Metal Finishing
The surface treatment of metals increases their durability and improves their appearance. A single product may undergo more than one surface treatment—for example, an auto body panel may be phosphated, primed and painted. This article deals with the processes used for surface treatment of metals and the methods used to reduce their environmental impact.
Operating a metal finishing business requires cooperation between company management, employees, government and the community to effectively minimize the environmental effect of the operations. Society is concerned with the amount and the long-term effects of pollution entering the air, water and land environment. Effective environmental management is established through detailed knowledge of all elements, chemicals, metals, processes and outputs.
Pollution prevention planning shifts the environmental management philosophy from reacting to problems to anticipating solutions focusing on chemical substitution, process change and internal recycling, using the following planning sequence:
- Initiate pollution prevention across all aspects of the business.
- Identify waste streams.
- Set priorities for action.
- Establish root cause of the waste.
- Identify and implement changes that reduce or eliminate the waste.
- Measure the results.
Continuous improvement is achieved by setting new priorities for action and repeating the sequence of actions.
Detailed process documentation will identify the waste streams and allow priorities to be set for waste reduction opportunities. Informed decisions about potential changes will encourage:
- easy and practical operational improvements
- process changes involving customers and suppliers
- changes to less harmful activities where possible
- reuse and recycling where change is not practical
- using landfilling of hazardous wastes only as a last resort.
Major processes and standard operating processes
Cleaning is required because all metal finishing processes require that parts to be finished be free from organic and inorganic soils, including oils, scale, buffing and polishing compounds. The three basic types of cleaners in use are solvents, vapour degreasers and alkaline detergents.
Solvents and vapour degreasing cleaning methods have been almost totally replaced by alkaline materials where the subsequent processes are wet. Solvents and vapour degreasers are still in use where parts must be clean and dry with no further wet processing. Solvents such as terpenes are in some instances replacing volatile solvents. Less toxic materials such as 1,1,1-trichloroethane have been substituted for more hazardous materials in vapour degreasing (although this solvent is being phased out as an ozone depleter).
Alkaline cleaning cycles usually include a soak immersion followed by an anodic electroclean, followed by a weak acid immersion. Non-etching, non-silicated cleaners are typically used to clean aluminium. The acids are typically sulphuric, hydrochloric and nitric.
Anodizing, an electrochemical process to thicken the oxide film on the metal surface (frequently applied to aluminium), treats the parts with dilute chromic or sulphuric acid solutions.
Conversion coating is used to provide a base for subsequent painting or to passivate for protection against oxidation. With chromating, parts are immersed in a hexavalent chrome solution with active organic and inorganic agents. For phosphating, parts are immersed in dilute phosphoric acid with other agents. Passivating is accomplished through immersion in nitric acid or nitric acid with sodium dichromate.
Electroless plating involves a deposition of metal without electricity. Copper or nickel electroless deposition is used in the manufacture of printed circuit boards.
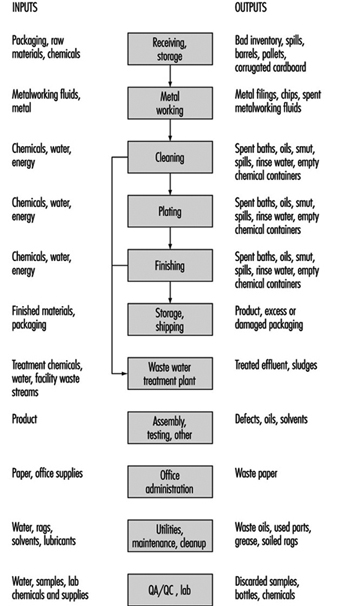
Electroplating involves the deposition of a thin coat of metal (zinc, nickel, copper, chromium, cadmium, tin, brass, bronze, lead, tin-lead, gold, silver and other metals such as platinum) on a substrate (ferrous or non-ferrous). Process baths include metals in solution in acid, alkaline neutral and alkaline cyanide formulations (see figure 1).
Figure 1. Inputs and outputs for a typical electroplating line
Chemical milling and etching are controlled dissolution immersion processes using chemical reagents and etchants. Aluminium is typically etched in caustic prior to anodizing or chemically brightened in a solution which could contain nitric, phosphoric and sulphuric acids.
Hot-dip coatings involve the application of metal to a workpiece by immersion in molten metal (zinc or tin galvanizing of steel).
Good management practices
Important safety, health and environmental improvements can be achieved through process improvements, such as:
- using counter-current rinsing and conductivity controls
- increasing drainage time
- using more or better wetting agents
- keeping process temperatures as high as possible to lower viscosity, thus increasing drag-out recovery (i.e., recovery of solution left on metal)
- using air agitation in rinsing to increase rinsing efficiency
- using plastic balls in plating tanks to reduce misting
- using improved filtration on plating tanks to reduce the frequency of purification treatment
- placing a curb around all process areas to contain spills
- using separate treatments for recoverable metals such as nickel
- installing recovery systems such as ion exchange, atmospheric evaporation, vacuum evaporation, electrolytic recovery, reverse osmosis and electrodialysis
- complementing drag-out recovery systems with reductions in drag-in of contaminants and improved cleaning systems
- using modern inventory controls to reduce waste and workplace hazards
- applying standard procedures (i.e., written procedures, regular operating reviews and sound operating logs) to provide the basis for a sound environmental management structure.
Environmental planning for specific wastes
Specific waste streams, usually spent plating solutions, can be reduced by:
- Filtration. Cartridge or diatomaceous earth filters can be used to remove the accumulation of solids, which reduce the efficiency of the process.
- Carbon treatment can be used to remove organic contaminants (most commonly applied in nickel plating, copper electroplating and zinc and cadmium plating).
- Purified water. The natural contaminants in water make-up and rinses (e.g., calcium, iron, magnesium, manganese, chlorine and carbonates) can be removed by using deionization, distillation or reverse osmosis. Improving rinse water efficiency reduces the volume of bath sludges requiring treatment.
- Cyanide bath carbonate freezing. Lowering the bath temperature to –3 °C crystallizes the carbonates formed in cyanide bath by the breakdown of cyanide, excessive anode current densities and the adsorption of carbon dioxide from the air and facilitates their removal.
- Precipitation. Removal of metal contaminants entering the bath as impurities in anodes can be achieved through precipitation with barium cyanide, barium hydroxide, calcium hydroxide, calcium sulphate or calcium cyanide.
- Hexavalent chrome alternatives. Hexavalent chromium can be replaced with trivalent chromium plating solutions for decorative plating. Chrome conversion coatings for paint pretreatments can sometimes be replaced by non-chrome conversion coatings or no-rinse chrome chemistries.
- Non-chelated process chemistries. Instead of chelators being added to process baths to control the concentration of free ions in the solution, non-chelated process chemistries can be used so that it may not be necessary to keep metals in solution. These metals can be allowed to precipitate and can be removed by continuous filtration.
- Non-cyanide process chemicals. Waste streams containing free cyanide are typically treated using hypochlorite or chlorine to accomplish oxidation, and complex cyanides are commonly precipitated using ferrous sulphate. Using non-cyanide process chemistries both eliminates a treatment step and reduces the sludge volume.
- Solvent degreasing. Hot alkaline cleaning baths can be used in place of solvent degreasing of workpieces before processing. The effectiveness of alkaline cleaners can be enhanced by applying electrocurrent or ultrasonics. The benefits of avoiding solvent vapours and sludges often outweigh any additional operating costs.
- Alkaline cleaners. Having to discard alkaline cleaners when the accumulation of oil, grease and soils from use reaches a level which impairs the cleaning efficiency of the bath can be avoided by using skimming devices to remove free-floating oils, settling devices or cartridge filters to remove particulates and oil-water coalescers and by using microfiltration or ultrafiltration to remove emulsified oils.
- Drag-out reduction. Reducing the volume of drag-out from process baths serves to reduce the amount of valuable process chemicals that contaminates the rinse water, which in turn reduces the amount of sludge that is generated by a conventional metal precipitation treatment process.
Several methods of reducing drag-out include:
- Process bath operating concentration. The chemical concentration should be kept as low as possible to minimize the viscosity (for quicker draining) and the quantity of chemicals (in the film).
- Process bath operating temperature. The viscosity of the process solution can be reduced by increasing the bath temperature.
- Wetting agents. The surface tension of the solution can be reduced by adding wetting agents to the process bath.
- Workpiece positioning. The workpiece should be positioned on the rack so that the adhering film drains freely and does not get trapped in grooves or cavities.
- Withdrawal or drainage time. The faster a workpiece is removed from the process bath, the thicker the film on the workpiece surface.
- Air knives. Blowing air at the workpiece as the workpiece rack is raised above the process tank can improve drainage and drying.
- Spray rinses. These can be used above heated baths so that the rinse flow rate equals the evaporation rate of the tank.
- Plating baths. Carbonates and organic contaminants should be removed to prevent accumulation of contamination that increases the viscosity of the plating bath.
- Drainage boards. The spaces between process tanks should be covered with drainage boards to capture process solutions and to return them to the process bath.
- Drag-out tanks. The workpieces should be placed in drag-out tanks (“static rinse” tanks) before the standard rinsing operation.
Drag-out recovery of chemicals uses a variety of technologies. These include:
- Evaporation. Atmospheric evaporators are most common, and vacuum evaporators offer energy savings.
- Ion exchange is used for chemical recovery of rinse water.
- Electrowinning. This is an electrolytic process whereby the dissolved metals in the solution are reduced and deposited on the cathode. The deposited metal is then recovered.
- Electrodialysis. This utilizes ion-permeable membranes and applied current in order to separate ionic species from the solution.
- Reverse osmosis. This utilizes a semi-permeable membrane to produce purified water and a concentrated ionic solution. High pressure is used to force the water through the membrane, while most dissolved salts are retained by the membrane.
Rinse water
Most of the hazardous waste produced in a metal finishing facility comes from waste water generated by the rinsing operations that follow cleaning and plating. By increasing rinse efficiency, a facility can significantly reduce waste water flow.
Two basic strategies improve rinsing efficiency. First, turbulence can be generated between the workpiece and the rinse water through spray rinses and rinse water agitation. Movement of the rack or forced water or air are used. Second, the contact time between the workpiece and the rinse water can be increased. Multiple rinse tanks set countercurrent in series will reduce the amount of rinse water used.
Industrial Coatings
The term coatings includes paints, varnishes, lacquers, enamels and shellacs, putties, wood fillers and sealers, paint and varnish removers, paint brush cleaners and allied paint products. Liquid coatings contain pigments and additives dispersed in a liquid binder and solvent mixture. Pigments are inorganic or organic compounds that provide coating colour and opacity and influence coating flow and durability. Pigments often contain heavy metals such as cadmium, lead, zinc, chromium and cobalt. The binder increases coating adhesiveness, cohesiveness and consistency and is the primary component that remains on the surface when coating is completed. Binders include a variety of oils, resins, rubbers and polymers. Additives such as fillers and extenders may be added to coatings to reduce manufacturing costs and increase coating durability.
The types of organic solvents used in coatings include aliphatic hydrocarbons, aromatic hydrocarbons, esters, ketones, glycol ethers and alcohols. Solvents disperse or dissolve the binders and decrease the coating viscosity and thickness. Solvents used in coatings formulations are hazardous because many are human carcinogens and are flammable or explosive. Most solvents contained in a coating evaporate when the coating cures, which generates volatile organic compound (VOC) emissions. VOC emissions are becoming increasingly regulated because of the negative effects on human health and the environment. Environmental concerns associated with conventional ingredients, coating application technologies and coating wastes are a driving force for developing pollution prevention alternatives.
Most coatings are used on architectural, industrial or special products. Architectural coatings are used in buildings and building products and for decorative and protective services such as varnishes to protect wood. Industrial facilities incorporate coating operations in various production processes. The automotive, metal can, farm machinery, coil coating, wood and metal furniture and fixtures, and household appliance industries are the major industrial coatings consumers.
Design of a coating formulation depends on the purpose of the coating application. Coatings provide aesthetics, and corrosion and surface protection. Cost, function, product safety, environmental safety, transfer efficiency and drying and curing speed determine formulations.
Coating processes
There are five operations comprising most coating processes: raw materials handling and preparation, surface preparation, coating, equipment cleaning and waste management.
Raw material handling and preparation
Raw material handling and preparation involves inventory storage, mixing operations, thinning and adjusting of coatings and raw material transfer through the facility. Monitoring and handling procedures and practices are needed to minimize the generation of wastes from spoilage, off specification and improper preparation that can result from excessive thinning and consequent wastage. Transfer, whether manual or through a piped system, must be scheduled to avoid spoilage.
Surface preparation
The type of surface preparation technique used depends on the surface being coated—previous preparation, amount of soil, grease, the coating to be applied and the surface finish required. Common preparation operations include degreasing, precoating or phosphating and coating removal. For metal finishing purposes, degreasing involves solvent wiping, cold cleaning or vapour degreasing with halogenated solvents, aqueous alkaline cleaning, semi-aqueous cleaning or aliphatic hydrocarbon cleaning to remove organic soil, dirt, oil and grease. Acid pickling, abrasive cleaning or flame cleaning are used to remove mill scale and rust.
The most common preparation operation for metal surfaces, other than cleaning, is phosphate coating, used to promote adhesion of organic coatings onto metal surfaces and retard corrosion. Phosphate coatings are applied by immersing or spraying metal surfaces with zinc, iron or manganese phosphate solution. Phosphating is a surface finishing process similar to electroplating, consisting of a series of process chemical and rinse baths in which parts are immersed to achieve the desired surface preparation. See the article “Surface treatment of metals” in this chapter.
Coating removal, chemical or mechanical, is conducted on surfaces that require recoating, repair or inspection. The most common chemical coating removal method is solvent stripping. These solutions usually contain phenol, methylene chloride and an organic acid to dissolve the coating from the coated surface. A final water wash to remove the chemicals can generate large quantities of wastewater. Abrasive blasting is the common mechanical process, a dry operation that uses compressed air to propel a blasting medium against the surface to remove the coating.
Surface preparation operations affect the quantity of waste from the specific preparation process. If the surface preparation is inadequate, resulting in poor coating, then removal of the coating and recoating adds to waste generation.
Coating
The coating operation involves transferring the coating to the surface and curing the coating on the surface. Most coating technologies fall into 1 of 5 basic categories: dip coating, roll coating, flow coating, spray coating, and the most common technique, air-atomized spray coating using solvent-based coatings.
Air-atomized spray coatings are usually conducted in a controlled environment because of solvent emissions and overspray. Overspray control devices are fabric filters or water walls, generating either used filters or wastewater from air scrubbing systems.
Curing is performed to convert the coating binder into a hard, tough, adherent surface. Curing mechanisms include: drying, baking or exposure to an electron beam or infrared or ultraviolet light. Curing generates significant VOCs from solvent-based coatings and poses a potential for explosion if the solvent concentrations rise above the lower explosive limit. Consequently, curing operations are equipped with air pollution control devices to prevent VOC emissions and for safety control to prevent explosions.
Environmental and health concerns, increased regulations affecting conventional coating formulations, high solvent costs and expensive hazardous waste disposal have created a demand for alternative coating formulations that contain less hazardous constituents and generate less waste when applied. Alternative coating formulations include:
- High-solid coatings, containing twice the amount of pigment and resin in the same volume of solvent as conventional coatings. Application lowers VOC emissions between 62 and 85% compared to conventional low-solid solvent-based coatings because the solvent content is reduced.
- Water-based coatings using water and an organic solvent mixture as the carrier with water used as the base. Compared to solvent-based coatings, water-based coatings generate between 80 and 95% less VOC emissions and spent solvents than conventional low-solid solvent-based coatings.
- Powder coatings containing no organic solvent, consisting of finely pulverized pigment and resin particles. They are either thermoplastic (high molecular weight resin for thick coatings) or thermosetting (low molecular weight compounds that form a thin layer before chemically cross-linking) powders.
Equipment cleaning
Equipment cleaning is a necessary, routine maintenance operation in coating processes. This creates significant amounts of hazardous waste, particularly if halogenated solvents are used for cleaning. Equipment cleaning for solvent-based coatings has traditionally been conducted manually with organic solvents to remove coatings from process equipment. Piping requires flushing with solvent in batches until clean. Coating equipment must be cleaned between product changes and after process shutdowns. The procedures and practices used will determine the level of waste generated from these activities.
Waste management
Several waste streams are generated by coating processes. Solid waste includes empty coating containers, coating sludge from overspray and equipment cleaning, spent filters and abrasive materials, dry coating and cleaning rags.
Liquid wastes include waste water from surface preparation, overspray control or equipment cleaning, off-specification or excess coating or surface preparation materials, overspray, spills and spent cleaning solutions. Onsite closed-loop recycling is becoming more popular for spent solvents as disposal costs rise. Water-based liquids are usually treated onsite prior to discharge to publicly owned treatment systems.
VOC emissions are generated by all conventional coating processes that use solvent-based coatings, requiring control devices such as carbon adsorption units, condensers or thermal catalytic oxidizers.