For the purposes of this article, the processes of the woodworking industry will be considered to start with the reception of converted timber from the sawmill and continue until the shipping of a finished wood article or product. Earlier stages in the handling of wood are dealt with in the chapters Forestry and Lumber industry.
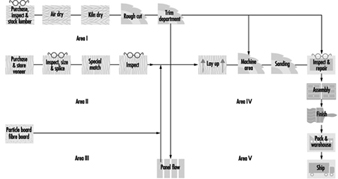
The woodworking industry produces furniture and a variety of building materials, ranging from plywood floors to shingles. This article covers the main stages in the processing of wood for the production of wooden products, which are machine working of natural wood or manufactured panels, assembly of machined parts and surface finishing (e.g., painting, staining, lacquering, veneering and so on). Figure 1 is a flow diagram for wood furniture manufacturing, which covers nearly the whole range of these processes.
Figure 1. Flow diagram for wood furniture manufacturing
Drying. Some furniture manufacturing facilities may purchase dried lumber, but others perform drying onsite using a drying kiln or oven, fired by a boiler. Usually wood waste is the fuel.
Machining. Once the lumber is dried, it is sawed and otherwise machined into the shape of the final furniture part, such as a table leg. In a normal plant, the wood stock moves from rough planer, to cutoff saw, to rip saw, to finish planer, to moulder, to lathe, to table saw, to band saw, to router, to shaper, to drill and mortiser, to carver and then to a variety of sanders.
Wood can be hand carved/worked with a variety of hand tools, including chisels, rasps, files, hand saws, sandpaper and the like.
In many instances, the design of furniture pieces requires bending of certain wooden parts. This occurs after the planing process, and usually involves the application of pressure in conjunction with a softening agent, such as water, and increased atmospheric pressure. After bending into the desired shape, the piece is dried to remove excess moisture.
Assembly. Wood furniture can either be finished and then assembled, or the reverse. Furniture made of irregularly shaped components is usually assembled and then finished.
The assembly process usually involves the use of adhesives (either synthetic or natural) in conjunction with other joining methods, such as nailing, followed by the application of veneers. Purchased veneers are trimmed to correct size and patterns, and bonded to purchased chipboard.
After assembly, the furniture part is examined to ensure a smooth surface for finishing.
Pre-finishing. After initial sanding, an even smoother surface is attained by spraying, sponging or dipping the furniture part with water to cause the wood fibres to swell and “raise”. After the surface has dried, a solution of glue or resin is applied and allowed to dry. The raised fibres are then sanded down to form a smooth surface.
If the wood contains rosin, which can interfere with the effectiveness of certain finishes, it may be derosinated by applying a mixture of acetone and ammonia. The wood is then bleached by spraying, sponging or dipping the wood into a bleaching agent such as hydrogen peroxide.
Surface finishing. Surface finishing may involve the use of a large variety of coatings. These coatings are applied after the product is assembled or in a flat line operation before assembly. Coatings could normally include fillers, stains, glazes, sealers, lacquers, paints, varnishes and other finishes. The coatings may be applied by spray, brush, pad, dip, roller or flow-coating machine.
Coatings can be either solvent based or water based. Paints may contain a wide variety of pigments, depending on the desired colour.
Hazards and Precautions
Machining safety
Woodworking manufacturing has many of the hazards to safety and health that are common to general industry, with a much larger proportion of extremely hazardous equipment and operations than most. Consequently, safety requires constant attention to safe work habits by employees, vigilant supervision, and maintenance of a safe work environment by employers.
Although in many instances woodworking machinery and equipment may be purchased without the necessary guards and other safety devices, it is management’s responsibility to provide adequate safeguards before such machinery and equipment is used. See also the articles “Routing machines” and “Wood planing machines”.
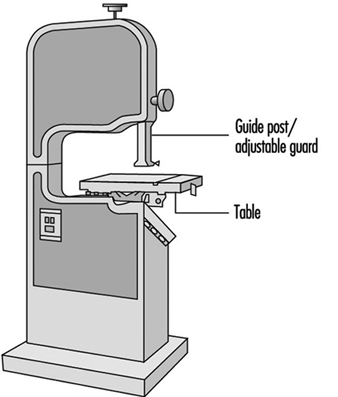
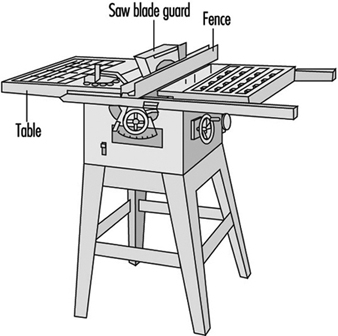
Sawing machines. Employees should be made aware of the safe operating practices necessary for the proper use of various woodworking saws (see figure 2 and figure 3).
Figure 2. Band saw
Specific guidelines are as follows:
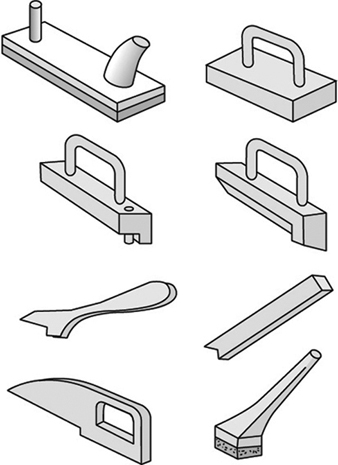
1. When feeding a table saw, hands must be kept out of the line of the cut. No guard can prevent a person’s hand from following the stock into the saw. When ripping with the fence gauge near the saw, a push stick or suitable jig must be used to complete the cut. See figure 4.
Figure 4. Push sticks
2. The saw blade must be positioned so as to minimize its protrusion above the stock; the lower the blade, the less chance for kickbacks. It is good practice to stand out of the line of the stock being ripped. A heavy leather apron or other guard for the abdomen is recommended.
3. Freehand sawing is always dangerous. The stock must always be held against a gauge or fence. See figure 3.
4. The saw must be appropriate for the job. For instance, it is an unsafe practice to rip with a table saw not equipped with a non-kickback device. Kickback aprons are recommended.
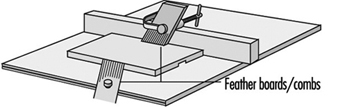
5. The dangerous practice of removing a hood guard because of narrow clearance on the gauge side can be avoided by clamping a filler board to the table between the gauge and the saw and using it to guide the stock. Employees must never be permitted to bypass guards. Combs, featherboards (see figure 5) or suitable jigs must be provided where standard guards cannot be used.
Figure 5. Featherboards & combs
6. Crosscutting long boards on a table saw should be avoided because the operator is required to use considerable hand pressure near the saw blade. Also, boards extending beyond the table may be struck by people or trucks. Long stock should be crosscut on a swing pull saw or radial arm saw with adequate supporting bench.
7. Work that should be done on special power-feed machines should not be done on general-purpose hand-fed machines.
8.To set a gauge of a table saw without taking off the guards, a permanent mark should designate the line of cut on the table top.
9. It is considered safe practice to bring equipment to a complete stop before adjusting blades or fences, and to disconnect the power source when changing blades.
10. A brush or stick should be used to clean sawdust and scrap from a saw.
A table saw is also called a variety saw because it can perform a wide variety of sawing functions. For this reason the operator should have a variety of guards, because no one guard can protect from every function. See figure 3.
Cutting machines. Cutting machines can also be hazardous if not adequately guarded and always used with respect and alertness. Cutting tools should be kept well sharpened and correctly balanced on their spindles.
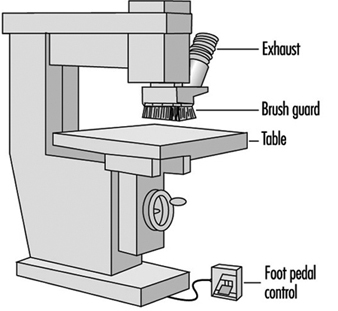
The router shown in figure 6 has a brush guard. Other routers may have a ring guard, a round guard that encircles the router bit. The purpose of guards is to keep the hands away from the cutting bit. Computer numerical controlled (CNC) routers may have several bits and are high production machines. On CNC machines the operator’s hands are kept further from the bit area. However, another problem is the high amount of wood dust. See also the article “Routing machines”.
Figure 6. Router
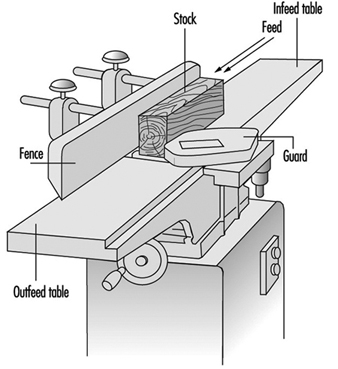
Guarding on a jointer or surface planing machine is mainly to keep the operator’s hands away from the revolving knives. The “mutton chop”-type guard allows only the portion of the knives which are cutting the stock to be exposed (see figure 7). The exposed portion of the knives behind the fence should also be guarded.
Figure 7. Jointer
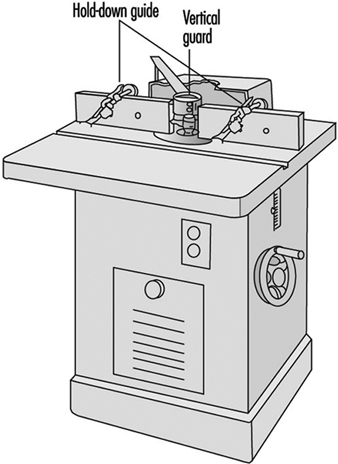
The shaper is a potentially very dangerous machine (see figure 8). If the shaper knives become separated from the above and below collars on the arbor, they can be thrown with great force. Also, stock must often be held close to the knives. This holding must be done with a fixture instead of by the operator’s hands. Featherboards can be used to hold the stock down against the table. Ring or saucer guards should be used whenever possible. A saucer guard is a round, flat, plastic disk that is mounted horizontally on the arbor above the shaper knives.
Figure 8. Shaper
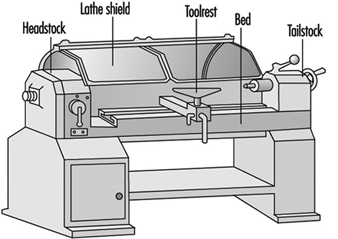
A lathe should be guarded by a hood guard because there is a danger of the stock being thrown from the machine. See figure 9. It is good practice for the hood to be interlocked with the motor so the lathe cannot be run unless the hood guard is in place.
Figure 9. Lathe
A ripsaw should have anti-kickback fingers installed to prevent the stock from reversing its direction and striking the operator. See figure 10. Also, the operator should wear a padded apron to lessen the impact if a kickback does occur.
Figure 10. Ripsaw
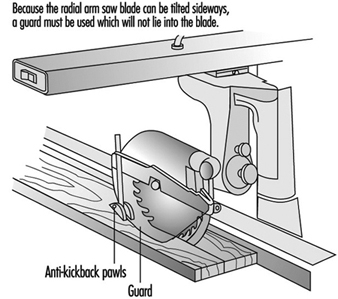
Because the radial arm saw blade can be tilted sideways, a guard must be used which will not lie into the blade. See figure 11.
Figure 11. Radial arm saw
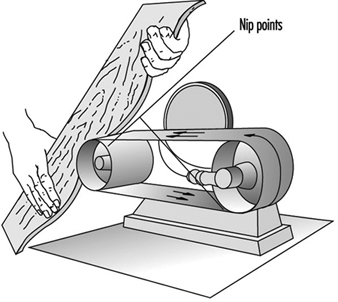
Sanding machines. Machined stock pieces are sanded down using belt, jitterbug, disc, drum or orbital sanders. Nip points are created in sanding belts. See figure 12. Often these nip points can be guarded with a hood which will also be part of a dust exhaust system.
Figure 12. Sander
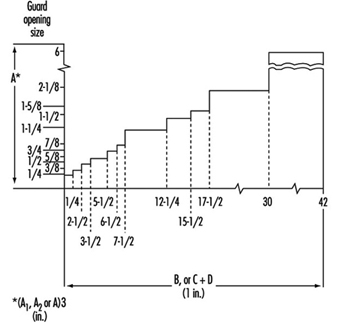
Machine guarding. Figure 13 illustrates that the opening between a guard and the point of contact must be decreased as the distance decreases.
Figure 13. Distance between guard & point of operation
Miscellaneous machine safety concerns. Care must be taken that the use of stock-clamping/holding devices do not create additional hazards.
Most woodworking machines create the necessity of the operator (and helper) wearing eye protection.
It is common practice for employees to blow dust off of themselves with compressed air. They should be cautioned to keep air pressure below 30 psi and to avoid blowing into eyes or open cuts.
Wood dust hazards
Machines that produce wood dust should be equipped with dust-collecting systems. If the exhaust system is inadequate to dispose of the wood dust, the operator may need to wear a dust respirator. The International Agency for Research on Cancer (IARC) has now determined that “there is sufficient evidence in humans for the carcinogenicity of wood dust”, and that “Wood dust is carcinogenic to humans (Group 1)”. Other studies indicate that wood dust may prove an irritant to the mucous membranes of the eyes, nose and throat. Some toxic woods are more actively pathogenic and may produce allergic reactions and occasionally pulmonary disorders and systemic poisoning. See table 1.
Table 1. Poisonous, allergenic and biologically active wood varieties
|
Scientific names |
Selected commercial names |
Family |
Health Impairment |
|
Abies alba Mill (A. pectinata D.C.) |
Silver fir |
Pinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Acacia spp. |
Australian blackwood |
Mimosaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Acer spp. |
Maple |
Aceraceae |
Dermatitis |
|
Afrormosia elata Harms. |
Afrormosia, kokrodua, asamala, obang, oleo pardo, bohele, mohole |
Papilionaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Afzelia africana Smith |
Doussié, afzelia, aligua, apa, chanfuta, lingue merbau, intsia, hintsy |
Caesalpinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Agonandra brasiliensis Miers |
Pao, marfim, granadillo |
Olacaceae |
Dermatitis |
|
Ailanthus altissima Mill |
Chinese sumac |
Simaroubaceae |
Dermatitis |
|
Albizzia falcata Backer |
Iatandza |
Mimosaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; |
|
Alnus spp. |
Common alder |
Betulaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Amyris spp. |
Venezuelan or West Indian sandalwood |
Rutaceae |
Dermatitis; toxic effects |
|
Anacardium occidentale L. |
Cashew |
Anacardiaceae |
Dermatitis |
|
Andira araroba Aguiar. (Vataireopsis araroba Ducke) |
Red cabbage tree |
Papilionaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Aningeria spp. |
Aningeria |
Sapotaceae |
Conjunctivitis-rhinitis; asthma |
|
Apuleia molaris spruce (A. leiocarpa MacBride) |
Redwood |
Caesalpinaceae |
Dermatitis; toxic effects |
|
Araucaria angustifolia O. Ktze |
Parana pine, araucaria |
Araucariaceae |
Toxic effects |
|
Aspidosperma spp. |
Red peroba |
Apocynaceae |
Dermatitis; conjunctivitis- |
|
Astrocaryum spp. |
Palm |
Palmaceae |
Dermatitis; toxic effects |
|
Aucoumea klaineana Pierre |
Gabon mahogany |
Burseraceae |
Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis |
|
Autranella congolensis |
Mukulungu, autracon, elang, bouanga, kulungu |
Sapotaceae |
Dermatitis |
|
Bactris spp. (Astrocaryum spp.) |
Palm |
Palmaceae |
Dermatitis; toxic effects |
|
Balfourodendron riedelianum Engl. |
Guatambu, gutambu blanco |
Rutaceae |
Dermatitis |
|
Batesia floribunda Benth. |
Acapu rana |
Caesalpinaceae |
Toxic effects |
|
Berberis vulgaris L. |
Barberry |
Berberidaceae |
Toxic effects |
|
Betula spp. |
Birch |
Betulaceae |
Dermatitis |
|
Blepharocarva involucrigera F. Muell. |
Rosebutternut |
Anacardiaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Bombax brevicuspe Sprague |
Kondroti, alone |
Bombacaceae |
Dermatitis |
|
Bowdichia spp. |
Black sucupira |
Papilionaceae |
Dermatitis |
|
Brachylaena hutchinsii Hutch. |
Muhuhu |
Compositae |
Dermatitis |
|
Breonia spp. |
Molompangady |
Rubiaceae |
Dermatitis |
|
Brosimum spp. |
Snakewood, letterwood, tigerwood |
Moraceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Brya ebenus DC. (Amerimnum ebenus Sw.) |
Brown ebony, green ebony, Jamaican ebony, tropical American ebony |
Papilionaceae |
Dermatitis |
|
Buxus sempervirens L. |
European boxwood, East London b., Cape b. |
Buxaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Caesalpinia echinata Lam. (Guilandina echinata Spreng.) |
Brasilwood |
Caesalpinaceae |
Dermatitis; toxic effects |
|
Callitris columellaris F. Muell. |
White cypress pine |
Cupressaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Calophyllum spp. |
Santa maria, jacareuba, kurahura, galba |
Guttiferae |
Dermatitis; toxic effects |
|
Campsiandra laurifolia Benth. |
Acapu rana |
Caesalpinaceae |
Toxic effects |
|
Carpinus betulus |
Hornbeam |
Betulaceae |
Dermatitis |
|
Cassia siamea Lamk. |
Tagayasan, muong ten, djohar |
Caesalpinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Castanea dentata Borkh |
Chestnut, sweet chestnut |
Fagaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Castanospermum australe A. Cunn. |
Black bean, Australian or Moreton Bay chestnut |
Papilionaceae |
Dermatitis |
|
Cedrela spp. (Toona spp.) |
Red cedar, Australian cedar |
Meliaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Cedrus deodara (Roxb. ex. Lamb.) G. Don |
Deodar |
Pinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Celtis brieyi De Wild. |
Diania |
Ulmaceae |
Dermatitis |
|
Chlorophora excelsa Benth. and Hook I. |
Iroko, gelbholz, yellowood, kambala, mvule, odum, moule, African teak, abang, tatajuba, fustic, mora |
Moraceae |
Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis |
|
Chloroxylon spp. |
Ceylon satinwood |
Rutaceae |
Dermatitis; toxic effects |
|
Chrysophyllum spp. |
Najara |
Sapotaceae |
Dermatitis |
|
Cinnamomum camphora Nees and Ebeim |
Asian camphorwood, cinnamon |
Lauraceae |
Toxic effects |
|
Cryptocarya pleurosperma White and Francis |
Poison walnut |
Lauraceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Dacrycarpus dacryoides (A. Rich.) de Laub. |
New Zealand white pine |
Podocarpaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Dacrydium cupressinum Soland |
Sempilor, rimu |
Podocarpaceae |
conjunctivitis-rhinitis; asthma |
|
Dactylocladus stenostachys Oliv. |
Jong kong, merebong, medang tabak |
Melastomaceae |
Toxic effects |
|
Dalbergia spp. |
Ebony |
Papilionaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; |
|
Dialium spp. |
Eyoum, eyum |
Caesalpinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Diospyros spp. |
Ebony, African ebony |
Ebenaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Dipterocarpus spp. |
Keruing, gurjum, yang, keruing |
Dipterocarpaceae |
Dermatitis |
|
Distemonanthus benthamianus Baill. |
Movingui, ayan, anyaran, Nigerian satinwood |
Caesalpinaceae |
Dermatitis |
|
Dysoxylum spp. |
Mahogany, stavewood, red bean |
Meliaceae |
dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
D. muelleri Benth. |
Rose mahogany |
||
|
Echirospermum balthazarii Fr. All. (Plathymenia reticulata Benth.) |
Vinhatico |
Mimosaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Entandophragma spp. |
Tiama |
Meliaceae |
Dermatitis; |
|
Erythrophloeum guineense G. Don |
Tali, missanda, eloun, massanda, sasswood, erun, redwater tree |
Caesalpinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Esenbeckia leiocarpa Engl. |
Guaranta |
Rutaceae |
Dermatitis |
|
Eucalyptus spp. |
|
Myrtaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Euxylophora paraensis Hub. |
Boxwood |
Rutaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Excoecaria africana M. Arg. (Spirostachys africana Sand) |
African sandalwood, tabootie, geor, aloewood, blind-your-eye |
Euphorbiaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Fagara spp. |
Yellow sanders, West Indian satinwood, atlaswood, olon, bongo, mbanza |
Rutaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Fagus spp. (Nothofagus spp.) |
Beech |
Fagaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Fitzroya cupressoides (Molina) Johnston |
Alerce |
Cupressaceae |
Dermatitis |
|
Flindersia australis R. Br. |
Australian teak, Queensland maple, maple |
Rutaceae |
Dermatitis |
|
Fraxinus spp. |
Ash |
Oleaceae |
Dermatitis |
|
Gluta spp. |
Rengas, gluta |
Anacardiaceae |
Dermatitis; toxic effects |
|
Gonioma kamassi E. Mey. |
Knysna boxwood, kamassi |
Apocynaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Gonystylus bancanus Baill. |
Ramin, melawis, akenia |
Gonystylaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis |
|
Gossweilerodendron balsamiferum (Verm.) Harms. |
Nigerian cedar |
Caesalpinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Grevillea robusta A. Cunn. |
Silky oak |
Proteaceae |
Dermatitis |
|
Guaiacum officinale L. |
Gaiac, lignum vitae |
Zygophyllaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Guarea spp. |
Bossé |
Meliaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Halfordia scleroxyla F. Muell. |
Saffron-heart |
Polygonaceae |
Dermatitis; allergic extrinsic alveolitis |
|
Hernandia spp. |
Mirobolan, topolite |
Hernandiaceae |
Dermatitis |
|
Hippomane mancinella L. |
Beach apple |
Euphorbiaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Illipe latifolia F. Muell. |
Moak, edel teak |
Sapotaceae |
Dermatitis |
|
Jacaranda spp. |
Jacaranda |
Bignoniaceae |
Dermatitis |
|
Juglans spp. |
Walnut |
Juglandaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Juniperus sabina L. |
|
Cupressaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Khaya antotheca C. DC. |
Ogwango, African mahogany, krala |
Meliaceae |
Dermatitis; allergic extrinsic alveolitis |
|
Laburnum anagyroides Medic. (Cytisus laburnum L.) |
Laburnum |
Papilionaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Larix spp. |
Larch |
Pinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Liquidambar styracifolia L. |
Amberbaum, satin-nussbaum |
Hamamelidaceae |
Dermatitis |
|
Liriodendron tulipifera L. |
American whitewood, tulip tree |
Magnoliaceae |
Dermatitis |
|
Lovoa trichilioides Harms. (L. klaineana Pierre) |
Dibetou, African walnut, apopo, tigerwood, side |
Meliaceae |
dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Lucuma spp. (Pouteria spp.) |
Guapeva, abiurana |
Sapotaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Maba ebenus Wight. |
Makassar-ebenholz |
Ebenaceae |
Dermatitis |
|
Machaerium pedicellatum Vog. |
Kingswood |
Papilionaceae |
Dermatitis |
|
Mansonia altissima A. Chev. |
Nigerian walnut |
Sterculiaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Melanoxylon brauna Schott |
Brauna, grauna |
Caesalpinaceae |
Dermatitis |
|
Microberlinia brazzavillensis A. Chev. |
African zebrawood |
Caesalpinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Millettia laurentii De Wild. |
Wenge |
Papilionaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; |
|
Mimusops spp. (Manilkara spp.) |
Muirapiranga |
Sapotaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; |
|
Mitragyna ciliata Aubr. and Pell. |
Vuku, African poplar |
Rubiaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; |
|
Nauclea diderrichii Merrill (Sarcocephalus diderrichii De Wild.) |
Bilinga, opepe, kussia, badi, West African boxwood |
Rubiaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Nesogordonia papaverifera R. Capuron |
Kotibé, danta, epro, otutu, ovové, aborbora |
Tiliaceae |
Toxic effects |
|
Ocotea spp. |
Stinkwood |
Lauraceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Paratecoma spp. |
|
Bignoniaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Parinarium spp. |
|
Rosaceae |
Dermatitis |
|
Peltogyne spp. |
Blue wood, purpleheart |
Caesalpinaceae |
Toxic effects |
|
Phyllanthus ferdinandi F.v.M. |
Lignum vitae, chow way, tow war |
Euphorbiaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Picea spp. |
European spruce, whitewood |
Pinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis |
|
Pinus spp. |
Pine |
Pinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Piptadenia africana Hook f. |
Dabema, dahoma, ekhimi |
Mimosaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Platanus spp. |
Plane |
Platanaceae |
Dermatitis |
|
Pometia spp. |
Taun |
Sapindaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Populus spp. |
Poplar |
Salicaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Prosopis juliflora D.C. |
Cashaw |
Mimosaceae |
Dermatitis |
|
Prunus spp. |
Cherry |
Rosaceae |
dermatitis; conjunctivitis-rhinitis; asthma |
|
Pseudomorus brunoniana Bureau |
White handlewood |
Moraceae |
Dermatitis; toxic effects |
|
Pseudotsuga douglasii Carr. (P. menziesii Franco) |
Douglas fir, red fir, Douglas spruce |
Pinaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Pterocarpus spp. |
African padauk, New Guinea rosewood, red sandalwood, red sanders, quassia wood |
Papilionaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Pycnanthus angolensis Warb. (P. kombo Warb.) |
Ilomba |
Myristicaceae |
Toxic effects |
|
Quercus spp. |
Oak |
Fagaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Raputia alba Engl. |
Arapoca branca, arapoca |
Rutaceae |
Dermatitis |
|
Rauwolfia pentaphylla Stapf. O. |
Peroba |
Apocynaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Sandoricum spp. |
Sentul, katon, kra-ton, ketjapi, thitto |
Meliaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Schinopsis lorentzii Engl. |
Quebracho colorado, red q., San Juan, pau mulato |
Anacardiaceae |
Dermatitis; toxic effects |
|
Semercarpus australiensis Engl. |
Marking nut |
Anacardiaceae |
Dermatitis; toxic effects |
|
Sequoia sempervirens Endl. |
Sequoia, California |
Taxodiaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Shorea spp. |
Alan, almon, red balau |
Dipterocarpaceae |
Dermatitis |
|
S. assamica Dyer |
Yellow lauan, white meranti |
||
|
Staudtia stipitata Warb. (S. gabonensis Warb.) |
Niové |
Myristicaceae |
Dermatitis |
|
Swietenia spp. |
Mahogany, Honduras mahogany, Tabasco m., baywood, American mahogany, |
Meliaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis; toxic effects |
|
Swintonia spicifera Hook. |
Merpauh |
Anacardiaceae |
Dermatitis |
|
Tabebuia spp. |
Araguan, ipé preto, lapacho |
Bignoniaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Taxus baccata L. |
Yew |
Taxaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis; toxic effects |
|
Tecoma spp. |
Green heart |
Bignoniaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Tectona grandis L. |
Teak, djati, kyun, teck |
Verbenaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; allergic extrinsic alveolitis |
|
Terminalia alata Roth. |
Indian laurel |
Combretaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Thuja occidentalis L. |
White cedar |
Cupressaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Tieghemella africana A. Chev. (Dumoria spp.) |
Makoré, douka, okola, ukola, makoré, abacu, baku, African cherry |
Sapotaceae |
Dermatitis; conjunctivitis-rhinitis; asthma; toxic effects |
|
Triplochiton scleroxylon K. Schum |
Obeche, samba, wawa, abachi, African whitewood, arere |
Sterculiaceae |
Dermatitis; conjunctivitis-rhinitis; asthma |
|
Tsuga heterophylla Sarg. |
Tsuga, Western hemlock |
Pinaceae |
Dermatitis |
|
Turraeanthus africana Pell. |
Avodiré |
Meliaceae |
Dermatitis; allergic extrinsic alveolitis |
|
Ulmus spp. |
Elm |
Ulmaceae |
Dermatitis |
|
Vitex ciliata Pell. |
Verbenaceae |
Dermatitis |
|
|
V. congolensis De Wild. and Th. Dur |
Difundu |
||
|
V. pachyphylla Bak. |
Evino |
||
|
Xylia dolabriformis Benth. |
Mimosaceae |
Conjunctivitis-rhinitis; |
|
|
X. xylocarpa Taub. |
Pyinkado |
asthma |
|
|
Zollernia paraensis Huber |
Santo wood |
Caesalpinaceae |
Dermatitis; toxic effects |
Source: Istituto del Legno, Florence, Italy.
Increased use of high-production CNC machinery such as routers, tenoners and lathes creates more wood dust and will require new dust-collection technology.
Dust control. Most dust in a woodworking production shop is removed by local exhaust systems. However, often there is a considerable accumulation of very fine dust that has settled on rafters and other structural members, especially in areas where sanding is done. This is a hazardous situation, with great potential for fire and explosion. A flash fire over dust-covered surfaces may be followed by explosions of increasing force. In order to minimize this probability, it would be wise to use a checklist. See sample checklist in box.
Assembly hazards
A wide range of adhesives is used in the bonding of veneers to manufactured panels, depending on the characteristics required of the final product. Apart from casein glue, natural adhesives are less widely employed and the greatest use is made of synthetic adhesives such as urea-formaldehyde. Synthetic adhesives may pose a hazard of skin disease or systemic intoxication, especially those which release free formaldehyde or organic solvents into the atmosphere. Adhesives should be handled in well ventilated premises and sources of vapour emission should be equipped with exhaust ventilation. Employees should be provided with gloves, protective creams, respirators and eye protection when necessary.
The moving parts, especially blades, of veneer slicing, jointing and clipping machines should be fully guarded. Two-hand controls may be necessary.
Finishing hazards
Surface finishing. Solvents used for carrying the sprayed pigments or for thinning can include a wide variety of volatile organic compounds which may reach toxic and explosive concentrations in the air. In addition, many pigments are toxic by inhalation of spray mist (e.g., lead, manganese and cadmium pigments). Wherever dangerous concentrations of vapour or mist can occur, use exhaust ventilation (e.g., spray painting in a booth) or use water sprays. All sources of ignition, including fires, electrical equipment and static electricity, should be eliminated before any operations begin.
An active hazardous material communication programme should be in place to alert employees to all hazards created by toxic, reactive, corrosive and/or ignitable finish, glue and solvent chemicals and the protective measures that should be taken. Eating in the presence of these chemicals should be prohibited. Proper storage of flammables and proper disposal of soiled rags and steel wool which could cause spontaneous ignition are imperative.
Fire prevention. In view of the highly flammable nature of wood (especially in the form of dust and shavings) and of the other items found in a woodworking plant (such as solvents, glues and coatings), the importance of fire prevention measures cannot be overemphasized. Measures include:
- installing automatic wood-dust and shaving collection equipment on saws, planers, moulders and so on, which transport the waste to storage silos pending disposal or recovery
- prohibiting smoking at the workplace and eliminating all sources of ignition (e.g., open flames)
- ensuring regular clean-up procedures of deposited dust and shavings
- adequate maintenance of machines to prevent occurrences such as the overheating of bearings
- installation of fire barriers, sprinkler systems, fire extinguishers, fire hoses and a crew trained to use this equipment
- proper storage of flammables
- explosion-proof electrical equipment where needed.
Environmental and Public Health Concerns
The production of finished products from wood can be done without long-range environmental damage. The harvesting of trees can be done in such a manner that new growth can replace what is cut. Major deforestation such as has been the case in rain forests can be discouraged. Waste products from the machining of wood (i.e., sawdust, wood chips) can be used in chipcore or as fuel.
While there are solid waste and process wastewater implications for the woodworking industry, the major concerns are the air emissions resulting from the use of waste wood as fuel and from solvent-intensive finishing operations. Wood-fired boilers are commonly used in drying operations, while many of the finishing materials are applied by spray. In both instances, engineering controls are required to reduce air-borne particulates and recover and/or incinerate the volatile compounds.
Controls should result in operators being exposed to less toxic chemicals as less hazardous substitutes are found. Use of water-based finishes instead of solvent-based will decrease fire hazards.