Basic Concepts and Definitions
At the worksite, industrial hygiene methodologies can measure and control only airborne chemicals, while other aspects of the problem of possible harmful agents in the environment of workers, such as skin absorption, ingestion, and non-work-related exposure, remain undetected and therefore uncontrolled. Biological monitoring helps fill this gap.
Biological monitoring was defined in a 1980 seminar, jointly sponsored by the European Economic Community (EEC), National Institute for Occupational Safety and Health (NIOSH) and Occupational Safety and Health Association (OSHA) (Berlin, Yodaiken and Henman 1984) in Luxembourg as “the measurement and assessment of agents or their metabolites either in tissues, secreta, excreta, expired air or any combination of these to evaluate exposure and health risk compared to an appropriate reference”. Monitoring is a repetitive, regular and preventive activity designed to lead, if necessary, to corrective actions; it should not be confused with diagnostic procedures.
Biological monitoring is one of the three important tools in the prevention of diseases due to toxic agents in the general or occupational environment, the other two being environmental monitoring and health surveillance.
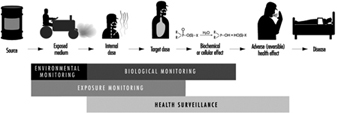
The sequence in the possible development of such disease may be schematically represented as follows: source-exposed chemical agent—internal dose—biochemical or cellular effect (reversible) —health effects—disease. The relationships among environmental, biological, and exposure monitoring, and health surveillance, are shown in figure 1.
Figure 1. The relationship between environmental, biological and exposure monitoring, and health surveillance
When a toxic substance (an industrial chemical, for example) is present in the environment, it contaminates air, water, food, or surfaces in contact with the skin; the amount of toxic agent in these media is evaluated via environmental monitoring.
As a result of absorption, distribution, metabolism, and excretion, a certain internal dose of the toxic agent (the net amount of a pollutant absorbed in or passed through the organism over a specific time interval) is effectively delivered to the body, and becomes detectable in body fluids. As a result of its interaction with a receptor in the critical organ (the organ which, under specific conditions of exposure, exhibits the first or the most important adverse effect), biochemical and cellular events occur. Both the internal dose and the elicited biochemical and cellular effects may be measured through biological monitoring.
Health surveillance was defined at the above-mentioned 1980 EEC/NIOSH/OSHA seminar as “the periodic medico-physiological examination of exposed workers with the objective of protecting health and preventing disease”.
Biological monitoring and health surveillance are parts of a continuum that can range from the measurement of agents or their metabolites in the body via evaluation of biochemical and cellular effects, to the detection of signs of early reversible impairment of the critical organ. The detection of established disease is outside the scope of these evaluations.
Goals of Biological Monitoring
Biological monitoring can be divided into (a) monitoring of exposure, and (b) monitoring of effect, for which indicators of internal dose and of effect are used respectively.
The purpose of biological monitoring of exposure is to assess health risk through the evaluation of internal dose, achieving an estimate of the biologically active body burden of the chemical in question. Its rationale is to ensure that worker exposure does not reach levels capable of eliciting adverse effects. An effect is termed “adverse” if there is an impairment of functional capacity, a decreased ability to compensate for additional stress, a decreased ability to maintain homeostasis (a stable state of equilibrium), or an enhanced susceptibility to other environmental influences.
Depending on the chemical and the analysed biological parameter, the term internal dose may have different meanings (Bernard and Lauwerys 1987). First, it may mean the amount of a chemical recently absorbed, for example, during a single workshift. A determination of the pollutant’s concentration in alveolar air or in the blood may be made during the workshift itself, or as late as the next day (samples of blood or alveolar air may be taken up to 16 hours after the end of the exposure period). Second, in the case that the chemical has a long biological half-life—for example, metals in the bloodstream—the internal dose could reflect the amount absorbed over a period of a few months.
Third, the term may also mean the amount of chemical stored. In this case it represents an indicator of accumulation which can provide an estimate of the concentration of the chemical in organs and/or tissues from which, once deposited, it is only slowly released. For example, measurements of DDT or PCB in blood could provide such an estimate.
Finally, an internal dose value may indicate the quantity of the chemical at the site where it exerts its effects, thus providing information about the biologically effective dose. One of the most promising and important uses of this capability, for example, is the determination of adducts formed by toxic chemicals with protein in haemoglobin or with DNA.
Biological monitoring of effects is aimed at identifying early and reversible alterations which develop in the critical organ, and which, at the same time, can identify individuals with signs of adverse health effects. In this sense, biological monitoring of effects represents the principal tool for the health surveillance of workers.
Principal Monitoring Methods
Biological monitoring of exposure is based on the determination of indicators of internal dose by measuring:
- the amount of the chemical, to which the worker is exposed, in blood or urine (rarely in milk, saliva, or fat)
- the amount of one or more metabolites of the chemical involved in the same body fluids
- the concentration of volatile organic compounds (solvents) in alveolar air
- the biologically effective dose of compounds which have formed adducts to DNA or other large molecules and which thus have a potential genotoxic effect.
Factors affecting the concentration of the chemical and its metabolites in blood or urine will be discussed below.
As far as the concentration in alveolar air is concerned, besides the level of environmental exposure, the most important factors involved are solubility and metabolism of the inhaled substance, alveolar ventilation, cardiac output, and length of exposure (Brugnone et al. 1980).
The use of DNA and haemoglobin adducts in monitoring human exposure to substances with carcinogenic potential is a very promising technique for measurement of low level exposures. (It should be noted, however, that not all chemicals that bind to macromolecules in the human organism are genotoxic, i.e., potentially carcinogenic.) Adduct formation is only one step in the complex process of carcinogenesis. Other cellular events, such as DNA repair promotion and progression undoubtedly modify the risk of developing a disease such as cancer. Thus, at the present time, the measurement of adducts should be seen as being confined only to monitoring exposure to chemicals. This is discussed more fully in the article “Genotoxic chemicals” later in this chapter.
Biological monitoring of effects is performed through the determination of indicators of effect, that is, those that can identify early and reversible alterations. This approach may provide an indirect estimate of the amount of chemical bound to the sites of action and offers the possibility of assessing functional alterations in the critical organ in an early phase.
Unfortunately, we can list only a few examples of the application of this approach, namely, (1) the inhibition of pseudocholinesterase by organophosphate insecticides, (2) the inhibition of d-aminolaevulinic acid dehydratase (ALA-D) by inorganic lead, and (3) the increased urinary excretion of d-glucaric acid and porphyrins in subjects exposed to chemicals inducing microsomal enzymes and/or to porphyrogenic agents (e.g., chlorinated hydrocarbons).
Advantages and Limitations of Biological Monitoring
For substances that exert their toxicity after entering the human organism, biological monitoring provides a more focused and targeted assessment of health risk than does environmental monitoring. A biological parameter reflecting the internal dose brings us one step closer to understanding systemic adverse effects than does any environmental measurement.
Biological monitoring offers numerous advantages over environmental monitoring and in particular permits assessment of:
- exposure over an extended time period
- exposure as a result of worker mobility in the working environment
- absorption of a substance via various routes, including the skin
- overall exposure as a result of different sources of pollution, both occupational and non-occupational
- the quantity of a substance absorbed by the subject depending on factors other than the degree of exposure, such as the physical effort required by the job, ventilation, or climate
- the quantity of a substance absorbed by a subject depending on individual factors that can influence the toxicokinetics of the toxic agent in the organism; for example, age, sex, genetic features, or functional state of the organs where the toxic substance undergoes biotransformation and elimination.
In spite of these advantages, biological monitoring still suffers today from considerable limitations, the most significant of which are the following:
- The number of possible substances which can be monitored biologically is at present still rather small.
- In the case of acute exposure, biological monitoring supplies useful information only for exposure to substances that are rapidly metabolized, for example, aromatic solvents.
- The significance of biological indicators has not been clearly defined; for example, it is not always known whether the levels of a substance measured on biological material reflect current or cumulative exposure (e.g., urinary cadmium and mercury).
- Generally, biological indicators of internal dose allow assessment of the degree of exposure, but do not furnish data that will measure the actual amount present in the critical organ
- Often there is no knowledge of possible interference in the metabolism of the substances being monitored by other exogenous substances to which the organism is simultaneously exposed in the working and general environment.
- There is not always sufficient knowledge on the relationships existing between the levels of environmental exposure and the levels of the biological indicators on the one hand, and between the levels of the biological indicators and possible health effects on the other.
- The number of biological indicators for which biological exposure indices (BEIs) exist at present is rather limited. Follow-up information is needed to determine whether a substance, presently identified as not capable of causing an adverse effect, may at a later time be shown to be harmful.
- A BEI usually represents a level of an agent that is most likely to be observed in a specimen collected from a healthy worker who has been exposed to the chemical to the same extent as a worker with an inhalation exposure to the TLV (threshold limit value) time-weighted average (TWA).
Information Required for the Development of Methods and Criteria for Selecting Biological Tests
Programming biological monitoring requires the following basic conditions:
- knowledge of the metabolism of an exogenous substance in the human organism (toxicokinetics)
- knowledge of the alterations that occur in the critical organ (toxicodynamics)
- existence of indicators
- existence of sufficiently accurate analytical methods
- possibility of using readily obtainable biological samples on which the indicators can be measured
- existence of dose-effect and dose-response relationships and knowledge of these relationships
- predictive validity of the indicators.
In this context, the validity of a test is the degree to which the parameter under consideration predicts the situation as it really is (i.e., as more accurate measuring instruments would show it to be). Validity is determined by the combination of two properties: sensitivity and specificity. If a test possesses a high sensitivity, this means that it will give few false negatives; if it possesses high specificity, it will give few false positives (CEC 1985-1989).
Relationship between exposure, internal dose and effects
The study of the concentration of a substance in the working environment and the simultaneous determination of the indicators of dose and effect in exposed subjects allows information to be obtained on the relationship between occupational exposure and the concentration of the substance in biological samples, and between the latter and the early effects of exposure.
Knowledge of the relationships between the dose of a substance and the effect it produces is an essential requirement if a programme of biological monitoring is to be put into effect. The evaluation of this dose-effect relationship is based on the analysis of the degree of association existing between the indicator of dose and the indicator of effect and on the study of the quantitative variations of the indicator of effect with every variation of indicator of dose. (See also the chapter Toxicology, for further discussion of dose-related relationships).
With the study of the dose-effect relationship it is possible to identify the concentration of the toxic substance at which the indicator of effect exceeds the values currently considered not harmful. Furthermore, in this way it may also be possible to examine what the no-effect level might be.
Since not all the individuals of a group react in the same manner, it is necessary to examine the dose-response relationship, in other words, to study how the group responds to exposure by evaluating the appearance of the effect compared to the internal dose. The term response denotes the percentage of subjects in the group who show a specific quantitative variation of an effect indicator at each dose level.
Practical Applications of Biological Monitoring
The practical application of a biological monitoring programme requires information on (1) the behaviour of the indicators used in relation to exposure, especially those relating to degree, continuity and duration of exposure, (2) the time interval between end of exposure and measurement of the indicators, and (3) all physiological and pathological factors other than exposure that can alter the indicator levels.
In the following articles the behaviour of a number of biological indicators of dose and effect that are used for monitoring occupational exposure to substances widely used in industry will be presented. The practical usefulness and limits will be assessed for each substance, with particular emphasis on time of sampling and interfering factors. Such considerations will be helpful in establishing criteria for selecting a biological test.
Time of sampling
In selecting the time of sampling, the different kinetic aspects of the chemical must be kept in mind; in particular it is essential to know how the substance is absorbed via the lung, the gastrointestinal tract and the skin, subsequently distributed to the different compartments of the body, biotransformed, and finally eliminated. It is also important to know whether the chemical may accumulate in the body.
With respect to exposure to organic substances, the collection time of biological samples becomes all the more important in view of the different velocity of the metabolic processes involved and consequently the more or less rapid excretion of the absorbed dose.
Interfering Factors
Correct use of biological indicators requires a thorough knowledge of those factors which, although independent of exposure, may nevertheless affect the biological indicator levels. The following are the most important types of interfering factors (Alessio, Berlin and Foà 1987).
Physiological factors including diet, sex and age, for example, can affect results. Consumption of fish and crustaceans may increase the levels of urinary arsenic and blood mercury. In female subjects with the same lead blood levels as males, the erythrocyte protoporphyrin values are significantly higher compared to those of male subjects. The levels of urinary cadmium increase with age.
Among the personal habits that can distort indicator levels, smoking and alcohol consumption are particularly important. Smoking may cause direct absorption of substances naturally present in tobacco leaves (e.g., cadmium), or of pollutants present in the working environment that have been deposited on the cigarettes (e.g., lead), or of combustion products (e.g., carbon monoxide).
Alcohol consumption may influence biological indicator levels, since substances such as lead are naturally present in alcoholic beverages. Heavy drinkers, for example, show higher blood lead levels than control subjects. Ingestion of alcohol can interfere with the biotransformation and elimination of toxic industrial compounds: with a single dose, alcohol can inhibit the metabolism of many solvents, for example, trichloroethylene, xylene, styrene and toluene, because of their competition with ethyl alcohol for enzymes which are essential for the breakdown of both ethanol and solvents. Regular alcohol ingestion can also affect the metabolism of solvents in a totally different manner by accelerating solvent metabolism, presumably due to induction of the microsome oxidizing system. Since ethanol is the most important substance capable of inducing metabolic interference, it is advisable to determine indicators of exposure for solvents only on days when alcohol has not been consumed.
Less information is available on the possible effects of drugs on the levels of biological indicators. It has been demonstrated that aspirin can interfere with the biological transformation of xylene to methylhippuric acid, and phenylsalicylate, a drug widely used as an analgesic, can significantly increase the levels of urinary phenols. The consumption of aluminium-based antacid preparations can give rise to increased levels of aluminium in plasma and urine.
Marked differences have been observed in different ethnic groups in the metabolism of widely used solvents such as toluene, xylene, trichloroethylene, tetrachloroethylene, and methylchloroform.
Acquired pathological states can influence the levels of biological indicators. The critical organ can behave anomalously with respect to biological monitoring tests because of the specific action of the toxic agent as well as for other reasons. An example of situations of the first type is the behaviour of urinary cadmium levels: when tubular disease due to cadmium sets in, urinary excretion increases markedly and the levels of the test no longer reflect the degree of exposure. An example of the second type of situation is the increase in erythrocyte protoporphyrin levels observed in iron-deficient subjects who show no abnormal lead absorption.
Physiological changes in the biological media—urine, for example—on which determinations of the biological indicators are based, can influence the test values. For practical purposes, only spot urinary samples can be obtained from individuals during work, and the varying density of these samples means that the levels of the indicator can fluctuate widely in the course of a single day.
In order to overcome this difficulty, it is advisable to eliminate over-diluted or over-concentrated samples according to selected specific gravity or creatinine values. In particular, urine with a specific gravity below 1010 or higher than 1030 or with a creatinine concentration lower than 0.5 g/l or greater than 3.0 g/l should be discarded. Several authors also suggest adjusting the values of the indicators according to specific gravity or expressing the values according to urinary creatinine content.
Pathological changes in the biological media can also considerably influence the values of the biological indicators. For example, in anaemic subjects exposed to metals (mercury, cadmium, lead, etc.) the blood levels of the metal may be lower than would be expected on the basis of exposure; this is due to the low level of red blood cells that transport the toxic metal in the blood circulation.
Therefore, when determinations of toxic substances or metabolites bound to red blood cells are made on whole blood, it is always advisable to determine the haematocrit, which gives a measure of the percentage of blood cells in whole blood.
Multiple exposure to toxic substances present in the workplace
In the case of combined exposure to more than one toxic substance present at the workplace, metabolic interferences may occur that can alter the behaviour of the biological indicators and thus create serious problems in interpretation. In human studies, interferences have been demonstrated, for example, in combined exposure to toluene and xylene, xylene and ethylbenzene, toluene and benzene, hexane and methyl ethyl ketone, tetrachloroethylene and trichloroethylene.
In particular, it should be noted that when biotransformation of a solvent is inhibited, the urinary excretion of its metabolite is reduced (possible underestimation of risk) whereas the levels of the solvent in blood and expired air increase (possible overestimation of risk).
Thus, in situations in which it is possible to measure simultaneously the substances and their metabolites in order to interpret the degree of inhibitory interference, it would be useful to check whether the levels of the urinary metabolites are lower than expected and at the same time whether the concentration of the solvents in blood and/or expired air is higher.
Metabolic interferences have been described for exposures where the single substances are present in levels close to and sometimes below the currently accepted limit values. Interferences, however, do not usually occur when exposure to each substance present in the workplace is low.
Practical Use of Biological Indicators
Biological indicators can be used for various purposes in occupational health practice, in particular for (1) periodic control of individual workers, (2) analysis of the exposure of a group of workers, and (3) epidemiological assessments. The tests used should possess the features of precision, accuracy, good sensitivity, and specificity in order to minimize the possible number of false classifications.
Reference values and reference groups
A reference value is the level of a biological indicator in the general population not occupationally exposed to the toxic substance under study. It is necessary to refer to these values in order to compare the data obtained through biological monitoring programmes in a population which is presumed to be exposed. Reference values should not be confused with limit values, which generally are the legal limits or guidelines for occupational and environmental exposure (Alessio et al. 1992).
When it is necessary to compare the results of group analyses, the distribution of the values in the reference group and in the group under study must be known because only then can a statistical comparison be made. In these cases, it is essential to attempt to match the general population (reference group) with the exposed group for similar characteristics such as, sex, age, lifestyle and eating habits.
To obtain reliable reference values one must make sure that the subjects making up the reference group have never been exposed to the toxic substances, either occupationally or due to particular conditions of environmental pollution.
In assessing exposure to toxic substances one must be careful not to include subjects who, although not directly exposed to the toxic substance in question, work in the same workplace, since if these subjects are, in fact, indirectly exposed, the exposure of the group may be in consequence underestimated.
Another practice to avoid, although it is still widespread, is the use for reference purposes of values reported in the literature that are derived from case lists from other countries and may often have been collected in regions where different environmental pollution situations exist.
Periodic monitoring of individual workers
Periodic monitoring of individual workers is mandatory when the levels of the toxic substance in the atmosphere of the working environment approach the limit value. Where possible, it is advisable to simultaneously check an indicator of exposure and an indicator of effect. The data thus obtained should be compared with the reference values and the limit values suggested for the substance under study (ACGIH 1993).
Analysis of a group of workers
Analysis of a group becomes mandatory when the results of the biological indicators used can be markedly influenced by factors independent of exposure (diet, concentration or dilution of urine, etc.) and for which a wide range of “normal” values exists.
In order to ensure that the group study will furnish useful results, the group must be sufficiently numerous and homogeneous as regards exposure, sex, and, in the case of some toxic agents, work seniority. The more the exposure levels are constant over time, the more reliable the data will be. An investigation carried out in a workplace where the workers frequently change department or job will have little value. For a correct assessment of a group study it is not sufficient to express the data only as mean values and range. The frequency distribution of the values of the biological indicator in question must also be taken into account.
Epidemiological assessments
Data obtained from biological monitoring of groups of workers can also be used in cross-sectional or prospective epidemiological studies.
Cross-sectional studies can be used to compare the situations existing in different departments of the factory or in different industries in order to set up risk maps for manufacturing processes. A difficulty that may be encountered in this type of application depends on the fact that inter-laboratory quality controls are not yet sufficiently widespread; thus it cannot be guaranteed that different laboratories will produce comparable results.
Prospective studies serve to assess the behaviour over time of the exposure levels so as to check, for example, the efficacy of environmental improvements or to correlate the behaviour of biological indicators over the years with the health status of the subjects being monitored. The results of such long-term studies are very useful in solving problems involving changes over time. At present, biological monitoring is mainly used as a suitable procedure for assessing whether current exposure is judged to be “safe,” but it is as yet not valid for assessing situations over time. A given level of exposure considered safe today may no longer be regarded as such at some point in the future.
Ethical Aspects
Some ethical considerations arise in connection with the use of biological monitoring as a tool to assess potential toxicity. One goal of such monitoring is to assemble enough information to decide what level of any given effect constitutes an undesirable effect; in the absence of sufficient data, any perturbation will be considered undesirable. The regulatory and legal implications of this type of information need to be evaluated. Therefore, we should seek societal discussion and consensus as to the ways in which biological indicators should best be used. In other words, education is required of workers, employers, communities and regulatory authorities as to the meaning of the results obtained by biological monitoring so that no one is either unduly alarmed or complacent.
There must be appropriate communication with the individual upon whom the test has been performed concerning the results and their interpretation. Further, whether or not the use of some indicators is experimental should be clearly conveyed to all participants.
The International Code of Ethics for Occupational Health Professionals, issued by the International Commission on Occupational Health in 1992, stated that “biological tests and other investigations must be chosen from the point of view of their validity for protection of the health of the worker concerned, with due regard to their sensitivity, their specificity and their predictive value”. Use must not be made of tests “which are not reliable or which do not have a sufficient predictive value in relation to the requirements of the work assignment”. (See the chapter Ethical Issues for further discussion and the text of the Code.)
Trends in Regulation and Application
Biological monitoring can be carried out for only a limited number of environmental pollutants on account of the limited availability of appropriate reference data. This imposes important limitations on the use of biological monitoring in evaluating exposure.
The World Health Organization (WHO), for example, has proposed health-based reference values for lead, mercury, and cadmium only. These values are defined as levels in blood and urine not linked to any detectable adverse effect.The American Conference of Governmental Industrial Hygienists (ACGIH) has established biological exposure indices (BEIs) for about 26 compounds; BEIs are defined as “values for determinants which are indicators of the degree of integrated exposure to industrial chemicals” (ACGIH 1995).