Cancer
Exposures to numerous substances designated by the International Agency for Research on Cancer (IARC) as known, probable and possible carcinogens may occur in pulp and paper operations. Asbestos, known to cause lung cancer and mesothelioma, is used to insulate pipes and boilers. Talc is used extensively as a paper additive, and can be contaminated with asbestos. Other paper additives, including benzidine-based dyes, formaldehyde and epichlorohydrin, are considered probable human carcinogens. Hexavalent chromium and nickel compounds, generated in stainless-steel welding, are known lung and nasal carcinogens. Wood dust has recently been classified by IARC as a known carcinogen, based mainly on evidence of nasal cancer among workers exposed to hardwood dust (IARC, 1995). Diesel exhaust, hydrazine, styrene, mineral oils, chlorinated phenols and dioxins, and ionizing radiation are other probable or possible carcinogens which may be present in mill operations.
Few epidemiological studies specific to pulp and paper operations have been conducted, and they indicate few consistent results. Exposure classifications in these studies have often used the broad industrial category “pulp and paper”, and even the most specific classifications grouped workers by types of pulping or large mill areas. The three cohort studies in the literature to date involved fewer than 4,000 workers each. Several large cohort studies are currently under way, and IARC is coordinating an international multicentric study likely to include data from more than 150,000 pulp and paper workers, allowing much more specific exposure analyses. This article will review the available knowledge from studies published to date. More detailed information may be obtained from earlier published reviews by IARC (1980, 1987, and 1995) and by Torén, Persson and Wingren (1996). Results for lung, stomach and haematological malignancies are summarized in table 1.
Table 1. Summary of studies on lung cancer, stomach cancer, lymphoma and leukaemia in pulp and paper workers
|
Process |
Location |
Type of |
Lung |
Stomach |
Lymphoma |
Leukaemia |
|
Sulphite |
Finland |
C |
0.9 |
1.3 |
X/X |
X |
|
Sulphite |
USA |
C |
1.1 |
0.7 |
— |
0.9 |
|
Sulphite |
USA |
C |
0.8 |
1.5 |
1.3/X |
0.7 |
|
Sulphite |
USA |
PM |
0.9 |
2.2* |
2.7*/X |
1.3 |
|
Sulphate |
Finland |
C |
0.9 |
0.9 |
0/0 |
X |
|
Sulphate |
USA |
C |
0.8 |
1.0 |
2.1/0 |
0.2 |
|
Sulphate |
USA |
PM |
1.1 |
1.9 |
1.1/4.1* |
1.7 |
|
Chlorine |
Finland |
C |
3.0* |
— |
— |
— |
|
Sulphite/paper |
Sweden |
CR |
— |
2.8* |
— |
— |
|
Paper dust |
Canada |
CR |
2.0* |
— |
— |
— |
|
Paper mill |
Finland |
C |
2.0* |
1.7 |
X/X |
— |
|
Paper mill |
Sweden |
C |
0.7* |
— |
— |
— |
|
Paper mill |
USA |
C |
0.8 |
2.0 |
— |
2.4 |
|
Paper mill |
Sweden |
CR |
1.6 |
— |
— |
— |
|
Paper mill |
USA |
PM |
1.3 |
0.9 |
X/1.4 |
1.4 |
|
Board mill |
Finland |
C |
2.2* |
0.6 |
X/X |
X |
|
Power plant |
Finland |
C |
0.5 |
2.1 |
— |
— |
|
Maintenance |
Finland |
C |
1.3 |
0.3* |
1.0/X |
1.5 |
|
Maintenance |
Sweden |
CR |
2.1* |
0.8 |
— |
— |
|
Pulp and paper |
USA |
C |
0.9 |
1.2 |
0.7/X |
1.8 |
|
Pulp and paper |
USA |
C |
0.8 |
1.2 |
1.7/X |
0.5 |
|
Pulp and paper |
Sweden |
CR |
0.8 |
1.3 |
1.8 |
1.1 |
|
Pulp and paper |
Sweden |
CR |
— |
— |
2.2/0 |
— |
|
Pulp and paper |
Sweden |
CR |
1.1 |
0.6 |
— |
— |
|
Pulp and paper |
USA |
CR |
1.2* |
— |
— |
— |
|
Pulp and paper |
USA |
CR |
1.1 |
— |
— |
— |
|
Pulp and paper |
USA |
CR |
— |
— |
—/4.0 |
— |
|
Pulp and paper |
Canada |
PM |
— |
1.2 |
3.8*/— |
— |
|
Pulp and paper |
USA |
PM |
1.5* |
0.5 |
4.4/4.5 |
2.3 |
|
Pulp and paper |
USA |
PM |
0.9 |
1.7* |
1.6/1.0 |
1.1 |
|
Pulp and paper |
USA |
PM |
0.9 |
1.2 |
1.5/1.9* |
1.4 |
|
Pulp and paper |
USA |
PM |
— |
1.7* |
1.4 |
1.6* |
C = cohort study, CR = case-referent study, PM = proportionate mortality study.
* Statistically significant. § = Where separately reported, NHL = non Hodgkin lymphoma and HD = Hodgkin’s disease. X = 0 or 1 case reported, no risk estimate calculated, — = No data reported.
A risk estimate exceeding 1.0 means the risk is increased, and a risk estimate below 1.0 indicates decreased risk.
Source: Adapted from Torén, Persson and Wingren 1996.
Respiratory System Cancers
Maintenance workers in paper and pulp mills experience an increased risk of lung cancer and malignant mesotheliomas, probably because of their exposure to asbestos. A Swedish study showed a threefold increased risk of pleural mesothelioma among pulp and paper workers (Malker et al. 1985). When the exposure was further analysed, 71% of the cases had been exposed to asbestos, the majority having worked in mill maintenance. Elevations in lung cancer risk among maintenance workers have also been shown in Swedish and Finnish pulp and paper mills (Torén, Sällsten and Järvholm 1991; Jäppinen et al. 1987).
In the same Finnish study, a twofold increased risk of lung cancer was also observed among both paper mill and board mill workers. The investigators made a subsequent study restricted to pulp mill workers exposed to chlorine compounds, and found a threefold increased risk of lung cancer.
Few other studies of pulp and paper workers have shown increased risks for lung cancer. A Canadian study showed an increased risk among those exposed to paper dust (Siemiatycki et al. 1986), and US and Swedish studies showed increased risks among paper mill workers (Milham and Demers 1984; Torén, Järvholm and Morgan 1989).
Gastro-intestinal Cancers
Increased risk of stomach cancer has been indicated in many studies, but the risks are not clearly associated with any one area; therefore the relevant exposure is unknown. Socio-economic status and dietary habits are also risk factors for stomach cancer, and might be confounders; these factors were not taken into account in any of the studies reviewed.
The association between gastric cancer and pulp and paper work was first seen in a US study in the 1970s (Milham and Demers 1984). The risk was found to be even higher, nearly doubled, when sulphite workers were examined separately. US sulphite and groundwood workers were also found in a later study to run an increased risk of stomach cancer (Robinson, Waxweiller and Fowler 1986). A risk of the same magnitude was found in a Swedish study among pulp and paper mill workers from an area where only sulphite pulp was produced (Wingren et al. 1991). American paper, paperboard and pulp mill workers in New Hampshire and Washington state ran an increased mortality from stomach cancer (Schwartz 1988; Milham 1976). The subjects were probably a mixture of sulphite, sulphate and paper mill workers. In a Swedish study, threefold increased mortality due to stomach cancer was found in a group comprising sulphite and paper mill workers (Wingren, Kling and Axelson 1985). The majority of pulp and paper studies reported excesses of stomach cancer, though some did not.
Due to the small number of cases, most studies of other gastrointestinal cancers are inconclusive. An increased risk of colon cancer among workers in the sulphate process and in paper board production has been reported in a Finnish study (Jäppinen et al. 1987), as well as among US pulp and paper workers (Solet et al. 1989). The incidence of biliary tract cancer in Sweden between 1961 and 1979 was linked with occupational data from the 1960 National Census (Malker et al. 1986). An increased incidence of cancer of the gallbladder among male paper mill workers was identified. Increased risks of pancreatic cancer have been observed in some studies of paper mill workers and sulphite workers (Milham and Demers 1984; Henneberger, Ferris and Monson 1989), as well as in the broad group of pulp and paper workers (Pickle and Gottlieb 1980; Wingren et al. 1991). These findings have not been substantiated in other studies.
Haematological Malignancies
The issue of lymphomas among pulp and paper mill workers was originally addressed in a US study from the 1960s, where a fourfold increased risk of Hodgkin’s disease was found among pulp and paper workers (Milham and Hesser 1967). In a subsequent study, the mortality among pulp and paper mill workers in the state of Washington between 1950 and 1971 was investigated, and a doubled risk of both Hodgkin’s disease and multiple myeloma was observed (Milham 1976). This study was followed by one analysing mortality among pulp and paper union members in the United States and Canada (Milham and Demers 1984). It showed almost a threefold increased risk for lymphosarcoma and reticulum cell sarcoma among sulphite workers, while sulphate workers had a fourfold increased risk of Hodgkin’s disease. In a US cohort study, sulphate workers were observed to have a twofold risk of lymphosarcoma and reticulosarcoma (Robinson, Waxweiller and Fowler 1986).
In many of the studies where it was possible to investigate the occurrence of malignant lymphomas, an increased risk has been found (Wingren et al. 1991; Persson et al. 1993). Since the increased risk occurs both in sulphate and sulphite mill workers, this points towards a common source of exposure. In the sorting and chipping departments, the exposures are rather similar. The workforce is exposed to wood dust, terpenes and other extractable compounds from the wood. In addition, both pulping processes bleach with chlorine, which has the potential to create chlorinated organic by-products, including small amounts of dioxins.
Compared with lymphomas, studies on leukaemias show less consistent patterns, and the risk estimates are lower.
Other Malignancies
Among US paper mill workers with presumed exposure to formaldehyde, four cases of urinary tract cancer were found after 30 years’ latency, although only one was expected (Robinson, Waxweiller and Fowler 1986). All of these individuals had worked in the paper-drying areas of the paper mills.
In a case-control study from Massachusetts, central nervous system tumours in childhood were associated with an unspecified paternal occupation as a paper and pulp mill worker (Kwa and Fine 1980). The authors regarded their observation as a random event. However, in three subsequent studies, increased risks were also found (Johnson et al. 1987; Nasca et al. 1988; Kuijten, Bunin and Nass 1992). In studies from Sweden and Finland, two- to threefold increased risks of brain tumours were observed among pulp and paper mill workers.
Injuries and Non-malignant Diseases
Injuries
Only limited statistics are available on accident rates in general in this industry. Compared to other manufacturing industries, the 1990 accident rate in Finland was below the average; in Canada, the rates from 1990 to 1994 were similar to other industries; in the United States, the 1988 rate was slightly above average; in Sweden and Germany, the rates were 25% and 70% above the average (ILO 1992; Workers’ Compensation Board of British Columbia 1995).
The most commonly encountered risk factors for serious and fatal accidents in the pulp and paper industry are the papermaking equipment itself and the extreme size and weight of pulp or paper bales and rolls. In a 1993 United States government study of occupational fatalities from 1979 to 1984 in pulp, paper and paperboard mills (US Department of Commerce 1993), 28% were due to workers being caught in or between rotating rolls or equipment (“nip-points”) and
18% were due to workers being crushed by falling or tumbling objects, especially rolls and bales. Other causes of multiple deaths included electrocution, hydrogen sulphide and other toxic gas inhalation, massive thermal/chemical burns and one case of heat exhaustion. The number of serious accidents associated with paper machines has been reported to decrease with the installation of newer equipment in some countries. In the converting sector, repetitive and monotonous work, and the use of mechanized equipment with higher speeds and forces, has become more common. Although no sector-specific data are available, it is expected that this sector will experience greater rates of over-exertion injuries associated with repetitive work.
Non-Malignant Diseases
The most well documented health problems encountered by pulp mill workers are acute and chronic respiratory disorders (Torén, Hagberg and Westberg 1996). Exposure to extremely high concentrations of chlorine, chlorine dioxide or sulphur dioxide may occur as a result of a leak or other process upset. Exposed workers may develop acute chemical-induced lung injury with severe inflammation of air passages and release of fluid into the air spaces, requiring hospitalization. The extent of damage depends on the duration and intensity of the exposure, and the specific gas involved. If the worker survives the acute episode, complete recovery may occur. However, in less intense exposure incidents (also usually as a result of process upsets or spills), acute exposure to chlorine or chlorine dioxide may trigger the subsequent development of asthma. This irritant-induced asthma has been recorded in numerous case reports and recent epidemiological studies, and current evidence indicates that it may persist for many years following the exposure incident. Workers similarly exposed who do not develop asthma may experience persistently increased nasal irritation, cough, wheezing and reduction in airflow rates. Workers most at risk for these exposure incidents include maintenance workers, bleach plant workers and construction workers at pulp mill sites. High levels of chlorine dioxide exposure also cause eye irritation and the sensation of seeing halos around lights.
Some mortality studies have indicated increased risk of death from respiratory disease among pulp mill workers exposed to sulphur dioxide and paper dust (Jäppinen and Tola 1990; Torén, Järvholm and Morgan 1989). Increased respiratory symptoms have also been reported in sulphite mill workers who are chronically exposed to low levels of sulphur dioxide (Skalpe 1964), although increased airflow obstruction is not normally reported among pulp mill populations in general. Symptoms of respiratory irritation are also reported by workers exposed to high air concentrations of terpenes in turpentine recovery processes often present at pulp mill sites. Soft paper dust has also been reported to be associated with increased asthma and chronic obstructive pulmonary disease (Torén, Hagberg and Westberg 1996).
Exposure to micro-organisms, especially around wood chip and waste piles, debarkers and sludge presses, creates an increased risk for hypersensitivity responses in the lungs. Evidence for this appears to be limited to isolated case reports of hypersensitivity pneumonitis, which can lead to chronic lung scarring. Bagassosis, or hypersensitivity pneumonitis associated with exposure to thermophylic micro-organisms and bagasse (a sugar cane by-product), is still seen in mills using bagasse for fibre.
Other respiratory hazards commonly encountered in the pulp and paper industry include stainless steel welding fumes and asbestos (see “Asbestos,” “Nickel” and “Chromium” elsewhere in the Encyclopaedia). Maintenance workers are the group most likely to be at risk from these exposures.
Reduced sulphur compounds (including hydrogen sulphide, dimethyl disulphides and mercaptans) are potent eye irritants and may cause headaches and nausea in some workers. These compounds have very low odour thresholds (ppb range) in individuals not previously exposed; however, among long-time workers in the industry, odour thresholds are considerably higher. Concentrations in the range of 50 to 200 ppm produce olfactory fatigue, and subjects can no longer detect the distinctive “rotten eggs” odour. At higher concentrations, exposure will result in unconsciousness, respiratory paralysis and death. Fatalities associated with exposure to reduced sulphur compounds in confined spaces have occurred at pulp mill sites.
Cardiovascular mortality has been reported to be increased in pulp and paper workers, with some exposure-response evidence suggesting a possible link with exposure to reduced sulphur compounds (Jäppinen 1987; Jäppinen and Tola 1990). However, other causes for this increased mortality may include noise exposure and shift work, both of which have been associated with increased risk for ischaemic heart disease in other industries.
Skin problems encountered by pulp and paper mill workers include acute chemical and thermal burns and contact dermatitis (both irritant and allergic). Pulp mill workers in kraft process mills frequently experience alkali burns to the skin as a result of contact with hot pulping liquors and calcium hydroxide slurries from the recovery process. Contact dermatitis is reported more frequently among paper mill and converting workers, as many of the additives, defoaming agents, biocides, inks and glues used in paper and paper-product making are primary skin irritants and sensitizers. Dermatitis may occur from exposure to the chemicals themselves or from handling freshly treated paper or paper products.
Noise is a significant hazard throughout the pulp and paper industry. The US Department of Labor estimated that noise levels over 85 dBA were found in over 75% of plants in the paper and allied products industries, compared to 49% of plants in manufacturing in general, and that over 40% of workers were exposed regularly to noise levels over 85 dBA (US Department of Commerce 1983). Noise levels around paper machines, chippers and recovery boilers tend to be well over 90 dBA. Conversion operations also tend to generate high noise levels. Reduction in worker exposure around paper machines is usually attempted by the use of enclosed control rooms. In converting, where the operator is usually stationed next to the machine, this type of control measure is seldom used. However where converting machines have been enclosed, this has resulted in decreased exposure to both paper dust and noise.
Excessive heat exposure is encountered by paper mill workers working in paper machine areas, with temperatures of 60°C being recorded, although no studies of the effects of heat exposure in this population are available in the published scientific literature.
Occupational Hazards and Controls
Table 1 provides an overview of the types of exposures which may be expected in each area of pulp and paper operations. Although exposures may be listed as specific to certain production processes, exposures to employees from other areas may also occur depending on weather conditions, proximity to sources of exposure, and whether they work in more than one process area (e.g., quality control, general labour pool and maintenance personnel).
Table 1. Potential health and safety hazards in pulp and paper production, by process area
|
Process area |
Safety hazards |
Physical hazards |
Chemical hazards |
Biological hazards |
|
Wood preparation |
||||
|
Log pond |
Drowning; mobile equipment; |
Noise; vibration; cold; heat |
Engine exhaust |
|
|
Wood room |
Nip points; slipping, falling |
Noise; vibration |
Terpenes and other wood extracts; wood dust |
Bacteria; fungi |
|
Chip screening |
Nip points; slipping, falling |
Noise; vibration |
Terpenes and other wood extracts; wood dust |
Bacteria; fungi |
|
Chip yard |
Nip points; mobile equipment |
Noise; vibration; cold; heat |
Engine exhaust; terpenes and other wood extracts; wood dust |
Bacteria; fungi |
|
Pulping |
||||
|
Stone groundwood |
Slipping, falling |
Noise; electric and magnetic fields; high humidity |
||
|
RMP, CMP, CTMP |
Slipping, falling |
Noise; electric and magnetic fields; high humidity |
Cooking chemicals and by-products; terpenes and other wood extracts; wood dust |
|
|
Sulphate pulping |
Slipping, falling |
Noise; high humidity; heat |
Acids and alkalis; cooking chemicals and by-products; reduced sulphur gases; terpenes |
|
|
Sulphate recovery |
Explosions; nip points; slipping, |
Noise; heat; steam |
Acids and alkalis; asbestos; ash; cooking chemicals and by-products; fuels; reduced |
|
|
Sulphite pulping |
Slipping, falling |
Noise; high humidity; heat |
Acids and alkalis; cooking chemicals and by-products; sulphur dioxide; terpenes and other wood extracts; wood dust |
|
|
Sulphite recovery |
Explosions; nip points; slipping, |
Noise; heat; steam |
Acids and alkalis; asbestos; ash; cooking chemicals and by-products; fuels; sulphur dioxide |
|
|
Repulping/de-inking |
Slipping, falling |
Acids and alkalis; bleaching chemicals and by- products; dyes and inks; pulp/paper dust; slimicides; solvents |
Bacteria |
|
|
Bleaching |
Slipping, falling |
Noise; high humidity; heat |
Bleaching chemicals and by-products; slimicides; terpenes and other wood extracts |
|
|
Sheet forming and |
||||
|
Pulp machine |
Nip points; slipping, falling |
Noise; vibration; high |
Acids and alkalis; bleaching chemicals and by-products; flocculant; pulp/paper dust; slimicides; solvents |
Bacteria |
|
Paper machine |
Nip points; slipping, falling |
Noise; vibration; high |
Acids and alkalis; bleaching chemicals and by-products; dyes and inks; flocculant; pulp/paper |
Bacteria |
|
Finishing |
Nip points; mobile equipment |
Noise |
Acids and alkalis; dyes and inks; flocculant; |
|
|
Warehouse |
Mobile equipment |
Fuels; engine exhaust; pulp/paper dust |
||
|
Other operations |
||||
|
Power generation |
Nip points; slipping, falling |
Noise; vibration; electric and |
Asbestos; ash; fuels; terpenes and other wood extracts; wood dust |
Bacteria; fungi |
|
Water treatment |
Drowning |
Bleaching chemicals and by-products |
Bacteria |
|
|
Effluent treatment |
Drowning |
Bleaching chemicals and by-products; flocculant; reduced sulphur gases |
Bacteria |
|
|
Chlorine dioxide |
Explosions; slipping, falling |
Bleaching chemicals and by-products |
Bacteria |
|
|
Turpentine recovery |
Slipping, falling |
Cooking chemicals and by-products; reduced sulphur gases; terpenes and other wood extracts |
||
|
Tall oil production |
Acids and alkalis; cooking chemicals and by-products; reduced sulphur gases; terpenes and other wood extracts |
RMP = refining mechanical pulping; CMP = chemi-mechanical pulping; CTMP = chemi-thermomechanical pulping.
Exposure to the potential hazards listed in table 1 is likely to depend on the extent of automation of the plant. Historically, industrial pulp and paper production was a semi-automatic process which required a great deal of manual intervention. In such facilities, operators would sit at open panels adjacent to the processes to view the effects of their actions. The valves at the top and bottom of a batch digester would be manually opened, and during the filling stages, gases in the digester would be displaced by the incoming chips (figure 1). Chemical levels would be adjusted based on experience rather than sampling, and process adjustments would be dependent on the skill and knowledge of the operator, which at times led to upsets. For example, over-chlorination of pulp would expose workers downstream to increased levels of bleaching agents. In most modern mills, progress from manually controlled to electronically controlled pumps and valves allows for remote operation. The demand for process control within narrow tolerances has required computers and sophisticated engineering strategies. Separate control rooms are used to isolate the electronic equipment from the pulp and paper production environment. Consequently, operators usually work in air-conditioned control rooms which offer refuge from the noise, vibration, temperature, humidity and chemical exposures inherent to mill operations. Other controls which have improved the working environment are described below.
Figure 1. Worker opening cap on manually controlled batch digester.
MacMillan Bloedel archives
Safety hazards including nip points, wet walking surfaces, moving equipment and heights are common throughout pulp and paper operations. Guards around moving conveyors and machinery parts, quick clean-up of spills, walking surfaces which allow drainage, and guard-rails on walkways adjacent to production lines or at height are all essential. Lock-out procedures must be followed for maintenance of chip conveyors, paper machine rolls and all other machinery with moving parts. Mobile equipment used in chip storage, dock and shipping areas, warehousing and other operations should have roll-over protection, good visibility and horns; traffic lanes for vehicles and pedestrians should be clearly marked and signed.
Noise and heat are also ubiquitous hazards. The major engineering control is operator enclosures, as described above, usually available in wood preparation, pulping, bleaching and sheet-forming areas. Air-conditioned enclosed cabs for mobile equipment used in chip pile and other yard operations are also available. Outside these enclosures, workers usually require hearing protection. Work in hot process or outdoor areas and in vessel maintenance operations requires workers to be trained to recognize symptoms of heat stress; in such areas, work scheduling should allow acclimatization and rest periods. Cold weather may create frostbite hazards in outdoor jobs, as well as foggy conditions near chip piles, which remain warm.
Wood, its extracts and associated micro-organisms are specific to wood preparation operations and the initial stages of pulping. Control of exposures will depend on the particular operation, and may include operator booths, enclosure and ventilation of saws and conveyors, as well as enclosed chip storage and low chip inventory. Use of compressed air to clear wood dust creates high exposures and should be avoided.
Chemical pulping operations present the opportunity for exposures to digestion chemicals as well as gaseous by-products of the cooking process, including reduced (kraft pulping) and oxidized (sulphite pulping) sulphur compounds and volatile organics. Gas formation may be influenced by a number of operating conditions: the wood species used; the quantity of wood pulped; the amount and concentration of white liquor applied; the amount of time required for pulping; and maximum temperature attained. In addition to automatic digester capping valves and operator control rooms, other controls for these areas include local exhaust ventilation at batch digesters and blow tanks, capable of venting at the rate the vessel’s gases are released; negative pressure in recovery boilers and sulphite-SO2 acid towers to prevent gas leaks; ventilated full or partial enclosures over post-digestion washers; continuous gas monitors with alarms where leaks may occur; and emergency response planning and training. Operators taking samples and conducting tests should be aware of the potential for acid and caustic exposure in process and waste streams, and the possibility of side reactions such as hydrogen sulphide gas (H2S) production if black liquor from kraft pulping comes into contact with acids (e.g., in sewers).
In chemical recovery areas, acidic and alkaline process chemicals and their by-products may be present at temperatures in excess of 800°C. Job responsibilities may require workers to come into direct contact with these chemicals, making heavy duty clothing a necessity. For example, workers rake the spattering molten smelt that collects at the base of the boilers, thereby risking chemical and thermal burns. Workers may be exposed to dust when sodium sulphate is added to concentrated black liquor, and any leak or opening will release noxious (and potentially fatal) reduced sulphur gases. The potential for a smelt water explosion always exists around the recovery boiler. Water leaks in the tube walls of the boiler have resulted in several fatal explosions. Recovery boilers should be shut down at any indication of a leak, and special procedures should be implemented for transferring the smelt. Loading of lime and other caustic materials should be done with enclosed and ventilated conveyors, elevators and storage bins.
In bleach plants, field operators may be exposed to the bleaching agents as well as chlorinated organics and other by-products. Process variables such as bleaching chemical strength, lignin content, temperature and pulp consistency are constantly monitored, with operators collecting samples and performing laboratory tests. Because of the hazards of many of the bleaching agents used, continuous alarm monitors should be in place, escape respirators should be issued to all employees, and operators should be trained in emergency response procedures. Canopy enclosures with dedicated exhaust ventilation are standard engineering controls found at the top of each bleaching tower and washing stage.
Chemical exposures in the machine room of a pulp or paper mill include chemical carry-over from the bleach plant, the papermaking additives and the chemical mixture in the waste water. Dusts (cellulose, fillers, coatings) and exhaust fumes from mobile equipment are present in the dry-end and the finishing operations. Cleaning between product runs may be done with solvents, acids and alkalis. Controls in this area may include complete enclosure over the sheet drier; ventilated enclosure of the areas where additives are unloaded, weighed and mixed; use of additives in liquid rather than powder form; use of water-based rather than solvent-based inks and dyes; and eliminating the use of compressed air to clean up trimmed and waste paper.
Paper production in recycled paper plants is generally dustier than conventional paper production using newly produced pulp. Exposure to micro-organisms can occur from the beginning (paper collection and separation) to the end (paper production) of the production chain, but exposure to chemicals is less important than in conventional paper production.
Pulp and paper mills employ an extensive maintenance group to service their process equipment, including carpenters, electricians, instrument mechanics, insulators, machinists, masons, mechanics, millwrights, painters, pipefitters, refrigeration mechanics, tinsmiths and welders. Along with their trade-specific exposures (see the Metal processing and metal working and Occupations chapters), these tradespeople may be exposed to any of the process-related hazards. As mill operations have become more automated and enclosed, the maintenance, cleaning and quality assurance operations have become the most highly exposed. Plant shutdowns to clean vessels and machines are of special concern. Depending on mill organization, these operations may be carried out by in-house maintenance or production personnel, although subcontracting to non-mill personnel, who may have less occupational health and safety support services, is common.
In addition to process exposures, pulp and paper mill operations entail some noteworthy exposures for maintenance personnel. Because pulping, recovery and boiler operations involve high heat, asbestos was used extensively to insulate pipes and vessels. Stainless steel is often used in vessels and pipes throughout pulping, recovery and bleaching operations, and to some extent in papermaking. Welding this metal is known to generate chromium and nickel fumes. During maintenance shut-downs, chromium-based sprays may be applied to protect the floor and walls of recovery boilers from corrosion during start-up operations. Process quality measurements in the production line are often made using infrared and radio-isotope gauges. Although the gauges are usually well shielded, instrument mechanics who service them may be exposed to radiation.
Some special exposures may also occur among employees in other mill-support operations. Power boiler workers handle bark, waste wood and sludge from the effluent treatment system. In older mills, workers remove ash from the bottom of the boilers and then reseal the boilers by applying a mixture of asbestos and cement around the boiler grate. In modern power boilers, this process is automated. When material is fed into the boiler at too high a moisture level, workers may be exposed to blow-backs of incomplete combustion products. Workers responsible for water treatment may be exposed to chemicals such as chlorine, hydrazine and various resins. Because of the reactivity of ClO2, the ClO2 generator is usually located in a restricted area and the operator is stationed in a remote control room with excursions to collect samples and service the saltcake filter. Sodium chlorate (a strong oxidizer) used to generate ClO2 can become dangerously flammable if it is allowed to spill on any organic or combustible material and then dry. All spills should be wetted down before any maintenance work may proceed, and all equipment should be thoroughly cleaned afterward. Wet clothing should be kept wet and separate from street clothing, until washed.
Chemical and By-product Production
Because many bleaching chemicals are reactive and hazardous to transport, they are produced on-site or nearby. Chlorine dioxide (ClO2), sodium hypochlorite (NaOCl) and peracids are always produced on-site, while chlorine (Cl2) and sodium hydroxide or caustic (NaOH) are usually produced off-site. Tall oil, a product derived from the resin and fatty acids that are extracted during kraft cooking, may be refined on- or off-site. Turpentine, a lighter fraction kraft by-product, is often collected and concentrated on-site, and refined elsewhere.
Chlorine Dioxide
Chlorine dioxide (ClO2) is a highly reactive greenish-yellow gas. It is toxic and corrosive, explodes at high concentrations (10%) and is quickly reduced to Cl2 and O2 in the presence of ultraviolet light. It must be prepared as a dilute gas and stored as a dilute liquid, making bulk transport impossible.
ClO2 is generated by reducing sodium chlorate (Na2ClO3) with either SO2, methanol, salt or hydrochloric acid. The gas leaving the reactor is condensed and stored as a 10% liquid solution. Modern ClO2 generators operate at 95% efficiency or greater, and the small amount of Cl2 that is produced will be collected or scrubbed out of the vent gas. Side reactions may occur depending on the purity of the feed chemicals, the temperature and other process variables. By-products are returned to the process and spent chemicals are neutralized and sewered.
Sodium Hypochlorite
Sodium hypochlorite (NaOCl) is produced by combining Cl2 with a dilute solution of NaOH. It is a simple, automated process that requires almost no intervention. The process is controlled by maintaining the caustic concentration such that the residual Cl2 in the process vessel is minimized.
Chlorine and Caustic
Chlorine (Cl2), used as a bleaching agent since the early 1800s, is a highly reactive, toxic, green-coloured gas which becomes corrosive when moisture is present. Chlorine is usually manufactured by the electrolysis of brine (NaCl) into Cl2 and NaOH at regional installations, and transported to the customer as a pure liquid. Three methods are used to produce Cl2 on an industrial scale: the mercury cell, the diaphragm cell, and the most recent development, the membrane cell. Cl2 is always produced at the anode. It is then cooled, purified, dried, liquefied and transported to the mill. At large or remote pulp mills, local facilities may be constructed, and the Cl2 can be transported as a gas.
The quality of NaOH depends on which of the three processes is used. In the older mercury cell method, the sodium and mercury combine to form an amalgam that is decomposed with water. The resulting NaOH is nearly pure. One of the shortcomings of this process is that mercury contaminates the workplace and has resulted in serious environmental problems. The NaOH produced from the diaphragm cell is removed with the spent brine and concentrated to allow the salt to crystallize and separate. Asbestos is used as the diaphragm. The purest NaOH is produced in membrane cells. A semi-permeable resin-based membrane allows sodium ions to pass through without the brine or chlorine ions, and combine with water added to the cathode chamber to form pure NaOH. Hydrogen gas is a by-product of each process. It is usually treated and used either in other processes or as fuel.
Tall Oil Production
Kraft pulping of highly-resinous species such as pine produces sodium soaps of resin and fatty acids. The soap is collected from black liquor storage tanks and from soap skimming tanks that are located in the evaporator train of the chemical recovery process. Refined soap or tall oil can be used as a fuel additive, dust control agent, road stabilizer, pavement binder and roofing flux.
At the processing plant, soap is stored in primary tanks to allow the black liquor to settle to the bottom. The soap rises and overflows into a second storage tank. Sulphuric acid and the decanted soap are fed into a reactor, heated to 100°C, agitated and then allowed to settle. After settling overnight, the crude tall oil is decanted into a storage vessel and allowed to sit for another day. The top fraction is considered dry crude tall oil and is pumped to storage, ready for shipment. The cooked lignin in the bottom fraction will become part of the subsequent batch. The spent sulphuric acid is pumped to a storage tank, and any entrained lignin is allowed to settle to the bottom. The lignin left in the reactor is concentrated for several cooks, dissolved in 20% caustic and returned to the primary soap tank. Periodically, the collected black liquor and the residual lignin from all sources are concentrated and burned as fuel.
Turpentine Recovery
Gases from the digesters and condensate from black liquor evaporators may be collected for recovery of turpentine. The gases are condensed, combined, then stripped of turpentine, which is recondensed, collected and sent to a decanter. The top fraction of the decanter is drawn off and sent to storage, while the bottom fraction is recycled to the stripper. Raw turpentine is stored separately from the rest of the collection system because it is noxious and flammable, and is usually processed off-site. All the non-condensable gases are collected and incinerated either in the power boilers, the lime kiln or a dedicated furnace. The turpentine can be processed for use in camphor, synthetic resins, solvents, flotation agents and insecticides.
Power Generation and Water Treatment
In addition to liquor recovery, pulp mills recover a significant portion of energy from burning waste materials and by-products of the process in power boilers. Materials such as bark, wood waste and dried sludge collected from effluent treatment systems may be burned to provide steam to power electrical generators.
Pulp and paper mills consume vast amounts of fresh water. A 1,000 tonne per day bleached kraft pulp mill may use more than 150 million litres of water a day; a paper mill even more. In order to prevent adverse effects on mill equipment and to maintain product quality, the incoming water must be treated to remove contaminants, bacteria and minerals. Several treatments are applied depending on the quality of the incoming water. Sedimentation beds, filters, flocculants, chlorine and ion exchange resins are all used to treat water before it is used in the process. Water that is used in the power and recovery boilers is further treated with oxygen scavengers and corrosion inhibitors such as hydrazine and morpholine to avoid deposits forming in the boiler tubes, to reduce metal corrosion, and to prevent carry-over of water to the steam turbine.
Sheet Production and Converting: Market Pulp, Paper, Paperboard
End products of pulp and paper mills depend on the pulping process, and may include market pulp and various types of paper or paperboard products. For example, the relatively weak mechanical pulp is converted into single-use products such as newspapers and tissue. Kraft pulp is converted into multi-use paper products such as high-quality writing paper, books and grocery bags. Sulphite pulp, which is primarily cellulose, can be used in a series of diverse end-products including specialty paper, rayon, photographic film, TNT, plastics, adhesives, and even ice cream and cake mixes. Chemi-mechanical pulps are exceptionally stiff, ideal for the structural support needed for corrugated container board. The fibres in pulp from recycled paper are usually shorter, less flexible and less water permeable, and can therefore not be used for high-quality paper products. Recycled paper is therefore mainly used for the production of soft paper products like tissue paper, toilet paper, paper towelling and napkins.
To produce market pulp, the pulp slurry is usually screened once more and its consistency adjusted (4 to 10%) before it is ready for the pulp machine. The pulp is then spread onto a travelling metal screen or plastic mesh (known as the “wire”) at the “wet end” of the pulp machine, where the operator monitors the speed of the moving wire and the water content of the pulp (figure 1; the presses and the cover of the drier can be seen in the upper left; in modern mills, operators spend a great deal of time in control rooms). Water and filtrate are drawn through the wire, leaving a web of fibres. The pulp sheet is passed through a series of rotating rolls (“presses”) that squeeze out water and air until the fibre consistency is 40 to 45%. The sheet is then floated through a multi-storey sequence of hot-air dryers until the consistency is 90 to 95%. Finally, the continuous pulp sheet is cut into pieces and stacked into bales. The pulp bales are compressed, wrapped and packaged into bundles for storage and transport.
Figure 1. Wet end of pulp machine showing fibre mat on the wire.
Canfor Library
Although similar in principle to making pulp sheets, paper making is considerably more complex. Some mills use a variety of different pulps to optimize paper quality (e.g., a mix of hardwood, softwood, kraft, sulphite, mechanical or recycled pulps). Depending on the type of pulp used, a series of steps is necessary prior to forming the paper sheet. Generally, dried market pulp is rehydrated, while high-consistency pulp from storage is diluted. Pulp fibres may be beaten to increase the fibre-bonding area and thereby improve paper sheet strength. The pulp is then blended with “wet-end” additives (table 1) and passed through a final set of screens and cleaners. The pulp is then ready for the paper machine.
Table 1. Papermaking additives
|
Additive |
Location applied |
Purpose and/or examples of specific agents |
|
Most commonly used additives |
||
|
Talc |
Wet end |
Pitch control (prevent deposition and accumulation |
|
Titanium dioxide |
Wet end |
Pigment (brighten sheet, improve printing) |
|
“Alum”(Al2(SO4)3) |
Wet end |
Precipitates rosin sizing onto fibres |
|
Rosin |
Wet end |
Internal sizing (resist liquid penetration) |
|
Clay (kaolin) |
Wet/dry |
Filler (make brighter, smoother, more opaque) |
|
Starch |
Wet/dry |
Surface sizing (resist liquid penetration) |
|
Dyes and |
Wet/dry |
e.g., acid, basic or direct dyes,colour lakes, |
|
Latex |
Dry end |
Adhesive (reinforce sheet, bind additives to paper, |
|
Other additives |
||
|
Slimicides |
Wet end |
e.g., thiones, thiazoles, thiocyanates, hiocarbamates, thiols, isothiazolinones, |
|
Defoamers |
Wet end |
e.g., pine oil, fuel oil, recycled oils, silicones, alcohols |
|
Wire treatment |
Wet end |
e.g., imidazoles, butyl diglycol, acetone, turpentine, |
|
Wet and dry |
Wet end |
e.g., formaldehyde resins, epichlorohydrin, glyoxal, |
|
Coatings, |
Dry end |
e.g., aluminium hydroxide, polyvinyl acetate, |
|
Others |
Wet/dry |
Corrosion inhibitors, dispersants, flameproofing, |
The flow spreader and headbox distribute a thin suspension (1 to 3%) of refined pulp onto a moving wire (similar to a pulp machine, only at a much higher speed, sometimes in excess of 55 km/h) which forms the fibres into a thin felted sheet. The sheet moves through a series of press rolls to the dryer section, where a series of steam-heated rolls evaporate most of the remaining water. Hydrogen bonds between the fibres have fully developed at this stage. Finally, the paper is calendered and reeled. Calendering is the process by which the paper surface is ironed smooth and its thickness reduced. The dried, calendered paper sheet is wound onto a reel, labelled and transported to the warehouse (figure 2; note waste paper under reel, and unenclosed operator control panel). “Dry-end” additives can be added before calendering on the paper machine or in separate “off-machine” coating operations in the converting sector of the industry.
Figure 2. Dry end of a paper machine showing full paper reel and operator using air slitter to cut end.
George Astrakianakis
A variety of chemicals are used in the papermaking process to provide the paper with specific surface characteristics and sheet properties. The most commonly used additives (table 1) are typically used at the per cent level, though some such as clay and talc may contribute as much as 40% to the dry weight of certain papers. Table 1 also indicates the diversity of chemical additives which may be used for specific production purposes and products; some of these are used at very low concentrations (e.g., slimicides are added to process water in parts per million).
The process of making paperboard is similar to that of making paper or pulp. A suspension of pulp and water is dispersed onto a travelling wire, the water is removed, and the sheet dried and stored as a roll. The process differs in the way that the sheet is formed to give thickness, in the combining of multiple layers, and in the drying process. Board can be made from single or multi-layered sheets with or without a core. The sheets are usually high-quality kraft pulp (or kraft and CTMP blend), while the core is made from either a blend of semi-chemical and low-cost recycled pulp or from entirely recycled pulp and other waste material. Coatings, vapour barriers and multiple layers are added according to the end use to protect the contents from water and physical damage.
Recycled Paper Operations
The use of waste or recycled paper as the raw material for pulp production has increased during the last several decades, and some paper plants depend almost completely on waste paper. In some countries, waste paper is separated from other household waste at the source before it is collected. In other countries separation by grade (e.g., corrugated board, newsprint, high-grade paper, mixed) takes place in special recycling plants.
Recycled paper can be repulped in a relatively mild process which uses water and sometimes NaOH. Small metal pieces and plastics may be separated during and/or after repulping, using a debris rope, cyclones or centrifugation. Filling agents, glues and resins are removed in a cleaning stage by blowing air through the pulp slurry, sometimes with the addition of flocculating agents. The foam contains the unwanted chemicals and is removed. The pulp can be de-inked using a series of washing steps which may or may not include the use of chemicals (i.e., surfactant fatty acid derivatives) to dissolve remaining impurities, and bleaching agents to whiten the pulp. Bleaching has the disadvantage that it may reduce fibre length and therefore lessen final paper quality. The bleaching chemicals used in recycled pulp production are usually similar to those used in brightening operations for mechanical pulps. After the repulping and de-inking operations, sheet production follows in a manner very similar to that using virgin fibre pulp.
Bleaching
Bleaching is a multi-stage process that refines and brightens raw pulp. The objective is to dissolve (chemical pulps) or modify (mechanical pulps) the brown-coloured lignin that was not removed during pulping, while maintaining the integrity of the pulp fibres. A mill produces customized pulp by varying the order, concentration and reaction time of the bleaching agents.
Each bleaching stage is defined by its bleaching agent, pH (acidity), temperature and duration (table 1). After each bleaching stage, the pulp may be washed with caustic to remove spent bleaching chemicals and dissolved lignin before it progresses to the next stage. After the last stage, the pulp is pumped through a series of screens and cleaners to remove any contaminants such as dirt or plastic. It is then concentrated and conveyed to storage.
Table 1. Bleaching agents and their conditions of use
|
Symbol |
Concentration |
pH |
Consistency* |
Temperature |
Time (h) |
|
|
Chlorine (Cl2) |
C |
2.5–8 |
2 |
3 |
20–60 |
0.5–1.5 |
|
Sodium hydroxide (NaOH) |
E |
1.5–4.2 |
11 |
10–12 |
<80 |
1–2 |
|
Chlorine dioxide (ClO2) |
D |
~1 |
0–6 |
10–12 |
60–75 |
2–5 |
|
Sodium hypochlorite (NaOCl) |
H |
1–2 |
9–11 |
10–12 |
30–50 |
0.5–3 |
|
Oxygen (O2) |
O |
1.2–1.9 |
7–8 |
25–33 |
90–130 |
0.3–1 |
|
Hydrogen peroxide (H2O2) |
P |
0.25 |
10 |
12 |
35–80 |
4 |
|
Ozone (O3) |
Z |
0.5–3.5 |
2–3 |
35–55 |
20–40 |
<0.1 |
|
Acid washing (SO2) |
A |
4–6 |
1.8–5 |
1.5 |
30–50 |
0.25 |
|
Sodium dithionite (NaS2O4) |
Y |
1–2 |
5.5–8 |
4–8 |
60–65 |
1–2 |
* Concentration of fibre in water solution.
Historically, the most common bleaching sequence used to produce market-grade bleached kraft pulp is based on the five-stage CEDED process (see table 1 for definition of symbols). The first two stages of bleaching complete the delignification process and are considered extensions of pulping. Because of environmental concerns about chlorinated organics in pulp mill effluents, many mills substitute chlorine dioxide (ClO2) for a portion of the chlorine (Cl2) used in the first bleaching stage (CDEDED) and use oxygen (O2) pre-treatment during the first caustic extraction (CDEODED). The current trend in Europe and North America is towards complete substitution with ClO2 (e.g., DEDED) or elimination of both Cl2 and ClO2. Where ClO2 is used, sulphur dioxide (SO2) is added during the final washing stage as an “antichlor” to stop the ClO2 reaction and to control the pH. Newly developed chlorine-free bleaching sequences (e.g., OAZQP, OQPZP, where Q = chelation) use enzymes, O2, ozone (O3), hydrogen peroxide (H2O2), peracids and chelating agents such as ethylene diamine tetracetic acid (EDTA). Totally chlorine-free bleaching had been adopted at eight mills worldwide by 1993. Because these newer methods eliminate the acidic bleaching steps, acid washing is a necessary addition to the initial stages of kraft bleaching to allow removal of metals bound to the cellulose.
Sulphite pulps are generally easier to bleach than kraft pulps because of their lower lignin content. Short bleaching sequences (e.g., CEH, DCEHD, P, HP, EPOP) can be used for most paper grades. For dissolving-grade sulphite pulps used in the production of rayon, cellophane and so on, both hemicellulose and lignin are removed, requiring more complex bleaching sequences (e.g., C1C2ECHDA). The final acid wash is both for metal control and antichlor purposes. The effluent load for dissolving-grade sulphite pulps is much greater because so much of the raw wood is consumed (typical yield 50%) and more water is used.
The term brightening is used to describe bleaching of mechanical and other high-yield pulps, because they are whitened by destroying chromophoric groups without dissolving the lignin. Brightening agents include H2O2 and/or sodium hydrosulphite (NaS2O4). Historically, zinc hydrosulphite (ZnS2O4) was commonly used, but has been largely eliminated because of its toxicity in effluent. Chelating agents are added before bleaching to neutralize any metal ions, thereby preventing the formation of coloured salts or the decomposition of H2O2. The effectiveness of mechanical pulp bleaching depends on the species of wood. Hardwoods (e.g., poplar and cottonwood) and softwoods (e.g., spruce and balsam) that are low in lignin and extractives can be bleached to a higher brightness level than the more resinous pine and cedar.
Pulping
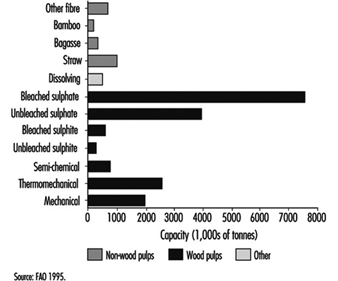
Pulping is the process by which the bonds within the wood structure are ruptured either mechanically or chemically. Chemical pulps can be produced by either alkaline (i.e., sulphate or kraft) or acidic (i.e., sulphite) processes. The highest proportion of pulp is produced by the sulphate method, followed by mechanical (including semi-chemical, thermomechanical and mechanical) and sulphite methods (figure 1). Pulping processes differ in the yield and quality of the product, and for chemical methods, in the chemicals used and the proportion that can be recovered for reuse.
Figure 1. Worldwide pulp capacities, by pulp type
Mechanical Pulping
Mechanical pulps are produced by grinding wood against a stone or between metal plates, thereby separating the wood into individual fibres. The shearing action breaks cellulose fibres, so that the resulting pulp is weaker than chemically separated pulps. The lignin connecting cellulose to hemicellulose is not dissolved; it merely softens, allowing the fibres to be ground out of the wood matrix. The yield (proportion of original wood in pulp) is usually greater than 85%. Some mechanical pulping methods also use chemicals (i.e., the chemi-mechanical pulps); their yields are lower since they remove more of the non-cellulosic materials.
In stone groundwood pulping (SGW), the oldest and historically most common mechanical method, fibres are removed from short logs by pressing them against a rotating abrasive cylinder. In refiner mechanical pulping (RMP, figure 2), which gained popularity after it became commercially viable in the 1960s, wood chips or sawdust are fed through the centre of a disc refiner, where they are shredded into finer pieces as they are pushed out through progressively narrower bars and grooves. (In figure 2, the refiners are enclosed in the middle of the picture and their large motors are on the left. Chips are supplied though the large diameter pipes, and pulp exits the smaller ones.) A modification of RMP is thermomechanical pulping (TMP), in which the chips are steamed before and during refining, usually under pressure.
Figure 2. Refiner mechanical pulping
One of the earliest methods of producing chemi-mechanical pulps involved pre-steaming logs before boiling them in chemical pulping liquors, then grinding them in stone grinders to produce “chemi-groundwood” pulps. Modern chemi-mechanical pulping uses disc refiners with chemical treatment (e.g., sodium bisulphite, sodium hydroxide) either prior to, during or after refining. Pulps produced in this manner are referred to either as chemi-mechanical pulps (CMP) or chemi-thermomechanical pulps (CTMP), depending on whether refining was carried out at atmospheric or elevated pressure. Specialized variations of CTMP have been developed and patented by a number of organizations.
Chemical Pulping and Recovery
Chemical pulps are produced by chemically dissolving the lignin between the wood fibres, thereby enabling the fibres to separate relatively undamaged. Because most of the non-fibrous wood components are removed in these processes, yields are usually in the order of 40 to 55%.
In chemical pulping, chips and chemicals in aqueous solution are cooked together in a pressure vessel (digester, figure 3) which can be operated on a batch or continuous basis. In batch cooking, the digester is filled with chips through a top opening, the digestion chemicals are added, and the contents cooked at elevated temperature and pressure. Once the cook is complete, the pressure is released, “blowing” the delignified pulp out of the digester and into a holding tank. The sequence is then repeated. In continuous digesting, pre-steamed chips are fed into the digester at a continuous rate. Chips and chemicals are mixed together in the impregnation zone at the top of the digester and then proceed through the upper cooking zone, the lower cooking zone, and the washing zone before being blown into the blow tank.
Figure 3. Continuous kraft digestor, with chip conveyor under construction
Canfor Library
The digesting chemicals are recovered in most chemical pulping operations today. The principal objectives are to recover and reconstitute digestion chemicals from the spent cooking liquor, and to recover heat energy by burning the dissolved organic material from the wood. The resulting steam and electricity supplies some, if not all, of the mill’s energy needs.
Sulphate Pulping and Recovery
The sulphate process produces a stronger, darker pulp than other methods and requires chemical recovery to compete economically. The method evolved from soda pulping (which uses only sodium hydroxide for digestion) and began to gain prominence in the industry from the 1930s to 1950s with the development of chlorine dioxide bleaching and chemical recovery processes, which also produced steam and power for the mill. The development of corrosion-proof metals, such as stainless steel, to handle the acidic and alkaline pulp mill environments also played a role.
The cooking mixture (white liquor) is sodium hydroxide (NaOH, “caustic”) and sodium sulphide (Na2S). Modern kraft pulping is usually carried out in continuous digesters often lined with stainless steel (figure 3). The temperature of the digester is raised slowly to approximately 170°C and held at that level for approximately 3 to 4 hours. The pulp (called brown stock because of its colour) is screened to remove uncooked wood, washed to remove the spent cooking mixture (now black liquor), and sent either to the bleach plant or to the pulp machine room. Uncooked wood is either returned to the digester or sent to the power boiler to be burned.
The black liquor collected from the digester and brown stock washers contains dissolved organic material whose exact chemical composition depends on the wood species pulped and the cooking conditions. The liquor is concentrated in evaporators until it contains less than 40% water, then sprayed into the recovery boiler. The organic component is consumed as fuel, generating heat which is recovered in the upper section of the furnace as high-temperature steam. The unburned inorganic component collects at the bottom of the boiler as a molten smelt. The smelt flows out of the furnace and is dissolved in a weak caustic solution, producing “green liquor” containing primarily dissolved Na2S and sodium carbonate (Na2CO3). This liquor is pumped to a recausticizing plant, where it is clarified, then reacted with slaked lime
(Ca(OH)2), forming NaOH and calcium carbonate (CaCO3). The white liquor is filtered and stored for subsequent use. CaCO3 is sent to a lime kiln, where it is heated to regenerate lime (CaO).
Sulphite Pulping and Recovery
Sulphite pulping dominated the industry from the late 1800s to the mid-1900s, but the method used during this era was limited by the types of wood which could be pulped and the pollution created by discharging untreated waste cooking liquor into waterways. Newer methods have overcome many of these problems, but sulphite pulping is now a small segment of the pulp market. Although sulphite pulping usually uses acid digestion, both neutral and basic variations exist.
The cooking liquor of sulphurous acid (H2SO3) and bisulphite ion (HSO3–) is prepared on-site. Elemental sulphur is burned to produce sulphur dioxide (SO2), which is passed up through an absorption tower that contains water and one of four alkaline bases (CaCO3, the original sulphite base, Na2CO3, magnesium hydroxide (Mg(OH)2) or ammonium hydroxide (NH4OH)) which produce the acid and ion and control their proportions. Sulphite pulping is usually carried out in brick-lined batch digesters. To avoid unwanted reactions, the digester is heated slowly to a maximum temperature of 130 to 140°C and the chips are cooked for a long time (6 to 8 hours). As the digester pressure increases, gaseous sulphur dioxide (SO2) is bled off and remixed with the raw cooking acid. When approximately 1 to 1.5 hours of cooking time remains, heating is discontinued and the pressure is decreased by bleeding off gas and steam. The pulp is blown into a holding tank, then washed and screened.
The spent digestion mixture, called red liquor, can be used for heat and chemical recovery for all but calcium-bisulphite-base operations. For ammonia-base sulphite pulping, the dilute red liquor is first stripped to remove residual SO2, then concentrated and burned. The flue gas containing SO2 is cooled and passed through an absorption tower where fresh ammonia combines with it to regenerate the cooking liquor. Finally, the liquor is filtered, fortified with fresh SO2 and stored. The ammonia cannot be recovered because it is converted into nitrogen and water in the recovery boiler.
In magnesium-base sulphite pulping, burning the concentrated pulping liquor gives magnesium oxide (MgO) and SO2, which are easily recovered. No smelt is produced in this process; rather MgO is collected from the flue gas and slaked with water to produce magnesium hydroxide (Mg(OH)2). SO2 is cooled and combined with the Mg(OH)2 in an absorption tower to reconstitute the cooking liquor. The magnesium bisulphite (Mg(HSO3)2) is then fortified with fresh SO2 and stored. Recovery of 80 to 90% of the cooking chemicals is possible.
Recovery of sodium-base sulphite cooking liquor is more complicated. Concentrated spent liquor is incinerated, and approximately 50% of the sulphur is converted into SO2. The remainder of the sodium and sulphur is collected at the bottom of the recovery boiler as a smelt of Na2S and Na2CO3. The smelt is dissolved to produce green liquor, which is converted to sodium bisulphite (NaHSO3) in several steps. The NaHSO3 is fortified and stored. The regeneration process produces reduced sulphur gases, in particular hydrogen sulphide (H2S).
The Generation and Transport of Hazardous Wastes: Social and Ethical Issues
Adapted from Soskolne 1997, with permission.
Hazardous wastes include, among other things, radioactive materials and chemicals. The movement of these substances from their source to other locations has been termed “toxic trade”. It was in the late 1980s that concern was raised about toxic trade, in particular with Africa (Vir 1989). This set the stage for the recently recognized issue of environmental justice, in some situations also known as environmental racism (Coughlin 1996).
Vir (1989) pointed out that as environmental safety laws became increasingly stringent in Europe and in the United States, and as the cost of disposal increased, “dumpers” or “waste merchants” began to turn their attention to poorer nations as potential and willing recipients of their waste products, providing a much needed source of revenue to these poorer countries. Some of these countries had been willing to take such waste at a fraction of the cost that developed nations would otherwise have had to pay for their disposal. To “nations that are drowning economically, this is an attractive deal” (Vir 1989).
Asante-Duah, Saccomanno and Shortreed (1992) show the exponential growth in the United States in the production of hazardous wastes since 1970, with the costs associated with treatment and disposal similarly increasing. They argue in favour of a controlled hazardous waste trade, one that is “regulated and informed”. They note that “countries generating small quantities of hazardous wastes should view the waste trade as an important economic option, as long as the waste recipients do not compromise their environmental sustainability”. Hazardous wastes will continue to be generated and there are countries for which an increase in some of these substances would not increase the risk to health of either present or future generations. It might therefore be economically efficient for such countries to accept waste.
There are others who argue that waste should be disposed of only at the source and not be transported at all (Puckett and Fogel 1994; Cray 1991; Southam News 1994). The latter argue from the position that science is incapable of providing any guarantees about the absence of risk.
One ethical principle that emerges from the foregoing argument is that of respect for autonomy (i.e., respect for persons), which also includes questions of national autonomy. The crucial question is one of the ability of a recipient country to adequately assess the level of risk associated with a shipment of hazardous waste. Assessment presupposes full disclosure of the contents of a shipment from the originating country and a level of local expertise to assess any potential impacts on the recipient country.
Because communities in developing countries are less likely to be informed about the potential risks associated with waste shipments, the NIMBY phenomenon (i.e., not in my backyard) so evident in the more affluent regions of the world is less likely to manifest in poorer regions. Furthermore, workers in developing regions of the world tend not to have the infrastructure related to worker protection, including information concerning the labelling of products with which they come into contact. Hence, workers in poorer nations involved in the management, storage and disposal of hazardous waste would lack the training to know how to protect themselves. Regardless of these ethical considerations, in the final analysis the economic benefits to be derived from accepting such waste shipments would need to be weighed against any potential harms that could arise in the short, medium and longer terms.
A second ethical principle emerging from the preceding argument is that of distributive justice, which involves question regarding who takes risks and who derives benefits. When there is an imbalance between those who take risks and those who derive benefits, the principle of distributive justice is not being honoured. It has often been financially poor labourers who have been exposed to hazards without any ability to enjoy the fruits of their efforts. This has occurred in the context of production of relatively expensive merchandise in the developing world for the benefit of first world markets. Another example related to the testing of new vaccines or drugs on people in developing countries who could never afford access to them in their own countries.
Towards Controlling the Transport of Hazardous Wastes
Because of the recognized need to better control the dumping of hazardous wastes, the Basel Convention was entered into by ministers of 33 countries in March 1989 (Asante-Duah, Saccomanno and Shortreed 1992). The Basel Convention addressed the transboundary movements of hazardous wastes and required the notification and consent of recipient countries before any waste shipments could take place.
Subsequently, the United Nations Environment Programme (UNEP) launched its Cleaner Production Programme, in close cooperation with governments and industry, to advocate low- and non-waste technologies (Rummel-Bulska 1993). In March 1994, a full ban was introduced on all transboundary movements of hazardous wastes from the 24 rich industrialized countries of the Organization for Economic Cooperation and Development (OECD) to other states that are not members of the OECD. The ban was immediate for wastes bound for final disposal and enters into force at the beginning of 1998 for all hazardous wastes that are said to be destined for recycling or recovery operations (Puckett and Fogel 1994). The countries most opposed to the introduction of a total ban were Australia, Canada, Japan and the United States. Despite this opposition from a handful of powerful industrial governments through the penultimate vote, the ban was finally agreed to by consensus (Puckett and Fogel 1994).
Greenpeace has stressed the primary prevention approach to solving the mounting waste crisis by addressing the root cause of the problem, namely minimizing waste generation through clean production technologies (Greenpeace 1994a). In making this point, Greenpeace identified major countries exporting hazardous wastes (Australia, Canada, Germany, the United Kingdom and the United States) and some countries importing them (Bangladesh, China (including Taiwan), India, Indonesia, Malaysia, Pakistan, the Philippines, the Republic of Korea, Sri Lanka and Thailand). In 1993, Canada, for example, had exported some 3.2 million kilograms of ash containing lead and zinc to India, the Republic of Korea and Taiwan, China, and 5.8 million kilograms of plastic waste to Hong Kong (Southam News 1994). Greenpeace (1993, 1994b) also addresses the extent of the problem in terms of specific substances and approaches to disposal.
Risk Assessment
Epidemiology is at the centre of human health risk assessment, which is invoked when concern is raised by a community about the consequences, if any, of exposure to hazardous and potentially toxic substances. The scientific method that epidemiology brings to the study of the environmental determinants of ill health can be fundamental to protecting unempowered communities, both from environmental hazards and from environmental degradation. Risk assessment conducted in advance of a shipment likely would fall into the legal trade arena; when conducted after a shipment has arrived, risk assessment would be undertaken to determine whether or not any health concerns were justified from what likely would have been an illegal shipment.
Among the concerns to the risk assessor would be hazard assessment, i.e., questions about what hazards, if any, exist and in what quantities and in what form they might be present. In addition, depending on the type of hazard, the risk assessor must make an exposure assessment to establish what possibilities there are for people to be exposed to the hazardous substance(s) through inhalation, skin absorption or ingestion (by contamination of the food chain or directly on foodstuffs).
In terms of trade, autonomy would require the informed consent of the parties in a voluntary and non-coercive milieu. However, it is hardly possible that non-coerciveness could ever pertain in such a circumstance by virtue of the financial need of an importing developing world country. The analogue here is the now accepted ethical guideline which does not permit the coercion of participants in research through payment for anything but direct costs (e.g., lost wages) for the time taken to participate in a study (CIOMS 1993). Other ethical issues involved here would include, on the one hand, truth in the presence of unknowns or in the presence of scientific uncertainty and, on the other hand, the principle of caveat emptor (buyer beware). The ethical principle of non-maleficence requires the doing of more good than harm. Here the short-term economic benefits of any trade agreement to accept toxic wastes must be weighed against the longer term damage to the environment, the public health and possibly also to future generations.
Finally, the principle of distributive justice requires recognition by the parties involved in a trade deal as to who would be deriving the benefits and who would be taking the risks in any trade deal. In the past, general practices for dumping waste and for locating hazardous waste sites in unempowered communities in the United States have led to the recognition of the concern now known as environmental justice or environmental racism (Coughlin 1996). In addition, questions of environmental sustainability and integrity have become central concerns in the public forum.
Acknowledgements: Dr. Margaret-Ann Armour, Department of Chemistry, University of Alberta, provided valuable references on the topic of toxic trade as well as materials from the November 1993 Pacific Basin “Conference on Hazardous Waste” at the University of Hawaii.
The Greenpeace office in Toronto, Ontario, Canada, was most helpful in providing copies of the Greenpeace references cited in this article.
" DISCLAIMER: The ILO does not take responsibility for content presented on this web portal that is presented in any language other than English, which is the language used for the initial production and peer-review of original content. Certain statistics have not been updated since the production of the 4th edition of the Encyclopaedia (1998)."