Introduction
Human exposure to pesticides has different characteristics according to whether it occurs during industrial production or use (table 1). The formulation of commercial products (by mixing active ingredients with other coformulants) has some exposure characteristics in common with pesticide use in agriculture. In fact, since formulation is typically performed by small industries which manufacture many different products in successive operations, the workers are exposed to each of several pesticides for a short time. In public health and agriculture, the use of a variety of compounds is generally the rule, although in some specific applications (for example, cotton defoliation or malaria control programmes) a single product may be used.
Table 1. Comparison of exposure characteristics during production and use of pesticides
|
Exposure on production |
Exposure on use |
|
|
Duration of exposure |
Continuous and prolonged |
Variable and intermittent |
|
Degree of exposure |
Fairly constant |
Extremely variable |
|
Type of exposure |
To one or few compounds |
To numerous compounds either in sequence or concomitantly |
|
Skin absorption |
Easy to control |
Variable according to work procedures |
|
Ambient monitoring |
Useful |
Seldom informative |
|
Biological monitoring |
Complementary to ambient monitoring |
Very useful when available |
Source: WHO 1982a, modified.
The measurement of biological indicators of exposure is particularly useful for pesticide users where the conventional techniques of exposure assessment through ambient air monitoring are scarcely applicable. Most pesticides are lipid-soluble substances that penetrate the skin. The occurrence of percutaneous (skin) absorption makes the use of biological indicators very important in assessing the level of exposure in these circumstances.
Organophosphate Insecticides
Biological indicators of effect:
Cholinesterases are the target enzymes accounting for organophosphate (OP) toxicity to insect and mammalian species. There are two principal types of cholinesterases in the human organism: acetylcholinesterase (ACHE) and plasma cholinesterase (PCHE). OP causes toxic effects in humans through the inhibition of synaptic acetylcholinesterase in the nervous system. Acetylcholinesterase is also present in red blood cells, where its function is unknown. Plasma cholinesterase is a generic term covering an inhomogeneous group of enzymes present in glial cells, plasma, liver and some other organs. PCHE is inhibited by OPs, but its inhibition does not produce known functional derangements.
Inhibition of blood ACHE and PCHE activity is highly correlated with intensity and duration of OP exposure. Blood ACHE, being the same molecular target as that responsible for acute OP toxicity in the nervous system, is a more specific indicator than PCHE. However, sensitivity of blood ACHE and PCHE to OP inhibition varies among the individual OP compounds: at the same blood concentration, some inhibit more ACHE and others more PCHE.
A reasonable correlation exists between blood ACHE activity and the clinical signs of acute toxicity (table 2). The correlation tends to be better as the rate of inhibition is faster. When inhibition occurs slowly, as with chronic low-level exposures, the correlation with illness may be low or totally non-existent. It must be noted that blood ACHE inhibition is not predictive for chronic or delayed effects.
Table 2. Severity and prognosis of acute OP toxicity at different levels of ACHE inhibition
|
ACHE inhibition (%) |
Level of poisoning |
Clinical symptoms |
Prognosis |
|
50–60 |
Mild |
Weakness, headache, dizziness, nausea, salivation, lacrimation, miosis, moderate bronchial spasm |
Convalescence in 1-3 days |
|
60–90 |
Moderate |
Abrupt weakness, visual disturbance, excess salivation, sweating, vomiting, diarrhoea, bradycardia, hypertonia, tremors of hands and head, disturbed gait, miosis, pain in the chest, cyanosis of the mucous membranes |
Convalescence in 1-2 weeks |
|
90–100 |
Severe |
Abrupt tremor, generalized convulsions, psychic disturbance, intensive cyanosis, lung oedema, coma |
Death from respiratory or cardiac failure |
Variations of ACHE and PCHE activities have been observed in healthy people and in specific physiopathological conditions (table 3). Thus, the sensitivity of these tests in monitoring OP exposure can be increased by adopting individual pre-exposure values as a reference. Cholinesterase activities after exposure are then compared with the individual baseline values. One should make use of population cholinesterase activity reference values only when pre-exposure cholinesterase levels are not known (table 4).
Table 3. Variations of ACHE and PCHE activities in healthy people and in selected physiopathological conditions
|
Condition |
ACHE activity |
PCHE activity |
|
Healthy people |
||
|
Interindividual variation1 |
10–18 % |
15–25 % |
|
Intraindividual variation1 |
3–7 % |
6% |
|
Sex differences |
No |
10–15 % higher in male |
|
Age |
Reduced up to 6 months old |
|
|
Body mass |
Positive correlation |
|
|
Serum cholesterol |
Positive correlation |
|
|
Seasonal variation |
No |
No |
|
Circadian variation |
No |
No |
|
Menstruation |
Decreased |
|
|
Pregnancy |
Decreased |
|
|
Pathological conditions |
||
|
Reduced activity |
Leukaemia, neoplasm |
Liver disease; uraemia; cancer; heart failure; allergic reactions |
|
Increased activity |
Polycythaemia; thalassaemia; other congenital blood dyscrasias |
Hyperthyroidism; other conditions of high metabolic rate |
1 Source: Augustinsson 1955 and Gage 1967.
Table 4. Cholinesterase activities of healthy people without exposure to OP measured with selected methods
|
Method |
Sex |
ACHE* |
PCHE* |
|
Michel1 (DpH/h) |
male female |
0.77±0.08 0.75±0.08 |
0.95±0.19 0.82±0.19 |
|
Titrimetric1 (mmol/min ml) |
male/female |
13.2±0.31 |
4.90±0.02 |
|
Ellman’s modified2 (UI/ml) |
male female |
4.01±0.65 3.45±0.61 |
3.03±0.66 3.03±0.68 |
* mean result, ± standard deviation.
Source: 1 Laws 1991. 2 Alcini et al. 1988.
Blood should preferably be sampled within two hours after exposure. Venipuncture is preferred to extracting capillary blood from a finger or earlobe because the sampling point can be contaminated with pesticide residing on the skin in exposed subjects. Three sequential samples are recommended to establish a normal baseline for each worker before exposure (WHO 1982b).
Several analytical methods are available for the determination of blood ACHE and PCHE. According to WHO, the Ellman spectrophotometric method (Ellman et al. 1961) should serve as a reference method.
Biological indicators of exposure.
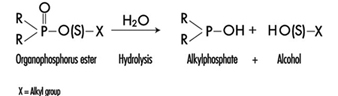
The determination in urine of metabolites that are derived from the alkyl phosphate moiety of the OP molecule or of the residues generated by the hydrolysis of the P–X bond (figure 1) has been used to monitor OP exposure.
Figure 1. Hydrolysis of OP insecticides
Alkyl phosphate metabolites.
The alkyl phosphate metabolites detectable in urine and the main parent compound from which they can originate are listed in table 5. Urinary alkyl phosphates are sensitive indicators of exposure to OP compounds: the excretion of these metabolites in urine is usually detectable at an exposure level at which plasma or erythrocyte cholinesterase inhibition cannot be detected. The urinary excretion of alkyl phosphates has been measured for different conditions of exposure and for various OP compounds (table 6). The existence of a relationship between external doses of OP and alkyl phosphate urinary concentrations has been established in a few studies. In some studies a significant relationship between cholinesterase activity and levels of alkyl phosphates in urine has also been demonstrated.
Table 5. Alkyl phosphates detectable in urine as metabolites of OP pesticides
|
Metabolite |
Abbreviation |
Principal parent compounds |
|
Monomethylphosphate |
MMP |
Malathion, parathion |
|
Dimethylphosphate |
DMP |
Dichlorvos, trichlorfon, mevinphos, malaoxon, dimethoate, fenchlorphos |
|
Diethylphosphate |
DEP |
Paraoxon, demeton-oxon, diazinon-oxon, dichlorfenthion |
|
Dimethylthiophosphate |
DMTP |
Fenitrothion, fenchlorphos, malathion, dimethoate |
|
Diethylthiophosphate |
DETP |
Diazinon, demethon, parathion,fenchlorphos |
|
Dimethyldithiophosphate |
DMDTP |
Malathion, dimethoate, azinphos-methyl |
|
Diethyldithiophosphate |
DEDTP |
Disulfoton, phorate |
|
Phenylphosphoric acid |
Leptophos, EPN |
Table 6. Examples of levels of urinary alkyl phosphates measured in various conditions of exposure to OP
|
Compound |
Condition of exposure |
Route of exposure |
Metabolite concentrations1 (mg/l) |
|
Parathion2 |
Nonfatal poisoning |
Oral |
DEP = 0.5 DETP = 3.9 |
|
Disulfoton2 |
Formulators |
Dermal/inhalation |
DEP = 0.01-4.40 DETP = 0.01-1.57 DEDTP = <0.01-.05 |
|
Phorate2 |
Formulators |
Dermal/inhalation |
DEP = 0.02-5.14 DETP = 0.08-4.08 DEDTP = <0.01-0.43 |
|
Malathion3 |
Sprayers |
Dermal |
DMDTP = <0.01 |
|
Fenitrothion3 |
Sprayers |
Dermal |
DMP = 0.01-0.42 DMTP = 0.02-0.49 |
|
Monocrotophos4 |
Sprayers |
Dermal/inhalation |
DMP = <0.04-6.3/24 h |
1 For abbreviations see table 27.12 [BMO12TE].
2 Dillon and Ho 1987.
3 Richter 1993.
4 van Sittert and Dumas 1990.
Alkyl phosphates are usually excreted in urine within a short time. Samples collected soon after the end of the workday are suitable for metabolite determination.
The measurement of alkyl phosphates in urine requires a rather sophisticated analytical method, based on derivatization of the compounds and detection by gas-liquid chromatography (Shafik et al. 1973a; Reid and Watts 1981).
Hydrolytic residues.
p-Nitrophenol (PNP) is the phenolic metabolite of parathion, methylparathion and ethyl parathion, EPN. The measurement of PNP in urine (Cranmer 1970) has been widely used and has proven to be successful in evaluating exposure to parathion. Urinary PNP correlates well with the absorbed dose of parathion. With PNP urinary levels up to 2 mg/l, the absorption of parathion does not cause symptoms, and little or no reduction of cholinesterase activities is observed. PNP excretion occurs rapidly and urinary levels of PNP become insignificant 48 hours after exposure. Thus, urine samples should be collected soon after exposure.
Carbamates
Biological indicators of effect.
Carbamate pesticides include insecticides, fungicides and herbicides. Insecticidal carbamate toxicity is due to the inhibition of synaptic ACHE, while other mechanisms of toxicity are involved for herbicidal and fungicidal carbamates. Thus, only exposure to carbamate insecticides can be monitored through the assay of cholinesterase activity in red blood cells (ACHE) or plasma (PCHE). ACHE is usually more sensitive to carbamate inhibitors than PCHE. Cholinergic symptoms have been usually observed in carbamate-exposed workers with a blood ACHE activity lower than 70% of the individual baseline level (WHO 1982a).
Inhibition of cholinesterases by carbamates is rapidly reversible. Therefore, false negative results can be obtained if too much time elapses between exposure and biological sampling or between sampling and analysis. In order to avoid such problems, it is recommended that blood samples be collected and analysed within four hours after exposure. Preference should be given to the analytical methods that allow the determination of cholinesterase activity immediately after blood sampling, as discussed for organophosphates.
Biological indicators of exposure.
The measurement of urinary excretion of carbamate metabolites as a method to monitor human exposure has so far been applied only to few compounds and in limited studies. Table 7 summarizes the relevant data. Since carbamates are promptly excreted in the urine, samples collected soon after the end of exposure are suitable for metabolite determination. Analytical methods for the measurements of carbamate metabolites in urine have been reported by Dawson et al. (1964); DeBernardinis and Wargin (1982) and Verberk et al. (1990).
Table 7. Levels of urinary carbamate metabolites measured in field studies
|
Compound |
Biological index |
Condition of exposure |
Environmental concentrations |
Results |
References |
|
Carbaryl |
a-naphthol a-naphthol a-naphthol |
formulators mixer/applicators unexposed population |
0.23–0.31 mg/m3 |
x=18.5 mg/l1 , max. excretion rate = 80 mg/day x=8.9 mg/l, range = 0.2–65 mg/l range = 1.5–4 mg/l |
WHO 1982a |
|
Pirimicarb |
metabolites I2 and V3 |
applicators |
range = 1–100 mg/l |
Verberk et al. 1990 |
1 Systemic poisonings have been occasionally reported.
2 2-dimethylamino-4-hydroxy-5,6-dimethylpyrimidine.
3 2-methylamino-4-hydroxy-5,6-dimethylpyrimidine.
x = standard deviation.
Dithiocarbamates
Biological indicators of exposure.
Dithiocarbamates (DTC) are widely used fungicides, chemically grouped in three classes: thiurams, dimethyldithiocarbamates and ethylene-bis-dithiocarbamates.
Carbon disulphide (CS2) and its main metabolite 2-thiothiazolidine-4-carboxylic acid (TTCA) are metabolites common to almost all DTC. A significant increase in urinary concentrations of these compounds has been observed for different conditions of exposure and for various DTC pesticides. Ethylene thiourea (ETU) is an important urinary metabolite of ethylene-bis-dithiocarbamates. It may also be present as an impurity in market formulations. Since ETU has been determined to be a teratogen and a carcinogen in rats and in other species and has been associated with thyroid toxicity, it has been widely applied to monitor ethylene-bis-dithiocarbamate exposure. ETU is not compound-specific, as it may be derived from maneb, mancozeb or zineb.
Measurement of the metals present in the DTC has been proposed as an alternative approach in monitoring DTC exposure. Increased urinary excretion of manganese has been observed in workers exposed to mancozeb (table 8).
Table 8. Levels of urinary dithiocarbamate metabolites measured in field studies
|
Compound |
Biological index |
Condition of exposure |
Environmental concentrations* ± standard deviation |
Results ± standard deviation |
References |
|
Ziram |
Carbon disulphide (CS2) TTCA1 |
formulators formulators |
1.03 ± 0.62 mg/m3 |
3.80 ± 3.70 mg/l 0.45 ± 0.37 mg/l |
Maroni et al. 1992 |
|
Maneb/Mancozeb |
ETU2 |
applicators |
range = < 0.2–11.8 mg/l |
Kurttio et al. 1990 |
|
|
Mancozeb |
Manganese |
applicators |
57.2 mg/m3 |
pre-exposure: 0.32 ± 0.23 mg/g creatinine; post-exposure: 0.53 ± 0.34 mg/g creatinine |
Canossa et al. 1993 |
* Mean result according to Maroni et al. 1992.
1 TTCA = 2-thiothiazolidine-4-carbonylic acid.
2 ETU = ethylene thiourea.
CS2, TTCA, and manganese are commonly found in urine of non-exposed subjects. Thus, the measurement of urinary levels of these compounds prior to exposure is recommended. Urine samples should be collected in the morning following the cessation of exposure. Analytical methods for the measurements of CS2, TTCA and ETU have been reported by Maroni et al. (1992).
Synthetic Pyrethroids
Biological indicators of exposure.
Synthetic pyrethroids are insecticides similar to natural pyrethrins. Urinary metabolites suitable for application in biological monitoring of exposure have been identified through studies with human volunteers. The acidic metabolite 3-(2,2’-dichloro-vinyl)-2,2’-dimethyl-cyclopropane carboxylic acid (Cl2CA) is excreted both by subjects orally dosed with permethrin and cypermethrin and the bromo-analogue (Br2CA) by subjects treated with deltamethrin. In the volunteers treated with cypermethrin, a phenoxy metabolite, 4-hydroxy-phenoxy benzoic acid (4-HPBA), has also been identified. These tests, however, have not often been applied in monitoring occupational exposures because of the complex analytical techniques required (Eadsforth, Bragt and van Sittert 1988; Kolmodin-Hedman, Swensson and Akerblom 1982). In applicators exposed to cypermethrin, urinary levels of Cl2CA have been found to range from 0.05 to 0.18 mg/l, while in formulators exposed to a-cypermethrin, urinary levels of 4-HPBA have been found to be lower than 0.02 mg/l.
A 24-hour urine collection period started after the end of exposure is recommended for metabolite determinations.
Organochlorines
Biological indicators of exposure.
Organochlorine (OC) insecticides were widely used in the 1950s and 1960s. Subsequently, the use of many of these compounds was discontinued in many countries because of their persistence and consequent contamination of the environment.
Biological monitoring of OC exposure can be carried out through the determination of intact pesticides or their metabolites in blood or serum (Dale, Curley and Cueto 1966; Barquet, Morgade and Pfaffenberger 1981). After absorption, aldrin is rapidly metabolized to dieldrin and can be measured as dieldrin in blood. Endrin has a very short half-life in blood. Therefore, endrin blood concentration is of use only in determining recent exposure levels. The determination of the urinary metabolite anti-12-hydroxy-endrin has also proven to be useful in monitoring endrin exposure (van Sittert and Tordoir 1987) .
Significant correlations between the concentration of biological indicators and the onset of toxic effects have been demonstrated for some OC compounds. Instances of toxicity due to aldrin and dieldrin exposure have been related to levels of dieldrin in blood above 200 μg/l. A blood lindane concentration of 20 μg/l has been indicated as the upper critical level as far as neurological signs and symptoms are concerned. No acute adverse effects have been reported in workers with blood endrin concentrations below 50 μg/l. Absence of early adverse effects (induction of liver microsomal enzymes) has been shown on repeated exposures to endrin at urinary anti-12-hydroxy-endrin concentrations below 130 μg/g creatinine and on repeated exposures to DDT at DDT or DDE serum concentrations below 250 μg/l.
OC may be found in low concentrations in the blood or urine of the general population. Examples of observed values are as follows: lindane blood concentrations up to 1 μg/l, dieldrin up to 10 μg/l, DDT or DDE up to 100 μg/l, and anti-12-hydroxy-endrin up to 1 μg/g creatinine. Thus, a baseline assessment prior to exposure is recommended.
For exposed subjects, blood samples should be taken immediately after the end of a single exposure. For conditions of long-term exposure, the time of collection of the blood sample is not critical. Urine spot samples for urinary metabolite determination should be collected at the end of exposure.
Triazines
Biological indicators of exposure.
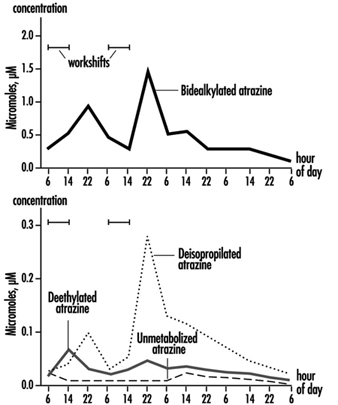
The measurement of urinary excretion of triazinic metabolites and the unmodified parent compound has been applied to subjects exposed to atrazine in limited studies. Figure 2 shows the urinary excretion profiles of atrazine metabolites of a manufacturing worker with dermal exposure to atrazine ranging from 174 to 275 μmol/workshift (Catenacci et al. 1993). Since other chlorotriazines (simazine, propazine, terbuthylazine) follow the same biotransformation pathway of atrazine, levels of dealkylated triazinic metabolites may be determined to monitor exposure to all chlorotriazine herbicides.
Figure 2. Urinary excretion profiles of atrazine metabolites
The determination of unmodified compounds in urine may be useful as a qualitative confirmation of the nature of the compound that has generated the exposure. A 24–hour urine collection period started at the beginning of exposure is recommended for metabolite determination.
Recently, by using an enzyme-linked immunosorbent assay (ELISA test), a mercapturic acid conjugate of atrazine has been identified as its major urinary metabolite in exposed workers. This compound has been found in concentrations at least 10 times higher than those of any dealkylated products. A relationship between cumulative dermal and inhalation exposure and total amount of the mercapturic acid conjugate excreted over a 10-day period has been observed (Lucas et al. 1993).
Coumarin Derivatives
Biological indicators of effect.
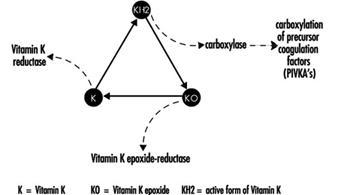
Coumarin rodenticides inhibit the activity of the enzymes of the vitamin K cycle in the liver of mammals, humans included (figure 3), thus causing a dose-related reduction of the synthesis of vitamin K-dependent clotting factors, namely factor II (prothrombin), VII, IX, and X. Anticoagulant effects appear when plasma levels of clotting factors have dropped below approximately 20% of normal.
Figure 3. Vitamin K cycle
These vitamin K antagonists have been grouped into so-called “first generation” (e.g., warfarin) and “second generation” compounds (e.g., brodifacoum, difenacoum), the latter characterized by a very long biological half-life (100 to 200 days).
The determination of prothrombin time is widely used in monitoring exposure to coumarins. However, this test is sensitive only to a clotting factor decrease of approximately 20% of normal plasma levels. The test is not suitable for detection of early effects of exposure. For this purpose, the determination of the prothrombin concentration in plasma is recommended.
In the future, these tests might be replaced by the determination of coagulation factor precursors (PIVKA), which are substances detectable in blood only in the case of blockage of the vitamin K cycle by coumarins.
With conditions of prolonged exposure, the time of blood collection is not critical. In cases of acute overexposure, biological monitoring should be carried out for at least five days after the event, in view of the latency of the anticoagulant effect. To increase the sensitivity of these tests, the measurement of baseline values prior to exposure is recommended.
Biological indicators of exposure.
The measurement of unmodified coumarins in blood has been proposed as a test to monitor human exposure. However, experience in applying these indices is very limited mainly because the analytical techniques are much more complex (and less standardized) in comparison with those required to monitor the effects on the coagulation system (Chalermchaikit, Felice and Murphy 1993).
Phenoxy Herbicides
Biological indicators of exposure.
Phenoxy herbicides are scarcely biotransformed in mammals. In humans, more than 95% of a 2,4-dichlorophenoxyacetic acid (2,4-D) dose is excreted unchanged in urine within five days, and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) and 4-chloro-2-methylphenoxyacetic acid (MCPA) are also excreted mostly unchanged via urine within a few days after oral absorption. The measurement of unchanged compounds in urine has been applied in monitoring occupational exposure to these herbicides. In field studies, urinary levels of exposed workers have been found to range from 0.10 to 8 μg/l for 2,4-D, from 0.05 to 4.5 μg/l for 2,4,5-T and from below 0.1 μg/l to 15 μg/l for MCPA. A 24-hour period of urine collection starting at the end of exposure is recommended for the determination of unchanged compounds. Analytical methods for the measurements of phenoxy herbicides in urine have been reported by Draper (1982).
Quaternary Ammonium Compounds
Biological indicators of exposure.
Diquat and paraquat are herbicides scarcely biotransformed by the human organism. Because of their high water solubility, they are readily excreted unchanged in urine. Urine concentrations below the analytical detection limit (0.01 μg/l) have been often observed in paraquat exposed workers; while in tropical countries, concentrations up to 0.73 μg/l have been measured after improper paraquat handling. Urinary diquat concentrations lower than the analytical detection limit (0.047 μg/l) have been reported for subjects with dermal exposures from 0.17 to 1.82 μg/h and inhalation exposures lower than 0.01 μg/h. Ideally, 24 hours sampling of urine collected at the end of exposure should be used for analysis. When this is impractical, a spot sample at the end of the workday can be used.
Determination of paraquat levels in serum is useful for prognostic purposes in case of acute poisoning: patients with serum paraquat levels up to 0.1 μg/l twenty-four hours after ingestion are likely to survive.
The analytical methods for paraquat and diquat determination have been reviewed by Summers (1980).
Miscellaneous Pesticides
4,6-Dinitro-o-cresol (DNOC).
DNOC is an herbicide introduced in 1925, but the use of this compound has been progressively decreased due to its high toxicity to plants and to humans. Since blood DNOC concentrations correlate to a certain extent with the severity of adverse health effects, the measure of unchanged DNOC in blood has been proposed for monitoring occupational exposures and for the evaluation of the clinical course of poisonings.
Pentachlorophenol.
Pentachlorophenol (PCP) is a wide-spectrum biocide with pesticidal action against weeds, insects, and fungi. Measurements of blood or urinary unchanged PCP have been recommended as suitable indices in monitoring occupational exposures (Colosio et al. 1993), because these parameters are significantly correlated with PCP body burden. In workers with prolonged exposure to PCP the time of blood collection is not critical, while urine spot samples should be collected on the morning after exposure.
A multiresidue method for the measurement of halogenated and nitrophenolic pesticides has been described by Shafik et al.(1973b).
Other tests proposed for the biological monitoring of pesticide exposure are listed in table 9.
Table 9. Other indices proposed in the literature for the biological monitoring of pesticide exposure
|
Compound |
Biological index |
|
|
Urine |
Blood |
|
|
Bromophos |
Bromophos |
Bromophos |
|
Captan |
Tetrahydrophtalimide |
|
|
Carbofuran |
3-Hydroxycarbofuran |
|
|
Chlordimeform |
4-Chloro-o-toluidine derivatives |
|
|
Chlorobenzilate |
p,p-1-Dichlorobenzophenone |
|
|
Dichloropropene |
Mercapturic acid metabolites |
|
|
Fenitrothion |
p-Nitrocresol |
|
|
Ferbam |
Thiram |
|
|
Fluazifop-Butyl |
Fluazifop |
|
|
Flufenoxuron |
Flufenoxuron |
|
|
Glyphosate |
Glyphosate |
|
|
Malathion |
Malathion |
Malathion |
|
Organotin compounds |
Tin |
Tin |
|
Trifenomorph |
Morpholine, triphenylcarbinol |
|
|
Ziram |
Thiram |
|
Conclusions
Biological indicators for monitoring pesticide exposure have been applied in a number of experimental and field studies.
Some tests, such as those for cholinesterase in blood or for selected unmodified pesticides in urine or blood, have been validated by extensive experience. Biological exposure limits have been proposed for these tests (table 10). Other tests, in particular those for blood or urinary metabolites, suffer from greater limitations because of analytical difficulties or because of limitations in interpretation of results.
Table 10. Recommended biological limit values (as of 1996)
|
Compound |
Biological index |
BEI1 |
BAT2 |
HBBL3 |
BLV4 |
|
ACHE inhibitors |
ACHE in blood |
70% |
70% |
70%, |
|
|
DNOC |
DNOC in blood |
20 mg/l, |
|||
|
Lindane |
Lindane in blood |
0.02mg/l |
0.02mg/l |
||
|
Parathion |
PNP in urine |
0.5mg/l |
0.5mg/l |
||
|
Pentachlorophenol (PCP) |
PCP in urine PCP in plasma |
2 mg/l 5 mg/l |
0.3mg/l 1 mg/l |
||
|
Dieldrin/Aldrin |
Dieldrin in blood |
100 mg/l |
|||
|
Endrin |
Anti-12-hydroxy-endrin in urine |
130 mg/l |
|||
|
DDT |
DDT and DDEin serum |
250 mg/l |
|||
|
Coumarins |
Prothrombin time in plasma Prothrombin concentration in plasma |
10% above baseline 60% of baseline |
|||
|
MCPA |
MCPA in urine |
0.5 mg/l |
|||
|
2,4-D |
2,4-D in urine |
0.5 mg/l |
1 Biological exposure indices (BEIs) are recommended by the American Conference of Governmental Industrial Hygienists (ACGIH 1995).
2 Biological tolerance values (BATs) are recommended by the German Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (DFG 1992).
3 Health-based biological limits (HBBLs) are recommended by a WHO Study Group (WHO 1982a).
4 Biological limit values (BLVs) are proposed by a Study Group of the Scientific Committee on Pesticides of the International Commission on Occupational Health (Tordoir et al. 1994). Assessment of working conditions is called for if this value is exceeded.
This field is in rapid development and, given the enormous importance of using biological indicators to assess exposure to these substances, new tests will be continuously developed and validated.