrified abrasive wheel mounted in a surface grinder and operating at a peripheral speed of 33 m/s
Eye injuries
Dust, abrasives, grains and splinters are a common hazard to the eyes in all dry-grinding operations. Effective eye protection by goggles or spectacles and fixed eye shields at the machine are essential; fixed eye shields are particularly useful when wheels are in intermittent use—for example, for tool grinding.
Fire
Grinding of magnesium alloys carries a high fire risk unless strict precautions are taken against accidental ignition and in the removal and drenching of dust. High standards of cleanliness and maintenance are required in all exhaust ducting to prevent risk of fire and also to keep ventilation working efficiently. Textile dust released from buffing operations is a fire hazard requiring good housekeeping and LEV.
Vibration
Portable and pedestal grinders carry a risk of hand-arm vibration syndrome (HAVS), also known as “white finger” from its most noticeable sign. Recommendations include limiting intensity and duration of exposure, redesigning tools, protective equipment and monitoring exposure and health.
Health hazards
Although modern grinding wheels do not themselves create the serious silicosis hazard associated in the past with sandstone wheels, highly dangerous silica dust may still be given off from the materials being ground—for example, sand castings. Certain resin-bonded wheels may contain fillers which create a dangerous dust. In addition, formaldehyde-based resins can emit formaldehyde during grinding. In any event, the volume of dust produced by grinding makes efficient LEV essential. It is more difficult to provide local exhaust for portable wheels, although some success in this direction has been achieved by use of low-volume, high-velocity capture systems. Prolonged work should be avoided and respiratory protective equipment provided if necessary. Exhaust ventilation is also required for most belt sanding, finishing, polishing and similar operations. With buffing in particular, combustible textile dust is a serious concern.
Protective clothing and good sanitary and washing facilities with showers should be provided, and medical supervision is desirable, especially for metal grinders.
Industrial Lubricants, Metal Working Fluids and Automotive Oils
The industrial revolution could not have occurred without the development of refined petroleum-based industrial oils, lubricants, cutting oils and greases. Prior to the discovery in the 1860s that a superior lubricant could be produced by distilling crude oil in a vacuum, industry depended on naturally occurring oils and animal fats such as lard and whale sperm oil for lubricating moving parts. These oils and animal products were especially susceptible to melting, oxidation and breakdown from exposure to heat and moisture produced by the steam engines which powered almost all industrial equipment at that time. The evolution of petroleum-based refined products has continued from the first lubricant, which was used to tan leather, to modern synthetic oils and greases with longer service life, superior lubricating qualities and better resistance to change under varying temperatures and climatic conditions.
Industrial Lubricants
All moving parts on machinery and equipment require lubrication. Although lubrication may be provided by dry materials such as Teflon or graphite, which are used in parts such as small electrical motor bearings, oils and greases are the most commonly used lubricants. As the complexity of the machinery increases, the requirements for lubricants and metal process oils become more stringent. Lubricating oils now range from clear, very thin oils used to lubricate delicate instruments, to thick, tar-like oils used on large gears such as those which turn steel mills. Oils with very specific requirements are used both in the hydraulic systems and to lubricate large computer-operated machine tools such as those used in the aerospace industry to produce parts with extremely close tolerances. Synthetic oils, fluids and greases, and blends of synthetic and petroleum-based oils, are used where extended lubricant life is desired, such as sealed-for-life electric motors, where the increased time between oil changes offsets the difference in cost; where extended temperature and pressure ranges exist, such as in aerospace applications; or where it is difficult and expensive to re-apply the lubricant.
Industrial Oils
Industrial oils such as spindle and lubricating oils, gear lubricants, hydraulic and turbine oils and transmission fluids are designed to meet specific physical and chemical requirements and to operate without discernible change for extended periods under varying conditions. Lubricants for aerospace use must meet entirely new conditions, including cleanliness, durability, resistance to cosmic radiation and the ability to operate in extremely cold and hot temperatures, without gravity and in a vacuum.
Transmissions, turbines and hydraulic systems contain fluids which transfer force or power, reservoirs to hold the fluids, pumps to move the fluids from one place to another and auxiliary equipment such as valves, piping, coolers and filters. Hydraulic systems, transmissions and turbines require fluids with specific viscosities and chemical stability to operate smoothly and provide the controlled transfer of power. The characteristics of good hydraulic and turbine oils include a high viscosity index, thermal stability, long life in circulating systems, deposit resistance, high lubricity, anti-foam capabilities, rust protection and good demulsibility.
Gear lubricants are designed to form strong, tenacious films which provide lubrication between gears under extreme pressure. The characteristics of gear oils include good chemical stability, demulsibility and resistance to viscosity increase and deposit formation. Spindle oils are thin, extremely clean and clear oils with lubricity additives. The most important characteristics for way oils—used to lubricate two flat sliding surfaces where there is high pressure and slow speed—are lubricity and tackiness to resist squeezing out and resistance to extreme pressure.
Cylinder and compressor oils combine the characteristics of both industrial and automotive oils. They should resist accumulation of deposits, act as a heat transfer agent (internal combustion engine cylinders), provide lubrication for cylinders and pistons, provide a seal to resist blow-back pressure, have chemical and thermal stability (especially vacuum pump oil), have a high viscosity index and resist water wash (steam-operated cylinders) and detergency.
Automotive Engine Oils
Manufacturers of internal combustion engines and organizations, such as the Society of Automotive Engineers (SAE) in the United States and Canada, have established specific performance criteria for automotive engine oils. Automotive gasoline and diesel engine oils are subjected to a series of performance tests to determine their chemical and thermal stability, corrosion resistance, viscosity, wear protection, lubricity, detergency and high and low temperature performance. They are then classified according to a code system which allows consumers to determine their suitability for heavy-duty use and for different temperatures and viscosity ranges.
Oils for automotive engines, transmissions and gear cases are designed with high viscosity indexes to resist changes in viscosity with temperature changes. Automotive engine oils are especially formulated to resist breakdown under heat as they lubricate internal combustion engines. Internal combustion engine oils must not be too thick to lubricate the internal moving parts when an engine starts up in cold weather, and they must not thin out as the engine heats up when operating. They should resist carbon build-up on valves, rings and cylinders and the formation of corrosive acids or deposits from moisture. Automotive engine oils contain detergents designed to hold carbon and metallic wear particles in suspension so that they can be filtered out as the oil circulates and not accumulate on internal engine parts and cause damage.
Cutting Fluids
The three types of cutting fluids used in industry are mineral oils, soluble oils and synthetic fluids. Cutting oils are typically a blend of high-quality, high-stability mineral oils of various viscosities together with additives to provide specific characteristics depending on the type of material being machined and the work performed. Soluble water-in-oil cutting fluids are mineral oils (or synthetic oils) which contain emulsifiers and special additives including defoamants, rust inhibitors, detergents, bactericides and germicides. They are diluted with water in varying ratios before being used. Synthetic cutting fluids are solutions of non-petroleum-based fluids, additives and water, rather than emulsions, some of which are fire resistant for machining specific metals. Semi-synthetic fluids contain 10 to 15% mineral oil. Some special fluids have both lubricating oil and cutting fluid characteristics due to the tendency of fluids to leak and intermix in certain machine tools such as multi-spindle, automatic screw machines.
The desired characteristics of cutting fluids depend on the composition of the metal being worked on, the cutting tool being used and the type of cutting, planing or shaping operation performed. Cutting fluids improve and enhance the metal working process by cooling and lubrication (i.e., protecting the edge of the cutting tool). For example, when working on a soft metal which creates a lot of heat, cooling is the most important criterion. Improved cooling is provided by using a light oil (such as kerosene) or water-based cutting fluid. Control of the built-up edge on cutting tools is provided by anti-weld or anti-wear additives such as sulphur, chlorine or phosphorus compounds. Lubricity, which is important when working on steel to overcome the abrasiveness of iron sulphide, is provided by synthetic and animal fats or sulphurized sperm oil additives.
Other Metal Working and Process Oils
Grinding fluids are designed to provide cooling and prevent metal build-up on grinding wheels. Their characteristics include thermal and chemical stability, rust protection (soluble fluids), preventing gummy deposits upon evaporation and a safe flashpoint for the work performed.
Quench oils, which require high stability, are used in metal treating to control the change of the molecular structure of steel as it cools. Quenching in lighter oil is used to case harden small, inexpensive steel parts. A slower quench rate is used to produce machine tool steels which are fairly hard on the outside with lower internal stress. A gapped or multi-phase quenching oil is used to treat high carbon and alloy steels.
Roll oils are specially formulated mineral or soluble oils which lubricate and provide a smooth finish to metal, particularly aluminium, copper and brass, as it goes through hot and cold rolling mills. Release oils are used to coat dies and moulds to facilitate the release of the formed metal parts. Tanning oils are still used in the felt and leather-making industry. Transformer oils are specially formulated dielectric fluids used in transformers and large electric breakers and switches.
Heat transfer oils are used in open or closed systems and may last up to 15 years in service. The primary characteristics are good thermal stability as systems operate at temperatures from 150 to 315°C, oxidation stability and high flashpoint. Heat transfer oils are normally too viscous to be pumped at ambient temperatures and must be heated to provide fluidity.
Petroleum solvents are used to clean parts by spraying, dripping or dipping. The solvents remove oil and emulsify dirt and metal particles. Rust preventive oils may be either solvent or water based. They are applied to stainless steel coils, bearings and other parts by dipping or spraying, and leave polarized or wax films on the metal surfaces for fingerprint and rust protection and water displacement.
Greases
Greases are mixtures of fluids, thickeners and additives used to lubricate parts and equipment which cannot be made oil-tight, which are hard to reach or where leaking or splashed liquid lubricants might contaminate products or create a hazard. They have a wide range of applications and performance requirements, from lubricating jet engine bearings at sub-zero temperatures to hot rolling mill gears, and resisting acid or water washout, as well as the continuous friction created by railroad car wheel roller bearings.
Grease is made by the blending of metallic soaps (salts of long-chained fatty acids) into a lubricating oil medium at temperatures of 205 to 315°C. Synthetic greases may use di-esters, silicone or phosphoric esters and polyalkyl glycols as fluids. The characteristics of the grease depend to a great extent upon the particular fluid, metallic element (e.g., calcium, sodium, aluminium, lithium and so on) in the soap and the additives used to improve performance and stability and to reduce friction. These additives include extreme-pressure additives which coat the metal with a thin layer of non-corrosive metallic sulphur compounds, lead naphthenate or zinc dithiophosphate, rust inhibitors, anti-oxidants, fatty acids for added lubricity, tackiness additives, colour dyes for identification and water inhibitors. Some greases may contain graphite or molybdenum fillers which coat the metallic parts and provide lubrication after the grease has run out or decomposed.
Industrial Lubricants, Grease and Automotive Engine Oil Additives
In addition to using high-quality lubricant base stocks with chemical and thermal stability and high viscosity indexes, additives are needed to enhance the fluid and provide specific characteristics required in industrial lubricants, cutting fluids, greases and automotive engine oils. The most commonly used additives include but are not limited to the following:
- Anti-oxidants. Oxidation inhibitors, such as 2,6-ditertiary butyl, paracresol and phenyl naphthylamine, reduce the rate of deterioration of oil by breaking up the long-chain molecules which form when exposed to oxygen. Oxidation inhibitors are used to coat metals such as copper, zinc and lead to prevent contact with the oil so they will not act as catalysts, speeding up oxidation and forming acids which attack other metals.
- Foam inhibitors. Defoamants, such as silicones and polyorganic silioxanes, are used in hydraulic oils, gear oils, transmission fluids and turbine oils to reduce surface film tension and remove air entrapped in the oil by pumps and compressors, in order to maintain constant hydraulic pressure and prevent cavitation.
- Corrosion inhibitors. Anti-rust additives, such as lead naphthenate and sodium sulphonate, are used to prevent rust from forming on metallic parts and systems where circulating oil has been contaminated with water or by moist air which entered system reservoirs as they cooled down when the equipment or machinery was not in use.
- Anti-wear additives. Anti-wear additives, such as tricresylphosphate, form polar compounds which are attracted to metal surfaces and provide a physical layer of additional protection in the event that the oil film is not sufficient.
- Viscosity index improvers. Viscosity index improvers help oils resist the effects of temperature changes. Unfortunately, their effectiveness diminishes with extended use. Synthetic oils are designed with very high viscosity indexes, allowing them to maintain their structure over wider temperature ranges and for much longer periods of time than mineral oils with viscosity index improver additives.
- Demulsifiers. Water inhibitors and special compounds separate water out of oil and prevent gum formation; they contain waxy oils which provide added lubricity. They are used where equipment is subject to water wash or where a large amount of moisture is present, such as in steam cylinders, air compressors and gear cases contaminated by soluble cutting fluids.
- Colour dyes. Dyes are used to assist users to identify different oils used for specific purposes, such as transmission fluids and gear oils, in order to prevent misapplication.
- Extreme pressure additives. Extreme pressure additives, such as non-corrosive sulphurized fatty compounds, zinc dithiophosphate and lead naphthenate, are used in automotive, gear and transmission oils to form coatings which protect metal surfaces when the protective oil film thins or is squeezed out and cannot prevent metal to metal contact.
- Detergents. Metal sulphonate and metal phenate detergents are used to hold dirt, carbon and metallic wear particles in suspension in hydraulic oils, gear oils, engine oils and transmission fluids. These contaminants are typically removed when the oil passes through a filter to prevent their being recirculated through the system where they could cause damage.
- Tackiness additives. Adhesive or tackiness additives are used to enable oils to adhere to and resist leakage from bearing assemblies, gear cases, large open gears on mills and construction equipment, and overhead machinery. Their tackiness diminishes with extended service.
- Emulsifiers. Fatty acids and fatty oils are used as emulsifiers in soluble oils to help form solutions with water.
- Lubricity additives. Fat, lard, tallow, sperm and vegetable oils are used to provide a higher degree of oiliness in cutting oils and some gear oils.
- Bactericides. Bactericides and germicides, such as phenol and pine oil, are added to soluble cutting oils to prolong the life of the fluid, maintain stability, reduce odours and prevent dermatitis.
Manufacturing Industrial Lubricants and Automotive Oils
Industrial lubricants and oils, grease, cutting fluids and automotive engine oils are manufactured in blending and packaging facilities, also called “lube plants” or “blending plants”. These facilities may be located either in or adjacent to refineries which produce lubricant base stocks, or they may be some distance away and receive the base stocks by marine tankers or barges, railroad tank cars or tank trucks. Blending and packaging plants blend and compound additives into lubricating oil base stocks to manufacture a wide range of finished products, which are then shipped in bulk or in containers.
The blending and compounding processes used to manufacture lubricants, fluids and greases depend on the age and sophistication of the facility, the equipment available, the types and formulation of the additives used and the variety and volume of products produced. Blending may require only physical mixing of base stocks and additive packages in a kettle using mixers, paddles or air agitation, or auxiliary heat from electric or steam coils may be needed to help dissolve and blend in the additives. Other industrial fluids and lubricants are produced automatically by mixing base stocks and pre-blended additive and oil slurries through manifold systems. Grease may be either batch produced or continuously compounded. Lube plants may compound their own additives from chemicals or purchase pre-packaged additives from specialty companies; a single plant may use both methods. When lube plants manufacture their own additives and additive packages, there may be a need for high temperatures and pressures in addition to chemical reactions and physical agitation to compound the chemicals and materials.
After production, fluids and lubricants may be held in the blending kettles or placed in holding tanks to ensure that the additives remain in suspension or solution, to allow time for testing to determine whether the product meets quality specifications and certification requirements, and to allow process temperatures to return to ambient levels before products are packaged and shipped. When testing is completed, finished products are released for bulk shipment or packaging into containers.
Finished products are shipped in bulk in railroad tank cars or in tank trucks directly to consumers, distributors or outside packaging plants. Finished products also are shipped to consumers and distributors in railroad box cars or package delivery trucks in a variety of containers, as follows:
- Metal, plastic and combination metal/plastic or plastic/fibre intermediate bulk containers, which range in size from 227 l to approximately 2,840 l, are shipped as individual units on built-in or separate pallets, stacked 1 or 2 high.
- Metal, fibre or plastic drums with a capacity of 208 l, 114 l or 180 kg are typically shipped 4 to a pallet.
- Metal or plastic drums with a capacity of 60 l or 54 kg, and 19 l or 16 kg metal or plastic pails, are stacked on pallets and banded or stretch wrapped to maintain stability.
- Metal or plastic containers with a capacity of 8 l or 4 l, 1 l plastic, metal and fibre bottles and cans and 2 kg grease cartridges are packaged in cartons which are stacked on pallets and banded or stretch wrapped for shipment.
Some blending and packaging plants may ship pallets of mixed products and mixed sizes of containers and packages directly to small consumers. For example, a single-pallet shipment to a service station could include 1 drum of transmission fluid, 2 kegs of grease, 8 cases of automotive engine oil and 4 pails of gear lubricant.
Product Quality
Lubricant product quality is important to keep machines and equipment operating properly and to produce quality parts and materials. Blending and packaging plants manufacture finished petroleum products to strict specifications and quality requirements. Users should maintain the level of quality by establishing safe practices for the handling, storage, dispensing and transfer of lubricants from their original containers or tanks to the dispensing equipment and to the point of application on the machine or equipment to be lubricated or the system to be filled. Some industrial facilities have installed centralized dispensing, lubrication and hydraulic systems which minimize contamination and exposure. Industrial oils, lubricants, cutting oils and grease will deteriorate from water or moisture contamination, exposure to excessively high or low temperatures, inadvertent mixing with other products and long-term storage which allows additive drop-out or chemical changes to occur.
Health and Safety
Because they are used and handled by consumers, finished industrial and automotive products must be relatively free of hazards. There is a potential for hazardous exposures when blending and compounding products, when handling additives, when using cutting fluids and when operating oil mist lubrication systems.
The chapter Oil and natural gas refineries in this Encyclopaedia gives information regarding potential hazards associated with auxiliary facilities at blending and packaging plants such as boiler rooms, laboratories, offices, oil-water separators and waste treatment facilities, marine docks, tank storage, warehouse operations, railroad tank car and tank truck loading racks and railroad box car and package truck loading and unloading facilities.
Safety
Manufacturing additives and slurries, batch compounding, batch blending and in-line blending operations require strict controls to maintain desired product quality and, along with the use of PPE, to minimize exposure to potentially hazardous chemicals and materials as well as contact with hot surfaces and steam. Additive drums and containers should be stored safely and kept tightly sealed until ready for use. Additives in drums and bags need to be handled properly to avoid muscular strain. Hazardous chemicals should be properly stored, and incompatible chemicals should not be stored where they can mix with one another. Precautions to be taken when operating filling and packaging machinery include using gloves and avoiding catching fingers in devices which crimp covers on kegs and pails. Machine guards and protective systems should not be removed, disconnected or by-passed to expedite work. Intermediate bulk containers and drums should be inspected before filling to make sure they are clean and suitable.
A confined-space permit system should be established for entry into storage tanks and blending kettles for cleaning, inspection, maintenance or repair. A lockout/tagout procedure should be established and implemented before working on packaging machinery, blending kettles with mixers, conveyors, palletizers and other equipment with moving parts.
Leaking drums and containers should be removed from the storage area and spills cleaned up to prevent slips and falls. Recycling, burning and disposal of waste, spilled and used lubricants, automotive engine oils and cutting fluids should be in accordance with government regulations and company procedures. Workers should use appropriate PPE when cleaning spills and handling used or waste products. Drained motor oil, cutting fluids or industrial lubricants which may be contaminated with gasoline and flammable solvents should be stored in a safe place away from sources of ignition, until proper disposal.
Fire protection
While the potential for fire is less in industrial and automotive lubricant blending and compounding than in refining processes, care must be taken when manufacturing metal working oils and greases due to the use of high blending and compounding temperatures and lower flashpoint products. Special precautions should be taken to prevent fires when products are dispensed or containers filled at temperatures above their flashpoints. When transferring flammable liquids from one container to another, proper bonding and grounding techniques should be applied to prevent static build-up and electrostatic discharge. Electrical motors and portable equipment should be properly classified for the hazards present in the area in which they are installed or used.
The potential for fire exists if a leaking product or vapour release in the lube blending and grease processing or storage areas reaches a source of ignition. The establishment and implementation of a hot-work permit system should be considered to prevent fires in blending and packaging facilities. Storage tanks installed inside buildings should be constructed, vented and protected in accordance with government requirements and company policy. Products stored on racks and in piles should not block fire protection systems, fire doors or exit routes.
Storage of finished products, both in bulk and in containers and packages, should be in accordance with recognized practices and fire prevention regulations. For example, flammable liquids and additives which are in solutions of flammable liquids may be stored in outside buildings or separate, specially designed inside or attached storage rooms. Many additives are stored in warm rooms (38 to 65°C) or in hot rooms (over 65°C) in order to keep the ingredients in suspension, to reduce the viscosity of thicker products or to provide for easier blending or compounding. These storage rooms should comply with electrical classification, drainage, ventilation and explosion venting requirements, especially when flammable liquids or combustible liquids are stored and dispensed at temperatures above their flashpoints.
Health
When blending, sampling and compounding, personal and respiratory protective equipment should be considered to prevent exposures to heat, steam, dusts, mists, vapours, fumes, metallic salts, chemicals and additives. Safe work practices, good hygiene and appropriate personal protection may be needed for exposure to oil mists, fumes and vapours, additives, noise and heat when conducting inspection and maintenance activities while sampling and handling hydrocarbons and additives during the production and packaging and when cleaning up spills and releases:
- Work shoes with oil- or slip-resistant soles should be worn for general work, and approved protective toe safety shoes with oil- or slip-resistant soles should be worn where hazards of foot injuries from rolling or falling objects or equipment exist.
- Safety goggles and respiratory protection may be needed for hazardous exposures to chemicals, dust or steam.
- Impervious gloves, aprons, footwear, face shields and chemical goggles should be worn when handling hazardous chemicals, additives and caustic solutions and when cleaning up spills.
- Head protection may be needed when working in pits or areas where the potential exists for injury to the head.
- Ready access to appropriate cleaning and drying facilities to handle splashes and spills should be provided.
Oil is a common cause of dermatitis, which can be controlled through the use of PPE and good personal hygiene practices. Direct skin contact with any formulated greases or lubricants should be avoided. Lighter oils such as kerosene, solvents and spindle oils defat the skin and cause rashes. Thicker products, such as gear oils and greases, block the pores of the skin, leading to folliculitis.
Health hazards due to microbial contamination of oil may be summarized as follows:
- Pre-existing skin conditions may be aggravated.
- Lubricant aerosols of respirable size may cause respiratory illness.
- Organisms may change the composition of the product so that it becomes directly injurious.
- Harmful bacteria from animals, birds or humans may be introduced.
Contact dermatitis may occur when employees are exposed to cutting fluids during production, work or maintenance and when they wipe oil-covered hands with rags embedded with minute metal particles. The metal causes small lacerations in the skin which may become infected. Water-based cutting fluids on skin and clothing may contain bacteria and cause infections, and the emulsifiers may dissolve fats from the skin. Oil folliculitis is caused by prolonged exposure to oil-based cutting fluids, such as from wearing oil-soaked clothing. Employees should remove and launder clothing that is soaked with oil before wearing it again. Dermatitis may also be caused by using soaps, detergents or solvents to clean the skin. Dermatitis is best controlled by good hygiene practices and minimizing exposure. Medical advice should be sought when dermatitis persists.
In the extensive review conducted as a basis for its criteria document, the US National Institute for Occupational Safety and Health (NIOSH) found an association between exposure to metal working fluids and the risk of developing cancer at several organ sites, including the stomach, pancreas, larynx and rectum (NIOSH 1996). The specific formulations responsible for the elevated cancer risks remain to be determined.
Occupational exposure to oil mists and aerosols is associated with a variety of non-malignant respiratory effects, including lipoid pneumonia, asthma, acute airways irritation, chronic bronchitis and impaired pulmonary function (NIOSH 1996).
Metal working fluids are readily contaminated by bacteria and fungi. They may affect the skin or, when inhaled as contaminated aerosols, they may have systemic effects.
Refinery processes such as hydrofinishing and acid treatment are used to remove aromatics from industrial lubricants, and the use of naphthenic base stocks has been restricted in order to minimize carcinogenicity. Additives introduced in blending and compounding may also create a potential risk to health. Exposures to chlorinated compounds and leaded compounds, such as those used in some gear lubricants and greases, cause irritation of the skin and may be potentially hazardous. Tri-orthocresyl phosphate has caused outbreaks of nerve palsies when lubricating oil was accidentally used for cooking. Synthetic oils consist mainly of sodium nitrite and triethanolamine and additives. Commercial triethanolamine contains diethanolamine, which can react with sodium nitrite to form a relatively weak carcinogen, N-nitrosodiethanolamine, which may create a hazard. Semi-synthetic lubricants present the hazards of both products, as well as the additives in their formulations.
Product safety information is important to employees of both manufacturers and users of lubricants, oils and greases. Manufacturers should have material safety data sheets (MSDSs) or other product information available for all of the additives and base stocks used in blending and compounding. Many companies have conducted epidemiological and toxicological testing to determine the degree of hazards associated with any acute and chronic health effects of their products. This information should be available to workers and users through warning labels and product safety information.
Surface Treatment of Metals
Adapted from the 3rd edition, Encyclopaedia of Occupational Health and Safety.
There is a wide variety of techniques for finishing the surfaces of metal products so that they resist corrosion, fit better and look better (see table 1). Some products are treated by a sequence of several of these techniques. This article will briefly describe some of those most commonly used.
Table 1. Summary of the hazards associated with the different metal treatment methods
|
Metal treatment method |
Hazards |
Precautions |
|
Electrolytic polishing |
Burns and irritation from caustic and corrosive chemicals |
Use appropriate personal protective equipment. Install effective exhaust ventilation. |
|
Electroplating |
Exposure to potentially cancer causing chromium and nickel; exposure to cyanides; burns and irritation from caustic and corrosive chemicals; electric shock; the process can be wet, causing slip and fall hazards; potential explosive dust generation; ergonomic hazards |
Use appropriate personal protective equipment. Install effective exhaust ventilation, often slotted, push-pull system. Clean up spills immediately. Install non-skid flooring. Use effective design of work procedures and stations to avoid ergonomic stress. |
|
Enamels and glazing |
Physical hazards from grinders, conveyers, mills; burn hazard from high temperature liquids and equipment; exposure to dusts that may cause lung disease |
Install proper machine guards, including interlocks. Use appropriate personal protective equipment. Install effective exhaust ventilation to avoid dust exposure. HEPA-filtered equipment may be necessary. |
|
Etching |
Exposure to hydrofluoric acid; burns and irritation from caustic and corrosive chemicals; burn hazard from high temperature liquids and equipment |
Implement a programme to avoid exposure to hydrofluoric acid. Use appropriate personal protective equipment. Install effective exhaust ventilation. |
|
Galvanizing |
Burn hazard from high temperature liquids, metals, and equipment; burns and irritation from caustic and corrosive chemicals; metal fume fever; potential lead exposure |
Use appropriate personal protective equipment. Install effective exhaust ventilation. Implement a lead exposure reduction/monitoring programme. |
|
Heat treatment |
Burn hazard from high temperature liquids, metals and equipment; burns and irritation from caustic and corrosive chemicals; possible explosive atmospheres of hydrogen; potential exposure to carbon monoxide; potential exposure to cyanides; fire hazard from oil quenching |
Use appropriate personal protective equipment. Install effective exhaust ventilation. Display signs warning of high temperature equipment and surfaces. Install systems to monitor the concentration of carbon monoxide. Install adequate fire-suppression systems. |
|
Metallizing |
Burn hazard from high temperature metals and equipment; possible explosive atmospheres of dust, acetylene; zinc metal fume fever |
Install adequate fire suppression systems. Properly separate chemicals and gases. Use appropriate personal protective equipment. Install effective exhaust ventilation. |
|
Phosphating |
Burns and irritation from caustic and corrosive chemicals |
Use appropriate personal protective equipment. Install effective exhaust ventilation. |
|
Plastics coating |
Exposure to chemical sensitizers |
Seek alternatives to sensitizers. Use appropriate personal protective equipment. Install effective exhaust ventilation. |
|
Priming |
Exposure to various solvents which are potentially toxic and flammable, exposure to chemical sensitizers, exposure to potentially carcinogenic chromium |
Seek alternatives to sensitizers. Use appropriate personal protective equipment. Install effective exhaust ventilation. Properly separate chemicals/gases. |
Before any of these techniques can be applied, the products must be thoroughly cleaned. A number of methods of cleaning are used, individually or in sequence. They include mechanical grinding, brushing and polishing (which produce metallic or oxidic dust—aluminium dust may be explosive), vapour degreasing, washing with organic grease solvents, “pickling” in concentrated acid or alkaline solutions and electrolytic degreasing. The last involves immersion in baths containing cyanide and concentrated alkali in which electrolytically formed hydrogen or oxygen remove the grease, resulting in “blank” metal surfaces that are free from oxides and grease. The cleaning is followed by adequate rinsing and drying of the product.
Proper design of the equipment and effective LEV will reduce some of the risk. Workers exposed to the hazard of splashes must be provided with protective goggles or eye shields and protective gloves, aprons and clothing. Showers and eyewash fountains should be nearby and in good working order, and splashes and spills should be washed away promptly. With electrolytic equipment, the gloves and shoes must be non-conducting, and other standard electrical precautions, such as the installation of ground fault circuit interrupters and lockout/tagout procedures should be followed.
Treatment Processes
Electrolytic polishing
Electrolytic polishing is used to produce a surface of improved appearance and reflectivity, to remove excess metal to accurately fit the required dimensions and to prepare the surface for inspection for imperfections. The process involves preferential anodic dissolution of high spots on the surface after vapour degreasing and hot alkaline cleaning. Acids are frequently used as the electrolyte solutions; accordingly, adequate rinsing is required afterwards.
Electroplating
Electroplating is a chemical or electrochemical process for applying a metallic layer to the product—for example, nickel to protect against corrosion, hard chromium to improve the surface properties or silver and gold to beautify it. Occasionally, non-metallic materials are used. The product, wired as the cathode, and an anode of the metal to be deposited are immersed in an electrolyte solution (which can be acidic, alkaline or alkaline with cyanide salts and complexes) and connected externally to a source of direct current. The positively charged cations of the metallic anode migrate to the cathode, where they are reduced to the metal and deposited as a thin layer (see figure 1). The process is continued until the new coating reaches the desired thickness, and the product is then washed, dried and polished.
Figure 1. Electroplating: Schematic representation
Anode: Cu → Cu+2 + 2e- ; Cathode: Cu+2 + 2e- → Cu
In electroforming, a process closely related to electroplating, objects moulded of, for example, plaster or plastic are made conductive by the application of graphite and then are connected as the cathode so that the metal is deposited on them.
In anodization, a process that has become increasingly important in recent years, products of aluminium (titanium and other metals are also used) are connected as the anode and immersed in dilute sulphuric acid. However, instead of the formation of positive aluminium ions and migrating for deposition on the cathode, they are oxidized by the oxygen atoms arising at the anode and become bound to it as an oxide layer. This oxide layer is partially dissolved by the sulphuric acid solution, making the surface layer porous. Subsequently, coloured or light-sensitive materials can be deposited in these pores, as in the fabrication of nameplates, for example.
Enamels and glazes
Vitreous enamel or porcelain enamel is used to give a high heat-, stain- and corrosion-resistant covering to metals, usually iron or steel, in a wide range of fabricated products including bath tubs, gas and electric cookers, kitchen ware, storage tanks and containers, and electrical equipment. In addition, enamels are used in the decoration of ceramics, glass, jewellery and decorative ornaments. The specialized use of enamel powders in the production of such ornamental ware as Cloisonné and Limoges has been known for centuries. Glazes are applied to pottery ware of all kinds.
The materials used in the manufacture of vitreous enamels and glazes include:
- refractories, such as quartz, feldspar and clay
- fluxes, such as borax (sodium borate decahydrate), soda ash (anhydrous sodium carbonate), sodium nitrate, fluorspar, cryolite, barium carbonate, magnesium carbonate, lead monoxide, lead tetroxide and zinc oxide
- colours, such as oxides of antimony, cadmium, cobalt, iron, nickel, manganese, selenium, vanadium, uranium and titanium
- opacifiers, such as oxides of antimony, titanium, tin and zirconium, and sodium antimoninate
- electrolytes, such as borax, soda ash, magnesium carbonate and sulphate, sodium nitrite and sodium aluminate
- flocculating agents, such as clay, gums, ammonium alginate, bentonite and colloidal silica.
The first step in all types of vitreous enamelling or glazing is the making of the frit, the enamel powder. This involves preparation of the raw materials, smelting and frit handing.
After careful cleaning of the metal products (e.g., shot blasting, pickling, degreasing), the enamel may be applied by a number of procedures:
- In the wet process, the object is dipped into the aqueous enamel slip, withdrawn and allowed to drain or, in “slushing”, the enamel slip is thicker and must be shaken from the object.
- In the dry process, the ground-coated object is heated to the enamelling temperature and then dry enamel powder is dusted through sieves onto it. The enamel sinters into place and, when the object is returned to the furnace, it melts down to a smooth surface.
- Spray application is being used increasingly, usually in a mechanized operation. It requires a cabinet under exhaust ventilation.
- Decorative enamels are usually applied by hand, using brushes or similar tools.
- Glazes for porcelain and pottery articles are usually applied by dipping or spraying. Although some dipping operations are being mechanized, pieces are usually dipped by hand in the domestic porcelain industry. The object is held in the hand, dipped into a large tub of glaze, the glaze is removed by a flick of the wrist and the object is placed in a dryer. An enclosed hood or cabinet with efficient exhaust ventilation should be provided when the glaze is sprayed.
The prepared objects are then “fired” in a furnace or kiln, which usually is gas fuelled.
Etching
Chemical etching produces a satin or matte finish. Most frequently, it is used as a pre-treatment prior to anodizing, lacquering, conversion coating, buffing or chemical brightening. It is most frequently applied to aluminium and stainless steel, but is also used for many other metals.
Aluminium is usually etched in alkaline solutions containing various mixtures of sodium hydroxide, potassium hydroxide, trisodium phosphate and sodium carbonate, together with other ingredients to prevent sludge formation. One of the most common processes uses sodium hydroxide at a concentration of 10 to 40 g/l maintained at a temperature of 50 to 85°C with an immersion time as long as 10 minutes.
The alkaline etching is usually preceded and followed by treatment in various mixtures of hydrochloric, hydrofluoric, nitric, phosphoric, chromic or sulphuric acid. A typical acid treatment involves immersions of 15 to 60 seconds in a mixture of 3 parts by volume of nitric acid and 1 part by volume of hydrofluoric acid that is maintained at a temperature of 20°C.
Galvanizing
Galvanizing applies a zinc coating to a variety of steel products to protect against corrosion. The product must be clean and oxide-free for the coating to adhere properly. This usually involves a number of cleaning, rinsing, drying or annealing processes before the product enters the galvanizing bath. In “hot dip” galvanizing, the product is passed through a bath of molten zinc; “cold” galvanizing is essentially electroplating, as described above.
Manufactured products are usually galvanized in a batch process, while the continuous strip method is used for steel strip, sheet or wire. Flux may be employed to maintain satisfactory cleaning of both the product and the zinc bath and to facilitate drying. A prefluxing step may be followed by an ammonium chloride flux cover on the surface of the zinc bath, or the latter may be used alone. In galvanizing pipe, the pipe is immersed in a hot solution of zinc ammonium chloride after cleaning and before the pipe enters the molten zinc bath. The fluxes decompose to form irritating hydrogen chloride and ammonia gas, requiring LEV.
The various types of continuous hot-dip galvanizing differ essentially in how the product is cleaned and whether the cleaning is done on-line:
- cleaning by flame oxidation of the surface oils with subsequent reduction in the furnace and annealing done in-line
- electrolytic cleaning done prior to in-line annealing
- cleaning by acid pickling and alkali cleaning, using a flux prior to the preheat furnace and annealing in a furnace before galvanizing
- cleaning by acid pickling and alkali cleaning, eliminating the flux and preheating in a reducing gas (e.g., hydrogen) prior to galvanizing.
The continuous galvanizing line for light-gauge strip steel omits pickling and the use of flux; it uses alkaline cleaning and maintains the clean surface of the strip by heating it in a chamber or furnace with a reducing atmosphere of hydrogen until it passes below the surface of the molten zinc bath.
Continuous galvanizing of wire requires annealing steps, usually with a molten lead pan in front of the cleaning and galvanizing tanks; air or water cooling; pickling in hot, dilute hydrochloric acid; rinsing; application of a flux; drying; and then galvanizing in the molten zinc bath.
A dross, an alloy of iron and zinc, settles to the bottom of the molten zinc bath and must be removed periodically. Various types of materials are floated on the surface of the zinc bath to prevent oxidation of the molten zinc. Frequent skimming is needed at the points of entry and exit of the wire or strip being galvanized.
Heat treatment
Heat treatment, the heating and cooling of a metal which remains in the solid state, is usually an integral part of the processing of metal products. It almost always involves a change in the crystalline structure of the metal which results in a modification of its properties (e.g., annealing to make the metal more malleable, heating and slow cooling to reduce hardness, heating and quenching to increase hardness, low-temperature heating to minimize internal stresses).
Annealing
Annealing is a “softening” heat treatment widely used to allow further cold working of the metal, improve machinability, stress-relieve the product before it is used and so on. It involves heating the metal to a specific temperature, holding it at that temperature for a specific length of time and allowing it to cool at a particular rate. A number of annealing techniques are used:
- Blue annealing, in which a layer of blue oxide is produced on the surface of iron-based alloys
- Bright annealing, which is carried out in a controlled atmosphere to minimize surface oxidation
- Close annealing or box annealing, a method in which both ferrous and non-ferrous metals are heated in a sealed metal container with or without a packing material and then slowly cooled
- Full annealing, usually carried out in a protective atmosphere, aimed at obtaining the maximum softness economically feasible
- Malleablizing, a special kind of anneal given to iron castings to make them malleable by transforming the combined carbon in the iron to fine carbon (i.e., graphite)
- Partial annealing, a low-temperature process to remove internal stresses induced in the metal by cold working
- Sub-critical or spheroidizing annealing, which produces improved machinability by allowing the iron carbide in the crystalline structure to acquire a spheroid shape.
Age-hardening
Age-hardening is a heat treatment often used on aluminium-copper alloys in which the natural hardening that takes place in the alloy is accelerated by heating to about 180°C for about 1 hour.
Homogenizing
Homogenizing, usually applied to ingots or powdered metal compacts, is designed to remove or greatly reduce segregation. It is achieved by heating to a temperature about 20°C below the metal’s melting point for about 2 hours or more and then quenching.
Normalizing
A process similar to full annealing, ensures the uniformity of the mechanical properties to be obtained and also produces greater toughness and resistance to mechanical loading.
Patenting
Patenting is a special type of annealing process that is usually applied to materials of small cross-section which are intended to be drawn (e.g., 0.6% carbon steel wire). The metal is heated in an ordinary furnace to above the transformation range and then passes from the furnace directly into, for example, a lead bath held at a temperature of about 170°C.
Quench-hardening and tempering
An increase in hardness can be produced in an iron-based alloy by heating to above the transformation range and rapidly cooling to room temperature by quenching in oil, water or air. The article is often too highly stressed to be put into service and, in order to increase its toughness, it is tempered by reheating to a temperature below the transformation range and allowing it to cool at the desired rate.
Martempering and austempering are similar processes except that the article is quenched, for example, in a salt or lead bath held at a temperature of 400°C.
Surface- and case-hardening
This is another heat-treatment process applied most frequently to iron-based alloys, which allows the surface of the object to remain hard while its core remains relatively ductile. It has a number of variations:
- Flame hardening involves hardening the surfaces of the object (e.g., gear teeth, bearings, slideways) by heating with a high-temperature gas torch and then quenching in oil, water or another suitable medium.
- Electrical induction hardening is similar to flame hardening except that the heating is produced by eddy currents induced in the surface layers.
- Carburizing increases the carbon content of the surface of an iron-based alloy by heating the object in a solid, liquid or gaseous carbonaceous medium (e.g., solid charcoal and barium carbonate, liquid sodium cyanide and sodium carbonate, gaseous carbon monoxide, methane and so on) at a temperature of about 900°C.
- Nitriding increases the nitrogen content of the surface of a special low-alloy cast iron or steel object by heating it in a nitrogenous medium, usually ammonia gas, at about 500 to 600°C.
- Cyaniding is a method of case-hardening in which the surface of a low-carbon steel object is enriched in both carbon and nitrogen simultaneously. It usually involves heating the object for 1 hour in a bath of molten 30% sodium cyanide at 870°C, and then quenching in oil or water.
- Carbo-nitriding is a gaseous process for the simultaneous absorption of carbon and nitrogen into the surface layer of steel by heating it to 800 to 875°C in an atmosphere of a carburizing gas (see above) and a nitriding gas (e.g., 2 to 5% anhydrous ammonia).
Metallizing
Metallizing, or metal spraying, is a technique for applying a protective metallic coating to a mechanically roughened surface by spraying it with molten droplets of metal. It is also used to build up worn or corroded surfaces and for salvaging badly-machined component parts. The process is widely known as Schooping, after the Dr. Schoop who invented it.
It uses the Schooping gun, a hand-held, pistol-shaped spray gun through which the metal in wire form is fed into a fuel gas/oxygen blowpipe flame which melts it and, using compressed air, sprays it onto the object. The heat source is a mixture of oxygen and either acetylene, propane or compressed natural gas. The coiled wire is usually straightened before being fed into the gun. Any metal that can be made into a wire may be used; the gun can also accept the metal in powder form.
Vacuum metallizing is a process in which the object is placed in a vacuum jar into which the coating metal is sprayed.
Phosphating
Phosphating is used mainly on mild and galvanized steel and aluminium to augment the adhesion and corrosion resistance of paint, wax and oil finishes. It is also used to form a layer which acts as a parting film in the deep drawing of sheet metal and improves its wear resistance. It essentially consists of allowing the metal surface to react with a solution of one or more phosphates of iron, zinc, manganese, sodium or ammonium. Sodium and ammonium phosphate solutions are used for combined cleaning and phosphating. The need to phosphate multi-metal objects and the desire to increase line speeds in automated operations have led to reducing reaction times by the addition of accelerators such as fluorides, chlorates, molybdates and nickel compounds to the phosphating solutions.To reduce crystal size and, consequently, increase the flexibility of zinc phosphate coatings, crystal refining agents such as tertiary zinc phosphate or titanium phosphate are added to the pre-treatment rinse.
The phosphating sequence typically includes the following steps:
- hot caustic cleaning
- brushing and rinsing
- further hot caustic cleaning
- conditioning water rinse
- spraying or dipping in hot solutions of acid phosphates
- cold water rinse
- warm chromic acid rinse
- another cold water rinse
- drying.
Priming
Organic paint primers are applied to metal surfaces to promote the adhesion of subsequently applied paints and to retard corrosion at the paint-metal interface. The primers usually contain resins, pigments and solvents and may be applied to the prepared metal surfaces by brush, spray, immersion, roller coating or electrophoresis.
The solvents may be any combination of aliphatic and aromatic hydrocarbons, ketones, esters, alcohols and ethers. The most commonly used resins are polyvinyl butynol, phenolic resins, drying oil alkyds, epoxidized oils, epoxyesters, ethyl silicates and chlorinated rubbers. In complex primers, cross-linking agents such as tetraethylene pentamine, pentaethylene hexamine, isocyanates and urea formaldehyde are used. Inorganic pigments used in primer formulations include lead, barium, chromium, zinc and calcium compounds.
Plastic coating
Plastic coatings are applied to metals in liquid form, as powders which are subsequently cured or sintered by heating, or in the form of fabricated sheets which are laminated to the metal surface with an adhesive. The most commonly used plastics include polyethylene, polyamides (nylons) and PVC. The latter may include plasticizers based on monomeric and polymeric esters and stabilizers such as lead carbonate, fatty acid salts of barium and cadmium, dibutyltin dilaurate, alkyltin mercaptides and zinc phosphate. Although generally of low toxicity and non-irritating, some of the plasticizers are skin sensitizers.
Hazards and Their Prevention
As might be deduced from the complexity of the processes outlined above, there is a large variety of safety and health hazards associated with the surface treatment of metals. Many are regularly encountered in manufacturing operations; others are presented by the uniqueness of the techniques and materials employed. Some are potentially life threatening. By and large, however, they can be prevented or controlled.
Workplace design
The workplace should be designed to allow the delivery of raw materials and supplies and the removal of the finished products without interfering with the ongoing processing. Since many of the chemicals are flammable or prone to react when mixed, proper separation in storage and in transit is essential. Many of the metal finishing operations involve liquids, and when leaks, spills or splashes of acids or alkalis occur they must be washed away promptly. Accordingly, adequately drained, slip-resistant floors must be provided. Housekeeping must be diligent to keep the work areas and other spaces clean and free from accumulations of materials. Systems for disposal of solid and liquid wastes and effluents from furnaces and exhaust ventilation must be designed with environmental concerns in mind.
Work stations and work assignments should use ergonomic principles to minimize strains, sprains, excessive fatigue and RSIs. Machine guards must have automatic lockout so the machine is de-energized if the guard is removed. Splash guards are essential. Because of the danger of splashes of hot acid and alkali solutions, eyewash fountains and whole-body showers must be installed within easy reach. Signs should be posted to warn other production and maintenance personnel of such dangers as chemical baths and hot surfaces.
Chemical assessment
All chemicals should be evaluated for potential toxicity and physical hazards, and less hazardous materials should be substituted where possible. However, since the less toxic material may be more flammable, the hazard of fire and explosion must also be considered. In addition, the chemical compatibility of materials must be considered. For example, mixing of nitrate and cyanide salts by accident could cause an explosion due to the strong oxidizing properties of nitrates.
Ventilation
Most of the metal coating processes require LEV that is strategically placed to draw the vapours or other contaminants away from the worker. Some systems push fresh air across the tank to “push” airborne contaminants to the exhaust side of the system. Fresh air intakes must be located away from exhaust vents so that potentially toxic gases are not recirculated.
Personal protective equipment
Processes should be engineered to prevent potentially toxic exposures, but since they cannot always be totally avoided, employees will have to be provided with appropriate PPE (e.g., goggles with or without face shields as appropriate, gloves, aprons or coveralls and shoes). Because many of the exposures involve hot corrosive or caustic solutions, the protective items should be insulated and chemical-resistant. If there is possible exposure to electricity, PPE should be non-conductive. PPE must be available in adequate quantity to allow contaminated, wet items to be cleaned and dried before re-using them. Insulated gloves and other protective clothing should be available where there is the risk of thermal burns from hot metal, furnaces and so on.
An important adjunct is the availability of wash-up facilities and clean lockers and dressing rooms, so that workers’ clothing remains uncontaminated and workers do not carry toxic materials back into their homes.
Employee training and supervision
Employee education and training are essential both when new to the job or when there have been changes in the equipment or the process. MSDSs must be provided for each of the chemical products which explain the chemical and physical hazards, in languages and at educational levels that ensure they will be understood by the workers. Competence testing and periodic retraining will assure that workers have retained the needed information. Close supervision is advisable to make sure that the proper procedures are being followed.
Selected hazards
Certain hazards are unique to the metal coating industry and deserve special consideration.
Alkaline and acid solutions
The heated alkaline and acid solutions used in cleaning and treatment of metals are particularly corrosive and caustic. They are irritating to the skin and mucous membranes and are especially dangerous when splashed into the eye. Eyewash fountains and emergency showers are essential. Proper protective clothing and goggles will guard against the inevitable splashes; when a splash reaches the skin, the area should be immediately and copiously rinsed with cool, clean water for at least 15 minutes; medical attention may be necessary, particularly when the eye is involved.
Care should be exercised when utilizing chlorinated hydrocarbons as phosgene may result from a reaction of the chlorinated hydrocarbon, acids and metals. Nitric and hydrofluoric acid are particularly dangerous when their gases are inhaled, because it may take 4 hours or more before the effects on the lungs become apparent. Bronchitis, pneumonitis and even potentially fatal pulmonary oedema may appear belatedly in a worker who apparently had no initial effect from the exposure. Prompt prophylactic medical treatment and, often, hospitalization are advisable for workers who have been exposed. Skin contact with hydrofluoric acid can cause severe burns without pain for several hours. Prompt medical attention is essential.
Dust
Metallic and oxidic dusts are a particular problem in grinding and polishing operations, and are most effectively removed by LEV as they are created. Ductwork should be designed to be smooth and air velocity should be sufficient to keep the particulates from settling out of the air stream. Aluminium and magnesium dust may be explosive and should be collected in a wet trap. Lead has become less of a problem with the decline of its use in ceramics and porcelain glazes, but it remains the ubiquitous occupational hazard and must always be guarded against. Beryllium and its compounds have received interest recently due to the possibility of carcinogenicity and chronic beryllium disease.
Certain operations present a risk of silicosis and pneumoconiosis: the calcining, crushing and drying of flint, quartz or stone; the sieving, mixing and weighing out of these substances in the dry state; and the charging of furnaces with such materials. They also represent a danger when they are used in a wet process and are splashed about the workplace and on workers’ clothing, to become dusts again when they dry out. LEV and rigorous cleanliness and personal hygiene are important preventive measures.
Organic solvents
Solvents and other organic chemicals used in degreasing and in certain processes are dangerous when inhaled. In the acute phase, their narcotic effects may lead to respiratory paralysis and death. In chronic exposure, toxicity of the central nervous system and liver and kidney damage are most frequent. Protection is provided by LEV with a safety zone of at least 80 to 100 cm between the source and the breathing area of the worker. Bench ventilation must also be installed to remove residual vapours from the finished workpieces. Defatting of the skin by organic solvents may be a precursor of dermatitis. Many solvents are also flammable.
Cyanide
Baths containing cyanides are frequently used in electrolytic degreasing, electroplating and cyaniding. Reaction with acid will form the volatile, potentially lethal hydrogen cyanide (prussic acid). The lethal concentration in air is 300 to 500 ppm. Fatal exposures may also result from skin absorption or ingestion of cyanides. Optimum cleanliness is essential for workers using cyanide. Food should not be eaten before washing, and should never be in the work area. Hands and clothing must be carefully cleaned following a potential cyanide exposure.
First aid measures for cyanide poisoning include transport into the open air, removal of contaminated clothing, copious washing of the exposed areas with water, oxygen therapy and inhalation of amyl nitrite. LEV and skin protection are essential.
Chromium and nickel
Chromic and nickel compounds used in galvanic baths in electroplating may be hazardous. Chromium compounds can cause burns, ulceration and eczema of the skin and mucosa and a characteristic perforation of the nasal septum. Bronchial asthma may occur. Nickel salts can cause obstinate allergic or toxic-irritative skin injury. There is evidence that both chromium and nickel compounds may be carcinogenic. LEV and skin protection are essential.
Furnaces and ovens
Special precautions are needed when working with the furnaces employed, for example, in the heat treatment of metals where components are handled at high temperatures and the materials used in the process may either be toxic or explosive or both. The gaseous media (atmospheres) in the furnace may react with the metal charge (oxidizing or reducing atmospheres) or they may be neutral and protective. Most of the latter contain up to 50% hydrogen and 20% carbon monoxide, which, in addition to being combustible, form highly explosive mixtures with air at elevated temperatures. The ignition temperature varies from 450 to 750 °C, but a local spark may cause ignition even at lower temperatures. The danger of explosion is greater when the furnace is being started up or shut down. Since a cooling furnace tends to suck in air (a particular danger when the fuel or power supply is interrupted), a supply of inert gas (e.g., nitrogen or carbon dioxide) should be available for purging when the furnace is shut down as well as when a protective atmosphere is introduced into a hot furnace.
Carbon monoxide is perhaps the greatest hazard from furnaces and ovens. Since it is colourless and odourless, it frequently reaches toxic levels before the worker becomes aware of it. Headache is one of the earliest symptoms of toxicity, and, therefore, a worker developing a headache on the job should immediately be removed into fresh air. Danger zones include recessed pockets in which the carbon monoxide may collect; it should be remembered that brickwork is porous and may retain the gas during normal purging and emit it when the purging is completed.
Lead furnaces may be dangerous since lead tends to vaporize quite rapidly at temperatures above 870°C. Accordingly, an effective fume extraction system is required. A pot breakage or failure may also be hazardous; a sufficiently large well or pit should be provided to capture the molten metal if this occurs.
Fire and explosion
Many of the compounds used in metal coating are flammable and, under certain circumstances, explosive. For the most part, the furnaces and drying ovens are gas fired, and special precautions such as flame-failure devices at burners, low-pressure cut-off valves in the supply lines and explosion relief panels in the structure of the stoves should be installed. In electrolytic operations, hydrogen formed in the process may collect at the surface of the bath and, if not exhausted, may reach explosive concentrations. Furnaces should be properly ventilated and burners protected from being clogged by dripping material.
Oil quenching is also a fire hazard, especially if the metal charge is not completely immersed. Quenching oils should have a high flashpoint, and their temperature should not exceed 27°C.
Compressed oxygen and fuel gas cylinders used in metallizing are fire and explosion hazards if not stored and operated properly. See the article “Welding and thermal cutting” in this chapter for detailed precautions.
As required by local ordinances, firefighting equipment, including alarms, should be provided and maintained in working order, and the workers drilled in using it properly.
Heat
The use of furnaces, open flames, ovens, heated solutions and molten metals inevitably presents the risk of excessive heat exposure, which is compounded in hot, humid climates and, particularly, by occlusive protective garments and gear. Complete air conditioning of a plant may not be economically feasible, but supplying cooled air in local ventilation systems is helpful. Rest breaks in cool surroundings and adequate fluid intake (fluids taken at the work station should be free of toxic contaminants) will help to avert heat toxicity. Workers and supervisors should be trained in the recognition of heat stress symptoms.
Conclusion
Surface treatment of metals involves a multiplicity of processes entailing a broad range of potentially toxic exposures, most of which can be prevented or controlled by the diligent application of well-recognized preventive measures.
Metal Reclamation
Metal reclamation is the process by which metals are produced from scrap. These reclaimed metals are not distinguishable from the metals produced from primary processing of an ore of the metal. However, the process is slightly different and the exposure could be different. The engineering controls are basically the same. Metal reclamation is very important to the world economy because of the depletion of raw materials and the pollution of the environment created by scrap materials.
Aluminium, copper, lead and zinc comprise 95% of the production in the secondary non-ferrous metal industry. Magnesium, mercury, nickel, precious metals, cadmium, selenium, cobalt, tin and titanium are also reclaimed. (Iron and steel are discussed in the chapter Iron and steel industry. See also the article “Copper, lead and zinc smelting and refining” in this chapter.)
Control Strategies
Emission/exposure control principles
Metal reclamation involves exposures to dust, fumes, solvents, noise, heat, acid mists and other potential hazardous materials and risks. Some process and/or material handling modifications may be feasible to eliminate or reduce the generation of emissions: minimizing handling, lowering pot temperatures, decreasing dross formation and surface generation of dust, and modifying plant layout to reduce material handling or re-entrainment of settled dust.
Exposure can be reduced in some cases if machines are selected to perform high-exposure tasks so that employees may be removed from the area. This can also reduce ergonomic hazards due to materials handling.
To prevent cross contamination of clean areas in the plant, it is desirable to isolate processes generating significant emissions. A physical barrier will contain emissions and reduce their spread. Thus, fewer people are exposed, and the number of emission sources contributing to exposure in any one area will be reduced. This simplifies exposure evaluations and makes the identification and control of major sources easier. Reclaim operations are often isolated from other plant operations.
Occasionally, it is possible to enclose or isolate a specific emission source. Because enclosures are seldom air tight, a negative draught exhaust system is often applied to the enclosure. One of the most common ways to control emissions is to provide local exhaust ventilation at the point of emission generation. Capturing emissions at their source reduces the potential for emissions to disperse into the air. It also prevents secondary employee exposure created by the re-entrainment of settled contaminants.
The capture velocity of an exhaust hood must be great enough to prevent fumes or dust from escaping the air flow into the hood. The air flow should have enough velocity to carry fume and dust particles into the hood and to overcome the disrupting effects of cross drafts and other random air movements. The velocity required to accomplish this will vary from application to application. The use of recirculation heaters or personal cooling fans which can overcome local exhaust ventilation should be restricted.
All exhaust or dilution ventilation systems also require replacement air (known also as “make-up” air systems). If the replacement air system is well designed and integrated into natural and comfort ventilation systems, more effective control of exposures can be expected. For example, replacement air outlets should be placed so clean air flows from the outlet across the employees, towards the emission source and to the exhaust. This technique is often used with supplied-air islands and places the employee between clean incoming air and the emission source.
Clean areas are intended to be controlled through direct emission controls and housekeeping. These areas exhibit low ambient contaminant levels. Employees in contaminated areas can be protected by supplied-air service cabs, islands, stand-by pulpits and control rooms, supplemented by personal respiratory protection.
The average daily exposure of workers can be reduced by providing clean areas such as breakrooms and lunchrooms that are supplied with fresh filtered air. By spending time in a relatively contaminant-free area, the employees’ time-weighted average exposure to contaminants can be reduced. Another popular application of this principle is the supplied-air island, where fresh filtered air is supplied to the breathing zone of the employee at the workstation.
Sufficient space for hoods, duct work, control rooms, maintenance activities, cleaning and equipment storage should be provided.
Wheeled-vehicles are significant sources of secondary emissions. Where wheeled-vehicle transport is used, emissions can be reduced by paving all surfaces, keeping surfaces free of accumulated dusty materials, reducing vehicle travel distances and speed, and by re-directing vehicle exhaust and cooling fan discharge. Appropriate paving material such as concrete should be selected after considering factors such as load, use and care of surface. Coatings may be applied to some surfaces to facilitate wash down of roadways.
All exhaust, dilution and make-up air ventilation systems must be properly maintained in order to effectively control air contaminants. In addition to maintaining general ventilation systems, process equipment must be maintained to eliminate spillage of material and fugitive emissions.
Work practice programme implementation
Although standards emphasize engineering controls as a means of achieving compliance, work practice controls are essential to a successful control programme. Engineering controls can be defeated by poor work habits, inadequate maintenance and poor housekeeping or personal hygiene. Employees who operate the same equipment on different shifts can have significantly different airborne exposures because of differences in these factors between shifts.
Work practice programmes, although often neglected, represent good managerial practice as well as good common sense; they are cost effective but require a responsible and cooperative attitude on the part of employees and line supervisors. The attitude of senior management toward safety and health is reflected in the attitude of line supervisors. Likewise, if supervisors do not enforce these programmes, employees attitudes may suffer. Fostering good health and safety attitudes can be accomplished through:
- a cooperative atmosphere in which employees participate in the programmes
- formal training and educational programmes
- emphasizing the plant safety and health programme. Motivating employees and obtaining their trust is necessary in order to have an effective programme.
Work practice programmes cannot be simply “installed”. Just as with a ventilation system, they must be maintained and continually checked to insure that they are functioning properly. These programmes are the responsibility of management and employees. Programmes should be established to teach, encourage and supervise “good” (i.e., low exposure) practices.
Personal protective equipment
Safety glasses with side shields, coveralls, safety shoes and work gloves should be routinely worn for all jobs. Those engaged in casting and melting, or in casting alloys, should wear aprons and hand protection made of leather or other suitable materials to protect against the splatter of molten metal.
In operations where engineering controls are not adequate to control dust or fume emissions, appropriate respiratory protection should be worn. If noise levels are excessive, and cannot be engineered out or noise sources cannot be isolated, hearing protection should be worn. There should also be a hearing conservation programme, including audiometric testing and training.
Processes
Aluminium
The secondary aluminium industry utilizes aluminium-bearing scrap to produce metallic aluminium and aluminium alloys. The processes used in this industry include scrap pre-treatment, remelting, alloying and casting. The raw material used by the secondary aluminium industry includes new and old scrap, sweated pig and some primary aluminium. New scrap consists of clippings, forging and other solids purchased from the aircraft industry, fabricators and other manufacturing plants. Borings and turnings are by-product of the machining of castings, rods and forging by the aircraft and automobile industry. Drosses, skimmings and slags are obtained from primary reduction plants, secondary smelting plants and foundries. Old scrap includes automobile parts, household items and airplane parts. The steps involved are as follows:
- Inspection and sorting. Purchased aluminium scrap undergoes inspection. Clean scrap requiring no pre-treatment is transported to storage or is charged directly into the smelting furnace. The aluminium that needs pre-treatment is manually sorted. Free iron, stainless steel, zinc, brass and oversized materials are removed.
- Crushing and screening. Old scrap, especially casting and sheet contaminated with iron, are inputs to this process. Sorted scrap is conveyed to a crusher or hammer mill where the material is shredded and crushed, and the iron is torn away from the aluminium. The crushed material is passed over vibrating screens to remove dirt and fines.
- Baling. Specially designed baling equipment is used to compact bulky aluminium scrap such as scrap sheet, castings and clippings.
- Shredding/classifying. Pure aluminium cable with steel reinforcement or insulation is cut with alligator-type shears, then granulated or further reduced in hammer mills to separate the iron core and plastic coating from the aluminium.
- Burning/drying. Borings and turning are pre-treated in order to remove cutting oils, greases, moisture and free iron. The scrap is crushed in a hammer mill or ring crusher, the moisture and organics are volatilized in a gas- or oil-fired rotary dryer, the dried chips are screened to remove aluminium fines, the remaining material is magnetically treated for iron removal, and the clean, dried borings are sorted in tote boxes.
- Hot-dross processing. Aluminium can be removed from the hot dross discharged from the refining furnace by batch fluxing with a salt-cryolite mixture. This process is carried out in a mechanically rotated, refractory-lined barrel. The metal is tapped periodically through a hole in its base.
- Dry milling. In the dry-milling process, cold aluminium-laden dross and other residues are processed by milling, screening and concentrating to obtain a product containing a minimum aluminium content of 60 to 70%. Ball mills, rod mills or hammer mills can be used to reduce the oxides and non-metallics to fine powders. Separation of dirt and other non-recoverables from the metal is achieved by screening, air classification and/or magnetic separation.
- Roasting. Aluminium foil backed with paper, gutta-percha or insulation is an input in this process. In the roasting process, carboneous materials associated with aluminium foils are charged and then separated from the metal product.
- Aluminium sweating. Sweating is a pyrometallurgical process which is used to recover aluminium from high-iron-content scrap. High-iron aluminium scrap, castings and dross are inputs in this process. Open-flame reverberatory furnaces with sloping hearths are generally employed. Separation is accomplished as aluminium and other low-melting constituents melt and trickle down the hearth, through a grate and into air-cooled moulds, collecting pots or holding wells. The product is termed “sweated pig”. The higher-melting materials including iron, brass and oxidation products formed during the sweating process are periodically tapped from the furnace.
- Reverberatory (chlorine) smelting-refining. Reverberatory furnaces are used to convert clean sorted scrap, sweated pigs or, in some cases, untreated scrap into specification alloys. The scrap is charged to the furnace by mechanical means. Materials are added for processing by batch or continuous feed. After the scrap is charged a flux is added to prevent contact with and subsequent oxidation of the melt by air (cover flux). Solvent fluxes are added which react with non-metallics, such as residues from burned coatings and dirt, to form insolubles which float to the surface as slag. Alloying agents are then added, depending on the specifications. Demagging is the process which reduces the magnesium content of the molten charge. When demagging with chlorine gas, chlorine is injected through carbon tubes or lances and reacts with magnesium and aluminium as it bubbles. In the skimming step impure semi-solid fluxes are skimmed off the surface of the melt.
- Reverberatory (fluorine) smelting-refining. This process is similar to the reverberatory (chlorine) smelting-refining process except that aluminium fluoride rather than chlorine is employed.
Table 1 lists exposure and controls for aluminium reclamation operations.
Table 1. Engineering/administrative controls for aluminium, by operation
|
Process equipment |
Exposure |
Engineering/administrative controls |
|
Sorting |
Torch desoldering—metal fumes such as lead and cadmium |
Local exhaust ventilation during desoldering; PPE—respiratory protection when desoldering |
|
Crushing/screening |
Non-specific dusts and aerosol, oil mists, metal particulates, and noise |
Local exhaust ventilation and general area ventilation, isolation of noise source; PPE—hearing protection |
|
Baling |
No known exposure |
No controls |
|
Burning/drying |
Non-specific particulate matter which may include metals, soot, and condensed heavy organics. Gases and vapours containing fluorides, sulphur dioxide, chlorides, carbon monoxide, hydrocarbons and aldehydes |
Local exhaust ventilation, general area ventilation, heat stress work/rest regimen, fluids, isolation of noise source; PPE—hearing protection |
|
Hot-dross processing |
Some fumes |
Local exhaust ventilation, general area ventilation |
|
Dry milling |
Dust |
Local exhaust ventilation, general area ventilation |
|
Roasting |
Dust |
Local exhaust ventilation, general area ventilation, heat stress work/rest regimen, fluids, isolation of noise source; PPE—hearing protection |
|
Sweating |
Metal fumes and particulates, non-specific gases and vapours, heat and noise |
Local exhaust ventilation, general area ventilation, heat stress work/rest regimen, fluids, isolation of noise source; PPE—hearing protection and respiratory protection |
|
Reverberatory (chlorine) smelting-refining |
Products of combustion, chlorine, hydrogen chlorides, metal chlorides, aluminium chlorides, heat and noise |
Local exhaust ventilation, general area ventilation, heat stress work/rest regimen, fluids, isolation of noise source; PPE—hearing protection and respiratory protection |
|
Reverberatory (fluorine) smelting-refining |
Products of combustion, fluorine, hydrogen flluorides, metal fluorides, aluminium fluorides, heat and noise |
Local exhaust ventilation, general area ventilation, heat stress work/rest regimen, fluids, isolation of noise source; PPE—hearing protection and respiratory protection |
Copper reclamation
The secondary copper industry utilizes copper-bearing scrap to produce metallic copper and copper based alloys. The raw materials used can be classified as new scrap produced in the fabrication of finished products or old scrap from obsolete worn out or salvaged articles. Old scrap sources include wire, plumbing fixtures, electrical equipment, automobiles and domestic appliances. Other materials with copper value include slags, drosses, foundry ashes and sweepings from smelters. The following steps are involved:
- Stripping and sorting. Scrap is sorted on the bases of its copper content and cleanliness. Clean scrap may be manually separated for charging directly to a melting and alloying furnace. Ferrous components can be separated magnetically. Insulation and lead cable coverings are stripped by hand or by specially designed equipment.
- Briquetting and crushing. Clean wire, thin plate, wire screen, borings, turnings and chips are compacted for easier handling. The equipment used includes hydraulic baling presses, hammer mills and ball mills.
- Shredding. The separation of copper wire from insulation is accomplished by reducing the size of the mixture. The shredded material is then sorted by air or hydraulic classification with magnetic separation of any ferrous materials.
- Grinding and gravity separation. This process accomplishes the same function as shredding but uses an aqueous separation medium and different input materials such as slags, drosses, skimmings, foundry ashes, sweepings and baghouse dust.
- Drying. Borings, turnings and chips containing volatile organic impurities such as cutting fluids, oils and greases are removed.
- Insulation burning. This process separates insulation and other coatings from copper wire by burning these materials in furnaces. The wire scrap is charged in batches to a primary ignition chamber or afterburner. Volatile combustion products are then passed through a secondary combustion chamber or baghouse for collection. Non-specific particulate matter is generated which may include smoke, clay and metal oxides. Gases and vapours may contain oxides of nitrogen, sulphur dioxide, chlorides, carbon monoxide, hydrocarbons and aldehydes.
- Sweating. The removal of low vapour-melting components from scrap is accomplished by heating the scrap to a controlled temperature which is just above the melting point of the metals to be sweated out. The primary metal, copper, is generally not the melted component.
- Ammonium carbonate leaching. Copper can be recovered from relatively clean scrap by leaching and dissolution in a basic ammonium carbonate solution. Cupric ions in an ammonia solution will react with metallic copper to produce cuprous ions, which can be reoxidized to the cupric state by air oxidation. After the crude solution is separated from the leach residue, the copper oxide is recovered by steam distillation.
- Steam distillation. Boiling the leached material from the carbonate leaching process precipitates the copper oxide. The copper oxide is then dried.
- Hydrothermal hydrogen reduction. Ammonium carbonate solution containing copper ions is heated under pressure in hydrogen, precipitating the copper as a powder. The copper is filtered, washed, dried and sintered under a hydrogen atmosphere. The powder is ground and screened.
- Sulphuric acid leaching. Scrap copper is dissolved in hot sulphuric acid to form a copper sulphate solution for feed to the electrowinning process. After digestion, the undissolved residue is filtered off.
- Converter smelting. Molten black copper is charged to converter, which is a pear-shaped or cylindrical steel shell lined refractory brick. Air is blown into the molten charges through nozzles called tuyères. The air oxidizes copper sulphide and other metals. A flux containing silica is added to react with the iron oxides to form an iron silicate slag. This slag is skimmed from the furnace, usually by tipping the furnace and then there is a secondary blow and skim. The copper from this process is called blister copper. The blister copper is generally further refined in a fire refining furnace.
- Fire refining. The blister copper from the converter is fire refined in a cylindrical tilting furnace, a vessel like a reverberatory furnace. The blister copper is charged to the refining vessel in an oxidizing atmosphere. The impurities are skimmed from the surface and a reducing atmosphere is created by the addition of green logs or natural gas. The resulting molten metal is then cast. If the copper is to be electrolytically refined, the refined copper will be cast as an anode.
- Electrolytic refining. The anodes from the fire refining process are placed in a tank containing sulphuric acid and a direct current. The copper from the anode is ionized and the copper ions are deposited on a pure copper starter sheet. As the anodes dissolve in the electrolyte the impurities settle to the bottom of the cell as a slime. This slime can be additionally processed to recover other metal values. The cathode copper produced is melted and cast into a variety of shapes.
Table 2 lists exposures and controls for copper reclamation operations.
Table 2. Engineering/administrative controls for copper, by operation
|
Process equipment |
Exposures |
Engineering/administrative controls |
|
Stripping and sorting |
Air contaminants from material handling and desoldering or scrap cutting |
Local exhaust ventilation, general area ventilation |
|
Briquetting and crushing |
Non-specific dusts and aerosol, oil mists, metal particulates and noise |
Local exhaust ventilation and general area ventilation, isolation of noise source; PPE—hearing protection and respiratory protection |
|
Shredding |
Non-specific dusts, wire insulation material, metal particulates and noise |
Local exhaust ventilation and general area ventilation, isolation of noise source; PPE—hearing protection and respiratory protection |
|
Grinding and gravity separation |
Non-specific dusts, metal particulates from fluxes, slags and drosses, and noise |
Local exhaust ventilation and general area ventilation, isolation of noise source; PPE—hearing protection and respiratory protection |
|
Drying |
Non-specific particulate matter, which may include metals, soot and condensed heavy organics |
Local exhaust ventilation, general area ventilation, work/rest regimen, fluids, isolation of noise source; PPE—hearing protection and respiratory protection |
|
Insulation burning |
Non-specific particulate matter which may include smoke, clay |
Local exhaust ventilation, general area ventilation, work/rest regimen, fluids, isolation of noise source; PPE—respiratory protection |
|
Sweating |
Metal fumes and particulates, non-specific gases, vapours and particulates |
Local exhaust ventilation, general area ventilation, work/rest regimen, fluids, isolation of noise source; PPE—hearing protection and respiratory protection |
|
Ammonium carbonate leaching |
Ammonia |
Local exhaust ventilation, general area ventilation; PPE—respiratory protection |
|
Steam distillation |
Ammonia |
Local exhaust ventilation, general area ventilation; PPE—glasses with side shields |
|
Hydrothermal hydrogen reduction |
Ammonia |
Local exhaust ventilation, general area ventilation; PPE—respiratory protection |
|
Sulphuric acid leaching |
Sulphuric acid mists |
Local exhaust ventilation, general area ventilation |
|
Converter smelting |
Volatile metals, noise |
Local exhaust ventilation, general area ventilation; PPE—respiratory protection and hearing protection |
|
Electric crucible smelting |
Particulate, sulphur and nitrogen oxides, soot, carbon monoxide, noise |
Local exhaust ventilation, general area ventilation; PPE—hearing protection |
|
Fire refining |
Sulphur oxides, hydrocarbons, particulates |
Local exhaust ventilation, general area ventilation; PPE—hearing protection |
|
Electrolytic refining |
Sulphuric acid and metals from sludge |
Local exhaust ventilation, general area ventilation |
Lead reclamation
Raw materials purchased by secondary lead smelters may require processing prior to being charged into a smelting furnace. This section discusses the most common raw materials which are purchased by secondary lead smelters and feasible engineering controls and work practices to limit employee exposure to lead from raw materials processing operations. It should be noted that lead dust can generally be found throughout lead reclamation facilities and that any vehicular air is likely to stir up lead dust which can then be inhaled or adhere to shoes, clothing, skin and hair.
Automotive batteries
The most common raw material at a secondary lead smelter is junk automotive batteries. Approximately 50% of the weight of a junk automotive battery will be reclaimed as metallic lead in the smelting and refining process. Approximately 90% of the automotive batteries manufactured today utilize a polypropylene box or case. The polypropylene cases are reclaimed by almost all secondary lead smelters due to the high economic value of this material. Most of these processes can generate metal fumes, in particular lead and antimony.
In automotive battery breaking there is a potential for forming arsine or stibine due to the presence of arsenic or antimony used as hardening agents in grid metal and the potential for having nascent hydrogen present.
The four most common processes for breaking automotive batteries are:
- high speed saw
- slow speed saw
- shear
- whole battery crushing (Saturn crusher or shredder or hammer mill).
The first three of these processes involve cutting the top off of the battery, then dumping the groups, or lead-bearing material. The fourth process involves crushing the entire battery in a hammer mill and separating the components by gravity separation.
Automotive battery separation takes place after automotive batteries have been broken in order that the lead-bearing material can be separated from the case material. Removing the case may generate acid mists. The most widely used techniques for accomplishing this task are:
- The manual technique. This is used by the vast majority of secondary lead smelters and remains the most widely used technique in small to mid-sized smelters. After the battery passes through the saw or shear, an employee manually dumps the groups or lead-bearing material into a pile and places the case and top of the battery into another pile or conveyance system.
- A tumbler device. Batteries are placed into a tumbler device after the tops have been sawed/sheared off to separate the groups from the cases. Ribs inside the tumbler dump the groups as it slowly rotates. Groups fall through the slots in the tumbler while the cases are conveyed to the far end and are collected as they exit. Plastic and rubber battery cases and tops are further processed after being separated from the lead bearing material.
- A sink/float process. The sink/float process typically is combined with the hammer mill or crushing process for battery breaking. Battery pieces, both lead bearing and cases, are placed in a series of tanks filled with water. Lead bearing material sinks to the bottom of the tanks and is removed by screw conveyor or drag chain while the case material floats and is skimmed off the tank surface.
Industrial batteries which were used to power mobile electric equipment or for other industrial uses are purchased periodically for raw material by most secondary smelters. Many of these batteries have steel cases which require removal by cutting the case open with a cutting torch or a hand-held gas powered saw.
Other purchased lead-bearing scrap
Secondary lead smelters purchase a variety of other scrap materials as raw materials for the smelting process. These materials include battery manufacturing plant scrap, drosses from lead refining, scrap metallic lead such as linotype and cable covering, and tetraethyl lead residues. These types of materials may be charged directly into smelting furnaces or mixed with other charge materials.
Raw material handling and transport
An essential part of the secondary lead smelting process is the handling, transportation and storage of raw material. Materials are transported by fork-lifts, front-end loaders or mechanical conveyors (screw, bucket elevator or belt). The primary method of material transporting in the secondary lead industry is mobile equipment.
Some common mechanical conveyance methods which are used by secondary lead smelters include: belt conveying systems that can be used to transport furnace feed material from storage areas to the furnace charring area; screw conveyors for transporting flue dust from the baghouse to an agglomeration furnace or a storage area or bucket elevators and drag chains/lines.
Smelting
The smelting operation at a secondary lead smelter involves the reduction of lead-bearing scrap into metallic lead in a blast furnace or reverberatory.
Blast furnaces are charged with lead-bearing material, coke (fuel) limestone and iron (flux). These materials are fed into the furnace at the top of the furnace shaft or through a charge door in the side of the shaft neat the top of the furnace. Some environmental hazards associated with blast furnace operations are metal fumes and particulates (especially lead and antimony), heat, noise and carbon monoxide. A variety of charge material conveying mechanisms are used in the secondary lead industry. The skip hoist is probably the most common. Other devices in use include vibratory hoppers, belt conveyors and bucket elevators.
Blast furnace tapping operations involve removing the molten lead and slag from the furnace into moulds or ladles. Some smelters tap metal directly into a holding kettle which keeps the metal molten for refining. The remaining smelters cast the furnace metal into blocks and allow the blocks to solidify.
Blast air for the combustion process enters the blast furnace through tuyères which occasionally begin to fill with accretions and must be physically punched, usually with a steel rod, to keep them from being obstructed. The conventional method to accomplish this task is to remove the cover of the tuyères and insert the steel rod. After the accretions have been punched, the cover is replaced.
Reverberatory furnaces are charged with lead-bearing raw material by a furnace charging mechanism. Reverberatory furnaces in the secondary lead industry typically have a sprung arch or hanging arch constructed of refractory brick. Many of the contaminants and physical hazards associated with reverberatory furnaces are similar to those of blast furnaces. Such mechanisms can be a hydraulic ram, a screw conveyor or other devices similar to those described for blast furnaces.
Reverberatory furnace tapping operations are very similar to blast-furnace tapping operations.
Refining
Lead refining in secondary lead smelters is conducted in indirect fired kettles or pots. Metal from the smelting furnaces is typically melted in the kettle, then the content of trace elements is adjusted to produce the desired alloy. Common products are soft (pure) lead and various alloys of hard (antimony) lead.
Virtually all secondary lead refining operations employ manual methods for adding alloying materials to the kettles and employ manual drossing methods. Dross is swept to the rim of the kettle and removed by shovel or large spoon into a container.
Table 3 lists exposures and controls for lead reclamation operations.
Table 3. Engineering/administrative controls for lead, by operation
|
Process equipment |
Exposures |
Engineering/administrative controls |
|
Vehicles |
Lead dust from roads and splashing water containing lead |
Water washdown and keeping areas wetted down. Operator training, prudent work practices and good housekeeping are key elements in minimizing lead emissions when operating mobile equipment. Enclose equipment and provide a positive pressure filtered air system. |
|
Conveyors |
Lead dust |
It is also preferable to equip belt conveyor systems with self-cleaning tail pulleys or belt wipes if they are used to transport furnace feed materials or flue dusts. |
|
Battery decasing |
Lead dust, acid mists |
Local exhaust ventilation, general area ventilation |
|
Charge preparation |
Lead dust |
Local exhaust ventilation, general area ventilation |
|
Blast furnace |
Metal fumes and particulates (lead, antimony), heat and noise, carbon monoxide |
Local exhaust ventilation, general area ventilation, work/rest regimen, fluids, isolation of noise source; PPE—respiratory protection and hearing protection |
|
Reverberatory furnace |
Metal fumes and particulates (lead, antimony), heat and noise |
Local exhaust ventilation, general area ventilation, work/rest regimen, fluids, isolation of noise source; PPE—respiratory protection and hearing protection |
|
Refining |
Lead particulates and possibly alloying metals and fluxing agents, noise |
Local exhaust ventilation, general area ventilation; PPE—hearing protection |
|
Casting |
Lead particulates and possibly alloying metals |
Local exhaust ventilation, general area ventilation |
Zinc reclamation
The secondary zinc industry utilizes new clippings, skimmings and ashes, die-cast skimmings, galvanizers’ dross, flue dust and chemical residue as sources of zinc. Most of the new scrap processed is zinc- and copper-based alloys from galvanizing and die-casting pots. Included in the old scrap category are old zinc engravers’ plates, die castings, and rod and die scrap. The processes are as follows:
- Reverberatory sweating. Sweating furnaces are used to separate zinc from other metals by controlling the furnace temperature. Scrap die-cast products, such as automobile grilles and licence plate frames, and zinc skins or residues are starting materials for the process. The scrap is charged to the furnace, flux is added and the contents melted. The high-melting residue is removed and the molten zinc flows out of the furnace directly to subsequent processes, such as melting, refining or alloying, or to collecting vessels. Metal contaminants include zinc, aluminium, copper, iron, lead, cadmium, manganese and chromium. Other contaminants are fluxing agents, sulphur oxides, chlorides and fluorides.
- Rotary sweating. In this process zinc scrap, die-cast products, residues and skimmings are charged to a direct-fired furnace and melted. The melt is skimmed, and zinc metal is collected in kettles situated outside the furnace. Unmeltable material, the slag, is then removed prior to recharging. The metal from this process is sent to distillation or alloying process. Contaminants are similar to those of reverberatory sweating.
- Muffle sweating and kettle (pot) sweating. In these processes zinc scrap, die-vapour-cast products, residues and skimmings are charged to the muffle furnace, the material sweated and the sweated zinc is sent to refining or alloying processes. The residue is removed by a shaker screen which separates the dross from the slag. Contaminants are similar to those of reverberatory sweating.
- Crushing/screening. Zinc residues are pulverized or crushed to break down physical bonds between metallic zinc and contaminant fluxes. The reduced material is then separated in a screening or pneumatic classification step. Crushing can produce zinc oxide and minor amounts of heavy metals and chlorides.
- Sodium carbonate leaching. Residues are chemically treated to leach out and convert zinc to zinc oxide. The scrap is first crushed and washed. In this step, the zinc is leached out of the material. The aqueous portion is treated with sodium carbonate, causing zinc to precipitate. The precipitate is dried and calcined to yield crude zinc oxide. The zinc oxide is then reduced to zinc metal. Various zinc salt contaminants can be produced.
- Kettle (pot), crucible, reverberatory, electric induction melting. The scrap is charged to the furnace and fluxes are added. The bath is agitated to form a dross that can be skimmed from the surface. After the furnace has been skimmed the zinc metal is poured into ladles or moulds. Zinc oxide fumes, ammonia and ammonium chloride, hydrogen chloride and zinc chloride can be produced.
- Alloying. The function of this process is to produce zinc alloys from pre-treated scrap zinc metal by adding to it in a refining kettle fluxes and alloying agents either in the solidified or molten form. The contents are then mixed, the dross skimmed, and the metal is cast into various shapes. Particulates containing zinc, alloying metals, chlorides, non-specific gases and vapours, as well as heat, are potential exposures.
- Muffle distillation. The muffle distillation process is used to reclaim zinc from alloys and to manufacture pure zinc ingots. The process is semi-continuous which involves charging molten zinc from a melting pot or sweating furnace to the muffle section and vaporizing the zinc and condensing the vaporized zinc and tapping from the condenser to moulds. The residue is removed periodically from the muffle.
- Retort distillation/oxidation and muffle distillation/oxidation. The product of the retort distillation/oxidation and muffle distillation/oxidation processes is zinc oxide. The process is similar to retort distillation through the vaporization step, but, in this process, the condenser is bypassed and combustion air is added. The vapour is discharged through an orifice into an air stream. Spontaneous combustion occurs inside a refractory vapour-lined chamber. The product is carried by the combustion gases and excess air into a baghouse where the product is collected. Excess air is present to insure complete oxidation and to cool the product. Each of these distillation processes can lead to zinc oxide fume exposures, as well as other metal particulate and oxides of sulphur exposure.
Table 4 lists exposures and controls for zinc reclamation operations.
Table 4. Engineering/administrative controls for zinc, by operation
|
Process equipment |
Exposures |
Engineering/administrative controls |
|
Reverberatory sweating |
Particulates containing zinc, aluminium, copper, iron, lead, cadmium, manganese and chromium, contaminants from fluxing agents, sulphur oxides, chlorides and fluorides |
Local exhaust ventilation, general area ventilation, heat stress–work/rest regimen, fluids |
|
Rotary sweating |
Particulates containing zinc, aluminium, copper, iron, lead, cadmium, manganese and chromium, contaminants from fluxing agents, sulphur oxides, chlorides and fluorides |
Local exhaust ventilation, general area ventilation, work/rest regimen, fluids |
|
Muffle sweating and kettle (pot) sweating |
Particulates containing zinc, aluminium, copper, iron, lead, cadmium, manganese and chromium, contaminants from fluxing agents, sulphur oxides, chlorides and fluorides |
Local exhaust ventilation, general area ventilation, work/rest regimen, fluids |
|
Crushing/screening |
Zinc oxide, minor amounts of heavy metals, chlorides |
Local exhaust ventilation, general area ventilation |
|
Sodium carbonate leaching |
Zinc oxide, sodium carbonate, zinc carbonate, zinc hydroxide, hydrogen chloride, zinc chloride |
Local exhaust ventilation, general area ventilation |
|
Kettle (pot) melting crucible, reverberatory, electric induction melting |
Zinc oxide fumes, ammonia, ammonia chloride, hydrogen chloride, zinc chloride |
Local exhaust ventilation, general area ventilation, work/rest regimen, fluids |
|
Alloying |
Particulates containing zinc, alloying metals, chlorides; non-specific gases and vapours; heat |
Local exhaust ventilation, general area ventilation, work/rest regimen, fluids |
|
Retort distillation, retort distillation/oxidation and muffle distillation |
Zinc oxide fumes, other metal particulates, oxides of sulphur |
Local exhaust ventilation, general area ventilation, work/rest regimen, fluids |
|
Graphite rod resistor distillation |
Zinc oxide fumes, other metal particulates, oxides of sulphur |
Local exhaust ventilation, general area ventilation, work/rest regimen, fluids |
Magnesium reclamation
Old scrap is obtained from sources such as scrap automobile and aircraft parts and old and obsolete lithographic plates, as well as some sludges from primary magnesium smelters. New scrap consists of clippings, turnings, borings, skimmings, slags, drosses and defective articles from sheet mills and fabrication plants. The greatest danger in handling magnesium is that of fire. Small fragments of the metal can readily be ignited by a spark or flame.
- Hand sorting. This process is used to separate magnesium and magnesium-alloy fractions from other metals present in the scrap. The scrap is spread out manually, sorted on the basis of weight.
- Open pot melting. This process is used to separate magnesium from contaminants in the sorted scrap. Scrap is added to a crucible, heated and a flux consisting of a mixture of calcium, sodium and potassium chlorides is added. The molten magnesium is then cast into ingots.
Table 5 lists exposures and controls for magnesium reclamation operations.
Table 5. Engineering/administrative controls for magnesium, by operation
|
Process equipment |
Exposures |
Engineering/administrative |
|
Scrap sorting |
Dust |
Water washdown |
|
Open pot melting |
Fumes and dust, a high potential for fires |
Local exhaust ventilation and general area ventilation and work practices |
|
Casting |
Dust and fumes, heat and a high potential for fires |
Local exhaust ventilation, general area ventilation, work/rest regimen, fluids |
Mercury reclamation
The major sources for mercury are dental amalgams, scrap mercury batteries, sludges from electrolytic processes that use mercury as a catalyst, mercury from dismantled chlor-alkali plants and mercury-containing instruments. Mercury vapour can contaminate each of these processes.
- Crushing. The crushing process is used to release residual mercury from metal, plastic and glass containers. After the containers are crushed, the contaminated liquid mercury is sent to the filtering process.
- Filtration. Insoluble impurities such as dirt are removed by passing the mercury-vapour bearing scrap through a filter media. The filtered mercury is fed to the oxygenation process and the solids which do not pass through the filters are sent to retort distillation.
- Vacuum distillation. Vacuum distillation is employed to refine contaminated mercury when the vapour pressures of the impurities are substantially lower than that of mercury. Mercury charge is vaporized in a heating pot and the vapours are condensed using a water-cooled condenser. Purified mercury is collected and sent to the bottling operation. The residue remaining in the heating pot is sent to the retorting process to recover the trace amounts of mercury that were not recovered in the vacuum distillation process.
- Solution purification. This process removes metallic and organic contaminants by washing the raw liquid mercury with a dilute acid. The steps involved are: leaching the raw liquid mercury with dilute nitric acid to separate metallic impurities; agitating the acid-mercury with compressed air to provide good mixing; decanting to separate the mercury from the acid; washing with water to remove the residual acid; and filtering the mercury in a medium such as activated carbon or silica gel to remove the last traces of moisture. In addition to mercury vapour there can be exposure to solvents, organic chemicals and acid mists.
- Oxygenation. This process refines the filtered mercury by removing metallic impurities by oxidation with sparging air. The oxidation process involves two steps, sparging and filtering. In the sparging step, contaminated mercury is agitated with air in a closed vessel to oxidize the metallic contaminants. After sparging, the mercury is filtered in a charcoal bed to remove the solid metal oxides.
- Retorting. The retorting process is used to produce pure mercury by volatilizing the mercury found in solid mercury-bearing scrap. The steps involved in retorting are: heating the scrap with an external heat source in a closed still pot or stack of trays to vaporize the mercury; condensing the mercury vapour in water-cooled condensers; collecting the condensed mercury in a collecting vessel.
Table 6 lists exposures and controls for mercury reclamation operations.
Table 6. Engineering/administrative controls for mercury, by operation
|
Process equipment |
Exposures |
Engineering/administrative controls |
|
Crushing |
Volatile mercury |
Local exhaust; PPE—respiratory protection |
|
Filtration |
Volatile mercury |
Local exhaust ventilation; PPE—respiratory protection |
|
Vacuum distillation |
Volatile mercury |
Local exhaust ventilation; PPE—respiratory protection |
|
Solution purification |
Volatile mercury, solvents, organics and acid mists |
Local exhaust ventilation, general area ventilation; PPE—respiratory protection |
|
Oxidation |
Volatile mercury |
Local exhaust ventilation; PPE—respiratory protection |
|
Retorting |
Volatile mercury |
Local exhaust ventilation; PPE—respiratory protection |
Nickel reclamation
The principal raw materials for nickel reclamation are nickel-, copper- and aluminium-vapour based alloys, which can be found as old or new scrap. Old scrap comprises alloys that are salvaged from machinery and airplane parts, while new scrap refers to sheet scrap, turnings and solids which are by-products of the manufacture of alloy products. The following steps are involved in nickel reclamation:
- Sorting. The scrap is inspected and manually separated from the non-metallic and non-nickel materials. Sorting produces dust exposures.
- Degreasing. Nickel scrap is degreased by using trichloroethylene. The mixture is filtrated or centrifuged to separate the nickel scrap. The spent solvent solution of trichloroethylene and grease goes through a solvent recovery system. There can be solvent exposure during degreasing.
- Smelting (electric arc or rotary reverberatory) furnace. Scrap is charged to an electric arc furnace and a reducing agent added, usually lime. The charge is melted and is either cast into ingots or sent directly to a reactor for additional refining. Fumes, dust, noise and heat exposures are possible.
- Reactor refining. The molten metal is introduced into a reactor where cold-base scrap and pig nickel are added, followed by lime and silica. Alloying materials such as manganese, columbium or titanium are then added to produce the desired alloy composition. Fumes, dust, noise and heat exposures are possible.
- Ingot casting. This process involves casting the molten metal from the smelting furnace or the refining reactor into ingots. The metal is poured into moulds and allowed to cool. The ingots are removed from the moulds. Heat and metal fume exposures are possible.
Exposures and control measures for nickel reclamation operations are listed in table 7.
Table 7. Engineering/administrative controls for nickel, by operation
|
Process equipment |
Exposures |
Engineering/administrative controls |
|
Sorting |
Dust |
Local exhaust and solvent substitution |
|
Degreasing |
Solvent |
Local exhaust ventilation and solvent substitution and/or recovery, general area ventilation |
|
Smelting |
Fumes, dust, noise, heat |
Local exhaust ventilation, work/rest regimen, fluids; PPE—respiratory protection and hearing protection |
|
Refining |
Fumes, dust, heat, noise |
Local exhaust ventilation, general area ventilation, work/rest regimen, fluids; PPE—respiratory protection and hearing protection |
|
Casting |
Heat, metal fumes |
Local exhaust ventilation, general area ventilation, work/rest regimen, fluids |
Precious metals reclamation
The raw materials for the precious metal industry consist of both old and new scrap. Old scrap includes electronic components from obsolete military and civilian equipment and scrap from the dental industry. New scrap is generated during the fabrication and manufacturing of precious metal products. The products are the elemental metals such as gold, silver, platinum and palladium. Precious metal processing includes the following steps:
- Hand sorting and shredding. Precious metal-bearing scrap is hand sorted and crushed and shredded in a hammer mill. Hammer mills are noisy.
- Incineration process. Sorted scrap is incinerated to remove paper, plastic and organic liquid contaminants. Organic chemicals, combustion gases and dust exposures are possible.
- Blast-furnace smelting. Treated scrap is charged to a blast furnace, along with coke, flux and recycled slag metal oxides. The charge is melted and slagged, producing black copper which contains the precious metals. The hard slag that is formed contains most of the slag impurities. Dust and noise may be present.
- Converter smelting. This process is designed to further purify the black copper by blowing air through the melt in a converter. Slag-containing metal contaminants are removed and recycled to the blast furnace. The copper bullion containing the precious metals is cast into moulds.
- Electrolytic refining. Copper bullion serves as the anode of an electrolytic cell. Pure copper thus plates out on the cathode while the precious metals fall to the bottom of the cell and are collected as slimes. The electrolyte used is copper sulphate. Acid mist exposures are possible.
- Chemical refining. The precious metal slime from the electrolytic refining process is chemically treated to recover the individual metals. Cyanide-based processes are used to recover gold and silver, which can also be recovered by dissolving them in aqua regia solution and/or nitric acid, followed by precipitation with ferrous sulphate or sodium chloride to recover the gold and silver, respectively. The platinum-group metals can be recovered by dissolving them in molten lead, which is then treated with nitric acid and leaves a residue from which the platinum-group metals can be selectively precipitated. The precious metal precipitates are then either melted or ignited in order to collect the gold and silver as grains and the platinum metals as sponge. There can be acid exposures.
Exposures and controls are listed, by operation, in table 8 (see also “Gold smelting and refining”).
Table 8. Engineering/administrative controls for precious metals, by operation
|
Process equipment |
Exposures |
Engineering/administrative controls |
|
Sorting and shredding |
Hammermill is a potential noise hazard |
Noise control material; PPE—hearing protection |
|
Incineration |
Organics, combustion gases and dust |
Local exhaust ventilation and general area ventilation |
|
Blast furnace smelting |
Dust, noise |
Local exhaust ventilation; PPE—hearing protection and respiratory protection |
|
Electrolytic refining |
Acid mists |
Local exhaust ventilation, general area ventilation |
|
Chemical refining |
Acid |
Local exhaust ventilation, general area ventilation; PPE—acid-resistant clothing, chemical goggles and face shield |
Cadmium reclamation
Old cadmium-bearing scrap includes cadmium-plated parts from junked vehicles and boats, household appliances, hardware and fasteners, cadmium batteries, cadmium contacts from switches and relays and other used cadmium alloys. New scrap is normally cadmium vapour bearing rejects and contaminated by-products from industries which handle the metals. The reclamation processes are:
- Pre-treatment. The scrap pre-treatment step involves the vapour degreasing of alloy scrap. Solvent vapours generated by heating recycled solvents are circulated through a vessel containing scrap alloys. The solvent and stripped grease are then condensed and separated with the solvent being recycled. There can be exposure to cadmium dust and solvents.
- Smelting/refining. In the smelting/refining operation, pre-treated alloy scrap or elemental cadmium scrap is processed to remove any impurities and produce cadmium alloy or elemental cadmium. Products of oil and gas combustion exposures and zinc and cadmium dust may be present.
- Retort distillation. Degreased scrap alloy is charged to a retort and heated to produce cadmium vapours which are subsequently collected in a condenser. The molten metal is then ready for casting. Cadmium dust exposures are possible.
- Melting/dezincing. Cadmium metal is charged to a melting pot and heated to the molten stage. If zinc is present in the metal, fluxes and chlorinating agents are added to remove the zinc. Among potential exposures are cadmium fumes and dust, zinc fumes and dust, zinc chloride, chlorine, hydrogen chloride and heat.
- Casting. The casting operation forms the desired product line from the purified cadmium alloy or cadmium metal produced in the previous step. Casting can produce cadmium dust and fumes and heat.
Exposures in cadmium reclamation processes and the necessary controls are summarized in table 9.
Table 9. Engineering/administrative controls for cadmium, by operation
|
Process equipment |
Exposures |
Engineering/administrative controls |
|
Scrap degreasing |
Solvents and cadmium dust |
Local exhaust and solvent substitution |
|
Alloy smelting/refining |
Products of oil and gas combustion, zinc fumes, cadmium dust and fumes |
Local exhaust ventilation and general area ventilation; PPE—respiratory protection |
|
Retort distillation |
Cadmium fumes |
Local exhaust ventilation; PPE—respiratory protection |
|
Melting/dezincing |
Cadmium fumes and dust, zinc fumes and dust, zinc chloride, chlorine, hydrogen chloride, heat stress |
Local exhaust ventilation, general area ventilation, work/rest regimen, fluids; PPE—respiratory protection |
|
Casting |
Cadmium dust and fumes, heat |
Local exhaust ventilation, general area ventilation, work/rest regimen, fluids; PPE—respiratory protection |
Selenium reclamation
Raw materials for this segment are used xerographic copying cylinders and scrap generated during the manufacture of selenium rectifiers. Selenium dusts may be present throughout. Distillation and retort smelting can produce combustion gases and dust. Retort smelting is noisy. Sulphur dioxide mist and acid mist are present in refining. Metal dusts can be produced from casting operations (see table 10).
Table 10. Engineering/administrative controls for selenium, by operation
|
Process equipment |
Exposures |
Engineering/administrative controls |
|
Scrap pretreatment |
Dust |
Local exhaust |
|
Retort smelting |
Combustion gases and dust, noise |
Local exhaust ventilation and general area ventilation; PPE—hearing protection; control of burner noise |
|
Refining |
SO2, acid mist |
Local exhaust ventilation; PPE—chemical goggles |
|
Distillation |
Dust and combustion products |
Local exhaust ventilation, general area ventilation |
|
Quenching |
Metal dust |
Local exhaust ventilation, general area ventilation |
|
Casting |
Selenium fumes |
Local exhaust ventilation, general area ventilation |
The reclamation processes are as follows:
- Scrap pre-treatment. This process separates selenium by mechanical processes such as the hammer mill or shot blasting.
- Retort smelting. This process purifies and concentrates pre-treated scrap in a retort distillation operation by melting the scrap and separating selenium from the impurities by distillation.
- Refining. This process achieves a purification of scrap selenium based on leaching with a suitable solvent such as aqueous sodium sulphite. Insoluble impurities are removed by filtration and the filtrate is treated to precipitate selenium.
- Distillation. This process produces a high vapour purity selenium. The selenium is melted, distilled and the selenium vapours are condensed and transferred as molten selenium to a product formation operation.
- Quenching. This process is used to produce purified selenium shot and powder. The selenium melt is used in producing a shot. The shot is then dried. The steps required to produce powder are the same, except that selenium vapour, rather than molten selenium, is the material which is quenched.
- Casting. This process is used to produce selenium ingots or other shapes from the molten selenium. These shapes are produced by pouring molten selenium into moulds of the proper size and shape and cooling and solidifying the melt.
Cobalt reclamation
The sources of cobalt scrap are super alloy grindings and turnings, and obsolete or worn engine parts and turbine blades. The processes of reclamation are:
- Hand sorting. Raw scrap is hand sorted to identify and separate the cobalt-base, nickel-base and non-processable components. This is a dusty operation.
- Degreasing. Sorted dirty scrap is charged to a degreasing unit where vapours of perchloroethylene are circulated. This solvent removes the grease and oil on the scrap. The solvent-oil-grease vapour mixture is then condensed and the solvent is recovered. Solvent exposures are possible.
- Blasting. Degreased scrap is blasted with grit to remove dirt, oxides and rust. Dusts can be present, depending on the grit used.
- Pickling and chemical treatment process. Scrap from the blasting operation is treated with acids to remove residual rust and oxide contaminants. Acid mists are a possible exposure.
- Vacuum melting. Cleaned scrap is charged to a vacuum furnace and melted by electric arc or induction furnace. There can be exposure to heavy metals.
- casting. Molten alloy is cast into ingots. Heat stress is possible.
See table 11 for a summary of exposures and controls for cobalt reclamation.
Table 11. Engineering/administrative controls for cobalt, by operation
|
Process equipment |
Exposures |
Engineering/administrative controls |
|
Hand sorting |
Dust |
Water washdown |
|
Degreasing |
Solvents |
Solvent recovery, local exhaust and solvent substitution |
|
Blasting |
Dust—toxicity dependent upon the grit used |
Local exhaust ventilation; PPE for physical hazard and respiratory protection depending on grit used |
|
Pickling and chemical treatment process |
Acid mists |
Local exhaust ventilation, general area ventilation; PPE—respiratory protection |
|
Vacuum melting |
Heavy metals |
Local exhaust ventilation, general area ventilation |
|
Casting |
Heat |
Local exhaust ventilation, general area ventilation, work/rest regimen, fluids |
Tin reclamation
The major sources of raw materials are tin-plated steel trimmings, rejects from tin-can manufacturing companies, rejected plating coils from the steel industry, tin drosses and sludges, solder drosses and sludges, used bronze and bronze rejects and metal type scrap. Tin dust and acid mists can be found in many of the processes.
- Dealuminization. In this process hot sodium hydroxide is used to leach aluminium from tin-can scrap by contacting the scrap with hot sodium hydroxide, separating the sodium aluminate solution from the scrap residue, pumping the sodium aluminate to a refining operation to recover soluble tin and recovering the dealuminized tin scrap for feed.
- Batch mixing. This process is a mechanical operation which prepares a feed suitable for charging to the smelting furnace by mixing drosses and sludges with a significant tin content.
- Chemical detinning. This process extracts the tin in scrap. A hot solution of sodium hydroxide and sodium nitrite or nitrate is added to dealuminized or raw scrap. Draining and pumping the solution to a refining/casting process are performed when the detinning reaction is complete. The detinned scrap is then washed.
- Dross smelting. This process is used to partially purify drosses and produce crude furnace metal by melting the charge, tapping the crude furnace metal and tapping the mattes and slags.
- Dust leaching and filtration. This process removes the zinc and chlorine values from flue dust by leaching with sulphuric acid to remove zinc and chlorine, filtering the resulting mixture to separate the acid and dissolved zinc and chlorine from the leached dust, drying the leached dust in a dryer and conveying the tin and lead rich dust back to the batch mixing process.
- Settling and leaf filtration. This process purifies the sodium stannate solution produced in the chemical detinning process. Impurities such as silver, mercury, copper, cadmium, some iron, cobalt and nickel are precipitated as sulphides.
- Evapocentrifugation. The sodium stannate is concentrated from the purified solution by evaporation, crystallization of sodium stannate and recovery of sodium stannate is by centrifugation.
- Electrolytic refining. This process produces cathodic-pure tin from the purified sodium stannate solution by passing the sodium stannate solution through electrolytic cells, removing the cathodes after the tin has been deposited and stripping the tin from the cathodes.
- Acidification and filtration. This process produces a hydrated tin oxide from the purified sodium stannate solution. This hydrated oxide can either be processed to produce the anhydrous oxide or smelted to produce elemental tin. The hydrated oxide is neutralized with sulphuric acid to form the hydrated tin oxide and filtered to separate the hydrate as filter cake.
- Fire refining. This process produces purified tin from the cathodic tin by melting the charge, removing the impurities as slag and dross, pouring the molten metal and casting the metallic tin.
- Smelting. This process is used to produce tin when electrolytic refining is not feasible. This is accomplished by reducing the hydrated tin oxide with a reducing agent, melting the tin metal formed, skimming the dross, pouring the molten tin and casting the molten tin.
- Calcining. This process converts the hydrated tin oxides to anhydrous stannic oxide by calcining the hydrate and removing and packaging the stannic oxides.
- Kettle refining. This process is used to purify crude furnace metal by charging a preheated kettle with it, drying the dross to remove the impurities as slag and matte, fluxing with sulphur to remove copper as matte, fluxing with aluminium to remove antimony and casting molten metal into desired shapes.
See table 12 for a summary of exposures and controls for tin reclamation.
Table 12. Engineering/administrative controls for tin, by operation
|
Process equipment |
Exposures |
Engineering/administrative controls |
|
Dealuminization |
Sodium hydroxide |
Local exhaust; PPE—chemical goggles and/or face shield |
|
Batch mixing |
Dust |
Local exhaust ventilation and general area ventilation |
|
Chemical detinning |
Caustic |
Local exhaust ventilation; PPE—chemical goggles and/or face shield |
|
Dross smelting |
Dust and heat |
Local exhaust ventilation, general area ventilation, work/rest regimen, fluids |
|
Dust leaching and filtration |
Dust |
Local exhaust ventilation, general area ventilation |
|
Settling and leaf filtration |
None identified |
None identified |
|
Evapocentrifugation |
None identified |
None identified |
|
Electrolytic refining |
Acid mist |
Local exhaust ventilation and general area ventilation; PPE—chemical goggles and/or face shield |
|
Acidification and filtration |
Acid mists |
Local exhaust ventilation and general area ventilation; PPE—chemical goggles and/or face shield |
|
Fire refining |
Heat |
Work/rest regimen, PPE |
|
Smelting |
Combustion gases, fumes and dust, heat |
Local exhaust ventilation and general area ventilation, work/rest regimen, PPE |
|
Calcining |
Dust, fumes, heat |
Local exhaust ventilation and general area ventilation work/rest regimen, PPE |
|
Kettle refining |
Dust, fumes, heat |
Local exhaust ventilation and general area ventilation, work/rest regimen, PPE |
Titanium reclamation
The two primary sources of titanium scrap are the home and titanium consumers. Home scrap which is generated by the milling and manufacturing of titanium products includes trim sheets, plank sheet, cuttings, turnings and borings. Consumer scrap consists of recycled titanium products. The reclamation operations include:
- Degreasing. In this process sized scrap is treated with vapourized organic solvent (e.g., trichloroethylene). Contaminant grease and oil are stripped from the scrap by the solvent vapour. The solvent is recirculated until it can no longer has an ability to degrease. Spent solvent can then be regenerated. The scrap can also be degreased by steam and detergent.
- Pickling. The acid-pickling process removes oxide scale from the degreasing operation by leaching with a solution of hydrochloric and hydrofluoric acids. The acid treatment scrap is washed with water and dried.
- Electrorefining. Electrorefining is a titanium scrap pre-treatment process which electro-refines scrap in a fused salt.
- Smelting. Pre-treated titanium scrap and alloying agents are melted in a electric-arc vacuum furnace to form a titanium alloy. The input materials include pre-treated titanium scrap and alloying materials such as aluminium, vanadium, molybdenum, tin, zirconium, palladium, columbium and chromium.
- Casting. Molten titanium is poured into moulds. The titanium solidifies into a bar called an ingot.
Controls for exposures in titanium reclamation procedures are listed in table 13.
Table 13. Engineering/administrative controls for titanium, by operation
|
Process equipment |
Exposures |
Engineering/administrative controls |
|
Solvent degreasing |
Solvent |
Local exhaust and solvent recovery |
|
Pickling |
Acids |
Face shields, aprons, long sleeves, safety glasses or goggles |
|
Electrorefining |
None known |
None known |
|
Smelting |
Volatile metals, noise |
Local exhaust ventilation and control of noise from burners; PPE—hearing protection |
|
Casting |
Heat |
PPE |
Environmental Issues in Metal Finishing and Industrial Coatings
Metal Finishing
The surface treatment of metals increases their durability and improves their appearance. A single product may undergo more than one surface treatment—for example, an auto body panel may be phosphated, primed and painted. This article deals with the processes used for surface treatment of metals and the methods used to reduce their environmental impact.
Operating a metal finishing business requires cooperation between company management, employees, government and the community to effectively minimize the environmental effect of the operations. Society is concerned with the amount and the long-term effects of pollution entering the air, water and land environment. Effective environmental management is established through detailed knowledge of all elements, chemicals, metals, processes and outputs.
Pollution prevention planning shifts the environmental management philosophy from reacting to problems to anticipating solutions focusing on chemical substitution, process change and internal recycling, using the following planning sequence:
- Initiate pollution prevention across all aspects of the business.
- Identify waste streams.
- Set priorities for action.
- Establish root cause of the waste.
- Identify and implement changes that reduce or eliminate the waste.
- Measure the results.
Continuous improvement is achieved by setting new priorities for action and repeating the sequence of actions.
Detailed process documentation will identify the waste streams and allow priorities to be set for waste reduction opportunities. Informed decisions about potential changes will encourage:
- easy and practical operational improvements
- process changes involving customers and suppliers
- changes to less harmful activities where possible
- reuse and recycling where change is not practical
- using landfilling of hazardous wastes only as a last resort.
Major processes and standard operating processes
Cleaning is required because all metal finishing processes require that parts to be finished be free from organic and inorganic soils, including oils, scale, buffing and polishing compounds. The three basic types of cleaners in use are solvents, vapour degreasers and alkaline detergents.
Solvents and vapour degreasing cleaning methods have been almost totally replaced by alkaline materials where the subsequent processes are wet. Solvents and vapour degreasers are still in use where parts must be clean and dry with no further wet processing. Solvents such as terpenes are in some instances replacing volatile solvents. Less toxic materials such as 1,1,1-trichloroethane have been substituted for more hazardous materials in vapour degreasing (although this solvent is being phased out as an ozone depleter).
Alkaline cleaning cycles usually include a soak immersion followed by an anodic electroclean, followed by a weak acid immersion. Non-etching, non-silicated cleaners are typically used to clean aluminium. The acids are typically sulphuric, hydrochloric and nitric.
Anodizing, an electrochemical process to thicken the oxide film on the metal surface (frequently applied to aluminium), treats the parts with dilute chromic or sulphuric acid solutions.
Conversion coating is used to provide a base for subsequent painting or to passivate for protection against oxidation. With chromating, parts are immersed in a hexavalent chrome solution with active organic and inorganic agents. For phosphating, parts are immersed in dilute phosphoric acid with other agents. Passivating is accomplished through immersion in nitric acid or nitric acid with sodium dichromate.
Electroless plating involves a deposition of metal without electricity. Copper or nickel electroless deposition is used in the manufacture of printed circuit boards.
Electroplating involves the deposition of a thin coat of metal (zinc, nickel, copper, chromium, cadmium, tin, brass, bronze, lead, tin-lead, gold, silver and other metals such as platinum) on a substrate (ferrous or non-ferrous). Process baths include metals in solution in acid, alkaline neutral and alkaline cyanide formulations (see figure 1).
Figure 1. Inputs and outputs for a typical electroplating line
Chemical milling and etching are controlled dissolution immersion processes using chemical reagents and etchants. Aluminium is typically etched in caustic prior to anodizing or chemically brightened in a solution which could contain nitric, phosphoric and sulphuric acids.
Hot-dip coatings involve the application of metal to a workpiece by immersion in molten metal (zinc or tin galvanizing of steel).
Good management practices
Important safety, health and environmental improvements can be achieved through process improvements, such as:
- using counter-current rinsing and conductivity controls
- increasing drainage time
- using more or better wetting agents
- keeping process temperatures as high as possible to lower viscosity, thus increasing drag-out recovery (i.e., recovery of solution left on metal)
- using air agitation in rinsing to increase rinsing efficiency
- using plastic balls in plating tanks to reduce misting
- using improved filtration on plating tanks to reduce the frequency of purification treatment
- placing a curb around all process areas to contain spills
- using separate treatments for recoverable metals such as nickel
- installing recovery systems such as ion exchange, atmospheric evaporation, vacuum evaporation, electrolytic recovery, reverse osmosis and electrodialysis
- complementing drag-out recovery systems with reductions in drag-in of contaminants and improved cleaning systems
- using modern inventory controls to reduce waste and workplace hazards
- applying standard procedures (i.e., written procedures, regular operating reviews and sound operating logs) to provide the basis for a sound environmental management structure.
Environmental planning for specific wastes
Specific waste streams, usually spent plating solutions, can be reduced by:
- Filtration. Cartridge or diatomaceous earth filters can be used to remove the accumulation of solids, which reduce the efficiency of the process.
- Carbon treatment can be used to remove organic contaminants (most commonly applied in nickel plating, copper electroplating and zinc and cadmium plating).
- Purified water. The natural contaminants in water make-up and rinses (e.g., calcium, iron, magnesium, manganese, chlorine and carbonates) can be removed by using deionization, distillation or reverse osmosis. Improving rinse water efficiency reduces the volume of bath sludges requiring treatment.
- Cyanide bath carbonate freezing. Lowering the bath temperature to –3 °C crystallizes the carbonates formed in cyanide bath by the breakdown of cyanide, excessive anode current densities and the adsorption of carbon dioxide from the air and facilitates their removal.
- Precipitation. Removal of metal contaminants entering the bath as impurities in anodes can be achieved through precipitation with barium cyanide, barium hydroxide, calcium hydroxide, calcium sulphate or calcium cyanide.
- Hexavalent chrome alternatives. Hexavalent chromium can be replaced with trivalent chromium plating solutions for decorative plating. Chrome conversion coatings for paint pretreatments can sometimes be replaced by non-chrome conversion coatings or no-rinse chrome chemistries.
- Non-chelated process chemistries. Instead of chelators being added to process baths to control the concentration of free ions in the solution, non-chelated process chemistries can be used so that it may not be necessary to keep metals in solution. These metals can be allowed to precipitate and can be removed by continuous filtration.
- Non-cyanide process chemicals. Waste streams containing free cyanide are typically treated using hypochlorite or chlorine to accomplish oxidation, and complex cyanides are commonly precipitated using ferrous sulphate. Using non-cyanide process chemistries both eliminates a treatment step and reduces the sludge volume.
- Solvent degreasing. Hot alkaline cleaning baths can be used in place of solvent degreasing of workpieces before processing. The effectiveness of alkaline cleaners can be enhanced by applying electrocurrent or ultrasonics. The benefits of avoiding solvent vapours and sludges often outweigh any additional operating costs.
- Alkaline cleaners. Having to discard alkaline cleaners when the accumulation of oil, grease and soils from use reaches a level which impairs the cleaning efficiency of the bath can be avoided by using skimming devices to remove free-floating oils, settling devices or cartridge filters to remove particulates and oil-water coalescers and by using microfiltration or ultrafiltration to remove emulsified oils.
- Drag-out reduction. Reducing the volume of drag-out from process baths serves to reduce the amount of valuable process chemicals that contaminates the rinse water, which in turn reduces the amount of sludge that is generated by a conventional metal precipitation treatment process.
Several methods of reducing drag-out include:
- Process bath operating concentration. The chemical concentration should be kept as low as possible to minimize the viscosity (for quicker draining) and the quantity of chemicals (in the film).
- Process bath operating temperature. The viscosity of the process solution can be reduced by increasing the bath temperature.
- Wetting agents. The surface tension of the solution can be reduced by adding wetting agents to the process bath.
- Workpiece positioning. The workpiece should be positioned on the rack so that the adhering film drains freely and does not get trapped in grooves or cavities.
- Withdrawal or drainage time. The faster a workpiece is removed from the process bath, the thicker the film on the workpiece surface.
- Air knives. Blowing air at the workpiece as the workpiece rack is raised above the process tank can improve drainage and drying.
- Spray rinses. These can be used above heated baths so that the rinse flow rate equals the evaporation rate of the tank.
- Plating baths. Carbonates and organic contaminants should be removed to prevent accumulation of contamination that increases the viscosity of the plating bath.
- Drainage boards. The spaces between process tanks should be covered with drainage boards to capture process solutions and to return them to the process bath.
- Drag-out tanks. The workpieces should be placed in drag-out tanks (“static rinse” tanks) before the standard rinsing operation.
Drag-out recovery of chemicals uses a variety of technologies. These include:
- Evaporation. Atmospheric evaporators are most common, and vacuum evaporators offer energy savings.
- Ion exchange is used for chemical recovery of rinse water.
- Electrowinning. This is an electrolytic process whereby the dissolved metals in the solution are reduced and deposited on the cathode. The deposited metal is then recovered.
- Electrodialysis. This utilizes ion-permeable membranes and applied current in order to separate ionic species from the solution.
- Reverse osmosis. This utilizes a semi-permeable membrane to produce purified water and a concentrated ionic solution. High pressure is used to force the water through the membrane, while most dissolved salts are retained by the membrane.
Rinse water
Most of the hazardous waste produced in a metal finishing facility comes from waste water generated by the rinsing operations that follow cleaning and plating. By increasing rinse efficiency, a facility can significantly reduce waste water flow.
Two basic strategies improve rinsing efficiency. First, turbulence can be generated between the workpiece and the rinse water through spray rinses and rinse water agitation. Movement of the rack or forced water or air are used. Second, the contact time between the workpiece and the rinse water can be increased. Multiple rinse tanks set countercurrent in series will reduce the amount of rinse water used.
Industrial Coatings
The term coatings includes paints, varnishes, lacquers, enamels and shellacs, putties, wood fillers and sealers, paint and varnish removers, paint brush cleaners and allied paint products. Liquid coatings contain pigments and additives dispersed in a liquid binder and solvent mixture. Pigments are inorganic or organic compounds that provide coating colour and opacity and influence coating flow and durability. Pigments often contain heavy metals such as cadmium, lead, zinc, chromium and cobalt. The binder increases coating adhesiveness, cohesiveness and consistency and is the primary component that remains on the surface when coating is completed. Binders include a variety of oils, resins, rubbers and polymers. Additives such as fillers and extenders may be added to coatings to reduce manufacturing costs and increase coating durability.
The types of organic solvents used in coatings include aliphatic hydrocarbons, aromatic hydrocarbons, esters, ketones, glycol ethers and alcohols. Solvents disperse or dissolve the binders and decrease the coating viscosity and thickness. Solvents used in coatings formulations are hazardous because many are human carcinogens and are flammable or explosive. Most solvents contained in a coating evaporate when the coating cures, which generates volatile organic compound (VOC) emissions. VOC emissions are becoming increasingly regulated because of the negative effects on human health and the environment. Environmental concerns associated with conventional ingredients, coating application technologies and coating wastes are a driving force for developing pollution prevention alternatives.
Most coatings are used on architectural, industrial or special products. Architectural coatings are used in buildings and building products and for decorative and protective services such as varnishes to protect wood. Industrial facilities incorporate coating operations in various production processes. The automotive, metal can, farm machinery, coil coating, wood and metal furniture and fixtures, and household appliance industries are the major industrial coatings consumers.
Design of a coating formulation depends on the purpose of the coating application. Coatings provide aesthetics, and corrosion and surface protection. Cost, function, product safety, environmental safety, transfer efficiency and drying and curing speed determine formulations.
Coating processes
There are five operations comprising most coating processes: raw materials handling and preparation, surface preparation, coating, equipment cleaning and waste management.
Raw material handling and preparation
Raw material handling and preparation involves inventory storage, mixing operations, thinning and adjusting of coatings and raw material transfer through the facility. Monitoring and handling procedures and practices are needed to minimize the generation of wastes from spoilage, off specification and improper preparation that can result from excessive thinning and consequent wastage. Transfer, whether manual or through a piped system, must be scheduled to avoid spoilage.
Surface preparation
The type of surface preparation technique used depends on the surface being coated—previous preparation, amount of soil, grease, the coating to be applied and the surface finish required. Common preparation operations include degreasing, precoating or phosphating and coating removal. For metal finishing purposes, degreasing involves solvent wiping, cold cleaning or vapour degreasing with halogenated solvents, aqueous alkaline cleaning, semi-aqueous cleaning or aliphatic hydrocarbon cleaning to remove organic soil, dirt, oil and grease. Acid pickling, abrasive cleaning or flame cleaning are used to remove mill scale and rust.
The most common preparation operation for metal surfaces, other than cleaning, is phosphate coating, used to promote adhesion of organic coatings onto metal surfaces and retard corrosion. Phosphate coatings are applied by immersing or spraying metal surfaces with zinc, iron or manganese phosphate solution. Phosphating is a surface finishing process similar to electroplating, consisting of a series of process chemical and rinse baths in which parts are immersed to achieve the desired surface preparation. See the article “Surface treatment of metals” in this chapter.
Coating removal, chemical or mechanical, is conducted on surfaces that require recoating, repair or inspection. The most common chemical coating removal method is solvent stripping. These solutions usually contain phenol, methylene chloride and an organic acid to dissolve the coating from the coated surface. A final water wash to remove the chemicals can generate large quantities of wastewater. Abrasive blasting is the common mechanical process, a dry operation that uses compressed air to propel a blasting medium against the surface to remove the coating.
Surface preparation operations affect the quantity of waste from the specific preparation process. If the surface preparation is inadequate, resulting in poor coating, then removal of the coating and recoating adds to waste generation.
Coating
The coating operation involves transferring the coating to the surface and curing the coating on the surface. Most coating technologies fall into 1 of 5 basic categories: dip coating, roll coating, flow coating, spray coating, and the most common technique, air-atomized spray coating using solvent-based coatings.
Air-atomized spray coatings are usually conducted in a controlled environment because of solvent emissions and overspray. Overspray control devices are fabric filters or water walls, generating either used filters or wastewater from air scrubbing systems.
Curing is performed to convert the coating binder into a hard, tough, adherent surface. Curing mechanisms include: drying, baking or exposure to an electron beam or infrared or ultraviolet light. Curing generates significant VOCs from solvent-based coatings and poses a potential for explosion if the solvent concentrations rise above the lower explosive limit. Consequently, curing operations are equipped with air pollution control devices to prevent VOC emissions and for safety control to prevent explosions.
Environmental and health concerns, increased regulations affecting conventional coating formulations, high solvent costs and expensive hazardous waste disposal have created a demand for alternative coating formulations that contain less hazardous constituents and generate less waste when applied. Alternative coating formulations include:
- High-solid coatings, containing twice the amount of pigment and resin in the same volume of solvent as conventional coatings. Application lowers VOC emissions between 62 and 85% compared to conventional low-solid solvent-based coatings because the solvent content is reduced.
- Water-based coatings using water and an organic solvent mixture as the carrier with water used as the base. Compared to solvent-based coatings, water-based coatings generate between 80 and 95% less VOC emissions and spent solvents than conventional low-solid solvent-based coatings.
- Powder coatings containing no organic solvent, consisting of finely pulverized pigment and resin particles. They are either thermoplastic (high molecular weight resin for thick coatings) or thermosetting (low molecular weight compounds that form a thin layer before chemically cross-linking) powders.
Equipment cleaning
Equipment cleaning is a necessary, routine maintenance operation in coating processes. This creates significant amounts of hazardous waste, particularly if halogenated solvents are used for cleaning. Equipment cleaning for solvent-based coatings has traditionally been conducted manually with organic solvents to remove coatings from process equipment. Piping requires flushing with solvent in batches until clean. Coating equipment must be cleaned between product changes and after process shutdowns. The procedures and practices used will determine the level of waste generated from these activities.
Waste management
Several waste streams are generated by coating processes. Solid waste includes empty coating containers, coating sludge from overspray and equipment cleaning, spent filters and abrasive materials, dry coating and cleaning rags.
Liquid wastes include waste water from surface preparation, overspray control or equipment cleaning, off-specification or excess coating or surface preparation materials, overspray, spills and spent cleaning solutions. Onsite closed-loop recycling is becoming more popular for spent solvents as disposal costs rise. Water-based liquids are usually treated onsite prior to discharge to publicly owned treatment systems.
VOC emissions are generated by all conventional coating processes that use solvent-based coatings, requiring control devices such as carbon adsorption units, condensers or thermal catalytic oxidizers.
" DISCLAIMER: The ILO does not take responsibility for content presented on this web portal that is presented in any language other than English, which is the language used for the initial production and peer-review of original content. Certain statistics have not been updated since the production of the 4th edition of the Encyclopaedia (1998)."