Beryllium disease is a systemic disorder involving multiple organs, with pulmonary manifestations being most prominent and common. It occurs on exposure to beryllium in its alloy form or in one of its various chemical compounds. Route of exposure is by inhalation and the disease can be either acute or chronic. Acute disease is extremely rare currently, and none has been reported since the first widespread industrial use of beryllium in the 1940s after industrial hygiene measures had been implemented to limit high-dose exposures. Chronic beryllium disease continues to be reported.
Beryllium, Alloys and Compounds
Beryllium, an industrial substance suspected of having carcinogenic potential, is notable for its lightness in weight, high tensile strength and corrosion resistance. Table 1 outlines the properties of beryllium and its compounds.
Table 1. Properties of beryllium and its compounds
|
Formula |
Specific |
Melting/boiling point (ºC) |
Solubility |
Description |
|
|
Beryllium (Be) |
9.01 (a.w.) |
1.85 |
1,298±5/2,970 |
— |
Grey to silver metal |
|
Beryllium oxide (BeO) |
25 |
3.02 |
2,530±30/— |
Soluble in acids and alkalis; insoluble in water |
White amorphous powder |
|
Beryllium fluoride1 (BeF2 ) |
47.02 |
1.99 |
Sublimes 800 °C |
Readily soluble in water; sparingly soluble in ethyl alcohol |
Hygroscopic solid |
|
Beryllium chloride2 (BeCl2 ) |
79.9 |
1.90 |
405/520 |
Very soluble in water; soluble in ethyl alcohol, benzene, ethyl ether and carbon disulphide |
White or slightly yellow deliquescent crystals |
|
Beryllium nitrate3 (Be(NO3 )2 ·3H2 O) |
187.08 |
1.56 |
60/142 |
Soluble in water and ethyl alcohol |
White to faintly yellow deliquescent crystals |
|
Beryllium nitride4 (Be3 N2 ) |
55.06 |
— |
2,200±100/— |
— |
Hard, refractory white crystals |
|
Beryllium sulphate |
177.2 |
1.71 |
100/— |
Soluble in water; insoluble in ethyl alcohol |
Colourless crystals |
1 Beryllium fluoride is made by the decompensation at 900–950 ºC of ammonium beryllium fluoride. Its main use is in the production of beryllium metal by reduction with magnesium.
2 Beryllium chloride is manufactured by passing chlorine over a mixture of beryllium oxide and carbon.
3 Beryllium nitrate is produced by the action of nitric acid on beryllium oxide. It is used as a chemical reagent and as a gas mantle hardener.
4 Beryllium nitride is prepared by heating beryllium metal powder in an oxygen-free, nitrogen atmosphere at 700–1,400 ºC. It is used in atomic energy reactions, including the production of the radioactive carbon isotope carbon-14.
5 Beryllium sulphate hydrate is produced by treating the fritted ore with concentrated suphuric acid.It is used in the production of metallic beryllium by the sulphate process.
Sources
Beryl (3BeO·Al2O3·6SiO2) is the chief commercial source of beryllium, the most abundant of the minerals containing high concentrations of beryllium oxide (10 to 13%). Major sources of beryl are to be found in Argentina, Brazil, India, Zimbabwe and the Republic of South Africa. In the United States, beryl is found in Colorado, South Dakota, New Mexico and Utah. Bertrandite, a low-grade ore (0.1 to 3%) with an acid-soluble beryllium content, is now being mined and processed in Utah.
Production
The two most important methods of extracting beryllium from the ore are the sulphate process and the fluoride process.
In the sulphate process, crushed beryl is melted in an arc furnace at 1,65°C and poured through a high-velocity water stream to form a frit. After heat treatment, the frit is ground in a ball mill and mixed with concentrated sulphuric acid to form a slurry, which is sprayed in the form of a jet into a directly heated, rotating sulphating mill. The beryllium, now in a water-soluble form, is leached from the sludge, and ammonium hydroxide is added to the leach liquor, which is then fed to a crystallizer where ammonium alum is crystallized out. Chelating agents are added to the liquor to hold iron and nickel in solution, sodium hydroxide is then added, and the sodium beryllate thus formed is hydrolyzed to precipitate beryllium hydroxide. The latter product may be converted to beryllium fluoride for reduction by magnesium to metallic beryllium, or to beryllium chloride for electrolytic reduction.
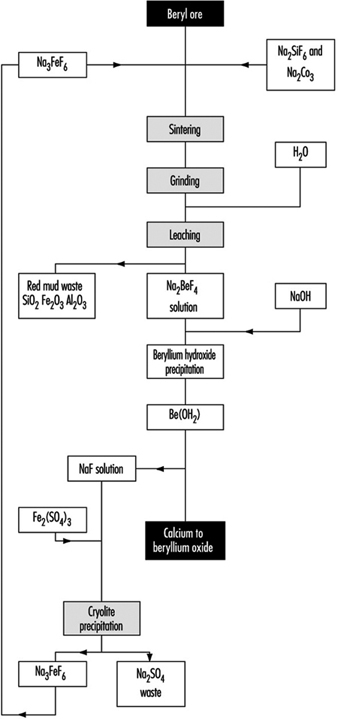
In the fluoride process (figure 1) a briquetted mixture of ground ore, sodium silicofluoride and soda ash is sintered in a rotating hearth furnace. The sintered material is crushed, milled and leached. Sodium hydroxide is added to the solution of beryllium fluoride thus obtained and the precipitate of beryllium hydroxide is filtered in a rotary filter. Metallic beryllium is obtained as in the previous process by the magnesium reduction of beryllium fluoride or by electrolysis of beryllium chloride.
Figure 1. Production of beryllium oxide by the fluoride process
Uses
Beryllium is used in alloys with a number of metals including steel, nickel, magnesium, zinc and aluminium, the most widely used alloy being beryllium-copper—properly called “a bronze”—which has a high tensile strength and a capacity for being hardened by heat treatment. Beryllium bronzes are used in non-spark tools, electrical switch parts, watch springs, diaphragms, shims, cams and bushings.
One of the largest uses of the metal is as a moderator of thermal neutrons in nuclear reactors and as a reflector to reduce the leakage of neutrons from the reactor core. A mixed uranium-beryllium source is often used as a neutron source. As a foil, beryllium is used as window material in x-ray tubes. Its lightness, high elastic modulus and heat stability make it an attractive material for the aircraft and aerospace industry.
Beryllium oxide is made by heating beryllium nitrate or hydroxide.
It is used in the manufacture of ceramics, refractory materials and other beryllium compounds. It was used for the manufacture of phosphors for fluorescent lamps until the incidence of beryllium disease in the industry caused its use for this purpose to be abandoned (in 1949 in the United States).
Hazards
Fire and health hazards are associated with processes involving beryllium. Finely divided beryllium powder will burn, the degree of combustibility being a function of particle size. Fires have occurred in dust filtration units and during the welding of ventilation ducting in which finely divided beryllium was present.
Beryllium and its compounds are highly toxic substances. Beryllium can affect all organ systems, although the primary organ involved is the lung. Beryllium causes systemic disease by inhalation and can distribute itself widely throughout the body after absorption from the lungs. Little beryllium is absorbed from the gastro-intestinal tract. Beryllium can cause skin irritation and its traumatic introduction into subcutaneous tissue can cause local irritation and granuloma formation.
Pathogenesis
Beryllium in all its forms, except for beryl ore, has been associated with disease. The route of entry is by inhalation and in the acute disease there is a direct toxic effect on both the nasopharyngeal mucosa and that of the entire tracheobronchial tree as well, causing oedema and inflammation. In the lung it causes an acute chemical pneumonitis. The major form of beryllium toxicity at this point in time is chronic beryllium disease. A beryllium-specific delayed type of hypersensitivity is the major pathway of chronic disease. The entry of beryllium into the system through the lungs leads to proliferation of specific CD+ lymphocytes, with beryllium acting as a specific antigen, either alone or as a hapten through an interleukin-2 (IL2) receptor pathway. Individual susceptibility to beryllium thus can be explained on the basis of the individual CD+ response. Release of lymphokines from the activated lymphocytes then can lead to granuloma formation and macrophage recruitment. Beryllium can be transported to sites outside the lung where it can cause granuloma formation. Beryllium is released slowly from different sites and it is excreted by the kidneys. This slow release can occur over a span of 20 to 30 years. The chronicity and latency of disease can probably be explained on the basis of the slow metabolism and release phenomenon. The immune mechanisms involved in the pathogenesis of beryllium disease also allow for specific approaches to diagnosis, which will be discussed below.
Histopathology
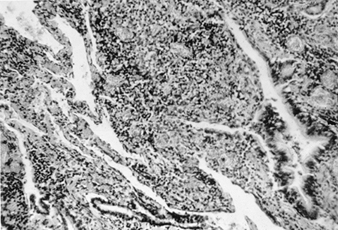
The primary pathological finding in beryllium disease is the formation of non-caseating granulomas in the lungs, lymph nodes and at other sites. Histopathological studies of lungs in patients with acute beryllium disease have shown a non-specific pattern of acute and subacute bronchitis and pneumonitis. In chronic beryllium disease, there are varying degrees of lymphocytic infiltration of the lung interstitium and non-caseating granuloma formation (figure 2).
Figure 2. Lung tissue in a patient with chronic beryllium disease
Both granulomas and round cell infiltration are visible
Many of the granulomas are located in the peribronchiolar areas. In addition, there can be histiocytes, plasma cells and giant cells with calcific inclusion bodies. If it is a case solely of granuloma formation, the long-term prognosis is better. The histology of the lung in chronic beryllium disease is indistinguishable from that of sarcoidosis. Non-caseating granulomas are also found in lymph nodes, liver, spleen, muscle and skin.
Clinical Manifestations
Skin injuries
Acid salts of beryllium cause allergic contact dermatitis. Such lesions may be erythematous, papular or papulovesicular, are commonly pruritic, and are found on exposed parts of the body. There is usually a delay of 2 weeks from first exposure to occurrence of the dermatitis, except in the case of heavy exposures, when an irritant reaction may be immediate. This delay is regarded as the time required to develop the hypersensitive state.
Accidental implantation of beryllium metal or crystals of a soluble beryllium compound in an abrasion, a crack in the skin or under the nail may cause an indurated area with central suppuration. Granulomas can also form at such sites.
Conjunctivitis and dermatitis may occur alone or together. In cases of conjunctivitis, periorbital oedema may be severe.
Acute disease
Beryllium nasopharyngitis is characterized by swollen and hyperaemic mucous membranes, bleeding points, fissures and ulceration. Perforation of the nasal septum has been described. Removal from exposure results in reversal of this inflammatory process within 3 to 6 weeks.
Involvement of the trachea and bronchial tree following exposure to higher levels of beryllium causes non-productive cough, substernal pain and moderate shortness of breath. Rhonchi and/or rales may be audible, and the x ray of the chest may show increased bronchovascular markings. The character and speed of onset and the severity of these signs and symptoms depend on the quality and quantity of exposure. Recovery is to be expected within 1 to 4 weeks if the worker is removed from further exposure.
The use of steroids is quite useful in countering the acute disease. No new cases of acute disease have been reported to the US Beryllium Case Registry in over 30 years. The Registry, which was started by Harriet Hardy in 1952, has almost 1,000 case records, among which are listed 212 acute cases. Almost all of these occurred in the fluorescent lamp manufacturing industry. Forty-four subjects with the acute disease subsequently developed chronic disease.
Chronic beryllium disease
Chronic beryllium disease is a pulmonary and systemic granulomatous disease caused by inhalation of beryllium. The latency of the disease can be from 1 to 30 years, most commonly occurring 10 to 15 years after first exposure. Chronic beryllium disease has a variable course with exacerbations and remissions in its clinical manifestations. However, the disease is usually progressive. There have been a few cases with chest x-ray abnormalities with a stable clinical course and without significant symptoms.
Exertional dyspnoea is the most common symptom of chronic beryllium disease. Other symptoms are cough, fatigue, weight loss, chest pain and arthralgias. Physical findings may be entirely normal or may include bibasilar crackles, lymphadenopathy, skin lesions, hepatosplenomegaly and clubbing. Signs of pulmonary hypertension may be present in severe, long-standing disease.
Renal stones and hyperuricaemia can occur in some patients and there have been rare reports of parotid gland enlargement and central nervous system involvement. The clinical manifestations of chronic beryllium disease are very similar to those of sarcoidosis.
Roentgenologic features
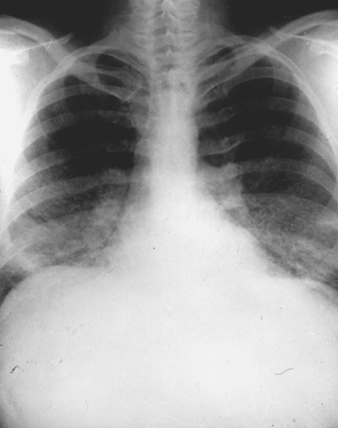
The x-ray pattern in chronic beryllium disease is non-specific and is similar to that which may be observed in sarcoidosis, idiopathic pulmonary fibrosis, tuberculosis, mycoses and dust disease (figure 3). Early in the course of the disease films may show granular, nodular or linear densities. These abnormalities may increase, decrease or remain unchanged, with or without fibrosis. Upper-lobe involvement is common. Hilar adenopathy, seen in approximately one-third of patients, is usually bilateral and accompanied by mottling of the lung fields. The absence of lung changes in the presence of adenopathy is a relative but not an absolute differential consideration in favour of sarcoidosis as opposed to chronic beryllium disease. Unilateral hilar adenopathy has been reported, but is quite rare.
Figure 3. Chest roentgenograph of a patient with chronic beryllium disease, showing diffuse fibronodular infiltrates and prominent hila
The x-ray picture does not correlate well with clinical status and does not reflect particular qualitative or quantitative aspects of the causal exposure.
Pulmonary function tests
Data from the Beryllium Case Registry show that 3 patterns of impairment may be found in chronic beryllium disease. Of 41 patients studied over a period of an average of 23 years after initial beryllium exposure, 20% had a restrictive defect, 36% had an interstitial defect (normal lung volumes and air flow rates but reduced diffusing capacity for carbon monoxide), 39% had an obstructive defect and 5% were normal. The obstructive pattern, which occurred in both smokers and non-smokers, was associated with granulomas in the peribronchial region. This study indicated that the pattern of impairment affects prognosis. Patients with interstitial defect fared best, with the least deterioration over a five-year interval. Patients with obstructive and restrictive defects experienced worsening of their impairment in spite of corticosteroid therapy.
Studies of lung function in beryllium extraction workers who were asymptomatic showed the presence of mild arterial hypoxaemia. This occurred usually within the first 10 years of exposure. In workers exposed to beryllium for 20 years or more there was a reduction in the forced vital capacity (FVC) and the forced expiratory volume in one second (FEV1). These findings suggest that the initial mild hypoxaemia could be due to the early alveolitis and that with further exposure and elapse of time the reduction in FEV1 and FVC could represent fibrosis and granuloma formation.
Other laboratory tests
Non-specific abnormal laboratory tests have been reported in chronic beryllium disease and include elevated sedimentation rate, erythrocytosis, increased gammaglobulin levels, hyperuricaemia and hypercalcaemia.
The Kveim skin test is negative in beryllium disease, whereas it may be positive in sarcoidosis. The angiotensin converting enzyme (ACE) level is usually normal in beryllium disease, but can be increased in 60% or more of patients with active sarcoidosis.
Diagnosis
Diagnosis of chronic beryllium disease for many years was based on the criteria developed through the Beryllium Case Registry, which included:
- a history of significant beryllium exposure
- evidence of lower respiratory tract disease
- abnormal chest x ray with interstitial fibronodular disease
- abnormal lung function tests with decreased carbon monoxide diffusing capacity (DLCO)
- pathological changes consistent with beryllium exposure in lung or thoracic lymph nodes
- the presence of beryllium in tissue.
Four of the six criteria had to be met and should have included either (1) or (6). Since the 1980s, advances in immunology have made it possible to make the diagnosis of beryllium disease without requiring tissue specimens for histological examination or beryllium analysis. The transformation of lymphocytes in blood in response to beryllium exposure (as in the lymphocyte transformation test, LTT) or lymphocytes from bronchoalveolar lavage (BAL) have been proposed by Newman et al. (1989) as useful diagnostic tools in making the diagnosis of beryllium disease in exposed subjects. Their data suggest that a positive blood LTT is indicative of sensitization. However, recent data show that the blood LTT does not correlate well with pulmonary disease. The BAL lymphocyte transformation correlates much better with abnormal pulmonary function and does not correlate well with concurrent abnormalities in the blood LTT. Thus, to make a diagnosis of beryllium disease, one needs a combination of clinical, radiological and lung function abnormalities and a positive LTT in the BAL. A positive blood LTT by itself is not diagnostic. Microprobe analysis of small tissue samples for beryllium is another recent innovation which could help in diagnosis of disease in small lung tissue samples obtained by transbronchial lung biopsy.
Sarcoidosis is the disorder most closely resembling chronic beryllium disease, and the differentiation may be difficult. Thus far, no cystic bone disease or involvement of the eye or tonsil has appeared in chronic beryllium disease. Similarly, the Kveim test is negative in beryllium disease. Skin testing to demonstrate beryllium sensitization is not recommended, in that the test itself is sensitizing, may possibly trigger systemic reactions in sensitized people and does not of itself establish that the presenting disease is necessarily beryllium related.
More sophisticated immunological approaches in differential diagnosis should allow for better differentiation from sarcoidosis in the future.
Prognosis
The prognosis of chronic beryllium disease has altered favourably during the years; it has been suggested that the longer delays in onset observed among beryllium workers may reflect lower exposure or lower beryllium body burden, resulting in a milder clinical course. Clinical evidence is that steroid therapy, if used when measurable disability first appears, in adequate doses for long enough periods, has improved the clinical status of many patients, allowing some of them to return to useful jobs. There is no clear evidence that steroids have cured chronic beryllium poisoning.
Beryllium and cancer
In animals, experimentally administered beryllium is a carcinogen, causing osteogenic sarcoma after intravenous injection in rabbits and lung cancer after inhalation in rats and monkeys. Whether beryllium may be a human carcinogen is a controversial issue. Some epidemiological studies have suggested an association, particularly after acute beryllium disease. This finding has been disputed by others. One can conclude that beryllium is carcinogenic in animals and there may be a link between lung cancer and beryllium in humans, particularly in those with the acute disease.
Safety and Health Measures
Safety and health precautions must cover the fire hazard as well as the much more serious toxicity danger.
Fire prevention
Arrangements must be made to prevent possible sources of ignition, such as the sparking or arcing of electrical apparatus, friction, and so forth, in the vicinity of finely divided beryllium powder. Equipment in which this powder has been present should be emptied and cleaned before acetylene or electrical welding apparatus is used on it. Oxide-free, ultrafine beryllium powder that has been prepared in inert gas is liable to ignite spontaneously on exposure to air.
Suitable dry powder—not water—should be used to extinguish a beryllium fire. Full personal protective equipment, including respiratory protective equipment, should be worn and firefighters should bathe afterwards and arrange for their clothing to be laundered separately.
Health protection
Beryllium processes must be conducted in a carefully controlled manner to protect both the worker and the general population. The main risk takes the form of airborne contamination and the process and plant should be designed to give rise to as little dust or fume as possible. Wet processes should be used instead of dry processes, and the ingredients of beryllium-containing preparations should be unified as aqueous suspensions instead of as dry powders; whenever possible the plant should be designed as groups of separate enclosed units. The permissible concentration of beryllium in the atmosphere is so low that enclosure must be applied even to wet processes, otherwise escaping splashes and spills can dry out and the dust can enter the atmosphere.
Operations from which dust may be evolved should be conducted in areas with maximum degree of enclosure consistent with the needs of manipulation. Some operations are performed in glove boxes, but many more are conducted in enclosures provided with exhaust ventilation similar to that installed in chemical fume cupboards. Machining operations may be ventilated by high-velocity, low-volume local exhaust systems or by hooded enclosures with exhaust ventilation.
To check the effectiveness of these precautionary measures atmosphere monitoring should be done in such a manner that the daily average exposure of workers to respirable beryllium can be calculated. The work area should be cleaned regularly by means of a proper vacuum cleaner or a wet mop. Beryllium processes should be segregated from the other operations in the factory.
Personal protective equipment should be provided for workers engaged in beryllium processes. Where they are fully employed in processes involving the manipulation of beryllium compounds or in processes associated with the extraction of the metal from the ore, provision should be made for a complete change of clothing so that the workers do not go home wearing clothing in which they have been working. Arrangements should be made for the safe laundering of such working clothes, and protective overalls should be provided even to laundry workers to ensure that they too are not exposed to risk. These arrangements should not be left to normal home laundering procedures. Cases of beryllium poisoning in the families of workers have been attributed to workers taking contaminated clothing home or wearing them in the home.
An occupational health standard of 2μg/m3, proposed in 1949 by a committee operating under the auspices of the US Atomic Energy Commission, continues to be widely observed. Existing interpretations generally permit fluctuations to a “ceiling” of 5μg/m3 as long as the time-weighted average is not exceeded. Additionally, an “acceptable maximum peak above the ceiling concentration for an eight-hour shift” of 25μg/m3 for up to 30 min is also permissible. These operational levels are achievable in current industrial practice, and there is no evidence of adverse health experience among persons working in an environment thus controlled. Because of a possible link between beryllium and lung cancer it has been suggested that the allowable limit be reduced to 1μg/m3, but no official action has been taken on this suggestion in the United States.
The population at risk for developing beryllium disease is that which in some manner deals with beryllium in its extraction or subsequent use. However, a few “neighbourhood” cases have been reported from a distance 1 to 2 km from beryllium extraction plants.
Pre-employment and periodical medical examinations of workers exposed to beryllium and its compounds are compulsory in a number of countries. Recommended evaluation includes an annual respiratory questionnaire, a chest x ray and lung function tests. With advances in immunology, the LTT may also become a routine evaluation, although at this time not enough data are available to recommend its use routinely. With evidence of beryllium disease, it is unwise to allow a worker to be exposed to beryllium further, even though the workplace meets the threshold criteria for beryllium concentration in the air.
Treatment
The major step in therapy is avoidance of further exposure to beryllium. Corticosteroids are the primary mode of therapy in chronic beryllium disease. Corticosteroids appear to alter the course of disease favourably but do not “cure” it.
Corticosteroids should be started on a daily basis with a relatively high dose of Prednisone of 0.5 to 1 mg per kg or more, and continued until improvement occurs or no further deterioration in clinical or lung function tests occurs. Usually this takes 4 to 6 weeks. Slow reduction of steroids is recommended, and eventually alternate-day therapy may be possible. Steroid therapy ordinarily becomes a lifelong necessity.
Other supportive measures such as supplemental oxygen, diuretics, digitalis and antibiotics (when infection exists) are indicated as the clinical condition of the patient would dictate. Immunization against influenza and pneumococcus should also be considered, as with any patient with chronic respiratory disease.