Children categories

53. Environmental Health Hazards (11)

53. Environmental Health Hazards
Chapter Editors: Annalee Yassi and Tord Kjellström
Table of Contents
Tables and Figures
Linkages between Environmental and Occupational Health
Annalee Yassi and Tord Kjellström
Food and Agriculture
Friedrich K. Käferstein
Industrial Pollution in Developing Countries
Niu Shiru
Developing Countries and Pollution
Tee L. Guidotti
Air Pollution
Isabelle Romieu
Land Pollution
Tee L. Guidotti and Chen Weiping
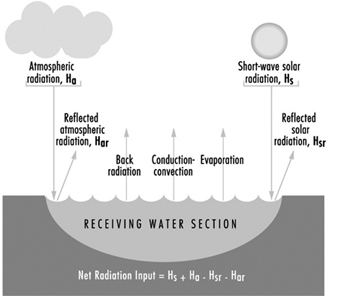
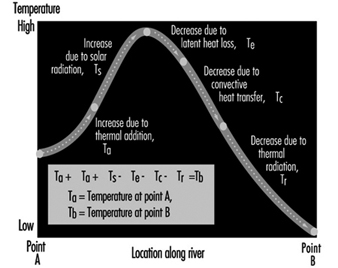
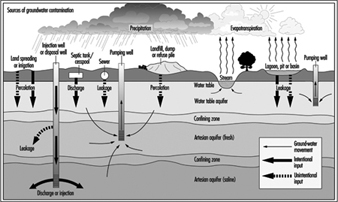
Water Pollution
Ivanildo Hespanhol and Richard Helmer
Energy and Health
L.D. Hamilton
Urbanization
Edmundo Werna
Global Climate Change and Ozone Depletion
Jonathan A. Patz
Species Extinction, Biodiversity Loss and Human Health
Eric Chivian
Tables
Click a link below to view table in article context.
1. Selected major "environmental disease" outbreaks
2. Foodborne-disease agents: epidemiology features
3. Major sources of outdoor air pollutants
4. Exposure-response relationship of PM10
5. Changes in ozone concentration: health outcomes
6. Morbidity & mortality: water-related diseases
7. Generating fuel electricity: health effects
8. Generating renewable electricity: health effects
9. Generating nuclear electricity: health effects
10. Housing & health
11. Urban infrastructure & health
12. Global status of major vector-borne diseases
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

54. Environmental Policy (10)

54. Environmental Policy
Chapter Editor: Larry R. Kohler
Table of Contents
Tables and Figures
Overview Occupational Safety and Health and the Environment - Two Sides of the Same Coin
Larry R. Kohler
Environment and the World of Work: An Integrated Approach to Sustainable Development, Environment and the Working Environment
Larry R. Kohler
Law and Regulations
Françoise Burhenne-Guilmin
International Environmental Conventions
David Freestone
Environmental Impact Assessments
Ron Bisset
Life-Cycle Assessment (Cradle-To-Grave)
Sven-Olof Ryding
Risk Assessment and Communication
Adrian V. Gheorghe and Hansjörg Seiler
Environmental Auditing - Definition and Methodology
Robert Coyle
Environmental Management Strategies and Workers’ Protection
Cecilia Brighi
Environmental Pollution Control: Making Pollution Prevention a Corporate Priority
Robert P. Bringer and Tom Zosel
Tables
Click a link below to view table in article context.
1. Scope of an environmental audit
2. Basic steps in environmental auditing
3. Voluntary agreements relevant to the environment
4. Environment-protection measures & collective agreements
5. Collective agreements on environment-protection
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

55. Environmental Pollution Control (11)

55. Environmental Pollution Control
Chapter Editors: Jerry Spiegel and Lucien Y. Maystre
Table of Contents
Tables and Figures
Environmental Pollution Control and Prevention
Jerry Spiegel and Lucien Y. Maystre
Air Pollution Management
Dietrich Schwela and Berenice Goelzer
Air Pollution: Modelling of Air Pollutant Dispersion
Marion Wichmann-Fiebig
Air Quality Monitoring
Hans-Ulrich Pfeffer and Peter Bruckmann
Air Pollution Control
John Elias
Water Pollution Control
Herbert C. Preul
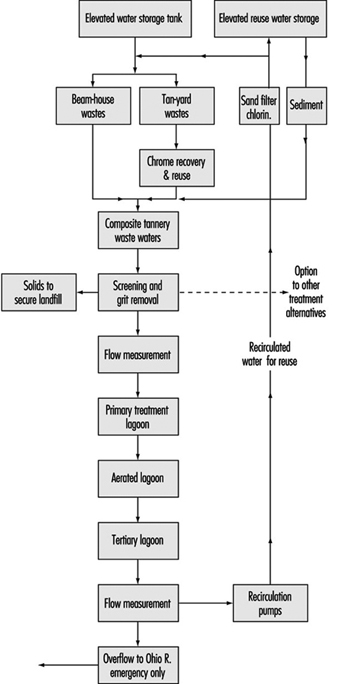
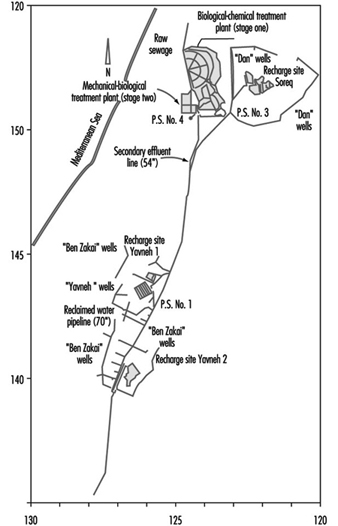
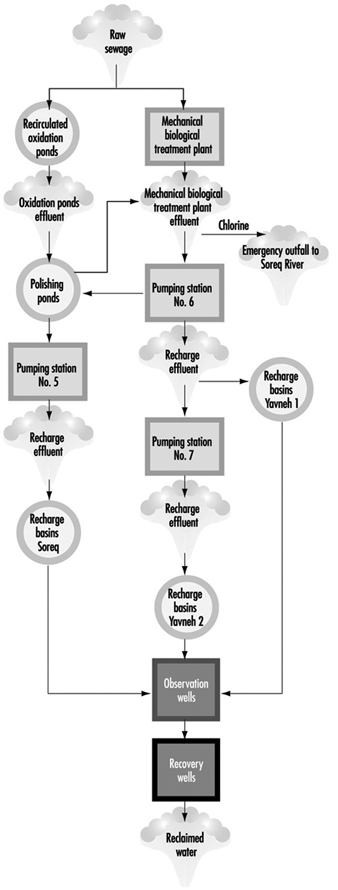
Dan Region Sewage Reclamation Project: A Case Study
Alexander Donagi
Principles of Waste Management
Lucien Y. Maystre
Solid Waste Management and Recycling
Niels Jorn Hahn and Poul S. Lauridsen
Case Study: Canadian Multimedia Pollution Control and Prevention on the Great Lakes
Thomas Tseng, Victor Shantora and Ian R. Smith
Cleaner Production Technologies
David Bennett
Tables
Click a link below to view table in article context.
1. Common atmospheric pollutants & their sources
2. Measurement planning parameters
3. Manual measurement procedures for inorganic gases
4. Automated measurement procedures for inorganic gases
5. Measurement procedures for suspended particulate
6. Long-distance measurement procedures
7. Chromatographic air quality measurement procedures
8. Systematic air quality monitoring in Germany
9. Steps in selecting pollution controls
10. Air quality standards for sulphur dioxide
11. Air quality standards for benzene
12. Examples of best available control technology
13. Industrial gas: cleaning methods
14. Sample emission rates for industrial processes
15. Wastewater treatment operations & processes
16. List of investigated parameters
17. Parameters investigated at the recovery wells
18. Sources of waste
19. Criteria for selection of substances
20. Reductions in releases of dioxin & furan in Canada
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.
International Environmental Conventions
The publicity surrounding the UN Conference on Environment and Development (UNCED), which took place in Rio de Janeiro in June 1992, confirmed the central place that global environmental concerns over issues such as global warming and loss of biological diversity have on the world political agenda. In fact, in the twenty years between the 1972 Stockholm Conference on the Human Environment and the 1992 UNCED there has been not only a major increase in awareness of the threats to the environment from human activities on both a local and global scale, but also a massive increase in the number of international legal instruments governing environmental issues. (There are large numbers of collections of environmental treaties: see, e.g., Burhenne 1974a, 1974b,1974c; Hohmann 1992; Molitor 1991. For a contemporary qualitative assessment see Sand 1992.)
It will be recalled that the two main sources of international law (as defined by the 1945 Statute of the International Court of Justice) are international conventions and international customary law (Article 38(1) of the Statute). International customary law derives from state practice repeated over time in the belief that it represents legal obligation. Although it is possible for new rules of custom to emerge relatively swiftly, the speed with which awareness of global environmental problems has reached the international political agenda has meant that customary law has tended to take second place to treaty or conventional law in the evolution of legal norms. Although certain basic principles, such as the equitable utilization of shared resources (Lac Lanoux Arbitration 1957) or the obligation not to allow activities which damage the environment of neighbouring states (Trail Smelter Arbitration 1939, 1941) can be attributed to judicial decisions derived from customary law, treaties have without doubt been the main method by which the international community has responded to the need to regulate activities which threaten the environment. Another important aspect of international environmental regulation is the development of “soft law”: non-binding instruments which lay down guidelines or desiderata for future action, or through which states commit themselves politically to meeting certain objectives. These soft law instruments sometimes develop into formal legal instruments or become linked to binding instruments as, for example, through decisions of the parties to a Convention. (On the significance of soft law in relation to international environmental law see Freestone 1994.) Many of the collections of international environmental law documents cited above include soft law instruments.
This article will give a brief overview of the main international environmental conventions. Although such a review inevitably concentrates on the main global conventions, the significant and growing web of regional and bilateral agreements should also be borne in mind. (For a systematic exposition of the whole body of international environmental law, see Kiss and Shelton 1991; Birnie and Boyle 1992. See also Churchill and Freestone 1991.)
Pre-Stockholm
Prior to the 1972 Stockholm Conference the majority of environmental conventions related to the conservation of wildlife. Of historical interest only are the very early bird protection conventions (e.g., the 1902 Convention for the Protection of Birds Useful to Agriculture; see further Lyster 1985). More significant in the longer term are the general nature conservation conventions, although the 1946 Washington Convention for the Regulation of Whaling (and its 1956 Protocol) is particularly noteworthy in this period—over time it has of course changed its focus from exploitation to conservation. A pioneering convention in conservation terms was the 1968 African Convention on Conservation of Nature and Natural Resources, Algiers, which despite its comprehensive and innovative approach to conservation made the mistake of many other conventions in not establishing an administrative structure to oversee its supervision. Also notable and considerably more successful is the 1971 Ramsar Convention on Wetlands of International Importance, especially as Waterfowl Habitat, which establishes a network of protected wetland areas in the territories of member states.
Other noteworthy developments in this period are the first global Oil Pollution Conventions. The 1954 International Convention for the Prevention of Pollution of the Sea by Oil (OILPOL) (amended 1962 and 1969) broke new ground by developing a regulatory framework for the carriage of oil by sea, but the first conventions to provide for emergency action and for compensation for oil pollution damage were developed directly in response to the world’s first major oil-tanker casualty—the wreck of the Liberian oil tanker Torrey Canyon off the coast of southwest England in 1967. The 1969 International Convention relating to Intervention on the High Seas in cases of Oil Pollution Damage authorized emergency action by coastal states outside territorial waters, and its fellows, the 1969 International Convention on Civil Liability for Oil Pollution Damage and the 1971 International Convention on the Establishment of an International Fund for Compensation for Oil Pollution Damage of Brussels, provided a basis for compensation claims against the owners and operators of oil tankers supplemented by an international compensation fund. (Note also the significant industry voluntary compensation schemes such as TOVALOP and CRISTAL; see further Abecassis and Jarashow 1985.)
From Stockholm to Rio
The years 1972 to 1992 witnessed an astonishing increase in the number and variety of international environmental law instruments. Much of this activity is directly attributable to the Stockholm Conference. Not only did the famous Conference Declaration (Declaration of the United Nations Conference on the Human Environment 1972) lay down certain principles, the majority of which were de lege ferenda (i.e., they stated what the law ought to be rather than what it was), but it also developed a 109-point Environmental Action Plan and a Resolution recommending institutional and financial implementation by the UN. The result of these recommendations was the establishment of the United Nations Environment Programme (UNEP), established by UN General Assembly Resolution (UNGA 1972) and based eventually in Nairobi. UNEP was directly responsible for the sponsoring of a number of key global environmental treaties and for the development of the important Regional Seas Programme, which has resulted in a network of some eight regional framework conventions protecting the marine environment, each with protocols developed to meet the special requirements of the region. A number of new regional programmes are still in the pipeline.
In order to provide an overview of the large number of environmental conventions developed during this period, they are divided into a number of groups: nature conservation; protection of the marine environment; and regulation of transboundary environmental impacts.
Conservation of nature and natural resources
This period saw the conclusion of a number of nature conservation treaties both at a global and regional level. At the global level, particularly noteworthy are the 1972 UNESCO Convention Concerning the Protection of the World Cultural and Natural Heritage, the 1973 Washington Convention on International Trade in Endangered Species (CITES) and the 1979 Bonn Convention on the Conservation of Migratory Species of Wild Animals. At a regional level the large number of treaties include the 1974 Nordic Convention on the Protection of the Environment, the 1976 Convention on Conservation of Nature in the South Pacific (Apia Convention, in Burhenne 1974a) and the 1979 Berne Convention on the Conservation of European Wildlife and Natural Habitats (European Treaty Series). Note also the 1979 EC Directive 79/409 on the conservation of wild birds (OJ 1979), now amended and supplemented by Directive 92/43 on the conservation of natural habitats and of wild flora and fauna (OJ 1992), the 1979 Convention for the Conservation and Management of the Vicuna and the 1985 ASEAN Agreement on the Conservation of Nature and Natural Resources (reproduced in Kiss and Shelton 1991). (Also of note are the treaties relating to the Antarctic—an area of global commons outside the jurisdiction of any state: the 1980 Canberra Convention on the Conservation of Antarctic Marine Living Resources, the 1988 Wellington Convention on the Regulation of Antarctic Mineral Resource Activities and the 1991 Protocol to the Antarctic Treaty on Environmental Protection, signed in Madrid.)
Protection of the marine environment
In 1973 the negotiations began of the Third UN Conference on the Law of the Sea (UNCLOS III). The nine years of UNCLOS negotiations culminated in the 1982 Montego Bay Convention on the Law of the Sea (LOSC), which included in its Part XII a general framework for the regulation of marine environmental issues including vessel and land-based sources of pollution and dumping, as well as laying down certain general duties regarding protection of the marine environment.
At a more detailed level, the International Maritime Organization (IMO) was responsible for the development of two major global conventions: the 1972 London Convention on the Prevention of Marine Pollution by Dumping of Wastes and Other Matter and the 1973 International Convention for the Prevention of Pollution from Ships, as amended in 1978 (MARPOL 1973/78), and a third relating to oil spills entitled the International Convention on Oil Pollution Preparedness, Response and Cooperation in 1990, establishes a global legal framework for collaboration and assistance in response to major oil spills. (Other Maritime Conventions which are not primarily environmental but are of relevance include the 1972 Convention on the International Regulations for Preventing Collisions at Sea (COLREG); the 1974 International Convention for the Safety of Life at Sea (SOLAS); the 1976 ILO Merchant Shipping (Minimum Standards) Convention (No. 147) and the 1978 Convention on Standards of Training, Certification and Watch Keeping for Sea Farers).
The 1972 London Convention adopted what has now become a common approach by listing substances (Annex I) which could not be dumped in the ocean; Annex II listed substances which could be dumped only with a permit. The regulatory structure, which requires signatory states to enforce these obligations against any vessels loading in their ports or their flag vessels anywhere in the world, has progressively tightened its regime to the extent that parties have now effectively ended the ocean dumping of industrial waste. The 1973/78 MARPOL Convention replaces the 1954 OILPOL Convention (above) and provides the main regulatory regime for pollution from vessels of all sorts, including oil tankers. MARPOL requires flag states to impose controls on the “operational discharges” of all controlled substances. The MARPOL regime was amended in 1978 so that it would progressively extend its regime over different forms of vessel sources pollution contained in the five Annexes. All the Annexes are now in force covering oil (Annex I), noxious liquid substances (Annex II), packaged waste (Annex III), sewage (Annex IV) and garbage (Annex V). Stricter standards are enforced within Special Areas agreed by the Parties.
At a regional level, the UNEP Regional Seas Programme provides a wide, although not comprehensive, network of marine protection treaties covering: the Mediterranean (Convention for the Protection of the Mediterranean Sea against Pollution, Barcelona, 16 February, 1976; protocols in 1976 (2), 1980 and 1982); Gulf (Kuwait Regional Convention for Co-operation on the Protection of the Marine Environment from Pollution, Kuwait, 24 April 1978; protocols in 1978, 1989 and 1990); West Africa (Convention for Co-operation in the Protection and Development of the Marine and Coastal Environment of the West and Central African Region (Abidjan, 23 March 1981), with a 1981 protocol); South East Pacific (Convention for the Protection of the Marine Environment and Coastal Areas of the South-East Pacific (Lima, 12 November 1981); protocols in 1981, 1983 (2) and 1989); Red Sea (Regional Convention for the Conservation of the Red Sea and Gulf of Aden Environment (Jeddah, 14 February 1982); protocol in 1982); Caribbean (Convention for the Protection and Development of the Marine Environment of the Wider Caribbean Region, (Cartagena des Indias, 24 March 1983); protocols in 1983 and 1990); East Africa (Convention for the Protection, Management and Development of the Marine and Coastal Environment of the East African Region (Nairobi, 21 June 1985); 2 protocols in 1985); and the South Pacific (Convention for the Protection of the Natural Resources and Environment of the South Pacific Region, (Noumea, 24 November 1986); 2 protocols in 1986)—with another six or so in various stages of planning. (For texts of all the above Conventions and their protocols, as well as details of developing programmes, see Sand 1987.) These treaties are supplemented by protocols covering a wide range of issues including regulation of land-based sources of pollution, ocean dumping, pollution from (and decommissioning of) off-shore oil rigs, specially protected areas and protection of wildlife.
Other regional regimes have been developed outside the UNEP framework, notably in the North East Atlantic, where a highly comprehensive network of regional instruments covers regulation of ocean dumping (1972 Oslo Convention for the Prevention of Marine Pollution by Dumping from Ships and Aircraft; protocols in 1983 and 1989), land-based sources of pollution (1974 Paris Convention for the Prevention of Marine Pollution from Land Based Sources; protocol in 1986), oil pollution monitoring and cooperation (1983 Bonn Agreement for Co-operation in Dealing with Pollution of the North Sea by Oil and other Harmful Substances: Amending Decision 1989), inspection of vessels for safety and protection of the marine environment (1982 Paris Memorandum of Understanding on Port State Control in Implementing Agreements on Maritime Safety and Protection of the Marine Environment, as well as nature conservation and fisheries. (See generally Freestone and IJlstra 1991. Note also the new 1992 Paris Convention for the Protection of the Marine Environment of the North-East Atlantic, which will replace the Oslo and Paris Conventions; text and analysis in Hey, IJlstra and Nollkaemper 1993.) In the Baltic the 1974 Helsinki Convention on the Protection of the Marine Environment of the Baltic Sea Area has recently been revised (for text and analysis of 1992 Convention see Ehlers 1993)), and a new Convention developed for the Black Sea Region (1992 Bucharest Convention on the Protection of the Black Sea; see also 1993 Odessa Ministerial Declaration on the Protection of the Black Sea.)
Transboundary impacts
Principle 21 of the Stockholm Declaration provided that States had “the responsibility to ensure that activities under their jurisdiction and control do not cause damage to the environment of other States or of areas beyond national jurisdiction”. Although this principle is now widely regarded as having become part of customary international law, the principle grosso modo requires considerable fine tuning to provide the basis for regulation of such activities. Addressing these issues, and largely in response to well publicized crises, international conventions have been developed to address issues such as long-range transboundary air pollution, protection of the ozone layer, notification and cooperation in response to nuclear accidents, transboundary movement of hazardous waste and global climate change.
Long-range transboundary air pollution
Long-range air pollution in Europe was first addressed by the 1979 Geneva Convention (Convention on Long-Range Transboundary Air Pollution). This, however, was a framework convention whose modestly expressed aims were “to limit and, as far as possible, gradually to reduce and prevent air pollution including long range transboundary pollution”. Substantive progress in regulating emissions of specific substances was made only with the development of the protocols, of which there are now four: the 1984 Geneva Protocol (Geneva Protocol on Long-term Financing of the Co-operative Programme for Monitoring and Evaluation of the Long-Range Transmission of Air Pollution in Europe) established a network of air quality monitoring stations; the 1985 Helsinki Protocol (on the Reduction of Sulphur Emissions) aimed to reduce sulphur emissions by 30% by 1993; the 1988 Sofia Protocol (Concerning the Control of Emissions of Nitrogen Oxides or their Transboundary Fluxes), now replaced by the Second Sulphur Protocol, Oslo, 1994, provided for a freeze on national emissions of nitrogen oxides at 1987 levels by 1994; and the 1991 Geneva Protocol (Concerning the Control of Emissions of Volatile Organic Compounds or their Transboundary Fluxes) provided a range of options for emission abatement of volatile organic compounds and fluxes.
Transboundary implications of nuclear accidents
World attention had been brought to the transboundary implications of nuclear accidents after the 1986 Chernobyl accident, but even prior to that, previous conventions had addressed a number of the issues relating to the risks from nuclear devices, including the 1961 Convention on Third Party Liability in the Field of Nuclear Energy (1960), and the Vienna Convention on Civil Liability for Nuclear Damage (1963). Note also the 1963 Treaty Banning Nuclear Weapon Tests in the Atmosphere, in Outer Space and Under Water. The 1980 Vienna Convention on the Physical Protection of Nuclear Material had attempted to establish standards for the protection of nuclear material from a number of threats, including terrorism. In the wake of Chernobyl two further conventions were agreed upon in 1986, on early notification of accidents (Vienna Convention on the Early Notification of a Nuclear Accident) and international cooperation in the event of such accidents (Vienna Convention on Assistance in the Case of a Nuclear Accident or Radiological Emergency).
Protection of the ozone layer
The 1985 Vienna Convention for the Protection of the Ozone Layer imposes general obligations on each party “in accordance with the means at their disposal and their capabilities” to:
a) cooperate by means of systematic observation, research and information exchange in order to better understand and assess the effects of human activities on the ozone layer and the effects on human health and the environment from modification of the ozone layer; (b) adopt appropriate legislative or administrative measures and cooperate in harmonizing appropriate policies to control, limit, reduce or prevent human activities under their jurisdiction or control should it be found that these activities have or are likely to have adverse effects resulting from modification or likely modification of the ozone layer; (c) cooperate in the formulation of agreed measures, procedures and standards for the implementation of the Convention, with a view to the adoption of protocols and annexes; (d) cooperate with competent international bodies to implement effectively the Convention and protocols to which they are party.
The Vienna Convention was supplemented by the 1987 Montreal Protocol on Substances that Deplete the Ozone Layer, itself adjusted and amended by the London Meeting of 1990 and most recently by the Copenhagen Meeting of November 1992. Article 2 of the Protocol requires parties to impose controls on ozone-depleting chemicals, namely CFCs, halons, other fully halogenated CFCs, carbon tetrachloride and 1,1,1-tri-chloroethane (methyl chloroform).
Article 5 provides an exemption from emissions restrictions for certain developing countries, “to meet (Their) basic domestic needs” for up to ten years, subject to certain provisos set out in Article 5(2) (3). The Protocol also provides for technical and financial cooperation for developing country parties claiming exemption under Article 5. A Multilateral Fund was agreed upon to assist such parties to research and meet their obligations (Article 10). In Copenhagen in November 1992, in the light of the 1991 Scientific Assessment of Ozone Depletion, which found that there was new evidence of ozone decreases in both hemispheres at middle and high latitudes, a number of new measures were agreed upon, subject of course to the general regime outlined above; delays under Article 5 are still possible for developing states. All parties were required to cease using halons by 1994, and CFCs, HBFCs, carbon tetrachloride and methyl chloroform by 1996. The use of HCFCs should be frozen by 1996, reduced 90% by 2015 and eliminated by 2030. Methyl bromide, still used as a fruit and grain preservative, was subjected to voluntary controls. Contracting parties agreed to “make every effort” to freeze its use by 1995 at 1991 levels. The overall aim was to stabilize atmospheric chlorine loading by the year 2000 and then reduce it to below critical levels by about 2060.
Transboundary movement of hazardous wastes
Following a series of notorious incidents in which shipments of hazardous waste from developed countries were found in uncontrolled and hazardous conditions in developing countries, the transboundary movement of hazardous wastes was made the subject of international regulation by the 1989 Basel Convention on the Control of Transboundary Movement of Hazardous Wastes and their Disposal (see also Kummer 1992). This Convention is premised upon the principle of prior informed consent on a state to state basis before the movement of such waste can take place. The Organization of African Unity has however gone further than this with its 1991 Bamako Convention on the Ban of the Import into Africa and the Control of Transboundary Movement and Management of Hazardous Wastes within Africa, which seeks to ban entirely the import of hazardous waste into Africa.
Environmental impact assessment (EIA) in a transboundary context
The 1991 Espoo Convention on Environmental Impact Assessment in a Transboundary Context sets out a framework for neighbourly relations. It extends the EIA concept, developed to date exclusively in the context of national planning laws and procedures, to the transboundary impacts of development projects and related procedures and decisions.
1992 and Post-Rio Conventions
The Rio UNCED prompted, or coincided with, a large number of new global and regional environment conventions, as well as a major declaration of principles for the future in the Rio Declaration on Environment and Development. In addition to the two conventions concluded at Rio—the Framework Convention on Climate Change and the Convention on Biological Diver-sity—new environmental conventions signed in 1992 included those regulating the use of international watercourses as well as the transboundary effects of industrial accidents. At a regional level 1992 saw the Helsinki Convention on the Protection and Use of the Baltic Sea Area (text and analysis in Ehlers 1993) and the Bucharest Convention on the Protection of the Black Sea against Pollution. Note also the 1993 Ministerial Declaration on the Protection of the Black Sea, which advocates a precautionary and holistic approach, and the Paris Convention for the Protection of the Marine Environment of the North East Atlantic (text and analysis in Hey, IJlstra and Nollkaemper 1993).
The United Nations Framework Convention on Climate Change (UNFCCC)
The UNFCCC, signed at Rio de Janeiro in June 1992 by some 155 states, is loosely modelled on the 1985 Vienna Convention. As its name suggests, it provides a framework within which more detailed obligations will be negotiated by the means of detailed protocols. The basic objective of the Convention is to achieve
stabilization of greenhouse gas concentrations in the atmosphere at a level that will prevent dangerous anthropogenic interference with the climate system ...hin a time-frame sufficient to allow ecosystems to adapt naturally to climate change, to ensure food production is not threatened and to enable economic development to proceed in a sustainable manner. (Article 2)
Two primary duties are imposed on all Parties by Article 4: (a) to develop, periodically update, publish and make available a national inventory of anthropogenic emissions by sources and removals by sinks of all greenhouse gases using comparable (and yet to be agreed upon) methodologies; and (b) to formulate, implement, publish and regularly update national and regional programmes of measures to mitigate climate change by addressing anthropogenic emissions by sources and removals by sinks of all greenhouse gases and measures to facilitate adequate adaptation to climate change. In addition developed country parties agree to a number of general obligations which will be made specific by more detailed protocols.
For example, to undertake to promote, and cooperate in, the development of technologies; to control, prevent or reduce anthropogenic emissions of greenhouse gases; to promote sustainable development and the conservation and enhancement of sinks and reservoirs including biomass, forests, oceans and other terrestrial, coastal and marine ecosystems; to cooperate in adaptation to impacts of climate change, by elaboration of plans for integrated coastal zone management, water resources and agriculture and for protection and rehabilitation of areas affected by, inter alia, floods; to promote and cooperate in the exchange of scientific, technological, socioeconomic and legal information relevant to climate, climate change and response strategies; and to promote and cooperate in relevant education, training and public awareness.
The Biological Diversity Convention
The objectives of the Convention on Biological Diversity, also approved at the 1992 UNCED in Rio de Janeiro, are to conserve biological diversity, the sustainable use of its components and the fair and equitable sharing of the benefits arising out of the utilization of genetic resources (Article 1) (for a useful critique, see Boyle 1993). Like the UNFCCC this convention too will be supplemented by protocols, but it establishes general obligations regarding conservation and sustainable use of natural resources, for identification and monitoring of biological diversity, for in situ and ex situ conservation, research and training as well as public education and awareness and EIA of activities likely to affect biodiversity. There are also general provisions relating to access to genetic resources and access to, and transfer of, relevant technology, including biotechnology, as well as international exchange of information and cooperation.
Regulation of the use of international watercourses
The 1992 Helsinki Convention on the Protection and Use of Transboundary Watercourses and International Lakes seeks to establish cooperative frameworks for joint monitoring and assessment, common research and development and information exchange between riparian states. It imposes basic duties on such states to prevent control and reduce transboundary impacts on such shared resources, particularly regarding water pollution, through proper management techniques, including EIA and contingency planning as well as through the adoption of low- or non-waste technology and reduction of pollution from point and diffuse sources.
The transboundary effects of industrial accidents
The Convention on the Transboundary Effects of Industrial Accidents, also signed in Helsinki in March 1992, covers the prevention of, preparedness for and response to industrial accidents capable of having a transboundary effect. The primary obligations are to cooperate and exchange information with other parties. The detailed system of thirteen annexes establishes systems to identify hazardous activities with transboundary implications, for the development of EIA with a transboundary dimension (in accordance with the 1991 Espoo Convention, above) for decisions on siting of potentially hazardous activities. It also provides for emergency preparedness and for access to information for the public as well as the other parties.
Conclusion
As this brief review should have demonstrated, over the last two decades there has been a major change in the attitude of the world community to environmental conservation and management. Part of that change has been a substantial increase in the numbers and the scope of international instruments addressing environmental concerns. The sheer number of instruments has been matched by new principles and institutions. The polluter pays principle, the precautionary principle (Churchill and Freestone 1991; Freestone and Hey 1996) and concern for the rights of future generations (Kiss, in Freestone and Hey 1996) are all reflected in the international conventions reviewed above. The role of the UN Environment Programme and the treaty secretariats established to service and monitor the burgeoning number of treaty regimes lead commentators to suggest that international environmental law, like, for example, the international law of human rights, has emerged as a new discrete branch of international law (Freestone 1994). UNCED played an important role in this, it has established a major agenda—much of which remains unfinished. Detailed protocols are still needed to add substance to the framework of the Climate Change Convention and, arguably, also to the Convention on Biological Diversity. Concern with the environmental impact of fishing in high seas areas led to the conclusion of the UN Agreement on Straddling Fish Stocks and Highly Migratory Fish Stocks was in 1995. Also held in 1995 was another UN Conference on Land Based Sources of Marine Pollution—now agreed to be the cause of more than 70% of all pollution of the oceans. The environmental dimensions of world trade as well as deforestation and desertification are also issues to be addressed for the future at a global level while progress continues to enhance our awareness of impacts of human activities on world eco-systems. The challenge for this emerging international environmental law is not simply to respond with an increase in the numbers of environmental instruments, but also to enhance their impact and effectiveness.
Environmental Impact Assessments
The term used as the title of this article, environmental impact assessments, has now been increasingly, but not universally, replaced with the term environmental assessments. A quick review of the reason for this change of name will help us define the essential nature of the activity described by these names, and one of the important factors behind opposition or reluctance to using the word impact.
In 1970, the National Environmental Policy Act (NEPA) became law in the United States, establishing environmental policy goals for the federal government, focusing on the need to take environmental factors into account in decision-making. It is, of course, easy to state a policy objective, but it is more difficult to achieve it. To ensure that the Act had “teeth”, legislators incorporated a provision requiring that the Federal government prepare an “Environmental Impact Statement” (EIS) for any proposed action “likely to significantly affect the quality of the human environment”. The content of this document was to be considered before a decision was made on whether the proposed action should be initiated. The work done to prepare the EIS became known as environmental impact assessment (EIA), because it involved the identification, prediction and evaluation of the impacts of the proposed federal action.
The word “impact”, in English, unfortunately is not a positive term. An impact is thought to be harmful (almost by definition). Therefore, as the practice of EIA spread beyond the United States to Canada, Europe, Southeast Asia and Australasia, many governments and their advisers wanted to move away from the negative aspects of impact, and so the term environmental assessment (EA) was born. EIA and EA are identical (except in the United States and those few countries which have adopted the US system, where EIA and EA have precise and different meanings). In this article only EIA will be referred to, although it should be remembered that all comments apply equally to EA, and both terms are in use internationally.
In addition to the use of the word impact, the context in which EIA was applied (particularly in the United States and Canada) was also influential on the perceptions of EIA which were (and in some cases still are) common amongst politicians, senior governmental officials and private and public-sector “developers”. In both the United States and Canada, land-use planning was weak and preparation of EISs or EIA reports were often “hijacked” by interested parties and almost became plan-making activities. This encouraged the production of large, multi-volume documents which were time-consuming and expensive to produce and, of course, virtually impossible to read and act upon! Sometimes projects were delayed while all this activity was in progress, causing irritation and financial costs to proponents and investors.
Also, in the first five to six years of its operation, NEPA gave rise to many court cases in which project opponents were able to challenge the adequacy of EISs on technical and sometimes procedural grounds. Again, this caused many delays to projects. However, as experience was gained and guidance was issued that was more clear and strict, the number of cases going to court declined significantly.
Unfortunately, the combined effect of these experiences was to give the distinct impression to many external observers that EIA was a well-intentioned activity which, unfortunately, had gone wrong and ended by being more of an obstacle than a help to development. To many people, it seemed an appropriate, if not entirely necessary, activity for self-indulgent developed countries, but for industrializing nations it was an expensive luxury they could not really afford.
Despite the adverse reaction in some places, globally the spread of EIA has proved irresistible. Starting in 1970 in the United States, EIA extended to Canada, Australia and to Europe. A number of developing countries—for example, the Philippines, Indonesia and Thailand—introduced EIA procedures before many Western European countries. Interestingly, the various development banks, such as the World Bank, were amongst the slowest organizations to introduce EIA into their decision-making systems. Indeed, it was only by the late 1980s and early 1990s that the banks and the bilateral aid agencies could be said to have caught up with the rest of the world. There is no sign that the rate at which EIA laws and regulations are being introduced into national decision-making systems is becoming slower. In fact, following the “Earth Summit” held in Rio de Janeiro in 1992, EIA has been used increasingly as international agencies and national governments attempt to meet the recommendations made in Rio regarding the need for sustainable development.
What is EIA?
How can we explain the ever-increasing popularity of EIA? What can it do for governments, private and public sector developers, workers, their families and the communities in which they live?
Before EIA, development projects such as highways, hydro-power dams, ports and industrial installations were assessed on technical, economic and, of course, political bases. Such projects have certain economic and social objectives to achieve, and decision-makers involved in issuing permits, licences or other types of authorization were interested in knowing whether the projects would achieve them (putting to one side those projects conceived and built for political purposes such as prestige). This required an economic study (usually cost-benefit analysis) and technical investigations. Unfortunately, these studies did not take account of environmental effects and, as time passed, more and more people became aware of the increasing damage caused to the environment by such development projects. In many cases, the unintended environmental and social impacts led to economic costs; for example, the Kariba Dam in Africa (on the border between Zambia and Zimbabwe) resulted in the resettlement of many villages into areas which were not suitable for the traditional agriculture practised by the people. In the resettled areas food became scarce and the government had to initiate emergency food supply operations. Other examples of unexpected “add-on” costs as well as environmental damage led to a growing realization that the traditional project appraisal techniques needed an additional dimension to reduce the chances of unexpected and unwelcome impacts.
The increasing awareness amongst governments, non-governmental organizations (NGOs) and members of the public of the unexpected economic penalties that could arise from major development projects coincided with a parallel growth in global understanding of the importance of the environment. In particular, concern focused on the implications of increasing population growth and the accompanying expansion in economic activities, and whether there might be environmental constraints to such growth. The importance of global biogeochemical and other processes for the maintenance of clean air and water as well as renewable resources such as food and timber were recognized increasingly. As a result, many were convinced that the environment could no longer be seen as a passive and never-ending deliverer of goods and a receiver of human wastes. It had to be seen as an active part of the development process which, if treated badly, could reduce the chances of achieving development objectives. This realization has led to the development and implementation of a number of procedures or practices to incorporate the environment into the development process by considering the extent to which it might be harmed or improved. One such procedure is EIA. The overall aim is to reduce the risk—for homo sapiens in general, and local groups in particular—that environmental damage will result in life-threatening consequences such as famines and floods.
Basically, EIA is a means of identifying, predicting and evaluating the environmental impacts of a proposed development action, and its alternatives, before a decision is made to implement it. The aim is to integrate EIA into the standard, pre-feasibility, feasibility, appraisal and design activities which are carried out to test whether a proposal will meet its objectives. By undertaking EIA work in parallel with these studies it should be possible to identify, early, the significant adverse impacts (and those which are beneficial) and to “design out”, as far as possible, the harmful impacts. Additionally, benefits can be enhanced. The outcome of any EIA should be a proposal which, in its location, design and method of construction or operation, is “environmentally friendly” in so far as its environmental implications are acceptable and any environmental deterioration is unlikely to cause difficulties. EIA is, therefore, a preventive tool, and medicine provides an appropriate analogy. In the field of community medicine it is better, and economically cheaper, to prevent illness rather than cure it. In the development process it is better to minimize environmental damage (while still achieving economic objectives) than to fund expensive clean-up or rehabilitation actions after damage has occurred.
Application of EIA
To what types of development activities does EIA apply? There is no standard or correct answer. Each country decides on the type and scale of activities to be subject to EIA; for example, a proposed 10 km road in a small tropical island may cause significant impacts, but a similar road in a large, semi-arid country with a low population density probably would be environmentally neutral. In all countries, EIA is applied to “physical” development projects according to national criteria; in some countries EIA is applied also to development plans, programmes and policies (such as sector development programmes for energy supply and national development plans) which might cause significant environmental impacts. Amongst the countries which apply EIA to these kinds of actions are the United States, the Netherlands and China. However, such countries are the exception to normal practice. Most EIAs are prepared for physical development projects, although there is no doubt that “strategic” EIAs will increase in importance in the future.
What kinds of impacts are analysed in EIAs? Again this varies from country to country, but to a lesser extent than in the case of the types of proposed activities subject to EIA. The usual answer given is “environmental” impacts, to which the inevitable response is likely to be, “Yes, but what is ‘environmental’?” Generally, most EIAs focus on the biophysical environment—that is, impacts on such factors as:
- water quality and quantity
- air quality
- ecosystems and ecological processes
- noise levels.
In some cases no other impacts are considered. However, the limitations of restricting EIA to biophysical impacts have been questioned and, increasingly, more and more EIAs are based on a broad concept of the environment and include, when appropriate, impacts on:
- local communities (“social” impacts)
- local economies
- health and safety
- landscapes
- cultural resources (archaeological or historical sites, environmental features with spiritual significance for local communities, etc.).
There are two reasons which help explain this wider definition of “environmental” impacts. First, it has been found to be socially and politically unacceptable to consider the impacts of a proposal on the biophysical environment and, at the same time, ignore the social, health and economic effects on local communities and inhabitants. This issue has been dominant in developed countries, especially those which have weak land-use planning systems into which social and economic objectives are incorporated.
In developing countries, this factor also exists and is joined by an additional, complementary explanation. The majority of the population in developing countries has a closer and, in many ways, more complex set of direct relationships with their environment than is the case in developed countries. This means that the way that local communities and their members interact with their environment can be changed by environmental, social and economic impacts. For example, in poor localities a major, new project such as a 2,400 MW power station will introduce a source of new labour opportunities and social infrastructure (schools, clinics) to provide for the large workforce needed. Basically, the income injected into the local economy makes the power station locality an island of prosperity in a sea of poverty. This attracts poor people to the area to try to improve their standard of living by trying to obtain a job and to use the new facilities. Not all will be successful. The unsuccessful will try to offer services to those employed, for example, by supplying firewood or charcoal. This will cause environmental stress, often at locations distant from the power station. Such impacts will occur in addition to the impacts caused by the influx of workers and their families who are directly employed at the station site. Thus, the main induced social effect of a project—in-migration—causes environmental impacts. If these socioeconomic implications were not analysed, then EISs would be in danger of failing to achieve one of their main objectives—that is, to identify, predict, evaluate and mitigate biophysical environmental impacts.
Virtually all project-related EIAs focus on the external environment, that is, the environment outside the site boundary. This reflects the history of EIA. As noted above it had its origins in the developed world. In these countries there is a strong legal framework for occupational health protection and it was inappropriate for EIA to focus on the internal, working environment as well as the external environment, as this would be a duplication of effort and misuse of scarce resources.
In many developing countries the opposite situation is often the reality. In such a context, it would seem appropriate for EIAs, particularly for industrial facilities, to consider the impacts on the internal environment. The main focus of considering such impacts as changes in internal air quality and noise levels is the health of workers. There are two other aspects which are important here. First, in poor countries the loss of a breadwinner through illness, injury or death can force the other members of a family to exploit natural resources to maintain income levels. If a number of families are affected then the cumulative impacts may be locally significant. Secondly, the health of family members can be affected, directly, by chemicals brought into the home on the clothes of workers. So there is a direct link between the internal and external environments. The inclusion of the internal environment in EIA has received little attention in the EIA literature and is conspicuous by its absence from EIA laws, regulations and guidelines. However, there is no logical or practical reason why, if local circumstances are appropriate, EIAs should not deal with the important issues of workers’ health and the possible external implications of a deterioration in the physical and mental well-being of workers.
Costs and Benefits of EIAs
Perhaps the most frequent issue raised by those who are either opposed to EIA or are neutral towards it concerns the cost. Preparation of EISs takes time and resources, and, in the end, this means money. It is important, therefore, to consider the economic aspects of EIA.
The main costs of introducing EIA procedures into a country fall on project investors or proponents, and central or local government (depending on the nature of the procedures). In virtually all countries, project investors or proponents pay for preparation of EIAs for their projects. Similarly, initiators (usually government agencies) of sectoral investment strategies and regional development plans pay for their EIAs. Evidence from developed and developing countries indicates that the cost of preparing EISs ranges from 0.1% to 1% of the capital cost of a project. This proportion can increase when mitigating measures recommended in the EISs are taken into account. The cost depends on the type of mitigation recommended. Obviously, resettling 5,000 families in such a way that their standard of living is maintained is a relatively costly exercise. In such cases the costs of the EIS and mitigation measures can rise to 15 to 20% of capital cost. In other cases it may be between 1 and 5%. Such figures may seem to be excessive and to indicate that EIA is a financial burden. There is no doubt that EIA costs money, but in the experience of the author no major projects have been halted because of the costs of EIA preparation, and in only a few cases have projects been made uneconomical because of the costs of necessary mitigating measures.
EIA procedures also impose costs to central or local governments which arise from the staff and other resources which need to be directed to managing the system and processing and reviewing the EISs. Again, the cost depends on the nature of the procedure and how many EISs are produced per year. The author is not aware of any calculations which attempt to provide an average figure for this cost.
To return to our medical analogy, prevention of illness requires a significant up-front investment to ensure future and possibly long-term dispersed benefits in terms of the health of the population, and EIA is no different. The financial benefits can be examined from the perspectives of the proponent as well as those of the government and the wider society. The proponent can benefit in a number of ways:
- prevention of delays in obtaining authorizations
- identification of mitigation measures involving recycling and recovery of components of waste streams
- creation of cleaner working environments
- identification of cheaper alternatives.
Not all of these will operate in all cases, but it is useful to consider the ways in which savings can accrue to the proponent.
In all countries various permits, permissions and authorizations are needed before a project can be implemented and operated. The authorization procedures take time, and this can be extended if there is opposition to a project and no formal mechanism exists by which concerns may be identified, considered and investigated. There seems little doubt that the days of passive populations welcoming all development as signs of inevitable economic and social progress are nearly over. All projects are subject to increasing local, national and international scrutiny—for example, the continuing opposition in India to the Sardar Sarovar (Narmada) complex of dams.
In this context, EIA provides a mechanism for public concerns to be addressed, if not eliminated. Studies in developed countries (such as the UK) have shown the potential for EIA to reduce the likelihood of delays in obtaining authorizations—and time is money! Indeed, a study by British Gas in the late 1970s showed that the average time taken to obtain authorization was shorter with EIA than for similar projects without EIA.
The add-on costs of mitigation have been mentioned, but it is worth considering the opposite situation. For facilities which produce one or more waste streams, the EIA may identify mitigation measures which reduce the waste load by use of recovery or recycling processes. In the former case recovery of a component from a waste stream might enable the proponent to sell it (if a market is available) and cover the costs of the recovery process or even make a profit. Recycling of an element such as water can reduce consumption, thus lowering expenditure on raw material inputs.
If an EIA has focused on the internal environment, then the working conditions should be better than would have been the case without the EIA. A cleaner, safer workplace reduces worker discontent, illness and absences. The overall effect is likely to be a more productive workforce, which again is a financial benefit to the proponent or operator.
Finally, the favoured option selected using solely technical and economic criteria may, in fact, not be the best alternative. In Botswana, a site had been selected for water to be stored before it was transported to Gaborone (the capital). An EIA was implemented and it was found, early in the EIA work, that the environmental impacts would be significantly adverse. During survey work, the EIA team identified an alternative site which they were given permission to include in the EIA. The alternative site comparison showed that the environmental impacts of the second option were much less severe. Technical and economic studies showed that the site met technical and economic criteria. In fact it was found that the second site could meet the original development objectives with less environmental damage and cost 50% less to build (IUCN and Government of the Republic of Botswana, undated). Unsurprisingly, the second option has been implemented, to the benefit not only to the proponent (a parastatal organization) but to the entire tax-paying population of Botswana. Such examples are likely to be uncommon, but do indicate the opportunity provided by EIA work to “test” various development options.
The main benefits of EIA procedures are dispersed amongst the component parts of society, such as government, communities and individuals. By preventing unacceptable environmental deterioration EIA helps to maintain the essential “life processes” upon which all human life and activities depend. This is a long-term and dispersed benefit. In specific instances, EIA can avoid localized environmental damage which would necessitate remedial measures (usually expensive) at a later date. The cost of remedial measures usually falls on local or central government and not the proponent or operator of the installation which caused the damage.
Recent events, especially since the Rio “Earth Summit”, are slowly changing the objectives of development activities. Until recently the objectives of development were to improve economic and social conditions in a specified area. Increasingly, the achievement of “sustainability” criteria or objectives is occupying a central place in the traditional hierarchy of objectives (which still remain relevant). The introduction of sustainability as an important, if not yet primary, objective in the development process will have a profound influence on the future existence of the sterile debate of “jobs versus environment” from which EIA has suffered. This debate had some meaning when environment was on the outside of the development process and looking in. Now the environment is becoming central and the debate is centred on mechanisms of having both jobs and a healthy environment linked in a sustainable manner. EIA still has a crucial and expanding contribution to make as one of the important mechanisms for moving towards, and achieving, sustainability.
Life-Cycle Assessment (Cradle-To-Grave)
The need to safeguard the environment for future generations makes it necessary not only to discuss the emerging environmental problems, but to make progress in identifying strategies that are cost-effective and environmentally sound to solve them and to take actions to enforce the measures that result from such discussion. There is ample evidence that enhancing the state of the environment as well as establishing policies to sustain the environment must take on greater priority within this generation and those that follow. While this belief is commonly held by governments, environmental groups, industry, academics and the general public, there is considerable debate on how to achieve improved environmental conditions without sacrificing current economic benefits. Furthermore, environmental protection has become an issue of great political importance, and ensuring ecological stability has been pushed to the top of many political agendas.
Past and present efforts to protect the environment are to a large extent characterized as single-issue approaches. Each problem has been dealt with on a case-by-case basis. With regard to problems caused by point-source pollution from easily identified emissions, this was an effective way of reducing environmental impacts. Today, the situation is more complex. Much pollution now originates from a large number of non-point sources easily transported from one country to another. Furthermore, each of us contributes to this total environmental pollution load through our daily patterns of living. The different non-point sources are difficult to identify, and the way in which they interact in impacting the environment is not well known.
The increasing environmental problems of more complex and global character will most likely entail great implications for several sectors of society in enforcing remedial actions. To be able to play a role in environmental protection, sound and universal policies must be applied jointly as an additional, multi-issue approach by all those actors taking part in the process—the scientists, trade unions, non-governmental organizations, companies and agencies of authority at the national and governmental levels, as well as the media. Therefore, it is important that all areas of sectoral interest be coordinated in their environmental ambitions, in order to get necessary interactions and responses to proposed solutions. It is likely that there may be a unanimous view with regard to the ultimate objectives of better environmental quality. However, it is equally likely that there may be disagreement about the pace, means and time required to achieve them.
Environmental protection has become a strategic issue of increasing importance for industry and the business sector, both in the siting of plants and in the technical performance of processes and products. Industrialists are increasingly becoming interested in being able to look holistically at the environmental consequences of their operations. Legislation is no longer the sole dimensioning factor following the growing importance of product-related environmental issues. The concepts of environmentally sound product development and environmentally friendly or “green” products are assuming wider acceptance among producers and consumers.
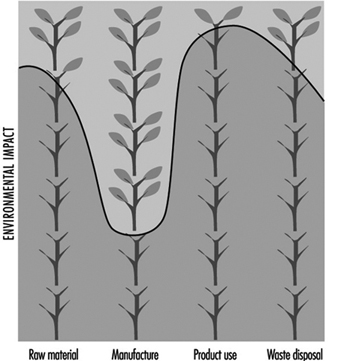
Indeed, this is a great challenge for industry; yet environmental criteria are often not considered at the beginning of the design of a product, when it may be easiest to avoid adverse impacts. Until recently, most environmental impacts were reduced through end-of-pipe controls and process design rather than product design. As a result, many companies spend too much time fixing problems instead of preventing them. A great deal of work, however, is needed to develop a suitable and accepted approach to incorporate environmental impacts into the various production stages and industrial activities—from raw material acquisition and manufacture to product use and final disposal.
The only known concept to deal with all these new complex issues seems to be a life-cycle approach to the problem. Life-cycle assessments (LCAs) have been widely recognized as an environmental management tool for the future, as product-related issues assume a more central role in the public debate. Although LCAs promise to be a valuable tool for programmes on cleaner production strategies and design for the environment, the concept is relatively new and will require future refinement to be accepted as a general tool for environmentally sound process and product development.
The Business Framework for Life-Cycle Assessment
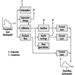
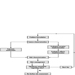
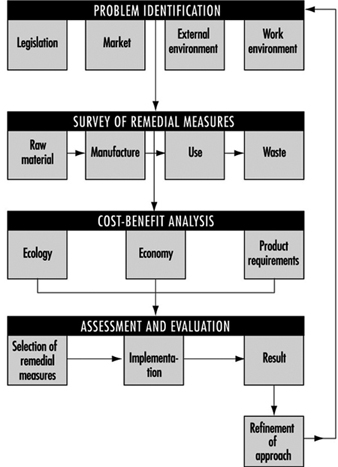
The necessary new approach to environmental protection in the business sector, to look at products and services in their totality, must be linked to development of a common, systematic and structured approach which enables relevant decisions to be made and priorities to be set. Such an approach must be flexible and expandable to cover various decision-making situations in industry as well as new input as science and technology progress. However, it should rest upon some basic principles and issues, for example: problem identification, survey of remedial measures, cost/benefit analysis and final assessment and evaluation (figure 1).
Figure 1. Outline of consecutive steps for setting priorities in decisions on environmental protection measures in industry
The problem identification ought to highlight different types of environmental problems and their causes. These judgements are multidimensional, taking into account various background conditions. There is indeed a close relationship between the work environment and the external environment. The ambition to safeguard the environment should therefore include two dimensions: to minimize the burden on the external environment following all kinds of human activities, and to promote the welfare of employees in terms of a well-planned and safe work environment.
A survey of potential remedial measures should include all the available practical alternatives for minimizing both pollutant emissions and the use of non-renewable natural resources. The technical solutions should be described, if possible, giving their expected value both in reducing resource use and pollution loads as well as in monetary terms. The cost/benefit analysis aims at producing a priority list by comparing the different identified approaches of remedial measures from the perspectives of product specifications and requirements to be met, economic feasibility and ecological efficiency. However, experience has shown that great difficulties often arise when seeking to express environmental assets in monetary terms.
The assessment and evaluation phase should be regarded as an integral part of the procedure of setting priorities to give the necessary input for the final judgement of the efficiency of the suggested remedial measures. The continuous exercise of assessment and evaluation following any measure that is implemented or enforced will give additional feedback for optimization of a general decision model for environmental priority strategies for product decision. The strategic value of such a model will likely increase in industry when it becomes gradually apparent that environmental priorities might be an equally important part of the future planning procedure for new processes or products. As LCA is a tool for identifying the environmental releases and evaluating the associated impacts caused by a process, product or activity, it will likely serve as the major vehicle for industry in their search for practical and user-friendly decision-making models for environmentally sound product development.
Concept of Life-Cycle Assessment
The concept of LCA is to evaluate the environmental effects associated with any given activity from the initial gathering of raw material from the earth until the point at which all residuals are returned to the earth. Therefore, the concept is often referred to as a “cradle-to-grave” assessment. While the practice of conducting life-cycle studies has existed since the early 1970s, there have been few comprehensive attempts to describe the full procedure in a manner that would facilitate understanding of the overall process, the underlying data requirements, the inherent assumptions and possibilities to make practical use of the methodology. However, since 1992 a number of reports have been published focusing on describing the various parts of a LCA from a theoretical viewpoint (Heijungs 1992; Vigon et al. 1992; Keoleian and Menerey 1993; Canadian Standards Association 1993; Society of Environmental Toxicology and Chemistry 1993). A few practical guides and handbooks have been published taking on the specific perspectives of product designers in making practical use of a complete LCA in environmentally sound product development (Ryding 1996).
LCA has been defined as an objective process to evaluate the environmental burdens associated with a process, product, activity or service system by identifying and quantifying energy and materials used and released to the environment in order to assess the impact of those energy and material uses and releases to the environment, and to evaluate and implement opportunities to effect environmental improvements. The assessment includes the entire life cycle of the process, product, activity or service system, encompassing extracting and processing raw materials, manu-facturing, transportation and distribution, use, reuse, maint-enance, recycling and final disposal.
The prime objectives of carrying out LCA are to provide as complete a picture as possible of the interactions of an activity with the environment, to contribute to the understanding of the overall and interdependent nature of environmental consequences of human activities and to provide decision-makers with information which identifies opportunities for environmental improvements.
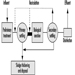
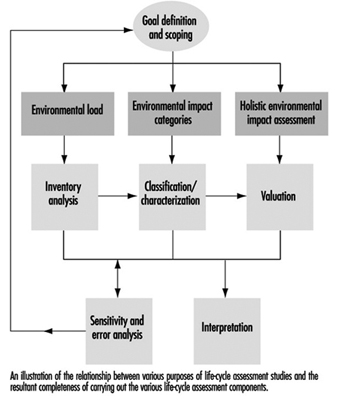
The LCA methodological framework is a stepwise calculation exercise comprising four components: goal definition and scoping, inventory analysis, impact assessment and interpretation. As one component of a broader methodology, none of these components alone can be described as an LCA. LCA ought to include all four. In many cases life-cycle studies focus on the inventory analysis and are usually referred to as LCI (life-cycle inventory).
Goal definition and scoping consists of a definition of the purpose and the system of the study—its scope, definition of the functional unit (the measure of performance which the system delivers), and the establishment of a procedure for quality assurance of the results.
When initiating an LCA study, it is of vital importance to clearly define the goal of the study, preferably in terms of a clear and unambiguous statement of the reason for carrying out the LCA, and the intended use of the results. A key consideration is to decide whether the results should be used for in-company applications to improve the environmental performance of an industrial process or a product, or whether the results should be used externally, for example, to influence public policy or consumer purchase choices.
Without setting a clear goal and purpose for the LCA study in advance, the inventory analysis and the impact assessment may be overdone, and the final results may not be properly used for practical decisions. Defining whether the results should focus on environmental loads, a specific environmental problem or a holistic environmental impact assessment will directly clarify whether to conduct an inventory analysis, classification/characterization or a valuation (figure 2). It is important to make all consecutive LCA components “visible” in order to make it easier for any user to choose the level of complexity they wish to use.
Figure 2. Purposes and completeness of life-cycle assessment
In many general programmes for cleaner production strategies, design for the environment or environmentally sound product development, the principal objective is often to lower the overall environmental impact during a product’s life cycle. To meet these demands it is sometimes necessary to arrive at a highly aggregated form of the environmental impact assessment which in turn emphasizes the need for identifying a general accepted valuation approach for a scoring system to weigh the different environmental effects against each other.
The scope of an LCA defines the system, boundaries, data requirements, assumptions and limitations. The scope should be defined well enough to ensure that the breadth and depth of analysis are compatible with and sufficient to address the stated purpose and all boundaries, and that assumptions are clearly stated, comprehensible and visible. However, as an LCA is an iterative process, it may be advisable in some cases not to permanently fix all aspects included in the scope. The use of sensitivity and error analysis is recommended to make possible the successive testing and validation of the purpose and scope of the LCA study versus the results obtained, in order to make corrections and set new assumptions.
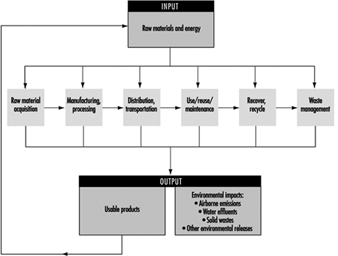
Inventory analysis is an objective, data-based process of quantifying energy and raw material requirements, air emissions, waterborne effluents, solid waste and other environmental releases throughout the life cycle of a process, product, activity or service system (figure 3).
Figure 3. Stepwise elements in a life-cycle inventory analysis.
The calculation of inputs and outputs in the inventory analysis refers to the system defined. In many cases, processing operations yield more than one output, and it is important to break down such a complex system into a series of separate sub-processes, each of which produces a single product. During the production of a construction material, pollutant emissions occur in each sub-process, from raw material acquisition to the final product. The total production process may be illustrated by a “process tree” where the stem may be seen as the main chain of flow of materials and energy, whereas the branches may illustrate sub-processes and the leaves the specific figures on pollutant emissions and so on. When added together, these sub-processes have the total characteristics of the original single system of co-products.
To estimate the accuracy of the data gained in the inventory analysis, a sensitivity and error analysis is recommended. All data used should therefore be “labelled” with relevant information not only as to reliability but also source, origin and so on, to facilitate future updating and refinement of the data (so-called meta-data). The use of a sensitivity and error analysis will identify the key data of great importance for the outcome of the LCA study that may need further efforts to increase its reliability.
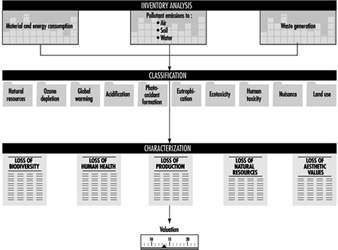
Impact assessment is a technical, qualitative and/or quantitative process to characterize and assess the effects of the environmental loading identified in the inventory component. The assessment should address both ecological and human health considerations, as well as other effects such as habitat modifications and noise pollution. The impact assessment component could be characterized as three consecutive steps—classification, characterization and valuation—all of which interpret the effects of environmental burdens identified in the inventory analysis, on different aggregated levels (figure 4). Classification is the step in which the inventory analyses are grouped together into a number of impact categories; characterization is the step in which analysis and quantification takes place, and, where possible, aggregation of the impacts within the given impact categories is carried out; valuation is the step in which the data of the different specific impact categories are weighted so that they can be compared amongst themselves to arrive at a further interpretation and aggregation of the data of the impact assessment.
Figure 4. Conceptual framework for the successive level of data aggregation in the impact assessment component
In the classification step, the impacts may be grouped in the general protection areas of resource depletion, ecological health and human health. These areas may be further divided into specific impact categories, preferably focusing on the environ-mental process involved, to allow a perspective consistent with current scientific knowledge about these processes.
There are various approaches to characterization—to relate data to no-observable-effect concentrations or to environmental standards, to model both exposure and effects and apply these models in a site-specific way, or to use equivalency factors for the different impact categories. A further approach is to normalize the aggregated data for each impact category to the actual magnitude of the impacts in some given area, to increase the comparability of the data from the different impact categories.
Valuation, with the aim of further aggregating the data of the impact assessment, is the LCA component that has probably generated the most heated debates. Some approaches, often referred to as decision theory techniques, are claimed to have the potential to make the valuation a rational, explicit method. Valuation principles may rest on scientific, political or societal judgements, and there are currently approaches available that cover all three perspectives. Of special importance is the use of sensitivity and error analysis. The sensitivity analysis enables the identification of those selected valuation criteria that may change the resultant priority between two process or product alternatives because of the uncertainties in the data. The error analysis may be used to indicate the likelihood of one alternative product being more environmentally benign than a competitor product.
Many are of the opinion that valuations have to be based largely on information about social values and preferences. However, no one has yet defined the specific requirements that a reliable and generally accepted valuation method should meet. Figure 5 lists some such specific requirements of potential value. However, it should be clearly emphasized that any valuation system for assessing the “seriousness” of environmental impacts of any human activity must be largely based on subjective value judgements. For such valuations it is probably not possible to establish criteria which are tenable in all situations worldwide.
Figure 5. List of suggested requirements to be met for a LCA valuation method
Interpretation of the results is a systematic evaluation of the needs and opportunities to reduce the environmental burden associated with energy and raw materials use and waste emissions throughout the whole life cycle of a product, process or activity. This assessment may include both quantitative and qualitative measures of improvements, such as changes in product design, raw material use, industrial processing, consumer demands and waste management.
Interpretation of the results is the component of an LCA in which options for reducing the environmental impacts or burdens of the processes or products under study are identified and evaluated. It deals with the identification, evaluation and selection of options for improvements in processes and product design, that is, technical redesign of a process or product to minimize the associated environmental burden while fulfilling the intended function and performance characteristics. It is important to guide the decision-maker regarding the effects of the existing uncertainties in the background data and the criteria used in achieving the results, to decrease the risk of making false conclusions regarding the processes and products under study. Again, a sensitivity and error analysis is needed to gain credibility for the LCA methodology as it provides the decision-maker with information on (1) key parameters and assumptions, which may need to be further considered and refined to strengthen the conclusions, and (2) the statistical significance of the calculated difference in total environmental burden between the process or product alternatives.
The interpretation component has been identified as the part of an LCA that is least documented. However, preliminary results from some large LCA studies carried out as comprehensive efforts by people from academia, consultancy firms and many companies all indicated that, from a general perspective, significant environmental burdens from products seem to be linked to the product use (figure 6). Hence, the potential seems to exist for industry-motivated initiatives to minimize environmental impacts through product development.
Figure 6. Outline of some general experiences of where in the life-cycles of products the major environmental burdens occur
A study on international experiences of environmentally sound product development based on LCA (Ryding 1994) indicated that promising general applications of LCA seem to be (1) for internal use by corporations to form the basis for providing guidance in long-term strategic planning concerning product design, but also (2) to some extent for use by regulatory agencies and authorities to suit general purposes of societal planning and decision-making. By developing and using LCA information regarding environmental effects that are both “upstream” and “downstream” of the particular activity under scrutiny, a new paradigm may be created for basing decisions in both corporate management and regulatory policy-making.
Conclusion
Knowledge about human threats to the environment seems to grow faster than our ability to solve them. Therefore, decisions in the environmental arena must often be taken with greater uncertainties present than those in other areas. Furthermore, very small safety margins usually exist. Present ecological and technical knowledge is not always sufficient to offer a complete, fool-proof strategy to safeguard the environment. It is not possible to gain full understanding of all ecological responses to environmental stress before taking action. However, the absence of complete, irrefutable scientific evidence should not discourage making decisions about and implementation of pollution abatement programmes. It is not possible to wait until all ecological questions are scientifically substantiated before taking action—the damage that may result through such delays could be irreversible. Hence, the meaning and scope of most problems is already known to a sufficient extent to justify action, and there is, in many cases, sufficient knowledge at hand to initiate effective remedial measures for most environmental problems.
Life-cycle assessment offers a new concept to deal with the future complex environmental issues. However, there are no shortcuts or simple answers to all questions posed. The rapidly emerging adoption of a holistic approach to combat environmental problems will most likely identify a lot of gaps in our knowledge about new aspects that need to be dealt with. Also, available data that may be used are in many cases intended for other purposes. Despite all difficulties, there is no argument for waiting to use LCA until it gets better. It is by no means hard to find difficulties and uncertainties in the present LCA concept, if one wants to use such arguments to justify an unwillingness to conduct an LCA. One has to decide whether it is worthwhile to seek a holistic life-cycle approach to environmental aspects despite all difficulties. The more LCA is used, the more knowledge will be gained about its structure, function and applicability, which will be the best guarantee for a feedback to ensure its successive improvement.
To make use of LCA today may be more a question of will and ambition than of undisputed knowledge. The whole idea of LCA ought to be to make the best use of present scientific and technical knowledge and to make use of the result in an intelligent and humble way. Such an approach will most likely gain credibility.
Risk Assessment and Communication
Government, industry and the community recognize the need to identify, assess and control the industrial risks (occupational and public) to people and the environment. Awareness of hazards and of the accidents that may result in significant loss of life and property have led to the development and application of systematic approaches, methods and tools for risk assessment and communication.
The risk assessment process involves: system description, the identification of hazards and the development of accident scenarios and outcomes for events associated with a process operation or a storage facility; the estimation of the effects or consequences of such hazardous events on people, property and the environment; the estimation of the probability or likelihood of such hazardous events occurring in practice and of their effects, accounting for the different operational and organizational hazard controls and practices; the quantification of ensuing risk levels outside the plant boundaries, in terms of both consequences and probabilities; and the assessment of such risk levels by reference to quantified risk criteria.
The process of quantified risk assessment is probabilistic in nature. Because major accidents may or may not occur over the entire life of a plant or a process, it is not appropriate to base the assessment process on the consequences of accidents in isolation. The likelihood or probability of such accidents actually occurring should be taken into account. Such probabilities and resultant risk levels should reflect the level of design, operational and organizational controls available at the plant. There are a number of uncertainties associated with the quantification of risk (e.g., mathematical models for consequence estimation, setting of probabilities for different accident scenarios, probability effects of such accidents). The risk assessment process should, in all cases, expose and recognize such uncertainties.
The main value of the quantified risk assessment process should not rest with the numerical value of the results (in isolation). The assessment process itself provides significant opportunities for the systematic identification of hazards and evaluation of risk. The risk assessment process provides for the identification and recognition of hazards and enables the allocation of relevant and appropriate resources to the hazards control process.
The objectives and uses of the hazard identification process (HIP) will determine in turn the scope of the analysis, the appropriate procedures and methods, and the personnel, expertise, funding and time required for the analysis, as well as the associated documentation necessary. Hazard identification is an efficient and necessary procedure to assist risk analysts and decision making for risk assessment and management of occupational safety and health. A number of major objectives may be identified:
- to establish what dangerous situations exist within a plant or a process operation
- to establish how these dangerous situations may come about
- to assist in the assessment of the safety of a hazardous installation.
The first general objective aims at extending the general understanding of the important issues and situations that might affect the risk analysis process for individual plants and processes; the synergy of individual hazards to the area study level has its special significance. Design and operational problems can be identified and a hazard classification scheme can be considered.
The second objective contains elements of risk assessment and deals with accident scenario development and interpretation of results. Consequence evaluation of various accidents and their impact propagation in time and space has special significance in the hazard identification phase.
The third objective aims at providing information that can later assist further steps in risk assessment and plant operations safety management. This may be in the form of improving the scenario specifications for risk analysis or identifying appropriate safety measures to comply with given risk criteria (e.g., individual or societal), or advice for emergency preparedness and accident management.
After defining objectives, the definition of the scope of the HIP study is the second most relevant element in the management, organization and implementation of the HIP. The scope of the HIP in a complex risk assessment study can be described mainly in terms of the following parameters: (1) potential sources of hazards (e.g., radioactive releases, toxic substances, fire, explosions); (2) plant or process damage states; (3) initiating events; (4) potential consequences; and (5) prioritization of hazards. The relevant factors that determine the extent to which these parameters are included in the HIP are: (a) the objectives and intended uses of the HIP; (b) the availability of appropriate information and data; and(c) the available resources and expertise. Hazard identification requires the consideration of all relevant information regarding the facility (e.g., plant, process). This might typically include: site and plant layout; detailed process information in the form of engineering diagrams and operating and maintenance conditions; the nature and quantities of materials being handled; operational, organizational and physical safeguards; and design standards.
In dealing with the external consequences of an accident, a number of such consequences may result (e.g., number of fatalities, number of people being hospitalized, various types of damage to the ecosystem, financial losses, etc.). The external consequences from an accident caused by the substance i for an identified activity j, can be calculated from the relationship:
Cij = Aa fa fm, where: Cij = number of fatalities per accident caused by the substance i for an identified activity j; A = affected area (ha); a = population density in populated areas within the affected zone (persons/ha); fa and fm are correction factors.
The consequences of (major) accidents to the environment are more difficult to estimate due to the variety of substances that can be involved, as well as the number of environmental impact indicators relevant in a given accident situation. Usually, a utility scale is associated with various environmental consequences; the relevant utility scale could include events related to incidents, accidents or catastrophic outcomes.
Evaluating monetary consequences of (potential) accidents requires a detailed estimate of possible consequences and their associated costs. A monetary value for special classes of consequences (e.g., loss of life or special biological habitats) is not always accepted a priori. The monetary evaluation of consequences should also include external costs, which are very often difficult to assess.
The procedures for identifying hazardous situations which may arise in process plants and equipment are generally considered to be the most developed and well established element in the assessment process of hazardous installations. It must be recognized that (1) the procedures and techniques vary in terms of comprehensiveness and level of detail, from comparative checklists to detailed structured logic diagrams, and (2) the procedures may apply at various stages of project formulation and implementation (from the early decision-making process to determine the location of a plant, through to its design, construction and operation).
Techniques for hazard identification essentially fall into three categories. The following indicates the most commonly used techniques within each category.
- Category 1: Comparative Methods: Process or System Checklist; Safety Audit Review; Relative Ranking (Dow and Mond Hazard Indices); Preliminary Hazard Analysis
- Category 2: Fundamental Methods: Hazard Operability Studies (HAZOP); “What If” Analysis; Failure Mode and Effect Analysis (FMEA)
- Category 3: Logic Diagrams Methods: Fault Tree Analysis; Event Tree Analysis.
Cause Consequence Analysis; Human Reliability Analysis
The appropriateness and relevancy of any one particular technique of hazard identification largely depend on the purpose for which the risk assessment is being undertaken. When further technical details are available one can combine them in the overall process for risk assessment of various hazards. Expert and engineering judgements can often be employed for further evaluation of risk for installations or processes. The primary principle is to first examine the plant or operations from the broadest viewpoint possible and systematically identify possible hazards. Elaborate techniques as a primary tool may cause problems and result in missing some obvious hazards. Sometimes it may be necessary to adopt more than one technique, depending on the level of detail required and whether the facility is a new proposed installation or an existing operation.
Probabilistic safety criteria (PSC) are associated with a rational decision-making process which requires the establishment of a consistent framework with standards to express the desired level of safety. Societal or group risks should be considered when assessing the acceptability of any hazardous industrial facility. A number of factors should be borne in mind when developing PSC based on societal risk, including public aversion to accidents with high consequences (i.e., the risk level chosen should decrease as the consequence increases). Whilst individual fatality risk levels include all components of risk (i.e., fires, explosions and toxicity), there may be uncertainties in correlating toxic concentrations with fatality risk levels. The interpretation of “fatal” should not rely on any one dose-effect relationship, but should involve a review of available data. The concept of societal risk implies that risk of higher consequences, with smaller frequency, are perceived as more important than those of smaller consequences with higher probabilities.
Irrespective of the numerical value of any risk criteria level for risk assessment purposes, it is essential that certain qualitative principles be adopted as yardsticks for risk assessment and safety management: (1) all “avoidable” risks should be avoided; (2) the risk from a major hazard should be reduced whenever practicable; (3) the consequences of more likely hazardous events should, wherever possible, be contained within the boundaries of the installation; and (4) where there is an existing high risk from a hazardous installation, additional hazardous developments should not be allowed if they add significantly to that existing risk.
In the 1990s an increasing importance has been given to risk communication, which has become a separate branch of risk science.
The main tasks in risk communication are:
- identifying controversial aspects of perceived risks
- presenting and explaining risk information
- influencing risk-related behaviour of individuals
- developing information strategies for emergency cases
- evolving cooperative/participative conflict resolution.
The scope and objectives of risk communication can differ, depending on the actors involved in the communication process as well as the functions and expectations they attribute to the communication process and its environment.
Individual and corporate actors in risk communication use manifold communicative means and channels. The main issues are health and environmental protection, safety improvement and risk acceptability.
According to general communication theory, communication can have the following functions:
- presentation of information
- appeal
- self-presentation
- definition of a relationship or decision path.
For the risk communication process in particular it can be helpful to distinguish between these functions. Depending on the function, different conditions for a successful communication process should be considered.
Risk communication can sometimes play the role of a simple presentation of facts. Information is a general need in a modern society. In environmental matters in particular there exist laws which, on the one hand, give the authorities the duty to inform the public and, on the other hand, give the public the right to know about the environmental and risk situation (e.g., the so-called Seveso Directive of the European Community and “Community Right-to-Know” legislation in the United States). Information can also be determined for a special public segment; for example, the employees in a factory must be informed about the risks they face within their workplace. In this sense risk communication must be:
- as neutral and objective as possible
- complete
- comprehensible for those who should get the information.
Appeals tend to incite someone to do something. In risk-related matters the following appeal functions can be distinguished:
- appeal to the general public or to a special segment of the public about risk prevention measures which could or should be taken (e.g., appeal to employees in a factory to take safety measures at work)
- appeal to the general public or to a special segment of the public about preventive measures for emergency cases
- appeal to the general public or to a special segment of the public about measures to be taken in case of an emergency situation (crisis management).
Appeal communication must be:
- as simple and comprehensible as possible, and as complete as necessary
- reliable; having confidence in the persons, authorities or other bodies which make the appeal is essential for the success of the appeal.
Self-presentation does not impart neutral information, but is mainly part of a persuasion or marketing strategy in order to improve the public image of an individual or to achieve public acceptance for a certain activity or to get public support for some kind of position. The criterion for the success of the communication is whether the public believes in the presentation. In a normative view, although the self-presentation aims at convincing someone, it should be honest and sincere.
These forms of communication are mainly of a one-way type. Communication aimed at reaching a decision or agreement is of a two-way or many-way type: there is not only one side which gives information—various actors are involved in a risk communication process and communicate with each other. This is the usual situation in a democratic society. Especially in risk- and environment-related matters communication is considered as an alternative regulatory instrument in complex situations, where easy solutions are not possible or accessible. Therefore the risky decisions with a relevant political importance have to be taken in a communicative atmosphere. Risk communication, in this sense, may include, among others, communication about highly politicized risk topics, but it may also mean, for example, the communication between an operator, the employees and the emergency services in order that the operator be best prepared in case of accident. Thus, depending on the scope and objective of the risk communication, different actors can participate in the communication process. The potential main actors in a risk communication environment are:
- the operator of a risky facility
- the potential victims of an undesired event (e.g., employees, neighbours)
- the regulatory authorities and appropriate political bodies
- the emergency services and general public
- interest groups
- the media
- insurers
- scientists and experts.
In a systems-theory approach all these categories of actors correspond to a certain social system and therefore have different codes of communication, different values and interests to be communicated. Very often it is not easy to find a common basis for a risk dialogue. Structures must be found in order to combine these different views and to achieve a practical result. Topics for such types of risk communication are, for example, a consensus decision about siting or not siting a hazardous plant in a certain region.
In all societies there exist legal and political procedures in order to deal with risk-related issues (e.g., parliamentary legislation, government or administrative decisions, legal procedures before a court, etc.). In many cases these existing procedures do not result in solutions that are entirely satisfactory for the peaceful settlement of risk disputes. Proposals reached by integrating elements of risk communication into the existing procedures have been found to improve the political decision process.
Two main issues have to be discussed when proposing risk communication procedures:
- the formal organization and legal significance of the process and of its results
- the structure of the communication process itself.
For the formal organization of risk communication there are various possibilities:
- The communication can take place inside or between existing bodies (e.g., between an agency of the central government, a local authority and existing interest groups).
- New bodies can be established specifically for the process of risk communications; various models have been developed (e.g., citizen juries, citizen panels, negotiation and mediation structures, mixed commissions consisting of operators, authorities and citizens). Most of these models are based on the idea of organizing a structured discourse in small groups. Significant differences of opinion exist about whether these groups should consist of experts, laymen, representatives of the political system, etc.
In any case the relationship between these communication structures and the existing legal and political decision-making bodies has to be clarified. Usually the result of a risk communication process has the effect of a non-binding recommendation to the deciding bodies.
Concerning the structure of the communication process, under the general rules of practical discourse, any argument is allowed if it fulfils the following conditions:
- adequate logical consistency
- sincerity (This means: The discourse should not be influenced by strategic or tactical thinking.)
- that the one who promotes an argument must be ready to accept the consequences of that argument also against himself or herself.
In the risk communication process various special rules and proposals have been developed in order to concretize these rules. Among these, the following rules are worth mentioning:
In the risk communication process a distinction must be made between:
- communicative claims
- cognitive claims
- normative claims
- expressive claims.
Correspondingly, differences of opinion can have various reasons, namely:
- differences in information
- differences in the understanding of facts
- differences in normative values.
It may be helpful to make clear through the risk communication process the level of differences and their significance. Various structural proposals have been made for improving the conditions for such a discourse and, at the same time, to help decision-makers to find fair and competent solutions—for example:
- For a fair discourse the result must be open-ended; if the aim is just to achieve acceptance for a decision that has already been made, it would not be sincere to open a discourse.
- If some solutions are simply not possible for factual, political or legal reasons, this must be clarified from the beginning.
- It may be helpful first to discuss not the alternatives, but the criteria which should be applied in evaluating the alternatives.
Effectiveness of risk communication can be defined as the degree to which an initial (undesired) situation is changed toward an intended state, as defined by initial goals. Procedural aspects are to be included in the evaluation of risk communication programmes. Such criteria include practicability (e.g., flexibility, adaptability, implementability) and costs (in terms of money, personnel and time) of the programme.
Environmental Auditing - Definition and Methodology
Origins of Environmental Auditing
Environmental safety and health auditing developed in the early 1970s, largely among companies operating in environmentally intensive sectors such as oils and chemicals. Since then environmental auditing has spread rapidly with a corresponding development of the approaches and techniques adopted. Several factors have influenced this growth.
- Industrial accidents. Major incidents such as the Bhopal, Chernobyl and Exxon-Valdez disasters have reminded companies that it is not sufficient to set corporate policies and standards on environmental health and safety matters without ensuring that they are being implemented. Audits can help reduce the risk of unpleasant surprises.
- Regulatory developments. Since the early 1970s regulations on environmental topics have increased substantially. This has made it steadily more difficult for a company to ascertain whether a specific plant in a particular country is complying with all of the relevant legislation.
- Public awareness. The public has become increasingly aware of, and vocal about, environmental and safety issues. Companies have had to demonstrate to the public that they are managing environmental risks effectively.
- Litigation. The growth of legislation has led to a corresponding explosion of litigation and liability claims, particularly in the United States. In Europe and elsewhere, there is growing emphasis on the responsibilities of individual directors and on making information available to the public.
What is an Environmental Audit?
It is important to draw the distinction between auditing and techniques such as environmental impact assessment (EIA). The latter assesses the potential environmental effects of a proposed facility. The essential purpose of an environmental audit is the systematic scrutiny of environmental performance throughout a company’s existing operations. At best, an audit is a comprehensive examination of management systems and facilities; at worst, it is a superficial review.
The term environmental audit means different things to different people. Terms such as assessment, survey and review are used to describe the same type of activity. Furthermore, some organizations consider that an “environmental audit” addresses only environmental matters, whereas others use the term to mean an audit of health, safety and environmental matters. Although there is no universal definition, auditing, as practised by many leading companies, follows the same basic philosophy and approach summarized by the broad definition adopted by the International Chambers of Commerce (ICC) in its publication Environmental Auditing (1989). The ICC defines environmental auditing as:
a management tool comprising a systematic, documented periodic and objective evaluation of how well environmental organization, management and equipment are performing, with the aim of helping safeguard the environment by:
(i) facilitating management control of environmental practices and
(ii) assessing compliance with company policies which would include meeting regulatory requirements.
The European Commission in its proposed regulation on environmental auditing also adopts the ICC definition of environmental audit.
Objectives of Environmental Auditing
The overall objective of environmental auditing is to help safeguard the environment and minimize risks to human health. Clearly, auditing alone will not achieve this goal (hence the use of the word help); it is a management tool. The key objectives of an environmental audit therefore are to:
- determine how well the environmental management systems and equipment are performing
- verify compliance with the relevant national, local or other laws and regulations
- minimize human exposure to risks from environmental, health and safety problems.
Scope of the Audit
As the prime objective of audits is to test the adequacy of existing management systems, they fulfil a fundamentally different role from the monitoring of environmental performance. Audits can address one topic, or a whole range of issues. The greater the scope of the audit, the greater will be the size of the audit team, the time spent onsite and the depth of investigation. Where international audits need to be carried out by a central team, there can be good reasons for covering more than one area while onsite to minimize costs.
In addition, the scope of an audit can vary from simple compliance testing to a more rigorous examination, depending on the perceived needs of the management. The technique is applied not only to operational environmental, health and safety management, but increasingly also to product safety and product quality management, and to areas such as loss prevention. If the intention of auditing is to help ensure that these broad areas are managed properly, then all of these individual topics must be reviewed. Items which may be addressed in audits, including environment, health, safety and product safety are shown in table 1.
Table 1. Scope of environmental audit
|
Environmental |
Safety |
Occupational Health |
Product Safety |
|
-Site history |
-Safety policy/procedures |
-Employee exposure to air contaminants |
-Product safety programme |
Although some companies have a regular (often annual) audit cycle, audits are primarily determined by need and priority. Thus not all facilities or aspects of a company will be assessed at the same frequency or to the same extent.
The Typical Audit Process
An audit is usually conducted by a team of people who will assemble factual information prior to and during a site visit, analyse the facts and compare them with the criteria for the audit, draw conclusions and report their findings. These steps are usually conducted within some kind of formal structure (an audit protocol), such that the process can be repeated reliably at other facilities and quality can be maintained. To ensure that an audit is effective, a number of key steps must be included. These are summarized and explained in table 2.
Table 2. Basic steps in environmental auditing
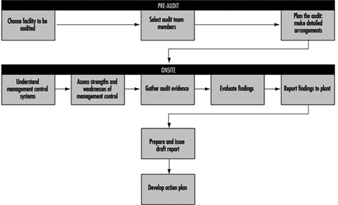
Basic Steps in Environmental Auditing
Criteria—what do you audit against?
An essential step in establishing an audit programme is to decide the criteria against which the audit will be conducted and to ensure that management throughout the organization knows what these criteria are. Typically criteria used for audits are:
- company policies and procedures on environmental matters
- applicable legislation and regulations
- good environmental management practice.
Pre-audit steps
Pre-audit steps include the administrative issues associated with planning the audit, selecting the personnel for the audit team (often from different parts of the company or from a specialized unit), preparing the audit protocol used by the organization and obtaining background information about the facility.
If auditing is new, the need for education of those involved in the audit process (the auditors or those being audited) should not be underestimated. This also applies to a multinational company extending an audit programme in its home country to subsidiaries abroad. In these situations, the time spent on explanation and education will pay dividends by ensuring that the audits are approached in a spirit of cooperation and are not seen as a threat by the local management.
When one major US company proposed extending its auditing programme to its operations in Europe, it was particularly concerned to ensure that the plants were properly briefed, that audit protocols were appropriate for European operations and that audit teams understood the relevant regulations. Pilot audits were conducted at selected plants. In addition, the audit process was introduced in a way that stressed the benefits of a cooperative rather than a “policing” approach.
Obtaining background information about a site and its processes can help to minimize the time spent onsite by the audit team and to focus its activities, thus saving resources.
The composition of the audit team will depend on the approach adopted by a particular organization. Where there is a lack of internal expertise, or where resources cannot be devoted to the audit activity, companies frequently use independent consultants to conduct the audits for them. Other companies employ a mix of in-house staff and external consultants on each team to ensure an “independent” view. Some large companies use only in-house staff for audits, and have environmental audit groups for this specific function. Many major companies have their own dedicated audit staff, but also include an independent consultant on many of the audits they carry out.
Onsite steps
- Understanding the internal controls. As a first step, it is necessary to develop an understanding of the controls that are in place or are thought to be in place. These will include assessing formal procedures and practices; record keeping and monitoring; inspection and maintenance programmes and physical controls for containing spills. The audit team gathers information on the various controls by observation, interviewing staff and the use of detailed questionnaires.
- Assessing strengths and weaknesses of internal controls. Evaluating the strengths and weaknesses of internal controls provides the rationale for conducting subsequent audit steps. Auditors will look for indicators such as clearly defined responsibilities, competence of personnel, appropriate documentation and records and systems of authorization. It is more important to determine whether the system is effective than whether it is sophisticated.
- Gathering audit evidence. The audit team attempts to verify that the steps and controls work as intended. Evidence may be collected through inquiry (e.g., asking a plant operator what he or she would do if there were a major chemical spill), observation (e.g., watching specific activities and operations in progress) and testing (checking records to confirm compliance with regulations).
- Recording audit findings. All the information obtained is recorded (usually on the audit protocol document and as working papers), and a comprehensive record of the audit and the state of the facility at the time is thus produced. Where a deficiency is found, it is noted as an audit “finding”.
- Evaluating the audit findings. The audit team integrates and evaluates the findings of the individual team members. There may also be common findings. For some observations, an informal discussion with the plant manager may be sufficient; for others, inclusion in the formal report will be appropriate.
Reporting the audit findings. This usually is done at a meeting with the plant management at the end of the team’s visit. Each finding and its significance can be discussed with the plant personnel. Prior to leaving the site, the audit team will often provide a written summary of findings for the plant management, to ensure that there are no surprises in the final report.
Post-audit steps
Following the onsite work, the next step is to prepare a draft report, which is reviewed by the plant management to confirm its accuracy. It is then distributed to senior management according to the requirements of the company.
The other key step is to develop an action plan to address the deficiencies. Some companies ask for recommendations for corrective action to be included in the formal audit report. The plant will then base its plan on implementing these recommendations. Other companies require the audit report to state the facts and the deficiencies, with no reference to how they should be corrected. It is then the responsibility of the plant management to devise the means of remedying the failings.
Once an audit programme is in place, future audits will include past reports—and progress in the implementation of any recommendations made therein—as part of their evidence.
Extending the Audit Process—Other Types of Audit
Although the most widespread use of environmental auditing is to assess the environmental performance of a company’s operations, there are variations on the theme. Other types of audit used in particular circumstances include the following:
- Pre-acquisition audits. Concern about potential liabilities has promoted the dramatic increase in environmental auditing prior to acquisition. Pre-acquisition audits are a means of identifying actual or potential problems, and taking these into account in the final negotiations of the deal. Time scales are often very short. However, the information obtained on past operations (perhaps before the present owner), current activities, past incidents and so on can be invaluable.
- Pre-sale audits. Less common than pre-acquisition audits, but becoming more popular, are audits conducted by the owner prior to selling a plant or a subsidiary company. A growing number of major organizations, such as the Dutch chemical company DSM and the Finnish conglomerate Neste, undertake pre-sale audits as part of corporate policy. The rationale is that the company will then know the status of environmental issues before the plant is sold, and can take action to remedy any problems if it feels that is appropriate. Equally important, it can present the results of an independent audit to a potential purchaser as confirmation of the situation. Should any environmental problems arise after the sale, a baseline has been established against which issues of liability can be decided.
Issues audits. Some organizations apply the audit technique to a specific issue that may have implications for the whole company, such as waste. The UK-based oil multinational BP has carried out audits examining the impact of ozone depletion and the implications of public concern about tropical deforestation.
Benefits of Environmental Auditing
If environmental auditing is implemented in a constructive way there are many benefits to be derived from the process. The auditing approach described in this paper will help to:
- safeguard the environment
- verify compliance with local and national laws
- indicate current or potential future problems that need to be addressed
- assess training programmes and provide data to assist in training
- enable companies to build on good environmental performance, give credit where appropriate and highlight deficiencies
- identify potential cost savings, such as from waste minimization
- assist the exchange and comparison of information between different plants or subsidiary companies
- demonstrate company commitment to environmental protection to employees, the public and the authorities.
Environmental Management Strategies and Workers' Protection
The Evolution of Environmental Response Strategies
In the past thirty years there has been a dramatic increase in environmental problems due to many different factors: demographic expansion (this pace is continuing, with an estimated 8 billion people by the year 2030), poverty, dominant economic models based on growth and quantity rather than quality, high consumption of natural resources driven particularly by industrial expansion, reduction of biological diversity especially as a result of increased agricultural production through monoculture, soil erosion, climate change, the unsustainable use of natural resources and the pollution of air, soils and water resources. However, the negative effects of human activity upon the environment have also accelerated the awareness and social perception of people in many countries, leading to changes in traditional approaches and response models.
Response strategies have been evolving: from no recognition of the problem, to ignoring the problem, to diluting and controlling pollution through a top-down approach—that is, the so-called end-of-pipe strategies. The 1970s marked the first widely relevant local environmental crises and the development of new awareness of environmental pollution. This led to the adoption of the first major series of national legislation, regulations and international conventions aimed at the control and regulation of pollution. This end-of-pipe strategy soon showed its failure, for it was directed in an authoritarian way to interventions related to the symptoms and not the causes of environmental problems. At the same time, industrial pollution also drew attention to the growing contradictions in philosophy between employers, workers and environmental groups.
The 1980s was the period of global environmental issues such as the Chernobyl disaster, acid rain, ozone depletion and the ozone hole, the greenhouse effect and climate change, and the growth in toxic wastes and their export. These events and the resulting problems enhanced public awareness and helped to generate support for new approaches and solutions focusing on environmental management tools and cleaner production strategies. Organizations such as UNEP, OECD, the European Union and many national institutions started to define the issue and work together within a more global framework based on principles of prevention, innovation, information, education and the participation of relevant stakeholders. As we entered the 1990s there was another dramatic increase in awareness that the environmental crisis was deepening, particularly in the developing world and in Central and Eastern Europe. This reached a critical threshold at the United Nations Conference on Environment and Development (UNCED) in Rio de Janeiro in 1992.
Today, the precautionary approach has become one of the most important factors necessary to take into account when assessing environmental policies and solutions. The precautionary approach suggests that even when there is scientific uncertainty or controversy on environmental problems and policies, decisions should reflect the need to take precautions to avoid future negative implications whenever economically, socially and technically feasible. The precautionary approach should be pursued when developing policies and regulations, and when planning and implementing projects and programmes.
In effect, both the preventive and precautionary approaches seek a more integrated approach to environmental action, shifting from an almost exclusive focus on the production process to the development of environmental management tools and techniques applicable to all forms of human economic activity and decision-making processes. Unlike pollution control, which implied a limited, react-and-retreat approach, the environmental management and cleaner production approach is aimed at the integration of a precautionary approach within broader strategies to create a process that will be assessed, monitored and continuously improved. To be effective, however, environmental management and cleaner production strategies need to be carefully implemented through the involvement of all stakeholders and at all levels of intervention.
These new approaches must not be considered as simply technical instruments related to the environment, but rather should be seen as holistic integrating approaches which will help to define new models of an environmentally and socially sound market economy. To be fully effective, these new approaches will also require a regulatory framework, incentive instruments and social consensus defined through the involvement of institutions, social partners and interested environmental and consumer organizations. If the scope of environmental management and cleaner production strategies is to lead to more sustainable socio-economic development scenarios, various factors will need to be taken into consideration in policy-setting, in the development and enforcement of standards and regulations, and in collective agreements and action plans, not only at the company or enterprise level, but at the local, national and international levels as well. Given the wide disparities in economic and social conditions around the world, the opportunities for success also will depend on local political, economic and social conditions.
Globalization, the liberalization of markets and structural adjustment policies, will also create new challenges to our capacity to analyse in an integrated fashion the economic, social and environmental implications of these complex changes within our societies, not the least of which will be the risk that these changes may lead to quite different power relationships and responsibilities, perhaps even ownership and control. Attention will need to be given to ensuring that these changes do not lead to the risk of powerlessness and paralysis in the development of environmental management and cleaner production technologies. On the other hand, this changing situation, in addition to its risks, also offers new opportunities to promote improvements in our present social, economic, cultural, political and environmental conditions. Such positive changes, however, will require a collaborative, participatory and flexible approach to managing change within our societies and within our enterprises. To avoid paralysis, we will need to take measures which will build confidence and emphasize a step-by-step, partial and gradual approach which will generate growing support and capacity aimed at facilitating more substantial changes in our conditions of life and work in future.
Main International Implications
As mentioned above, the new international situation is characterized by the liberalization of markets, the elimination of trade barriers, new information technologies, rapid and enormous daily capital transfers and the globalization of production, especially through multinational enterprises. Deregulation and competitiveness are the dominant criteria for investment strategies. These changes also, however, facilitate the delocalization of plants, the fragmentation of production processes and the establishment of special Export Processing Zones, which exempt industries from labour and environmental regulations and other obligations. Such effects may promote excessively low labour costs and consequently higher profits for industry, but this is frequently accompanied by situations of deplorable human and environmental exploitation. In addition, in the absence of regulations and controls, obsolete plants, technologies and equipment are being exported just as dangerous chemicals and substances which have been banned, withdrawn or severely restricted in one country for environmental or safety reasons are also being exported, particularly to developing countries.
In order to respond to these issues, it is of particular importance that the new World Trade Organization (WTO) rules are defined so as to promote socially and environmentally acceptable trade. This means that WTO, in order to ensure fair competition, should require all countries to fulfil basic international labour standards (e.g., basic ILO Conventions) and environmental conventions and regulations. Moreover, guidelines such as those prepared by OECD on technology transfer and regulations should be effectively implemented in order to avoid the export of highly polluting and unsafe production systems.
International factors to be considered include:
- international trade in equipment and plants
- financial mechanisms and technical assistance
- WTO regulations
- raw material pricing
- tax systems
- transfer of technology and know-how
- transboundary migration of pollution
- multinational companies’ production strategies
- development and implementation of international conventions, agreements, guidelines and regulations
- involvement of international organizations of employers, workers and relevant environmental groups.
Developing and other countries in need of assistance should be given special financial assistance, reduction in taxes, incentives and technical assistance to help them implement the above-mentioned basic labour and environmental regulations and to introduce cleaner production technologies and products. An innovative approach which deserves further attention in the future is the development of codes of conduct negotiated by certain companies and their trade unions with a view to promoting the respect of basic social rights and environmental rules. A unique role in the assessment of the process at the international level is being played by the ILO, given its tripartite structure, and in strict coordination with other United Nations agencies and international financial institutions responsible for international aid and financial assistance.
Main National and Local Implications
An appropriate general regulatory framework also has to be defined at both the national and local level in order to develop appropriate environmental management procedures. This will require a decision-making process which links budgetary, fiscal, industrial, economic, labour and environmental policies, and also provides for the full consultation and participation of the social actors most concerned (i.e., employers, trade union organizations, environmental and consumer groups). Such a systematic approach would include linkages between different programmes and policies, for example:
- The taxation system should provide incentives which will encourage the penetration of environmentally sound goods and raw materials into the market and penalize those products, economic activities and collective or individual behaviour which are environmentally unsound.
- Adequate policies and resources should be available to promote research and development of environmentally and socially sound technologies, production processes and infrastructure.
- Advisory, information and training centres for cleaner production technologies should be established to assist enterprises, especially small- and medium-sized enterprises, to procure, adapt and use the technologies safely and effectively.
National and local industrial policies should be designed and implemented in full consultation with trade union organizations so that business policies and labour policies can match social and environmental needs. Direct negotiations and consultations at the national level with trade unions can help to prevent potential conflicts arising from safety, health and environmental implications of new industrial policies. Such negotiations at the national level, however, should be matched by negotiations and consultations at the level of individual companies and enterprises so as to ensure that adequate controls, incentives and assistance are also available at the workplace.
In summary, national and local factors to be considered include:
- national and local regulations, guidelines, agreements and policies
- industrial relations procedures
- involvement of social partners (trade unions and employers’ organizations), environmental NGOs and consumer organizations in all decision-making processes
- industrial policies
- raw material pricing policies
- trade policies
- tax systems
- incentives for research and development
- incentives for introduction of innovative environmental management initiatives
- integration of health and safety procedures/standards
- establishment of advisory, information and training centres for the dissemination of cleaner production technologies
- assistance for overcoming obstacles (conceptual, organizational, technical, skills and financial) to the introduction of new technologies, policies, regulations.
Environmental Management at Company Level
Environmental management within a given company, enterprise or other economic structure requires an ongoing assessment and consideration of environmental effects—at the workplace (i.e., the working environment) and outside the plant gates (i.e., the external environment)—as regards the full range of activities and decisions related to operations. It implies, as well, the consequent modification of the organization of work and production processes to respond efficiently and effectively to those environmental effects.
It is necessary for enterprises to foresee potential environmental consequences of a given activity, process or product from the earliest planning stages in order to ensure the implementation of adequate, timely and participatory response strategies. The objective is to make industry and other economic sectors economically, socially and environmentally sustainable. Most certainly, in many cases there still will need to be a transition period which will require pollution control and remediation activities. Therefore, environmental management should be seen as a composite process of prevention and control that aims to bring company strategies in line with environmental sustainability. To do this, companies will need to develop and implement procedures within their overall management strategy to assess cleaner production processes and to audit environmental performance.
Environmental management and cleaner production will lead to a range of benefits that will not only effect environmental performance but may also lead to improvements in:
- health and safety of workers
- rates of absenteeism
- preventing and resolving conflict with workers and communities
- promoting a cooperative climate within the company
- the company public image
- the market penetration of new green products
- efficient use of energy and raw materials
- waste management, including the safe disposal of wastes
- the productivity and quality of products.
Companies should not simply focus on evaluating company conformity with existing legislation and regulations but should define possible environmental targets to be reached through a time-bound, step-by-step process which would include:
- the definition of company environmental objectives and policy
- the definition of short-, medium- and long-term strategies
- the adoption of a cradle-to-grave approach
- the allocation of appropriate budget resources
- the integration of health and safety within environmental audit procedures
- the participation of workers and trade union representatives in the analysis and decision-making process
- the establishment of an environmental audit team with worker representatives.
There are many different approaches to assessing activities, and the following are important potential components of any such programme:
- definition of flow diagrams for each operational unit
- monitoring of process inputs by operational unit—for example, water, energy, raw materials used, number of workers involved, health, safety and environmental risk assessment, organization of work
- monitoring of process outputs by operational unit—for example, quantification of products/byproducts, waste water, gaseous emissions, solid wastes for disposal on and off site
- adoption of company targets
- feasibility analysis of potential barriers (economic, technical, environmental, social) and adoption of consequent programmes
- adoption and implementation of an information strategy
- adoption and implementation of training strategy to promote worker awareness and full participation
- monitoring and evaluation of performance/results.
Industrial Relations and Environmental Management
While in some countries basic trade union rights are still not recognized and workers are prevented from protecting their health and safety and working conditions and improving environmental performance, in various other countries the participatory approach to company environmental sustainability has been tried with good results. In the last ten years, the traditional approach of industrial relations has shifted more and more to include not only health and safety issues and programmes reflecting national and international regulations in this area, but also has begun to integrate environmental issues into the industrial relations mechanisms. Partnerships between employers and trade union representatives at company, sector and national level have been defined, according to different situations, through collective agreements and sometimes also have been covered in regulations and consultation procedures set up by local or national authorities to manage environmental conflicts. See table 1, table 2 and table 3.
Table 1. Actors involved in voluntary agreements relevant to the environment
|
Country |
Employer/ |
Employer/ |
Employer/ |
Employer/ |
|
Netherlands |
X |
X |
X |
|
|
Belgium |
X |
X |
||
|
Denmark |
X |
X |
X |
X |
|
Austria |
X |
|||
|
Germany |
X |
X |
X |
|
|
United Kingdom |
X |
X |
||
|
Italy |
X |
X |
X |
X |
|
France |
X |
X |
||
|
Spain |
X |
X |
||
|
Greece |
X |
X |
Source: Hildebrandt and Schmidt 1994.
Table 2. Scope of application voluntary agreements on environment-protection measures between parties to collective agreements
|
Country |
National |
Branch (regional) |
Plant |
|
Netherlands |
X |
X |
X |
|
Belgium |
X |
X |
|
|
Denmark |
X |
X |
X |
|
Austria |
X |
||
|
Germany |
X |
X |
|
|
United Kingdom |
X |
||
|
Italy |
X |
X |
X |
|
France |
|||
|
Spain |
X |
X |
|
|
Greece |
X |
Source: Hildebrandt and Schmidt 1994.
Table 3. Nature of agreements on environment protection measures between parties to collective agreements
|
Country |
Joint declarations, |
Branch-level |
Agreements on plant |
|
Netherlands |
X |
X |
X |
|
Belgium |
X |
X |
|
|
Denmark |
X |
X |
X |
|
Austria |
X |
||
|
Germany |
X |
X |
X |
|
United Kingdom |
X |
||
|
Italy |
X |
X |
X |
|
France |
X |
X |
|
|
Spain |
X |
||
|
Greece |
X |
Source: Hildebrandt and Schmidt 1994.
Pollution Remediation: Cleaning Up
Cleaning up contaminated sites is a procedure which has become increasingly evident and costly since the 1970s, when awareness was enhanced about the serious cases of soil and water contamination from accumulated chemical wastes, abandoned industrial sites and so on. These contaminated sites have been generated from such activities as the following:
- waste disposal sites (industrial and public)
- abandoned industrial sites (e.g., chemical, metal processing)
- mining activities
- agricultural sites
- major accidents
- incinerator sites
- industrial water discharges
- small and medium enterprise zones.
The design of a remediation/clean-up plan requires complex technical activities and procedures which must be accompanied by the definition of clear management responsibilities and consequent liability. Such initiatives should be carried out in the context of harmonized national legislation, and provide for the participation of interested populations, for the definition of clear conflict resolution procedures and for the avoidance of possible socio-environmental dumping effects. Such regulations, agreements and plans should clearly encompass not only natural biotic and abiotic resources such as water, air, soil or flora and fauna but should also include cultural heritage, other visual aspects of landscapes and damage to physical persons and properties. A restrictive definition of environment will consequently reduce the definition of environmental damage and therefore limit actual remediation of sites. At the same time, it should also be possible not only for the subjects directly affected by damages to be granted certain rights and protection, but it also should be possible for collective group action to be taken to protect collective interests in order to ensure the restoration of previous conditions.
Conclusion
Significant action will be required to respond to our rapidly changing environmental situation. The focus of this article has been on the need for action to be taken to improve the environmental performance of industry and other economic activities. To do this efficiently and effectively, workers and their trade unions must play an active role not only at the enterprise level, but as well within their local communities and at the national level. Workers must be seen and actively mobilized as key partners in meeting future environment and sustainable development objectives. The ability of workers and their trade unions to contribute as partners in this process of environmental management is not dependent simply on their own capacity and awareness—although efforts are indeed needed and underway to increase their capacity—but it will also depend on the commitment of management and communities to create an enabling environment which promotes the development of new forms of collaboration and participation in the future.
Environmental Pollution Control: Making Pollution Prevention a Corporate Priority
Seeing the possibilities and making them happen is what pollution prevention is all about. It is a commitment to products and processes that have a minimal impact on the environment.
Pollution prevention is not a new idea. It is the manifestation of an environmental ethic that was practised by the original inhabitants of many cultures, including Native Americans. They lived in harmony with their environment. It was the source of their shelter, their food and the very foundation of their religion. Although their environment was exceedingly harsh, it was treated with honour and respect.
As nations developed and the Industrial Revolution advanced, a very different attitude toward the environment emerged. Society came to view the environment as an endless source of raw materials and a convenient dumping ground for wastes.
Early Efforts to Reduce Waste
Even so, some industries have practised a type of pollution prevention since the first chemical processes were developed. Initially, industry focused on efficiency or increasing process yield through waste reduction, rather than specifically preventing pollution by keeping wastes from entering the environment. However, the end result of both activities is the same—less material waste is released to the environment.
An early example of pollution prevention under another guise was practised in a German sulphuric acid production facility during the 1800s. Process improvements at the plant reduced the amount of sulphur dioxide emitted per pound of product produced. These actions were most likely labelled as efficiency or quality improvements. Only recently has the concept of pollution prevention been directly associated with this type of process change.
Pollution prevention as we know it today began to emerge in the mid-1970s in response to the growing volume and complexity of environmental requirements. The US Environmental Protection Agency (EPA) was created then. The first efforts at pollution reduction were mostly installations of end-of-pipe or costly add-on pollution control equipment. Eliminating the source of a pollution problem was not a priority. When it occurred, it was more a matter of profit or efficiency than an organized effort to protect the environment.
Only recently have businesses adopted a more specific environmental point of view and kept track of progress. However, the processes by which businesses approach pollution prevention can differ significantly.
Prevention versus Control
In time, the focus began to change from pollution control to pollution prevention. It became apparent that the scientists who invent the products, engineers who design the equipment, process experts who operate the manufacturing facilities, marketers who work with customers to improve product environmental performance, sales representatives who bring environmental concerns from customers back to the laboratory for solutions and office employees who work to reduce paper usage all can help reduce the environmental impact of operations or activities under their control.
Developing effective pollution prevention programmes
In state-of-the-art pollution prevention, pollution prevention programmes as well as specific pollution prevention technologies must be examined. Both the overall pollution prevention programme and the individual pollution prevention technologies are equally important in achieving environmental benefit. While the development of technologies is an absolute requirement, without the organizational structure to support and implement those technologies, the environmental benefits will never be fully achieved.
The challenge is to obtain total corporate participation in pollution prevention. Some companies have implemented pollution prevention at every level of their organization through well organized, detailed programmes. Perhaps the three most widely recognized of these in the United States are 3M’s Pollution Prevention Pays (3P) programme, Chevron’s Save Money and Reduce Toxics (SMART) and Dow Chemical’s Waste Reduction Always Pays (WRAP).
The goal of such programmes is to reduce waste as much as technologically possible. But relying on source reduction alone is not always technically feasible. Recycling and reuse also must be part of the pollution prevention effort, as they are in the above programmes. When every employee is asked not only to make processes as efficient as possible, but also to find a productive use for every by-product or residual stream, pollution prevention becomes an integral part of the corporate culture.
In late 1993, The Business Roundtable in the US released the results of a pollution prevention benchmark study of successful efforts. The study identified best-in-class facility pollution prevention programmes and highlighted elements necessary to fully integrate pollution prevention into company operations. Included were facilities from Proctor & Gamble (P&G), Intel, DuPont, Monsanto, Martin Marietta and 3M.
Pollution prevention initiatives
The study found that successful pollution prevention programmes in these companies shared the following elements:
- top management support
- involvement of all employees
- recognition of accomplishments
- facilities had freedom to choose the best method to reach corporate goals
- transfer of information between facilities
- measurement of results
- all included recycling and reuse of waste.
In addition, the study found that each of the facilities had advanced from concentrating on pollution prevention in the manufacturing process to integrating pollution prevention in pre-manufacturing decisions. Pollution prevention had become a core corporate value.
Top management support is a necessity for a fully operational pollution prevention programme. Top officials at both the corporate and facility levels must send a strong message to all employees that pollution prevention is an integral part of their jobs. This must begin at the chief executive officer (CEO) level because that person sets the tone for all corporate activities. Speaking out publicly and within the company gets the message heard.
The second reason for success is employee involvement. Technical and manufacturing people are most involved in develop-ing new processes or product formulations. But employees in every position can be involved in waste reduction through reuse, reclamation and recycling as part of pollution prevention. Employees know the possibilities in their area of responsibility much better than environmental professionals. In order to spur employee involvement, the company must educate employees about the challenge the company faces. For example, articles on environmental issues in the corporate newsletter can increase employee awareness.
Recognition of accomplishments can be done in many ways. The CEO of 3M presents a special environmental leadership award not only to employees who contribute to the company’s goals, but also to those who contribute to community environmental efforts. In addition, environmental achievements are recognized in annual performance reviews.
Measuring results is extremely important because that is the driving force for employee action. Some facilities and corporate programmes measure all wastes, while others focus on Toxic Release Inventory (TRI) emissions or on other measurements which best fit within their corporate culture and their specific pollution prevention programmes.
Environmental Programme Examples
Over the course of 20 years, pollution prevention has become imbedded in 3M’s culture. 3M management pledged to go beyond government regulations, in part by developing environmental management plans that merge environmental goals with business strategy. The 3P programme focused on preventing pollution, not control.
The idea is to stop pollution before it starts, and seek out prevention opportunities at all stages of a product’s life, not just at the end. Successful companies recognize that prevention is more environmentally effective, more technically sound and less costly than conventional control procedures, which do not eliminate the problem. Pollution prevention is economical, because if pollution is avoided in the first place, it does not have to be dealt with later.
3M employees have developed and implemented more than 4,200 pollution prevention projects since the inception of the 3P programme. Over the past 20 years, these projects have resulted in the elimination of more than 1.3 billion pounds of pollutants and saved the company $750 million.
Between 1975 and 1993, 3M reduced the amount of energy needed per unit of production by 3,900 BTUs, or 58%. The annual energy savings for 3M in the United States alone totals 22 trillion BTUs each year. This is enough energy to heat, cool and light more than 200,000 homes in the United States and eliminates more than 2 million tons of carbon dioxide. And in 1993, 3M facilities in the United Sates recovered and recycled more solid waste (199 million pounds) than they sent to landfills (198 million pounds).
Pollution Prevention Technologies
The concept of designing for the environment is becoming important, but technologies used for pollution prevention are as diverse as the companies themselves. In general, this concept can be realized through technical innovation in four areas:
- product reformulation—developing nonpolluting or lesspolluting products or processes by using different raw materials
- process modification—changing manufacturing processes so they become nonpolluting or less polluting
- equipment redesign—modifying equipment to perform better under specific operating conditions or to make use of available resources
- resource recovery—recycling by-products for sale or for use by other companies or for use in the company’s other products or processes.
Concentrated efforts in each of these areas can mean new and safer products, cost savings and greater customer satisfaction.
Product reformulation can be the most difficult. Many of the attributes which make materials ideal for their intended uses may also contribute to problems for the environment. One example of product reformulation led a team of scientists to eliminate the ozone-depleting chemical methyl chloroform from a fabric protector product. This new water-based product greatly reduces the use of solvents and gives the company a competitive edge in the marketplace.
In making medication tablets for the pharmaceutical industry, employees developed a new water-based coating solution for the solvent-based coating solution that had been used to coat the tablets. The change cost $60,000, but eliminated the need to spend $180,000 for pollution control equipment, saves $150,000 in material cost and prevents 24 tons a year of air pollution.
An example of process modification resulted in a move away from hazardous chemicals to thoroughly clean copper sheeting prior to using it to make electric products. In the past, the sheeting was cleaned by a spray with ammonium persulphate, phosphoric acid and sulphuric acid—all hazardous chemicals. This procedure has been replaced by one that employs a light citric acid solution, a nonhazardous chemical. The process change eliminated the generation of 40,000 pounds of hazardous waste per year and saves the company about $15,000 per year in raw material and disposal costs.
Redesigning equipment also reduces waste. In the resin product area, a company regularly sampled a particular liquid phenolic resin by using a tap on the process flow line. Some of the product was wasted before and after the sample was collected. By installing a simple funnel under the sample tape and a pipe leading back to the process, the company now takes samples without any loss of product. This prevents about 9 tons of waste per year, saves about $22,000, increases the yield and decreases the disposal cost, all for a capital cost of about $1,000.
Resource recovery, the productive use of waste material, is extremely important in pollution prevention. One brand of wool soap pads is now made entirely of post-consumer recycled plastic soda bottles. In the first two years of this new product, the company used in excess of a million pounds of this recycled material to make soap pads. This is the equivalent of more than 10 million two-litre soda bottles. Also, waste rubber trimmed from floor mats in Brazil is used to make sandals. In 1994 alone, the plant recovered about 30 tons of material, enough to make more than 120,000 pairs of sandals.
In another example, Post-it(T) Recycled Paper Notes use 100% recycled paper. One ton of recycled paper alone saves 3 cubic yards of landfill space, 17 trees, 7,000 gallons of water and 4,100 kilowatt hours of energy, enough to heat the average home for six months.
Life-Cycle Analysis
Life-Cycle Analysis or a similar process is in place at every successful company. This means that each phase of a product’s life cycle from development through manufacturing, use and disposal offers opportunities for environmental improvement. The response to such environmental challenges has led to products with strong environmental claims throughout industry.
For example, P&G was the first commercial-goods manufacturer to develop concentrated detergents which require 50 to 60% smaller packaging than the previous formula. P&G also manufacturers refills for more than 57 brands in 22 countries. Refills typically cost less and save up to 70% in solid waste.
Dow has developed a new highly effective herbicide that is non-toxic. It is less risky for people and animals and is applied in ounces rather than pounds per acre. Using biotechnology, Monsanto developed a potato plant that is resistant to insects, so it reduced the need for chemical insecticides. Another herbicide from Monsanto helps restore the natural habitat of wetlands by controlling weeds in a safer way.
Commitment to a Cleaner Environment
It is critical that we approach pollution prevention on a comprehensive scale, including commitment to both programmatic and technological improvements. Increasing efficiency or process yield and reducing waste production has long been a practice of the manufacturing industry. However, only within the last decade have these activities focused more directly on pollution prevention. Substantial efforts are now aimed at improving source reduction as well as tailoring processes to separate, recycle and reuse by-products. All these are proven pollution prevention tools.
Environmental Pollution Control and Prevention
Over the course of the twentieth century, growing recognition of the environmental and public health impacts associated with anthropogenic activities (discussed in the chapter Environmental Health Hazards) has prompted the development and application of methods and technologies to reduce the effects of pollution. In this context, governments have adopted regulatory and other policy measures (discussed in the chapter Environmental Policy) to minimize negative effects and ensure that environmental quality standards are achieved.
The objective of this chapter is to provide an orientation to the methods that are applied to control and prevent environmental pollution. The basic principles followed for eliminating negative impacts on the quality of water, air or land will be introduced; the shifting emphasis from control to prevention will be considered; and the limitations of building solutions for individual environmental media will be examined. It is not enough, for example, to protect air by removing trace metals from a flue gas only to transfer these contaminants to land through improper solid waste management practices. Integrated multimedia solutions are required.
The Pollution Control Approach
The environmental consequences of rapid industrialization have resulted in countless incidents of land, air and water resources sites being contaminated with toxic materials and other pollutants, threatening humans and ecosystems with serious health risks. More extensive and intensive use of materials and energy has created cumulative pressures on the quality of local, regional and global ecosystems.
Before there was a concerted effort to restrict the impact of pollution, environmental management extended little beyond laissez-faire tolerance, tempered by disposal of wastes to avoid disruptive local nuisance conceived of in a short-term perspective. The need for remediation was recognized, by exception, in instances where damage was determined to be unacceptable. As the pace of industrial activity intensified and the understanding of cumulative effects grew, a pollution control paradigm became the dominant approach to environmental management.
Two specific concepts served as the basis for the control approach:
- the assimilative capacity concept, which asserts the existence of a specified level of emissions into the environment which does not lead to unacceptable environmental or human health effects
- the principle of control concept, which assumes that environmental damage can be avoided by controlling the manner, time and rate at which pollutants enter the environment
Under the pollution control approach, attempts to protect the environment have especially relied on isolating contaminants from the environment and using end-of-pipe filters and scrubbers. These solutions have tended to focus on media-specific environmental quality objectives or emission limits, and have been primarily directed at point source discharges into specific environmental media (air, water, soil).
Applying Pollution Control Technologies
Application of pollution control methods has demonstrated considerable effectiveness in controlling pollution problems - particularly those of a local character. Application of appropriate technologies is based on a systematic analysis of the source and nature of the emission or discharge in question, of its interaction with the ecosystem and the ambient pollution problem to be addressed, and the development of appropriate technologies to mitigate and monitor pollution impacts.
In their article on air pollution control, Dietrich Schwela and Berenice Goelzer explain the importance and implications of taking a comprehensive approach to assessment and control of point sources and non-point sources of air pollution. They also highlight the challenges - and opportunities - that are being addressed in countries that are undergoing rapid industrialization without having had a strong pollution control component accompanying earlier development.
Marion Wichman-Fiebig explains the methods that are applied to model air pollutant dispersion to determine and characterize the nature of pollution problems. This forms the basis for understanding the controls that are to be put into effect and for evaluating their effectiveness. As the understanding of potential impacts has deepened, appreciation of effects has expanded from the local to the regional to the global scale.
Hans-Ulrich Pfeffer and Peter Bruckmann provide an introduction to the equipment and methods that are used to monitor air quality so that potential pollution problems can be assessed and the effectiveness of control and prevention interventions can be evaluated.
John Elias provides an overview of the types of air pollution controls that can be applied and the issues that must be addressed in selecting appropriate pollution control management options.
The challenge of water pollution control is addressed by Herbert Preul in an article which explains the basis whereby the earth’s natural waters may become polluted from point, non-point and intermittent sources; the basis for regulating water pollution; and the different criteria that can be applied in determining control programmes. Preul explains the manner in which discharges are received in water bodies, and may be analysed and evaluated to assess and manage risks. Finally, an overview is provided of the techniques that are applied for large-scale wastewater treatment and water pollution control.
A case study provides a vivid example of how wastewater can be reused - a topic of considerable significance in the search for ways that environmental resources can be used effectively, especially in circumstances of scarcity. Alexander Donagi provides a summary of the approach that has been pursued for the treatment and groundwater recharge of municipal wastewater for a population of 1.5 million in Israel.
Comprehensive Waste Management
Under the pollution control perspective, waste is regarded as an undesirable by-product of the production process which is to be contained so as to ensure that soil, water and air resources are not contaminated beyond levels deemed to be acceptable. Lucien Maystre provides an overview of the issues that must be addressed in managing waste, providing a conceptual link to the increasingly important roles of recycling and pollution prevention.
In response to extensive evidence of the serious contamination associated with unrestricted management of waste, governments have established standards for acceptable practices for collection, handling and disposal to ensure environmental protection. Particular attention has been paid to the criteria for environmentally safe disposal through sanitary landfills, incineration and hazardous-waste treatment.
To avoid the potential environmental burden and costs associated with the disposal of waste and promote a more thorough stewardship of scarce resources, waste minimization and recycling have received growing attention. Niels Hahn and Poul Lauridsen provide a summary of the issues that are addressed in pursuing recycling as a preferred waste management strategy, and consider the potential worker exposure implications of this.
Shifting Emphasis to Pollution Prevention
End-of-pipe abatement risks transferring pollution from one medium to another, where it may either cause equally serious environmental problems, or even end up as an indirect source of pollution to the same medium. While not as expensive as remediation, end-of-pipe abatement can contribute significantly to the costs of production processes without contributing any value. It also typically is associated with regulatory regimes which add other sets of costs associated with enforcing compliance.
While the pollution control approach has achieved considerable success in producing short-term improvements for local pollution problems, it has been less effective in addressing cumulative problems that are increasingly recognized on regional (e.g., acid rain) or global (e.g., ozone depletion) levels.
The aim of a health-oriented environmental pollution control programme is to promote a better quality of life by reducing pollution to the lowest level possible. Environmental pollution control programmes and policies, whose implications and priorities vary from country to country, cover all aspects of pollution (air, water, land and so on) and involve coordination among areas such as industrial development, city planning, water resources development and transportation policies.
Thomas Tseng, Victor Shantora and Ian Smith provide a case study example of the multimedia impact that pollution has had on a vulnerable ecosystem subjected to many stresses - the North American Great Lakes. The limited effectiveness of the pollution control model in dealing with persistent toxins that dissipate through the environment is particularly examined. By focusing on the approach being pursued in one country and the implications that this has for international action, the implications for actions that address prevention as well as control are illustrated.
As environmental pollution control technologies have become more sophisticated and more expensive, there has been a growing interest in ways to incorporate prevention in the design of industrial processes - with the objective of eliminating harmful environmental effects while promoting the competitiveness of industries. Among the benefits of pollution prevention approaches, clean technologies and toxic use reduction is the potential for eliminating worker exposure to health risks.
David Bennett provides an overview of why pollution prevention is emerging as a preferred strategy and how it relates to other environmental management methods. This approach is central to implementing the shift to sustainable development which has been widely endorsed since the release of the United Nations Commission on Trade and Development in 1987 and reiterated at the Rio United Nations Conference on Environment and Development (UNCED) Conference in 1992.
The pollution prevention approach focuses directly on the use of processes, practices, materials and energy that avoid or minimize the creation of pollutants and wastes at source, and not on “add-on” abatement measures. While corporate commitment plays a critical role in the decision to pursue pollution prevention (see Bringer and Zoesel in Environmental policy), Bennett draws attention to the societal benefits in reducing risks to ecosystem and human health—and the health of workers in particular. He identifies principles that can be usefully applied in assessing opportunities for pursuing this approach.
Air Pollution Management
Air pollution management aims at the elimination, or reduction to acceptable levels, of airborne gaseous pollutants, suspended particulate matter and physical and, to a certain extent, biological agents whose presence in the atmosphere can cause adverse effects on human health (e.g., irritation, increase of incidence or prevalence of respiratory diseases, morbidity, cancer, excess mortality) or welfare (e.g., sensory effects, reduction of visibility), deleterious effects on animal or plant life, damage to materials of economic value to society and damage to the environment (e.g., climatic modifications). The serious hazards associated with radioactive pollutants, as well as the special procedures required for their control and disposal, also deserve careful attention.
The importance of efficient management of outdoor and indoor air pollution cannot be overemphasized. Unless there is adequate control, the multiplication of pollution sources in the modern world may lead to irreparable damage to the environment and mankind.
The objective of this article is to give a general overview of the possible approaches to the management of ambient air pollution from motor vehicle and industrial sources. However, it is to be emphasized from the very beginning that indoor air pollution (in particular, in developing countries) might play an even larger role than outdoor air pollution due to the observation that indoor air pollutant concentrations are often substantially higher than outdoor concentrations.
Beyond considerations of emissions from fixed or mobile sources, air pollution management involves consideration of additional factors (such as topography and meteorology, and community and government participation, among many others) all of which must be integrated into a comprehensive programme. For example, meteorological conditions can greatly affect the ground-level concentrations resulting from the same pollutant emission. Air pollution sources may be scattered over a community or a region and their effects may be felt by, or their control may involve, more than one administration. Furthermore, air pollution does not respect any boundaries, and emissions from one region may induce effects in another region by long-distance transport.
Air pollution management, therefore, requires a multidisciplinary approach as well as a joint effort by private and governmental entities.
Sources of Air Pollution
The sources of man-made air pollution (or emission sources) are of basically two types:
- stationary, which can be subdivided into area sources such as agricultural production, mining and quarrying, industrial, point and area sources such as manufacturing of chemicals, nonmetallic mineral products, basic metal industries, power generation and community sources (e.g., heating of homes and buildings, municipal waste and sewage sludge incinerators, fireplaces, cooking facilities, laundry services and cleaning plants)
- mobile, comprising any form of combustion-engine vehicles (e.g., light-duty gasoline powered cars, light- and heavy-duty diesel powered vehicles, motorcycles, aircraft, including line sources with emissions of gases and particulate matter from vehicle traffic).
In addition, there are also natural sources of pollution (e.g., eroded areas, volcanoes, certain plants which release great amounts of pollen, sources of bacteria, spores and viruses). Natural sources are not discussed in this article.
Types of Air Pollutants
Air pollutants are usually classified into suspended particulate matter (dusts, fumes, mists, smokes), gaseous pollutants (gases and vapours) and odours. Some examples of usual pollutants are presented below:
Suspended particulate matter (SPM, PM-10) includes diesel exhaust, coal fly-ash, mineral dusts (e.g., coal, asbestos, limestone, cement), metal dusts and fumes (e.g., zinc, copper, iron, lead) and acid mists (e.g., sulphuric acid), fluorides, paint pigments, pesticide mists, carbon black and oil smoke. Suspended particulate pollutants, besides their effects of provoking respiratory diseases, cancers, corrosion, destruction of plant life and so on, can also constitute a nuisance (e.g., accumulation of dirt), interfere with sunlight (e.g., formation of smog and haze due to light scattering) and act as catalytic surfaces for reaction of adsorbed chemicals.
Gaseous pollutants include sulphur compounds (e.g., sulphur dioxide (SO2) and sulphur trioxide (SO3)), carbon monoxide, nitrogen compounds (e.g., nitric oxide (NO), nitrogen dioxide (NO2), ammonia), organic compounds (e.g., hydrocarbons (HC), volatile organic compounds (VOC), polycyclic aromatic hydrocarbons (PAH), aldehydes), halogen compounds and halogen derivatives (e.g., HF and HCl), hydrogen sulphide, carbon disulphide and mercaptans (odours).
Secondary pollutants may be formed by thermal, chemical or photochemical reactions. For example, by thermal action sulphur dioxide can oxidize to sulphur trioxide which, dissolved in water, gives rise to the formation of sulphuric acid mist (catalysed by manganese and iron oxides). Photochemical reactions between nitrogen oxides and reactive hydrocarbons can produce ozone (O3), formaldehyde and peroxyacetyl nitrate (PAN); reactions between HCl and formaldehyde can form bis-chloromethyl ether.
While some odours are known to be caused by specific chemical agents such as hydrogen sulphide (H2S), carbon disulphide (CS2) and mercaptans (R-SH or R1-S-R2) others are difficult to define chemically.
Examples of the main pollutants associated with some industrial air pollution sources are presented in table 1 (Economopoulos 1993).
Table 1. Common atmospheric pollutants and their sources
|
Category |
Source |
Emitted pollutants |
|
Agriculture |
Open burning |
SPM, CO, VOC |
|
Mining and |
Coal mining Crude petroleum Non-ferrous ore mining Stone quarrying |
SPM, SO2, NOx, VOC SO2 SPM, Pb SPM |
|
Manufacturing |
Food, beverages and tobacco Textiles and leather industries Wood products Paper products, printing |
SPM, CO, VOC, H2S SPM, VOC SPM, VOC SPM, SO2, CO, VOC, H2S, R-SH |
|
Manufacture |
Phthalic anhydride Chlor-alkali Hydrochloric acid Hydrofluoric acid Sulphuric acid Nitric acid Phosphoric acid Lead oxide and pigments Ammonia Sodium carbonate Calcium carbide Adipic acid Alkyl lead Maleic anhydride and Fertilizer and Ammonium nitrate Ammonium sulphate Synthetic resins, plastic Paints, varnishes, lacquers Soap Carbon black and printing ink Trinitrotoluene |
SPM, SO2, CO, VOC Cl2 HCl HF, SiF4 SO2, SO3 NOx SPM, F2 SPM, Pb SPM, SO2, NOx, CO, VOC, NH3 SPM, NH3 SPM SPM, NOx, CO, VOC Pb CO, VOC SPM, NH3 SPM, NH3, HNO3 VOC SPM, VOC, H2S, CS2 SPM, VOC SPM SPM, SO2, NOx, CO, VOC, H2S SPM, SO2, NOx, SO3, HNO3 |
|
Petroleum refineries |
Miscellaneous products |
SPM, SO2, NOx, CO, VOC |
|
Non-metallic mineral |
Glass products Structural clay products Cement, lime and plaster |
SPM, SO2, NOx, CO, VOC, F SPM, SO2, NOx, CO, VOC, F2 SPM, SO2, NOx, CO |
|
Basic metal industries |
Iron and steel Non-ferrous industries |
SPM, SO2, NOx, CO, VOC, Pb SPM, SO2, F, Pb |
|
Power generation |
Electricity, gas and steam |
SPM, SO2, NOx, CO, VOC, SO3, Pb |
|
Wholesale and |
Fuel storage, filling operations |
VOC |
|
Transport |
SPM, SO2, NOx, CO, VOC, Pb |
|
|
Community services |
Municipal incinerators |
SPM, SO2, NOx, CO, VOC, Pb |
Source: Economopoulos 1993
Clean Air Implementation Plans
Air quality management aims at the preservation of environmental quality by prescribing the tolerated degree of pollution, leaving it to the local authorities and polluters to devise and implement actions to ensure that this degree of pollution will not be exceeded. An example of legislation within this approach is the adoption of ambient air quality standards based, very often, on air quality guidelines (WHO 1987) for different pollutants; these are accepted maximum levels of pollutants (or indicators) in the target area (e.g., at ground level at a specified point in a community) and can be either primary or secondary standards. Primary standards (WHO 1980) are the maximum levels consistent with an adequate safety margin and with the preservation of public health, and must be complied with within a specific time limit; secondary standards are those judged to be necessary for protection against known or anticipated adverse effects other than health hazards (mainly on vegetation) and must be complied “within a reasonable time”. Air quality standards are short-, medium- or long-term values valid for 24 hours per day, 7 days per week, and for monthly, seasonal or annual exposure of all living subjects (including sensitive subgroups such as children, the elderly and the sick) as well as non-living objects; this is in contrast to maximum permissible levels for occupational exposure, which are for a partial weekly exposure (e.g., 8 hours per day, 5 days per week) of adult and supposedly healthy workers.
Typical measures in air quality management are control measures at the source, for example, enforcement of the use of catalytic converters in vehicles or of emission standards in incinerators, land-use planning and shut-down of factories or reduction of traffic during unfavourable weather conditions. The best air quality management stresses that the air pollutant emissions should be kept to a minimum; this is basically defined through emission standards for single sources of air pollution and could be achieved for industrial sources, for example, through closed systems and high-efficiency collectors. An emission standard is a limit on the amount or concentration of a pollutant emitted from a source. This type of legislation requires a decision, for each industry, on the best means of controlling its emissions (i.e., fixing emission standards).
The basic aim of air pollution management is to derive a clean air implementation plan (or air pollution abatement plan) (Schwela and Köth-Jahr 1994) which consists of the following elements:
- description of area with respect to topography, meteorology and socioeconomy
- emissions inventory
- comparison with emission standards
- air pollutant concentrations inventory
- simulated air pollutant concentrations
- comparison with air quality standards
- inventory of effects on public health and the environment
- causal analysis
- control measures
- cost of control measures
- cost of public health and environmental effects
- cost-benefit analysis (costs of control vs. costs of efforts)
- transportation and land-use planning
- enforcement plan; resource commitment
- projections for the future on population, traffic, industries and fuel consumption
- strategies for follow-up.
Some of these issues will be described below.
Emissions Inventory; Comparison with Emission Standards
The emissions inventory is a most complete listing of sources in a given area and of their individual emissions, estimated as accurately as possible from all emitting point, line and area (diffuse) sources. When these emissions are compared with emission standards set for a particular source, first hints on possible control measures are given if emission standards are not complied with. The emissions inventory also serves to assess a priority list of important sources according to the amount of pollutants emitted, and indicates the relative influence of different sources—for example, traffic as compared to industrial or residential sources. The emissions inventory also allows an estimate of air pollutant concentrations for those pollutants for which ambient concentration measurements are difficult or too expensive to perform.
Air Pollutant Concentrations Inventory; Comparison with Air Quality Standards
The air pollutant concentrations inventory summarizes the results of the monitoring of ambient air pollutants in terms of annual means, percentiles and trends of these quantities. Compounds measured for such an inventory include the following:
- sulphur dioxide
- nitrogen oxides
- suspended particulate matter
- carbon monoxide
- ozone
- heavy metals (Pb, Cd, Ni, Cu, Fe, As, Be)
- polycyclic aromatic hydrocarbons: benzo(a)pyrene, benzo(e)pyrene, benzo(a)anthracene, dibenzo(a,h)anthracene, benzoghi)perylene, coronen
- volatile organic compounds: n-hexane, benzene, 3-methyl-hexane, n-heptane, toluene, octane, ethyl-benzene xylene (o-,m-,p-), n-nonane, isopropylbenzene, propylbenezene, n-2-/3-/4-ethyltoluene, 1,2,4-/1,3,5-trimethylbenzene, trichloromethane, 1,1,1 trichloroethane, tetrachloromethane, tri-/tetrachloroethene.
Comparison of air pollutant concentrations with air quality standards or guidelines, if they exist, indicates problem areas for which a causal analysis has to be performed in order to find out which sources are responsible for the non-compliance. Dispersion modelling has to be used in performing this causal analysis (see “Air pollution: Modelling of air pollutant dispersion”). Devices and procedures used in today’s ambient air pollution monitoring are described in “Air quality monitoring”.
Simulated Air Pollutant Concentrations; Comparison with Air Quality Standards
Starting from the emissions inventory, with its thousands of compounds which cannot all be monitored in the ambient air for economy reasons, use of dispersion modelling can help to estimate the concentrations of more “exotic” compounds. Using appropriate meteorology parameters in a suitable dispersion model, annual averages and percentiles can be estimated and compared to air quality standards or guidelines, if they exist.
Inventory of Effects on Public Health and the Environment; Causal Analysis
Another important source of information is the effects inventory (Ministerium für Umwelt 1993), which consists of results of epidemiological studies in the given area and of effects of air pollution observed in biological and material receptors such as, for example, plants, animals and construction metals and building stones. Observed effects attributed to air pollution have to be causally analysed with respect to the component responsible for a particular effect—for example, increased prevalence of chronic bronchitis in a polluted area. If the compound or compounds have been fixed in a causal analysis (compound-causal analysis), a second analysis has to be performed to find out the responsible sources (source-causal analysis).
Control Measures; Cost of Control Measures
Control measures for industrial facilities include adequate, well-designed, well-installed, efficiently operated and maintained air cleaning devices, also called separators or collectors. A separator or collector can be defined as an “apparatus for separating any one or more of the following from a gaseous medium in which they are suspended or mixed: solid particles (filter and dust separators), liquid particles (filter and droplet separator) and gases (gas purifier)”. The basic types of air pollution control equipment (discussed further in “Air pollution control”) are the following:
- for particulate matter: inertial separators (e.g., cyclones); fabric filters (baghouses); electrostatic precipitators; wet collectors (scrubbers)
- for gaseous pollutants: wet collectors (scrubbers); adsorption units (e.g., adsorption beds); afterburners, which can be direct-fired (thermal incineration) or catalytic (catalytic combustion).
Wet collectors (scrubbers) can be used to collect, at the same time, gaseous pollutants and particulate matter. Also, certain types of combustion devices can burn combustible gases and vapours as well as certain combustible aerosols. Depending on the type of effluent, one or a combination of more than one collector can be used.
The control of odours that are chemically identifiable relies on the control of the chemical agent(s) from which they emanate (e.g., by absorption, by incineration). However, when an odour is not defined chemically or the producing agent is found at extremely low levels, other techniques may be used, such as masking (by a stronger, more agreeable and harmless agent) or counteraction (by an additive which counteracts or partially neutralizes the offensive odour).
It should be kept in mind that adequate operation and maintenance are indispensable to ensure the expected efficiency from a collector. This should be ensured at the planning stage, both from the know-how and financial points of view. Energy requirements must not be overlooked. Whenever selecting an air cleaning device, not only the initial cost but also operational and maintenance costs should be considered. Whenever dealing with high-toxicity pollutants, high efficiency should be ensured, as well as special procedures for maintenance and disposal of waste materials.
The fundamental control measures in industrial facilities are the following:
Substitution of materials. Examples: substitution of less toxic solvents for highly toxic ones used in certain industrial processes; use of fuels with lower sulphur content (e.g., washed coal), therefore giving rise to less sulphur compounds and so on.
Modification or change of the industrial process or equipment. Examples: in the steel industry, a change from raw ore to pelleted sintered ore (to reduce the dust released during ore handling); use of closed systems instead of open ones; change of fuel heating systems to steam, hot water or electrical systems; use of catalysers at the exhaust air outlets (combustion processes) and so on.
Modifications in processes, as well as in plant layout, may also facilitate and/or improve the conditions for dispersion and collection of pollutants. For example, a different plant layout may facilitate the installation of a local exhaust system; the performance of a process at a lower rate may allow the use of a certain collector (with volume limitations but otherwise adequate). Process modifications that concentrate different effluent sources are closely related to the volume of effluent handled, and the efficiency of some air-cleaning equipment increases with the concentration of pollutants in the effluent. Both the substitution of materials and the modification of processes may have technical and/or economic limitations, and these should be considered.
Adequate housekeeping and storage. Examples: strict sanitation in food and animal product processing; avoidance of open storage of chemicals (e.g., sulphur piles) or dusty materials (e.g., sand), or, failing this, spraying of the piles of loose particulate with water (if possible) or application of surface coatings (e.g., wetting agents, plastic) to piles of materials likely to give off pollutants.
Adequate disposal of wastes. Examples: avoidance of simply piling up chemical wastes (such as scraps from polymerization reactors), as well as of dumping pollutant materials (solid or liquid) in water streams. The latter practice not only causes water pollution but can also create a secondary source of air pollution, as in the case of liquid wastes from sulphite process pulp mills, which release offensive odorous gaseous pollutants.
Maintenance. Example: well maintained and well-tuned internal combustion engines produce less carbon monoxide and hydrocarbons.
Work practices. Example: taking into account meteorological conditions, particularly winds, when spraying pesticides.
By analogy with adequate practices at the workplace, good practices at the community level can contribute to air pollution control - for example, changes in the use of motor vehicles (more collective transportation, small cars and so on) and control of heating facilities (better insulation of buildings in order to require less heating, better fuels and so on).
Control measures in vehicle emissions are adequate and efficient mandatory inspection and maintenance programmes which are enforced for the existing car fleet, programmes of enforcement of the use of catalytic converters in new cars, aggressive substitution of solar/battery-powered cars for fuel-powered ones, regulation of road traffic, and transportation and land use planning concepts.
Motor vehicle emissions are controlled by controlling emissions per vehicle mile travelled (VMT) and by controlling VMT itself (Walsh 1992). Emissions per VMT can be reduced by controlling vehicle performance - hardware, maintenance - for both new and in-use cars. Fuel composition of leaded gasoline may be controlled by reducing lead or sulphur content, which also has a beneficial effect on decreasing HC emissions from vehicles. Lowering the levels of sulphur in diesel fuel as a means to lower diesel particulate emission has the additional beneficial effect of increasing the potential for catalytic control of diesel particulate and organic HC emissions.
Another important management tool for reducing vehicle evaporative and refuelling emissions is the control of gasoline volatility. Control of fuel volatility can greatly lower vehicle evaporative HC emissions. Use of oxygenated additives in gasoline lowers HC and CO exhaust as long as fuel volatility is not increased.
Reduction of VMT is an additional means of controlling vehicle emissions by control strategies such as
- use of more efficient transportation modes
- increasing the average number of passengers per car
- spreading congested peak traffic loads
- reducing travel demand.
While such approaches promote fuel conservation, they are not yet accepted by the general population, and governments have not seriously tried to implement them.
All these technological and political solutions to the motor vehicle problem except substitution of electrical cars are increasingly offset by growth in the vehicle population. The vehicle problem can be solved only if the growth problem is addressed in an appropriate way.
Cost of Public Health and Environmental Effects; Cost-Benefit Analysis
The estimation of the costs of public health and environmental effects is the most difficult part of a clean air implementation plan, as it is very difficult to estimate the value of lifetime reduction of disabling illnesses, hospital admission rates and hours of work lost. However, this estimation and a comparison with the cost of control measures is absolutely necessary in order to balance the costs of control measures versus the costs of no such measure undertaken, in terms of public health and environmental effects.
Transportation and Land-Use Planning
The pollution problem is intimately connected to land-use and transportation, including issues such as community planning, road design, traffic control and mass transportation; to concerns of demography, topography and economy; and to social concerns (Venzia 1977). In general, the rapidly growing urban aggregations have severe pollution problems due to poor land-use and transportation practices. Transportation planning for air pollution control includes transportation controls, transportation policies, mass transit and highway congestion costs. Transportation controls have an important impact on the general public in terms of equity, repressiveness and social and economic disruption - in particular, direct transportation controls such as motor vehicle constraints, gasoline limitations and motor vehicle emission reductions. Emission reductions due to direct controls can be reliably estimated and verified. Indirect transportation controls such as reduction of vehicle miles travelled by improvement of mass transit systems, traffic flow improvement regulations, regulations on parking lots, road and gasoline taxes, car-use permissions and incentives for voluntary approaches are mostly based on past trial-and-error experience, and include many uncertainties when trying to develop a viable transportation plan.
National action plans incurring indirect transportation controls can affect transportation and land-use planning with regard to highways, parking lots and shopping centres. Long-term planning for the transportation system and the area influenced by it will prevent significant deterioration of air quality and provide for compliance with air quality standards. Mass transit is consistently considered as a potential solution for urban air pollution problems. Selection of a mass transit system to serve an area and different modal splits between highway use and bus or rail service will ultimately alter land-use patterns. There is an optimum split that will minimize air pollution; however, this may not be acceptable when non-environmental factors are considered.
The automobile has been called the greatest generator of economic externalities ever known. Some of these, such as jobs and mobility, are positive, but the negative ones, such as air pollution, accidents resulting in death and injury, property damage, noise, loss of time, and aggravation, lead to the conclusion that transportation is not a decreasing cost industry in urbanized areas. Highway congestion costs are another externality; lost time and congestion costs, however, are difficult to determine. A true evaluation of competing transportation modes, such as mass transportation, cannot be obtained if travel costs for work trips do not include congestion costs.
Land-use planning for air pollution control includes zoning codes and performance standards, land-use controls, housing and land development, and land-use planning policies. Land-use zoning was the initial attempt to accomplish protection of the people, their property and their economic opportunity. However, the ubiquitous nature of air pollutants required more than physical separation of industries and residential areas to protect the individual. For this reason, performance standards based initially on aesthetics or qualitative decisions were introduced into some zoning codes in an attempt to quantify criteria for identifying potential problems.
The limitations of the assimilative capacity of the environment must be identified for long-term land-use planning. Then, land-use controls can be developed that will prorate the capacity equitably among desired local activities. Land-use controls include permit systems for review of new stationary sources, zoning regulation between industrial and residential areas, restriction by easement or purchase of land, receptor location control, emission-density zoning and emission allocation regulations.
Housing policies aimed at making home ownership available to many who could otherwise not afford it (such as tax incentives and mortgage policies) stimulate urban sprawl and indirectly discourage higher-density residential development. These policies have now proven to be environmentally disastrous, as no consideration was given to the simultaneous development of efficient transportation systems to serve the needs of the multitude of new communities being developed. The lesson learnt from this development is that programmes impacting on the environment should be coordinated, and comprehensive planning undertaken at the level where the problem occurs and on a scale large enough to include the entire system.
Land-use planning must be examined at national, provincial or state, regional and local levels to adequately ensure long-term protection of the environment. Governmental programmes usually start with power plant siting, mineral extraction sites, coastal zoning and desert, mountain or other recreational development. As the multiplicity of local governments in a given region cannot adequately deal with regional environmental problems, regional governments or agencies should coordinate land development and density patterns by supervising the spatial arrangement and location of new construction and use, and transportation facilities. Land-use and transportation planning must be interrelated with enforcement of regulations to maintain the desired air quality. Ideally, air pollution control should be planned for by the same regional agency that does land-use planning because of the overlapping externalities associated with both issues.
Enforcement Plan, Resource Commitment
The clean air implementation plan should always contain an enforcement plan which indicates how the control measures can be enforced. This implies also a resource commitment which, according to a polluter pays principle, will state what the polluter has to implement and how the government will help the polluter in fulfilling the commitment.
Projections for the Future
In the sense of a precautionary plan, the clean air implementation plan should also include estimates of the trends in population, traffic, industries and fuel consumption in order to assess responses to future problems. This will avoid future stresses by enforcing measures well in advance of imagined problems.
Strategies for Follow-up
A strategy for follow-up of air quality management consists of plans and policies on how to implement future clean air implementation plans.
Role of Environmental Impact Assessment
Environmental impact assessment (EIA) is the process of providing a detailed statement by the responsible agency on the environmental impact of a proposed action significantly affecting the quality of the human environment (Lee 1993). EIA is an instrument of prevention aiming at consideration of the human environment at an early stage of the development of a programme or project.
EIA is particularly important for countries which develop projects in the framework of economic reorientation and restructuring. EIA has become legislation in many developed countries and is now increasingly applied in developing countries and economies in transition.
EIA is integrative in the sense of comprehensive environmental planning and management considering the interactions between different environmental media. On the other hand, EIA integrates the estimation of environmental consequences into the planning process and thereby becomes an instrument of sustainable development. EIA also combines technical and participative properties as it collects, analyses and applies scientific and technical data with consideration of quality control and quality assurance, and stresses the importance of consultations prior to licensing procedures between environmental agencies and the public which could be affected by particular projects. A clean air implementation plan can be considered as a part of the EIA procedure with reference to the air.
Air Pollution: Modelling of Air Pollutant Dispersion
The aim of air pollution modelling is the estimation of outdoor pollutant concentrations caused, for instance, by industrial production processes, accidental releases or traffic. Air pollution modelling is used to ascertain the total concentration of a pollutant, as well as to find the cause of extraordinary high levels. For projects in the planning stage, the additional contribution to the existing burden can be estimated in advance, and emission conditions may be optimized.
Figure 1. Global Environmental Monitoring System/Air pollution management
Depending on the air quality standards defined for the pollutant in question, annual mean values or short-time peak concentrations are of interest. Usually concentrations have to be determined where people are active - that is, near the surface at a height of about two metres above the ground.
Parameters Influencing Pollutant Dispersion
Two types of parameters influence pollutant dispersion: source parameters and meteorological parameters. For source parameters, concentrations are proportional to the amount of pollutant which is emitted. If dust is concerned, the particle diameter has to be known to determine sedimentation and deposition of the material (VDI 1992). As surface concentrations are lower with greater stack height, this parameter also has to be known. In addition, concentrations depend on the total amount of the exhaust gas, as well as on its temperature and velocity. If the temperature of the exhaust gas exceeds the temperature of the surrounding air, the gas will be subject to thermal buoyancy. Its exhaust velocity, which can be calculated from the inner stack diameter and the exhaust gas volume, will cause a dynamic momentum buoyancy. Empirical formulae may be used to describe these features (VDI 1985; Venkatram and Wyngaard 1988). It has to be stressed that it is not the mass of the pollutant in question but that of the total gas that is responsible for the thermal and dynamic momentum buoyancy.
Meteorological parameters which influence pollutant dispersion are wind speed and direction, as well as vertical thermal stratification. The pollutant concentration is proportional to the reciprocal of wind speed. This is mainly due to the accelerated transport. Moreover, turbulent mixing increases with growing wind speed. As so-called inversions (i.e., situations where temperature is increasing with height) hinder turbulent mixing, maximum surface concentrations are observed during highly stable stratification. On the contrary, convective situations intensify vertical mixing and therefore show the lowest concentration values.
Air quality standards - for example, annual mean values or 98 percentiles - are usually based on statistics. Hence, time series data for the relevant meteorological parameters are needed. Ideally, statistics should be based on ten years of observation. If only shorter time series are available, it should be ascertained that they are representative for a longer period. This can be done, for example, by analysis of longer time series from other observations sites.
The meteorological time series used also has to be representative of the site considered - that is, it must reflect the local characteristics. This is specially important concerning air quality standards based on peak fractions of the distribution, like 98 percentiles. If no such time series is at hand, a meteorological flow model may be used to calculate one from other data, as will be described below.
International Monitoring Programmes
International agencies such as the World Health Organization (WHO), the World Meteorological Organization (WMO) and the United Nations Environment Programme (UNEP) have instituted monitoring and research projects in order to clarify the issues involved in air pollution and to promote measures to prevent further deterioration of public health and environmental and climatic conditions.
The Global Environmental Monitoring System GEMS/Air (WHO/ UNEP 1993) is organized and sponsored by WHO and UNEP and has developed a comprehensive programme for providing the instruments of rational air pollution management (see figure 55.1.[EPC01FE] The kernel of this programme is a global database of urban air pollutant concentrations of sulphur dioxides, suspended particulate matter, lead, nitrogen oxides, carbon monoxide and ozone. As important as this database, however, is the provision of management tools such as guides for rapid emission inventories, programmes for dispersion modelling, population exposure estimates, control measures, and cost-benefit analysis. In this respect, GEMS/Air provides methodology review handbooks (WHO/UNEP 1994, 1995), conducts global assessments of air quality, facilitates review and validation of assessments, acts as a data/information broker, produces technical documents in support of all aspects of air quality management, facilitates the establishment of monitoring, conducts and widely distributes annual reviews, and establishes or identifies regional collaboration centres and/or experts to coordinate and support activities according to the needs of the regions. (WHO/UNEP 1992, 1993, 1995)The Global Atmospheric Watch (GAW) programme (Miller and Soudine 1994) provides data and other information on the chemical composition and related physical characteristics of the atmosphere, and their trends, with the objective of understanding the relationship between changing atmospheric composition and changes of global and regional climate, the long-range atmospheric transport and deposition of potentially harmful substances over terrestrial, fresh-water and marine ecosystems, and the natural cycling of chemical elements in the global atmosphere/ocean/biosphere system, and anthropogenic impacts thereon. The GAW programme consists of four activity areas: the Global Ozone Observing System (GO3OS), global monitoring of background atmospheric composition, including the Background Air Pollution Monitoring Network (BAPMoN); dispersion, transport, chemical transformation and deposition of atmospheric pollutants over land and sea on different time and space scales; exchange of pollutants between the atmosphere and other environmental compartments; and integrated monitoring. One of the most important aspects of the GAW is the establishment of Quality Assurance Science Activity Centres to oversee the quality of the data produced under GAW.
Concepts of Air Pollution Modelling
As mentioned above, dispersion of pollutants is dependent on emission conditions, transport and turbulent mixing. Using the full equation which describes these features is called Eulerian dispersion modelling (Pielke 1984). By this approach, gains and losses of the pollutant in question have to be determined at every point on an imaginary spatial grid and in distinct time steps. As this method is very complex and computer time consuming, it usually cannot be handled routinely. However, for many applications, it may be simplified using the following assumptions:
- no change of emission conditions with time
- no change of meteorological conditions during transport
- wind speeds above 1 m/s.
In this case, the equation mentioned above can be solved analytically. The resulting formula describes a plume with Gaussian concentration distribution, the so called Gaussian plume model (VDI 1992). The distribution parameters depend on meteorological conditions and downwind distance as well as on stack height. They have to be determined empirically (Venkatram and Wyngaard 1988). Situations where emissions and/or meteorological parameters vary by a considerable amount in time and/or space may be described by the Gaussian puff model (VDI 1994). Under this approach, distinct puffs are emitted in fixed time steps, each following its own path according to the current meteorological conditions. On its way, each puff grows according to turbulent mixing. Parameters describing this growth, again, have to be determined from empirical data (Venkatram and Wyngaard 1988). It has to be stressed, however, that to achieve this objective, input parameters must be available with the necessary resolution in time and/or space.
Concerning accidental releases or single case studies, a Lagrangian or particle model (VDI Guideline 3945, Part 3) is recommended. The concept thereby is to calculate the paths of many particles, each of which represents a fixed amount of the pollutant in question. The individual paths are composed of transport by the mean wind and of stochastic disturbances. Due to the stochastic part, the paths do not fully agree, but depict the mixture by turbulence. In principle, Lagrangian models are capable of considering complex meteorological conditions - in particular, wind and turbulence; fields calculated by flow models described below can be used for Lagrangian dispersion modelling.
Dispersion Modelling in Complex Terrain
If pollutant concentrations have to be determined in structured terrain, it may be necessary to include topographic effects on pollutant dispersion in modelling. Such effects are, for example, transport following the topographic structure, or thermal wind systems like sea breezes or mountain winds, which change wind direction in the course of the day.
If such effects take place on a scale much larger than the model area, the influence may be considered by using meteorological data which reflect the local characteristics. If no such data are available, the three-dimensional structure impressed on the flow by topography can be obtained by using a corresponding flow model. Based on these data, dispersion modelling itself may be carried out assuming horizontal homogeneity as described above in the case of the Gaussian plume model. However, in situations where wind conditions change significantly inside the model area, dispersion modelling itself has to consider the three-dimensional flow affected by the topographic structure. As mentioned above, this may be done by using a Gaussian puff or a Lagrangian model. Another way is to perform the more complex Eulerian modelling.
To determine wind direction in accord with the topographically structured terrain, mass consistent or diagnostic flow modelling may be used (Pielke 1984). Using this approach, the flow is fitted to topography by varying the initial values as little as possible and by keeping its mass consistent. As this is an approach which leads to quick results, it may also be used to calculate wind statistics for a certain site if no observations are available. To do this, geostrophic wind statistics (i.e., upper air data from rawinsondes) are used.
If, however, thermal wind systems have to be considered in more detail, so called prognostic models have to be used. Depending on the scale and the steepness of the model area, a hydrostatic, or the even more complex non-hydrostatic, approach is suitable (VDI 1981). Models of this type need much computer power, as well as much experience in application. Determination of concentrations based on annual means, in general, are not possible with these models. Instead, worst case studies can be performed by considering only one wind direction and those wind speed and stratification parameters which result in the highest surface concentration values. If those worst case values do not exceed air quality standards, more detailed studies are not necessary.
Figure 2. Topographic structure of a model region
Figure 2, figure 3 and figure 4 demonstrate how the transport and dispension of pollutants can be presented in relation to the influence of terrain and wind climatologies derived from consideration of surface and geostrophic wind frequencies.
Figure 3. Surface frequency distributions as determined from geostrophic frequency distribution
Figure 4. Annual mean pollutant concentrations for a hypothetical region calculated from the geostrophic frequency distribution for heterogeneous wind fields
Dispersion Modelling in Case of Low Sources
Considering air pollution caused by low sources (i.e., stack heights on the order of building height or emissions of road traffic) the influence of the surrounding buildings has to be considered. Road traffic emissions will be trapped to a certain amount in street canyons. Empirical formulations have been found to describe this (Yamartino and Wiegand 1986).
Pollutants emitted from a low stack situated on a building will be captured in the circulation on the lee side of the building. The extent of this lee circulation depends on the height and width of the building, as well as on wind speed. Therefore, simplified approaches to describe pollutant dispersion in such a case, based solely on the height of a building, are not generally valid. The vertical and horizontal extent of the lee circulation has been obtained from wind tunnel studies (Hosker 1985) and can be implemented in mass consistent diagnostic models. As soon as the flow field has been determined, it can be used to calculate the transport and turbulent mixing of the pollutant emitted. This can be done by Lagrangian or Eulerian dispersion modelling.
More detailed studies - concerning accidental releases, for instance - can be performed only by using non-hydrostatic flow and dispersion models instead of a diagnostic approach. As this, in general, demands high computer power, a worst case approach as described above is recommended in advance of a complete statistical modelling.
More...
Air Quality Monitoring
Air quality monitoring means the systematic measurement of ambient air pollutants in order to be able to assess the exposure of vulnerable receptors (e.g., people, animals, plants and art works) on the basis of standards and guidelines derived from observed effects, and/or to establish the source of the air pollution (causal analysis).
Ambient air pollutant concentrations are influenced by the spatial or time variance of emissions of hazardous substances and the dynamics of their dispersion in the air. As a consequence, marked daily and annual variations of concentrations occur. It is practically impossible to determine in a unified way all these different variations of air quality (in statistical language, the population of air quality states). Thus, ambient air pollutant concentrations measurements always have the character of random spatial or time samples.
Measurement Planning
The first step in measurement planning is to formulate the purpose of the measurement as precisely as possible. Important questions and fields of operation for air quality monitoring include:
Area measurement:
- representative determination of exposure in one area (general air monitoring)
- representative measurement of pre-existing pollution in the area of a planned facility (permit, TA Luft (Technical instruction, air))
- smog warning (winter smog, high ozone concentrations)
- measurements in hot spots of air pollution to estimate maximum exposure of receptors (EU-NO2 guideline, measurements in street canyons, in accordance with the German Federal Immission Control Act)
- checking the results of pollution abatement measures and trends over time
- screening measurements
- scientific investigations - for example, the transport of air pollution, chemical conversions, calibrating dispersion calculations.
Facility measurement:
- measurements in response to complaints
- ascertaining sources of emissions, causal analysis
- measurements in cases of fires and accidental releases
- checking success of reduction measures
- monitoring factory fugitive emissions.
The goal of measurement planning is to use adequate measurement and assessment procedures to answer specific questions with sufficient certainty and at minimum possible expense.
An example of the parameters that should be used for measurement planning is presented in table 1, in relation to an assessment of air pollution in the area of a planned industrial facility. Recognizing that formal requirements vary by jurisdiction, it should be noted that specific reference here is made to German licensing procedures for industrial facilities.
Table 1. Parameters for measurement planning in measuring ambient air pollution concentrations (with example of application)
|
Parameter |
Example of application: Licensing procedure for |
|
Statement of the question |
Measurement of prior pollution in the licensing procedure; representative random probe measurement |
|
Area of measurement |
Circle around location with radius 30 times actual chimney height (simplified) |
|
Assessment standards (place and time dependent): characteristic values to be |
Threshold limits IW1 (arithmetic mean) and IW2 (98th percentile) of TA Luft (Technical instruction, air); calculation of I1 (arithmetic mean) and I2 (98th percentile) from measurements taken for 1 km2 (assessment surface) to be compared with IW1 and IW2 |
|
Ordering, choice and density |
Regular scan of 1km2, resulting in “random” choice of measurement sites |
|
Measurement time period |
1 year, at least 6 months |
|
Measurement height |
1.5 to 4 metres above ground |
|
Measurement frequency |
52 (104) measurements per assessment area for gaseous pollutants, depending on the height of the pollution |
|
Duration of each measurement |
1/2 hour for gaseous pollutants, 24 hours for suspended dust, 1 month for dust precipitation |
|
Measurement time |
Random choice |
|
Measured object |
Air pollution emitted from the planned facility |
|
Measurement procedure |
National standard measurement procedure (VDI guidelines) |
|
Necessary certainty of measurement results |
High |
|
Quality requirements, quality control, calibration, maintenance |
VDI guidelines |
|
Recording of measurement data, validation, archiving, assessment |
Calculation of quantity of data I1V and I2V for every assessment area |
|
Costs |
Depend on measurement area and objectives |
The example in table 1 shows the case of a measurement network that is supposed to monitor the air quality in a specific area as representatively as possible, to compare with designated air quality limits. The idea behind this approach is that a random choice of measurement sites is made in order to cover equally locations in an area with varying air quality (e.g., living areas, streets, industrial zones, parks, city centres, suburbs). This approach may be very costly in large areas due to the number of measurement sites necessary.
Another conception for a measurement network therefore starts with measurement sites that are representatively selected. If measurements of differing air quality are conducted in the most important locations, and the length of time that the protected objects remain in these “microenvironments” is known, then the exposure can be determined. This approach can be extended to other microenvironments (e.g., interior rooms, cars) in order to estimate the total exposure. Diffusion modelling or screening measurements can help in choosing the right measurement sites.
A third approach is to measure at the points of presumed highest exposure (e.g., for NO2 and benzene in street canyons). If assessment standards are met at this site, there is sufficient probability that this will also be the case for all other sites. This approach, by focusing on critical points, requires relatively few measurement sites, but these must be chosen with particular care. This particular method risks overestimating real exposure.
The parameters of measurement time period, assessment of the measurement data and measurement frequency are essentially given in the definition of the assessment standards (limits) and the desired level of certainty of the results. Threshold limits and the peripheral conditions to be considered in measurement planning are related. By using continuous measurement procedures, a resolution that is temporally almost seamless can be achieved. But this is necessary only in monitoring peak values and/or for smog warnings; for monitoring annual mean values, for example, discontinuous measurements are adequate.
The following section is dedicated to describing the capabilities of measurement procedures and quality control as a further parameter important to measurement planning.
Quality Assurance
Measurements of ambient air pollutant concentrations can be costly to conduct, and results can affect significant decisions with serious economic or ecological implications. Therefore, quality assurance measures are an integral part of the measurement process. Two areas should be distinguished here.
Procedure-oriented measures
Every complete measurement procedure consists of several steps: sampling, sample preparation and clean-up; separation, detection (final analytical step); and data collection and assessment. In some cases, especially with continuous measurement of inorganic gases, some steps of the procedure can be left out (e.g., separation). Comprehensive adherence to procedures should be strived for in conducting measurements. Procedures that are standardized and thus comprehensively documented should be followed, in the form of DIN/ISO standards, CEN standards or VDI guidelines.
User-oriented measures
Using standardized and proven equipment and procedures for ambient air pollutant concentration measurement cannot alone ensure acceptable quality if the user does not employ adequate methods of quality control. The standards series DIN/EN/ISO 9000 (Quality Management and Quality Assurance Standards), EN 45000 (which defines the requirements for testing laboratories) and ISO Guide 25 (General Requirements for the Competence of Calibration and Testing Laboratories) are important for user-oriented measures to ensure quality.
Important aspects of user quality control measures include:
- acceptance and practice of the content of the measures in the sense of good laboratory practice (GLP)
- correct maintenance of measurement equipment, qualified measures to eliminate disruptions and ensure repairs
- carrying out calibrations and regular checking to ensure proper functioning
- carrying out interlaboratory testing.
Measurement Procedures
Measurement procedures for inorganic gases
A wealth of measurement procedures exists for the broad range of inorganic gases. We will differentiate between manual and automatic methods.
Manual procedures
In the case of manual measurement procedures for inorganic gases, the substance to be measured is normally adsorbed during the sampling in a solution or solid material. In most cases a photometric determination is made after an appropriate colour reaction. Several manual measurement procedures have special significance as reference procedures. Because of the relatively high personnel cost, these manual procedures are conducted only rarely for field measurements today, when alternative automatic procedures are available. The most important procedures are briefly sketched in table 2.
Table 2. Manual measurement procedures for inorganic gases
|
Material |
Procedure |
Execution |
Comments |
|
SO2 |
TCM procedure |
Absorption in tetrachloromercurate solution (wash bottle); reaction with formaldehyde and pararosaniline to red-violet sulphonic acid; photometric determination |
EU-reference measurement procedure; |
|
SO2 |
Silica gel procedure |
Removal of interfering substances by concentrated H3PO4; adsorption on silica gel; thermal desorption in H2-stream and reduction to H2S; reaction to molybdenum-blue; photometric determination |
DL = 0.3 µg SO2; |
|
NO2 |
Saltzman procedure |
Absorption in reaction solution while forming a red azo dye (wash bottle); photometric determination |
Calibration with sodium nitrite; |
|
O3 |
Potassium iodide |
Formation of iodine from aqueous potassium iodide solution (wash bottle); photometric determination |
DL = 20 µg/m3; |
|
F– |
Silver bead procedure; |
Sampling with dust preseparator; enrichment of F– on sodium carbonate-coated silver beads; elution and measurement with ion-sensitive lanthanum fluoride-electrode chain |
Inclusion of an undetermined portion of particulate fluoride immissions |
|
F– |
Silver bead procedure; |
Sampling with heated membrane filter; enrichment of F– on sodium carbonate-coated silver beads; determination by electrochemical (variant 1) or photometric (alizarin-complexone) procedure |
Danger of lower findings due to partial sorption of gaseous fluoride immissions on membrane filter; |
|
Cl– |
Mercury rhodanide |
Absorption in 0.1 N sodium hydroxide solution (wash bottle); reaction with mercury rhodanide and Fe(III) ions to iron thiocyanato complex; photometric determination |
DL = 9 µg/m3 |
|
Cl2 |
Methyl-orange procedure |
Bleaching reaction with methyl-orange solution (wash bottle); photometric determination |
DL = 0.015 mg/m3 |
|
NH3 |
Indophenol procedure |
Absorption in dilute H2SO4 (Impinger/wash bottle); conversion with phenol and hypochlorite to indophenol dye; photometric determination |
DL = 3 µg/m3 (impinger); partial |
|
NH3 |
Nessler procedure |
Absorption in dilute H2SO4 (Impinger/wash bottle); distillation and reaction with Nessler’s reagent, photometric determination |
DL = 2.5 µg/m3 (impinger); partial |
|
H2S |
Molybdenum-blue |
Absorption as silver sulphide on glass beads treated with silver sulphate and potassium hydrogen sulphate (sorption tube); released as hydrogen sulphide and conversion to molybdenum blue; photometric determination |
DL = 0.4 µg/m3 |
|
H2S |
Methylene blue procedure |
Absorption in cadmium hydroxide suspension while forming CdS; conversion to methylene blue; photometric determination |
DL = 0.3 µg/m3 |
DL = detection limit; s = standard deviation; rel. s = relative s.
A special sampling variant, used primarily in connection with manual measurement procedures, is the diffusion separation tube (denuder). The denuder technique is aimed at separating the gas and particle phases by using their different diffusion rates. Thus, it is often used on difficult separation problems (e.g., ammonia and ammonium compounds; nitrogen oxides, nitric acid and nitrates; sulphur oxides, sulphuric acid and sulphates or hydrogen halides/halides). In the classic denuder technique, the test air is sucked through a glass tube with a special coating, depending on the material(s) to be collected. The denuder technique has been further developed in many variations and also partially automated. It has greatly expanded the possibilities of differentiated sampling, but, depending on the variant, it can be very laborious, and proper utilization requires a great deal of experience.
Automated procedures
There are numerous different continuous measuring monitors on the market for sulphur dioxide, nitrogen oxides, carbon monoxide and ozone. For the most part they are used particularly in measurement networks. The most important features of the individual methods are collected in table 3.
Table 3. Automated measurement procedures for inorganic gases
|
Material |
Measuring principle |
Comments |
|
SO2 |
Conductometry reaction of SO2 with H2O2 in dilute H2SO4; measurement of increased conductivity |
Exclusion of interferences with selective filter (KHSO4/AgNO3) |
|
SO2 |
UV fluorescence; excitationof SO2 molecules with UV radiation (190–230 nm); measurement of fluorescence radiation |
Interferences, e.g., by hydrocarbons, |
|
NO/NO2 |
Chemiluminescence; reaction of NO with O3 to NO2; detection of chemiluminescence radiation with photomultiplier |
NO2 only indirectly measurable; use of converters for reduction of NO2 to NO; measurement of NO and NOx |
|
CO |
Non-dispersive infrared absorption; |
Reference: (a) cell with N2; (b) ambient air after removal of CO; (c) optical removal of CO absorption (gas filter correlation) |
|
O3 |
UV absorption; low-pressure Hg lamp as radiation source (253.7 nm); registration of UV absorption in accordance with Lambert-Beer’s law; detector: vacuum photodiode, photosensitive valve |
Reference: ambient air after removal of ozone (e.g., Cu/MnO2) |
|
O3 |
Chemiluminescence; reaction of O3 with ethene to formaldehyde; detection of chemiluminescence radiation with |
Good selectivity; ethylene necessary as reagent gas |
It should be emphasized here that all automatic measurement procedures based on chemical-physical principles must be calibrated using (manual) reference procedures. Since automatic equipment in measurement networks often runs for extended periods of time (e.g., several weeks) without direct human supervision, it is indispensable that their correct functioning is regularly and automatically checked. This generally is done using zero and test gases that can be produced by several methods (preparation of ambient air; pressurized gas cylinders; permeation; diffusion; static and dynamic dilution).
Measurement procedures for dust-forming air pollutants and its composition
Among particulate air pollutants, dustfall and suspended particulate matter (SPM) are differentiated. Dustfall consists of larger particles, which sink to the ground because of their size and thickness. SPM includes the particle fraction that is dispersed in the atmosphere in a quasi-stable and quasi-homogenous manner and therefore remains suspended for a certain time.
Measurement of suspended particulate matter and metallic compounds in SPM
As is the case with measurements of gaseous air pollutants, continuous and discontinuous measurement procedures for SPM can be differentiated. As a rule, SPM is first separated on glass fibre or membrane filters. It follows a gravimetric or radiometric determination. Depending on the sampling, a distinction can be made between a procedure to measure the total SPM without fractionation according to the size of the particles and a fractionation procedure to measure the fine dust.
The advantages and disadvantages of fractionated suspended dust measurements are disputed internationally. In Germany, for example, all threshold limits and assessment standards are based on total suspended particulates. This means that, for the most part, only total SPM measurements are performed. In the United States, on the contrary, the so-called PM-10 procedure (particulate matter £ 10μm) is very common. In this procedure, only particles with an aerodynamic diameter up to 10 μm are included (50 per cent inclusion portion), which are inhalable and can enter the lungs. The plan is to introduce the PM-10 procedure into the European Union as a reference procedure. The cost for fractionated SPM measurements is considerably higher than for measuring total suspended dust, because the measuring devices must be fitted with special, expensively constructed sampling heads that require costly maintenance. Table 4 contains details on the most important SPM measurement procedures.
Table 4. Measurement procedures for suspended particulate matter (SPM)
|
Procedure |
Measuring principle |
Comments |
|
Small filter device |
Non-fractionated sampling; air flow rate 2.7–2.8 m3/h; filter diameter 50 mm; gravimetric analysis |
Easy handling; control clock; |
|
LIB device |
Non-fractionated sampling; air flow rate 15-16 m3/h; filter diameter 120 mm; gravimetric analysis |
Separation of large dust |
|
High-Volume-Sampler |
Inclusion of particles up to approx. 30 µm diameter; air flow rate approx. 100 m3/h; filter diameter 257 mm; gravimetric analysis |
Separation of large dust |
|
FH 62 I |
Continuous, radiometric dust measuring device; non-fractionating sampling; air flow rate 1 or 3 m3/h; registration of dust mass separated on a filter band by measuring attenuation of β-radiation (krypton 85) in passage through exposed filter (ionization chamber) |
Gravimetric calibration by dusting of single filters; device also operable with PM-10 preseparator |
|
BETA dust meter F 703 |
Continuous, radiometric dust measuring device; non-fractionated sampling; air flow rate 3 m3/h; registration of dust mass separated on a filter band by measuring attenuation of β-radiation (carbon 14) in passage through exposed filter (Geiger Müller counter tube) |
Gravimetric calibration by dusting of single filters; device also operable with PM-10 preseparator |
|
TEOM 1400 |
Continuous dust measuring device; non-fractionated sampling; air flow rate 1 m3/h; dust collected on a filter, which is part of a self-resonating, vibrating system, in side stream (3 l/min); registration of the frequency lowering by increased dust load on the filter |
Relationship between frequency
|
Recently, automatic filter changers have also been developed that hold a larger number of filters and supply them to the sampler, one after another, at timed intervals. The exposed filters are stored in a magazine. The detection limits for filter procedures lie between 5 and 10 μg/m3 of dust, as a rule.
Finally, the black smoke procedure for SPM measurements has to be mentioned. Coming from Britain, it has been incorporated into EU guidelines for SO2 and suspended dust. In this procedure, the blackening of the coated filter is measured with a reflex photometer after the sampling. The black smoke values that are thus photometrically obtained are converted into gravimetric units (μg/m3) with the help of a calibration curve. Since this calibration function depends to a high degree on the composition of the dust, especially its soot content, the conversion into gravimetric units is problematic.
Today, metal compounds are often routinely determined in suspended dust immission samples. In general, the collection of the suspended dust on filters is followed by a chemical dissolution of the separated dusts, since the most common final analytical steps presuppose converting the metallic and metalloid compounds in an aqueous solution. In practice, the most important methods by far are atom absorption spectroscopy (AAS) and spectroscopy with plasma excitation (ICP-OES). Other procedures for determining metallic compounds in suspended dust are x-ray fluorescence analysis, polarography and neutron activation analysis. Although metallic compounds have been measured for more than a decade now as a component of SPM in outside air at certain measurement sites, important unanswered questions remain. Thus the conventional sampling by separating the suspended dust on filters assumes that the separation of the heavy metal compounds on the filter is complete. However, earlier indications have been found in the literature questioning this. The results are very heterogeneous.
A further problem lies in the fact that different compound forms, or single compounds of the respective elements, cannot be distinguished in the analysis of metallic compounds in suspended dust using the conventional measurement procedures. While in many cases adequate total determinations can be made, a more thorough differentiation would be desirable with certain especially carcinogenic metals (As, Cd, Cr, Ni, Co, Be). There are often big differences in the carcinogenic effects of elements and their individual compounds (e.g., chromium compounds in oxidation levels III and VI - only those in level VI are carcinogenic). In such cases a specific measurement of the individual compounds (species analysis) would be desirable. Despite the significance of this problem, only first attempts at species analysis are being made in measurement technique.
Measurement of dustfall and metallic compounds in dustfall
Two fundamentally different methods are used to collect dustfall:
- sampling in collecting vessels
- sampling on adhesive surfaces.
A popular procedure for measuring dustfall (deposited dust) is the so-called Bergerhoff procedure. In this procedure the entire atmospheric precipitation (dry and wet depositions) is collected over 30± 2 days in vessels about 1.5 to 2.0 metres above the ground (bulk deposition). Then the collecting vessels are taken to the lab and prepared (filtered, water evaporated, dried, weighed). The result is calculated on the basis of the surface area of the collecting vessel and exposure time in grams per square meter and day (g/m2d). The relative detection limit is 0.035 g/m2d.
Additional procedures for collecting dustfall include the Liesegang-Löbner device and methods which collect the deposited dust on adhesive foils.
All measurement results for dustfall are relative values that depend on the apparatus used, as the dust separation is influenced by the flow conditions at the device and other parameters. The differences in the measurement values obtained with the different procedures can reach 50 per cent.
Also important is the composition of the deposited dust, such as the content of lead, cadmium and other metallic compounds. The analytical procedures used for this are basically the same as those used for suspended dust.
Measuring special materials in dust form
Special materials in dust form include asbestos and soot. Collecting fibres as air pollutants is important since asbestos has been classified as a confirmed carcinogenic material. Fibres with a diameter of D ≤ 3μm and a length of L ≥ 5μm, where L:D ≥ 3, are considered carcinogenic. Measurement procedures for fibrous materials consist of counting, under the microscope, fibres that have been separated on filters. Only electron microscopic procedures can be considered for outside air measurements. The fibres are separated on gold-coated porous filters. Prior to assessment in an electron scan microscope, the sample is freed of organic substances through plasma incineration right on the filter. The fibres are counted on part of the filter surface, randomly chosen and classified by geometry and type of fibre. With the help of energy dispersive x-ray analysis (EDXA), asbestos fibres, calcium sulphate fibres and other inorganic fibres can be differentiated on the basis of elemental composition. The entire procedure is extremely expensive and requires the greatest care to achieve reliable results.
Soot in the form of particles emitted by diesel motors has become relevant since diesel soot was also classified as carcinogenic. Because of its changing and complex composition and because of the fact that various constituents are also emitted from other sources, there is no measurement procedure specific to diesel soot. Nevertheless, in order to say something concrete about the concentrations in ambient air, soot is conventionally defined as elemental carbon, as a part of total carbon. It is measured after sampling and an extraction step and/or thermal desorption. Determination of the carbon content ensues through burning in an oxygen stream and coulometric titration or non-dispersive IR detection of the carbon dioxide formed in the process.
The so-called aethalometer and the photoelectric aerosol sensor are also used for measuring soot, in principle.
Measuring Wet Depositions
Together with dry deposition, wet deposition in rain, snow, fog and dew constitute the most important means by which harmful materials enter the ground, water or plant surfaces from the air.
In order to clearly distinguish the wet deposition in rain and snow (fog and dew present special problems) from the measurement of total deposition (bulk deposition, see section “Measurement of dustfall and metallic compounds” above) and dry deposition, rain catchers, whose collection opening is covered when there is no rain (wet-only sampler), are used for sampling. With rain sensors, which mostly work on the principle of conductivity changes, the cover is opened when it starts to rain and closed again when the rain stops.
The samples are transferred through a funnel (open area approx. 500 cm2 and more) into a darkened and if possible insulated collection container (of glass or polyethylene for inorganic components only).
In general, analysing the collected water for inorganic components can be done without sample preparation. The water should be centrifuged or filtered if it is visibly cloudy. The conductivity, pH value and important anions (NO3 – , SO4 2– , Cl–) and cations (Ca2+, K+, Mg2+, Na+, NH4 + and so on) are routinely measured. Unstable trace compounds and intermediate states like H2O2 or HSO3 – are also measured for research purposes.
For analysis, procedures are used that are generally available for aqueous solutions such as conductometry for conductivity, electrodes for pH values, atom adsorption spectroscopy for cations (see section “Measuring special materials in dust form”, above) and, increasingly, ion exchange chromatography with conductivity detection for anions.
Organic compounds are extracted from rain water with, for example, dichloromethane, or blown out with argon and adsorbed with Tenax tubes (only highly volatile materials). The materials are then subjected to a gas chromatographic analysis (see “Measurement procedures for organic air pollutants”, below).
Dry deposition correlates directly with ambient air concentrations. The concentration differences of airborne harmful materials in rain, however, are relatively small, so that for measuring wet deposition, wide-mesh measuring networks are adequate. Examples include the European EMEP measurement network, in which the entry of sulphate and nitrate ions, certain cations and precipitation pH values are collected in approximately 90 stations. There are also extensive measurement networks in North America.
Optical Long-Distance Measurement Procedures
Whereas the procedures described up to now catch air pollution at one point, optical long-distance measuring procedures measure in an integrated manner over light paths of several kilometres or they determine the spatial distribution. They use the absorption characteristics of gases in the atmosphere in the UV, visible or IR spectral range and are based on the Lambert-Beer law, according to which the product of light path and concentration are proportional to the measured extinction. If the sender and receiver of the measuring installation change the wavelength, several components can be measured in parallel or sequentially with one device.
In practice, the measurement systems identified in table 5 play the biggest role.
Table 5. Long-distance measurement procedures
|
Procedure |
Application |
Advantages, disadvantages |
|
Fourier |
IR range (approx. 700–3,000 cm–1), several hundred metres light path. |
+ Multi-component system |
|
Differential |
Light path to several km; measures SO2, NO2, benzene, HNO3; monitors linear and surface sources, used in measuring networks |
+ Easy to handle |
|
Long-distance |
Research area, in low-pressure cuvettes for OH- |
+ High sensitivity (to ppt) |
|
Differential |
Monitors surface sources, large surface immission measurements |
+ Measurements of spatial |
LIDAR = Light detection and ranging; DIAL = differential absorption LIDAR.
Measurement Procedures for Organic Air Pollutants
The measurement of air pollution containing organic components is complicated primarily by the range of materials in this class of compounds. Several hundred individual components with very different toxicological, chemical and physical characteristics are covered under the general title “organic air pollutants” in the emissions registers and air quality plans of congested areas.
Especially due to the great differences in potential impact, collecting relevant individual components has more and more taken the place of previously used summation procedures (e.g., Flame Ionization Detector, total carbon procedure), the results of which cannot be assessed toxicologically. The FID method, however, has retained a certain significance in connection with a short separation column to separate out methane, which is photochemically not very reactive, and for collecting the precursor volatile organic compounds (VOC) for the formation of photo-oxidants.
The frequent necessity of separating the complex mixtures of the organic compounds into relevant individual components makes measuring it virtually an exercise in applied chromatography. Chromatographic procedures are the methods of choice when the organic compounds are sufficiently stable, thermally and chemically. For organic materials with reactive functional groups, separate procedures that use the functional groups’ physical characteristics or chemical reactions for detection continue to hold their ground.
Examples include using amines to convert aldehydes to hydrazones, with subsequent photometric measurement; derivatization with 2,4-dinitrophenylhydrazine and separation of the 2,4-hydrazone that is formed; or forming azo-dyes with p-nitroaniline for detecting phenols and cresols.
Among chromatographic procedures, gas chromatography (GC) and high-pressure liquid chromatography (HPLC) are most frequently employed for separating the often complex mixtures. For gas chromatography, separation columns with very narrow diameters (approx. 0.2 to 0.3 mm, and approx. 30 to 100 m long), so-called high-resolution capillary columns (HRGC), are almost exclusively utilized today. A series of detectors are available for finding the individual components after the separation column, such as the above-mentioned FID, the ECD (electron capture detector, specifically for electrophilic substitutes such as halogen), the PID (photo-ionization detector, which is especially sensitive to aromatic hydrocarbons and other p-electron systems), and the NPD (thermo-ionic detector specifically for nitrogen and phosphorus compounds). The HPLC uses special through-flow detectors which, for example, are designed as the through-flow cuvette of a UV spectrometer.
Especially effective, but also especially expensive, is the use of a mass spectrometer as a detector. Really certain identification, especially with unknown mixtures of compounds, is often possible only through the mass spectrum of the organic compound. The qualitative information of the so-called retention time (time the material remains in the column) that is contained in the chromatogram with conventional detectors is supplemented with the specific detection of the individual components by mass fragmentograms with high detection sensitivity.
Sampling must be considered before the actual analysis. The choice of sampling method is determined primarily by volatility, but also by expected concentration range, polarity and chemical stability. Furthermore, with non-volatile compounds, a choice must be made between concentration and deposition measurements.
Table 6 provides an overview of common procedures in air monitoring for active enrichment and chromatographic analysis of organic compounds, with examples of applications.
Table 6. Overview of common chromatographic air quality measurement procedures of organic compounds (with examples of applications)
|
Material group |
Concentration |
Sampling, preparation |
Final analytical step |
|
Hydrocarbons C1–C9 |
μg/m3 |
Gas mice (rapid sampling), gas-tight syringe, cold trapping in front of capillary column (focusing), thermal desorption |
GC/FID |
|
Low-boiling hydrocarbons, highly |
ng/m3–μg/m3 |
Evacuated, passivated high-grade steel cylinder (also for clean air measurements) |
GC/FID/ECD/PID |
|
Organic compounds in boiling point |
μg/m3 |
Adsorption on activated carbon, (a) desorption with CS2 (b) desorption with solvents (c) headspace analysis |
Capillary |
|
Organic compounds in boiling point |
ng/m3–μg/m3 |
Adsorption on organic polymers (e.g., Tenax) or molecular carbon sieve (carbopack), thermal desorption with cold trapping in front of capillary column (focusing) or solvent extraction |
Capillary |
|
Modification for low-boiling |
ng/m3–μg/m3 |
Adsorption on cooled polymers (e.g. thermogradient tube), cooled to –120 ºC, use of carbopack |
Capillary |
|
High boiling organic compounds |
fg/m3–ng/m3 |
Sampling on filters (e.g., small filter device or high volume sampler) with subsequent polyurethane cartridges for gaseous portion, solvent desorption of filter and polyurethane, various purification and preparatory steps, for PAH also sublimation |
Capillary |
|
High boiling organic compounds, |
fg/m3–ng/m3 |
Adsorption on organic polymers (e.g., polyurethane foam cylinder) with prior filters (e.g., glass fibre) or inorg. adsorp. (e.g., silica gel), extraction with solvents, various purification and preparatory steps, (including multicolumn chromatography), derivatizing for chlorophenols |
HRGC/ECD |
|
High boiling organic compounds |
ng/m3 |
Separation of aerosols on glass fibre filters (e.g., high or low volume sampler) or dust collection on standardized surfaces, extraction with solvents (for deposition also of remaining filtered water), various purification and preparation steps |
HRGC/MS |
GC = gas chromatography; GCMS = GC/mass spectroscopy; FID = flame ionization detector; HRGC/ECD = high resolution GC/ECD; ECD = electron capture detector; HPLC = high performance liquid chromatography. PID = photo-ionization detector.
Deposition measurements of organic compounds with low volatility (e.g., dibenzodioxins and dibenzofurans (PCDD/PCDF), polycyclic aromatic hydrocarbons (PAH)) are gaining in importance from the perspective of environmental impact. Since food is the main source of human intake, airborne material transferred onto food plants is of great significance. There is, however, evidence that material transfer by way of particulate deposition is less important than dry deposition of quasi-gaseous compounds.
For measuring total deposition, standardized devices for dust precipitation are used (e.g., Bergerhoff procedure), which have been slightly modified by darkening as a protection against the entry of strong light. Important technical measurement problems, such as the resuspension of already separated particles, evaporation or possible photolytic decomposition, are now being systematically researched in order to improve the less-than-optimal sampling procedures for organic compounds.
Olfactometric Investigations
Olfactometric immission investigations are used in monitoring to quantify odour complaints and to determine baseline pollution in licensing procedures. They serve primarily to assess whether existing or anticipated odours should be classified as significant.
In principle, three methodological approaches can be differentiated:
- measurement of the emission concentration (number of odour units) with an olfactometer and subsequent dispersion modelling
- measurement of individual components (e.g., NH3) or mixtures of compounds (e.g., gas chromatography of gases from landfills), if these adequately characterize the odour
- odour determinations by means of inspections.
The first possibility combines emission measurement with modelling and, strictly speaking, cannot be classified under the term air quality monitoring. In the third method, the human nose is used as the detector with significantly reduced precision as compared to physical-chemical methods.
Details of inspections, measurement plans and assessing the results are contained, for example, in the environmental protection regulations of some German states.
Screening Measurement Procedures
Simplified measurement procedures are sometimes used for preparatory studies (screening). Examples include passive samplers, test tubes and biological procedures. With passive (diffusive) samplers, the material to be tested is collected with freely flowing processes such as diffusion, permeation or adsorption in simple forms of collectors (tubes, plaques) and enriched in impregnated filters, meshes or other adsorption media. So-called active sampling (sucking the sample air through a pump) thus does not occur. The enriched quantity of material, analytically determined according to definite exposure time, is converted into concentration units on the basis of physical laws (e.g., of diffusion) with the help of collection time and the collector’s geometric parameters. The methodology stems from the field of occupational health (personal sampling) and indoor air measurement, but it is increasingly being used for ambient air pollutant concentration measurements. An overview can be found in Brown 1993.
Detector tubes are often used for sampling and quick preparatory analysis of gases. A certain test air volume is sucked through a glass tube that is filled with an adsorptive reagent that corresponds with the test objective. The contents of the tube change colour depending on the concentration of the material to be determined that is present in the test air. Small testing tubes are often used in the field of workplace monitoring or as a quick procedure in cases of accidents, such as fires. They are not used for routine ambient air pollutant concentration measurements due to the generally too high detection limits and too limited selectivity. Detector testing tubes are available for numerous materials in various concentration ranges.
Among the biological procedures, two methods have become accepted in routine monitoring. With the standardized lichen exposure procedure, the mortality rate of the lichen is determined over the exposure time of 300 days. In another procedure, French pasture grass is exposed for 14±1 days. Then the amount of growth is determined. Both procedures serve as summary determinations of air pollutant concentration effects.
Air Quality Monitoring Networks
Around the world, the most varied types of air quality networks are utilized. A distinction should be drawn between measurement networks, consisting of automatic, computer-controlled measuring stations (measurement containers), and virtual measurement networks, which only define the measurement locations for various types of air pollutant concentration measurements in the form of a preset grid. Tasks and conceptions of measurement networks were discussed above.
Continuous monitoring networks
Continuously operating measurement networks are based on automatic measuring stations, and serve primarily for air quality monitoring of urban areas. Measured are air pollutants such as sulphur dioxide (SO2), dust, nitrogen monoxide (NO), nitrogen dioxide (NO2), carbon monoxide (CO), ozone (O3), and to an extent also the sum of the hydrocarbons (free methane, CnHm) or individual organic components (e.g., benzene, toluene, xylenes). In addition, depending on need, meteorological parameters such as wind direction, wind speed, air temperature, relative humidity, precipitation, global radiation or radiation balance are included.
The measuring equipment operated in measurement stations generally consists of an analyser, a calibration unit, and control and steering electronics, which monitors the whole measuring equipment and contains a standardized interface for data collection. In addition to the measurement values, the measuring equipment supplies so-called status signals on errors and the operating status. The calibration of the devices is automatically checked by computer at regular intervals.
As a rule, the measurement stations are connected with fixed data lines, dial connections or other data transfer systems to a computer (process computer, workstation or PC, depending on the scope of the system) in which the measurement results are entered, processed and displayed. The measurement network computers and, if necessary, specially trained personnel monitor continuously whether various threshold limits are exceeded. In this manner critical air quality situations can be recognized at any time. This is very important, especially for monitoring critical smog situations in winter and summer (photo-oxidants) and for current public information.
Measurement networks for random sample measurements
Beyond the telemetric measurement network, other measuring systems for monitoring air quality are used to varying extents. Examples include (occasionally partially automated) measurement networks to determine:
- dust deposition and its components
- suspended dust (SPM) and its components
- hydrocarbons and chlorinated hydrocarbons
- low volatile organic materials (dioxins, furans, polychlorinated biphenyls).
A series of substances measured in this manner have been classified as carcinogens, such as cadmium compounds, PAHs or benzene. Monitoring them is therefore particularly important.
To provide an example of a comprehensive programme, table 7 summarizes the air quality monitoring that is systematically conducted in North Rhine-Westphalia, which with 18 million inhabitants is the most populous state in Germany.
Table 7. Systematic air quality monitoring in North-Rhine-Westphalia (Germany)
|
Continuous measuring |
Partially automated |
Discontinuous measuring |
|
Sulphur dioxide |
SPM composition: |
Benzene and other |
Air Pollution Control
Management of Air Pollution
The objective of a manager of an air pollution control system is to ensure that excessive concentrations of air pollutants do not reach a susceptible target. Targets could include people, plants, animals and materials. In all cases we should be concerned with the most sensitive of each of these groups. Air pollutants could include gases, vapours, aerosols and, in some cases, biohazardous materials. A well designed system will prevent a target from receiving a harmful concentration of a pollutant.
Most air pollution control systems involve a combination of several control techniques, usually a combination of technological controls and administrative controls, and in larger or more complex sources there may be more than one type of technological control.
Ideally, the selection of the appropriate controls will be made in the context of the problem to be solved.
- What is emitted, in what concentration?
- What are the targets? What is the most susceptible target?
- What are acceptable short-term exposure levels?
- What are acceptable long-term exposure levels?
- What combination of controls must be selected to ensure that the short-term and long-term exposure levels are not exceeded?
Table 1 describes the steps in this process.
Table 1. Steps in selecting pollution controls
|
Step 1: |
The first part is to determine what will be released from the stack. |
|
Step 2: |
All susceptible targets should be identified. This includes people, animals, plants and materials. In each case, the most susceptible member of each group must be identified. For example, asthmatics near a plant that emits isocyanates. |
|
Step 3: |
An acceptable level of exposure for the most sensitive target group must |
|
Step 4: |
Step 1 identifies the emissions, and Step 3 determines the acceptable |
* When setting exposure levels in Step 3, it must be remembered that these exposures are total exposures, not just those from the plant. Once the acceptable level has been established, background levels, and contributions from other plants just be subtracted to determine the maximum amount that the plant can emit without exceeding the acceptable exposure level. If this is not done, and three plants are allowed to emit at the maximum amount, the target groups will be exposed to three times the acceptable level.
** Some materials such as carcinogens do not have a threshold below which no harmful effects will occur. Therefore, as long as some of the material is allowed to escape to the environment, there will be some risk to the target populations. In this case a no effect level cannot be set (other than zero). Instead, an acceptable level of risk must be established. Usually this is set in the range of 1 adverse outcome in 100,000 to 1,000,000 exposed persons.
Some jurisdictions have done some of the work by setting standards based on the maximum concentration of a contaminant that a susceptible target can receive. With this type of standard, the manager does not have to carry out Steps 2 and 3, since the regulating agency has already done this. Under this system, the manager must establish only the uncontrolled emission standards for each pollutant (Step 1), and then determine what controls are necessary to meet the standard (Step 4).
By having air quality standards, regulators can measure individual exposures and thus determine whether anyone is exposed to potentially harmful levels. It is assumed that the standards set under these conditions are low enough to protect the most susceptible target group. This is not always a safe assumption. As shown in table 2, there can be a wide variation in common air quality standards. Air quality standards for sulphur dioxide range from 30 to 140 μg/m3. For less commonly regulated materials this variation can be even larger (1.2 to 1,718 μg/m3), as shown in table 3 for benzene. This is not surprising given that economics can play as large a role in standard setting as does toxicology. If a standard is not set low enough to protect susceptible populations, no one is well served. Exposed populations have a feeling of false confidence, and can unknowingly be put at risk. The emitter may at first feel that they have benefited from a lenient standard, but if effects in the community require the company to redesign their controls, or install new controls, costs could be higher than doing it correctly the first time.
Table 2. Range of air quality standards for a commonly controlled air contaminant (sulphur dioxide)
|
Countries and territories |
Long-term sulphur dioxide |
|
Australia |
50 |
|
Canada |
30 |
|
Finland |
40 |
|
Germany |
140 |
|
Hungary |
70 |
|
Taiwan |
133 |
Table 3. Range of air quality standards for a less commonly controlled air contaminant (benzene)
|
City/State |
24-hour air quality standard for |
|
Connecticut |
53.4 |
|
Massachusetts |
1.2 |
|
Michigan |
2.4 |
|
North Carolina |
2.1 |
|
Nevada |
254 |
|
New York |
1,718 |
|
Philadelphia |
1,327 |
|
Virginia |
300 |
The levels were standardized to an averaging time of 24 hours to assist in the comparisons.
(Adapted from Calabrese and Kenyon 1991.)
Sometimes this stepwise approach to selecting air pollution controls is short circuited, and the regulators and designers go directly to a “universal solution”. One such method is best available control technology (BACT). It is assumed that by using the best combination of scrubbers, filters and good work practices on an emission source, a level of emissions low enough to protect the most susceptible target group would be achieved. Frequently, the resulting emission level will be below the minimum required to protect the most susceptible targets. This way all unnecessary exposures should be eliminated. Examples of BACT are shown in table 4.
Table 4. Selected examples of best available control technology (BACT) showing the control method used and estimated efficiency
|
Process |
Pollutant |
Control method |
Estimated efficiency |
|
Soil remediation |
Hydrocarbons |
Thermal oxidizer |
99 |
|
Kraft pulp mill |
Particulates |
Electrostatic |
99.68 |
|
Production of fumed |
Carbon monoxide |
Good practice |
50 |
|
Automobile painting |
Hydrocarbons |
Oven afterburner |
90 |
|
Electric arc furnace |
Particulates |
Baghouse |
100 |
|
Petroleum refinery, |
Respirable particulates |
Cyclone + Venturi |
93 |
|
Medical incinerator |
Hydrogen chloride |
Wet scrubber + dry |
97.5 |
|
Coal-fired boiler |
Sulphur dioxide |
Spray dryer + |
90 |
|
Waste disposal by |
Particulates |
Cyclone + condenser |
95 |
|
Asphalt plant |
Hydrocarbons |
Thermal oxidizer |
99 |
BACT by itself does not ensure adequate control levels. Although this is the best control system based on gas cleaning controls and good operating practices, BACT may not be good enough if the source is a large plant, or if it is located next to a sensitive target. Best available control technology should be tested to ensure that it is indeed good enough. The resulting emission standards should be checked to determine whether or not they may still be harmful even with the best gas cleaning controls. If emission standards are still harmful, other basic controls, such as selecting safer processes or materials, or relocating in a less sensitive area, may have to be considered.
Another “universal solution” that bypasses some of the steps is source performance standards. Many jurisdictions establish emission standards that cannot be exceeded. Emission standards are based on emissions at the source. Usually this works well, but like BACT they can be unreliable. The levels should be low enough to maintain the maximum emissions low enough to protect susceptible target populations from typical emissions. However, as with best available control technology, this may not be good enough to protect everyone where there are large emission sources or nearby susceptible populations. If this is the case, other procedures must be used to ensure the safety of all target groups.
Both BACT and emission standards have a basic fault. They assume that if certain criteria are met at the plant, the target groups will be automatically protected. This is not necessarily so, but once such a system is passed into law, effects on the target become secondary to compliance with the law.
BACT and source emission standards or design criteria should be used as minimum criteria for controls. If BACT or emission criteria will protect the susceptible targets, then they can be used as intended, otherwise other administrative controls must be used.
Control Measures
Controls can be divided into two basic types of controls - technological and administrative. Technological controls are defined here as the hardware put on an emission source to reduce contaminants in the gas stream to a level that is acceptable to the community and that will protect the most sensitive target. Administrative controls are defined here as other control measures.
Technological controls
Gas cleaning systems are placed at the source, before the stack, to remove contaminants from the gas stream before releasing it to the environment. Table 5 shows a brief summary of the different classes of gas cleaning system.
Table 5. Gas cleaning methods for removing harmful gases, vapours and particulates from industrial process emissions
|
Control method |
Examples |
Description |
Efficiency |
|
Gases/Vapours |
|||
|
Condensation |
Contact condensers |
The vapour is cooled and condensed to a liquid. This is inefficient and is used as a preconditioner to other methods |
80+% when concentration >2,000 ppm |
|
Absorption |
Wet scrubbers (packed |
The gas or vapour is collected in a liquid. |
82–95% when concentration <100 ppm |
|
Adsorption |
Carbon |
The gas or vapour is collected on a solid. |
90+% when concentration <1,000 ppm |
|
Incineration |
Flares |
An organic gas or vapour is oxidized by heating it to a high temperature and holding it at that temperature for a |
Not recommended when |
|
Particulates |
|||
|
Inertial |
Cyclones |
Particle-laden gases are forced to change direction. The inertia of the particle causes them to separate from the gas stream. This is inefficient and is used as a |
70–90% |
|
Wet scrubbers |
Venturi |
Liquid droplets (water) collect the particles by impaction, interception and diffusion. The droplets and their particles are then separated from the gas stream. |
For 5 μm particles, 98.5% at 6.8 w.g.; |
|
Electrostatic |
Plate-wire |
Electrical forces are used to move the particles out of the gas stream onto collection plates |
95–99.5% for 0.2 μm particles |
|
Filters |
Baghouse |
A porous fabric removes particulates from the gas stream. The porous dust cake that forms on the fabric then actually |
99.9% for 0.2 μm particles |
The gas cleaner is part of a complex system consisting of hoods, ductwork, fans, cleaners and stacks. The design, performance and maintenance of each part affects the performance of all other parts, and the system as a whole.
It should be noted that system efficiency varies widely for each type of cleaner, depending on its design, energy input and the characteristics of the gas stream and the contaminant. As a result, the sample efficiencies in table 5 are only approximations. The variation in efficiencies is demonstrated with wet scrubbers in table 5. Wet scrubber collection efficiency goes from 98.5 per cent for 5 μm particles to 45 per cent for 1 μm particles at the same pressure drop across the scrubber (6.8 in. water gauge (w.g.)). For the same size particle, 1 μm, efficiency goes from 45 per cent efficiency at 6.8 w.g. to 99.95 at 50 w.g. As a result, gas cleaners must be matched to the specific gas stream in question. The use of generic devices is not recommended.
Waste disposal
When selecting and designing gas cleaning systems, careful consideration must be given to the safe disposal of the collected material. As shown in table 6, some processes produce large amounts of contaminants. If most of the contaminants are collected by the gas cleaning equipment there can be a hazardous waste disposal problem.
Table 6. Sample uncontrolled emission rates for selected industrial processes
|
Industrial source |
Emission rate |
|
100 ton electric furnace |
257 tons/year particulates |
|
1,500 MM BTU/hr oil/gas turbine |
444 lb/hr SO2 |
|
41.7 ton/hr incinerator |
208 lb/hr NOx |
|
100 trucks/day clear coat |
3,795 lb/week organics |
In some cases the wastes may contain valuable products that can be recycled, such as heavy metals from a smelter, or solvent from a painting line. The wastes can be used as a raw material for another industrial process - for example, sulphur dioxide collected as sulphuric acid can be used in the manufacture of fertilizers.
Where the wastes cannot be recycled or reused, disposal may not be simple. Not only can the volume be a problem, but they may be hazardous themselves. For example, if the sulphuric acid captured from a boiler or smelter cannot be reused, it will have to be further treated to neutralize it before disposal.
Dispersion
Dispersion can reduce the concentration of a pollutant at a target. However, it must be remembered that dispersion does not reduce the total amount of material leaving a plant. A tall stack only allows the plume to spread out and be diluted before it reaches ground level, where susceptible targets are likely to exist. If the pollutant is primarily a nuisance, such as an odour, dispersion may be acceptable. However if the material is persistent or cumulative, such as heavy metals, dilution may not be an answer to an air pollution problem.
Dispersion should be used with caution. Local meteorological and ground surface conditions must be taken into consideration. For example, in colder climates, particularly with snow cover, there can be frequent temperature inversions that can trap pollutants close to the ground, resulting in unexpectedly high exposures. Similarly, if a plant is located in a valley, the plumes may move up and down the valley, or be blocked by surrounding hills so that they do not spread out and disperse as expected.
Administrative controls
In addition to the technological systems, there is another group of controls that must be considered in the overall design of an air pollution control system. For the large part, they come from the basic tools of industrial hygiene.
Substitution
One of the preferred occupational hygiene methods for controlling environmental hazards in the workplace is to substitute a safer material or process. If a safer process or material can be used, and harmful emissions avoided, the type or efficacy of controls becomes academic. It is better to avoid the problem than it is to try to correct a bad first decision. Examples of substitution include the use of cleaner fuels, covers for bulk storage and reduced temperatures in dryers.
This applies to minor purchases as well as the major design criteria for the plant. If only environmentally safe products or processes are purchased, there will be no risk to the environment, indoors or out. If the wrong purchase is made, the remainder of the programme consists of trying to compensate for that first decision. If a low-cost but hazardous product or process is purchased it may need special handling procedures and equipment, and special disposal methods. As a result, the low-cost item may have only a low purchase price, but a high price to use and dispose of it. Perhaps a safer but more expensive material or process would have been less costly in the long run.
Local ventilation
Controls are required for all the identified problems that cannot be avoided by substituting safer materials or methods. Emissions start at the individual worksite, not the stack. A ventilation system that captures and controls emissions at the source will help protect the community if it is properly designed. The hoods and ducts of the ventilation system are part of the total air pollution control system.
A local ventilation system is preferred. It does not dilute the contaminants, and provides a concentrated gas stream that is easier to clean before release to the environment. Gas cleaning equipment is more efficient when cleaning air with higher concentrations of contaminants. For example, a capture hood over the pouring spout of a metal furnace will prevent contaminants from getting into the environment, and deliver the fumes to the gas cleaning system. In table 5 it can be seen that cleaning efficiencies for absorption and adsorption cleaners increase with the concentration of the contaminant, and condensation cleaners are not recommended for low levels (<2,000 ppm) of contaminants.
If pollutants are not caught at the source and are allowed to escape through windows and ventilation openings, they become uncontrolled fugitive emissions. In some cases, these uncontrolled fugitive emissions can have a significant impact on the immediate neighbourhood.
Isolation
Isolation - locating the plant away from susceptible targets - can be a major control method when engineering controls are inadequate by themselves. This may be the only means of achieving an acceptable level of control when best available control technology (BACT) must be relied on. If, after applying the best available controls, a target group is still at risk, consideration must be given to finding an alternate site where sensitive populations are not present.
Isolation, as presented above, is a means of separating an individual plant from susceptible targets. Another isolation system is where local authorities use zoning to separate classes of industries from susceptible targets. Once industries have been separated from target populations, the population should not be allowed to relocate next to the facility. Although this seems like common sense, it isn’t employed as often as it should be.
Work procedures
Work procedures must be developed to ensure that equipment is used properly and safely, without risk to workers or the environment. Complex air pollution systems must be properly maintained and operated if they are to do their job as intended. An important factor in this is staff training. Staff must be trained in how to use and maintain the equipment to reduce or eliminate the amount of hazardous materials emitted to the workplace or the community. In some cases BACT relies on good practice to ensure acceptable results.
Real time monitoring
A system based on real time monitoring is not popular, and is not commonly used. In this case, continuous emission and meteorological monitoring can be combined with dispersion modelling to predict downwind exposures. When the predicted exposures approach the acceptable levels, the information is used to reduce production rates and emissions. This is an inefficient method, but may be an acceptable interim control method for an existing facility.
The converse of this to announce warnings to the public when conditions are such that excessive concentrations of contaminants may exist, so that the public can take appropriate action. For example, if a warning is sent out that atmospheric conditions are such that sulphur dioxide levels downwind of a smelter are excessive, susceptible populations such as asthmatics would know not to go outside. Again, this may be an acceptable interim control until permanent controls are installed.
Real time atmospheric and meteorological monitoring is sometimes used to avoid or reduce major air pollution events where multiple sources may exist. When it becomes evident that excessive air pollution levels are likely, the personal use of cars may be restricted and major emitting industries shut down.
Maintenance/housekeeping
In all cases the effectiveness of the controls depends on proper maintenance; the equipment has to operate as intended. Not only must the air pollution controls be maintained and used as intended, but the processes generating potential emissions must be maintained and operated properly. An example of an industrial process is a wood chip dryer with a failing temperature controller; if the dryer is operated at too high a temperature, it will emit more materials, and perhaps a different type of material, from the drying wood. An example of gas cleaner maintenance affecting emissions would be a poorly maintained baghouse with broken bags, which would allow particulates to pass through the filter.
Housekeeping also plays an important part in controlling total emissions. Dusts that are not quickly cleaned up inside the plant can become re-entrained and present a hazard to staff. If the dusts are carried outside of the plant, they are a community hazard. Poor housekeeping in the plant yard could present a significant risk to the community. Uncovered bulk materials, plant wastes or vehicle-raised dusts can result in pollutants being carried on the winds into the community. Keeping the yard clean, using proper containers or storage sites, is important in reducing total emissions. A system must be not only designed properly, but used properly as well if the community is to be protected.
A worst case example of poor maintenance and housekeeping would be the lead recovery plant with a broken lead dust conveyor. The dust was allowed to escape from the conveyor until the pile was so high the dust could slide down the pile and out a broken window. Local winds then carried the dust around the neighbourhood.
Equipment for Emission Sampling
Source sampling can be carried out for several reasons:
- To characterize the emissions. To design an air pollution control system, one must know what is being emitted. Not only the volume of gas, but the amount, identity and, in the case of particulates, size distribution of the material being emitted must be known. The same information is necessary to catalogue total emissions in a neighbourhood.
- To test equipment efficiency. After an air pollution control system has been purchased, it should be tested to ensure that it is doing the intended job.
- As part of a control system. When emissions are continuously monitored, the data can be used to fine tune the air pollution control system, or the plant operation itself.
- To determine compliance. When regulatory standards include emission limits, emission sampling can be used to determine compliance or non-compliance with the standards.
The type of sampling system used will depend on the reason for taking the samples, costs, availability of technology, and training of staff.
Visible emissions
Where there is a desire to reduce the soiling power of the air, improve visibility or prevent the introduction of aerosols into the atmosphere, standards may be based on visible emissions.
Visible emissions are composed of small particles or coloured gases. The more opaque a plume is, the more material is being emitted. This characteristic is evident to the sight, and trained observers can be used to assess emission levels. There are several advantages to using this method of assessing emission standards:
- No expensive equipment is required.
- One person can make many observations in a day.
- Plant operators can quickly assess the effects of process changes at low cost.
- Violators can be cited without time-consuming source testing.
- Questionable emissions can be located and the actual emissions then determined by source testing as described in the following sections.
Extractive sampling
A much more rigorous sampling method calls for a sample of the gas stream to be removed from the stack and analysed. Although this sounds simple, it does not translate into a simple sampling method.
The sample should be collected isokinetically, especially when particulates are being collected. Isokinetic sampling is defined as sampling by drawing the sample into the sampling probe at the same velocity that the material is moving in the stack or duct. This is done by measuring the velocity of the gas stream with a pitot tube and then adjusting the sampling rate so that the sample enters the probe at the same velocity. This is essential when sampling for particulates, since larger, heavier particles will not follow a change in direction or velocity. As a result the concentration of larger particles in the sample will not be representative of the gas stream and the sample will be inaccurate.
A sample train for sulphur dioxide is shown in figure 1. It is not simple, and a trained operator is required to ensure that a sample is collected properly. If something other than sulphur dioxide is to be sampled, the impingers and ice bath can be removed and the appropriate collection device inserted.
Figure 1. A diagram of an isokinetic sampling train for sulphur dioxide
Extractive sampling, particularly isokinetic sampling, can be very accurate and versatile, and has several uses:
- It is a recognized sampling method with adequate quality controls, and thus can be used to determine compliance with standards.
- The potential accuracy of the method makes it suitable for performance testing of new control equipment.
- Since samples can be collected and analysed under controlled laboratory conditions for many components, it is useful for characterizing the gas stream.
A simplified and automated sampling system can be connected to a continuous gas (electrochemical, ultraviolet-photometric or flame ionization sensors) or particulate (nephelometer) analyzer to continuously monitor emissions. This can provide documentation of the emissions, and instantaneous operating status of the air pollution control system.
In situ sampling
Emissions can also be sampled in the stack. Figure 2 is a representation of a simple transmissometer used to measure materials in the gas stream. In this example, a beam of light is projected across the stack to a photocell. The particulates or coloured gas will absorb or block some of the light. The more material, the less light will get to the photocell. (See figure 2.)
Figure 2. A simple transmissometer to measure particulates in a stack
By using different light sources and detectors such as ultraviolet light (UV), gases transparent to visible light can be detected. These devices can be tuned to specific gases, and thus can measure gas concentration in the waste stream.
An in situ monitoring system has an advantage over an extractive system in that it can measure the concentration across the entire stack or duct, whereas the extractive method measures concentrations only at the point from which the sample was extracted. This can result in significant error if the sample gas stream is not well mixed. However, the extractive method offers more methods of analysis, and thus perhaps can be used in more applications.
Since the in situ system provides a continuous readout, it can be used to document emissions, or to fine tune the operating system.
Water Pollution Control
This article is intended to provide the reader with an understanding of currently available technology for approaching water pollution control, building on the discussion of trends and occurrence provided by Hespanhol and Helmer in the chapter Environmental Health Hazards. The following sections address the control of water pollution problems, first under the heading “Surface Water Pollution Control” and then under the heading “Groundwater Pollution Control”.
Surface Water Pollution Control
Definition of water pollution
Water pollution refers to the qualitative state of impurity or uncleanliness in hydrologic waters of a certain region, such as a watershed. It results from an occurrence or process which causes a reduction in the utility of the earth’s waters, especially as related to human health and environmental effects. The pollution process stresses the loss of purity through contamination, which further implies intrusion by or contact with an outside source as the cause. The term tainted is applied to extremely low levels of water pollution, as in their initial corruption and decay. Defilement is the result of pollution and suggests violation or desecration.
Hydrologic waters
The earth’s natural waters may be viewed as a continuously circulating system as shown in figure 1, which provides a graphic illustration of waters in the hydrologic cycle, including both surface and subsurface waters.
Figure 1. The hydrologic cycle
As a reference for water quality, distilled waters (H2O) represent the highest state of purity. Waters in the hydrologic cycle may be viewed as natural, but are not pure. They become polluted from both natural and human activities. Natural degradation effects may result from a myriad of sources - from fauna, flora, volcano eruptions, lightning strikes causing fires and so on, which on a long-term basis are considered to be prevailing background levels for scientific purposes.
Human-made pollution disrupts the natural balance by superimposing waste materials discharged from various sources. Pollutants may be introduced into the waters of the hydrologic cycle at any point. For example: atmospheric precipitation (rainfall) may become contaminated by air pollutants; surface waters may become polluted in the runoff process from watersheds; sewage may be discharged into streams and rivers; and groundwaters may become polluted through infiltration and underground contamination.
Figure 2 shows a distribution of hydrologic waters. Pollution is then superimposed on these waters and may therefore be viewed as an unnatural or unbalanced environmental condition. The process of pollution may occur in waters of any part of the hydrologic cycle, and is more obvious on the earth’s surface in the form of runoff from watersheds into streams and rivers. However groundwater pollution is also of major environmental impact and is discussed following the section on surface water pollution.
Figure 2. Distribution of precipitation
Watershed sources of water pollution
Watersheds are the originating domain of surface water pollution. A watershed is defined as an area of the earth’s surface on which hydrologic waters fall, accumulate, are used, disposed of, and eventually are discharged into streams, rivers or other bodies of water. It is comprised of a drainage system with ultimate runoff or collection in a stream or river. Large river watersheds are usually referred to as drainage basins. Figure 3 is a representation of the hydrologic cycle on a regional watershed. For a region, the disposition of the various waters can be written as a simple equation, which is the basic equation of hydrology as written by Viessman, Lewis and Knapp (1989); typical units are mm/year:
P - R - G - E - T = ±S
where:
P = precipitation (i.e., rainfall, snowfall, hail)
R = runoff or watershed surface flow
G = groundwater
E = evaporation
T = transpiration
S = surface storage
Figure 3. Regional hydrologic cycle
Precipitation is viewed as the initiating form in the above hydrologic budget. The term runoff is synonymous with stream flow. Storage refers to reservoirs or detention systems which collect waters; for example, a human-made dam (barrage) on a river creates a reservoir for purposes of water storage. Groundwater collects as a storage system and may flow from one location to another; it may be influent or effluent in relation to surface streams. Evaporation is a water surface phenomenon, and transpiration is associated with transmission from biota.
Although watersheds may vary greatly in size, certain drainage systems for water pollution designation are classified as urban or non-urban (agricultural, rural, undeveloped) in character. Pollution occurring within these drainage systems originates from the following sources:
Point sources: waste discharges into a receiving water body at a specific location, at a point such as a sewer pipe or some type of concentrated system outlet.
Non-point (dispersed) sources: pollution entering a receiving water body from dispersed sources in the watershed; uncollected rainfall runoff water drainage into a stream is typical. Non-point sources are also sometimes referred to as “diffuse” waters; however, the term dispersed is seen as more descriptive.
Intermittent sources: from a point or source which discharges under certain circumstances, such as with overloaded conditions; combined sewer overflows during heavy rainfall runoff periods are typical.
Water pollutants in streams and rivers
When deleterious waste materials from the above sources are discharged into streams or other bodies of water, they become pollutants which have been classified and described in a previous section. Pollutants or contaminants which enter a body of water can be further divided into:
- degradable (non-conservative) pollutants: impurities which eventually decompose into harmless substances or which may be removed by treatment methods; that is, certain organic materials and chemicals, domestic sewage, heat, plant nutrients, most bacteria and viruses, certain sediments
- non-degradable (conservative) pollutants: impurities which persist in the water environment and do not reduce in concentration unless diluted or removed through treatment; that is, certain organic and inorganic chemicals, salts, colloidal suspensions
- hazardous waterborne pollutants: complex forms of deleterious wastes including toxic trace metals, certain inorganic and organic compounds
- radionuclide pollutants: materials which have been subjected to a radioactive source.
Water pollution control regulations
Broadly applicable water pollution control regulations are generally promulgated by national governmental agencies, with more detailed regulations by states, provinces, municipalities, water districts, conservation districts, sanitation commissions and others. At the national and state (or province) levels, environmental protection agencies (EPAs) and ministries of health are usually charged with this responsibility. In the discussion of regulations below, the format and certain portions follow the example of the water quality standards currently applicable for the US State of Ohio.
Water quality use designations
The ultimate goal in the control of water pollution would be zero discharge of pollutants to water bodies; however, complete achievement of this objective is usually not cost effective. The preferred approach is to set limitations on waste disposal discharges for the reasonable protection of human health and the environment. Although these standards may vary widely in different jurisdictions, use designations for specific bodies of water are commonly the basis, as briefly addressed below.
Water supplies include:
- public water supply: waters which with conventional treatment will be suitable for human consumption
- agricultural supply: waters suitable for irrigation and livestock watering without treatment
- industrial/commercial supply: waters suitable for industrial and commercial uses with or without treatment.
Recreational activities include:
- bathing waters: waters which during certain seasons are suitable for swimming as approved for water quality along with protective conditions and facilities
- primary contact: waters which during certain seasons are suitable for full body contact recreation such as swimming, canoeing and underwater diving with minimal threat to public health as a result of water quality
- secondary contact: waters which during certain seasons are suitable for partial body contact recreation such as, but not limited to, wading, with minimal threat to public health as a result of water quality.
Public water resources are categorized as water bodies which lie within park systems, wetland, wildlife areas, wild, scenic and recreational rivers and publicly owned lakes, and waters of exceptional recreational or ecological significance.
Aquatic life habitats
Typical designations will vary according to climates, but relate to conditions in water bodies for supporting and maintaining certain aquatic organisms, especially various species of fish. For example, use designations in a temperate climate as subdivided in regulations for the State of Ohio Environmental Protection Agency (EPA) are listed below without detailed descriptions:
- warmwater
- limited warmwater
- exceptional warmwater
- modified warmwater
- seasonal salmonid
- coldwater
- limited resource water.
Water pollution control criteria
Natural waters and wastewaters are characterized in terms of their physical, chemical and biological composition. The principal physical properties and the chemical and biological constituents of wastewater and their sources are a lengthy list, reported in a textbook by Metcalf and Eddy (1991). Analytical methods for these determinations are given in a widely used manual entitled Standard Methods for the Examination of Water and Waste Water by the American Public Health Association (1995).
Each designated water body should be controlled according to regulations which may be comprised of both basic and more detailed numerical criteria as briefly discussed below.
Basic freedom from pollution. To the extent practical and possible, all bodies of water should attain the basic criteria of the “Five Freedoms from Pollution”:
- free from suspended solids or other substances that enter the waters as a result of human activity and that will settle to form putrid or otherwise objectionable sludge deposits, or that will adversely affect aquatic life
- free from floating debris, oil, scum and other floating materials entering the waters as a result of human activity in amounts sufficient to be unsightly or cause degradation
- free from materials entering the waters as a result of human activity, producing colour, odour or other conditions in such degree as to create a nuisance
- free from substances entering the waters as a result of human activity, in concentrations that are toxic or harmful to human, animal or aquatic life and/or are rapidly lethal in the mixing zone
- free from nutrients entering the waters as a result of human activity, in concentrations that create nuisance growths of aquatic weeds and algae.
Water quality criteria are numerical limitations and guidelines for the control of chemical, biological and toxic constituents in bodies of water.
With over 70,000-plus chemical compounds in use today it is impractical to specify the control of each. However, criteria for chemicals can be established on the basis of limitations as they first of all relate to three major classes of consumption and exposure:
Class 1: Chemical criteria for protection of human health are of first major concern and should be set according to recommendations from governmental health agencies, the WHO and recognized health research organizations.
Class 2: Chemical criteria for control of agricultural water supply should be based on recognized scientific studies and recommendations which will protect against adverse effects on crops and livestock as a result of crop irrigation and livestock watering.
Class 3: Chemical criteria for protection of aquatic life should be based on recognized scientific studies regarding the sensitivity of these species to specific chemicals and also as related to human consumption of fish and sea foods.
Wastewater effluent criteria relate to limitations on pollutant constituents present in wastewater effluents and are a further method of control. They may be set as related to the water use designations of bodies of water and as they relate to the above classes for chemical criteria.
Biological criteria are based on water body habitat conditions which are needed to support aquatic life.
Organic content of wastewaters and natural waters
The gross content of organic matter is most important in characterizing the pollutional strength of both wastewater and natural waters. Three laboratory tests are commonly used for this purpose:
Biochemical oxygen demand (BOD): five-day BOD (BOD5) is the most widely used parameter; this test measures the dissolved oxygen used by micro-organisms in the biochemical oxidation of organic matter over this period.
Chemical oxygen demand (COD): this test is to measure the organic matter in municipal and industrial wastes that contain compounds that are toxic to biological life; it is a measure of the oxygen equivalent of the organic matter that can be oxidized.
Total organic carbon (TOC): this test is especially applicable to small concentrations of organic matter in water; it is a measure of the organic matter that is oxidized to carbon dioxide.
Antidegradation policy regulations
Antidegradation policy regulations are a further approach for preventing the spread of water pollution beyond certain prevailing conditions. As an example, the Ohio Environmental Protection Agency Water Quality Standards antidegradation policy consists of three tiers of protection:
Tier 1: Existing uses must be maintained and protected. No further water quality degradation is allowed that would interfere with existing designated uses.
Tier 2: Next, water quality better than that needed to protect uses must be maintained unless it is shown that a lower water quality is necessary for important economic or social development, as determined by the EPA Director.
Tier 3: Finally, the quality of water resource waters must be maintained and protected. Their existing ambient water quality is not to be degraded by any substances determined to be toxic or to interfere with any designated use. Increased pollutant loads are allowed to be discharged into water bodies if they do not result in lowering existing water quality.
Water pollution discharge mixing zones and waste load allocation modelling
Mixing zones are areas in a body of water which allow for treated or untreated wastewater discharges to attain stabilized conditions, as illustrated in figure 4 for a flowing stream. The discharge is initially in a transitory state which becomes progressively diluted from the source concentration to the receiving water conditions. It is not to be considered as a treatment entity and may be delineated with specific restrictions.
Figure 4. Mixing zones
Typically, mixing zones must not:
- interfere with migration, survival, reproduction or growth of aquatic species
- include spawning or nursery areas
- include public water supply intakes
- include bathing areas
- constitute more than 1/2 the width of a stream
- constitute more than 1/2 the cross-sectional area of a stream mouth
- extend downstream for a distance more than five times the stream width.
Waste load allocation studies have become important because of the high cost of nutrient control of wastewater discharges to avoid instream eutrophication (defined below). These studies generally employ the use of computer models for simulation of water quality conditions in a stream, particularly with regard to nutrients such as forms of nitrogen and phosphorous, which affect the dissolved oxygen dynamics. Traditional water quality models of this type are represented by the US EPA model QUAL2E, which has been described by Brown and Barnwell (1987). A more recent model proposed by Taylor (1995) is the Omni Diurnal Model (ODM), which includes a simulation of the impact of rooted vegetation on instream nutrient and dissolved oxygen dynamics.
Variance provisions
All water pollution control regulations are limited in perfection and therefore should include provisions which allow for judgemental variance based on certain conditions which may prevent immediate or complete compliance.
Risk assessment and management as related to water pollution
The above water pollution control regulations are typical of worldwide governmental approaches for achieving compliance with water quality standards and wastewater effluent discharge limits. Generally these regulations have been set on the basis of health factors and scientific research; where some uncertainty exists as to possible effects, safety factors often are applied. Implementation of certain of these regulations may be unreasonable and exceedingly costly for the public at large as well as for private enterprise. Therefore there is a growing concern for more efficient allocation of resources in achieving goals for water quality improvement. As previously pointed out in the discussion of hydrologic waters, pristine purity does not exist even in naturally occurring waters.
A growing technological approach encourages assessment and management of ecological risks in the setting of water pollution regulations. The concept is based on an analysis of the ecological benefits and costs in meeting standards or limits. Parkhurst (1995) has proposed the application of aquatic ecological risk assessment as an aid in setting water pollution control limits, particularly as applicable for the protection of aquatic life. Such risk assessment methods may be applied to estimate the ecological effects of chemical concentrations for a broad range of surface water pollution conditions including:
- point source pollution
- non-point source pollution
- existing contaminated sediments in stream channels
- hazardous wastes sites as related to water bodies
- analysis of existing water pollution control criteria.
The proposed method consists of three tiers; as shown in figure 5 which illustrates the approach.
Figure 5. Methods for conducting risk assessment for successive tiers of analysis. Tier 1: Screening level; Tier 2: Quantification of potentially significant risks ; Tier 3: Site-specific risk quantification
Water pollution in lakes and reservoirs
Lakes and reservoirs provide for the volumetric storage of watershed inflow and may have long flushing time periods as compared with the rapid inflow and outflow for a reach in a flowing stream. Therefore they are of special concern with regard to the retention of certain constituents, especially nutrients including forms of nitrogen and phosphorous which promote eutrophication. Eutrophication is a natural ageing process in which the water content becomes organically enriched, leading to the domination of undesirable aquatic growth, such as algae, water hyacinth and so on. The eutrophic process tends to decrease aquatic life and has detrimental dissolved oxygen effects. Both natural and cultural sources of nutrients may promote the process, as illustrated by Preul (1974) in figure 6, showing a schematic listing of nutrient sources and sinks for Lake Sunapee, in the US State of New Hampshire.
Figure 6. Schematic listing of nutrient (nitrogen and phosphorus) sources and sinks for Lake Sunapee, New Hampshire (US)
Lakes and reservoirs, of course, can be sampled and analysed to determine their trophic status. Analytical studies usually start with a basic nutrient balance such as the following:
(lake influent nutrients) = (lake effluent nutrients) + (nutrient retention in lake)
This basic balance can be further expanded to include the various sources shown in figure 6.
Flushing time is an indication of the relative retention aspects of a lake system. Shallow lakes, such as Lake Erie, have relatively short flushing times and are associated with advanced eutrophication because shallow lakes often are more conducive to aquatic plant growth. Deep lakes such as Lake Tahoe and Lake Superior have very long flushing periods, which are usually associated with lakes with minimal eutrophication because up to the present time, they have not been overloaded and also because their extreme depths are not conducive to extensive aquatic plant growth except in the epilimnion (upper zone). Lakes in this category are generally classified as oligotrophic, on the basis that they are relatively low in nutrients and support minimal aquatic growth such as algae.
It is of interest to compare the flushing times of some major US lakes as reported by Pecor (1973) using the following calculation basis:
lake flushing time (LFT) = (lake storage volume)/(lake outflow)
Some examples are: Lake Wabesa (Michigan), LFT=0.30 years; Houghton Lake (Michigan), 1.4 years; Lake Erie, 2.6 years; Lake Superior, 191 years; Lake Tahoe, 700 years.
Although the relationship between the process of eutrophication and nutrient content is complex, phosphorous is typically recognized as the limiting nutrient. Based on fully mixed conditions, Sawyer (1947) reported that algal blooms tend to occur if nitrogen values exceed 0.3 mg/l and phosphorous exceeds 0.01 mg/l. In stratified lakes and reservoirs, low dissolved oxygen levels in the hypoliminion are early signs of eutrophication. Vollenweider (1968, 1969) has developed critical loading levels of total phosphorous and total nitrogen for a number of lakes based on nutrient loadings, mean depths and trophic states. For a comparison of work on this subject, Dillon (1974) has published a critical review of Vollenweider’s nutrient budget model and other related models. More recent computer models are also available for simulating nitrogen/phosphorous cycles with temperature variations.
Water pollution in estuaries
An estuary is an intermediate passageway of water between the mouth of a river and a sea coast. This passageway is comprised of a river mouth channel reach with river inflow (fresh water) from upstream and outflow discharge on the downstream side into a constantly changing tailwater level of sea water (salt water). Estuaries are continuously affected by tidal fluctuations and are among the most complex bodies of water encountered in water pollution control. The dominant features of an estuary are variable salinity, a salt wedge or interface between salt and fresh water, and often large areas of shallow, turbid water overlying mud flats and salt marshes. Nutrients are largely supplied to an estuary from the inflowing river and combine with the sea water habitat to provide prolific production of biota and sea life. Especially desired are seafoods harvested from estuaries.
From a water pollution standpoint, estuaries are individually complex and generally require special investigations employing extensive field studies and computer modelling. For a further basic understanding, the reader is referred to Reish 1979, on marine and estuarine pollution; and to Reid and Wood 1976, on the ecology of inland waters and estuaries.
Water pollution in marine environments
Oceans may be viewed as the ultimate receiving water or sink, since wastes carried by rivers finally discharge into this marine environment. Although oceans are vast bodies of salt water with seemingly unlimited assimilation capacity, pollution tends to blight coastlines and further affects marine life.
Sources of marine pollutants include many of those encountered in land-based wastewater environments plus more as related to marine operations. A limited list is given below:
- domestic sewage and sludge, industrial wastes, solid wastes, shipboard wastes
- fishery wastes, sediments and nutrients from rivers and land runoff
- oil spills, offshore oil exploration and production wastes, dredge operations
- heat, radioactive wastes, waste chemicals, pesticides and herbicides.
Each of the above requires special handling and methods of control. The discharge of domestic sewage and sewage sludges through ocean outfalls is perhaps the major source of marine pollution.
For current technology on this subject, the reader is referred to the book on marine pollution and its control by Bishop (1983).
Techniques for reducing pollution in wastewater discharges
Large-scale wastewater treatment is typically carried out by municipalities, sanitary districts, industries, commercial enterprises and various pollution control commissions. The purpose here is to describe contemporary methods of municipal wastewater treatment and then to provide some insights regarding treatment of industrial wastes and more advanced methods.
In general, all processes of wastewater treatment may be grouped into physical, chemical or biological types, and one or more of these may be employed to achieve a desired effluent product. This classification grouping is most appropriate in the understanding of wastewater treatment approaches and is tabulated in table 1.
Table 1. General classification of wastewater treatment operations and processes
|
Physical Operations |
Chemical Processes |
Biological Processes |
|
Flow measurement |
Precipitation |
Aerobic action |
Contemporary methods of wastewater treatment
The coverage here is limited and is intended to provide a conceptual overview of current wastewater treatment practices around the world rather than detailed design data. For the latter, the reader is referred to Metcalf and Eddy 1991.
Municipal wastewaters along with some intermingling of industrial/commercial wastes are treated in systems commonly employing primary, secondary and tertiary treatment as follows:
Primary treatment system: Pre-treat ® Primary settling ® Disinfection (chlorination) ® Effluent
Secondary treatment system: Pre-treat ® Primary settling ® Biological unit ® Second settling ® Disinfection (chlorination) ® Effluent to stream
Tertiary treatment system: Pre-treat ® Primary settling ® Biological unit ® Second settling ® Tertiary unit ® Disinfection (chlorination) ® Effluent to stream
Figure 7 further shows a schematic diagram of a conventional wastewater treatment system. Overview descriptions of the above processes follow.
Figure 7. Schematic diagram of conventional wastewater treatment
Primary treatment
The basic objective of primary treatment for municipal wastewaters, including domestic sewage intermingled with some industrial/commercial wastes, is to remove suspended solids and clarify the wastewater, to make it suitable for biological treatment. After some pre-treatment handling such as screening, grit removal and comminution, the main process of primary sedimentation is the settling of the raw wastewater in large settling tanks for periods up to several hours. This process removes from 50 to 75% of the total suspended solids, which are drawn off as an underflow sludge collected for separate treatment. The overflow effluent from the process then is directed for secondary treatment. In certain cases, chemicals may be employed to improve the degree of primary treatment.
Secondary treatment
The portion of the organic content of the wastewater which is finely suspended or dissolved and not removed in the primary process, is treated by secondary treatment. The generally accepted forms of secondary treatment in common use include trickling filters, biological contactors such as rotating discs, activated sludge, waste stabilization ponds, aerated pond systems and land application methods, including wetland systems. All of these systems will be recognized as employing biological processes of some form or another. The most common of these processes are briefly discussed below.
Biological contactor systems. Trickling filters are one of the earliest forms of this method for secondary treatment and are still widely used with some improved methods of application. In this treatment, the effluent from the primary tanks is applied uniformly onto a bed of media, such as rock or synthetic plastic media. Uniform distribution is accomplished typically by trickling the liquid from perforated piping rotated over the bed intermittently or continuously according to the desired process. Depending on the rate of organic and hydraulic loadings, trickling filters can remove up to 95% of the organic content, usually analysed as biochemical oxygen demand (BOD). There are numerous other more recent biological contactor systems in use which can provide treatment removals in the same range; some of these methods offer special advantages, particularly applicable in certain limiting conditions such as space, climate and so on. It is to be noted that a following secondary settling tank is considered to be a necessary part of completing the process. In secondary settling, some so-called humus sludge is drawn off as an underflow, and the overflow is discharged as a secondary effluent.
Activated sludge. In the most common form of this biological process, primary treated effluent flows into an activated sludge unit tank containing a previously existing biological suspension called activated sludge. This mixture is referred to as mixed liquor suspended solids (MLSS) and is provided a contact period typically ranging from several hours up to 24 hours or more, depending on the desired results. During this period the mixture is highly aerated and agitated to promote aerobic biological activity. As the process finalizes, a portion of the mixture (MLSS) is drawn off and returned to the influent for continuation of the biological activation process. Secondary settling is provided following the activated sludge unit for the purpose of settling out the activated sludge suspension and discharging a clarified overflow as an effluent. The process is capable of removing up to about 95% of the influent BOD.
Tertiary treatment
A third level of treatment may be provided where a higher degree of pollutant removal is required. This form of treatment may typically include sand filtration, stabilization ponds, land disposal methods, wetlands and other systems which further stabilize the secondary effluent.
Disinfection of effluents
Disinfection is commonly required to reduce bacteria and pathogens to acceptable levels. Chlorination, chlorine dioxide, ozone and ultraviolet light are the most commonly used processes.
Overall wastewater treatment plant efficiency
Wastewaters include a broad range of constituents which generally are classified as suspended and dissolved solids, inorganic constituents and organic constituents.
The efficiency of a treatment system can be measured in terms of the percentage removal of these constituents. Common parameters of measurement are:
- BOD: biochemical oxygen demand, measured in mg/l
- COD: chemical oxygen demand, measured in mg/l
- TSS: total suspended solids, measured in mg/l
- TDS: total dissolved solids, measured in mg/l
- nitrogen forms: including nitrate and ammonia, measured in mg/l (nitrate is of particular concern as a nutrient in eutrophication)
- phosphate: measured in mg/l (also of particular concern as a nutrient in eutrophication)
- pH: degree of acidity, measured as a number from 1 (most acid) to 14 (most alkaline)
- coliform bacteria counts: measured as most probable number per 100 ml (Escherichia and fecal coliform bacteria are most common indicators).
Industrial wastewater treatment
Types of industrial wastes
Industrial (non-domestic) wastes are numerous and vary greatly in composition; they may be highly acidic or alkaline, and often require a detailed laboratory analysis. Specialized treatment may be necessary to render them innocuous before discharge. Toxicity is of great concern in the disposal of industrial wastewaters.
Representative industrial wastes include: pulp and paper, slaughterhouse, brewery, tannery, food processing, cannery, chemical, petroleum, textile, sugar, laundry, meat and poultry, hog feeding, rendering and many others. The initial step in treatment design development is an industrial waste survey, which provides data on variations in flow and waste characteristics. Undesirable waste characteristics as listed by Eckenfelder (1989) can be summarized as follows:
- soluble organics causing depletion of dissolved oxygen
- suspended solids
- trace organics
- heavy metals, cyanide and toxic organics
- colour and turbidity
- nitrogen and phosphorus
- refractory substances resistant to biodegradation
- oil and floating material
- volatile materials.
The US EPA has further defined a list of toxic organic and inorganic chemicals with specific limitations in granting discharge permits. The list includes more than 100 compounds and is too long to reprint here, but may be requested from the EPA.
Treatment methods
The handling of industrial wastes is more specialized than the treatment of domestic wastes; however, where amenable to biological reduction, they are usually treated using methods similar to those previously described (secondary/tertiary biological treatment approaches) for municipal systems.
Waste stabilization ponds are a common method of organic wastewater treatment where sufficient land area is available. Flow-through ponds are generally classified according to their bacterial activity as aerobic, facultative or anaerobic. Aerated ponds are supplied with oxygen by diffused or mechanical aeration systems.
Figure 8 and figure 9 show sketches of waste stabilization ponds.
Figure 8. Two-cell stabilization pond: cross sectional diagram
Figure 9. Aerated lagoon types: schematic diagram
Pollution prevention and waste minimization
When industrial waste in-plant operations and processes are analysed at their source, they often can be controlled so as to prevent significant polluting discharges.
Recirculation techniques are important approaches in pollution prevention programmes. A case study example is a recycling plan for a leather tannery wastewater effluent published by Preul (1981), which included chrome recovery/reuse along with the complete recirculation of all tannery wastewaters with no effluent to any stream except in emergencies. The flow diagram for this system is shown in figure 10.
Figure 10. Flow diagram for tannery wastewater effluent recycling system
For more recent innovations in this technology, the reader is referred to a publication on pollution prevention and waste minimization by the Water Environment Federation (1995).
Advanced methods of wastewater treatment
A number of advanced methods are available for higher degrees of removal of pollution constituents as may be required. A general listing includes:
filtration (sand and multimedia)
chemical precipitation
carbon adsorption
electrodialysis
distillation
nitrification
algae harvesting
reclamation of effluents
micro-straining
ammonia stripping
reverse osmosis
ion exchange
land application
denitrification
wetlands.
The most appropriate process for any situation must be determined on the basis of the quality and quantity of the raw wastewater, the receiving water requirements and, of course, costs. For further reference, see Metcalf and Eddy 1991, which includes a chapter on advanced wastewater treatment.
Advanced wastewater treatment case study
The case study of the Dan Region Sewage Reclamation Project discussed elsewhere in this chapter provides an excellent example of innovative methods for wastewater treatment and reclamation.
Thermal pollution
Thermal pollution is a form of industrial waste, defined as deleterious increases or reductions in normal water temperatures of receiving waters caused by the disposal of heat from human-made facilities. The industries producing major waste heat are fossil fuel (oil, gas and coal) and nuclear power generating plants, steel mills, petroleum refineries, chemical plants, pulp and paper mills, distilleries and laundries. Of particular concern is the electric power generating industry which supplies energy for many countries (e.g., about 80% in the US).
Impact of waste heat on receiving waters
Influence on waste assimilation capacity
- Heat increases biological oxidation.
- Heat decreases oxygen saturation content of water and decreases rate of natural reoxygenation.
- The net effect of heat is generally detrimental during warm months of year.
- Winter effect may be beneficial in colder climates, where ice conditions are broken up and surface aeration is provided for fish and aquatic life.
Influence on aquatic life
Many species have temperature tolerance limits and need protection, particularly in heat affected reaches of a stream or body of water. For example, cold water streams usually have the highest type of sport fish such as trout and salmon, whereas warm waters generally support coarse fish populations, with certain species such as pike and bass fish in intermediate temperature waters.
Figure 11. Heat exchange at the boundaries of a receiving water cross section
Thermal analysis in receiving waters
Figure 11 illustrates the various forms of natural heat exchange at the boundaries of a receiving water. When heat is discharged to a receiving water such as a river, it is important to analyse the river capacity for thermal additions. The temperature profile of a river can be calculated by solving a heat balance similar to that used in calculating dissolved oxygen sag curves. The principal factors of the heat balance are illustrated in figure 12 for a river reach between points A and B. Each factor requires an individual calculation dependent on certain heat variables. As with a dissolved oxygen balance, the temperature balance is simply a summation of temperature assets and liabilities for a given section. Other more sophisticated analytical approaches are available in the literature on this subject. The results from the heat balance calculations can be used in establishing heat discharge limitations and possibly certain use constraints for a body of water.
Figure 12. River capacity for thermal additions
Thermal pollution control
The main approaches for the control of thermal pollution are:
- improved power plant operation efficiencies
- cooling towers
- isolated cooling ponds
- consideration of alternative methods of power generation such as hydro-power.
Where physical conditions are favourable within certain environmental limits, hydro-electric power should be considered as an alternative to fossil-fuel or nuclear power generation. In hydro-electric power generation, there is no disposal of heat and there is no discharge of waste waters causing water pollution.
Groundwater Pollution Control
Importance of groundwater
Since the world’s water supplies are widely extracted from aquifers, it is most important that these sources of supply be protected. It is estimated that more than 95% of the earth’s available fresh water supply is underground; in the United States approximately 50% of the drinking water comes from wells, according to the 1984 US Geological Survey. Because underground water pollution and movement are of subtle and unseen nature, less attention sometimes is given to the analysis and control of this form of water degradation than to surface water pollution, which is far more obvious.
Figure 13. Hydrologic cycle and sources of groundwater contamination
Sources of underground pollution
Figure 13 shows the hydrologic cycle with superimposed sources of groundwater contamination. A complete listing of the potential sources of underground pollution is extensive; however, for illustration the most obvious sources include:
- industrial waste discharges
- polluted streams in contact with aquifers
- mining operations
- solid and hazardous waste disposal
- underground storage tanks such as for petroleum
- irrigation systems
- artificial recharge
- sea water encroachment
- spills
- polluted ponds with permeable bottoms
- disposal wells
- septic tank tile fields and leaching pits
- improper well drilling
- agricultural operations
- roadway de-icing salts.
Specific pollutants in underground contamination are further categorized as:
- undesirable chemical constituents (typical, not complete list) - organic and inorganic (e.g., chloride, sulphate, iron, manganese, sodium, potassium)
- total hardness and total dissolved solids
- toxic constituents (typical, not complete list) - nitrate, arsenic, chromium, lead, cyanide, copper, phenols, dissolved mercury
- undesirable physical characteristics - taste, colour and odour
- pesticides and herbicides - chlorinated hydrocarbons and others
- radioactive materials - various forms of radioactivity
- biological - bacteria, viruses, parasites and so on
- acid (low pH) or caustic (high pH).
Of the above, nitrates are of special concern in both ground waters and surface waters. In groundwater supplies, nitrates can cause the disease methaemoglobinaemia (infant cyanosis). They further cause detrimental eutrophication effects in surface waters and occur in a wide range of water resources, as reported by Preul (1991). Preul (1964, 1967, 1972) and Preul and Schroepfer (1968) have also reported on the underground movement of nitrogen and other pollutants.
Pollution travel in underground domain
Groundwater movement is exceedingly slow and subtle as compared with the travel of surface waters in the hydrologic cycle. For a simple understanding of the travel of ordinary groundwater under ideal steady flow conditions, Darcy’s Law is the basic approach for the evaluation of groundwater movement at low Reynolds numbers (R):
V = K(dh/dl)
where:
V = velocity of groundwater in aquifer, m/day
K = coefficient of permeability of aquifer
(dh/dl) = hydraulic gradient which represents the driving force for movement.
In pollutant travel underground, ordinary groundwater (H2O) is generally the carrying fluid and can be calculated to move at a rate according to the parameters in Darcy’s Law. However, the rate of travel or velocity of a pollutant, such as an organic or inorganic chemical, may be different due to advection and hydrodynamic dispersion processes. Certain ions move slower or faster than the general rate of groundwater flow as a result of reactions within the aquifer media, so that they can be categorized as “reacting” or “non-reacting”. Reactions are generally of the following forms:
- physical reactions between the pollutant and the aquifer and/or the transporting liquid
- chemical reactions between the pollutant and the aquifer and/or the transporting liquid
- biological actions on the pollutant.
The following are typical of reacting and non-reacting underground pollutants:
- reacting pollutants - chromium, ammonium ion, calcium, sodium, iron and so on; cations in general; biological constituents; radioactive constituents
- non-reacting pollutants - chloride, nitrate, sulphate and so on; certain anions; certain pesticide and herbicide chemicals.
At first, it might seem that reacting pollutants are the worst type, but this may not always be the case because the reactions detain or retard pollutant travel concentrations whereas non-reacting pollutant travel may be largely uninhibited. Certain “soft” domestic and agricultural products are now available which biologically degrade after a period of time and therefore avoid the possibility of groundwater contamination.
Aquifer remediation
Prevention of underground pollution is obviously the best approach; however, uncontrolled existence of polluted groundwater conditions usually is made known after its occurrence, such as by complaints from water well users in the area. Unfortunately, by the time the problem is recognized, severe damage may have occurred and remediation is necessary. Remediation may require extensive hydro-geological field investigations with laboratory analyses of water samples in order to establish the extent of pollutant concentrations and travel plumes. Often existing wells can be used in initial sampling, but severe cases may require extensive borings and water samplings. These data can then be analysed to establish current conditions and to make future condition predictions. The analysis of groundwater contamination travel is a specialized field often requiring the use of computer models to better understand the groundwater dynamics and to make predictions under various constraints. A number of two- and three-dimensional computer models are available in the literature for this purpose. For more detailed analytical approaches, the reader is referred to the book by Freeze and Cherry (1987).
Pollution prevention
The preferred approach for the protection of groundwater resources is pollution prevention. Although drinking water standards generally apply to the use of groundwater supplies, the raw water supplies require protection from contamination. Governmental entities such as ministries of health, natural resources agencies, and environmental protection agencies are generally responsible for such activities. Groundwater pollution control efforts are largely directed at protection of aquifers and the prevention of pollution.
Pollution prevention requires land-use controls in the form of zoning and certain regulations. Laws may apply to the prevention of specific functions as particularly applicable to point sources or actions which potentially may cause pollution. Control by land-use zoning is a groundwater protection tool which is most effective at the municipal or county level of government. Aquifer and wellhead protection programmes as discussed below are leading examples of pollution prevention.
An aquifer protection programme requires establishing the boundaries of the aquifer and its recharge areas. Aquifers may be of an unconfined or confined type, and therefore need to be analysed by a hydrologist to make this determination. Most major aquifers are generally well known in developed countries, but other areas may require field investigations and hydrogeologic analysis. The key element of the programme in the protection of the aquifer from water quality degradation is control of land use over the aquifer and its recharge areas.
Wellhead protection is a more definitive and limited approach which applies to the recharge area contributing to a particular well. The US federal government by amendments passed in 1986 to the Safe Drinking Water Act (SDWA) (1984) now requires that specific wellhead protection areas be established for public supply wells. The wellhead protection area (WHPA) is defined in the SDWA as “the surface and subsurface area surrounding a water well or well field, supplying a public water supply system, through which contaminants are reasonably likely to move toward and reach such water well or well field.” The main objective in the WHPA programme, as outlined by the US EPA (1987), is the delineation of well protection areas based on selected criteria, well operation and hydrogeologic considerations.
Dan Region Sewage Reclamation Project: A Case Study
Conception and Design
The Dan Region Reclamation Project of municipal wastewater is the biggest project of its kind in the world. It consists of facilities for treatment and groundwater recharge of municipal wastewater from the Dan Region Metropolitan Area - an eight-city conglomerate centred around Tel Aviv, Israel, with a combined population of about 1.5 million inhabitants. The project was created for the purpose of collection, treatment and disposal of municipal wastewater. The reclaimed effluent, after a relatively long detention period in the underground aquifer, is pumped for unrestricted agricultural use, irrigating the arid Negev (the southern part of Israel). A general scheme of the project is given in figure 1. The project was established in the 1960s, and has been growing continuously. At present, the system collects and treats about 110 x 106 m3 per year. Within a few years, at its final stage, the system will handle 150 to 170 x 106 m3 per year.
Figure 1. Dan Region Sewage Reclamation Plant: layout
Sewage treatment plants are known to create a multitude of environmental and occupational health problems. The Dan Region project is a unique system of national importance that combines national benefit together with considerable saving of water resources, high treatment efficiency and production of inexpensive water, without creating excessive occupational hazards.
Throughout the design, installation and routine operation of the system, careful consideration has been given to water sanitation and occupational hygiene concerns. All necessary precautions have been taken to ensure that the reclaimed wastewater will be practically as safe as regular drinking water, in the event that people accidentally drink or swallow it. Similarly, appropriate attention has been given to the issue of reducing to the minimum any potential exposure to accidents or other biological, chemical or physical hazards that may affect either the workers at the wastewater treatment plant proper or other workers engaged in the disposal and agricultural use of the reclaimed water.
At Stage One of the project, the wastewater was biologically treated by a system of facultative oxidation ponds with recirculation and additional chemical treatment by a lime-magnesium process, followed by detention of the high-pH effluent in “polishing ponds”. The partially treated effluent was recharged to the regional groundwater aquifer by means of the Soreq spreading basins.
At Stage Two, the wastewater conveyed to the treatment plant undergoes mechanical-biological treatment by means of an activated-sludge process with nitrification-denitrification. The secondary effluent is recharged to the groundwater by means of the spreading basins Yavneh 1 and Yavneh 2.
The complete system consists of a number of different elements complementing each other:
- a wastewater treatment plant system, comprised of an activated-sludge plant (the biomechanical plant), which treats most of the wastes, and of a system of oxidation and polishing ponds used mostly for treatment of excess sewage flows
- a groundwater recharge system for the treated effluent, which consists of spreading basins, at two different sites (Yavneh and Soreq), that are intermittently flooded; the absorbed effluent passes through the soil’s unsaturated zone and through a portion of the aquifer, and creates a special zone that is dedicated to complementary effluent treatment and seasonal storage, which is called SAT (soil-aquifer-treatment)
- networks of observation wells (53 wells all together) which surround the recharge basins and allow the monitoring of the efficiency of the treatment process
- networks of recovery wells (a total of 74 active wells in 1993) which surround the recharge sites
- a special and separate reclaimed water conveyance main for unrestricted irrigation of agricultural areas in the Negev; this main is called “The Third Negev Line”, and it complements the water supply system to the Negev, which includes another two major fresh water supply main lines
- a setup for chlorination of the effluent, which consists, at present, of three chlorination sites (two more to be added in the future)
- six operational reservoirs along the conveyance system, which regulate the amounts of water pumped and consumed along the system
- an effluent distribution system, composed of 13 major pressure zones, along the effluent main, that supply the treated water to the consumers
- a comprehensive monitoring system which supervises and controls the complete operation of the project.
Description of the Reclamation System
The general scheme of the reclamation system is presented in figure 1 and the flow diagram in figure 2. The system consists of the following segments: wastewater treatment plant, water recharge fields, recovery wells, conveyance and distribution system, chlorination setup and a comprehensive monitoring system.
Figure 2. Flow diagram of Dan Region Project
The wastewater treatment plant
The wastewater treatment plant of the Dan Region Metropolitan Area receives the domestic wastes of the eight cities in the region, and also handles part of their industrial wastes. The plant is located within the Rishon-Lezion sand dunes and is based mostly on secondary treatment of the wastes by the activated-sludge method. Some of the wastes, mostly during peak-flow discharges, are treated in another, older system of oxidation ponds occupying an area of 300 acres. The two systems together can handle, at present, about 110 x 106 m3 per year.
The recharge fields
The treatment plant effluents are pumped into three different sites located within the regional sand dunes, where they are spread on the sand and percolate downward into the underground aquifer for temporary storage and for additional time-dependent treatment. Two of the spreading basins are used for recharge of the mechanical-biological treatment-plant effluent. These are Yavneh 1 (60 acres, located 7 km to the south of the plant) and Yavneh 2 (45 acres, 10 km south of the plant); the third basin is used for recharge of a mixture of the oxidation ponds effluent and a certain fraction from the biomechanical treatment plant that is required in order to improve the quality of the effluent to the necessary level. This is the Soreq site, which has an area of about 60 acres and is located to the east of the ponds.
The recovery wells
Around the recharge sites there are networks of observation wells through which the recharged water is re-pumped. Not all of the 74 wells in operation in 1993 were active during the whole project. In 1993 a total of about 95 million cubic metres of water were recovered from the system’s wells and pumped into the Third Negev Line.
The conveyance and distribution systems
The water pumped from the various recovery wells is collected into the conveyance and distribution system of the Third Line. The conveyance system is composed of three sections, having a combined length of 87 km and a diameter ranging from 48 to 70 inches. Along the conveyance system six different operational reservoirs, “floating” on the main line, were constructed, in order to regulate the water flow of the system. The operational volume of these reservoirs ranges from 10,000 m3 to 100,000 m3.
The water flowing in the Third Line system was supplied to the customers in 1993 through a system of 13 major pressure zones. Numerous water consumers, mostly farms, are connected to these pressure zones.
The chlorination system
The purpose of the chlorination that is carried out in the Third Line is “breakage of the human connection”, which means elimination of any possibility for existence of micro-organisms of human origin in Third Line water. Throughout the course of monitoring it was found that there is a considerable increase of fecal micro-organisms during the stay of the reclaimed water in the water reservoirs. Therefore it was decided to add more chlorination points along the line, and by 1993 three separate chlorination points were routinely operating. Two more chlorination points are to be added to the system in the near future. The residual chlorine ranges between 0.4 and 1.0 mg/l of free chlorine. This method, whereby low concentrations of free chlorine are maintained at various points along the system rather than a single massive dose at the beginning of the line, secures the breakage of the human connection, and at the same time enables fish to live in the reservoirs. In addition, this chlorination method will disinfect the water in the downstream sections of the conveyance and distribution system, in the event that pollutants entered the system at a point downstream from the initial chlorination point.
The monitoring system
Operation of the reclamation system of the Third Negev Line is dependent upon routine functioning of a monitoring setup which is supervised and controlled by a professional and independent scientific entity. This body is the Research and Development Institute of the Technion - Israel Institute of Technology, in Haifa, Israel.
The establishment of an independent monitoring system has been a mandatory requirement of the Israeli Ministry of Health, the local legal authority according to the Israeli Public Health Ordinance. The need for establishing this monitoring setup stems from the facts that:
- This wastewater reclamation project is the biggest one in the world.
- It comprises some non-routine elements that have not as yet been experimented with.
- The reclaimed water is to be used for unlimited irrigation of agricultural crops.
The major role of the monitoring system is therefore to secure the chemical and sanitary quality of the water supplied by the system and to issue warnings regarding any change in the water quality. In addition, the monitoring setup is conducting a follow-up of the complete Dan Region reclamation project, also investigating certain aspects, such as the routine operation of the plant and the chemico-biological quality of its water. This is necessary in order to determine the adaptability of the Third Line water for unlimited irrigation, not only from the sanitary aspect but also from the agricultural viewpoint.
The preliminary monitoring layout was designed and prepared by the Mekoroth Water Co., the major Israeli water supplier and the operator of the Dan Region project. A specially appointed steering committee has been reviewing the monitoring programme on a periodic basis, and has been modifying it according to the accumulated experience gained through the routine operation. The monitoring programme dealt with the various sampling points along the Third Line system, the various investigated parameters and the sampling frequency. The preliminary programme referred to various segments of the system, namely the recovery wells, conveyance line, reservoirs, a limited number of consumer connections, as well as the presence of potable water wells in the vicinity of the plant. The list of parameters included within the monitoring schedule of the Third Line is given in table 1.
Table 1. List of investigated parameters
|
Ag |
Silver |
μg/l |
|
Al |
Aluminium |
μg/l |
|
ALG |
Algae |
No./100 ml |
|
ALKM |
Alkalinity as CaCO3 |
mg/l |
|
As |
Arsenic |
μg/l |
|
B |
Boron |
mg/l |
|
Ba |
Barium |
μg/l |
|
BOD |
Biochemical oxygen demand |
mg/l |
|
Br |
Bromide |
mg/l |
|
Ca |
Calcium |
mg/l |
|
Cd |
Cadmium |
μg/l |
|
Cl |
Chloride |
mg/l |
|
CLDE |
Chlorine demand |
mg/l |
|
CLRL |
Chlorophile |
μg/l |
|
CN |
Cyanides |
μg/l |
|
Co |
Cobalt |
μg/l |
|
COLR |
Colour (platinum cobalt) |
|
|
COD |
Chemical oxygen demand |
mg/l |
|
Cr |
Chromium |
μg/l |
|
Cu |
Copper |
μg/l |
|
DO |
Dissolved oxygen as O2 |
mg/l |
|
DOC |
Dissolved organic carbon |
mg/l |
|
DS10 |
Dissolved solids at 105 ºC |
mg/l |
|
DS55 |
Dissolved solids at 550 ºC |
mg/l |
|
EC |
Electrical conductivity |
μmhos/cm |
|
ENTR |
Enterococcus |
No./100 ml |
|
F– |
Fluoride |
mg/l |
|
FCOL |
Faecal coliforms |
No./100 ml |
|
Fe |
Iron |
μg/l |
|
HARD |
Hardness as CaCO3 |
mg/l |
|
HCO3 – |
Bicarbonate as HCO3 – |
mg/l |
|
Hg |
Mercury |
μg/l |
|
K |
Potassium |
mg/l |
|
Li |
Lithium |
μg/l |
|
MBAS |
Detergents |
μg/l |
|
Mg |
Magnesium |
mg/l |
|
Mn |
Manganese |
μg/l |
|
Mo |
Molybdenum |
μg/l |
|
Na |
Sodium |
mg/l |
|
NH4 + |
Ammonia as NH4 + |
mg/l |
|
Ni |
Nickel |
μg/l |
|
NKJT |
Kjeldahl nitrogen total |
mg/l |
|
NO2 |
Nitrite as NO2 – |
mg/l |
|
NO3 |
Nitrate as NO3 – |
mg/l |
|
ODOR |
Odour-threshold odour number |
|
|
OG |
Oil and grease |
μg/l |
|
Pb |
Lead |
μg/l |
|
PHEN |
Phenols |
μg/l |
|
PHFD |
pH measured at field |
|
|
PO4 |
Phosphate as PO4 –2 |
mg/l |
|
PTOT |
Total phosphorus as P |
mg/l |
|
RSCL |
Residual free chlorine |
mg/l |
|
SAR |
Sodium adsorption ratio |
|
|
Se |
Selenium |
μg/l |
|
Si |
Silica as H2SiO3 |
mg/l |
|
Sn |
Tin |
μg/l |
|
SO4 |
Sulphate |
mg/l |
|
Sr |
Strontium |
μg/l |
|
SS10 |
Suspended solids at 100 ºC |
mg/l |
|
SS55 |
Suspended solids at 550 ºC |
mg/l |
|
STRP |
Streptococcus |
No./100 ml |
|
T |
Temperature |
ºC |
|
TCOL |
Total coliforms |
No./100 ml |
|
TOTB |
Total bacteria |
No./100 ml |
|
TS10 |
Total solids at 105 ºC |
mg/l |
|
TS55 |
Total solids at 550 ºC |
mg/l |
|
TURB |
Turbidity |
NTU |
|
UV |
UV (absorb. at 254 nm)(/cm x 10) |
|
|
Zn |
Zinc |
μg/l |
Recovery wells monitoring
The sampling programme of the recovery wells is based upon a bi-monthly or tri-monthly measurement of a few “indicator-parameters” (table 2). When the chlorides concentration at the sampled well exceeds by more than 15% the initial chlorides level of the well, it is interpreted as a “significant” increase of the share of the recovered effluent within the underground aquifer water, and the well is transferred into the next category of sampling. Here, 23 “characteristic-parameters” are determined, once every three months. In some of the wells, once a year, a complete water investigation, including 54 various parameters, is carried out.
Table 2. The various parameters investigated at the recovery wells
|
Group A |
Group B |
Group C |
|
Indicator parameters |
Characteristic Parameters |
Complete-Test Parameters |
|
1. Chlorides |
Group A and: |
Groups A+B and: |
Conveyance system monitoring
The conveyance system, the length of which is 87 km, is monitored at seven central points along the wastewater line. At these points 16 different parameters are sampled once per month. These are: PHFD, DO, T, EC, SS10, SS55, UV, TURB, NO3 +, PTOT, ALKM, DOC, TOTB, TCOL, FCOL and ENTR. Parameters which are not expected to change along the system are measured at two sampling points only - at the beginning and at the end of the conveyance line. These are: Cl, K, Na, Ca, Mg, HARD, B, DS, SO4 –2, NH4 +, NO2 – and MBAS. At those two sampling points, once a year, various heavy metals are sampled (Zn, Sr, Sn, Se, Pb, Ni, Mo, Mn, Li, Hg, Fe, Cu, Cr, Co, Cd, Ba, As, Al, Ag).
Reservoirs monitoring
The monitoring setup of the Third Line reservoirs is based mostly on examination of a limited number of parameters which serve as indicators of biological development in the reservoirs, and for pinpointing the entry of external pollutants. Five reservoirs are sampled, once per month, for: PHFD, T, DO, Total SS, Volatile SS, DOC, CLRL, RSCL, TCOL, FCOL, STRP and ALG. At these five reservoirs Si is also sampled, once per two months. All these parameters are also sampled at another reservoir, Zohar B, at a frequency of six times per year.
Summary
The Dan Region Reclamation Project supplies high-quality reclaimed water for unrestricted irrigation of the Israeli Negev.
Stage One of this project is in partial operation since 1970 and in full operation since 1977. From 1970 to 1993, a total raw sewage amount of 373 million cubic metres (MCM) was conveyed to the facultative oxidation ponds, and a total water amount of 243 MCM was pumped from the aquifer in the period 1974–1993 and supplied to the South of the country. Part of the water was lost, mostly due to evaporation and seepage from the ponds. In 1993 these losses amounted to about 6.9% of the raw sewage conveyed to the Stage One plant (Kanarek 1994).
The mechanical-biological treatment plant, Stage Two of the project, has been in operation since 1987. During the 1987-1993 period of operation a total raw sewage amount of 478 MCM was conveyed to the mechanical-biological treatment plant. In 1993 about 103 MCM of water (95 MCM reclaimed water plus 8 MCM potable water) were conveyed through the system, and used for unlimited irrigation of the Negev.
The recovery-wells water represents the underground aquifer water quality. The aquifer water quality is changing all the time as a result of the percolation of effluent into it. The aquifer water quality approaches that of the effluent for those parameters that are not influenced by the Soil-Aquifer Treatment (SAT) processes, while parameters that are affected by the passage through the soil layers (e.g., turbidity, suspended solids, ammonia, dissolved organic carbon and so on) show considerably lower values. Noteworthy is the chloride content of the aquifer water, which increased within a recent four-year period by 15 to 26%, as evidenced by the changing water quality in the recovery wells. This change indicates the continuous replacement of aquifer water by effluent having a considerably higher chloride content.
The quality of the water in the six reservoirs of the Third Line system is influenced by biological and chemical changes that occur within the open reservoirs. The oxygen content is increased, as a result of photosynthesis of algae and due to dissolution of atmospheric oxygen. Concentrations of various types of bacteria are also increased as a result of random pollution by various water fauna residing near the reservoirs.
The quality of the water supplied to the customers along the system is dependent upon the quality of water from the recovery wells and the reservoirs. Mandatory chlorination of the system’s water constitutes an additional safeguard against erroneous use of the water as potable water. Comparison of the Third Line water data with the requirements of the Israeli Ministry of Health regarding quality of wastewater to be used for unlimited agricultural use shows that most of the time the water quality fully satisfies the requirements.
In conclusion it might be said that the Third Line wastewater recovery and utilization system has been a successful environmental and national Israeli project. It has solved the problem of sanitary disposal of the Dan Region sewage and at the same time it has increased the national water balance by a factor of about 5%. In an arid country such as Israel, where water supply, especially for agricultural use, is quite limited, this is a real contribution.
The costs of the recharge operation and maintenance of the reclaimed water, in 1993, was about 3 US cents per m3 (0.093 NIS/m3).
The system has been operating since the late 1960s under strict surveillance of the Israeli Ministry of Health and of Mekoroth’s occupational safety and hygiene department. There have been no reports of any occupational disease resulting from the operation of this intricate and comprehensive system.
" DISCLAIMER: The ILO does not take responsibility for content presented on this web portal that is presented in any language other than English, which is the language used for the initial production and peer-review of original content. Certain statistics have not been updated since the production of the 4th edition of the Encyclopaedia (1998)."