After its discovery by Roentgen in 1895, the x ray was introduced so rapidly into the diagnosis and treatment of disease that injuries from excessive radiation exposure began to be encountered almost immediately in pioneer radiation workers, who had yet to become aware of the dangers (Brown 1933). The first such injuries were predominantly skin reactions on the hands of those working with the early radiation equipment, but within a decade many other types of injury also had been reported, including the first cancers attributed to radiation (Stone 1959).
Throughout the century since these early findings, study of the biological effects of ionizing radiation has received continuing impetus from the growing uses of radiation in medicine, science and industry, as well as from the peaceful and military applications of atomic energy. As a result, the biological effects of radiation have been investigated more thoroughly than those of virtually any other environmental agent. The evolving knowledge of radiation effects has been influential in shaping measures for the protection of human health against many other environmental hazards as well as radiation.
Nature and Mechanisms of the Biological Effects of Radiation
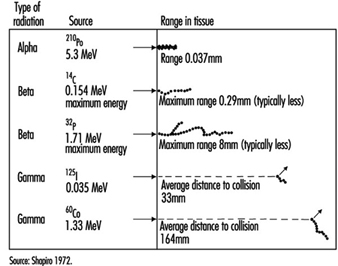
Energy deposition. In contrast to other forms of radiation, ionizing radiation is capable of depositing enough localized energy to dislodge electrons from the atoms with which it interacts. Thus, as radiation collides randomly with atoms and molecules in passing through living cells, it gives rise to ions and free radicals which break chemical bonds and cause other molecular changes that injure the affected cells. The spatial distribution of the ionizing events depends on the radiation weighting factor, w R of the radiation (see table 1 and figure 1).
Table 1. Radiation weighting factors wR
|
Type and energy range |
wR 1 |
|
Photons, all energies |
1 |
|
Electrons and muons, all energies2 |
1 |
|
Neutrons, energy <10 keV |
5 |
|
10 keV to 100 keV |
10 |
|
>100 keV to 2 MeV |
20 |
|
>2 MeV to 20 MeV |
10 |
|
>20 MeV |
5 |
|
Protons, other than recoil protons, energy >2 MeV |
5 |
|
Alpha particles, fission fragments, heavy nuclei |
20 |
1 All values relate to the radiation incident on the body or, for internal sources, emitted from the source.
2 Excluding Auger electrons emitted from nuclei bound to DNA.
Figure 1. Differences among various types of ionizing radiation in penetrating power in tissue
Effects on DNA. Any molecule in the cell may be altered by radiation, but DNA is the most critical biological target because of the limited redundancy of the genetic information it contains. An absorbed dose of radiation large enough to kill the average dividing cell—2 gray (Gy)—suffices to cause hundreds of lesions in its DNA molecules (Ward 1988). Most such lesions are reparable, but those produced by a densely ionizing radiation (for example, a proton or an alpha particle) are generally less reparable than those produced by a sparsely ionizing radiation (for example, an x ray or a gamma ray) (Goodhead 1988). Densely ionizing (high LET) radiations, therefore, typically have a higher relative biological effectiveness (RBE) than sparsely ionizing (low LET) radiations for most forms of injury (ICRP 1991).
Effects on genes. Damage to DNA that remains unrepaired or is misrepaired may be expressed in the form of mutations, the frequency of which appears to increase as a linear, non-threshold function of the dose, approximately 10–5 to 10–6 per locus per Gy (NAS 1990). The fact that the mutation rate appears to be proportional to the dose is interpreted to signify that traversal of the DNA by a single ionizing particle may, in principle, suffice to cause a mutation (NAS 1990). In Chernobyl accident victims, the dose-response relationship for glycophorin mutations in bone marrow cells closely resembles that observed in atomic bomb survivors (Jensen, Langlois and Bigbee 1995).
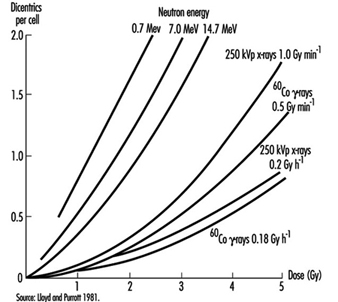
Effects on chromosomes. Radiation damage to the genetic apparatus may also cause changes in chromosome number and structure, the frequency of which has been observed to increase with the dose in radiation workers, atomic bomb survivors, and others exposed to ionizing radiation. The dose-response relationship for chromosome aberrations in human blood lymphocytes (figure 2) has been characterized well enough so that the frequency of aberrations in such cells can serve as a useful biological dosimeter (IAEA 1986).
Figure 2. Frequency of dicentric chromosome aberrations in human lymphocytes in relation to dose, dose rate, and quality of irradiation in vitro
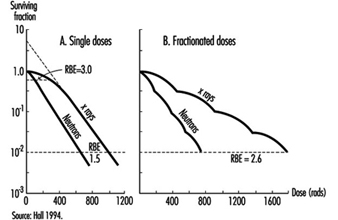
Effects on cell survival. Among the earliest reactions to irradiation is the inhibition of cell division, which appears promptly after exposure, varying both in degree and duration with the dose (figure 3). Although the inhibition of mitosis is characteristically transitory, radiation damage to genes and chromosomes may be lethal to dividing cells, which are highly radiosensitive as a class (ICRP 1984). Measured in terms of proliferative capacity, the survival of dividing cells tends to decrease exponentially with increasing dose, 1 to 2 Gy generally sufficing to reduce the surviving population by about 50% (figure 4).
Figure 3. Mitotic inhibition induced by x rays in rat corneal epithelial cells
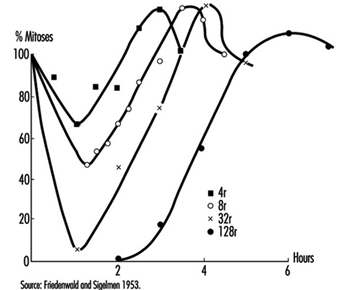
Figure 4. Typical dose-survival curves for mammalian cells exposed to x rays and fast neutrons
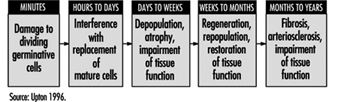
Effects on tissues. Mature, non-dividing cells are relatively radioresistant, but the dividing cells in a tissue are radiosensitive and may be killed in sufficient numbers by intensive irradiation to cause the tissue to become atrophic (figure 5). The rapidity of such atrophy depends on cell population dynamics within the affected tissue; that is, in organs characterized by slow cell turnover, such as the liver and vascular endothelium, the process is typically much slower than in organs characterized by rapid cell turnover, such as the bone marrow, epidermis and intestinal mucosa (ICRP 1984). It is noteworthy, moreover, that if the volume of tissue irradiated is sufficiently small, or if the dose is accumulated gradually enough, the severity of injury may be greatly reduced by the compensatory proliferation of surviving cells.
Figure 5. Characteristic sequence of events in the pathogenesis of nonstochastic effects of ionizing radiation
Clinical Manifestations of Injury
Types of effects. Radiation effects encompass a wide variety of reactions, varying markedly in their dose-response relationships, clinical manifestations, timing and prognosis (Mettler and Upton 1995). The effects are often subdivided, for convenience, into two broad categories: (1) heritable effects, which are expressed in the descendants of exposed individuals, and (2) somatic effects, which are expressed in exposed individuals themselves. The latter include acute effects, which occur relatively soon after irradiation, as well as late (or chronic) effects, such as cancer, which may not appear until months, years or decades later.
Acute effects. The acute effects of radiation result predominantly from the depletion of progenitor cells in affected tissues (figure 5) and can be elicited only by doses that are large enough to kill many such cells (for example, table 2). For this reason, such effects are viewed as nonstochastic, or deterministic, in nature (ICRP 1984 and 1991), in contradistinction to the mutagenic and carcinogenic effects of radiation, which are viewed as stochastic phenomena resulting from random molecular alterations in individual cells that increase as linear-nonthreshold functions of the dose (NAS 1990; ICRP 1991).
Table 2. Approximate threshold doses of conventionally fractionated therapeutic x-radiation for clinically detrimental nonstochastic effects in various tissues
|
Organ |
Injury at 5 years |
Threshold |
Irradiation |
|
Skin |
Ulcer, severe fibrosis |
55 |
100 cm2 |
|
Oral mucosa |
Ulcer, severe fibrosis |
60 |
50 cm2 |
|
Oesophagus |
Ulcer, stricture |
60 |
75 cm2 |
|
Stomach |
Ulcer, perforation |
45 |
100 cm2 |
|
Small intestine |
Ulcer, stricture |
45 |
100 cm2 |
|
Colon |
Ulcer, stricture |
45 |
100 cm2 |
|
Rectum |
Ulcer, stricture |
55 |
100 cm2 |
|
Salivary glands |
Xerostomia |
50 |
50 cm2 |
|
Liver |
Liver failure, ascites |
35 |
whole |
|
Kidney |
Nephrosclerosis |
23 |
whole |
|
Urinary bladder |
Ulcer, contracture |
60 |
whole |
|
Testes |
Permanent sterility |
5-15 |
whole |
|
Ovary |
Permanent sterility |
2-3 |
whole |
|
Uterus |
Necrosis, perforation |
>100 |
whole |
|
Vagina |
Ulcer, fistula |
90 |
5 cm2 |
|
Breast, child |
Hypoplasia |
10 |
5 cm2 |
|
Breast, adult |
Atrophy, necrosis |
>50 |
whole |
|
Lung |
Pneumonitis, fibrosis |
40 |
lobe |
|
Capillaries |
Telangiectasis, fibrosis |
50-60 |
s |
|
Heart |
Pericarditis, pancarditis |
40 |
whole |
|
Bone, child |
Arrested growth |
20 |
10 cm2 |
|
Bone, adult |
Necrosis, fracture |
60 |
10 cm2 |
|
Cartilage, child |
Arrested growth |
10 |
whole |
|
Cartilage, adult |
Necrosis |
60 |
whole |
|
Central nervous system (brain) |
Necrosis |
50 |
whole |
|
Spinal cord |
Necrosis, transection |
50 |
5 cm2 |
|
Eye |
Panophthalmitis, haemorrhage |
55 |
whole |
|
Cornea |
Keratitis |
50 |
whole |
|
Lens |
Cataract |
5 |
whole |
|
Ear (inner) |
Deafness |
>60 |
whole |
|
Thyroid |
Hypothyroidism |
45 |
whole |
|
Adrenal |
Hypoadrenalism |
>60 |
whole |
|
Pituitary |
Hypopituitarism |
45 |
whole |
|
Muscle, child |
Hypoplasia |
20-30 |
whole |
|
Muscle, adult |
Atrophy |
>100 |
whole |
|
Bone marrow |
Hypoplasia |
2 |
whole |
|
Bone marrow |
Hypoplasia, fibrosis |
20 |
localized |
|
Lymph nodes |
Atrophy |
33-45 |
s |
|
Lymphatics |
Sclerosis |
50 |
s |
|
Foetus |
Death |
2 |
whole |
* Dose causing effect in 1-5 per cent of exposed persons.
Source: Rubin and Casarett 1972.
Acute injuries of the types that were prevalent in pioneer radiation workers and early radiotherapy patients have been largely eliminated by improvements in safety precautions and treatment methods. Nevertheless, most patients treated with radiation today still experience some injury of the normal tissue that is irradiated. In addition, serious radiation accidents continue to occur. For example, some 285 nuclear reactor accidents (excluding the Chernobyl accident) were reported in various countries between 1945 and 1987, irradiating more than 1,350 persons, 33 of them fatally (Lushbaugh, Fry and Ricks 1987). The Chernobyl accident alone released enough radioactive material to require the evacuation of tens of thousands of people and farm animals from the surrounding area, and it caused radiation sickness and burns in more than 200 emergency personnel and fire-fighters, injuring 31 fatally (UNSCEAR 1988). The long-term health effects of the radioactive material released cannot be predicted with certainty, but estimates of the resulting risks of carcinogenic effects, based on nonthreshold dose-incidence models (discussed below), imply that up to 30,000 additional cancer deaths may occur in the population of the northern hemisphere during the next 70 years as a result of the accident, although the additional cancers in any given country are likely to be too few to be detectable epidemiologically (USDOE 1987).
Less catastrophic, but far more numerous, than reactor accidents have been accidents involving medical and industrial gamma ray sources, which also have caused injuries and loss of life. For example, the improper disposal of a caesium-137 radiotherapy source in Goiânia, Brazil, in 1987, resulted in the irradiation of dozens of unsuspecting victims, four of them fatally (UNSCEAR 1993).
A comprehensive discussion of radiation injuries is beyond the scope of this review, but acute reactions of the more radiosensitive tissues are of widespread interest and are, therefore, described briefly in the following sections.
Skin. Cells in the germinal layer of the epidermis are highly radiosensitive. As a result, rapid exposure of the skin to a dose of 6 Sv or more causes erythema (reddening) in the exposed area, which appears within a day or so, typically lasts a few hours, and is followed two to four weeks later by one or more waves of deeper and more prolonged erythema, as well as by epilation (hair loss). If the dose exceeds 10 to 20 Sv, blistering, necrosis and ulceration may ensue within two to four weeks, followed by fibrosis of the underlying dermis and vasculature, which may lead to atrophy and a second wave of ulceration months or years later (ICRP 1984).
Bone marrow and lymphoid tissue. Lymphocytes also are highly radiosensitive; a dose of 2 to 3 Sv delivered rapidly to the whole body can kill enough of them to depress the peripheral lymphocyte count and impair the immune response within hours (UNSCEAR 1988). Haemopoietic cells in the bone marrow are similarly radiosensitive and are depleted sufficiently by a comparable dose to cause granulocytopenia and thrombocytopenia to ensue within three to five weeks. Such reductions in granulocyte and platelet counts may be severe enough after a larger dose to result in haemorrhage or fatal infection (table 3).
Table 3. Major forms and features of the acute radiation syndrome
|
Time after |
Cerebral form |
Gastro- |
Hemopoietic form |
Pulmonary form |
|
First day |
nausea |
nausea |
nausea |
nausea |
|
Second week |
nausea |
|||
|
Third to sixth |
weakness |
|||
|
Second to eighth |
cough |
Source: UNSCEAR 1988.
Intestine. Stem cells in the epithelium lining the small bowel also are extremely radiosensitive, acute exposure to 10 Sv depleting their numbers sufficiently to cause the overlying intestinal villi to become denuded within days (ICRP 1984; UNSCEAR 1988). Denudation of a large area of the mucosa can result in a fulminating, rapidly fatal dysentery-like syndrome (table 3).
Gonads. Mature spermatozoa can survive large doses (100 Sv), but spermatogonia are so radiosensitive that as little as 0.15 Sv delivered rapidly to both testes suffices to cause oligospermia, and a dose of 2 to 4 Sv can cause permanent sterility. Oocytes, likewise, are radiosensitive, a dose of 1.5 to 2.0 Sv delivered rapidly to both ovaries causing temporary sterility, and a larger dose, permanent sterility, depending on the age of the woman at the time of exposure (ICRP 1984).
Respiratory tract. The lung is not highly radiosensitive, but rapid exposure to a dose of 6 to 10 Sv can cause acute pneumonitis to develop in the exposed area within one to three months. If a large volume of lung tissue is affected, the process may result in respiratory failure within weeks, or may lead to pulmonary fibrosis and cor pulmonale months or years later (ICRP 1984; UNSCEAR 1988).
Lens of the eye. Cells of the anterior epithelium of the lens, which continue to divide throughout life, are relatively radiosensitive. As a result, rapid exposure of the lens to a dose exceeding 1 Sv may lead within months to the formation of a microscopic posterior polar opacity; and 2 to 3 Sv received in a single brief exposure—or 5.5 to 14 Sv accumulated over a period of months—may produce a vision-impairing cataract (ICRP 1984).
Other tissues. In comparison with the tissues mentioned above, other tissues of the body are generally appreciably less radiosensitive (for example, table 2); however, the embryo constitutes a notable exception, as discussed below. Noteworthy also is the fact that the radiosensitivity of every tissue is increased when it is in a rapidly growing state (ICRP 1984).
Whole-body radiation injury. Rapid exposure of a major part of the body to a dose in excess of 1 Gy can cause the acute radiation syndrome. This syndrome includes: (1) an initial prodromal stage, characterized by malaise, anorexia, nausea and vomiting, (2) an ensuing latent period, (3) a second (main) phase of illness and (4) ultimately, either recovery or death (table 3). The main phase of the illness typically takes one of the following forms, depending on the predominant locus of radiation injury: (1) haematological, (2) gastro-intestinal, (3) cerebral or (4) pulmonary (table 3).
Localized radiation injury. Unlike the clinical manifestations of acute whole-body radiation injury, which typically are dramatic and prompt, the reaction to sharply localized irradiation, whether from an external radiation source or from an internally deposited radionuclide, tends to evolve slowly and to produce few symptoms or signs unless the volume of tissue irradiated and/or the dose are relatively large (for example, table 3).
Effects of radionuclides. Some radionuclides - for example, tritium (3H), carbon-14 (14C) and cesium-137 (137Cs) - tend to be distributed systemically and to irradiate the body as a whole, whereas other radionuclides are characteristically taken up and concentrated in specific organs, producing injuries that are correspondingly localized. Radium (Ra) and strontium-90
(90Sr), for example, are deposited predominantly in bone and thus injure skeletal tissues primarily, whereas radioactive iodine concentrates in the thyroid gland, the primary site of any resulting injury (Stannard 1988; Mettler and Upton 1995).
Carcinogenic Effects
General features. The carcinogenicity of ionizing radiation, first manifested early in this century by the occurrence of skin cancers and leukaemias in pioneer radiation workers (Upton 1986), has since been documented extensively by dose-dependent excesses of many types of neoplasms in radium-dial painters, underground hardrock miners, atomic bomb survivors, radiotherapy patients and experimentally irradiated laboratory animals (Upton 1986; NAS 1990).
The benign and malignant growths induced by irradiation characteristically take years or decades to appear and exhibit no known features by which they can be distinguished from those produced by other causes. With few exceptions, moreover, their induction has been detectable only after relatively large dose equivalents (0.5 Sv), and it has varied with the type of neoplasm as well as the age and sex of those exposed (NAS 1990).
Mechanisms. The molecular mechanisms of radiation carcinogenesis remain to be elucidated in detail, but in laboratory animals and cultured cells the carcinogenic effects of radiation have been observed to include initiating effects, promoting effects, and effects on the progression of neoplasia, depending on the experimental conditions in question (NAS 1990). The effects also appear to involve the activation of oncogenes and/or the inactivation or loss of tumor-suppressor genes in many, if not all, instances. In addition, the carcinogenic effects of radiation resemble those of chemical carcinogens in being similarly modifiable by hormones, nutritional variables and other modifying factors (NAS 1990). It is noteworthy, moreover, that the effects of radiation may be additive, synergistic or mutually antagonistic with those of chemical carcinogens, depending on the specific chemicals and exposure conditions in question (UNSCEAR 1982 and 1986).
Dose-effect relationship. Existing data do not suffice to describe the dose-incidence relationship unambiguously for any type of neoplasm or to define how long after irradiation the risk of the growth may remain elevated in an exposed population. Any risks attributable to low-level irradiation can, therefore, be estimated only by extrapolation, based on models incorporating assumptions about such parameters (NAS 1990). Of various dose-effect models that have been used to estimate the risks of low-level irradiation, the one that has been judged to provide the best fit to the available data is of the form:
![]()
where R0 denotes the age-specific background risk of death from a specific type of cancer, D the radiation dose, f(D) a function of dose that is linear-quadratic for leukaemia and linear for some other types of cancer, and g(b) is a risk function dependent on other parameters, such as sex, age at exposure and time after exposure (NAS 1990).
Non-threshold models of this type have been applied to epidemiological data from the Japanese atomic-bomb survivors and other irradiated populations to derive estimates of the lifetime risks of different forms of radiation-induced cancer (for example, table 4). Such estimates must be interpreted with caution, however, in attempting to predict the risks of cancer attributable to small doses or doses that are accumulated over weeks, months or years, since experiments with laboratory animals have shown the carcinogenic potency of x rays and gamma rays to be reduced by as much as an order of magnitude when the exposure is greatly prolonged. In fact, as has been emphasized elsewhere (NAS 1990), the available data do not exclude the possibility that there may be a threshold in the millisievert (mSv) dose equivalent range, below which radiation may lack carcinogenicity.
Table 4. Estimated lifetime risks of cancer attributable to 0.1 Sv rapid irradiation
|
Type or site of cancer |
Excess cancer deaths per 100,000 |
|
|
(No.) |
(%)* |
|
|
Stomach |
110 |
18 |
|
Lung |
85 |
3 |
|
Colon |
85 |
5 |
|
Leukaemia (excluding CLL) |
50 |
10 |
|
Urinary bladder |
30 |
5 |
|
Oesophagus |
30 |
10 |
|
Breast |
20 |
1 |
|
Liver |
15 |
8 |
|
Gonads |
10 |
2 |
|
Thyroid |
8 |
8 |
|
Osteosarcoma |
5 |
5 |
|
Skin |
2 |
2 |
|
Remainder |
50 |
1 |
|
Total |
500 |
2 |
* Percentage increase in “background” expectation for a non-irradiated population.
Source: ICRP 1991.
It is also noteworthy that the estimates tabulated are based on population averages and are not necessarily applicable to any given individual; that is, susceptibility to certain types of cancer (for example, cancers of the thyroid and breast) is substantially higher in children than in adults, and susceptibility to certain cancers is also increased in association with some hereditary disorders, such as retinoblastoma and the nevoid basal cell carcinoma syndrome (UNSCEAR 1988, 1994; NAS 1990). Such differences in susceptibility notwithstanding, population-based estimates have been proposed for use in compensation cases as a basis for gauging the probability that a cancer arising in a previously irradiated person may have been caused by the exposure in question (NIH 1985).
Low-dose risk assessment. Epidemiological studies to ascertain whether the risks of cancer from low-level exposure to radiation actually vary with dose in the manner predicted by the above estimates have been inconclusive thus far. Populations residing in areas of elevated natural background radiation levels manifest no definitely attributable increases in cancer rates (NAS 1990; UNSCEAR 1994); conversely, a few studies have even suggested an inverse relationship between background radiation levels and cancer rates, which has been interpreted by some observers as evidence for the existence of beneficial (or hormetic) effects of low-level irradiation, in keeping with the adaptive responses of certain cellular systems (UNSCEAR 1994). The inverse relationship is of questionable significance, however, since it has not persisted after controlling for the effects of confounding variables (NAS 1990). Likewise in today’s radiation workers—except for certain cohorts of underground hardrock miners (NAS 1994; Lubin, Boice and Edling 1994)—the rates of cancers other than leukaemia are no longer detectably increased (UNSCEAR 1994), thanks to advances in radiation protection; furthermore, the rates of leukaemia in such workers are consistent with the estimates tabulated above (IARC 1994). In summary, therefore, the data available at present are consistent with the estimates tabulated above (table 4), which imply that less than 3% of cancers in the general population are attributable to natural background radiation (NAS 1990; IARC 1994), although up to 10% of lung cancers may be attributable to indoor radon (NAS 1990; Lubin, Boice and Edling 1994).
High levels of radioactive fallout from a thermonuclear weapons test at Bikini in 1954 have been observed to cause a dose-dependent increase in the frequency of thyroid cancer in Marshall Islanders who received large doses to the thyroid gland in childhood (Robbins and Adams 1989). Similarly, children living in areas of Belarus and the Ukraine contaminated by radionuclides released from the Chernobyl accident have been reported to show an increased incidence of thyroid cancer (Prisyazhuik, Pjatak and Buzanov 1991; Kasakov, Demidchik and Astakhova 1992), but the findings are at variance with those of the International Chernobyl Project, which found no excess of benign or malignant thyroid nodules in children living in the more heavily contaminated areas around Chernobyl (Mettler, Williamson and Royal 1992). The basis for the discrepancy, and whether the reported excesses may have resulted from heightened surveillance alone, remain to be determined. In this connection, it is noteworthy that children of south-western Utah and Nevada who were exposed to fallout from nuclear weapons tests in Nevada during the 1950s have shown increase in the frequency of any type of thyroid cancer (Kerber et al. 1993), and the prevalence of acute leukaemia appears to have been elevated in such children dying between 1952 and 1957, the period of greatest exposure to fallout (Stevens et al. 1990).
The possibility that excesses of leukaemia among children residing in the vicinity of nuclear plants in the United Kingdom may have been caused by radioactivity released from the plants has also been suggested. The releases, however, are estimated to have increased the total radiation dose to such children by less than 2%, from which it is inferred that other explanations are more likely (Doll, Evans and Darby 1994). An ineffective aetiology for the observed clusters of leukaemia is implied by the existence of comparable excesses of childhood leukaemia at sites in the UK that lack nuclear facilities but otherwise resemble nuclear sites in having similarly experienced large influxes of population in recent times (Kinlen 1988; Doll, Evans and Darby 1994). Another hypothesis—namely, that the leukaemias in question may have been caused by occupational irradiation of the fathers of the affected children—also has been suggested by the results of a case-control study (Gardner et al. 1990), but this hypothesis is generally discounted for reasons that are discussed in the section to follow.
Heritable Effects
Heritable effects of irradiation, although well documented in other organisms, have yet to be observed in humans. For example, intensive study of more than 76,000 children of the Japanese atomic-bomb survivors, carried out over four decades, has failed to disclose any heritable effects of radiation in this population, as measured by untoward pregnancy outcomes, neonatal deaths, malignancies, balanced chromosomal rearrangements, sex-chromosome aneuploidy, alterations of serum or erythrocyte protein phenotypes, changes in sex ratio or disturbances in growth and development (Neel, Schull and Awa 1990). Consequently, estimates of the risks of heritable effects of radiation must rely heavily on extrapolation from findings in the laboratory mouse and other experimental animals (NAS 1990; UNSCEAR 1993).
From the available experimental and epidemiological data, it is inferred that the dose required to double the rate of heritable mutations in human germ cells must be at least 1.0 Sv (NAS 1990; UNSCEAR 1993). On this basis, it is estimated that less than 1% of all genetically determined diseases in the human population can be attributed to natural background irradiation (table 5).
Table 5. Estimated frequencies of heritable disorders attributable to natural background ionizing irradiation
|
Type of disorder |
Natural prevalence |
Contribution from natural background |
|
|
First generation |
Equilibrium |
||
|
Autosomal |
180,000 |
20-100 |
300 |
|
X-linked |
400 |
<1 |
<15 |
|
Recessive |
2,500 |
<1 |
very slow increase |
|
Chromosomal |
4,400 |
<20 |
very slow increase |
|
Congenital |
20,000-30,000 |
30 |
30-300 |
|
Other disorders of complex aetiology: |
|||
|
Heart disease |
600,000 |
not estimated4 |
not estimated4 |
|
Cancer |
300,000 |
not estimated4 |
not estimated4 |
|
Selected others |
300,000 |
not estimated4 |
not estimated4 |
1 Equivalent to » 1 mSv per year, or » 30 mSv per generation (30 years).
2 Values rounded.
3 After hundreds of generations, the addition of unfavorable radiation-induced mutations eventually becomes balanced by their loss from the population, resulting in a genetic "equilibrium".
4 Quantitative risk estimates are lacking because of uncertainty about the mutational component of the disease(s) indicated.
Source: National Research Council 1990.
The hypothesis that the excess of leukaemia and non-Hodgkin’s lymphoma in young people residing in the village of Seascale resulted from heritable oncogenic effects caused by the occupational irradiation of the children’s fathers at the Sellafield nuclear installation has been suggested by the results of a case-control study (Gardner et al. 1990), as noted above. Arguments against this hypothesis, however, are:
- the lack of any comparable excess in larger numbers of children born outside Seascale to fathers who had received similar, or even larger, occupational doses at the same nuclear plant (Wakeford et al. 1994a)
- the lack of similar excesses in French (Hill and LaPlanche 1990), Canadian (McLaughlin et al. 1993) or Scottish (Kinlen, Clarke and Balkwill 1993) children born to fathers with comparable occupational exposures
- the lack of excesses in the children of atomic-bomb survivors (Yoshimoto et al. 1990)
- the lack of excesses in US counties containing nuclear plants (Jablon, Hrubec and Boice 1991)
- the fact that the frequency of radiation-induced mutations implied by the interpretation is far higher than established rates (Wakeford et al. 1994b).
On balance, therefore, the available data fail to support the paternal gonadal irradiation hypothesis (Doll, Evans and Darby 1994; Little, Charles and Wakeford 1995).
Effects of Prenatal Irradiation
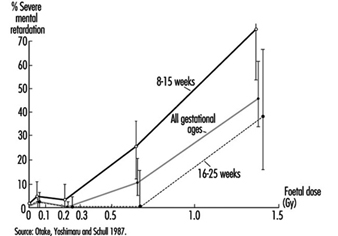
Radiosensitivity is relatively high throughout prenatal life, but the effects of a given dose vary markedly, depending on the developmental stage of the embryo or foetus at the time of exposure (UNSCEAR 1986). During the pre-implantation period, the embryo is most susceptible to killing by irradiation, while during critical stages in organogenesis it is susceptible to the induction of malformations and other disturbances of development (table 6). The latter effects are dramatically exemplified by the dose-dependent increase in the frequency of severe mental retardation (figure 6) and the dose-dependent decrease in IQ test scores in atomic-bomb survivors who were exposed between the eighth and fifteenth weeks (and, to a lesser extent, between the sixteenth and twenty-fifth weeks) (UNSCEAR 1986 and 1993).
Table 6. Major developmental abnormalities produced by prenatal irradiation
|
Brain |
||
|
Anencephaly |
Porencephaly |
Microcephaly* |
|
Encephalocoele |
Mongolism* |
Reduced medulla |
|
Cerebral atrophy |
Mental retardation* |
Neuroblastoma |
|
Narrow aqueduct |
Hydrocephalus* |
Dilatation of ventricles* |
|
Spinal cord anomalies* |
Cranial nerve anomalies |
|
|
Eyes |
||
|
Anophthalmia |
Microphthalmia* |
Microcornia* |
|
Coloboma* |
Deformed iris |
Absence of lens |
|
Absence of retina |
Open eyelids |
Strabismus* |
|
Nystagmus* |
Retinoblastoma |
Hypermetropia |
|
Glaucoma |
Cataract* |
Blindness |
|
Chorioretinitis* |
Partial albinism |
Ankyloblepharon |
|
Skeleton |
||
|
General stunting |
Reduced size of skull |
Skull deformities* |
|
Head ossification defects* |
Vaulted cranium |
Narrow head |
|
Cranial blisters |
Cleft palate* |
Funnel chest |
|
Dislocation of hip |
Spina bifida |
Deformed tail |
|
Deformed feet |
Club foot* |
Digital anomalies* |
|
Calcaneo valgus |
Odontogenesis imperfecta* |
Tibial exostosis |
|
Amelanogenesis* |
Scleratomal necrosis |
|
|
Miscellaneous |
||
|
Situs inversus |
Hydronephrosis |
Hydroureter |
|
Hydrocoele |
Absence of kidney |
Gonadal anomalies* |
|
Congenital heart disease |
Facial deformities |
Pituitary disturbances |
|
Deformities of ears |
Motor disturbances |
Dermatomal necrosis |
|
Myotomal necrosis |
Abnormalities in skinpigmentation |
|
* These abnormalities have been observed in humans exposed prenatally to large doses of radiation and have, therefore, been tentatively attributed to irradiation.
Source: Brill and Forgotson 1964.
Susceptibility to the carcinogenic effects of radiation also appears to be relatively high throughout the prenatal period, judging from the association between childhood cancer (including leukaemia) and prenatal exposure to diagnostic x rays reported in case-control studies (NAS 1990). The results of such studies imply that prenatal irradiation may cause a 4,000% per Sv increase in the risk of leukaemia and other childhood cancers (UNSCEAR 1986; NAS 1990), which is a far larger increase than is attributable to postnatal irradiation (UNSCEAR 1988; NAS 1990). Although, paradoxically, no excess of childhood cancer was recorded in A-bomb survivors irradiated prenatally (Yoshimoto et al. 1990), as noted above, there were too few such survivors to exclude an excess of the magnitude in question.
Figure 6. The frequency of severe mental retardation in relation to radiation dose in prenatally irradiated atomic bomb survivors
Summary and Conclusions
The adverse effects of ionizing radiation on human health are widely diverse, ranging from rapidly fatal injuries to cancers, birth defects, and hereditary disorders that appear months, years or decades later. The nature, frequency and severity of effects depend on the quality of the radiation in question as well as on the dose and conditions of exposure. Most such effects require relatively high levels of exposure and are, therefore, encountered only in accident victims, radiotherapy patients, or other heavily irradiated persons. The genotoxic and carcinogenic effects of ionizing radiation, by contrast, are presumed to increase in frequency as linear non-threshold functions of the dose; hence, although the existence of thresholds for these effects cannot be excluded, their frequency is assumed to increase with any level of exposure. For most effects of radiation, the sensitivity of exposed cells varies with their rate of proliferation and inversely with their degree of differentiation, the embryo and growing child being especially vulnerable to injury.