Human biological monitoring uses samples of body fluids or other easily obtainable biological material for the measurement of exposure to specific or nonspecific substances and/or their metabolites or for the measurement of the biological effects of this exposure. Biological monitoring allows one to estimate total individual exposure through different exposure pathways (lungs, skin, gastrointestinal tract) and different sources of exposure (air, diet, lifestyle or occupation). It is also known that in complex exposure situations, which are very often encountered in workplaces, different exposing agents may interact with one another, either enhancing or inhibiting the effects of the individual compounds. And since individuals differ in their genetic constitution, they exhibit variability in their response to chemical exposures. Thus, it may be more reasonable to look for early effects directly in the exposed individuals or groups than to try to predict potential hazards of the complex exposure patterns from data pertaining to single compounds. This is an advantage of genetic biomonitoring for early effects, an approach employing techniques that focus on cytogenetic damage, point mutations, or DNA adducts in surrogate human tissue (see the article “General principles” in this chapter).
What Is Genotoxicity?
Genotoxicity of chemical agents is an intrinsic chemical character, based on the agent’s electrophilic potential to bind with such nucleophilic sites in the cellular macromolecules as deoxyribonucleic acid, DNA, the carrier of hereditary information. Genotoxicity is thus toxicity manifested in the genetic material of cells.
The definition of genotoxicity, as discussed in a consensus report (IARC 1992), is broad, and includes both direct and indirect effects in DNA: (1) the induction of mutations (gene, chromosomal, genomial, recombinational) that at the molecular level are similar to events known to be involved in carcinogenesis, (2) indirect surrogate events associated with mutagenesis (e.g., unscheduled DNA synthesis (UDS) and sister chromatid exchange (SCE), or (3) DNA damage (e.g., the formation of adducts), which may eventually lead to mutations.
Genotoxicity, Mutagenicity And Carcinogenicity
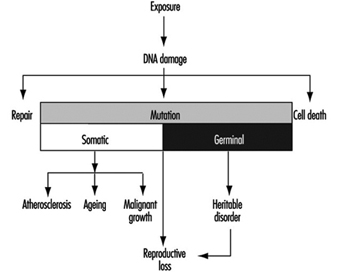
Mutations are permanent hereditary changes in the cell lines, either horizontally in the somatic cells or vertically in the germinal (sex) cells of the body. That is, mutations may affect the organism itself through changes in body cells, or they may be passed on to other generations through alteration of the sex cells. Genotoxicity thus preceeds mutagenicity although most of genotoxicity is repaired and is never expressed as mutations. Somatic mutations are induced at the cellular level and in the event that they lead to cell death or malignancies, may become manifest as various disorders of tissues or of the organism itself. Somatic mutations are thought to be related to ageing effects or to the induction of atherosclerotic plaques (see figure 1 and the chapter on Cancer).
Figure 1. Schematic view of the scientific paradigm in genetic toxicology and human health effects
Mutations in the germ cell line may be transferred to the zygote—the fertilized egg cell—and be expressed in the offspring generation (see also the chapter Reproductive System). The most important mutational disorders found in the newborn are induced by malsegregation of chromosomes during gametogenesis (the development of germ cells) and result in severe chromosomal syndromes (e.g., trisomy 21 or Down’s syndrome, and monosomy X or Turner’s syndrome).
The paradigm of genotoxicology from exposure to anticipated effects may be simplified as shown in figure 1.
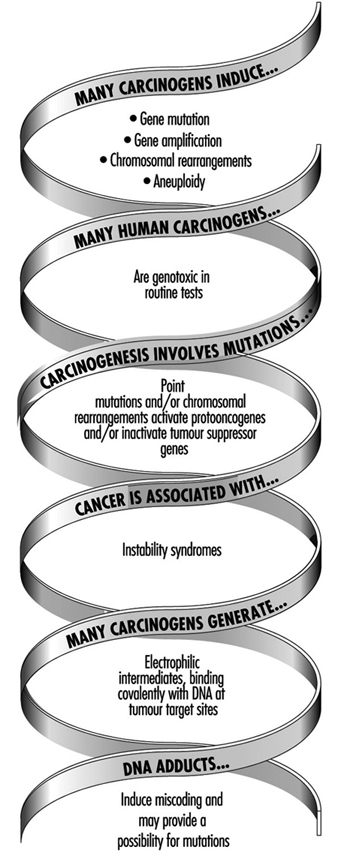
The relationship of genotoxicity to carcinogenicity is well supported by various indirect research facts, as shown in figure 2.
Figure 2. The interrelationships of genotoxicity and carcinogenicity
This correlation provides the basis for applying biomarkers of genotoxicity to be used in human monitoring as indicators of cancer hazard.
Genetic Toxicity in Hazard Identification
The role of genetic changes in carcinogenesis underscores the importance of genetic toxicity testing in the identification of potential carcinogens. Various short-term test methods have been developed which are able to detect some of the endpoints in genotoxicity supposedly relevant in carcinogenesis.
Several extensive surveys have been performed to compare the carcinogenicity of chemicals with results obtained by examining them in short-term tests. The general conclusion has been that since no single validated test can provide information on all of the above-mentioned genetic end-points; it is necessary to test each chemical in more than one assay. Also, the value of short-term tests of genetic toxicity for prediction of chemical carcinogenicity has been discussed and reviewed repeatedly. On the basis of such reviews, a working group at the International Agency for Research on Cancer (IARC) concluded that most human carcinogens give positive results in routinely used short-term tests such as the Salmonella assay and the chromosome aberration assays (table 1). However, it must be realized that the epigenetic carcinogens—such as hormonally active compounds which can increase genotoxic activity without themselves being genotoxic—cannot be detected by short-term tests, which measure only the intrinsic genotoxic activity of a substance.
Table 1. Genotoxicity of chemicals evaluated in Supplements 6 and 7 to the IARC Monographs (1986)
|
Carcinogenicity classification |
Ratio of evidence for genotoxicity/carcinogenicity |
% |
|
1: human carcinogens |
24/30 |
80 |
|
2A: probable human carcinogens |
14/20 |
70 |
|
2B: possible human carcinogens |
72/128 |
56 |
|
3: not classifiable |
19/66 |
29 |
Genetic Biomonitoring
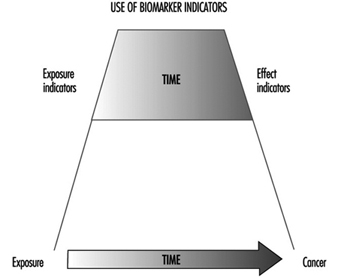
Genetic monitoring utilizes genetic toxicology methods for biological monitoring of genetic effects or assessment of genotoxic exposure in a group of individuals with defined exposure at a worksite or through environment or lifestyle. Thus, genetic monitoring has the potential for early identification of genotoxic exposures in a group of persons and enables identification of high-risk populations and thus priorities for intervention. Use of predictive biomarkers in an exposed population is warranted to save time (as compared with epidemiological techniques) and to prevent unnecessary end effects, namely cancer (figure 3).
Figure 3. The predictiveness of biomarkers enables preventive actions to be taken to decrease risks to health in human populations
The methods currently used for biomonitoring of genotoxic exposure and early biological effects are listed in table 2. The samples used for biomonitoring must meet several criteria, including the necessity that they be both easily obtainable and comparable with the target tissue.
Table 2. Biomarkers in genetic monitoring of genotoxicity exposure and the most commonly used cell/tissue samples.
|
Marker of genetic monitoring |
Cell/tissue samples |
|
Chromosomal aberrations (CA) |
Lymphocytes |
|
Sister chromatid exchanges (SCE) |
Lymphocytes |
|
Micronuclei (MN) |
Lymphocytes |
|
Point mutations (e.g., HPRT gene) |
Lymphocytes and other tissues |
|
DNA adducts |
DNA isolated from cells/tissues |
|
Protein adducts |
Haemoglobin, albumin |
|
DNA strand breaks |
DNA isolated from cells/tissues |
|
Oncogene activation |
DNA or specific proteins isolated |
|
Mutations/oncoproteins |
Various cells and tissues |
|
DNA repair |
Isolated cells from blood samples |
The types of molecularly recognisable DNA damage include the formation of DNA adducts and reorganization of the DNA sequence. These kinds of damage can be detected by measurements of DNA adducts using various techniques, for example, either 32P-postlabelling or the detection of monoclonal antibodies to DNA adducts. Measurement of DNA strand breaks is conventionally carried out using alkaline elution or unwinding assays. Mutations may be detected by sequencing the DNA of a specific gene, for example, the HPRT gene.
Several methodological reports have appeared that discuss the techniques of table 2 in detail (CEC 1987; IARC 1987, 1992, 1993).
Genotoxicity can also be monitored indirectly through the measurement of protein adducts, that is, in haemoglobin instead of DNA, or the monitoring of DNA repair activity. As a measuring strategy, the monitoring activity may be either one time or continuous. In all cases the results must be applied to the development of safe working conditions.
Cytogenetic Biomonitoring
A theoretical and empirical rationale links cancer to chromosome damage. Mutational events altering the activity or expression of growth-factor genes are key steps in carcinogenesis. Many types of cancers have been associated with specific or nonspecific chromosomal aberrations. In several hereditary human diseases, chromosome instability is associated with increased susceptibility to cancer.
Cytogenetic surveillance of people exposed to carcinogenic and/or mutagenic chemicals or radiation can bring to light effects on the genetic material of the individuals concerned. Chromosomal aberration studies of people exposed to ionizing radiation have been applied for biological dosimetry for decades, but well-documented positive results are as yet available only for a limited number of chemical carcinogens.
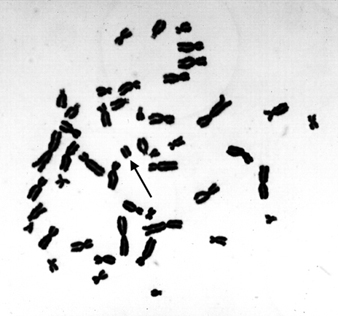
Microscopically recognizable chromosomal damage includes both structural chromosomal aberrations (CA), in which a gross change in the morphology (shape) of a chromosome has occurred, and by sister chromatid exchanges (SCE). SCE is the symmetrical exchange of chromosomal materials between two sister chromatids. Micronuclei (MN) can arise either from acentric chromosome fragments or from lagging whole chromosomes. These types of changes are illustrated in figure 4.
Figure 4. Human lymphocyte chromosomes at metaphase, revealing an induced chromosome mutation (arrow pointing to an acentric fragment)
Peripheral blood lymphocytes in humans are suitable cells to be used in surveillance studies because of their easy accessibility and because they can integrate exposure over a relatively long lifespan. Exposure to a variety of chemical mutagens may result in increased frequencies of CAs and/or SCEs in blood lymphocytes of exposed individuals. Also, the extent of damage is roughly correlated with exposure, although this has been shown with only a few chemicals.
When cytogenetic tests on peripheral blood lymphocytes show that the genetic material has been damaged, the results can be used to estimate risk only at the level of the population. An increased frequency of CAs in a population should be considered an indication of increased risk to cancer, but cytogenetic tests do not, as such, allow individual risk prediction of cancer.
The health significance of somatic genetic damage as seen through the narrow window of a sample of peripheral blood lymphocytes has little or no significance to the health of an individual, since most of the lymphocytes carrying genetic damage die and are replaced.
Problems and their Control in Human Biomonitoring Studies
Rigorous study design is necessary in the application of any human biomonitoring method, since many interindividual factors that are not related to the specific chemical exposure(s) of interest may affect the biological responses studied. Since human biomonitoring studies are tedious and difficult in many respects, careful preplanning is very important. In performing human cytogenetic studies, experimental confirmation of the chromosome damaging potential of the exposing agent(s) should always be an experimental prerequisite.
In cytogenetic biomonitoring studies, two major types of variations have been documented. The first includes technical factors associated with slide-reading discrepancies and with culture conditions, specifically with the type of medium, temperature, and concentration of chemicals (such as bromodeoxyuridine or cytochalasin-B). Also, sampling times can alter chromosome aberration yields, and possibly also findings of SCE incidence, through changes in subpopulations of T- and B-lymphocytes. In micronucleus analyses, methodological differences (e.g., use of binucleated cells induced by cytochalasin-B) quite clearly affect the scoring results.
The lesions induced in the DNA of lymphocytes by chemical exposure that lead to formation of structural chromosome aberrations, sister chromatid exchange, and micronuclei must persist in vivo until the blood is withdrawn and then in vitro until the cultured lymphocyte begins DNA synthesis. It is, therefore, important to score cells directly after the first division (in the case of chromosome aberrations or micronuclei) or after the second division (sister chromatid exchanges) in order to obtain the best estimate of induced damage.
Scoring constitutes an extremely important element in cytogenetic biomonitoring. Slides must be randomized and coded to avoid scorer bias as far as possible. Consistent scoring criteria, quality control and standardized statistical analyses and reporting should be maintained. The second category of variability is due to conditions associated with the subjects, such as age, sex, medication and infections. Individual variations can also be caused by genetic susceptibility to environmental agents.
It is critical to obtain a concurrent control group that is matched as closely as possible on internal factors such as sex and age as well as on factors such as smoking status, viral infections and vaccinations, alcohol and drug intake, and exposure to x-rays. Additionally, it is necessary to obtain qualitative (job category, years exposed) and quantitative (e.g., breathing zone air samples for chemical analysis and specific metabolites, if possible) estimates or exposure to the putative genotoxic agent(s) in the workplace. Special consideration should be paid to proper statistical treatment of the results.
Relevancy of genetic biomonitoring to cancer risk assessment
The number of agents repeatedly shown to induce cytogenetic changes in humans is still relatively limited, but most known carcinogens induce damage in lymphocyte chromosomes.
The extent of damage is a function of exposure level, as has been shown to be the case with, for example, vinyl chloride, benzene, ethylene oxide, and alkylating anticancer agents. Even if the cytogenetic end points are not very sensitive or specific as regards the detection of exposures occurring in present-day occupational settings, positive results of such tests have often prompted implementation of hygienic controls even in the absence of direct evidence relating somatic chromosomal damage to adverse health outcomes.
Most experience with application of cytogenetic biomonitoring derives from “high exposure” occupational situations. Very few exposures have been confirmed by several independent studies, and most of these have been performed using chromosomal aberration biomonitoring. The database of the International Agency for Research on Cancer lists in its updated volumes 43–50 of the IARC Monographs a total of 14 occupational carcinogens in groups 1, 2A or 2B, for which there is positive human cytogenetic data available that are in most cases supported by corresponding animal cytogenetics (table 3). This limited database suggests that there is a tendency for carcinogenic chemicals to be clastogenic, and that clastogenicity tends to be associated with known human carcinogens. Quite clearly, however, not all carcinogens induce cytogenetic damage in humans or experimental animals in vivo. Cases in which the animal data are positive and the human findings are negative may represent differences in exposure levels. Also, the complex and long-term human exposures at work may not be comparable with short-term animal experiments.
Table 3. Proven, probable and possible human carcinogens for which occupational exposure exists and for which cytogenetic end points have been measured in both humans and experimental animals
|
Cytogenic findings1 |
||||||
|
Humans |
Animals |
|||||
|
Agent/exposure |
CA |
SCE |
MN |
CA |
SCE |
MN |
|
GROUP 1, Human carcinogens |
||||||
|
Arsenic and arsenic compounds |
? |
? |
|
+ |
|
+ |
|
Asbestos |
|
? |
|
– |
|
– |
|
Benzene |
+ |
|
|
+ |
+ |
+ |
|
Bis(chloromethyl)ether and chloromethyl methyl ether (technical grade) |
(+) |
|
|
– |
|
|
|
Cyclophosphamide |
+ |
+ |
|
+ |
+ |
+ |
|
Hexavalent chromium compounds |
+ |
+ |
|
+ |
+ |
+ |
|
Melphalan |
+ |
+ |
|
+ |
|
|
|
Nickel compounds |
+ |
– |
|
? |
|
|
|
Radon |
+ |
|
|
– |
|
|
|
Tobacco smoke |
+ |
+ |
+ |
|
+ |
|
|
Vinyl chloride |
+ |
? |
|
+ |
+ |
+ |
|
GROUP 2A, Probable human carcinogens |
||||||
|
Acrylonitrile |
– |
|
|
– |
|
– |
|
Adriamycin |
+ |
+ |
|
+ |
+ |
+ |
|
Cadmium and cadmium compounds |
– |
(–) |
|
– |
|
|
|
Cisplatin |
|
+ |
|
+ |
+ |
|
|
Epichlorohydrin |
+ |
|
|
? |
+ |
– |
|
Ethylene dibromide |
– |
– |
|
– |
+ |
– |
|
Ethylene oxide |
+ |
+ |
+ |
+ |
+ |
+ |
|
Formaldehyde |
? |
? |
|
– |
|
– |
|
GROUP 2B, Possible human carcinogens |
||||||
|
Chlorophenoxy herbicides (2,4-D and 2,4,5-T) |
– |
– |
|
+ |
+ |
– |
|
DDT |
? |
|
|
+ |
|
– |
|
Dimethylformamide |
(+) |
|
|
|
– |
– |
|
Lead compounds |
? |
? |
|
? |
– |
? |
|
Styrene |
+ |
? |
+ |
? |
+ |
+ |
|
2,3,7,8-Tetrachlorodibenzo-para-dioxin |
? |
|
|
– |
– |
– |
|
Welding fumes |
+ |
+ |
|
– |
– |
|
1 CA, chromosomal aberration; SCE, sister chromatid exchange; MN, micronuclei.
(–) = negative relationship for one study; – = negative relationship;
(+) = positive relationship for one study; + = positive relationship;
? = inconclusive; blank area = not studied
Source: IARC, 1987; updated through volumes 43–50 of IARC monographs.
Studies of genotoxicity in exposed humans include various end points other than chromosomal end points, such as DNA damage, DNA repair activity, and adducts in DNA and in proteins. Some of these end points may be more relevant than others for the prediction of carcinogenic hazard. Stable genetic changes (e.g., chromosomal rearrangements, deletions, and point mutations) are highly relevant, since these types of damage are known to be related to carcinogenesis. The significance of DNA adducts is dependent upon their chemical identification and evidence that they result from the exposure. Some endpoints, such as SCE, UDS, SSB, DNA strand breakage, are potential indicators and/or markers of genetic events; however, their value is reduced in the absence of a mechanistic understanding of their ability to lead to genetic events. Clearly, the most relevant genetic marker in humans would be the induction of a specific mutation that has been directly associated with cancer in rodents exposed to the agent under study (figure 5).
Figure 5. Relevance of different genetic biomonitoring effects for potential cancer risk
Ethical Considerations for Genetic Biomonitoring
Rapid advances in molecular genetic techniques, the enhanced speed of sequencing of the human genome, and the identification of the role of tumour suppressor genes and proto-oncogenes in human carcinogenesis, raise ethical issues in the interpretation, communication, and use of this kind of personal information. Quickly improving techniques for the analysis of human genes will soon allow the identification of yet more inherited susceptibility genes in healthy, asymptomatic individuals (US Office of Technology Assessment 1990), lending themselves to be used in genetic screening.
Many questions of social and ethical concern will be raised if the application of genetic screening soon becomes a reality. Already at present roughly 50 genetic traits of metabolism, enzyme polymorphisms, and DNA repair are suspected for specific disease sensitivities, and a diagnostic DNA test is available for about 300 genetic diseases. Should any genetic screening at all be performed at the workplace? Who is to decide who will undergo testing, and how will the information be used in employment decisions? Who will have access to the information obtained from genetic screening, and how will the results be communicated to the person(s) involved? Many of these questions are strongly linked to social norms and prevailing ethical values. The main objective must be the prevention of disease and human suffering, but respect must be accorded to the individual’s own will and ethical premises. Some of the relevant ethical questions which must be answered well before the outset of any workplace biomonitoring study are given in table 4 and are also discussed in the chapter Ethical Issues.
Table 4. Some ethical principles relating to the need to know in occupational genetic biomonitoring studies
|
Groups to whom information is given |
|||
|
Information given |
Persons studied |
Occupational health unit |
Employer |
|
What is being studied |
|||
|
Why is the study performed |
|||
|
Are there risks involved |
|||
|
Confidentiality issues |
|||
|
Preparedness for possible hygienic improvements, exposure reductions indicated |
|||
Time and effort must be put into the planning phase of any genetic biomonitoring study, and all necessary parties—the employees, employers, and the medical personnel of the collaborating workplace—must be well-informed before the study, and the results made known to them after the study as well. With proper care and reliable results, genetic biomonitoring can help to ensure safer workplaces and improve workers’ health.