Children categories

1. Blood (3)

1. Blood
Chapter Editor: Bernard D. Goldstein
Table of Contents
Tables
Haematopoietic and Lymphatic System
Bernard D. Goldstein
Leukaemia, Malignant Lymphomas and Multiple Myeloma
Timo Partanen, Paolo Boffetta, Elisabete Weiderpass
Agents or Work Conditions Affecting the Blood
Bernard D. Goldstein
Tables
Click a link below to view the table in the article context.

2. Cancer (4)

2. Cancer
Chapter Editor: Paolo Boffetta
Table of Contents
Tables
Introduction
Neil Pearce, Paolo Boffetta and Manolis Kogevinas
Occupational Carcinogens
Paolo Boffetta, Rodolfo Saracci, Manolis Kogevinas, Julian Wilbourn and Harri Vainio
Environmental Cancer
Bruce K. Armstrong and Paolo Boffetta
Prevention
Per Gustavsson
Tables
Click a link below to view table in article context.
- Occupational cancer: Key facts
- Estimated proportions of cancer (PAR) attributable to occupations
- Evaluation of evidence of carcinogenicity in the IARC Monographs
- IARC Monograph programme classification groups
- Group 1-Chemicals carcinogenic to humans
- Group 2A—Chemicals probably carcinogenic to humans
- Group 2B—Chemicals possibly carcinogenic to humans
- Pesticides evaluated in IARC Monographs, Volumes 1-63 (1972-1995)
- Drugs evaluated in IARC Monographs, Volumes 1-63 (1972-1995)
- Environmental agents/exposures known or suspected of human cancer
- Industries, occupations, exposures presenting a carcinogenic risk
- Industries, occs., exps. with cancer excess not definitive carcinogens
- Registered population variations of incidence of some common cancers

3. Cardiovascular System (7)

3. Cardiovascular System
Chapter Editors: Lothar Heinemann and Gerd Heuchert
Table of Contents
Tables and Figures
Introduction
Lothar Heinemann and Gerd Heuchert
Cardiovascular Morbidity and Mortality in the Workforce
Gottfried Enderlein and Lothar Heinemann
The Risk Factor Concept in Cardiovascular Disease
Lothar Heinemann, Gottfried Enderlein and Heide Stark
Rehabilitation and Prevention Programmes
Lothar Heinemann and Gottfried Enderlein
Physical, Chemical and Biological Hazards
Physical Factors
Heide Stark and Gerd Heuchert
Chemical Hazardous Materials
Ulrike Tittelbach and Wolfram Dietmar Schneider
Biological Hazards
Regina Jäckel, Ulrike Tittelbach and Wolfram Dietmar Schneider
Tables
Click a link below to view table in article context
- Mortality from cardiovascular diseases
- Mortality rates, special cardiovascular diagnosis groups
- Rate of disease and reduced work ability
- Work associated with cardiovascular hazards
- Occupation-related infection and disease
Figures
Point to a thumbnail to see figure caption, click to see the figure in the article context.

4. Digestive System (6)

4. Digestive System
Chapter Editor: Heikki Savolainen
Table of Contents
Figures
Digestive system
G. Frada
Mouth and teeth
F. Gobbato
Liver
George Kazantzis
Peptic ulcer
K. S. Cho
Liver cancer
Timo Partanen, Timo Kauppinen, Paolo Boffetta and Elisabete Weiderpass
Pancreatic cancer
Timo Partanen, Timo Kauppinen, Paolo Boffetta and Elisabete Weiderpass
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

5. Mental Health (8)

5. Mental Health
Chapter Editors: Joseph J. Hurrell, Lawrence R. Murphy, Steven L. Sauter and Lennart Levi
Table of Contents
Tables and Figures
Work and Mental Health
Irene L.D. Houtman and Michiel A.J. Kompier
Work-related Psychosis
Craig Stenberg, Judith Holder and Krishna Tallur
Mood and Affect
Depression
Jay Lasser and Jeffrey P. Kahn
Work-related Anxiety
Randal D. Beaton
Post-traumatic Stress Disorder and its Relationship to Occupational Health and Injury Prevention
Mark Braverman
Stress and Burnout and their Implication in the Work Environment
Herbert J. Freudenberger
Cognitive Disorders
Catherine A. Heaney
Karoshi: Death from Overwork
Takashi Haratani
Tables
Click a link below to view table in article context.
1. Schematic overview of management strategies & examples
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

6. Musculoskeletal System (14)

6. Musculoskeletal System
Chapter Editors: Hilkka Riihimäki and Eira Viikari-Juntura
Table of Contents
Tables and Figures
Overview
Hilkka Riihimäki
Muscles
Gisela Sjøgaard
Tendons
Thomas J. Armstrong
Bones and Joints
David Hamerman
Intervertebral Discs
Sally Roberts and Jill P.G. Urban
Low-back Region
Hilkka Riihimäki
Thoracic Spine Region
Jarl-Erik Michelsson
Neck
Åsa Kilbom
Shoulder
Mats Hagberg
Elbow
Eira Viikari-Juntura
Forearm, Wrist and Hand
Eira Viikari-Juntura
Hip and Knee
Eva Vingård
Leg, Ankle and Foot
Jarl-Erik Michelsson
Other Diseases
Marjatta Leirisalo-Repo
Tables
Click a link below to view table in article context.
- Structure-function of joint components
- Prevalence of back disorders, in Finns over 30 years
- Reducing the risks for low-back pain at work
- Classification-low-back disorders (Quebec Task Force)
- Permissible motions for head in prolonged driving
- Incidence of epicondylitis in various populations
- Incidence of tenosynovitis/peritendinitis
- Primary osteoarthrosis of the hip in Malmö, Sweden
- Guidelines for the treatment of rheumatoid arthritis
- Infections known to trigger reactive arthritis
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

7. Nervous System (9)

7. Nervous System
Chapter Editor: Donna Mergler
Table of Contents
Tables and Figures
Nervous System: Overview
Donna Mergler and José A. Valciukas
Anatomy and Physiology
José A. Valciukas
Chemical Neurotoxic Agents
Peter Arlien-Søborg and Leif Simonsen
Manifestations of Acute and Early Chronic Poisoning
Donna Mergler
Preventing Neurotoxicity at Work
Barry Johnson
Clinical Syndromes Associated with Neurotoxicity
Robert G. Feldman
Measuring Neurotoxic Deficits
Donna Mergler
Diagnosis
Anna Maria Seppäläinen
Occupational Neuroepidemiology
Olav Axelson
Tables
Click a link below to view table in article context.
- Names & main functions of each pair of cranial nerves
- Grouping neurotoxic effects as to neurotoxicity
- Gases associated with neurotoxic effects
- Neurotoxic metals & their inorganic compounds
- Neurotoxic monomers
- Organic solvents associated with neurotoxicity
- Classes of common neurotoxic pesticides
- Other chemicals associated with neurotoxicity
- Chronic symptoms checklist
- Neuro-functional effects of exposures to some neurotoxins
- Chemical exposures & associated neurotoxic syndromes
- Some “core” batteries for assessing early neurotoxic effects
- Decision tree for neurotoxic disease
- Consistent neuro-functional effects of worksite exposures to some leading neurotoxic substances
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

8. Renal-Urinary System (2)

8. Renal-Urinary System
Chapter Editor: George P. Hemstreet
Table of Contents
Tables and Figures
Renal-Urinary Systems
George P. Hemstreet
Renal-Urinary Cancers
Timo Partanen, Harri Vainio, Paolo Boffetta and Elisabete Weiderpass
Tables
Click a link below to view table in article context.
- Drug-metabolism enzymes in kidney
- The most common causes of haematuria, by age & sex
- Criteria for biomarker selection
- Potential biomarkers linked to cell injury
- Acute renal insufficiency & occupation
- Segments of the nephron affected by selected toxicants
- Applications of urinary cytology
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

9. Reproductive System (9)

9. Reproductive System
Chapter Editor: Grace Kawas Lemasters
Table of Contents
Tables and Figures
Reproductive System: Introduction
Lowell E. Sever
Introduction to Male and Female Reproductive Function
Donald R. Mattison
Male Reproductive System and Toxicology
Steven Schrader and Grace Kawas Lemasters
Structure of the Female Reproductive System and Target Organ Vulnerability
Donald R. Mattison
Maternal Occupational Exposures and Adverse Pregnancy Outcomes
Grace Kawas Lemasters
Preterm Delivery and Work
Nicole Mamelle
Occupational and Environmental Exposures to the Newborn
Mary S. Wolff and Patrisha M. Woolard
Maternity Protection in Legislation
Marie-Claire Séguret
Pregnancy and US Work Recommendations
Leon J. Warshaw
Tables
Click a link below to view table in article context.
1. Exposures with multiple adverse endpoints
2. Epidemiological studies of paternal effects on pregnancy outcome
3. Potential female reproductive toxicants
4. Definition of foetal loss & infant death
5. Factors for small for gestational age and foetal loss
6. Identified sources of occupational fatigue
7. Relative risks & fatigue indices for preterm delivery
8. Prematurity risk by number of occupational fatigue indices
9. Relative risks and changes in working conditions
10. Newborn exposure sources and levels
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

10. Respiratory System (18)

10. Respiratory System
Chapters Editors: Alois David and Gregory R. Wagner
Table of Contents
Tables and Figures
Structure and Function
Morton Lippmann
Lung Function Examination
Ulf Ulfvarson and Monica Dahlqvist
Diseases Caused by Respiratory Irritants and Toxic Chemicals
David L.S. Ryon and William N. Rom
Occupational Asthma
George Friedman-Jimenez and Edward L. Petsonk
Diseases Caused by Organic Dusts
Ragnar Rylander and Richard S. F. Schilling
Beryllium Disease
Homayoun Kazemi
Pneumoconioses: Definition
Alois David
ILO International Classification of Radiographs of Pneumoconioses
Michel Lesage
Aetiopathogenesis of Pneumoconioses
Patrick Sébastien and Raymond Bégin
Silicosis
John E. Parker and Gregory R. Wagner
Coal Workers’ Lung Diseases
Michael D. Attfield, Edward L. Petsonk and Gregory R. Wagner
Asbestos-Related Diseases
Margaret R. Becklake
Hard Metal Disease
Gerolamo Chiappino
Respiratory System: The Variety of Pneumoconioses
Steven R. Short and Edward L. Petsonk
Chronic Obstructive Pulmonary Disease
Kazimierz Marek and Jan E. Zejda
Health Effects of Man-Made Fibres
James E. Lockey and Clara S. Ross
Respiratory Cancer
Paolo Boffetta and Elisabete Weiderpass
Occupationally Acquired Infections of the Lung
Anthony A. Marfin, Ann F. Hubbs, Karl J. Musgrave, and John E. Parker
Tables
Click a link below to view table in article context.
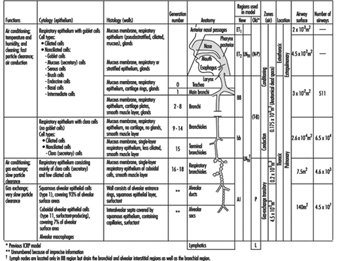
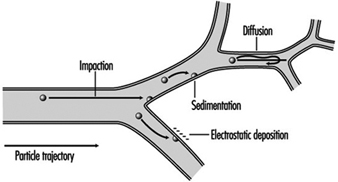
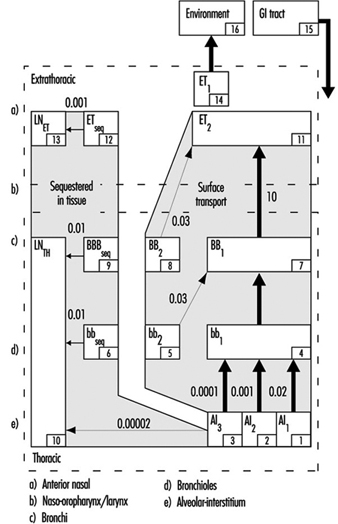
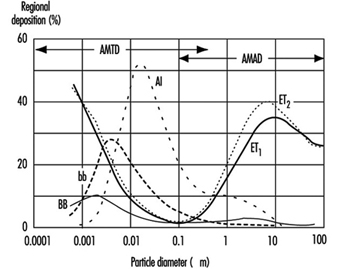
1. Respiratory tract regions & particle deposition models
2. Inhalable, thoracic & respirable dust criteria
3. Summary of respiratory irritants
4. Mechanisms of lung injury by inhaled substances
5. Compounds capable of lung toxicity
6. Medical case definition of occupational asthma
7. Steps in diagnostic evaluation of asthma in the workplace
8. Sensitizing agents that can cause occupational asthma
9. Examples of sources of hazards of exposure to organic dust
10. Agents in organic dusts with potential biological activity
11. Diseases induced by organic dusts & their ICD codes
12. Diagnostic criteria for byssinosis
13. Properties of beryllium & its compounds
14. Description of standard radiographs
15. ILO 1980 Classification: Radiographs of Pneumoconioses
16. Asbestos-related diseases & conditions
17. Main commercial sources, products & uses of asbestos
18. Prevalence of COPD
19. Risk factors implicated in COPD
20. Loss of ventilatory function
21. Diagnostic classification, chronic bronchitis & emphysema
22. Lung function testing in COPD
23. Synthetic fibres
24. Established human respiratory carcinogens (IARC)
25. Probable human respiratory carcinogens (IARC)
26. Occupationally acquired respiratory infectious diseases
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

11. Sensory Systems (8)

11. Sensory Systems
Chapter Editor: Heikki Savolainen
Table of Contents
Tables and Figures
The Ear
Marcel-André Boillat
Chemically-Induced Hearing Disorders
Peter Jacobsen
Physically-Induced Hearing Disorders
Peter L. Pelmear
Equilibrium
Lucy Yardley
Vision and Work
Paule Rey and Jean-Jacques Meyer
Taste
April E. Mott and Norman Mann
Smell
April E. Mott
Cutaneous Receptors
Robert Dykes and Daniel McBain
Tables
Click a link below to view table in article context.
1. Typical calculation of functional loss from an audiogram
2. Visual requirements for different activities
3. Recommended illuminance values for the lighting design
4. Visual requirements for a driving licence in France
5. Agents/processes reported to alter the taste system
6. Agents/processes associated with olfactory abnormalities
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

12. Skin Diseases (7)

12. Skin Diseases
Chapter Editor: Louis-Philippe Durocher
Table of Contents
Tables and Figures
Overview: Occupational Skin Diseases
Donald J. Birmingham
Non-Melanocytic Skin Cancer
Elisabete Weiderpass, Timo Partanen, Paolo Boffetta
Malignant Melanoma
Timo Partanen, Paolo Boffetta, Elisabete Weiderpass
Occupational Contact Dermatitis
Denis Sasseville
Prevention of Occupational Dermatoses
Louis-Phillipe Durocher
Occupational Nail Dystrophy
C.D. Calnan
Stigmata
H. Mierzecki
Tables
Click a link below to view table in article context.
1. Occupations at risk
2. Types of contact dermatitis
3. Common irritants
4. Common skin allergens
5. Predisposing factors for occupational dermatitis
6. Examples of skin irritants & sensitizers with occupations
7. Occupational dermatoses in Quebec in 1989
8. Risk factors & their effects on the skin
9. Collective measures (group approach) to prevention
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

13. Systemic Conditions (3)

13. Systemic Conditions
Chapter Editor: Howard M. Kipen
Table of Contents
Figures
Systemic Conditions: An Introduction
Howard M. Kipen
Sick Building Syndrome
Michael J. Hodgson
Multiple Chemical Sensitivities
Mark R. Cullen
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.
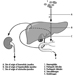
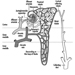
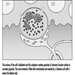
Structure of the Female Reproductive System and Target Organ Vulnerability
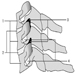
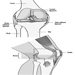
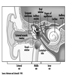
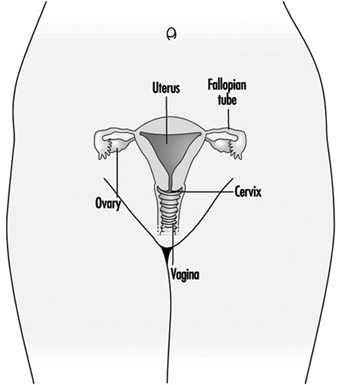
Figure 1. The female reproductive system.
The female reproductive system is controlled by components of the central nervous system, including the hypothalamus and pituitary. It consists of the ovaries, the fallopian tubes, the uterus and the vagina (Figure 1). The ovaries, the female gonads, are the source of oocytes and also synthesize and secrete oestrogens and progestogens, the major female sex hormones. The fallopian tubes transport oocytes to and sperm from the uterus. The uterus is a pear-shaped muscular organ, the upper part of which communicates through the fallopian tubes to the abdominal cavity, while the lower part is contiguous through the narrow canal of the cervix with the vagina, which passes to the exterior. Table 1 summarizes compounds, clinical manifestations, site and mechanisms of action of potential reproductive toxicants.
Table 1. Potential female reproductive toxicants
| Compound | Clinical manifestation | Site | Mechanism/target |
| Chemical reactivity | |||
| Alkylating agents |
Altered menses Amenorrhoea Ovarian atrophy Decreased fertility Premature menopause |
Ovary Uterus |
Granulosa cell cytotoxicity Oocyte cytotoxicity Endometrial cell cytotoxicity |
| Lead | Abnormal menses Ovarian atrophy Decreased fertility |
Hypothalamus Pituitary Ovary |
Decreased FSH Decreased progesterone |
| Mercury | Abnormal menses | Hypothalamus Ovary |
Altered gonadotrophin production and secretion Follicle toxicity Granulosa cell proliferation |
| Cadmium | Follicular atresia Persistent diestrus |
Ovary Pituitary Hypothalamus |
Vascular toxicity Granulosa cell cytotoxicity Cytotoxicity |
| Structural similarity | |||
| Azathioprine | Reduced follicle numbers | Ovary Oogenesis |
Purine analog Disruption of DNA/RNA synthesis |
| Chlordecone | Impaired fertility | Hypothalamus | Oestrogen agonist |
| DDT | Altered menses | Pituitary | FSH, LH disruption |
| 2,4-D | Infertility | ||
| Lindane | Amenorrhoea | ||
| Toxaphene | Hypermenorrhoea | ||
| PCBs, PBBs | Abnormal menses | FSH, LH disruption | |
Source: From Plowchalk, Meadows and Mattison 1992. These compounds are suggested to be direct-acting reproductive toxicants based primarily on toxicity testing in experimental animals.
The Hypothalamus and Pituitary
The hypothalamus is located in the diencephalon, which sits on top of the brainstem and is surrounded by the cerebral hemispheres. The hypothalamus is the principal intermediary between the nervous and the endocrine systems, the two major control systems of the body. The hypothalamus regulates the pituitary gland and hormone production.
The mechanisms by which a chemical might disrupt the reproductive function of the hypothalamus generally include any event that could modify the pulsatile release of gonadotrophin releasing hormone (GnRH). This may involve an alteration in either the frequency or the amplitude of GnRH pulses. The processes susceptible to chemical injury are those involved in the synthesis and secretion of GnRH—more specifically, transcription or translation, packaging or axonal transport, and secretory mechanisms. These processes represent sites where direct-acting chemically reactive compounds might interfere with hypothalmic synthesis or release of GnRH. An altered frequency or amplitude of GnRH pulses could result from disruptions in stimulatory or inhibitory pathways that regulate the release of GnRH. Investigations of the regulation of the GnRH pulse generator have shown that catecholamines, dopamine, serotonin, γ-aminobutyric acid, and endorphins all have some potential for altering the release of GnRH. Therefore, xenobiotics that are agonists or antagonists of these compounds could modify GnRH release, thus interfering with communication with the pituitary.
Prolactin, follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are three protein hormones secreted by the anterior pituitary that are essential for reproduction. These play a critical role in maintaining the ovarian cycle, governing follicle recruitment and maturation, steroidogenesis, completion of ova maturation, ovulation and luteinization.
The precise, finely tuned control of the reproductive system is accomplished by the anterior pituitary in response to positive and negative feedback signals from the gonads. The appropriate release of FSH and LH during the ovarian cycle controls normal follicular development, and the absence of these hormones is followed by amenorrhoea and gonadal atrophy. The gonadotrophins play a critical role in initiating changes in the morphology of ovarian follicles and in their steroidal microenvironments through the stimulation of steroid production and the induction of receptor populations. Timely and adequate release of these gonadotrophins is also essential for ovulatory events and a functional luteal phase. Because gonadotrophins are essential for ovarian function, altered synthesis, storage or secretion may seriously disrupt reproductive capacity. Interference with gene expression—whether in transcription or translation, post-translational events or packaging, or secretory mechanisms—may modify the level of gonadotrophins reaching the gonads. Chemicals that act by means of structural similarity or altered endocrine homeostasis might produce effects by interference with normal feedback mechanisms. Steroid-receptor agonists and antagonists might initiate an inappropriate release of gonadotrophins from the pituitary, thereby inducing steroid-metabolizing enzymes, reducing steroid half-life and subsequently the circulating level of steroids reaching the pituitary.
The Ovary
The ovary in primates is responsible for the control of reproduction through its principal products, oocytes and steroid and protein hormones. Folliculogenesis, which involves both intraovarian and extraovarian regulatory mechanisms, is the process by which oocytes and hormones are produced. The ovary itself has three functional subunits: the follicle, the oocyte and the corpus luteum. During the normal menstrual cycle, these components, under the influence of FSH and LH, function in concert to produce a viable ovum for fertilization and a suitable environment for implantation and subsequent gestation.
During the preovulatory period of the menstrual cycle, follicle recruitment and development occur under the influence of FSH and LH. The latter stimulates the production of androgens by thecal cells, whereas the former stimulates the aromatization of androgens into oestrogens by the granulosa cells and the production of inhibin, a protein hormone. Inhibin acts at the anterior pituitary to decrease the release of FSH. This prevents excess stimulation of follicular development and allows continuing development of the dominant follicle—the follicle destined to ovulate. Oestrogen production increases, stimulating both the LH surge (resulting in ovulation) and the cellular and secretory changes in the vagina, cervix, uterus and oviduct that enhance spermatozoa viability and transport.
In the postovulatory phase, thecal and granulosa cells remaining in the follicular cavity of the ovulated ovum, form the corpus luteum and secrete progesterone. This hormone stimulates the uterus to provide a proper environment for implantation of the embryo if fertilization occurs. Unlike the male gonad, the female gonad has a finite number of germ cells at birth and is therefore uniquely sensitive to reproductive toxicants. Such exposure of the female can lead to decreased fecundity, increased pregnancy wastage, early menopause or infertility.
As the basic reproductive unit of the ovary, the follicle maintains the delicate hormonal environment necessary to support the growth and maturation of an oocyte. As previously noted, this complex process is known as folliculogenesis and involves both intraovarian and extraovarian regulation. Numerous morphological and biochemical changes occur as a primordial follicle progresses to a pre-ovulatory follicle (which contains a developing oocyte), and each stage of follicular growth exhibits unique patterns of gonadotrophin sensitivity, steroid production and feedback pathways. These characteristics suggest that a number of sites are available for xenobiotic interaction. Also, there are different follicle populations within the ovary, which further complicates the situation by allowing for differential follicle toxicity. This creates a situation in which the patterns of infertility induced by a chemical agent would depend on the follicle type affected. For example, toxicity to primordial follicles would not produce immediate signs of infertility but would ultimately shorten the reproductive lifespan. On the other hand, toxicity to antral or preovulatory follicles would result in an immediate loss of reproductive function. The follicle complex is composed of three basic components: granulosa cells, thecal cells and the oocyte. Each of these components has characteristics that may make it uniquely susceptible to chemical injury.
Several investigators have explored methodology for screening xenobiotics for granulosa cell toxicity by measuring the effects on progesterone production by granulosa cells in culture. Oestradiol suppression of progesterone production by granulosa cells has been utilized to verify granulosa cell responsiveness. The pesticide p,p’-DDT and its o,p’-DDT isomer produce supression of progesterone production apparently with potencies equal to that of oestradiol. By contrast, the pesticides malathion, arathion and dieldrin and the fungicide hexachlorobenzene are without effect. Further detailed analysis of isolated granulosa cell responses to xenobiotics is needed to define the utility of this assay system. The attractiveness of isolated systems such as this is economy and ease of use; however, it is important to remember that granulosa cells represent only one component of the reproductive system.
Thecal cells provide precursors for steroids synthesized by granulosa cells. Thecal cells are believed to be recruited from ovarian stroma cells during follicle formation and growth. Recruitment may involve stromal cellular proliferation as well as migration to regions around the follicle. Xenobiotics that impair cell proliferation, migration and communication will impact on thecal cell function. Xenobiotics that alter thecal androgen production may also impair follicle function. For example, the androgens metabolized to oestrogens by granulosa cells are provided by thecal cells. Alterations in thecal cell androgen production, either increases or decreases, are expected to have a significant effect on follicle function. For example, it is believed that excess production of androgens by thecal cells will lead to follicle atresia. In addition, impaired production of androgens by thecal cells may lead to decreased poestrogen production by granulosa cells. Either circumstance will clearly impact on reproductive performance. At resent, little is known about thecal cell vulnerability to xenobiotics.
Although there is a acuity of information defining the vulnerability of ovarian cells to xenobiotics, there are data clearly demonstrating that oocytes can be damaged or destroyed by such agents. Alkylating agents destroy oocytes in humans and experimental animals. Lead produces ovarian toxicity. Mercury and cadmium also produce ovarian damage that may be mediated through oocyte toxicity.
Fertilization to Implantation
Gametogenesis, release and union of male and female germ cells are all preliminary events leading to a zygote. Sperm cells deposited in the vagina must enter the cervix and move through the uterus and into the fallopian tube to meet the ovum. penetration of ovum by sperm and the merging of their respective DNA comprise the process of fertilization. After fertilization cell division is initiated and continues during the next three or four days, forming a solid mass of cells called a morula. The cells of the morula continue to divide, and by the time the developing embryo reaches the uterus it is a hollow ball called a blastocyst.
Following fertilization, the developing embryo migrates through the fallopian tube into the uterus. The blastocyst enters the uterus and implants in the endometrium approximately seven days after ovulation. At this time the endometrium is in the postovulatory phase. Implantation enables the blastocyst to absorb nutrients or toxicants from the glands and blood vessels of the endometrium.
Maternal Occupational Exposures and Adverse Pregnancy Outcomes
Paid employment among women is growing worldwide. For example, almost 70% of women in the United States are employed outside the home during their predominant childbearing years (ages 20 to 34). Furthermore, since the 1940s there has been an almost linear trend in synthetic organic chemical production, creating a more hazardous environment for the pregnant worker and her offspring.
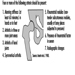
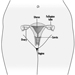
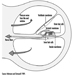
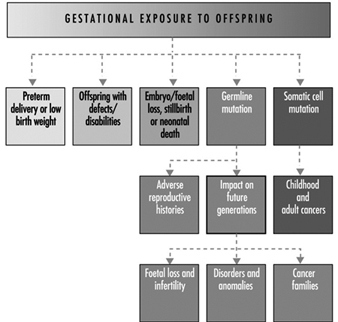
Ultimately, a couple’s reproductive success depends on a delicate physiochemical balance within and between the father, the mother and the foetus. Metabolic changes occurring during a pregnancy can increase exposure to hazardous toxicants for both worker and concetus. These metabolic changes include increased pulmonary absorption, increased cardiac output, delayed gastric emptying, increased intestinal motility and increased body fat. As shown in figure 1, exposure of the concetus can produce varying effects depending on the phase of development—early or late embryogenesis or the foetal period.
Figure 1. Consequences of maternal exposure to toxicants on the offspring.
Transport time of a fertilized ovum before implantation is between two and six days. During this early stage the embryo may be exposed to chemical compounds that penetrate into the uterine fluids. Absorption of xenophobic compounds may be accompanied by degenerative changes, alteration in the blastocystic protein profile or failure to implant. Insult during this period is likely to lead to a spontaneous abortion. Based on experimental data, it is thought that the embryo is fairly resistant to teratogenic insult at this early stage because the cells have not initiated the complex sequence of chemical differentiation.
The period of later embryogenesis is characterized by differentiation, mobilization and organization of cells and tissue into organ rudiments. Early pathogenesis may induce cell death, failed cellular interaction, reduced biosynthesis, impaired morphogenic movement, mechanical disruption, adhesions or oedema (Paul 1993). The mediating factors that determine susceptibility include route and level of exposure, pattern of exposure and foetal and maternal genotype. Extrinsic factors such as nutritional deficiencies, or the additive, synergistic or antagonistic effects associated with multiple exposures may further impact the response. Untoward responses during late embryogenesis may culminate in spontaneous abortion, gross structural defects, foetal loss, growth retardation or developmental abnormalities.
The foetal period extends from embryogenesis to birth and is defined as beginning at 54 to 60 gestational days, with the concetus having a crown-rum length of 33 mm. The distinction between the embryonic and foetal period is somewhat arbitrary. The foetal period is characterized developmentally by growth, histogenesis and functional maturation. Toxicity may be manifested by a reduction in cell size and number. The brain is still sensitive to injury; myelination is incomplete until after birth. Growth retardation, functional defects, disruption in the pregnancy, behavioural effects, translacental carcinogenesis or death may result from toxicity during the foetal period. This article discusses the biological, sociological and epidemiological effects of maternal environmental/occupational exposures.
Embryonic/Foetal Loss
The developmental stages of the zygote, defined in days from ovulation (DOV), proceed from the blastocyst stage at days 15 to 20 (one to six DOV), with implantation occurring on day 20 or 21 (six or seven DOV), to the embryonic period from days 21 to 62 (seven to 48 DOV), and the foetal period from day 63 (49+ DOV) until the designated period of viability, ranging from 140 to 195 days. Estimates of the probability of pregnancy termination at one of these stages depend on both the definition of foetal loss and the method used to measure the event. Considerable variability in the definition of early versus late foetal loss exists, ranging from the end of week 20 to week 28. The definitions of foetal and infant death recommended by the World Health Organization (1977) are listed in table 1. In the United States the gestational age setting the lower limit for stillbirths is now widely accepted to be 20 weeks.
Table 1. Definition of foetal loss and infant death
| Spontaneous abortion | ≤500 g or 20-22 weeks or 25 cm length |
| Stillbirth | 500 g (1000 g International) nonviable |
| Early neonatal death | Death of a live-born infant ≤7 days (168 hours) |
| Late neonatal death | 7 days to ≤28 days |
Source: World Health Organization 1977.
Because the majority of early aborted foetuses have chromosomal anomalies, it has been suggested that for research purposes a finer distinction should be made—between early foetal loss, before 12 weeks’ gestation, and later foetal loss (Källén 1988). In examining late foetal losses it also may be appropriate to include early neonatal deaths, as the cause may be similar. WHO defines early neonatal death as the death of an infant aged seven days or younger and late neonatal death as occurring between seven and 29 days. In studies conducted in developing countries, it is important to distinguish between prepartum and intrapartum deaths. Because of problematic deliveries, intrapartum deaths account for a large portion of stillbirths in less developed countries.
In a review by Kline, Stein and Susser (1989) of nine retrospective or cross-sectional studies, the foetal loss rates before 20 weeks’ gestation ranged from 5.5 to 12.6%. When the definition was expanded to include losses u to 28 weeks’ gestation, the foetal loss rate varied between 6.2 and 19.6%. The rates of foetal loss among clinically recognized pregnancies in four prospective studies, however, had a relatively narrow range of 11.7 to 14.6% for the gestational period u to 28 weeks. This lower rate, seen in prospective versus retrospective or cross-sectional designs, may be attributable to differences in underlying definitions, misreporting of induced abortions as spontaneous or misclassification of delayed or heavy menses as foetal loss.
When occult abortions or early “chemical” losses identified by an elevated level of human chorionic gonadotrohins (hCG) are included, the total spontaneous abortion rate jumps dramatically. In a study using hCG methods, the incidence of post-implantation subclinical loss of fertilized ova was 22% (Wilcox et al. 1988). In these studies urinary hCG was measured with immunoradiometric assay using a detection antibody. The assay originally used by Wilcox employed a now extinct high affinity, polyclonal rabbit antibody. More recent studies have used an inexhaustible monoclonal antibody that requires less than 5 ml of urine for replicate samples. The limiting factor for use of these assays in occupational field studies is not only the cost and resources needed to coordinate collection, storage and analysis of urine samples but the large population needed. In a study of early pregnancy loss in women workers exposed to video display terminals (VDTs), approximately 7,000 women were screened in order to acquire a usable population of 700 women. This need for ten times the population size in order to achieve an adequate sample stems from reduction in the available number of women because of ineligibility due to age, sterility and the enrollment exclusively of women who are using either no contraceptives or relatively ineffective forms of contraception.
More conventional occupational studies have used recorded or questionnaire data to identify spontaneous abortions. Recorded data sources include vital statistics and hospital, private practitioner and outpatient clinic records. Use of record systems identifies only a subset of all foetal losses, principally those that occur after the start of prenatal care, typically after two to three missed periods. Questionnaire data are collected by mail or in personal or telephone interviews. By interviewing women to obtain reproductive histories, more complete documentation of all recognized losses is possible. Questions that are usually included in reproductive histories include all pregnancy outcomes; prenatal care; family history of adverse pregnancy outcomes; marital history; nutritional status; re-pregnancy weight; height; weight gain; use of cigarettes, alcohol and prescription and nonprescription drugs; health status of the mother during and prior to a pregnancy; and exposures at home and in the workplace to physical and chemical agents such as vibration, radiation, metals, solvents and pesticides. Interview data on spontaneous abortions can be a valid source of information, particularly if the analysis includes those of eight weeks’ gestation or later and those that occurred within the last 10 years.
The principal physical, genetic, social and environmental factors associated with spontaneous abortion are summarized in table 2. To ensure that the observed exposure-effect relationship is not due to a confounding relationship with another risk factor, it is important to identify the risk factors that may be associated with the outcome of interest. Conditions associated with foetal loss include syphilis, rubella, genital Mycolasma infections, herpes simplex, uterine infections and general hyperpyrexia. One of the most important risk factors for clinically recognized spontaneous abortion is a history of pregnancy ending in foetal loss. Higher gravidity is associated with increased risk, but this may not be independent of a history of spontaneous abortion. There are conflicting interpretations of gravidity as a risk factor because of its association with maternal age, reproductive history and heterogeneity of women at different gravidity ranks. Rates of spontaneous abortion are higher for women younger than 16 and older than 36 years. After adjusting for gravidity and a history of pregnancy loss, women older than 40 were shown to have twice the risk of foetal loss of younger women. The increased risk for older women has been associated with an increase in chromosomal anomalies, particularly trisomy. possiblemale-mediated effects associated with foetal loss have been recently reviewed (Savitz, Sonnerfeld and Olshaw 1994). A stronger relationship was shown with paternal exposure to mercury and anaesthetic gases, as well as a suggestive but inconsistent relationship with exposure to lead, rubber manufacturing, selected solvents and some pesticides.
Table 2. Factors associated with small for gestational age and foetal loss
| Small for gestational age | |
| Physical-genetic | Environmental-social |
| Preterm delivery Multiple births Malformed foetus Hypertension Placental or cord anomaly Maternal medical history History of adverse pregnancy outcomes Race Chromosome anomalies Sex Maternal height, weight, weight gain Paternal height Parity Length of gestation Short interval between pregnancies |
Malnutrition Low income/poor education Maternal smoking Maternal alcohol consumption Occupational exposure Psychosocial stress Altitude History of infections Marijuana use |
| Foetal loss | |
| Physical-genetic | Environmental-social |
| Higher gravidity Maternal age Birth order Race Repeat spontaneous abortion Insulin dependent diabetes Uterine disorders Twinning Immunological factor Hormonal factors |
Socio-economic status Smoking history Prescribed and recreational drugs Alcohol use Poor nutrition Infections/maternal fever Spermicides Employment factors Chemical exposure Irradiation |
Employment status may be a risk factor regardless of a specific physical or chemical hazard and may act as a confounder in assessment of occupational exposure and spontaneous abortion. Some investigators suggest that women who stay in the workforce are more likely to have an adverse pregnancy history and as a result are able to continue working; others believe this group is an inherently more fit subpopulation due to higher incomes and better prenatal care.
Congenital Anomalies
During the first 60 days after conception, the developing infant may be more sensitive to xenobiotic toxicants than at any other stage in the life cycle. Historically, terata and congenital malformations referred to structural defects resent at birth that may be gross or microscopic, internal or external, hereditary or nonhereditary, single or multiple. Congenital anomaly, however, is more broadly defined as including abnormal behaviour, function and biochemistry. Malformations may be single or multiple; chromosomal defects generally produce multiple defects, whereas single-gene changes or exposure to environmental agents may cause either single defects or a syndrome.
The incidence of malformations depends on the status of the concetus—live birth, spontaneous abortus, stillbirth. Overall, the abnormality rate in spontaneous abortuses is approximately 19%, a tenfold increase in what is seen in the live born (Sheard, Fantel and Fitsimmons 1989). A 32% rate of anomalies was found among stillborn foetuses weighing more than 500 g. The incidence of major defects in live births is about 2.24% (Nelson and Holmes 1989). The prevalence of minor defects ranges between 3 and 15% (averaging about 10%). Birth anomalies are associated with genetic factors (10.1%), multifactorial inheritance (23%), uterine factors (2.5%), twinning (0.4%) or teratogens (3.2%). The causes of the remaining defects are unknown. Malformation rates are approximately 41% higher for boys than for girls and this is explained by the significantly higher rate of anomalies for male genital organs.
One challenge in studying malformations is deciding how to group defects for analysis. Anomalies can be classified by several parameters, including seriousness (major, minor), pathogenesis (deformation, disruption), associated versus isolated, anatomic by organ system, and aetiological (e.g., chromosomal, single gene defects or teratogen induced). Often, all malformations are combined or the combination is based either on major or minor categorization. A major malformation can be defined as one that results in death, requires surgery or medical treatment or constitutes a substantial physical or psychological handicap. The rationale for combining anomalies into large groups is that the majority arise, at approximately the same time period, during organogenesis. Thus, by maintaining larger sample sizes, the total number of cases is increased with a concomitant increase in the statistical power. If, however, the exposure effect is specific to a particular type of malformation (e.g., central nervous system), such grouping may mask the effect. Alternatively, malformations may be grouped by organ system. Though this method may be an improvement, certain defects may dominate the class, such as varus deformities of the feet in the musculoskeletal system. Given a sufficiently large sample, the optimal approach is to divide the defects into embryologically or pathogenetically homogenous groups (Källén 1988). Considerations should be given to the exclusion or inclusion of certain malformations, such as those that are likely caused by chromosomal defects, autosomal dominant conditions or malposition in utero. Ultimately, in analysing congenital anomalies, a balance has to be maintained between maintaining precision and compromising statistical power.
A number of environmental and occupational toxicants have been associated with congenital anomalies in offspring. One of the strongest associations is maternal consumption of food contaminated with methylmercury causing morphological, central nervous system and neurobehavioural abnormalities. In Japan, the cluster of cases was linked to consumption of fish and shellfish contaminated with mercury derived from the effluent of a chemical factory. The most severely affected offspring developed cerebral palsy. Maternal ingestion of polychlorinated biphenyl’s (CBs) from contaminated rice oil gave rise to babies with several disorders, including growth retardation, dark brown skin pigmentation, early eruption of teeth, gingival hyperplasia, wide sagittal suture, facial oedema and exophthalmoses. Occupations involving exposures to mixtures have been linked with a variety of adverse outcomes. The offspring of women working in the ul and aer industry, in either laboratory work or jobs involving “conversions” or aer refinement, also had increased risk of central nervous system, heart and oral cleft defects. Women working in industrial or construction work with unspecified exposures had a 50% increase in central nervous system defects, and women working in transportation and communication had two times the risk of having a child with an oral cleft. Veterinarians represent a unique group of health care personnel exposed to anaesthetic gases, radiation, trauma from animal kicks, insecticides and zoonotic diseases. Though no difference was found in the rate of spontaneous abortions or in birth weight of the offspring between female veterinarians and female lawyers, there was a significant excess of birth defects among veterinarians (Schenker et al. 1990). Lists of known, possible and unlikely teratogens are available as well as computer databases and risk lines for obtaining current information on potential teratogens (Paul 1993). Evaluating congenital anomalies in an occupational cohort is particularly difficult, however, because of the large sample size needed for statistical power and our limited ability to identify specific exposures occurring during a narrow window of time, primarily the first 55 days of gestation.
Small for Gestational Age
Among the many factors linked with infant survival, physical underdevelopment associated with low birth weight (LBW) resents one of the greatest risks. Significant weight gain of the foetus does not begin until the second trimester. The concetus weighs 1 g at eight weeks, 14 g at 12 weeks, and reaches 1.1 kg at 28 weeks. An additional 1.1 kg is gained every six weeks thereafter until term. The normal newborn weighs approximately 3,200 g at term. The newborn’s weight is dependent on its rate of growth and its gestational age at delivery. An infant that is growth retarded is said to be small for gestational age (SGA). If an infant is delivered prior to term it will have a reduced weight but will not necessarily be growth retarded. Factors associated with a preterm delivery are discussed elsewhere, and the focus of this discussion is on the growth-retarded newborn. The terms SGA and LBW will be used interchangeably. A low birth-weight infant is defined as an infant weighing less than 2,500 g, a very low birth weight is defined as less than 1,500 g, and extremely low birth weight is one that is less than 1,000 g (WHO 1969).
When examining causes of reduced growth, it is important to distinguish between asymmetrical and symmetrical growth retardation. Asymmetrical growth retardation, i.e., where the weight is affected more than the skeletal structure, is primarily associated with a risk factor operating during late pregnancy. On the other hand, symmetrical growth retardation may more likely be associated with an aetiology that operates over the entire period of gestation (Kline, Stein and Susser 1989). The difference in rates between asymmetrical and symmetrical growth retardation is especially apparent when comparing developing and developed countries. The rate of growth retardation in developing countries is 10 to 43%, and is primarily symmetrical, with the most important risk factor being poor nourishment. In developed countries foetal growth retardation is usually much lower, 3 to 8%, and is generally asymmetrical with a multifactorial aetiology. Hence, worldwide, the proportion of low birth-weight infants defined as intrauterine growth retarded rather than preterm varies dramatically. In Sweden and the United States, the proportion is approximately 45%, while in developing countries, such as India, the proportion varies between approximately 79 and 96% (Villar and Belizan 1982).
Studies of the Dutch famine showed that starvation confined to the third trimester depressed foetal growth in an asymmetric pattern, with birth weight being primarily affected and head circumference least affected (Stein, Susser and Saenger 1975). Asymmetry of growth also has been observed in studies of environmental exposures. In a study of 202 expectant mothers residing in neighbourhoods at high risk for lead exposures, prenatal maternal blood samples were collected between the sixth and the 28th week of gestation (Bornschein, Grote and Mitchell 1989). Blood lead levels were associated with both a decreased birth weight and length, but not head circumference, after adjustment for other relevant risk factors including length of gestation, socioeconomic status and use of alcohol or cigarettes. The finding of maternal blood lead as a factor in birth length was seen entirely in Caucasian infants. The birth length of Caucasian infants decreased approximately 2.5 cm per log unit increment in maternal blood lead. Care should be given to selection of the outcome variable. If only birth weight had been selected for study, the finding of the effects of lead on other growth parameters might have been missed. Also, if Caucasians and African Americans had been pooled in the above analysis, the differential effects on Caucasians, perhaps due to genetic differences in the storage and binding capacity of lead, may have been missed. A significant confounding effect also was observed between prenatal blood lead and maternal age and the birth weight of the offspring after adjustment for other covariables. The findings indicate that for a 30-year-old woman with an estimated blood lead level of about 20 mg/dl, the offspring weighed proximately 2,500 g compared with proximately 3,000 g for a 20-year-old with similar lead levels. The investigators speculated that this observed difference may indicate that older women are more sensitive to the additional insult of lead exposure or that older women may have had higher total lead burden from greater numbers of years of exposure or higher ambient lead levels when they were children. Another factor may be increased blood pressure. Nonetheless, the important lesson is that careful examination of high-risk subpopulations by age, race, economic status, daily living habits, sex of the offspring and other genetic differences may be necessary in order to discover the more subtle effects of exposures on foetal growth and development.
Risk factors associated with low birth weight are summarized in Table 5. Social class as measured by income or education persists as a risk factor in situations in which there are no ethnic differences. Other factors that may be operating under social class or race may include cigarette smoking, physical work, prenatal care and nutrition. Women between the ages of 25 and 29 are least likely to deliver a growth-retarded offspring. Maternal smoking increases the risk of low birth-weight offspring by about 200% for heavy smokers. Maternal medical conditions associated with LBW include placental abnormalities, heart disease, viral pneumonia, liver disease, re-eclamsia, eclamsia, chronic hypertension, weight gain and hyeremesis. An adverse pregnancy history of foetal loss, preterm delivery or prior LBW infant increases the risk of a current preterm low birth-weight infant two- to fourfold. An interval between births of less than a year triples the risk of having a low birth-weight offspring. Chromosomal anomalies associated with abnormal growth include Down’s syndrome, trisomy 18 and most malformation syndromes.
Smoking cigarettes is one of the primary behaviours most directly linked with lower weight offspring. Maternal smoking during pregnancy has been shown to increase the risk of a low birth-weight offspring two to three times and to cause an overall weight deficit of between 150 and 400 g. Nicotine and carbon monoxide are considered the most likely causative agents since both are rapidly and referentially transferred across the placenta. Nicotine is a powerful vasoconstrictor, and significant differences in the size of umbilical vessels of smoking mothers have been demonstrated. Carbon monoxide levels in cigarette smoke range from 20,000 to 60,000 m. Carbon monoxide has an affinity for haemoglobin 210 times that of oxygen, and because of lower arterial oxygen tension the foetus is especially compromised. Others have suggested that these effects are not due to smoking but are attributable to characteristics of smokers. Certainly occupations with potential carbon monoxide exposure, such as those associated with ul and aer, blast furnaces, acetylene, breweries, carbon black, coke ovens, garages, organic chemical synthesizers and petroleum refineries should be considered possible high risk occupations for pregnant employees.
Ethanol is also a widely used and researched agent associated with foetal growth retardation (as well as congenital anomalies). In a prospective study of 9,236 births, it was found that maternal alcohol consumption of more than 1.6 oz per day was associated with an increase in stillbirths and growth-retarded infants (Kaminski, Rumeau and Schwartz 1978). Smaller infant length and head circumference also are related to maternal alcohol ingestion.
In evaluating the possible effects of exposures on birth weight, some problematic issues must be considered. preterm delivery should be considered as a possible mediating outcome and the potential effects on gestational age considered. In addition, pregnancies having longer gestational length also have a longer opportunity for exposure. If enough women work late in pregnancy, the longest cumulative exposure may be associated with the oldest gestational ages and heaviest babies purely as an artifact. There are a number of procedures that can be used to overcome this problem including a variant of the Cox life-table regression model, which has the ability to handle time-dependent covariables.
Another problem centres on how to define lowered birth weight. Often studies define lower birth weight as a dichotomous variable, less than 2,500 g. The exposure, however, must have a very powerful effect in order to produce a drastic drop in the infant’s weight. Birth weight defined as a continuous variable and analysed in a multiple regression model is more sensitive for detecting subtle effects. The relative paucity of significant findings in the literature in relationship to occupational exposures and SGA infants may, in art, be caused by ignoring these design and analysis issues.
Conclusions
Studies of adverse pregnancy outcomes must characterize exposures during a fairly narrow window of time. If the woman has been transferred to another job or laid off work during a critical period of time such as organogenesis, the exposure-effect relationship can be severely altered. Therefore, the investigator is held to a high standard of identifying the woman’s exposure during a critical small time period as compared with other studies of chronic diseases, where errors of a few months or even years may have minimal impact.
Uterine growth retardation, congenital anomaly and spontaneous abortions are frequently evaluated in occupational exposure studies. There is more than one approach available to assess each outcome. These end-points are of public health importance due to both the psychological and the financial costs. Generally, nonsecificity in the exposure-outcome relationships has been observed, e.g., with exposure to lead, anaesthetic gases and solvents. Because of the potential for nonsecificity in the exposure-effect relationship, studies should be designed to assess several end-points associated with a range of possible mechanisms.
Preterm Delivery and Work
The reconciliation of work and maternity is an important public health issue in industrialized countries, where more than 50% of women of child-bearing age work outside the home. Working women, unions, employers, politicians and clinicians are all searching for ways of preventing work-induced unfavourable reproductive outcomes. Women want to continue working while pregnant, and may even consider their physician’s advice about lifestyle modifications during pregnancy to be overprotective and unnecessarily restrictive.
physiological Consequences of pregnancy
At this point, it would be useful to review a few of the physiological consequences of pregnancy that may interfere with work.
A pregnant woman undergoes profound changes which allow her to adapt to the needs of the foetus. Most of these changes involve the modification of physiological functions that are sensitive to changes of posture or physical activity—the circulatory system, the respiratory system and water balance. As a result, physically active pregnant women may experience unique physiological and physiopathological reactions.
The main physiological, anatomical, and functional modifications undergone by pregnant women are (Mamelle et al. 1982):
- An increase in peripheral oxygen demand, leading to modification of the respiratory and circulatory systems. Tidal volume begins to increase in the third month and may amount to 40% of re-pregnancy values by the end of the pregnancy. The resultant increase in gas exchange may increase the hazard of the inhalation of toxic volatiles, while hyperventilation related to increased tidal volume may cause shortness of breath on exertion.
- Cardiac output increases from the very beginning of pregnancy, as a result of an increase in blood volume. This reduces the heart’s ability to adapt to exertion and also increases venous pressure in the lower limbs, rendering standing for long periods difficult.
- Anatomical modifications during pregnancy, including exaggeration of dorsolumbar lordosis, enlargement of the polygon of support and increases in abdominal volume, affect static activities.
- A variety of other functional modifications occur during pregnancy. Nausea and vomiting result in fatigue; daytime sleepiness results in inattention; mood changes and feelings of anxiety may lead to interpersonal conflicts.
- Finally, it is interesting to note that the daily energy requirements during pregnancy are equivalent to the requirements of two to four hours of work.
Because of these profound changes, occupational exposures may have special consequences in pregnant women and may result in unfavourable pregnancy outcomes.
Epidemiological Studies of Working Conditions and preterm Delivery
Although there are many possible unfavourable pregnancy outcomes, we review here the data on preterm delivery, defined as the birth of a child before the 37th week of gestation. preterm birth is associated with low birth weight and with significant complications for the newborn. It remains a major public health concern and is an ongoing reoccupation among obstetricians.
When we began research in this field in the mid-1980s, there was relatively strong legislative protection of pregnant women’s health in France, with prenatal maternity leave mandated to start six weeks prior to the due date. Although the preterm delivery rate has fallen from 10 to 7% since then, it appeared to have levelled off. Because medical prevention had apparently reached the limit of its powers, we investigated risk factors likely to be amenable to social intervention. Our hypotheses were as follows:
- Is working per se a risk factor for preterm birth?
- Are certain occupations associated with an increased risk of preterm delivery?
- Do certain working conditions constitute a hazard to the pregnant woman and foetus?
- Are there social preventive measures which could help reduce the risk of preterm birth?
Our first study, conducted in 1977–78 in two hospital maternity wards, examined 3,400 women, of whom 1,900 worked during pregnancy and 1,500 remained at home (Mamelle, Laumon and Lazar 1984). The women were interviewed immediately after delivery and asked to describe their home and work lifestyle during pregnancy as accurately as possible.
We obtained the following results:
Work per se
The mere fact of working outside the home cannot be considered to be a risk factor for preterm delivery, since women remaining at home exhibited a higher prematurely rate than did women who worked outside the home (7.2 versus 5.8%).
Working conditions
An excessively long work week appears to be a risk factor, since there was a regular increase in preterm delivery rate with the number of work hours. Retail-sector workers, medical social workers, specialized workers and service personnel were at higher risk of preterm delivery than were office workers, teachers, management, skilled workers or supervisors. The prematurely rates in the two groups were 8.3 and 3.8% respectively.
Table 1. Identified sources of occupational fatigue
| Occupational fatigue index | “HIGH” index if: |
| Posture | Standing for more than 3 hours per day |
| Work on machines | Work on industrial conveyor belts; independent work on industrial machines with strenuous effort |
| Physical load | Continuous or periodical physical effort; carrying loads of more than 10kg |
| Mental load | Routine work; varied tasks requiring little attention without stimulation |
| Environment | Significant noise level; cold temperature; very wet atmosphere; handling of chemical substances |
Source: Mamelle, Laumon and Lazar 1984.
Task analysis allowed identification of five sources of occupation fatigue: posture, work with industrial machines, physical workload, mental workload and the work environment. Each of these sources of occupational fatigue constitutes a risk factor for preterm delivery (see tables 1 and 2).
Table 2. Relative risks (RR) and fatigue indices for preterm delivery
| Index | Low index % | High index % | RR | Statistical significance |
| Posture | 4.5 | 7.2 | 1.6 | Significant |
| Work on machines | 5.6 | 8.8 | 1.6 | Significant |
| Physical load | 4.1 | 7.5 | 1.8 | Highly significant |
| Mental load | 4.0 | 7.8 | 2.0 | Highly significant |
| Environment | 4.9 | 9.4 | 1.9 | Highly significant |
Source: Mamelle, Laumon and Lazar 1984.
Exposure to multiple sources of fatigue may result in unfavourable pregnancy outcomes, as evidenced by the significant increase of the rate of preterm delivery with an increased number of sources of fatigue (table 3). Thus, 20% of women had concomitant exposure to at least three sources of fatigue, and experienced a preterm delivery rate twice as high as other women. Occupational fatigue and excessively long work weeks exert cumulative effects, such that women who experience intense fatigue during long work weeks exhibit an even higher prematurely rate. preterm delivery rates increase further if the woman also has a medical risk factor. The detection of occupational fatigue is therefore even more important than the detection of medical risk factors.
Table 3. Relative risk of prematurity according to number of occupational fatigue indices
| Number of high fatigue indices |
Proportion of exposed women % |
Estimated relative risk |
| 0 | 24 | 1.0 |
| 1 | 28 | 2.2 |
| 2 | 25 | 2.4 |
| 3 | 15 | 4.1 |
| 4-5 | 8 | 4.8 |
Source: Mamelle, Laumon and Lazar 1984
European and North American studies have confirmed our results, and our fatigue scale has been shown to be reproducible in other surveys and countries.
In a case-control follow-u study conducted in France a few years later in the same maternity wards (Mamelle and Munoz 1987) , only two of the five previously defined indices of fatigue were significantly related to preterm delivery. It should however be noted that women had a greater opportunity to sit down and were withdrawn from physically demanding tasks as a result of preventive measures implemented in the workplaces during this period. The fatigue scale nevertheless remained a predictor of preterm delivery in this second study.
In a study in Montreal, Quebec (McDonald et al. 1988), 22,000 pregnant women were interviewed retrospectively about their working conditions. Long work weeks, alternating shift work and carrying heavy loads were all shown to exert significant effects. The other factors studied did not appear to be related to preterm delivery, although there appears to be a significant association between preterm delivery and a fatigue scale based on the total number of sources of fatigue.
With the exception of work with industrial machines, no significant association between working conditions and preterm delivery was found in a French retrospective study of a representative sample of 5,000 pregnant women (Saurel-Cubizolles and Kaminski 1987). However, a fatigue scale inspired by our own was found to be significantly associated with preterm delivery.
In the United States, Homer, Beredford and James (1990), in a historical cohort study, confirmed the association between physical workload and an increased risk of preterm delivery. Teitelman and co-workers (1990), in a prospective study of 1,200 pregnant women, whose work was classified as sedentary, active or standing, on the basis of job description, demonstrated an association between work in a standing position and preterm delivery.
Barbara Luke and co-workers (in press) conducted a retrospective study of US nurses who worked during pregnancy. Using our occupational risk scale, she obtained similar results to ours, that is, an association between preterm delivery and long work weeks, standing work, heavy workload and unfavourable work environment. In addition, the risk of preterm delivery was significantly higher among women with concomitant exposure to three or four sources of fatigue. It should be noted that this study included over half of all nurses in the United States.
Contradictory results have however been reported. These may be due to small sample sizes (Berkowitz 1981), different definitions of prematurely (Launer et al. 1990) and classification of working conditions on the basis of job description rather than actual workstation analysis (Klebanoff, Shiono and Carey 1990). In some cases, workstations have been characterized on a theoretical basis only—by the occupational physician, for example, rather than by the women themselves (peoples-Shes et al. 1991). We feel that it is important to take subjective fatigue—that is, fatigue as it is described and experienced by women—into account in the studies.
Finally, it is possible that the negative results are related to the implementation of preventive measures. This was the case in the prospective study of Ahlborg, Bodin and Hogstedt (1990), in which 3,900 active Swedish women completed a self-administered questionnaire at their first prenatal visit. The only reported risk factor for preterm delivery was carrying loads weighing more than 12 kg more often than 50 times per week, and even then the relative risk of 1.7 was not significant. Ahlborg himself points out that preventive measures in the form of aid maternity leave and the right to perform less tiring work during the two months receding their due date had been implemented for pregnant women engaged in tiring work. Maternity leaves were five times as frequent among women who described their work as tiring and involving the carrying of heavy loads. Ahlborg concludes that the risk of preterm delivery may have been minimized by these preventive measures.
preventive Interventions: French Examples
Are the results of aetiological studies convincing enough for preventive interventions to be applied and evaluated? The first question which must be answered is whether there is a public health justification for the application of social preventive measures designed to reduce the rate of preterm delivery.
Using data from our previous studies, we have estimated the proportion of preterm births caused by occupational factors. Assuming a rate of preterm delivery of 10% in populations exposed to intense fatigue and a rate of 4.5% in non-exposed populations, we estimate that 21% of premature births are caused by occupational factors. Reducing occupational fatigue could therefore result in the elimination of one-fifth of all preterm births in French working women. This is ample justification for the implementation of social preventive measures.
What preventive measures can be applied? The results of all the studies lead to the conclusion that working hours can be reduced, fatigue can be lessened through workstation modification, work breaks can be allowed and prenatal leave can be lengthened. Three cost-equivalent alternatives are available:
- reducing the work week to 30 hours starting from the 20th week of gestation
- prescribing a work break of one week each month starting in the 20th week of gestation
- beginning prenatal leave at the 28th week of gestation.
It is relevant to recall here that French legislation provides the following preventive measures for pregnant women:
- guaranteed employment after childbirth
- reduction of the workday by 30 to 60 minutes, applied through collective agreements
- workstation modification in cases of incompatibility with pregnancy
- work breaks during pregnancy, prescribed by attending physicians
- prenatal maternity leave six weeks prior to the due date, with a further two weeks available in case of complications
- postnatal maternity leave of ten weeks.
A one-year prospective observational study of 23,000 women employed in 50 companies in the Rhône-Ales region of France (Bertucat, Mamelle and Munoz 1987) examined the effect of tiring work conditions on preterm delivery. Over the period of the study, 1,150 babies were born to the study population. We analysed the modifications of working conditions to accommodate pregnancy and the relation of these modifications to preterm delivery (Mamelle, Bertucat and Munoz 1989), and observed that:
- Workstation modification was reformed for only 8% of women.
- 33% of women worked their normal shifts, with the others having their workday reduced by 30 to 60 minutes.
- 50% of women took at least one work break, apart from their prenatal maternity leave; fatigue was the cause in one-third of cases.
- 90% of women stopped working before their legal maternity leave began and obtained at least the two weeks leave allowed for in the case of complications of pregnancy; fatigue was the cause in half the cases.
- In all, given the legal prenatal leave period of six weeks prior to the due date (with an additional two weeks available in some cases), the real duration of prenatal maternity leave was 12 weeks in this population of women subjected to tiring work conditions.
Do these modifications of work have any effect on the outcome of pregnancy? Workstation modification and the slight reduction of the workday (30 to 60 min) were both associated with non-significant reductions of the risk of preterm delivery. We believe that further reductions of the work week would have a greater effect (table 4).
Table 4. Relative risks of prematurity associated with modifications in working conditions
| Modifications in working conditions |
Number of women | Preterm birth rates (%) |
Relative risk (95% confidence intervals) |
| Change in work situation | |||
| No Yes |
1,062 87 |
6.2 3.4 |
0.5 (0.2-1.6) |
| Reduction of weekly working hours | |||
| No Yes |
388 761 |
7.7 5.1 |
0.7 (0.4-1.1) |
| Episodes of sick leave1 | |||
| No Yes |
357 421 |
8.0 3.1 |
0.4 (0.2-0.7) |
| Increase of antenatal maternity leave1 | |||
| None or only additional 2 weeks Yes |
487 291 |
4.3 7.2 |
1.7 (0.9-3.0) |
1 In a reduced sample of 778 women with no previous or present obstetric pathology.
Source: Mamelle, Bertucat and Munoz 1989.
To analyse the relation between prenatal leave, work breaks and preterm delivery, it is necessary to discriminate between preventive and curative work breaks. This requires restriction of the analysis to women with uncomplicated pregnancies. Our analysis of this subgroup revealed a reduction of the preterm delivery rate among women who took work breaks during their pregnancy, but not in those who took prolonged prenatal leave (Table 9).
This observational study demonstrated that women who work in tiring conditions take more work breaks during their pregnancies than do other women, and that these breaks, particularly when motivated by intense fatigue, are associated with reductions of the risk of preterm delivery (Mamelle, Bertucat and Munoz 1989).
Choice of preventive Strategies in France
As epidemiologists, we would like to see these observations verified by experimental preventive studies. We must however ask ourselves which is more reasonable: to wait for such studies or to recommend social measures aimed at preventing preterm delivery now?
The French Government recently decided to include a “work and pregnancy guide”, identical to our fatigue scale, in each pregnant woman’s medical record. Women can thus calculate their fatigue score for themselves. If work conditions are arduous, they may ask the occupational physician or the person responsible for occupational safety in their company to implement modifications aimed at alleviating their workload. Should this be refused, they can ask their attending physician to prescribe rest weeks during their pregnancy, and even to prolong their prenatal maternity leave.
The challenge is now to identify preventive strategies that are well adapted to legislation and social conditions in every country. This requires a health economics approach to the evaluation and comparison of preventive strategies. Before any preventive measure can be considered generally applicable, many factors have to be taken into consideration. These include effectiveness, of course, but also low cost to the social security system, resultant job creation, women’s references and the acceptability to employers and unions.
This type of problem can be resolved using multicriteria methods such as the Electra method. These methods allow both the classification of preventive strategies on the basis of each of a series of criteria, and the weighting of the criteria on the basis of political considerations. Special importance can thus be given to low cost to the social security system or to the ability of women to choose, for example (Mamelle et al. 1986). While the strategies recommended by these methods vary depending on the decision makers and political options, effectiveness is always maintained from the public health standpoint.
Occupational and Environmental Exposures to the Newborn
Environmental hazards pose a special risk for infants and young children. Children are not “little adults”, either in the way they absorb and eliminate chemicals or in their response to toxic exposures. Neonatal exposures may have a greater impact because the body surface area is disproportionately large and metabolic capacity (or the ability to eliminate chemicals) is relatively underdeveloped. At the same time, the potential toxic effects are greater, because the brain, the lungs and the immune system are still developing during the early years of life.
Opportunities for exposure exist at home, in day care facilities and on playgrounds:
- Young children can absorb environmental agents from the air (by inhalation) or through the skin.
- Ingestion is a major route of exposure, especially when children begin to exhibit hand-to-mouth activity.
- Substances on the hair, clothes or hands of the parents can be transferred to the young child.
- Breast milk is another potential source of exposure for infants, although the potential benefits of nursing far outweigh the potential toxic effects of chemicals in breast milk.
For a number of the health effects discussed in connection with neonatal exposures, it is difficult to distinguish prenatal from postnatal events. Exposures taking lace before birth (through the placenta) can continue to be manifest in early childhood. Both lead and environmental tobacco smoke have been associated with deficits in cognitive development and lung function both before and after birth. In this review, we have attempted to focus on postnatal exposures and their effects on the health of very young children.
Lead and Other Heavy Metals
Among the heavy metals, lead (b) is the most important elemental exposure for humans in both environmental and occupational circumstances. Significant occupational exposures occur in battery manufacture, smelters, soldering, welding, construction and paint removal. parents employed in these industries have long been known to bring dust home on their clothes that can be absorbed by their children. The primary route of absorption by children is through ingestion of lead-contaminated paint chips, dust and water. Respiratory absorption is efficient, and inhalation becomes a significant exposure pathway if an aerosol of lead or alkyl lead is resent (Clement International Corporation 1991).
Lead poisoning can damage virtually every organ system, but current levels of exposure have been associated chiefly with neurological and developmental changes in children. In addition, renal and haematological disease have been observed among both adults and children intensely exposed to lead. Cardiovascular disease as well as reproductive dysfunction are known sequelae of lead exposure among adults. Subclinical renal, cardiovascular and reproductive effects are suspected to arise from lower, chronic lead exposure, and limited data support this idea. Animal data support human findings (Sager and Girard 1994).
In terms of measurable dose, neurological effects range from IQ deficits at low exposures (blood lead = 10 μg/dl) to enceha-loathy (80 μg/dl). Levels of concern in children in 1985 were 25 μg/dl, which was lowered to 10 μg/dl in 1993.
Neonatal exposure, as it resulted from dust brought home by working parents, was described as “fouling the nest” by Chisholm in 1978. Since that time, preventive measures, such as showering and changing clothing before leaving the workplace, have reduced the take-home dust burden. However, occupationally derived lead is still an important potential source of neonatal exposure today. A survey of children in Denmark found that blood lead was approximately twice as high among children of exposed workers than in homes with only non-occupational exposures (Grandjean and Bach 1986). Exposure of children to occupationally derived lead has been documented among electric cable splicers (Rinehart and Yanagisawa 1993) and capacitor manufacturing workers (Kaye, Novotny and Tucker 1987).
Non-occupational sources of environmental lead exposure continue to be a serious hazard to young children. Since the gradual ban of tetraethyl lead as a fuel additive in the United States (in 1978), average blood lead levels in children have declined from 13 to 3 μg/dl (Pirkle et al. 1994). paint chips and paint dust are now the principal cause of childhood lead poisoning in the United States (Roer 1991). For example in one report, younger children (neonates aged less than 11 months) with excessive lead in their blood were at greatest risk of exposure through dust and water while older children (aged 24 months) were at risk more from ingestion of paint chips (ica) (Shannon and Graef 1992). Lead abatement through paint removal has been successful in protecting children from exposure to dust and paint chips (Farfel, Chisholm and Rohde 1994). Ironically, workers engaged in this enterprise have been shown to carry lead dust home on their clothes. In addition, it has been noted that the continuing exposure of young children to lead disproportionately affects economically disadvantaged children (Brody et al. 1994; Goldman and Carra 1994). art of this inequity arises from the poor condition of housing; as early as 1982, it was shown that the extent of deterioration of housing was directly related to blood lead levels in children (Clement International Corporation 1991).
Another potential source of occupationally derived exposure for the neonate is lead in breast milk. Higher levels of lead in breast milk have been linked to both occupational and environmental sources (Ryu, Ziegler and Fomon 1978; Dabeka et al. 1986). The concentrations of lead in milk are small relative to blood (approximately 1/5 to 1/2) (Wolff 1993), but the large volume of breast milk ingested by an infant can add milligram quantities to the body burden. In comparison, there is normally less than 0.03 mg b in the circulating blood of an infant and the usual intake is less than 20 mg per day (Clement International Corporation 1991). Indeed, absorption from breast milk is reflected in the blood lead level of infants (Rabinowitz, Leviton and Needleman 1985; Ryu et al. 1983; Ziegler et al. 1978). It should be noted that normal lead levels in breast milk are not excessive, and lactation contributes an amount similar to that from other sources of infant nutrition. By comparison, a small paint chi could contain more than 10 mg (10,000 mg) of lead.
Developmental decrements in children have been linked with both prenatal and postnatal exposures to lead. prenatal exposure is thought to be responsible for lead-related deficits in mental and behavioural development that have been found in children until the age of two to four years (Landrigan and Cambell 1991; Bellinger et al. 1987). The effects of postnatal lead exposure, such as that experienced by the neonate from occupational sources, may be detected in children from ages two to six and even later. Among these are problem behaviour and lower intelligence (Bellinger et al. 1994). These effects are not confined only to high exposures; they have been observed at relatively low levels, e.g., where blood lead levels are in the range of 10 mg/dl (Needleman and Bellinger 1984).
Mercury (Hg) exposure from the environment may occur as inorganic and organic (mainly methyl) forms. Recent occupational exposures to mercury have been found among workers in thermometer manufacture and in repair of high-voltage equipment containing mercury. Other occupations with potential exposures include painting, dentistry, plumbing and chlorine manufacture (Agency for Toxic Substance and Disease Registry 1992).
prenatal and postnatal mercury poisoning has been well documented among children. Children are more susceptible to effects of methylmercury than adults. This is largely because the developing human central nervous system is so “remarkably sensitive” to methylmercury, an effect also seen at low levels in animals (Clarkson, Nordberg and Sager 1985). Methylmercury exposures in children arise chiefly from ingestion of contaminated fish or from breast milk, while elemental mercury is derived from occupational exposures. Household exposure incidental to occupational exposure has been noted (Zirschky and Wetherell 1987). Accidental exposures in the home have been reported in recent years in domestic industries (Meeks, Keith and Tanner 1990; Rowens et al. 1991) and in an accidental sill of metallic mercury (Florentine and Sanfilio 1991). Elemental mercury exposure occurs mainly by inhalation, while alkyl mercury can be absorbed by ingestion, inhalation or dermal contact.
In the best-studied episode of poisoning, sensory and motor dysfunction and mental retardation were found following very high exposures to methylmercury either in utero or from breast milk (Bakir et al. 1973). Maternal exposures resulted from ingestion of methylmercury that had been used as a fungicide on grain.
pesticides and Related Chemicals
Several hundred million tons of pesticides are produced worldwide each year. Herbicides, fungicides and insecticides are employed mainly in agriculture by developed countries to improve crop yield and quality. Wood preservatives are a much smaller, but still a major, art of the market. Home and garden use represents a relatively minor proportion of total consumption, but from the point of view of neonatal toxicity, domestic poisonings are perhaps the most numerous. Occupational exposure is also a potential source of indirect exposure to infants if a parent is involved in work that uses pesticides. Exposure to pesticides is possible through dermal absorption, inhalation and ingestion. More than 50 pesticides have been declared carcinogenic in animals (McConnell 1986).
Organochlorine pesticides include aromatic compounds, such as DDT (bis(4-chlorohenyl)-1,1,1-trichloroethane), and cyclodienes, such as dieldrin. DDT came into use in the early 1940s as an effective means to eliminate mosquitoes carrying malaria, an application that is still widely employed today in developing countries. Lindane is an organochlorine used widely to control body lice and in agriculture, especially in developing countries. olychlorinated bihenyls (CBs), another fat-soluble organochlorine mixture used since the 1940s, pose a potential health risk to young children exposed through breast milk and other contaminated foods. Both lindane and CBs are discussed separately in this chapter. olybrominated bihenyls (BBs) also have been detected in breast milk, almost exclusively in Michigan. Here, a fire-retardant inadvertently mixed into livestock feed in 1973-74 became widely dispersed across the state through dairy and meat products.
Chlordane has been used as a pesticide and as a termiticide in houses, where it is effective for decades, no doubt because of its persistence. Exposure to this chemical can be from dietary and direct respiratory or dermal absorption. Levels in human milk in Japan could be related both to diet and to how recently homes had been treated. Women living in homes treated more than two years earlier had chlordane levels in milk three times those of women living in untreated homes (Taguchi and Yakushiji 1988).
Diet is the main source of persistent organochlorines, but smoking, air and water may also contribute to exposure. This class of pesticides, also termed halogenated hydrocarbons, is quite persistent in the environment, since these are lipophilic, resistant to metabolism or biodegradation and exhibit low volatility. Several hundreds of m have been found in human and animal fat among those with highest exposures. Because of their reproductive toxicity in wildlife and their tendency to bioaccumulate, organochlorines have been largely banned or restricted in developed countries.
At very high doses, neurotoxicity has been observed with organochlorines, but potential long-term health effects are of more concern among humans. Although chronic health effects have not been widely documented, heatotoxicity, cancer and reproductive dysfunction have been found in experimental animals and in wildlife. Health concerns arise mainly from observations in animal studies of carcinogenesis and of profound changes in the liver and the immune system.
Organohoshates and carbamates are less persistent than the organochlorines and are the most widely used class of insecticides internationally. pesticides of this class are degraded relatively quickly in the environment and in the body. A number of the organohoshates and carbamates exhibit high acute neurotoxicity and in certain cases chronic neurotoxicity as well. Dermatitis is also a widely reported symptom of pesticide exposure.
The petroleum-based products used to apply some pesticides are also of potential concern. Chronic effects including haematooietic and other childhood cancers have been associated with parental or residential exposures to pesticides, but the epidemiological data are quite limited. Nevertheless, based on the data from animal studies, exposures to pesticides should be avoided.
For the newborn, a wide spectrum of exposure possibilities and toxic effects have been reported. Among children who required hospitalization for acute poisoning, most had inadvertently ingested pesticide products, while a significant number had been exposed while laying on sprayed carets (Casey, Thomson and Vale 1994; Zwiener and Ginsburg 1988). Contamination of workers’ clothing by pesticide dust or liquid has long been recognized. Therefore, this route provides ample opportunity for home exposures unless workers take proper hygienic precautions after work. For example, an entire family had elevated levels of chlordecone (Keone) in their blood, attributed to home laundering of a worker’s clothes (Grandjean and Bach 1986). Household exposure to TCDD (dioxin) has been documented by the occurrence of chloracne in the son and wife of two workers exposed in the aftermath of an explosion (Jensen, Sneddon and Walker 1972).
Most of the possible exposures to infants arise from pesticide applications within and around the home (Lewis, Fortmann and Camann 1994). Dust in home carets has been found to be extensively contaminated with numerous pesticides (Fenske et al. 1994). Much of reported home contamination has been attributed to flea extermination or to lawn and garden application of pesticides (Davis, Bronson and Garcia 1992). Infant absorption of chloryrifos after treatment of homes for fleas has been predicted to exceed safe levels. Indeed, indoor air levels following such fumigation procedures do not always rapidly diminish to safe levels.
Breast milk is a potential source of pesticide exposure for the neonate. Human milk contamination with pesticides, especially the organochlorines, has been known for decades. Occupational and environmental exposures can lead to significant pesticide contamination of breast milk (D’Ercole et al. 1976; McConnell 1986). Organochlorines, which in the past have been resent in breast milk at excessive levels, are declining in developed countries, paralleling the decline in adipose concentrations that has occurred after restriction of these compounds. Therefore, DDT contamination of human milk is now highest in developing countries. There is little evidence of organohoshates in breast milk. This may be attributable to properties of water solubility and raid metabolism of these compounds in the body.
Ingestion of water contaminated with pesticides is also a potential health risk for the neonate. This problem is most renounced where infant formula must be reared using water. Otherwise, commercial infant formulae are relatively free of contaminants (National Research Council 1993). Food contamination with pesticides may also lead to infant exposure. Contamination of commercial milk, fruits and vegetables with pesticides exists at very low levels even in developed countries where regulation and monitoring are most vigorous (The Referee 1994). Although milk comprises most of the infant diet, fruits (especially ales) and vegetables (especially carrots) are also consumed in a significant amount by young children and therefore represent a possible source of pesticide exposure.
In the industrialized countries, including the United States and western Europe, most of the organochlorine pesticides, including DDT, chlordane, dieldrin and lindane, have been either banned, suspended or restricted since the 1970s (Maxcy Rosenau-Last 1994). pesticides still used for agricultural and non-agricultural purposes are regulated in terms of their levels in foods, water and pharmaceutical products. As a result of this regulation, the levels of pesticides in adipose tissue and human milk have significantly declined over the past four decades. However, the organochlorines are still widely used in developing countries, where, for example, lindane and DDT are among the most frequently employed pesticides for agricultural use and for malaria control (Awumbila and Bokuma 1994).
Lindane
Lindane is the γ-isomer and active ingredient of the technical grade of benzene hexachloride (BHC). BHC, also known as hexachlorocyclohexane (HCH), contains 40 to 90% of other isomers— α, β and δ. This organochlorine has been used as an agricultural and non-agricultural pesticide throughout the world since 1949. Occupational exposures may occur during the manufacture, formulation and application of BHC. Lindane as a pharmaceutical reparation in creams, lotions and shampoos is also widely used to treat scabies and body lice. Because these skin conditions commonly occur among infants and children, medical treatment can lead to absorption of BHC by infants through the skin. Neonatal exposure can also occur by inhalation of vapour or dust that may be brought home by a parent or that may linger after home use. Dietary intake is also a possible means of exposure to infants since BHC has been detected in human milk, dairy products and other foods, as have many organochlorine insecticides. Exposure through breast milk was more prevalent in the United States prior to the ban on the commercial production of lindane. According to the IARC (International Agency for Research on Cancer 1987), it is possible that hexachlorocyclohexane is carcinogenic to humans. However, evidence for adverse health outcomes among infants has been reported chiefly as effects on the neurological and haematooietic systems.
Household exposure to lindane has been described in the wife of a pesticide formulator, demonstrating the potential for similar neonatal exposures. The wife had 5 ng/ml of γ-BHC in her blood, a concentration lower than that of her husband (table 1) (Starr et al. 1974). presumably, γ-BHC was brought into the home on the body and/or clothes of the worker. Levels of γ-BHC in the woman and her husband were higher than those reported in children treated with lotion containing 0.3 to 1.0% BHC.
BHC in breast milk exists mainly as the β-isomer (Smith 1991). The half-life of the γ-isomer in the human body is approximately one day, while the β-isomer accumulates.
Table 1. Potential sources and levels of exposure to newborns
| Source of exposure | g-BHC in blood (ng/ml; ppb) |
|
| Occupational exposures | Low exposures High exposures |
5 36 |
| Adult male | Attempted suicide | 1300 |
| Child | Acute poisoning | 100-800 |
| Children | 1% BHC lotion (average) | 13 |
| Case report of home exposure1 | Husband Wife |
17 5 |
| Unexposed populations since1980 | Yugoslavia Africa Brazil India |
52 72 92 752 |
1Starr et al. (1974); other data from Smith (1991).
2Largely b-isomer.
Dermal absorption of lindane from pharmaceutical products is a function of the amount applied to the skin and duration of exposure. Compared with adults, infants and young children appear to be more susceptible to the toxic effects of lindane (Clement International Corporation 1992). One reason may be that dermal absorption is enhanced by increased permeability of the infant’s skin and a large surface-to-volume ratio. Levels in the neonate may persist longer because the metabolism of BHC is less efficient in infants and young children. In addition, exposure in neonates may be increased by licking or mouthing treated areas (Kramer et al. 1990). A hot shower or bath before dermal application of medical products may facilitate dermal absorption, thereby exacerbating toxicity.
In a number of reported cases of accidental lindane poisoning, overt toxic effects have been described, some in young children. In one case, a two-month-old infant died after multiple exposures to 1% lindane lotion, including a full-body application following a hot bath (Davies et al. 1983).
Lindane production and use is restricted in most developed countries. Lindane is still used extensively in other countries for agricultural purposes, as noted in a study of pesticide use on farms in Ghana, where lindane accounted for 35 and 85% of pesticide use for farmers and herdsmen, respectively (Awumbila and Bokuma 1994).
olychlorinated bihenyls
olychlorinated bihenyls were used from the mid-1940s until the late 1970s as insulating fluids in electrical capacitors and transformers. Residues are still resent in the environment because of pollution, which is due largely to improper disposal or accidental sills. Some equipment still in use or stored remains a potential source of contamination. An incident has been reported in which children had detectable levels of CBs in their blood following exposure while laying with capacitors (Wolff and Schecter 1991). Exposure in the wife of an exposed worker has also been reported (Fishbein and Wolff 1987).
In two studies of environmental exposures, re- and postnatal exposure to CBs has been associated with small but significant effects in children. In one study, slightly impaired motor development was detected among children whose mothers had immediate postnatal breast milk CB levels in the upper 95th percentile of the study group (Rogan et al. 1986). In the other, sensory deficits (as well as smaller gestational size) were seen among children with blood levels in approximately the to 25% (Jacobson et al. 1985; Fein et al. 1984). These exposure levels were in the upper range for the studies (above 3 m in mother’s milk (fat basis) and above 3 ng/ml in children’s blood), yet these are not excessively high. Common occupational exposures result in levels ten to 100 times higher (Wolff 1985). In both studies, effects were attributed to prenatal exposure. Such results however sound a cautionary note for unduly exposing neonates to such chemicals both pre- and postnatally.
Solvents
Solvents are a group of volatile or semi-volatile liquids that are used mainly to dissolve other substances. Exposure to solvents can occur in manufacturing processes, for example hexane exposure during distillation of petroleum products. For most persons, exposures to solvents will arise while these are being used on the job or in the home. Common industrial applications include dry cleaning, degreasing, painting and paint removal, and printing. Within the home, direct contact with solvents is possible during use of products such as metal cleaners, dry cleaning products, paint thinners or sprays.
The major routes of exposure for solvents in both adults and infants are through respiratory and dermal absorption. Ingestion of breast milk is one means of neonatal exposure to solvents derived from the parent’s work. Because of the brief half-life of most solvents, their duration in breast milk will be similarly short. However, following maternal exposure, some solvents will be resent in breast milk at least for a short time (at least one half-life). Solvents that have been detected in breast milk include tetrachloroethylene, carbon disulhide and halothane (an anaesthetic). A detailed review of potential infant exposure to tetrachloroethylene (TCE) has concluded that levels in breast milk can easily exceed recommended health risk guidelines (Schreiber 1993). Excess risk was highest for infants whose mothers might be exposed in the workplace (58 to 600 per million persons). For the highest non-occupational exposures, excess risks of 36 to 220 per 10 million persons were estimated; such exposures can exist in homes directly above dry-cleaners. It was further estimated that milk concentrations of TCE would return to “normal” (re-exposure) levels four to eight weeks after cessation of exposure.
Non-occupational exposures are possible for the infant in the home where solvents or solvent-based products are used. Indoor air has very low, but consistently detectable, levels of solvents like tetrachloroethylene. Water may also contain volatile organic compounds of the same type.
Mineral Dusts and Fibres: Asbestos, Fibreglass, Rock Wool, Zeolites, Talc
Mineral dust and fibre exposure in the workplace causes respiratory disease, including lung cancer, among workers. Dust exposure is a potential problem for the newborn if a parent carries articles into the home on the clothes or body. With asbestos, fibres from the workplace have been found in the home environment, and resulting exposures of family members have been termed bystander or family exposures. Documentation of familial asbestos disease has been possible because of the occurrence of a signal tumour, mesothelioma, that is primarily associated with asbestos exposure. Mesothelioma is a cancer of the leura or eritoneum (linings of lung and abdomen, respectively) that occurs following a long latency period, typically 30 to 40 years after the first asbestos exposure. The aetiology of this disease appears to be related only to the length of time after initial exposure, not to intensity or duration, nor to age at first exposure (Nicholson 1986; Otte, Sigsgaard and Kjaerulff 1990). Respiratory abnormalities have also been attributed to bystander asbestos exposure (Grandjean and Bach 1986). Extensive animal experiments support the human observations.
Most cases of familial mesothelioma have been reported among wives of exposed miners, millers, manufacturers and insulators. However, a number of childhood exposures have also been associated with disease. Quite a few of these children had initial contact that occurred at an early age (Dawson et al. 1992; Anderson et al. 1976; Roggli and Longo 1991). For example, in one investigation of 24 familial contacts with mesothelioma who lived in a crocidolite asbestos mining town, seven cases were identified whose ages were 29 to 39 years at diagnosis or death and whose initial exposure had occurred at less than one year of age (n=5) or at three years (n=2) (Hansen et al. 1993).
Exposure to asbestos is clearly causative for mesothelioma, but an epigenetic mechanism has been further pro[osed to account for unusual clustering of cases within certain families. Thus, the occurrence of mesothelioma among 64 persons in 27 families suggests a genetic trait that may render certain individuals more sensitive to the asbestos insult leading to this disease (Dawson et al. 1992; Bianchi, Brollo and Zuch 1993). However, it also has been suggested that exposure alone may provide an adequate explanation for the reported familial aggregation (Alderson 1986).
Other inorganic dusts associated with occupational disease include fibreglass, zeolites and talc. Both asbestos and fibreglass have been widely used as insulating materials. pulmonary fibrosis and cancer are associated with asbestos and much less clearly with fibreglass. Mesothelioma has been reported in areas of Turkey with indigenous exposures to natural zeolites. Exposures to asbestos may also arise from non-occupational sources. Diaers (“naies”) constructed from asbestos fibre were implicated as a source of childhood asbestos exposure (Li, Dreyfus and Antman 1989); however, parental clothing was not excluded as a source of asbestos contact in this report. Asbestos also has been found in cigarettes, hairdryers, floor tiles and some types of talcum powder. Its use has been eliminated in many countries. However, an important consideration for children is residual asbestos insulation in schools, which has been widely investigated as a potential public health problem.
Environmental Tobacco Smoke
Environmental tobacco smoke (ETS) is a combination of exhaled smoke and smoke emitted from the smoldering cigarette. Although ETS is not itself a source of occupational exposure that may affect the neonate, it is reviewed here because of its potential to cause adverse health effects and because it provides a good example of other aerosol exposures. Exposure of a non-smoker to ETS is often described as passive or involuntary smoking. prenatal exposure to ETS is clearly associated with deficits or impairments in foetal growth. It is difficult to distinguish postnatal outcomes from effects of ETS in the prenatal period, since parental smoking is rarely confined to one time or the other. However, there is evidence to support a relationship of postnatal exposure to ETS with respiratory illness and impaired lung function. The similarity of these findings to experiences among adults strengthens the association.
ETS has been well characterized and extensively studied in terms of human exposure and health effects. ETS is a human carcinogen (US Environmental protection Agency 1992). ETS exposure can be assessed by measuring levels of nicotine, a component of tobacco, and cotinine, its major metabolite, in biological fluids including saliva, blood and urine. Nicotine and cotinine have also been detected in breast milk. Cotinine has also been found in the blood and urine of infants who were exposed to ETS only by breast-feeding (Charlton 1994; National Research Council 1986).
Exposure of the neonate to ETS has been clearly established to result from paternal and maternal smoking in the home environment. Maternal smoking provides the most significant source. For example, in several studies urinary cotinine in children has been shown to correlate with the number of cigarettes smoked by the mother per day (Marbury, Hammon and Haley 1993). The major routes of ETS exposure for the neonate are respiratory and dietary (through breast milk). Day care centers represent another potential exposure situation; many child care facilities do not have a no-smoking policy (Sockrider and Coultras 1994).
Hospitalization for respiratory illness occurs more often among newborns whose parents smoke. In addition, the duration of hospital visits is longer among infants exposed to ETS. In terms of causation, ETS exposure has not been associated with specific respiratory diseases. There is evidence, however, that passive smoking increases the severity of re-existing illnesses such as bronchitis and asthma (Charlton 1994; Chilmonczyk et al. 1993; Rylander et al. 1993). Children and infants exposed to ETS also have higher frequencies of respiratory infections. In addition, smoking parents with respiratory illnesses can transmit airborne infections to infants by coughing.
Children exposed to ETS postnatally show small deficits in lung function which appear to be independent of prenatal exposures (Frischer et al. 1992). Although the ETS-related changes are small (0.5% decrement per year of forced expiratory volume), and while these effects are not clinically significant, they suggest changes in the cells of the developing lung that may portend later risk. parental smoking has also been associated with increased risk of otitis media, or middle ear effusion, in children from infancy to age nine. This condition is a common cause of deafness among children which can cause delays in educational progress. Associated risk is supported by studies attributing one-third of all cases of otitis media to parental smoking (Charlton 1994).
Radiation Exposures
Ionizing radiation exposure is an established health hazard which is generally the result of intense exposure, either accidental or for medical purposes. It can be damaging to highly proliferative cells, and can therefore be very harmful to the developing foetus or neonate. Radiation exposures that result from diagnostic x rays are generally very low level, and considered to be safe. A potential household source of exposure to ionizing radiation is radon, which exists in certain geographic areas in rock formations.
prenatal and postnatal effects of radiation include mental retardation, lower intelligence, growth retardation, congenital malformations and cancer. Exposure to high doses of ionizing radiation is also associated with increased prevalence of cancer. Incidence for this exposure is dependent upon dose and age. In fact, the highest relative risk observed for breast cancer (~9) is among women who were exposed to ionizing radiation at a young age.
Recently, attention has focused on the possible effects of non-ionizing radiation, or electromagnetic fields (EMF). The basis of a relationship between EMF exposure and cancer is not yet known, and the epidemiological evidence is still unclear. However, in several international studies an association has been reported between EMF and leukaemia and male breast cancer.
Childhood exposure to excessive sunlight has been associated with skin cancer and melanoma (Marks 1988).
Childhood Cancer
Although specific substances have not been identified, parental occupational exposures have been linked to childhood cancer. The latency period for developing childhood leukaemia can be two to 10 years following the onset of exposure, indicating that exposures in utero or in the early postnatal period may be implicated in the cause of this disease. Exposure to a number of organochlorine pesticides (BHC, DDT, chlordane) has been tentatively associated with leukaemia, although these data have not been confirmed in more detailed studies. Moreover, elevated risk of cancer and leukaemia has been reported for children whose parents engage in work that involves pesticides, chemicals and fumes (O’Leary et al. 1991). Similarly, risk of Ewing’s bone sarcoma in children was associated with parental occupations in agriculture or exposure to herbicides and pesticides (Holly et al. 1992).
Summary
Many nations attempt to regulate safe levels of toxic chemicals in ambient air and food products and in the workplace. Nevertheless, opportunities for exposure abound, and children are particularly susceptible to both absorption and to effects of toxic chemicals. It has been noted that “many of the 40,000 child lives lost in the developing world every day are a consequence of environmental abuses reflected in unsafe water supplies, disease, and malnutrition” (Schaefer 1994). Many environmental exposures are avoidable. Therefore, prevention of environmental diseases takes high priority as a defence against adverse health effects among children.
Maternity Protection in Legislation
During pregnancy, exposure to certain health and safety hazards of the job or the working environment may have adverse effects on the health of a woman worker and her unborn child. Before and after giving birth, she also needs a reasonable amount of time off from her job to recuperate, breast-feed and bond with her child. Many women want and need to be able to return to work after childbirth; this is increasingly recognized as a basic right in a world where the participation of women in the labour force is continuously increasing and approaching that of men in many countries. As most women need to support themselves and their families, continuity of income during maternity leave is vital.
Over time, governments have enacted a range of legislative measures to protect women workers during pregnancy and at childbirth. A feature of more recent measures is the prohibition of discrimination in employment on the grounds of pregnancy. Another trend is to provide the right for mothers and fathers to share leave entitlements after the birth so that either may care for the child. Collective bargaining in many countries contributes to the more effective application of such measures and often improves upon them. Employers also lay an important role in furthering maternity protection through the terms of individual contracts of employment and enterprise policies.
The Limits of Protection
Laws providing maternity protection for working women are usually restricted to the formal sector, which may represent a small proportion of economic activity. These do not apply to women working in unregistered economic activities in the informal sector, who in many countries represent the majority of working women. While there is a trend worldwide to improve and extend maternity protection, how to protect the large segment of the population living and working outside the formal economy remains a major challenge.
In most countries, labour legislation provides maternity protection for women employed in industrial and non-industrial enterprises in the private and often also the public sector. Homeworkers, domestic employees, own-account workers and workers in enterprises employing only family members are frequently excluded. Since many women work in small firms, the relatively frequent exclusion of undertakings which employ less than a certain number of workers (e.g., five permanent workers in the Republic of Korea) is of concern.
Many women workers in precarious employment, such as temporary workers, or casual workers in Ireland, are excluded from the scope of labour legislation in a number of countries. Depending on the number of hours they work, part-time workers may also be excluded. Other groups of women may be excluded, such as women managers (e.g., Singapore, Switzerland), women whose earnings exceed a certain maximum (e.g., Mauritius) or women who are paid by results (e.g., the Philippines). In rare cases, unmarried women (e.g., teachers in Trinidad and Tobago) do not qualify for maternity leave. However, in Australia (federal), where parental leave is available to employees and their spouses, the term “spouse” is defined to include a de facto spouse. Where age limits are set (e.g., in Israel, women below the age of 18) they usually do not exclude very many women as they are normally fixed below or above the prime child-bearing ages.
Public servants are often covered by special rules, which may provide for more favourable conditions than those applicable to the private sector. For example, maternity leave may be longer, cash benefits may correspond to the full salary instead of a percentage of it, parental leave is more likely to be available, or the right to reinstatement may be more clearly established. In a significant number of countries, conditions in the public service can act as an agent of progress since collective bargaining agreements in the private sector are often negotiated along the lines of public service maternity protection rules.
Similar to labour legislation, social security laws may limit their application to certain sectors or categories of workers. While this legislation is often more restrictive than the corresponding labour laws in a country, it may provide access to maternity cash benefits to groups not covered by labour laws, such as self-employed women or women who work with their self-employed husbands. In many developing countries, owing to a lack of resources, social security legislation may only apply to a limited number of sectors.
Over the decades, however, the coverage of legislation has been extended to more economic sectors and categories of workers. Yet, while an employee may be covered by a law, the enjoyment of certain benefits, in particular maternity leave and cash benefits, may depend on certain eligibility requirements. Thus, while most countries protect maternity, working women do not enjoy a universal right to such protection.
Maternity Leave
Time off work for childbirth can vary from a few weeks to several months, often divided into two parts, before and after the birth. A period of employment prohibition may be stipulated for a part or the whole of the entitlement to ensure that women have sufficient rest. Maternity leave is commonly extended in case of illness, preterm or late birth, and multiple births, or shortened in case of miscarriage, stillbirth or infant death.
Normal duration
Under the ILO’s Maternity protection Convention, 1919 (No. 3), “a woman shall not be permitted to work during the six weeks following her confinement; [and] shall have the right to leave her work if she produces a medical certificate stating that her confinement will probably take lace within six weeks”. The Maternity protection Convention (Revised), 1952 (No. 103), confirms the 12-week leave, including an employment prohibition for six weeks after the birth, but does not prescribe the use of the remaining six weeks. The Maternity protection Recommendation, 1952 (No. 95), suggests a 14-week leave. The Maternity protection Recommendation, 2000 (No. 191) suggests a 18-week leave [Edited, 2011]. Most of the countries surveyed meet the 12-week standard, and at least one-third grant longer periods.
A number of countries afford a possibility of choice in the distribution of maternity leave. In some, the law does not prescribe the distribution of maternity leave (e.g., Thailand), and women are entitled to start the leave as early or as late as they wish. In another group of countries, the law indicates the number of days to be taken after confinement; the balance can be taken either before or after the birth.
Other countries do not allow flexibility: the law provides for two periods of leave, before and after confinement. These periods may be equal, especially where the total leave is relatively short. Where the total leave entitlement exceeds 12 weeks, the prenatal period is often shorter than the postnatal period (e.g., in Germany six weeks before and eight weeks after the birth).
In a relatively small number of countries (e.g., Benin, Chile, Italy), the employment of women is prohibited during the whole period of maternity leave. In others, a period of compulsory leave is prescribed, often after confinement (e.g., Barbados, Ireland, India, Morocco). The most common requirement is a six-week compulsory period after birth. Over the past decade, the number of countries providing for some compulsory leave before the birth has increased. On the other hand, in some countries (e.g., Canada) there is no period of compulsory leave, as it is felt that the leave is a right that should be freely exercised, and that time off should be organized to suit the individual woman’s needs and preferences.
Eligibility for maternity leave
The legislation of most countries recognizes the right of women to maternity leave by stating the amount of leave to which women are entitled; a woman needs only to be employed at the time of going on leave to be eligible for the leave. In a number of countries, however, the law requires women to have been employed for a minimum period prior to the date on which they absent themselves. This period ranges from 13 weeks in Ontario or Ireland to two years in Zambia.
In several countries, women must have worked a certain number of hours in the week or month to be entitled to maternity leave or benefits. When such thresholds are high (as in Malta, 35 hours per week), they can result in excluding a large number of women, who form the majority of part-time workers. In a number of countries, however, thresholds have been lowered recently (e.g., in Ireland, from 16 to eight hours per week).
A small number of countries limit the number of times a woman may request maternity leave over a given period (for example two years), or restrict eligibility to a certain number of pregnancies, either with the same employer or throughout the woman’s life (e.g., Egypt, Malaysia). In Zimbabwe, for example, women are eligible for maternity leave once in every 24 months and for a maximum of three times during the period that they work for the same employer. In other countries, the women who have more than the prescribed number of children are eligible for maternity leave, but not for cash benefits (e.g., Thailand), or are eligible for a shorter period of leave with benefits (e.g., Sri Lanka: 12 weeks for the first two children, six weeks for the third and subsequent children). The number of countries that limit eligibility for maternity leave or benefits to a certain number of pregnancies, children or surviving children (between two and four) appears to be growing, although it is by no means certain that the duration of maternity leave is a decisive factor in motivating decisions about family size.
Advance notice to the employer
In most countries, the only requirement for women to be entitled to maternity leave is the presentation of a medical certificate. Elsewhere, women are also required to give their employer notice of their intention to take maternity leave. The period of notice ranges from as soon as the pregnancy is known (e.g., Germany) to one week before going on leave (e.g., Belgium). Failure to meet the notice requirement may lose women their right to maternity leave. Thus, in Ireland, information regarding the timing of maternity leave is to be supplied as soon as reasonably practicable, but not later than four weeks before the commencement of the leave. An employee loses her entitlement to maternity leave if she fails to satisfy this requirement. In Canada (federal), the notice requirement is waived where there is a valid reason why the notice cannot be given; at provincial level, the notice period ranges from four months to two weeks. If the notice period is not complied with, a woman worker is still entitled to the normal maternity leave in Manitoba; she is entitled to shorter periods (usually six weeks as opposed to 17 or 18) in most other provinces. In other countries, the law does not clarify the consequences of failing to give notice.
Cash Benefits
Most women cannot afford to forfeit their income during maternity leave; if they had to, many would not use all their leave. Since the birth of healthy children benefits the whole nation, as a matter of equity, employers should not bear the full cost of their workers’ absences. Since 1919, ILO standards have held that during maternity leave, women should receive cash benefits, and that these should be paid out of public funds or through a system of insurance. Convention No. 103 requires that contributions due under a compulsory social insurance scheme be paid based on the total number of men and women employed by the undertakings concerned, without distinction based on sex. Although in a few countries, maternity benefits represent only a relatively small percentage of wages, the level of two-thirds called for in Convention No. 103 is reached in several and exceeded in many others. In more than half of the countries surveyed, maternity benefits constitute 100% of insured wages or of full wages.
Many social security laws may provide a specific maternity benefit, thus recognizing maternity as a contingency in its own right. Others provide that during maternity leave, a worker will be entitled to sickness or unemployment benefits. Treating maternity as a disability or the leave as a period of unemployment could be considered unequal treatment since, in general, such benefits are only available during a certain period, and women who use them in connection with maternity may find they do not have enough left to cover actual sickness or unemployment periods later. Indeed, when the 1992 European Council Directive was drafted, a proposal that during maternity leave women would receive sickness benefits was strongly challenged; it was argued that in terms of equal treatment between men and women, maternity needed to be recognized as independent grounds for obtaining benefits. As a compromise, the maternity allowance was defined as guaranteeing an income at least equivalent to what the worker concerned would receive in the event of sickness.
In nearly 80 of the countries surveyed, benefits are paid by national social security schemes, and in over 40, these are at the expense of the employer. In about 15 countries, the responsibility for financing maternity benefits is shared between social security and the employer. Where benefits are financed jointly by social security and the employer, each may be required to pay half (e.g., Costa Rica), although other percentages may be found (e.g., Honduras: two-thirds by social security and one-third by the employer). Another type of contribution may be required of employers: when the amount of maternity benefit paid by social security is based on a statutory insurable income and represents a low percentage of a woman’s full wage, the law sometimes provides that the employer will pay the balance between the woman’s salary and the maternity benefit paid by the social security fund (e.g., in Burkina Faso). Voluntary additional payment by the employer is a feature of many collective agreements, and also of individual employment contracts. The involvement of employers in the payment of cash maternity benefits may be a realistic solution to the problem posed by the lack of other funds.
Protection of the Health of Pregnant and Nursing Women
In line with the requirements of the Maternity protection Recommendation, 1952 (No. 95), many countries provide for various measures to protect the health of pregnant women and their children, seeking to minimize fatigue by the reorganization of working time or to protect women against dangerous or unhealthy work.
In a few countries (e.g., the Netherlands, Panama), the law specifies an obligation of the employer to organize work so that it does not affect the outcome of the pregnancy. This approach, which is in line with modern occupational health and safety practice, permits matching the needs of individual women with the corresponding preventive measures, and is therefore most satisfactory. Much more generally, protection is sought through prohibiting or limiting work which may be harmful to the health of the mother or child. Such a prohibition may be worded in general terms or may apply to certain types of hazardous work. However, in Mexico, the prohibition of employing women in unhealthy or dangerous work does not apply if the necessary health protection measures have, in the opinion of the competent authority, been taken; nor does it apply to women in managerial positions or those who possess a university degree or technical diploma, or the necessary knowledge and experience to carry on the work.
In many countries, the law provides that pregnant women and nursing mothers may not be allowed to do work that is “beyond their strength”, which “involves hazards”, “is dangerous to their health or that of their child”, or “requires a physical effort unsuited to their condition”. The application of such a general prohibition, however, can present problems: how, and by whom, shall it be determined that a job is beyond a person’s strength? By the worker concerned, the employer, the labour inspector, the occupational health physician, the woman’s own doctor? Differences in appreciation might lead to a woman being kept away from work which she could in fact do, while another might not be removed from work which is too taxing.
Other countries list, sometimes in great detail, the type of work that is prohibited to pregnant women and nursing mothers (e.g., Austria, Germany). The handling of loads is frequently regulated. Legislation in some countries specifically prohibits exposure to certain chemicals (e.g., benzene), biological agents, lead and radiation. Underground work is prohibited in Japan during pregnancy and one year after confinement. In Germany, piece-rate work and work on an assembly line with a fixed pace are prohibited. In a few countries, pregnant workers may not be assigned to work outside their permanent place of residence (e.g., Ghana, after the fourth month). In Austria, smoking is not permitted in places where pregnant women are working.
In a number of countries (e.g., Angola, Bulgaria, Haiti, Germany), the employer is required to transfer the worker to suitable work. Often, the worker must retain her former salary even if the salary of the post to which she is transferred is lower. In the Lao people’s Democratic Republic, the woman keeps her former salary during a three-month period, and is then paid at the rate corresponding to the job she is actually performing. In the Russian Federation, where a suitable post is to be given to a woman who can no longer perform her work, she retains her salary during the period in which a new post is found. In certain cases (e.g., Romania), the difference between the two salaries is paid by social security, an arrangement which is to be referred, since the cost of maternity protection should not, as far as feasible, be borne by individual employers.
Transfer may also be available from work that is not dangerous in itself but which a medical practitioner has certified to be harmful to a particular woman’s state of health (e.g., France). In other countries, a transfer is possible at the request of the worker concerned (e.g., Canada, Switzerland). Where the law enables the employer to suggest a transfer, if there is a disagreement between the employer and the worker, an occupational physician will determine whether there is any medical need for changing jobs and whether the worker is fit to take up the job that has been suggested to her.
A few countries clarify the fact that the transfer is temporary and that the worker must be reassigned to her former job when she returns from maternity leave or at a specified time thereafter (e.g., France). Where a transfer is not possible, some countries provide that the worker will be granted sick leave (e.g., Seychelles) or, as was discussed above, that maternity leave will start early (e.g., Iceland).
Non-discrimination
Measures are taken in a growing number of countries to ensure that women do not suffer discrimination on account of pregnancy. Their aim is to ensure that pregnant women are considered for employment and treated during employment on an equal basis with men and with other women, and in particular are not demoted, do not lose seniority or are not denied promotion solely on the grounds of pregnancy. It is now more and more common for national legislation to prohibit discrimination on account of sex. Such a prohibition could be and indeed has been in many cases interpreted by the courts as a prohibition to discriminate on account of pregnancy. The European Court of Justice has followed this approach. In a 1989 judgement, the Court ruled that an employer who dismisses or refuses to recruit a woman because she is pregnant is in breach of Directive 76/207/EEC of the European Council on equal treatment. This judgement was important in clarifying the fact that sex discrimination exists when employment decisions are made on the basis of pregnancy even though the law does not specifically cite pregnancy as prohibited grounds for discrimination. It is customary in sex equality cases to compare the treatment given to a woman with the treatment given to a hypothetical man. The Court ruled that such comparison was not called for in the case of a pregnant woman, since pregnancy was unique to women. Where unfavourable treatment is made on grounds of pregnancy, there is by definition discrimination on grounds of sex. This is consistent with the position of the ILO Committee of Exerts on the Application of Conventions and Recommendations concerning the scope of the Discrimination (Employment and Occupation) Convention, 1958 (No. 111), which notes the discriminatory nature of distinctions on the basis of pregnancy, confinement and related medical conditions (ILO 1988).
A number of countries provide for an explicit prohibition of discrimination on the grounds of pregnancy (e.g., Australia, Italy, US, Venezuela). Other countries define discrimination on grounds of sex to include discrimination on grounds of pregnancy or absence on maternity leave (e.g., Finland). In the US, protection is further ensured through treating pregnancy as a disability: in undertakings with more than 15 workers, discrimination is prohibited against pregnant women, women at childbirth and women who are affected by related medical conditions; and policies and practices in connection with pregnancy and related matters must be applied on the same terms and conditions as applied to other disabilities.
In several countries, the law contains precise requirements which illustrate instances of discrimination on the grounds of pregnancy. For example, in the Russian Federation, an employer may not refuse to hire a woman because she is pregnant; if a pregnant woman is not hired, the employer must state in writing the reasons for not recruiting her. In France, it is unlawful for an employer to take pregnancy into account in refusing to employ a woman, in terminating her contract during a period of probation or in ordering her transfer. It is also unlawful for the employer to seek to determine whether an applicant is pregnant, or to cause such information to be sought. Similarly, women cannot be required to reveal the fact that they are pregnant, whether they apply for a job or are employed in one, except when they request to benefit from any law or regulation governing the protection of pregnant women.
Transfers unilaterally and arbitrarily imposed on a pregnant woman can constitute discrimination. In Bolivia, as in other countries in the region, a woman is protected against involuntary transfer during pregnancy and up to a year after the birth of her child.
The issue of combining the right of working women to health protection during pregnancy and their right not to suffer discrimination poses a special difficulty at the time of recruitment. Should a pregnant applicant reveal her condition, especially one who applies for a position involving work which is prohibited to pregnant women? In a 1988 judgement, the Federal Labour Court of Germany held that a pregnant woman applying for a job involving exclusively night work, which is prohibited to pregnant women under German legislation, should inform a potential employer of her condition. The judgement was overruled by the European Court of Justice as being contrary to the 1976 EC Directive on equal treatment. The Court found that the Directive precluded an employment contract from being held to be void on account of the statutory prohibition of night work, or from being avoided by the employer on account of a mistake on his or her part as to an essential personal characteristic of the woman at the time of the conclusion of the contract. The employee’s inability, due to pregnancy, to perform the work for which she was being recruited was temporary since the contract was not concluded with a fixed term. It would therefore be contrary to the objective of the Directive to hold it invalid or void because of such an inability.
Employment Security
Many women have lost their jobs because of a pregnancy. Nowadays, although the extent of protection varies, employment security is a significant component of maternity protection policies.
International labour standards address the issue in two different ways. The maternity protection Conventions prohibit dismissal during maternity leave and any extension thereof, or at such time as a notice of dismissal would expire during the leave under the terms of Convention No. 3, Article 4 and Convention No. 103, Article 6. Dismissal on grounds that might be regarded as legitimate is not considered to be permitted during this period (ILO 1965). In the event that a woman has been dismissed before going on maternity leave, the notice should be suspended for the time she is absent and continue after her return. The Maternity protection Recommendation, 1952 (No. 95), calls for the protection of a pregnant woman’s employment from the date the employer is informed of the pregnancy until one month after her return from maternity leave. It identifies cases of serious fault by the employed woman, the shutting down of the undertaking and the expiry of a fixed-term contract as legitimate grounds for dismissal during the protected period. The Termination of Employment Convention, 1982 (No. 158; Article 5(d)–(e)), does not prohibit dismissal, but provides that pregnancy or absence from work on maternity leave shall not constitute valid reasons for termination of employment.
At the level of the European Union, the 1992 Directive prohibits dismissal from the beginning of pregnancy until the end of the maternity leave, save in exceptional cases not connected with the worker’s condition.
Usually, countries provide for two sets of rules regarding dismissal. Dismissal with notice applies in such cases as the closure of the enterprise, redundancy and where, for a variety of reasons, the worker is unable to perform the work for which he or she has been recruited or fails to perform such work to the employer’s satisfaction. Dismissal without notice is used to terminate the services of a worker who is guilty of gross negligence, serious misconduct or other grave instances of behaviour, usually comprehensively listed in the legislation.
Where dismissal with notice is concerned, it is clear that employers could arbitrarily decide that pregnancy is incompatible with a worker’s tasks and dismiss her on grounds of pregnancy. Those who wish to avoid their obligations to pregnant women, or even simply do not like to have pregnant women around the workplace, could find a pretext to dismiss workers during pregnancy even if, in view of the existence of non-discrimination rules, they would refrain from using pregnancy as grounds for dismissal. Many people agree that it is legitimate to protect workers against such discriminatory decisions: the prohibition of dismissal with notice on grounds of pregnancy or during pregnancy and maternity leave is often viewed as a measure of equity and is in force in many countries.
The ILO Committee of Exerts on the Application of Conventions and Recommendations considers that protection against dismissal does not preclude an employer from terminating an employment relationship because he or she has detected a serious fault on the part of a woman employee: rather, when there are reasons such as this to justify dismissal, the employer is obliged to extend the legal period of notice by any period required to complete the period of protection under the Conventions. This is the situation, for example, in Belgium, where an employer who has legal grounds for dismissing a woman cannot do so while she is on maternity leave, but can serve notice so that it expires after the woman returns from leave.
The protection of pregnant women against dismissal in case of closure of the undertaking or economic retrenchment poses a similar problem. It is indeed a burden for a firm which ceases operation to continue to pay the salary of a person who is not working for them any more, even for a short period. However, recruitment prospects are often bleaker for women who are pregnant than for women who are not, or for men, and pregnant women particularly need the emotional and financial security of continuing to be employed. Where women may not be dismissed during pregnancy, they can put off looking for a job until after the birth. In fact, where legislation provides for the order in which various categories of workers to be retrenched are to be dismissed, pregnant women are among those to be dismissed last or next to last (e.g., Ethiopia).
Leave and Benefits for Fathers and Parents
Going beyond the protection of the health and employment status of pregnant and nursing women, many countries provide for paternity leave (a short period of leave at or about the time of birth). Other forms of leave are linked to the needs of children. One type is adoption leave, and another is leave to facilitate child-rearing. Many countries foresee the latter type of leave, but use different approaches. One group provides for time off for the mother of very young children (optional maternity leave), while another provides additional leave for both parents (parental education leave). The view that both the father and mother need to be available to care for young children is also reflected in integrated parental leave systems, which provide a long period of leave available to both parents.
Pregnancy and US Work Recommendations
Changes in family life over recent decades have had dramatic effects on the relationship between work and pregnancy. These include the following:
- Women, particularly those of childbearing age, continue to enter the labour force in considerable numbers.
- A tendency has developed on the part of many of these women to defer starting their families until they are older, by which time they have often achieved positions of responsibility and become important members of the productive apparatus.
- At the same time, there is an increasing number of teenage pregnancies, many of which are high-risk pregnancies.
- Reflecting increasing rates of separation, of divorce and of choices of alternative lifestyles, as well as an increase in the number of families in which both parents must work, financial pressures are forcing many women to continue working for as long as possible during pregnancy.
The impact of pregnancy-related absences and lost or impaired productivity, as well as concern over the health and well-being of both the mothers and their infants, have led employers to become more proactive in dealing with the problem of pregnancy and work. Where employers pay all or part of health insurance premiums, the prospect of avoiding the sometimes staggering costs of complicated pregnancies and neonatal problems is a potent incentive. Certain responses are dictated by laws and government regulations, for example, guarding against potential occupational and environmental hazards and providing maternity leave and other benefits. Others are voluntary: prenatal education and care programmers, modified work arrangements such as flex-time and other work schedule arrangements, dependant care and other benefits.
Management of pregnancy
Of primary importance to the pregnant woman—and to her employer—whether or not she continues working during her pregnancy, is access to a professional health management programme designed to identify and avert or minimize risks to the mother and her foetus, thus enabling her to remain on the job without concern. At each of the scheduled prenatal visits, the physician or midwife should evaluate medical information (childbearing and other medical history, current complaints, physical examinations and laboratory tests) and information about her job and work environment, and develop appropriate recommendations.
It is important that health professionals not rely on the simple job descriptions pertaining to their patients’ work, as these are often inaccurate and misleading. The job information should include details concerning physical activity, chemical and other exposures and emotional stress, most of which can be provided by the woman herself. In some instances, however, input from a supervisor, often relayed by the safety department or the employee health service (where there is one), may be needed to provide a more complete picture of hazardous or trying work activities and the possibility of controlling their potential for harm. This can also serve as a check on patients who inadvertently or deliberately mislead their physicians; they may exaggerate the risks or, if they feel it is important to continue working, may understate them.
Recommendations for Work
Recommendations regarding work during pregnancy fall into three categories:
The woman may continue to work without changes in her activities or the environment. This is applicable in most instances. After extensive deliberation, the Task Force on the Disability of pregnancy comprising obstetrical health professionals, occupational physicians and nurses, and women’s representatives assembled by ACOG (the American College of Obstetricians and Gynecologists) and NIOSH (the National Institute for Occupational Safety and Health) concluded that “the normal woman with an uncomplicated pregnancy who is in a job that presents no greater hazards than those encountered in normal daily life in the community, may continue to work without interruption until the onset of labor and may resume working several weeks after an uncomplicated delivery” (Isenman and Warshaw, 1977).
The woman may continue to work, but only with certain modifications in the work environment or her work activities. These modifications would be either “desirable” or “essential” (in the latter case, she should stop work if they cannot be made).
The woman should not work. It is the physician’s or midwife’s judgement that any work would probably be detrimental to her health or to that of the developing foetus.
The recommendations should not only detail the needed job modifications but should also stipulate the length of time they should be in effect and indicate the date for the next professional examination.
Non-medical Considerations
The recommendations suggested above are based entirely on considerations of the health of the mother and her foetus in relation to job requirements. They do not take into account the burden of such off-the-job activities as commuting to and from the workplace, housework and care of other children and family members; these may sometimes be even more demanding than those of the job. When modification or restriction of activities is called for, one should consider the question whether it should be implemented on the job, in the home or both.
In addition, recommendations for or against continuing work may form the basis of a variety of non-medical considerations, for example, eligibility for benefits, paid versus unpaid leave or guaranteed job retention. A critical issue is whether the woman is considered disabled. Some employers categorically consider all pregnant workers to be disabled and strive to eliminate them from the workforce, even though many are able to continue to work. Other employers assume that all pregnant employees tend to magnify any disability in order to be eligible for all available benefits. And some even challenge the notion that a pregnancy, whether or not it is disabling, is a matter for them to be concerned about at all. Thus, disability is a complex concept which, although fundamentally based on medical findings, involves legal and social considerations.
Pregnancy and Disability
In many jurisdictions, it is important to distinguish between the disability of pregnancy and pregnancy as a period in life that calls for special benefits and dispensations. The disability of pregnancy falls into three categories:
- Disability following delivery. From a purely medical standpoint, recovery following the termination of pregnancy through an uncomplicated delivery lasts only a few weeks, but conventionally it extends to six or eight weeks because that is when most obstetricians customarily schedule their first postnatal check-up. However, from a practical and sociological point of view, a longer leave is considered by many to be desirable in order to enhance family bonding, to facilitate breast-feeding, and so on.
- Disability resulting from medical complications. Medical complications such as eclamsia, threatened abortion, cardiovascular or renal problems and so on, will dictate periods of reduced activity or even hospitalization that will last as long as the medical condition persists or until the woman has recovered from both the medical problem and the pregnancy.
- Disability reflecting the necessity of avoiding exposure to toxicity hazards or abnormal physical stress. Because of the greater sensitivity of the foetus to many environmental hazards, the pregnant woman may be considered disabled even though her own health might not be in danger of being compromised.
Conclusion
The challenge of balancing family responsibilities and work outside the home is not new to women. What may be new is a modern society that values the health and well-being of women and their offspring while confronting women with the dual challenges of achieving personal fulfillment through employment and coping with the economic pressures of maintaining an acceptable standard of living. The increasing number of single parents and of married couples both of whom must work suggest that work-family issues cannot be ignored. Many employed women who become pregnant simply must continue to work.
Whose responsibility is it to meet the needs of these individuals? Some would argue that it is purely a personal problem to be dealt with entirely by the individual or the family. Others consider it a societal responsibility and would enact laws and provide financial and other benefits on a community-wide basis.
How much should be loaded on the employer? This depends largely on the nature, the location and often the size of the organization. The employer is driven by two sets of considerations: those imposed by laws and regulations (and sometimes by the need to meet demands won by organized labour) and those dictated by social responsibility and the practical necessity of maintaining optimal productivity. In the last analysis, it hinges on lacing a high value on human resources and acknowledging the interdependence of work responsibilities and family commitments and their sometimes counterbalancing effects on health and productivity.
Structure and Function
The respiratory system extends from the breathing zone just outside of the nose and mouth through the conductive airways in the head and thorax to the alveoli, where respiratory gas exchange takes place between the alveoli and the capillary blood flowing around them. Its prime function is to deliver oxygen (O2) to the gas-exchange region of the lung, where it can diffuse to and through the walls of the alveoli to oxygenate the blood passing through the alveolar capillaries as needed over a wide range of work or activity levels. In addition, the system must also: (1) remove an equal volume of carbon dioxide entering the lungs from the alveolar capillaries; (2) maintain body temperature and water vapour saturation within the lung airways (in order to maintain the viability and functional capacities of the surface fluids and cells); (3) maintain sterility (to prevent infections and their adverse consequences); and (4) eliminate excess surface fluids and debris, such as inhaled particles and senescent phagocytic and epithelial cells. It must accomplish all of these demanding tasks continuously over a lifetime, and do so with high efficiency in terms of performance and energy utilization. The system can be abused and overwhelmed by severe insults such as high concentrations of cigarette smoke and industrial dust, or by low concentrations of specific pathogens which attack or destroy its defence mechanisms, or cause them to malfunction. Its ability to overcome or compensate for such insults as competently as it usually does is a testament to its elegant combination of structure and function.
Mass Transfer
The complex structure and numerous functions of the human respiratory tract have been summarized concisely by a Task Group of the International Commission on Radiological Protection (ICRP 1994), as shown in figure 1. The conductive airways, also known as the respiratory dead space, occupy about 0.2 litres. They condition the inhaled air and distribute it, by convective (bulk) flow, to the approximately 65,000 respiratory acini leading off the terminal bronchioles. As tidal volumes increase, convective flow dominates gas exchange deeper into the respiratory bronchioles. In any case, within the respiratory acinus, the distance from the convective tidal front to alveolar surfaces is short enough so that efficient CO2-O2 exchange takes place by molecular diffusion. By contrast, airborne particles, with diffusion coefficients smaller by orders of magnitude than those for gases, tend to remain suspended in the tidal air, and can be exhaled without deposition.
Figure 1. Morphometry, cytology, histology, function and structure of the respiratory tract and regions used in the 1994 ICRP dosimetry model.
A significant fraction of the inhaled particles do deposit within the respiratory tract. The mechanisms accounting for particle deposition in the lung airways during the inspiratory phase of a tidal breath are summarized in figure 2. Particles larger than about 2 mm in aerodynamic diameter (diameter of a unit density sphere having the same terminal settling (Stokes) velocity) can have significant momentum and deposit by impaction at the relatively high velocities present in the larger airways. Particles larger than about 1 mm can deposit by sedimentation in the smaller conductive airways, where flow velocities are very low. Finally, particles with diameters between 0.1 and 1 mm, which have a very low probability of depositing during a single tidal breath, can be retained within the approximately 15% of the inspired tidal air that is exchanged with residual lung air during each tidal cycle. This volumetric exchange occurs because of the variable time-constants for airflow in the different segments of the lungs. Due to the much longer residence times of the residual air in the lungs, the low intrinsic particle displacements of 0.1 to 1 mm particles within such trapped volumes of inhaled tidal air become sufficient to cause their deposition by sedimentation and/or diffusion over the course of successive breaths.
Figure 2. Mechanisms for particle deposition in lung airways
The essentially particle-free residual lung air that accounts for about 15% of the expiratory tidal flow tends to act like a clean-air sheath around the axial core of distally moving tidal air, such that particle deposition in the respiratory acinus is concentrated on interior surfaces such as airway bifurcations, while interbranch airway walls have little deposition.
The number of particles deposited and their distribution along the respiratory tract surfaces are, along with the toxic properties of the material deposited, the critical determinants of pathogenic potential. The deposited particles can damage the epithelial and/or the mobile phagocytic cells at or near the deposition site, or can stimulate the secretion of fluids and cell-derived mediators that have secondary effects on the system. Soluble materials deposited as, on, or within particles can diffuse into and through surface fluids and cells and be rapidly transported by the bloodstream throughout the body.
Aqueous solubility of bulk materials is a poor guide to particle solubility in the respiratory tract. Solubility is generally greatly enhanced by the very large surface-to-volume ratio of particles small enough to enter the lungs. Furthermore, the ionic and lipid contents of surface fluids within the airways are complex and highly variable, and can lead to either enhanced solubility or to rapid precipitation of aqueous solutes. Furthermore, the clearance pathways and residence times for particles on airway surfaces are very different in the different functional parts of the respiratory tract.
The revised ICRP Task Group’s clearance model identifies the principal clearance pathways within the respiratory tract that are important in determining the retention of various radioactive materials, and thus the radiation doses received by respiratory tissues and other organs after translocation. The ICRP deposition model is used to estimate the amount of inhaled material that enters each clearance pathway. These discrete pathways are represented by the compartment model shown in figure 3. They correspond to the anatomic compartments illustrated in Figure 1, and are summarized in table 1, along with those of other groups providing guidance on the dosimetry of inhaled particles.
Figure 3. Compartment model to represent time-dependent particle transport from each region in 1994 ICRP model
Table 1. Respiratory tract regions as defined in particle deposition models
| Anatomic structures included | ACGIH Region | ISO and CEN Regions | 1966 ICRP Task Group Region | 1994 ICRP Task Group Region |
| Nose, nasopharynx Mouth, oropharynx, laryngopharynx |
Head airways (HAR) | Extrathoracic (E) | Nasopharynx (NP) | Anterior nasal passages (ET1 ) All other extrathoracic (ET2 ) |
| Trachea, bronchi | Tracheobronchial (TBR) | Tracheobronchial (B) | Tracheobronchial (TB) | Trachea and large bronchi (BB) |
| Bronchioles (to terminal bronchioles) | Bronchioles (bb) | |||
| Respiratory bronchioles, alveolar ducts, alveolar sacs, alveoli |
Gas exchange (GER) | Alveolar (A) | Pulmonary (P) | Alveolar-interstitial (AI) |
Extrathoracic airways
As shown in figure 1, the extrathoracic airways were partitioned by ICRP (1994) into two distinct clearance and dosimetric regions: the anterior nasal passages (ET1) and all other extrathoracic airways (ET2)—that is, the posterior nasal passages, the naso- and oropharynx, and the larynx. Particles deposited on the surface of the skin lining the anterior nasal passages (ET1) are assumed to be subject only to removal by extrinsic means (nose blowing, wiping and so on). The bulk of material deposited in the naso-oropharynx or larynx (ET2) is subject to fast clearance in the layer of fluid that covers these airways. The new model recognizes that diffusional deposition of ultrafine particles in the extrathoracic airways can be substantial, while the earlier models did not.
Thoracic airways
Radioactive material deposited in the thorax is generally divided between the tracheobronchial (TB) region, where deposited particles are subject to relatively fast mucociliary clearance, and the alveolar-interstitial (AI) region, where the particle clearance is much slower.
For dosimetry purposes, the ICRP (1994) divided deposition of inhaled material in the TB region between the trachea and bronchi (BB), and the more distal, small airways, the bronchioles (bb). However, the subsequent efficiency with which cilia in either type of airways are able to clear deposited particles is controversial. In order to be certain that doses to bronchial and bronchiolar epithelia would not be underestimated, the Task Group assumed that as much as half the number of particles deposited in these airways is subject to relatively “slow” mucociliary clearance. The likelihood that a particle is cleared relatively slowly by the mucociliary system appears to depend on its physical size.
Material deposited in the AI region is subdivided among three compartments (AI1, AI2 and AI3) that are each cleared more slowly than TB deposition, with the subregions cleared at different characteristic rates.
Figure 4. Fractional deposition in each region of respiratory tract for reference light worker (normal nose breather) in 1994 ICRP model.
Figure 4 depicts the predictions of the ICRP (1994) model in terms of the fractional deposition in each region as a function of the size of the inhaled particles. It reflects the minimal lung deposition between 0.1 and 1 mm, where deposition is determined largely by the exchange, in the deep lung, between tidal and residual lung air. Deposition increases below 0.1 mm as diffusion becomes more efficient with decreasing particle size. Deposition increases with increasing particle size above 1 mm as sedimentation and impaction become increasingly effective.
Less complex models for size-selective deposition have been adopted by occupational health and community air pollution professionals and agencies, and these have been used to develop inhalation exposure limits within specific particle size ranges. Distinctions are made between:
- those particles that are not aspirated into the nose or mouth and therefore represent no inhalation hazard
- the inhalable (also known as inspirable) particulate mass (IPM)—those that are inhaled and are hazardous when deposited anywhere within the respiratory tract
- the thoracic particulate mass (TPM)—those that penetrate the larynx and are hazardous when deposited anywhere within the thorax and
- the respirable particulate mass (RPM)—those particles that penetrate through the terminal bronchioles and are hazardous when deposited within the gas-exchange region of the lungs.
In the early 1990s there has been an international harmonization of the quantitative definitions of IPM, TPM and RPM. The size-selective inlet specifications for air samplers meeting the criteria of the American Conference of Governmental Industrial Hygienists (ACGIH 1993), the International Organization for Standardization (ISO 1991) and the European Standardization Committee (CEN 1991) are enumerated in table 2. They differ from the deposition fractions of ICRP (1994), especially for larger particles, because they take the conservative position that protection should be provided for those engaged in oral inhalation, and thereby bypass the more efficient filtration efficiency of the nasal passages.
Table 2. Inhalable, thoracic and respirable dust criteria of ACGIH, ISO and CEN, and PM10 criteria of US EPA
| Inhalable | Thoracic | Respirable | PM10 | ||||
| Particle aero- dynamic diameter (mm) |
Inhalable Particulate Mass (IPM) (%) |
Particle aero- dynamic diameter (mm) |
Thoracic Particulate Mass (TPM) (%) |
Particle aero- dynamic diameter (mm) |
Respirable Particulate Mass (RPM) (%) |
Particle aero- dynamic diameter (mm) |
Thoracic Particulate Mass (TPM) (%) |
| 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 |
| 1 | 97 | 2 | 94 | 1 | 97 | 2 | 94 |
| 2 | 94 | 4 | 89 | 2 | 91 | 4 | 89 |
| 5 | 87 | 6 | 80.5 | 3 | 74 | 6 | 81.2 |
| 10 | 77 | 8 | 67 | 4 | 50 | 8 | 69.7 |
| 20 | 65 | 10 | 50 | 5 | 30 | 10 | 55.1 |
| 30 | 58 | 12 | 35 | 6 | 17 | 12 | 37.1 |
| 40 | 54.5 | 14 | 23 | 7 | 9 | 14 | 15.9 |
| 50 | 52.5 | 16 | 15 | 8 | 5 | 16 | 0 |
| 100 | 50 | 18 | 9.5 | 10 | 1 | ||
| 20 | 6 | ||||||
| 25 | 2 | ||||||
The US Environmental Protection Agency (EPA 1987) standard for ambient air particle concentration is known as PM10, that is, particulate matter less than 10 mm in aerodynamic diameter. It has a sampler inlet criterion that is similar (functionally equivalent) to TPM but, as shown in Table 2, somewhat different numerical specifications.
Air Pollutants
Pollutants can be dispersed in air at normal ambient temperatures and pressures in gaseous, liquid and solid forms. The latter two represent suspensions of particles in air and were given the generic term aerosols by Gibbs (1924) on the basis of analogy to the term hydrosol, used to describe dispersed systems in water. Gases and vapours, which are present as discrete molecules, form true solutions in air. Particles consisting of moderate to high vapour pressure materials tend to evaporate rapidly, because those small enough to remain suspended in air for more than a few minutes (i.e., those smaller than about 10 mm) have large surface-to-volume ratios. Some materials with relatively low vapour pressures can have appreciable fractions in both vapour and aerosol forms simultaneously.
Gases and vapours
Once dispersed in air, contaminant gases and vapours generally form mixtures so dilute that their physical properties (such as density, viscosity, enthalpy and so on) are indistinguishable from those of clean air. Such mixtures may be considered to follow ideal gas law relationships. There is no practical difference between a gas and a vapour except that the latter is generally considered to be the gaseous phase of a substance that can exist as a solid or liquid at room temperature. While dispersed in air, all molecules of a given compound are essentially equivalent in their size and probabilities of capture by ambient surfaces, respiratory tract surfaces and contaminant collectors or samplers.
Aerosols
Aerosols, being dispersions of solid or liquid particles in air, have the very significant additional variable of particle size. Size affects particle motion and, hence, the probabilities of physical phenomena such as coagulation, dispersion, sedimentation, impaction onto surfaces, interfacial phenomena and light-scattering properties. It is not possible to characterize a given particle by a single size parameter. For example, a particle’s aerodynamic properties depend on density and shape as well as linear dimensions, and the effective size for light scattering is dependent on refractive index and shape.
In some special cases, all of the particles are essentially the same in size. Such aerosols are considered to be monodisperse. Examples are natural pollens and some laboratory-generated aerosols. More typically, aerosols are composed of particles of many different sizes and hence are called heterodisperse or polydisperse. Different aerosols have different degrees of size dispersion. It is, therefore, necessary to specify at least two parameters in characterizing aerosol size: a measure of central tendency, such as a mean or median, and a measure of dispersion, such as an arithmetic or geometric standard deviation.
Particles generated by a single source or process generally have diameters following a log-normal distribution; that is, the logarithms of their individual diameters have a Gaussian distribution. In this case, the measure of dispersion is the geometric standard deviation, which is the ratio of the 84.1 percentile size to the 50 percentile size. When more than one source of particles is significant, the resulting mixed aerosol will usually not follow a single log-normal distribution, and it may be necessary to describe it by the sum of several distributions.
Particle characteristics
There are many properties of particles other than their linear size that can greatly influence their airborne behaviour and their effects on the environment and health. These include:
Surface. For spherical particles, the surface varies as the square of the diameter. However, for an aerosol of given mass concentration, the total aerosol surface increases with decreasing particle size. For non-spherical or aggregate particles, and for particles with internal cracks or pores, the ratio of surface to volume can be much greater than for spheres.
Volume. Particle volume varies as the cube of the diameter; therefore, the few largest particles in an aerosol tend to dominate its volume (or mass) concentration.
Shape. A particle’s shape affects its aerodynamic drag as well as its surface area and therefore its motion and deposition probabilities.
Density. A particle’s velocity in response to gravitational or inertial forces increases as the square root of its density.
Aerodynamic diameter. The diameter of a unit-density sphere having the same terminal settling velocity as the particle under consideration is equal to its aerodynamic diameter. Terminal settling velocity is the equilibrium velocity of a particle that is falling under the influence of gravity and fluid resistance. Aerodynamic diameter is determined by the actual particle size, the particle density and an aerodynamic shape factor.
Types of aerosols
Aerosols are generally classified in terms of their processes of formation. Although the following classification is neither precise nor comprehensive, it is commonly used and accepted in the industrial hygiene and air pollution fields.
Dust. An aerosol formed by mechanical subdivision of bulk material into airborne fines having the same chemical composition. Dust particles are generally solid and irregular in shape and have diameters greater than 1 mm.
Fume. An aerosol of solid particles formed by condensation of vapours formed by combustion or sublimation at elevated temperatures. The primary particles are generally very small (less than 0.1 mm) and have spherical or characteristic crystalline shapes. They may be chemically identical to the parent material, or may be composed of an oxidation product such as metal oxide. Since they may be formed in high number concentrations, they often rapidly coagulate, forming aggregate clusters of low overall density.
Smoke. An aerosol formed by condensation of combustion products, generally of organic materials. The particles are generally liquid droplets with diameters less than 0.5 mm.
Mist. A droplet aerosol formed by mechanical shearing of a bulk liquid, for example, by atomization, nebulization, bubbling or spraying. The droplet size can cover a very large range, usually from about 2 mm to greater than 50 mm.
Fog. An aqueous aerosol formed by condensation of water vapour on atmospheric nuclei at high relative humidities. The droplet sizes are generally greater than 1 mm.
Smog. A popular term for a pollution aerosol derived from a combination of smoke and fog. It is now commonly used for any atmospheric pollution mixture.
Haze. A submicrometer-sized aerosol of hygroscopic particles that take up water vapour at relatively low relative humidities.
Aitken or condensation nuclei (CN). Very small atmospheric particles (mostly smaller than 0.1 mm) formed by combustion processes and by chemical conversion from gaseous precursors.
Accumulation mode. A term given to the particles in the ambient atmosphere ranging from 0.1 to about 1.0 mm in diameter. These particles generally are spherical (having liquid surfaces), and form by coagulation and condensation of smaller particles that derive from gaseous precursors. Being too large for rapid coagulation and too small for effective sedimentation, they tend to accumulate in the ambient air.
Coarse particle mode. Ambient air particles larger than about 2.5 mm in aerodynamic diameter and generally formed by mechanical processes and surface dust resuspension.
Biological Responses of the Respiratory System to Air Pollutants
Responses to air pollutants range from nuisance to tissue necrosis and death, from generalized systemic effects to highly specific attacks on single tissues. Host and environmental factors serve to modify the effects of inhaled chemicals, and the ultimate response is the result of their interaction. The main host factors are:
- age—for example, older people, especially those with chronically reduced cardiovascular and respiratory function, who may not be able to cope with additional pulmonary stresses
- state of health—for example, concurrent disease or dysfunction
- nutritional status
- immunological status
- sex and other genetic factors—for example, enzyme-related differences in biotransformation mechanisms, such as deficient metabolic pathways, and inability to synthesize certain detoxification enzymes
- psychological state—for example, stress, anxiety and
- cultural factors—for example, cigarette smoking, which may affect normal defences, or may potentiate the effect of other chemicals.
The environmental factors include the concentration, stability and physicochemical properties of the agent in the exposure environment and the duration, frequency and route of exposure. Acute and chronic exposures to a chemical may result in different pathological manifestations.
Any organ can respond in only a limited number of ways, and there are numerous diagnostic labels for the resultant diseases. The following sections discuss the broad types of responses of the respiratory system which may occur following exposure to environmental pollutants.
Irritant response
Irritants produce a pattern of generalized, non-specific tissue inflammation, and destruction may result at the area of contaminant contact. Some irritants produce no systemic effect because the irritant response is much greater than any systemic effect, while some also have significant systemic effects following absorption—for example, hydrogen sulphide absorbed via the lungs.
At high concentrations, irritants may cause a burning sensation in the nose and throat (and usually also in the eyes), pain in the chest and coughing producing inflammation of the mucosa (tracheitis, bronchitis). Examples of irritants are gases such as chlorine, fluorine, sulphur dioxide, phosgene and oxides of nitrogen; mists of acids or alkali; fumes of cadmium; dusts of zinc chloride and vanadium pentoxide. High concentrations of chemical irritants may also penetrate deep into the lungs and cause lung oedema (the alveoli are filled with liquid) or inflammation (chemical pneumonitis).
Highly elevated concentrations of dusts which have no chemical irritative properties can also mechanically irritate bronchi and, after entering the gastrointestinal tract, may also contribute to stomach and colon cancer.
Exposure to irritants may result in death if critical organs are severely damaged. On the other hand, the damage may be reversible, or it may result in permanent loss of some degree of function, such as impaired gas-exchange capacity.
Fibrotic response
A number of dusts lead to the development of a group of chronic lung disorders termed pneumoconioses. This general term encompasses many fibrotic conditions of the lung, that is, diseases characterized by scar formation in the interstitial connective tissue. Pneumoconioses are due to the inhalation and subsequent selective retention of certain dusts in the alveoli, from which they are subject to interstitial sequestration.
Pneumoconioses are characterized by specific fibrotic lesions, which differ in type and pattern according to the dust involved. For example, silicosis, due to the deposition of crystalline-free silica, is characterized by a nodular type of fibrosis, while a diffuse fibrosis is found in asbestosis, due to asbestos-fibre exposure. Certain dusts, such as iron oxide, produce only altered radiology (siderosis) with no functional impairment, while the effects of others range from a minimal disability to death.
Allergic response
Allergic responses involve the phenomenon known as sensitization. Initial exposure to an allergen results in the induction of antibody formation; subsequent exposure of the now “sensitized” individual results in an immune response—that is, an antibody-antigen reaction (the antigen is the allergen in combination with an endogenous protein). This immune reaction may occur immediately following exposure to the allergen, or it may be a delayed response.
The primary respiratory allergic reactions are bronchial asthma, reactions in the upper respiratory tract which involve the release of histamine or histamine-like mediators following immune reactions in the mucosa, and a type of pneumonitis (lung inflammation) known as extrinsic allergic alveolitis. In addition to these local reactions, a systemic allergic reaction (anaphylactic shock) may follow exposure to some chemical allergens.
Infectious response
Infectious agents can cause tuberculosis, anthrax, ornithosis, brucellosis, histoplasmosis, Legionnaires’ disease and so on.
Carcinogenic response
Cancer is a general term for a group of related diseases characterized by the uncontrolled growth of tissues. Its development is due to a complex process of interacting multiple factors in the host and the environment.
One of the great difficulties in attempting to relate exposure to a specific agent to cancer development in humans is the long latent period, typically from 15 to 40 years, between onset of exposure and disease manifestation.
Examples of air pollutants that can produce cancer of the lungs are arsenic and its compounds, chromates, silica, particles containing polycyclic aromatic hydrocarbons and certain nickel-bearing dusts. Asbestos fibres can cause bronchial cancer and mesothelioma of the pleura and peritoneum. Deposited radioactive particles may expose lung tissue to high local doses of ionizing radiation and be the cause of cancer.
Systemic response
Many environmental chemicals produce a generalized systemic disease due to their effects upon a number of target sites. Lungs are not only the target for many harmful agents but the site of entry of toxic substances which pass through the lungs into the bloodstream without any damage to the lungs. However, when distributed by the blood circulation to various organs, they can damage them or cause general poisoning and have systemic effects. This role of the lungs in occupational pathology is not the subject of this article. However, the effect of finely dispersed particulates (fumes) of several metal oxides which are often associated with an acute systemic syndrome known as metal fume fever should be mentioned.
Lung Function Examination
Lung function may be measured in a number of ways. However, the aim of the measurements has to be clear before the examination, in order to interpret the results correctly. In this article we will discuss lung function examination with special regard to the occupational field. It is important to remember the limitations in different lung function measurements. Acute temporary lung function effects may not be discernible in case of exposure to fibrogenic dust like quartz and asbestos, but chronic effects on lung function after long-term (>20 years) exposure may be. This is due to the fact that chronic effects occur years after the dust is inhaled and deposited in the lungs. On the other hand, acute temporary effects of organic and inorganic dust, as well as mould, welding fumes and motor exhaust, are well suited to study. This is due to the fact that the irritative effect of these dusts will occur after a few hours of exposure. Acute or chronic lung function effects also may be discernible in cases of exposure to concentrations of irritating gases (nitrogen dioxide, aldehydes, acids and acid chlorides) in the vicinity of well documented exposure limit values, especially if the effect is potentiated by particulate air contamination.
Lung function measurements have to be safe for the examined subjects, and the lung function equipment has to be safe for the examiner. A summary of the specific requirements for different kinds of lung function equipment are available (e.g., Quanjer et al. 1993). Of course, the equipment must be calibrated according to independent standards. This may be difficult to achieve, especially when computerized equipment is being used. The result of the lung function test is dependent on both the subject and the examiner. To provide satisfactory results from the examination, technicians have to be well trained, and able to instruct the subject carefully and also encourage the subject to carry out the test properly. The examiner should also have knowledge about the airways and lungs in order to interpret the results from the recordings correctly.
It is recommended that the methods used have a fairly high reproducibility both between and within subjects. Reproducibility may be measured as the coefficient of variation, that is, the standard deviation multiplied by 100 divided by the mean value. Values below 10% in repeated measurements on the same subject are deemed acceptable.
In order to determine if the measured values are pathological or not, they must be compared with prediction equations. Usually the prediction equations for spirometric variables are based on age and height, stratified for sex. Men have on the average higher lung function values than women, of the same age and height. Lung function decreases with age and increases with height. A tall subject will therefore have higher lung volume than a short subject of the same age. The outcome from prediction equations may differ considerably between different reference populations. The variation in age and height in the reference population will also influence the predicted values. This means, for example, that a prediction equation must not be used if age and/or height for the examined subject are outside the ranges for the population that is the basis for the prediction equation.
Smoking will also diminish lung function, and the effect may be potentiated in subjects who are occupationally exposed to irritating agents. Lung function used to be considered as not being pathological if the obtained values are within 80% of the predicted value, derived from a prediction equation.
Measurements
Lung function measurements are carried out to judge the condition of the lungs. Measurements may either concern single or multiple measured lung volumes, or the dynamic properties in the airways and lungs. The latter is usually determined through effort-dependent manoeuvres. The conditions in the lungs may also be examined with regard to their physiological function, that is, diffusion capacity, airway resistance and compliance (see below).
Measurements concerning ventilatory capacity are obtained by spirometry. The breathing manoeuvre is usually performed as a maximal inspiration followed by a maximal expiration, vital capacity (VC, measured in litres). At least three technically satisfactory recordings (i.e., full inspiration and expiration effort and no observed leaks) should be done, and the highest value reported. The volume may be directly measured by a water-sealed or a low-resistive bell, or indirectly measured by pneumotachography (i.e., integration of a flow signal over time). It is important here to note that all measured lung volumes should be expressed in BTPS, that is, body temperature and ambient pressure saturated with water vapour.
Forced expired vital capacity (FVC, in litres) is defined as a VC measurement performed with a maximally forced expiratory effort. Due to the simplicity of the test and the relatively inexpensive equipment, the forced expirogram has become a useful test in the monitoring of lung function. However, this has resulted in many poor recordings, of which the practical value is debatable. In order to carry out satisfactory recordings, the updated guideline for the collection and use of the forced expirogram, published by the American Thoracic Society in 1987, may be useful.
Instantaneous flows may be measured on flow-volume or flow-time curves, while time average flows or times are derived from the spirogram. Associated variables which can be calculated from the forced expirogram are forced expired volume in one second (FEV1, in litres per second), in percentage of FVC (FEV1%), peak flow (PEF, l/s), maximal flows at 50% and 75% of forced vital capacity (MEF50 and MEF25, respectively). An illustration of the derivation of FEV1 from the forced expirogram is outlined in figure 1. In healthy subjects, maximal flow rates at large lung volumes (i.e., at the beginning of expiration) reflect mainly the flow characteristics of the large airways while those at small lung volumes (i.e., the end of expiration) are usually held to reflect the characteristics of the small airways, figure 2. In the latter the flow is laminar, while in the large airways it may be turbulent.
Figure 1. Forced expiratory spirogram showing the derivation of FEV1 and FVC according to the extrapolation principle.
Figure 2. Flow-volume curve showing the derivation of peak expiratory flow (PEF), maximal flows at 50% and 75% of forced vital capacity (![]() and
and ![]() , respectively).
, respectively).
PEF may also be measured by a small portable device such as the one developed by Wright in 1959. An advantage with this equipment is that the subject may carry out serial measurements—for example, at the workplace. To get useful recordings, however, it is necessary to instruct the subjects well. Moreover, one should keep in mind that measurements of PEF with, for example, a Wright meter and those measured by conventional spirometry should not be compared due to the different blow techniques.
The spirometric variables VC, FVC and FEV1 show a reasonable variation between individuals where age, height and sex usually explain 60 to 70% of the variation. Restrictive lung function disorders will result in lower values for VC, FVC and FEV1. Measurements of flows during expiration show a great individual variation, since the measured flows are both effort and time dependent. This means, for example, that a subject will have extremely high flow in case of diminished lung volume. On the other hand, the flow may be extremely low in case of very high lung volume. However, the flow is usually decreased in case of a chronic obstructive disease (e.g., asthma, chronic bronchitis).
Figure 3. A principal outline of the equipment for determination of total lung capacity (TLC) according to the helium dilution technique.
The proportion of residual volume (RV), that is, the volume of air which still is in the lungs after a maximal expiration, can be determined by gas dilution or by body plethysmography. The gas dilution technique requires less complicated equipment and is therefore more convenient to use in studies carried out at the workplace. In figure 3, the principle for the gas dilution technique has been outlined. The technique is based on dilution of an indicator gas in a rebreathing circuit. The indicator gas must be sparingly soluble in biological tissues so that it is not taken up by the tissues and blood in the lung. Hydrogen was initially used, but because of its ability to form explosive mixtures with air it was replaced by helium, which is easily detected by means of the thermal conductivity principle.
The subject and the apparatus form a closed system, and the initial concentration of the gas is thus reduced when it is diluted into the gas volume in the lungs. After equilibration, the concentration of indicator gas is the same in the lungs as in the apparatus, and functional residual capacity (FRC) can be calculated by means of a simple dilution equation. The volume of the spirometer (including the addition of the gas mixture into the spirometer) is denoted by VS, VL is the volume of the lung, Fi is the initial gas concentration and Ff is the final concentration.
FRC = VL = [(VS · Fi) / Ff] – VS
Two to three VC manoeuvres are carried out to provide a reliable base for the calculation of TLC (in litres). The subdivisions of the different lung volumes are outlined in figure 4.
Figure 4. Spirogram labelled to show the subdivisions of the total capacity.
Due to change in the elastic properties of the airways, RV and FRC increase with age. In chronic obstructive diseases, increased values of RV and FRC are usually observed, while VC is decreased. However, in subjects with badly ventilated lung areas—for example, subjects with emphysema—the gas dilution technique may underestimate RV, FRC and also TLC. This is due to the fact that the indicator gas will not communicate with closed-off airways, and therefore the decrease in the indicator gas concentration will give erroneously small values.
Figure 5. A principal outline of the recording of airway closure and the slope of the alveolar plateau (%![]() ).
).
Measures of airway closure and gas distribution in the lungs can be obtained in one and the same manoeuvre by the single breath wash-out technique, figure 5. The equipment consists of a spirometer connected to a bag-in-box system and a recorder for continuous measurements of nitrogen concentration. The manoeuvre is carried out by means of a maximal inspiration of pure oxygen from the bag. In the beginning of the expiration, the nitrogen concentration increases as a result of emptying the subject’s deadspace, containing pure oxygen. The expiration continues with the air from the airways and alveoli. Finally, air from the alveoli, containing 20 to 40% nitrogen, is expired. When the expiration from the basal parts of the lungs increases, the nitrogen concentration will rise abruptly in case of airway closure in dependent lung regions, figure 5. This volume above RV, at which airways close during an expiration, is usually expressed as closing volume (CV) in percentage of VC (CV%). Distribution of the inspired air in the lungs is expressed as the slope of the alveolar plateau (%N2 or phase III, %N2/l). It is obtained by taking the difference in nitrogen concentration between the point when 30% of the air is exhaled and the point for airway closure, and dividing this by the corresponding volume.
Ageing as well as chronic obstructive disorders will result in increased values for both CV% and phase III. However, not even healthy subjects have a uniform gas distribution in the lungs, resulting in slightly elevated values for phase III, that is, 1 to 2% N2/l. The variables CV% and phase III are considered to reflect the conditions in the peripheral small airways with an internal diameter about 2 mm. Normally, the peripheral airways contribute to a small part (10 to 20%) of the total airway resistance. Quite extensive changes which are not detectable by conventional lung function tests like dynamic spirometry, may occur, for example, as a result of an exposure to irritating substances in the air in the peripheral airways. This suggests that airway obstruction begins in the small airways. Results from studies also have shown alterations in CV% and phase III before any changes from the dynamic and static spirometry have occurred. These early changes may go into remission when exposure to hazardous agents has ceased.
The transfer factor of the lung (mmol/min; kPa) is an expression of the diffusion capacity of oxygen transport into the pulmonary capillaries. The transfer factor can be determined using single or multiple breath techniques; the single breath technique is considered to be most suitable in studies at the workplace. Carbon monoxide (CO) is used since the back pressure of CO is very low in the peripheral blood, in contrast to that of oxygen. The uptake of CO is assumed to follow an exponential model, and this assumption can be used to determine the transfer factor for the lung.
Determination of TLCO (transfer factor measured with CO) is carried out by means of a breathing manoeuvre including a maximal expiration, followed by a maximal inspiration of a gas mixture containing carbon monoxide, helium, oxygen and nitrogen. After a breath-holding period, a maximal exhalation is done, reflecting the content in the alveolar air, Figure 10. Helium is used for the determination of the alveolar volume (VA). Assuming that the dilution of CO is the same as for helium, the initial concentration of CO, before the diffusion has started, can be calculated. TLCO is calculated according to the equation outlined below, where k depends on the dimensions of the component terms, t is the effective time for breath-holding and log is base 10 logarithm. Inspired volume is denoted Vi and the fractions F of CO and helium are denoted by i and a for inspired and alveolar, respectively.
TLCO = k Vi (Fa,He/Fi,He) log (Fi,CO Fa,He/Fa,CO Fi,He) (t)-1
Figure 6. A principal outline of the recording of transfer factor
The size of TLCO will depend on a variety of conditions—for example, the amount of available haemoglobin, the volume of ventilated alveoli and perfused lung capillaries and their relation to each other. Values for TLCO decrease with age and increase with physical activity and increased lung volumes. Decreased TLCO will be found in both restrictive and obstructive lung disorders.
Compliance (l/kPa) is a function, inter alia, of the elastic property of the lungs. The lungs have an intrinsic tendency to collaborate—that is, to collapse. The power to keep the lungs stretched will depend on the elastic lung tissue, the surface tension in the alveoli, and the bronchial musculature. On the other hand, the chest wall tends to expand at lung volumes 1 to 2 litres above the FRC level. At higher lung volumes, power has to be applied to further expand the chest wall. At the FRC level, the corresponding tendency in the lungs is balanced by the tendency to expand. The FRC level is therefore denoted by the resting level of the lung.
The compliance of the lung is defined as the change in volume divided by the change in transpulmonary pressure, that is, the difference between the pressures in the mouth (atmospheric) and in the lung, as the result of a breathing manoeuvre. Measurements of the pressure in the lung are not easily carried out and are therefore replaced by measurements of the pressure in the oesophagus. The pressure in the oesophagus is almost the same as the pressure in the lung, and it is measured with a thin polyethylene catheter with a balloon covering the distal 10 cm. During inspiratory and expiratory manoeuvres, the changes in volume and pressure are recorded by means of a spirometer and pressure transducer, respectively. When the measurements are performed during tidal breathing, dynamic compliance can be measured. Static compliance is obtained when a slow VC manoeuvre is carried out. In the latter case, the measurements are carried out in a body plethysmograph, and the expiration is intermittently interrupted by means of a shutter. However, measurements of compliance are cumbersome to perform when examining exposure effects on lung function at the worksite, and this technique is considered to be more appropriate in the laboratory.
A decreased compliance (increased elasticity) is observed in fibrosis. To cause a change in volume, large changes in pressure are required. On the other hand, a high compliance is observed, for example, in emphysema as the result of loss of elastic tissue and therefore also elasticity in the lung.
The resistance in the airways essentially depends on the radius and length of the airways but also on air viscosity. The airway resistance (RL in (kPa/l) /s), can be determined by use of a spirometer, pressure transducer and a pneumotachograph (to measure the flow). The measurements may also be carried out using a body plethysmograph to record the changes in flow and pressure during panting manoeuvres. By administration of a drug intended to cause broncho-constriction, sensitive subjects, as a result of their hyperreactive airways, may be identified. Subjects with asthma usually have increased values for RL.
Acute and Chronic Effects of Occupational Exposure on Pulmonary Function
Lung function measurement may be used to disclose an occupational exposure effect on the lungs. Pre-employment examination of lung function should not be used to exclude job-seeking subjects. This is because the lung function of healthy subjects varies within wide limits and it is difficult to draw a borderline below which it can safely be stated that the lung is pathological. Another reason is that the work environment should be good enough to allow even subjects with slight lung function impairment to work safely.
Chronic effects on the lungs in occupationally exposed subjects may be detected in a number of ways. The techniques are designed to determine historical effects, however, and are less suitable to serve as guidelines to prevent lung function impairment. A common study design is to compare the actual values in exposed subjects with the lung function values obtained in a reference population without occupational exposure. The reference subjects may be recruited from the same (or nearby) workplaces or from the same city.
Multivariate analysis has been used in some studies to assess differences between exposed subjects and matched unexposed referents. Lung function values in exposed subjects may also be standardized by means of a reference equation based on lung function values in the unexposed subjects.
Another approach is to study the difference between the lung function values in exposed and unexposed workers after adjustment for age and height with the use of external reference values, calculated by means of a prediction equation based on healthy subjects. The reference population may also be matched to the exposed subjects according to ethnic group, sex, age, height and smoking habits in order to further control for those influencing factors.
The problem is, however, to decide if a decrease is large enough to be classified as pathological, when external reference values are being used. Although the instruments in the studies have to be portable and simple, attention must be paid both to the sensitivity of the chosen method for detecting small anomalies in airways and lungs and the possibility of combining different methods. There are indications that subjects with respiratory symptoms, such as exertion dyspnoea, are at a higher risk of having an accelerated decline in lung function. This means that the presence of respiratory symptoms is important and so should not be neglected.
The subject may also be followed-up by spirometry, for example, once a year, for a number of years, in order to give a warning against the development of illness. There are limitations, however, since this will be very time-consuming and the lung function may have deteriorated permanently when the decrease can be observed. This approach therefore must not be an excuse for delay in carrying out measures in order to decrease harmful concentrations of air pollutants.
Finally, chronic effects on lung function may also be studied by examining the individual changes in lung function in exposed and unexposed subjects over a number of years. One advantage of the longitudinal study design is that the intersubject variability is eliminated; however, the design is considered to be time-consuming and expensive.
Susceptible subjects may also be identified by comparing their lung function with and without exposure during working shifts. In order to minimize possible effects of diurnal variations, lung function is measured at the same time of day on one unexposed and one exposed occasion. The unexposed condition can be obtained, for example, by occasionally moving the worker to an uncontaminated area or by use of a suitable respirator during a whole shift, or in some cases by performing lung function measurements in the afternoon of a worker’s day off.
One special concern is that repeated, temporary effects can result in chronic effects. An acute temporary lung function decrease may not only be a biological exposure indicator but also a predictor of a chronic lung function decrement. Exposure to air pollutants may result in discernible acute effects on lung function, although the mean values of the measured air pollutants are below the hygienic limit values. The question thus arises, whether these effects really are harmful in the long run. This question is hard to answer directly, especially since the air pollution in workplaces often has a complex composition and the exposure cannot be described in terms of mean concentrations of single compounds. The effect of an occupational exposure is also partly due to the sensitivity of the individual. This means that some subjects will react sooner or to a larger extent than others. The underlying pathophysiological ground for an acute, temporary decrease in lung function is not fully understood. The adverse reaction upon exposure to an irritating air contaminant is, however, an objective measurement, in contrast to subjective experiences like symptoms of different origin.
The advantage of detecting early changes in airways and lungs caused by hazardous air pollutants is obvious—the prevailing exposure may be reduced in order to prevent more severe illnesses. Therefore, an important aim in this respect is to use the measurements of acute temporary effects on lung function as a sensitive early warning system that can be used when studying groups of healthy working people.
Monitoring of Irritants
Irritation is one of the most frequent criteria for setting exposure limit values. It is, however, not certain that compliance with an exposure limit based on irritation will protect against irritation. It should be considered that an exposure limit for an air contaminant usually contains at least two parts—a time-weighted average limit (TWAL) and a short-term exposure limit (STEL), or at least rules for exceeding the time-weighted average limit, “excursion limits”. In the case of highly irritating substances, such as sulphur dioxide, acrolein and phosgene, it is important to limit the concentration even during very short periods, and it has therefore been common practice to fix occupational exposure limit values in the form of ceiling limits, with a sampling period that is kept as short as the measuring facilities will allow.
Time-weighted average limit values for an eight-hour day combined with rules for excursion above these values are given for most of the substances in the American Conference of Governmental Industrial Hygienists (ACGIH) threshold limit value (TLV) list. The TLV list of 1993-94 contains the following statement concerning excursion limits for exceeding limit values:
“For the vast majority of substances with a TLV-TWA, there is not enough toxicological data available to warrant a STEL = short-term exposure limit). Nevertheless, excursions above the TLV-TWA should be controlled even where the eight-hour TWA is within recommended limits.”
Exposure measurements of known air contaminants and comparison with well documented exposure limit values should be carried out on a routine basis. There are, however, many situations when the determination of compliance with exposure limit values is not enough. This is the case in the following circumstances (inter alia):
- when the limit value is too high to safeguard against irritation
- when the irritant is unknown
- when the irritant is a complex mixture and there is no suitable indicator known.
As advocated above, the measurement of acute, temporary effects on lung function can be used in these cases as a warning against over-exposure to irritants.
In cases (2) and (3), acute, temporary effects on lung function may be applicable also in testing the efficiency of control measures to decrease exposure to air contamination or in scientific investigations, for example, in attributing biological effects to components of air contaminants. A number of examples follow in which acute, temporary lung function effects have been successfully employed in occupational health investigations.
Studies of Acute, Temporary Lung Function Effects
Work-related, temporary decrease of lung function over a work shift was recorded in cotton workers at the end of 1950. Later, several authors reported work-related, acute, temporary changes of lung function in hemp and textile workers, coal miners, workers exposed to toluene di-isocyanate, fire-fighters, rubber processing workers, moulders and coremakers, welders, ski waxers, workers exposed to organic dust and irritants in water-based paints.
However, there are also several examples where measurements before and after exposure, usually during a shift, have failed to demonstrate any acute effects, despite a high exposure. This is probably due to the effect of normal circadian variation, mainly in lung function variables depending on the size of airway calibre. Thus the temporary decrease in these variables must exceed the normal circadian variation to be recognized. The problem may be circumvented, however, by measuring lung function at the same time of the day at each study occasion. By using the exposed employee as his or her own control, the interindividual variation is further decreased. Welders were studied in this way, and although the mean difference between unexposed and exposed FVC values was less than 3% in 15 examined welders, this difference was significant at the 95% confidence level with a power of more than 99%.
The reversible transient effects on the lungs can be used as an exposure indicator of complicated irritating components. In the study cited above, particles in the work environment were crucial for the irritating effects on the airways and lungs. The particles were removed by a respirator consisting of a filter combined with a welding helmet. The results indicated that the effects on the lungs were caused by the particles in welding fumes, and that the use of a particulate respirator might prevent this effect.
Exposure to diesel exhaust also gives measurable irritative effects on the lungs, shown as an acute, temporary lung function decrease. Mechanical filters mounted on the exhaust pipes of trucks used in loading operations by stevedores relieved subjective disorders and reduced the acute, temporary lung function decrease observed when no filtration was done. The results thus indicate that the presence of particles in the work environment does play a role in the irritative effect on airways and lungs, and that it is possible to assess the effect by measurements of acute changes in lung function.
A multiplicity of exposures and a continually changing work environment may present difficulties in discerning the causal relationship of the different agents existing in a work environment. The exposure scenario in sawmills is an illuminating example. It is not possible (e.g., for economical reasons) to carry out exposure measurements of all possible agents (terpenes, dust, mould, bacteria, endotoxin, mycotoxins, etc.) in this work environment. A feasible method may be to follow the development of lung function longitudinally. In a study of sawmill workers in the wood-trimming department, lung function was examined before and after a working week, and no statistically significant decrease was found. However, a follow-up study carried out a few years later disclosed that those workers who actually had a numerical decrease in lung function during a working week also had an accelerated long-term decline in lung function. This may indicate that vulnerable subjects can be detected by measuring changes in lung function during a working week.
Diseases Caused by Respiratory Irritants and Toxic Chemicals
The presence of respiratory irritants in the workplace can be unpleasant and distracting, leading to poor morale and decreased productivity. Certain exposures are dangerous, even lethal. In either extreme, the problem of respiratory irritants and inhaled toxic chemicals is common; many workers face a daily threat of exposure. These compounds cause harm by a variety of different mechanisms, and the extent of injury can vary widely, depending on the degree of exposure and on the biochemical properties of the inhalant. However, they all have the characteristic of nonspecificity; that is, above a certain level of exposure virtually all persons experience a threat to their health.
There are other inhaled substances that cause only susceptible individuals to develop respiratory problems; such complaints are most appropriately approached as diseases of allergic and immunological origin. Certain compounds, such as isocyanates, acid anhydrides and epoxy resins, can act not only as non-specific irritants in high concentrations, but can also predispose certain subjects to allergic sensitization. These compounds provoke respiratory symptoms in sensitized individuals at very low concentrations.
Respiratory irritants include substances that cause inflammation of the airways after they are inhaled. Damage may occur in the upper and lower airways. More dangerous is acute inflammation of the pulmonary parenchyma, as in chemical pneumonitis or non-cardiogenic pulmonary oedema. Compounds that can cause parenchymal damage are considered toxic chemicals. Many inhaled toxic chemicals also act as respiratory irritants, warning us of their danger with their noxious odour and symptoms of nose and throat irritation and cough. Most respiratory irritants are also toxic to the lung parenchyma if inhaled in sufficient amount.
Many inhaled substances have systemic toxic effects after being absorbed by inhalation. Inflammatory effects on the lung may be absent, as in the case of lead, carbon monoxide or hydrogen cyanide. Minimal lung inflammation is normally seen in the inhalation fevers (e.g., organic dust toxic syndrome, metal fume fever and polymer fume fever). Severe lung and distal organ damage occurs with significant exposure to toxins such as cadmium and mercury.
The physical properties of inhaled substances predict the site of deposition; irritants will produce symptoms at these sites. Large particles (10 to 20mm) deposit in the nose and upper airways, smaller particles (5 to 10mm) deposit in the trachea and bronchi, and particles less than 5mm in size may reach the alveoli. Particles less than 0.5mm are so small they behave like gases. Toxic gases deposit according to their solubility. A water-soluble gas will be adsorbed by the moist mucosa of the upper airway; less soluble gases will deposit more randomly throughout the respiratory tract.
Respiratory Irritants
Respiratory irritants cause non-specific inflammation of the lung after being inhaled. These substances, their sources of exposure, physical and other properties, and effects on the victim are outlined in Table 1. Irritant gases tend to be more water soluble than gases more toxic to the lung parenchyma. Toxic fumes are more dangerous when they have a high irritant threshold; that is, there is little warning that the fume is being inhaled because there is little irritation.
Table 1. Summary of respiratory irritants
|
Chemical |
Sources of exposure |
Important properties |
Injury produced |
Dangerous exposure level under 15 min (PPM) |
|
Acetaldehyde |
Plastics, synthetic rubber industry, combustion products |
High vapour pressure; high water solubility |
Upper airway injury; rarely causes delayed pulmonary oedema |
|
|
Acetic acid, organic acids |
Chemical industry, electronics, combustion products |
Water soluble |
Ocular and upper airway injury |
|
|
Acid anhydrides |
Chemicals, paints, and plastics industries; components of epoxy resins |
Water soluble, highly reactive, may cause allergic sensitization |
Ocular, upper airway injury, bronchospasm; pulmonary haemorrhage after massive exposure |
|
|
Acrolein |
Plastics, textiles, pharmaceutical manufacturing, combustion products |
High vapour pressure, intermediate water solubility, extremely irritating |
Diffuse airway and parenchymal injury |
|
|
Ammonia |
Fertilizers, animal feeds, chemicals, and pharmaceuticals manufacturing |
Alkaline gas, very high water solubility |
Primarily ocular and upper airway burn; massive exposure may cause bronchiectasis |
500 |
|
Antimony trichloride, antimony penta-chloride |
Alloys, organic catalysts |
Poorly soluble, injury likely due to halide ion |
Pneumonitis, non-cardiogenic pulmonary oedema |
|
|
Beryllium |
Alloys (with copper), ceramics; electronics, aerospace and nuclear reactor equipment |
Irritant metal, also acts as an antigen to promote a long-term granulomatous response |
Acute upper airway injury, tracheobronchitis, chemical pneumonitis |
25 μg/m3 |
|
Boranes (diborane) |
Aircraft fuel, fungicide manufacturing |
Water soluble gas |
Upper airway injury, pneumonitis with massive exposure |
|
|
Hydrogen bromide |
Petroleum refining |
Upper airway injury, pneumonitis with massive exposure |
||
|
Methyl bromide |
Refrigeration, produce fumigation |
Moderately soluble gas |
Upper and lower airway injury, pneumonitis, CNS depression and seizures |
|
|
Cadmium |
Alloys with Zn and Pb, electroplating, batteries, insecticides |
Acute and chronic respiratory effects |
Tracheobronchitis, pulmonary oedema (often delayed onset over 24–48 hours); chronic low level exposure leads to inflammatory changes and emphysema |
100 |
|
Calcium oxide, calcium hydroxide |
Lime, photography, tanning, insecticides |
Moderately caustic, very high doses required for toxicity |
Upper and lower airway inflammation, pneumonitis |
|
|
Chlorine |
Bleaching, formation of chlorinated compounds, household cleaners |
Intermediate water solubilty |
Upper and lower airway inflammation, pneumonitis and non-cardiogenic pulmonary oedema |
5–10 |
|
Chloroacetophenone |
Crowd control agent, “tear gas” |
Irritant qualities are used to incapacitate; alkylating agent |
Ocular and upper airway inflammation, lower airway and parenchymal injury with masssive exposure |
1–10 |
|
o-Chlorobenzomalo- nitrile |
Crowd control agent, “tear gas” |
Irritant qualities are used to incapacitate |
Ocular and upper airway inflammation, lower airway injury with massive exposure |
|
|
Chloromethyl ethers |
Solvents, used in manufacture of other organic compounds |
Upper and lower airway irritation, also a respiratory tract carcinogen |
||
|
Chloropicrin |
Chemical manufacturing, fumigant component |
Former First World War gas |
Upper and lower airway inflammation |
15 |
|
Chromic acid (Cr(IV)) |
Welding, plating |
Water soluble irritant, allergic sensitizer |
Nasal inflammation and ulceration, rhinitis, pneumonitis with massive exposure |
|
|
Cobalt |
High temperature alloys, permanent magnets, hard metal tools (with tungsten carbide) |
Non-specific irritant, also allergic sensitizer |
Acute bronchospasm and/or pneumonitis; chronic exposure can cause lung fibrosis |
|
|
Formaldehyde |
Manufacture of foam insulation, plywood, textiles, paper, fertilizers, resins; embalming agents; combustion products |
Highly water soluble, rapidly metabolized; primarily acts via sensory nerve stimulation; sensitization reported |
Ocular and upper airway irritation; bronchospasm in severe exposure; contact dermatitis in sensitized persons |
3 |
|
Hydrochloric acid |
Metal refining, rubber manufacturing, organic compound manufacture, photographic materials |
Highly water soluble |
Ocular and upper airway inflammation, lower airway inflammation only with massive exposure |
100 |
|
Hydrofluoric acid |
Chemical catalyst, pesticides, bleaching, welding, etching |
Highly water soluble, powerful and rapid oxidant, lowers serum calcium in massive exposure |
Ocular and upper airway inflammation, tracheobronchitis and pneumonitis with massive exposure |
20 |
|
Isocyanates |
Polyurethane production; paints; herbicide and insecticide products; laminating, furniture, enamelling, resin work |
Low molecular weight organic compounds, irritants, cause sensitization in susceptible persons |
Ocular, upper and lower inflammation; asthma, hypersensitivity pneumonitis in sensitized persons |
0.1 |
|
Lithium hydride |
Alloys, ceramics, electronics, chemical catalysts |
Low solubility, highly reactive |
Pneumonitis, non-cardiogenic pulmonary oedema |
|
|
Mercury |
Electrolysis, ore and amalgam extraction, electronics manufacture |
No respiratory symptoms with low level, chronic exposure |
Ocular and respiratory tract inflammation, pneumonitis, CNS, kidney and systemic effects |
1.1 mg/m3 |
|
Nickel carbonyl |
Nickel refining, electroplating, chemical reagents |
Potent toxin |
Lower respiratory irritation, pneumonitis, delayed systemic toxic effects |
8 μg/m3 |
|
Nitrogen dioxide |
Silos after new grain storage, fertilizer making, arc welding, combustion products |
Low water solubility, brown gas at high concentration |
Ocular and upper airway inflammation, non-cardiogenic pulmonary oedema, delayed onset bronchiolitis |
50 |
|
Nitrogen mustards; sulphur mustards |
Military gases |
Causes severe injury, vesicant properties |
Ocular, upper and lower airway inflammation, pneumonitis |
20mg/m3 (N) 1 mg/m3 (S) |
|
Osmium tetroxide |
Copper refining, alloy with iridium, catalyst for steroid synthesis and ammonia formation |
Metallic osmium is inert, tetraoxide forms when heated in air |
Severe ocular and upper airway irritation; transient renal damage |
1 mg/m3 |
|
Ozone |
Arc welding, copy machines, paper bleaching |
Sweet smelling gas, moderate water solubility |
Upper and lower airway inflammation; asthmatics more susceptible |
1 |
|
Phosgene |
Pesticide and other chemical manufacture, arc welding, paint removal |
Poorly water soluble, does not irritate airways in low doses |
Upper airway inflammation and pneumonitis; delayed pulmonary oedema in low doses |
2 |
|
Phosphoric sulphides |
Production of insecticides, ignition compounds, matches |
Ocular and upper airway inflammation |
||
|
Phosphoric chlorides |
Manufacture of chlorinated organic compounds, dyes, gasoline additives |
Form phosphoric acid and hydrochloric acid on contact with mucosal surfaces |
Ocular and upper airway inflammation |
10 mg/m3 |
|
Selenium dioxide |
Copper or nickel smelting, heating of selenium alloys |
Strong vessicant, forms selenious acid (H2SeO3) on mucosal surfaces |
Ocular and upper airway inflammation, pulmonary oedema in massive exposure |
|
|
Hydrogen selenide |
Copper refining, sulphuric acid production |
Water soluble; exposure to selenium compounds gives rise to garlic odour breath |
Ocular and upper airway inflammation, delayed pulmonary oedema |
|
|
Styrene |
Manufacture of polystyrene and resins, polymers |
Highly irritating |
Ocular, upper and lower airway inflammation, neurological impairments |
600 |
|
Sulphur dioxide |
Petroleum refining, pulp mills, refrigeration plants, manufacturing of sodium sulphite |
Highly water soluble gas |
Upper airway inflammation, bronchoconstriction, pneumonitis on massive exposure |
100 |
|
Titanium tetrachloride |
Dyes, pigments, sky writing |
Chloride ions form HCl on mucosa |
Upper airway injury |
|
|
Uranium hexafluoride |
Metal coat removers, floor sealants, spray paints |
Toxicity likely from chloride ions |
Upper and lower airway injury, bronchospasm, pneumonitis |
|
|
Vanadium pentoxide |
Cleaning oil tanks, metallurgy |
Ocular, upper and lower airway symptoms |
70 |
|
|
Zinc chloride |
Smoke grenades, artillery |
More severe than zinc oxide exposure |
Upper and lower airway irritation, fever, delayed onset pneumonitis |
200 |
|
Zirconium tetrachloride |
Pigments, catalysts |
Chloride ion toxicity |
Upper and lower airway irritation, pneumonitis |
This condition is thought to result from persistent inflammation with reduction of epithelial cell layer permeability or reduced conductance threshold for subepithelial nerve endings.Adapted from Sheppard 1988; Graham 1994; Rom 1992; Blanc and Schwartz 1994; Nemery 1990; Skornik 1988.
The nature and extent of the reaction to an irritant depends on the physical properties of the gas or aerosol, the concentration and time of exposure, and on other variables as well, such as temperature, humidity and the presence of pathogens or other gases (Man and Hulbert 1988). Host factors such as age (Cabral-Anderson, Evans and Freeman 1977; Evans, Cabral-Anderson and Freeman 1977), prior exposure (Tyler, Tyler and Last 1988), level of antioxidants (McMillan and Boyd 1982) and presence of infection may play a role in determining the pathological changes seen. This wide range of factors has made it difficult to study the pathogenic effects of respiratory irritants in a systematic way.
The best understood irritants are those which inflict oxidative injury. The majority of inhaled irritants, including the major pollutants, act by oxidation or give rise to compounds that act in this way. Most metal fumes are actually oxides of the heated metal; these oxides cause oxidative injury. Oxidants damage cells primarily by lipid peroxidation, and there may be other mechanisms. On a cellular level, there is initially a fairly specific loss of ciliated cells of the airway epithelium and of Type I alveolar epithelial cells, with subsequent violation of the tight junction interface between epithelial cells (Man and Hulbert 1988; Gordon, Salano and Kleinerman 1986; Stephens et al. 1974). This leads to subepithelial and submucosal damage, with stimulation of smooth muscle and parasympathetic sensory afferent nerve endings causing bronchoconstriction (Holgate, Beasley and Twentyman 1987; Boucher 1981). An inflammatory response follows (Hogg 1981), and the neutrophils and eosinophils release mediators that cause further oxidative injury (Castleman et al. 1980). Type II pneumocytes and cuboidal cells act as stem cells for repair (Keenan, Combs and McDowell 1982; Keenan, Wilson and McDowell 1983).
Other mechanisms of lung injury eventually involve the oxidative pathway of cellular damage, particularly after damage to the protective epithelial cell layer has occurred and an inflammatory response has been elicited. The most commonly described mechanisms are outlined in table 2.
Table 2. Mechanisms of lung injury by inhaled substances
|
Mechanism of injury |
Example compounds |
Damage that occurs |
|
Oxidation |
Ozone, nitrogen dioxide, sulphur dioxide, chlorine, oxides |
Patchy airway epithelial damage, with increased permeability and exposure of nerve fibre endings; loss of cilia from ciliated cells; necrosis of type I pneumocytes; free radical formation and subsequent protein binding and lipid peroxidation |
|
Acid formation |
Sulphur dioxide, chlorine, halides |
Gas dissolves in water to form acid that damages epithelial cells via oxidation; action mainly on upper airway |
|
Alkali formation |
Ammonia, calcium oxide, hydroxides |
Gas dissolves in water to form alkaline solution that may cause tissue liquefaction; predominant upper airway damage, lower airway in heavy exposures |
|
Protein binding |
Formaldehyde |
Reactions with amino acids lead to toxic intermediates with damage to the epithelial cell layer |
|
Afferent nerve stimulation |
Ammonia, formaldehyde |
Direct nerve ending stimulation provokes symptoms |
|
Antigenicity |
Platinum, acid anhydrides |
Low molecular weight molecules serve as haptens in sensitized persons |
|
Stimulation of host inflammatory response |
Copper and zinc oxides, lipoproteins |
Stimulation of cytokines and inflammatory mediators without apparent direct cellular damage |
|
Free radical formation |
Paraquat |
Promotion of formation or retardation of clearance of superoxide radicals, leading to lipid peroxidation and oxidative damage |
|
Delayed particle clearance |
Any prolonged inhalation of mineral dust |
Overwhelming of mucociliary escalators and alveolar macrophage systems with particles, leading to a non-specific inflammatory response |
Workers exposed to low levels of respiratory irritants may have subclinical symptoms traceable to mucous membrane irritation, such as watery eyes, sore throat, runny nose and cough. With significant exposure, the added feeling of shortness of breath will often prompt medical attention. It is important to secure a good medical history in order to determine the likely composition of the exposure, the quantity of exposure, and the period of time during which the exposure took place. Signs of laryngeal oedema, including hoarseness and stridor, should be sought, and the lungs should be examined for signs of lower airway or parenchymal involvement. Assessment of the airway and lung function, together with chest radiography, are important in short-term management. Laryngoscopy may be indicated to evaluate the airway.
If the airway is threatened, the patient should undergo intubation and supportive care. Patients with signs of laryngeal oedema should be observed for at least 12 hours to insure that the process is self-limited. Bronchospasm should be treated with b-agonists and, if refractory, intravenous corticosteroids. Irritated oral and ocular mucosa should be thoroughly irrigated. Patients with crackles on examination or chest radiograph abnormalities should be hospitalized for observation in view of the possibility of pneumonitis or pulmonary oedema. Such patients are at risk of bacterial superinfection; nevertheless, no benefit has been demonstrated by using prophylactic antibiotics.
The overwhelming majority of patients who survive the initial insult recover fully from irritant exposures. The chances for long-term sequelae are more likely with greater initial injury. The term reactive airway dysfunction syndrome (RADS) has been applied to the persistence of asthma-like symptoms following acute exposure to respiratory irritants (Brooks, Weiss and Bernstein 1985).
High-level exposures to alkalis and acids can cause upper and lower respiratory tract burns that lead to chronic disease. Ammonia is known to cause bronchiectasis (Kass et al. 1972); chlorine gas (which becomes HCl in the mucosa) is reported to cause obstructive lung disease (Donelly and Fitzgerald 1990; Das and Blanc 1993). Chronic low-level exposures to irritants may cause continued ocular and upper airway symptoms (Korn, Dockery and Speizer 1987), but deterioration of lung function has not been conclusively documented. Studies of the effects of chronic low-level irritants on airway function are hampered by a lack of long-term follow-up, confounding by cigarette smoking, the “healthy worker effect,” and the minimal, if any, actual clinical effect (Brooks and Kalica 1987).
After a patient recovers from the initial injury, regular follow-up by a physician is needed. Clearly, there should be an effort to investigate the workplace and evaluate respiratory precautions, ventilation and containment of the culprit irritants.
Toxic Chemicals
Chemicals toxic to the lung include most of the respiratory irritants given enough high exposure, but there are many chemicals that cause significant parenchymal lung injury despite possessing low to moderate irritant properties. These compounds work their effects by mechanisms reviewed in Table 3 and discussed above. Pulmonary toxins tend to be less water soluble than upper airway irritants. Examples of lung toxins and their sources of exposure are reviewed in table 3.
Table 3. Compounds capable of lung toxicity after low to moderate exposure
|
Compound |
Sources of exposure |
Toxicity |
|
Acrolein |
Plastics, textiles, pharmaceutical manufacturing, combustion products |
Diffuse airway and parenchymal injury |
|
Antimony trichloride; antimony |
Alloys, organic catalysts |
Pneumonitis, non-cardiogenic pulmonary oedema |
|
Cadmium |
Alloys with zinc and lead, electroplating, batteries, insecticides |
Tracheobronchitis, pulmonary oedema (often delayed onset over 24–48 hours), kidney damage: tubule proteinuria |
|
Chloropicrin |
Chemical manufacturing, fumigant components |
Upper and lower airway inflammation |
|
Chlorine |
Bleaching, formation of chlorinated compounds, household cleaners |
Upper and lower airway inflammation, pneumonitis and non-cardiogenic pulmonary oedema |
|
Hydrogen sulphide |
Natural gas wells, mines, manure |
Ocular, upper and lower airway irritation, delayed pulmonary oedema, asphyxiation from systemic tissue hypoxia |
|
Lithium hydride |
Alloys, ceramics, electronics, chemical catalysts |
Pneumonitis, non-cardiogenic pulmonary oedema |
|
Methyl isocyanate |
Pesticide synthesis |
Upper and lower respiratory tract irritation, pulmonary oedema |
|
Mercury |
Electrolysis, ore and amalgam extraction, electronics manufacture |
Ocular and respiratory tract inflammation, pneumonitis, CNS, kidney and systemic effects |
|
Nickel carbonyl |
Nickel refining, electroplating, chemical reagents |
Lower respiratory irritation, pneumonitis, delayed systemic toxic effects |
|
Nitrogen dioxide |
Silos after new grain storage, fertilizer making, arc welding; combustion products |
Ocular and upper airway inflammation, non-cardiogenic pulmonary oedema, delayed onset bronchiolitis |
|
Nitrogen mustards, sulphur |
Military agents, vesicants |
Ocular and respiratory tract inflammation, pneumonitis |
|
Paraquat |
Herbicides (ingested) |
Selective damage to type-2 pneumocytes leading to RADS, pulmonary fibrosis; renal failure, GI irritation |
|
Phosgene |
Pesticide and other chemical manufacture, arc welding, paint removal |
Upper airway inflammation and pneumonitis; delayed pulmonary oedema in low doses |
|
Zinc chloride |
Smoke grenades, artillery |
Upper and lower airway irritation, fever, delayed onset pneumonitis |
One group of inhalable toxins are termed asphyxiants. When present in high enough concentrations, the asphyxiants, carbon dioxide, methane and nitrogen, displace oxygen and in effect suffocate the victim. Hydrogen cyanide, carbon monoxide and hydrogen sulphide act by inhibiting cellular respiration despite adequate delivery of oxygen to the lung. Non-asphyxiant inhaled toxins damage target organs, causing a wide variety of health problems and mortality.
The medical management of inhaled lung toxins is similar to the management of respiratory irritants. These toxins often do not elicit their peak clinical effect for several hours after exposure; overnight monitoring may be indicated for compounds known to cause delayed onset pulmonary oedema. Since the therapy of systemic toxins is beyond the scope of this chapter, the reader is referred to discussions of the individual toxins elsewhere in this Encyclopaedia and in further texts on the subject (Goldfrank et al. 1990; Ellenhorn and Barceloux 1988).
Inhalation Fevers
Certain inhalation exposures occurring in a variety of different occupational settings may result in debilitating flu-like illnesses lasting a few hours. These are collectively referred to as inhalation fevers. Despite the severity of the symptoms, the toxicity seems to be self-limited in most cases, and there are few data to suggest long-term sequelae. Massive exposure to inciting compounds can cause a more severe reaction involving pneumonitis and pulmonary oedema; these uncommon cases are considered more complicated than simple inhalation fever.
The inhalation fevers have in common the feature of nonspecificity: the syndrome can be produced in nearly anyone, given adequate exposure to the inciting agent. Sensitization is not required, and no previous exposure is necessary. Some of the syndromes exhibit the phenomenon of tolerance; that is, with regular repeated exposure the symptoms do not occur. This effect is thought to be related to an increased activity of clearance mechanisms, but has not been adequately studied.
Organic Dust Toxic Syndrome
Organic dust toxic syndrome (ODTS) is a broad term denoting the self-limited flu-like symptoms that occur following heavy exposure to organic dusts. The syndrome encompasses a wide range of acute febrile illnesses that have names derived from the specific tasks that lead to dust exposure. Symptoms occur only after a massive exposure to organic dust, and most individuals so exposed will develop the syndrome.
Organic dust toxic syndrome has previously been called pulmonary mycotoxicosis, owing to its putative aetiology in the action of mould spores and actinomycetes. With some patients, one can culture species of Aspergillus, Penicillium, and mesophilic and thermophilic actinomycetes (Emmanuel, Marx and Ault 1975; Emmanuel, Marx and Ault 1989). More recently, bacterial endotoxins have been proposed to play at least as large a role. The syndrome has been provoked experimentally by inhalation of endotoxin derived from Enterobacter agglomerans, a major component of organic dust (Rylander, Bake and Fischer 1989). Endotoxin levels have been measured in the farm environment, with levels ranging from 0.01 to 100μg/m3. Many samples had a level greater than 0.2μg/m3, which is the level where clinical effects are known to occur (May, Stallones and Darrow 1989). There is speculation that cytokines, such as IL-1, may mediate the systemic effects, given what is already known about the release of IL-1 from alveolar macrophages in the presence of endotoxin (Richerson 1990). Allergic mechanisms are unlikely given the lack of need for sensitization and the requirement for high dust exposure.
Clinically, the patient will usually present symptoms 2 to 8 hours after exposure to (usually mouldy) grain, hay, cotton, flax, hemp or wood chips, or upon manipulation of pigs (Do Pico 1992). Often symptoms begin with eye and mucous membrane irritation with dry cough, progressing to fever, and malaise, chest tightness, myalgias and headache. The patient appears ill but otherwise normal upon physical examination. Leukocytosis frequently occurs, with levels as high as 25,000 white blood corpuscles (WBC)/mm3. The chest radiograph is almost always normal. Spirometry may reveal a modest obstructive defect. In cases where fibre optic bronchoscopy was performed and bronchial washings were obtained, an elevation of leukocytes was found in the lavage fluid. The percentage of neutrophils was significantly higher than normal (Emmanuel, Marx and Ault 1989; Lecours, Laviolette and Cormier 1986). Bronchoscopy 1 to 4 weeks after the event shows a persistently high cellularity, predominantly lymphocytes.
Depending on the nature of the exposure, the differential diagnosis may include toxic gas (such as nitrogen dioxide or ammonia) exposure, particularly if the episode occurred in a silo. Hypersensitivity pneumonitis should be considered, especially if there are significant chest radiograph or pulmonary function test abnormalities. The distinction between hypersensitivity pneumonitis (HP) and ODTS is important: HP will require strict exposure avoidance and has a worse prognosis, whereas ODTS has a benign and self-limited course. ODTS is also distinguished from HP because it occurs more frequently, requires higher levels of dust exposure, does not induce the release of serum precipitating antibodies, and (initially) does not give rise to the lymphocytic alveolitis that is characteristic of HP.
Cases are managed with antipyretics. A role for steroids has not been advocated given the self-limited nature of the illness. Patients should be educated about massive exposure avoidance. The long-term effect of repeated occurrences is thought to be negligible; however, this question has not been adequately studied.
Metal Fume Fever
Metal fume fever (MFF) is another self-limited, flu-like illness that develops after inhalation exposure, in this instance to metal fumes. The syndrome most commonly develops after zinc oxide inhalation, as occurs in brass foundries, and in smelting or welding galvanized metal. Oxides of copper and iron also cause MFF, and vapours of aluminium, arsenic, cadmium, mercury, cobalt, chromium, silver, manganese, selenium and tin have been occasionally implicated (Rose 1992). Workers develop tachyphalaxis; that is, symptoms appear only when the exposure occurs after several days without exposure, not when there are regular repeated exposures. An eight-hour TLV of 5 mg/m3 for zinc oxide has been established by the US Occupational Safety and Health Administration (OSHA), but symptoms have been elicited experimentally after a two-hour exposure at this concentration (Gordon et al. 1992).
The pathogenesis of MFF remains unclear. The reproducible onset of symptoms regardless of the individual exposed argues against a specific immune or allergic sensitization. The lack of symptoms associated with histamine release (flushing, itching, wheezing, hives) also militates against the likelihood of an allergic mechanism. Paul Blanc and co-workers have developed a model implicating cytokine release (Blanc et al. 1991; Blanc et al.1993). They measured the levels of tumour necrosis factor (TNF), and of the interleukins IL-1, IL-4, IL-6 and IL-8 in the fluid lavaged from the lungs of 23 volunteers experimentally exposed to zinc oxide fumes (Blanc et al. 1993). The volunteers developed elevated levels of TNF in their bronchoalveolar lavage (BAL) fluid 3 hours after exposure. Twenty hours later, high BAL fluid levels of IL-8 (a potent neutrophil attractant) and an impressive neutrophilic alveolitis were observed. TNF, a cytokine capable of causing fever and stimulating immune cells, has been shown to be released from monocytes in culture that are exposed to zinc (Scuderi 1990). Accordingly, the presence of increased TNF in the lung accounts for the onset of symptoms observed in MFF. TNF is known to stimulate the release of both IL-6 and IL-8, in a time period that correlated with the peaks of the cytokines in these volunteers’ BAL fluid. The recruitment of these cytokines may account for the ensuing neutrophil alveolitis and flu-like symptoms that characterize MFF. Why the alveolitis resolves so quickly remains a mystery.
Symptoms begin 3 to 10 hours after exposure. Initially, there may be a sweet metallic taste in the mouth, accompanied by a worsening dry cough and shortness of breath. Fever and shaking chills often develop, and the worker feels ill. The physical examination is otherwise unremarkable. Laboratory evaluation shows a leukocytosis and a normal chest radiograph. Pulmonary function studies may show a slightly reduced FEF25-75 and DLCO levels (Nemery 1990; Rose 1992).
With a good history the diagnosis is readily established and the worker can be treated symptomatically with antipyretics. Symptoms and clinical abnormalities resolve within 24 to 48 hours. Otherwise, bacterial and viral aetiologies of the symptoms must be considered. In cases of extreme exposure, or exposures involving contamination by toxins such as zinc chloride, cadmium or mercury, MFF may be a harbinger of a clinical chemical pneumonitis that will evolve over the next 2 days (Blount 1990). Such cases can exhibit diffuse infiltrates on a chest radiograph and signs of pulmonary oedema and respiratory failure. While this possibility should be considered in the initial evaluation of an exposed patient, such a fulminant course is unusual and not characteristic of uncomplicated MFF.
MFF does not require a specific sensitivity of the individual for the metal fumes; rather, it indicates inadequate environmental control. The exposure problem should be addressed to prevent recurrent symptoms. Although the syndrome is considered benign, the long-term effects of repeated bouts of MFF have not been adequately investigated.
Polymer Fume Fever
Polymer fume fever is a self-limited febrile illness similar to MFF, but caused by inhaled pyrolysis products of fluoropolymers, including polytetrafluoroethane (PTFE; trade names Teflon, Fluon, Halon). PTFE is widely used for its lubricant, thermal stability and electrical insulative properties. It is harmless unless heated above 30°C, when it starts to release degradation products (Shusterman 1993). This situation occurs when welding materials coated with PTFE, heating PTFE with a tool edge during high speed machining, operating moulding or extruding machines (Rose 1992) and rarely during endotracheal laser surgery (Rom 1992a).
A common cause of polymer fume fever was elicited after a period of classic public health detective work in the early 1970s (Wegman and Peters 1974; Kuntz and McCord 1974). Textile workers were developing self-limited febrile illnesses with exposures to formaldehyde, ammonia and nylon fibre; they did not have exposure to fluoropolymer fumes but handled crushed polymer. After finding that exposure levels of the other possible aetiological agents were within acceptable limits, the fluoropolymer work was examined more closely. As it turned out, only cigarette smokers working with the fluoropolymer were symptomatic. It was hypothesized that the cigarettes were being contaminated with fluoropolymer on the worker’s hands, then the product was combusted on the cigarette when it was smoked, exposing the worker to toxic fumes. After banning cigarette smoking in the workplace and setting strict handwashing rules, no further illnesses were reported (Wegman and Peters 1974). Since then, this phenomenon has been reported after working with waterproofing compounds, mould-release compounds (Albrecht and Bryant 1987) and after using certain kinds of ski wax (Strom and Alexandersen 1990).
The pathogenesis of polymer fume fever is not known. It is thought to be similar to the other inhalation fevers owing to its similar presentation and apparently non-specific immune response. There have been no human experimental studies; however, rats and birds both develop severe alveolar epithelial damage on exposure to PTFE pyrolysis products (Wells, Slocombe and Trapp 1982; Blandford et al. 1975). Accurate measurement of pulmonary function or BAL fluid changes has not been done.
Symptoms appear several hours after exposure, and a tolerance or tachyphalaxis effect is not there as seen in MFF. Weakness and myalgias are followed by fever and chills. Often there is chest tightness and cough. Physical examination is usually otherwise normal. Leukocytosis is often seen, and the chest radiograph is usually normal. Symptoms resolve spontaneously in 12 to 48 hours. There have been a few cases of persons developing pulmonary oedema after exposure; in general, PTFE fumes are thought to be more toxic than zinc or copper fumes in causing MFF (Shusterman 1993; Brubaker 1977). Chronic airways dysfunction has been reported in persons who have had multiple episodes of polymer fume fever (Williams, Atkinson and Patchefsky 1974).
The diagnosis of polymer fume fever requires a careful history with high clinical suspicion. After ascertaining the source of the PTFE pyrolysis products, efforts must be made to prevent further exposure. Mandatory handwashing rules and the elimination of smoking in the workplace has effectively eliminated cases related to contaminated cigarettes. Workers who have had multiple episodes of polymer fume fever or associated pulmonary oedema should have long-term medical follow-up.
Occupational Asthma
Asthma is a respiratory disease characterized by airway obstruction that is partially or completely reversible, either spontaneously or with treatment; airway inflammation; and increased airway responsiveness to a variety of stimuli (NAEP 1991). Occupational asthma (OA) is asthma that is caused by environmental exposures in the workplace. Several hundred agents have been reported to cause OA. Pre-existing asthma or airway hyper-responsiveness, with symptoms worsened by work exposure to irritants or physical stimuli, is usually classified separately as work-aggravated asthma (WAA). There is general agreement that OA has become the most prevalent occupational lung disease in developed countries, although estimates of actual prevalence and incidence are quite variable. It is clear, however, that in many countries asthma of occupational aetiology causes a largely unrecognized burden of disease and disability with high economic and non-economic costs. Much of this public health and economic burden is potentially preventable by identifying and controlling or eliminating the workplace exposures causing the asthma. This article will summarize current approaches to recognition, management and prevention of OA. Several recent publications discuss these issues in more detail (Chan-Yeung 1995; Bernstein et al. 1993).
Magnitude of the Problem
Prevalences of asthma in adults generally range from 3 to 5%, depending on the definition of asthma and geographic variations, and may be considerably higher in some low-income urban populations. The proportion of adult asthma cases in the general population that is related to the work environment is reported to range from 2 to 23%, with recent estimates tending towards the higher end of the range. Prevalences of asthma and OA have been estimated in small cohort and cross-sectional studies of high-risk occupational groups. In a review of 22 selected studies of workplaces with exposures to specific substances, prevalences of asthma or OA, defined in various ways, ranged from 3 to 54%, with 12 studies reporting prevalences over 15% (Becklake, in Bernstein et al. 1993). The wide range reflects real variation in actual prevalence (due to different types and levels of exposure). It also reflects differences in diagnostic criteria, and variation in the strength of the biases, such as “survivor bias” which may result from exclusion of workers who developed OA and left the workplace before the study was conducted. Population estimates of incidence range from 14 per million employed adults per year in the United States to 140 per million employed adults per year in Finland (Meredith and Nordman 1996). Ascertainment of cases was more complete and methods of diagnosis were generally more rigorous in Finland. The evidence from these different sources is consistent in its implication that OA is often under-diagnosed and/or under-reported and is a public health problem of greater magnitude than generally recognized.
Causes of Occupational Asthma
Over 200 agents (specific substances, occupations or industrial processes) have been reported to cause OA, based on epidemiological and/or clinical evidence. In OA, airway inflammation and bronchoconstriction can be caused by immunological response to sensitizing agents, by direct irritant effects, or by other non-immunological mechanisms. Some agents (e.g., organophosphate insecticides) may also cause bronchoconstriction by direct pharmacological action. Most of the reported agents are thought to induce a sensitization response. Respiratory irritants often worsen symptoms in workers with pre-existing asthma (i.e., WAA) and, at high exposure levels, can cause new onset of asthma (termed reactive airways dysfunction syndrome (RADS) or irritant-induced asthma) (Brooks, Weiss and Bernstein 1985; Alberts and Do Pico 1996).
OA may occur with or without a latency period. Latency period refers to the time between initial exposure and development of symptoms, and is highly variable. It is often less than 2 years, but in around 20% of cases is 10 years or longer. OA with latency is generally caused by sensitization to one or more agents. RADS is an example of OA without latency.
High molecular weight sensitizing agents (5,000 daltons (Da) or greater) often act by an IgE-dependent mechanism. Low molecular weight sensitizing agents (less than 5,000 Da), which include highly reactive chemicals like isocyanates, may act by IgE-independent mechanisms or may act as haptens, combining with body proteins. Once a worker becomes sensitized to an agent, re-exposure (frequently at levels far below the level that caused sensitization) results in an inflammatory response in the airways, often accompanied by increases in airflow limitation and non-specific bronchial responsiveness (NBR).
In epidemiological studies of OA, workplace exposures are consistently the strongest determinants of asthma prevalence, and the risk of developing OA with latency tends to increase with estimated intensity of exposure. Atopy is an important and smoking a somewhat less consistent determinant of asthma occurrence in studies of agents that act through an IgE-dependent mechanism. Neither atopy nor smoking appears to be an important determinant of asthma in studies of agents acting through IgE-independent mechanisms.
Clinical Presentation
The symptom spectrum of OA is similar to non-occupational asthma: wheeze, cough, chest tightness and shortness of breath. Patients sometimes present cough-variant or nocturnal asthma. OA can be severe and disabling, and deaths have been reported. Onset of OA occurs due to a specific job environment, so identifying exposures that occurred at the time of onset of asthmatic symptoms is key to an accurate diagnosis. In WAA, workplace exposures cause a significant increase in frequency and/or severity of symptoms of pre-existing asthma.
Several features of the clinical history may suggest occupational aetiology (Chan-Yeung 1995). Symptoms frequently worsen at work or at night after work, improve on days off, and recur on return to work. Symptoms may worsen progressively towards the end of the workweek. The patient may note specific activities or agents in the workplace that reproducibly trigger symptoms. Work-related eye irritation or rhinitis may be associated with asthmatic symptoms. These typical symptom patterns may be present only in the initial stages of OA. Partial or complete resolution on weekends or vacations is common early in the course of OA, but with repeated exposures, the time required for recovery may increase to one or two weeks, or recovery may cease to occur. The majority of patients with OA whose exposures are terminated continue to have symptomatic asthma even years after cessation of exposure, with permanent impairment and disability. Continuing exposure is associated with further worsening of asthma. Brief duration and mild severity of symptoms at the time of cessation of exposure are good prognostic factors and decrease the likelihood of permanent asthma.
Several characteristic temporal patterns of symptoms have been reported for OA. Early asthmatic reactions typically occur shortly (less than one hour) after beginning work or the specific work exposure causing the asthma. Late asthmatic reactions begin 4 to 6 hours after exposure begins, and can last 24 to 48 hours. Combinations of these patterns occur as dual asthmatic reactions with spontaneous resolution of symptoms separating an early and late reaction, or as continuous asthmatic reactions with no resolution of symptoms between phases. With exceptions, early reactions tend to be IgE mediated, and late reactions tend to be IgE independent.
Increased NBR, generally measured by methacholine or histamine challenge, is considered a cardinal feature of occupational asthma. The time course and degree of NBR may be useful in diagnosis and monitoring. NBR may decrease within several weeks after cessation of exposure, although abnormal NBR commonly persists for months or years after exposures are terminated. In individuals with irritant-induced occupational asthma, NBR is not expected to vary with exposure and/or symptoms.
Recognition and Diagnosis
Accurate diagnosis of OA is important, given the substantial negative consequences of either under- or over-diagnosis. In workers with OA or at risk of developing OA, timely recognition, identification and control of the occupational exposures causing the asthma improve the chances of prevention or complete recovery. This primary prevention can greatly reduce the high financial and human costs of chronic, disabling asthma. Conversely, since a diagnosis of OA may obligate a complete change of occupation, or costly interventions in the workplace, accurately distinguishing OA from asthma that is not occupational can prevent unnecessary social and financial costs to both employers and workers.
Several case definitions of OA have been proposed, appropriate in different circumstances. Definitions found valuable for worker screening or surveillance (Hoffman et al. 1990) may not be entirely applicable for clinical purposes or compensation. A consensus of researchers has defined OA as “a disease characterized by variable airflow limitation and/or airway hyper-responsiveness due to causes and conditions attributable to a particular occupational environment and not to stimuli encountered outside the workplace” (Bernstein et al. 1993). This definition has been operationalized as a medical case definition, summarized in table 1 (Chan-Yeung 1995).
Table 1. ACCP medical case definition of occupational asthma
Criteria for diagnosis of occupational asthma1 (requires all 4, A-D):
(A) Physician diagnosis of asthma and/or physiological evidence of airways hyper-responsiveness
(B) Occupational exposure preceded onset of asthmatic symptoms1
(C) Association between symptoms of asthma and work
(D) Exposure and/or physiological evidence of relation of asthma to workplace environment (Diagnosis of OA requires one or more of D2-D5, likely OA requires only D1)
(1) Workplace exposure to agent reported to give rise to OA
(2) Work-related changes in FEV1 and/or PEF
(3) Work-related changes in serial testing for non-specific bronchial responsiveness (e.g., Methacholine Challenge Test)
(4) Positive specific bronchial challenge test
(5) Onset of asthma with a clear association with a symptomatic exposure to an inhaled irritant in the workplace (generally RADS)
Criteria for diagnosis of RADS (should meet all 7):
(1) Documented absence of preexisting asthma-like complaints
(2) Onset of symptoms after a single exposure incident or accident
(3) Exposure to a gas, smoke, fume, vapour or dust with irritant properties present in high concentration
(4) Onset of symptoms within 24 hours after exposure with persistence of symptoms for at least 3 months
(5) Symptoms consistent with asthma: cough, wheeze, dyspnoea
(6) Presence of airflow obstruction on pulmonary function tests and/or presence of non-specific bronchial hyper-responsiveness (testing should be done shortly after exposure)
(7) Other pulmonary diseases ruled out
Criteria for diagnosis of work-aggravated asthma (WAA):
(1) Meets criteria A and C of ACCP Medical Case Definition of OA
(2) Pre-existing asthma or history of asthmatic symptoms, (with active symptoms during the year prior to start of employment or exposure of interest)
(3) Clear increase in symptoms or medication requirement, or documentation of work-related changes in PEFR or FEV1 after start of employment or exposure of interest
1 A case definition requiring A, C and any one of D1 to D5 may be useful in surveillance for OA, WAA and RADS.
Source: Chan-Yeung 1995.
Thorough clinical evaluation of OA can be time consuming, costly and difficult. It may require diagnostic trials of removal from and return to work, and often requires the patient to reliably chart serial peak expiratory flow (PEF) measurements. Some components of the clinical evaluation (e.g., specific bronchial challenge or serial quantitative testing for NBR) may not be readily available to many physicians. Other components may simply not be achievable (e.g., patient no longer working, diagnostic resources not available, inadequate serial PEF measurements). Diagnostic accuracy is likely to increase with the thoroughness of the clinical evaluation. In each individual patient, decisions on the extent of medical evaluation will need to balance costs of the evaluation with the clinical, social, financial and public health consequences of incorrectly diagnosing or ruling out OA.
In consideration of these difficulties, a stepped approach to diagnosis of OA is outlined in table 2. This is intended as a general guide to facilitate accurate, practical and efficient diagnostic evaluation, recognizing that some of the suggested procedures may not be available in some settings. Diagnosis of OA involves establishing both the diagnosis of asthma and the relation between asthma and workplace exposures. After each step, for each patient, the physician will need to determine whether the level of diagnostic certainty achieved is adequate to support the necessary decisions, or whether evaluation should continue to the next step. If facilities and resources are available, the time and cost of continuing the clinical evaluation are usually justified by the importance of making an accurate determination of the relationship of asthma to work. Highlights of diagnostic procedures for OA will be summarized; details can be found in several of the references (Chan-Yeung 1995; Bernstein et al. 1993). Consultation with a physician experienced in OA may be considered, since the diagnostic process may be difficult.
Table 2. Steps in diagnostic evaluation of asthma in the workplace
Step 1 Thorough medical and occupational history and directed physical examination.
Step 2 Physiologic evaluation for reversible airway obstruction and/or non specific bronchial hyper-responsiveness.
Step 3 Immunologic assessment, if appropriate.
Assess Work Status:
Currently working: Proceed to Step 4 first.
Not currently working, diagnostic trial of return to work feasible: Step 5 first, then Step 4.
Not currently working, diagnostic trial of return to work not feasible: Step 6.
Step 4 Clinical evaluation of asthma at work or diagnostic trial of return to work.
Step 5 Clinical evaluation of asthma away from work or diagnostic trial of removal from work.
Step 6 Workplace challenge or specific bronchial challenge testing. If available for suspected causal exposures, this step may be performed prior to Step 4 for any patient.
This is intended as a general guide to facilitate practical and efficient diagnostic evaluation. It is recommended that physicians who diagnose and manage OA refer to current clinical literature as well.
RADS, when caused by an occupational exposure, is usually considered a subclass of OA. It is diagnosed clinically, using the criteria in Table 6. Patients who have experienced significant respiratory injury due to high-level irritant inhalations should be evaluated for persistent symptoms and presence of airflow obstruction shortly after the event. If the clinical history is compatible with RADS, further evaluation should include quantitative testing for NBR, if not contra-indicated.
WAA may be common, and may cause a substantial preventable burden of disability, but little has been published on diagnosis, management or prognosis. As summarized in Table 6, WAA is recognized when asthmatic symptoms preceded the suspected causal exposure but are clearly aggravated by the work environment. Worsening at work can be documented either by physiological evidence or through evaluation of medical records and medication use. It is a clinical judgement whether patients with a history of asthma in remission, who have recurrence of asthmatic symptoms that otherwise meet the criteria for OA, are diagnosed with OA or WAA. One year has been proposed as a sufficiently long asymptomatic period that the onset of symptoms is likely to represent a new process caused by the workplace exposure, although no consensus yet exists.
Step 1: Thorough medical and occupational history anddirected physical examination
Initial suspicion of possible OA in appropriate clinical and workplace situations is key, given the importance of early diagnosis and intervention in improving prognosis. The diagnosis of OA or WAA should be considered in all asthmatic patients in whom symptoms developed as a working adult (especially recent onset), or in whom the severity of asthma has substantially increased. OA should also be considered in any other individuals who have asthma-like symptoms and work in occupations in which they are exposed to asthma-causing agents or who are concerned that their symptoms are work-related.
Patients with possible OA should be asked to provide a thorough medical and occupational/environmental history, with careful documentation of the nature and date of onset of symptoms and diagnosis of asthma, and any potentially causal exposures at that time. Compatibility of the medical history with the clinical presentation of OA described above should be evaluated, especially the temporal pattern of symptoms in relation to work schedule and changes in work exposures. Patterns and changes in patterns of use of asthma medications, and the minimum period of time away from work required for improvement in symptoms should be noted. Prior respiratory diseases, allergies/atopy, smoking and other toxic exposures, and a family history of allergy are pertinent.
Occupational and other environmental exposures to potential asthma-causing agents or processes should be thoroughly explored, with objective documentation of exposures if possible. Suspected exposures should be compared with a comprehensive list of agents reported to cause OA (Harber, Schenker and Balmes 1996; Chan-Yeung and Malo 1994; Bernstein et al. 1993; Rom 1992b), although inability to identify specific agents is not uncommon and induction of asthma by agents not previously described is possible as well. Some illustrative examples are shown in table 3. Occupational history should include details of current and relevant past employment with dates, job titles, tasks and exposures, especially current job and job held at time of onset of symptoms. Other environmental history should include a review of exposures in the home or community that could cause asthma. It is helpful to begin the exposure history in an open-ended way, asking about broad categories of airborne agents: dusts (especially organic dusts of animal, plant or microbial origin), chemicals, pharmaceuticals and irritating or visible gases or fumes. The patient may identify specific agents, work processes or generic categories of agents that have triggered symptoms. Asking the patient to describe step by step the activities and exposures involved in the most recent symptomatic workday can provide useful clues. Materials used by co-workers, or those released in high concentration from a spill or other source, may be relevant. Further information can often be obtained on product name, ingredients and manufacturer name, address and phone number. Specific agents can be identified by calling the manufacturer or through a variety of other sources including textbooks, CD ROM databases, or Poison Control Centers. Since OA is frequently caused by low levels of airborne allergens, workplace industrial hygiene inspections which qualitatively evaluate exposures and control measures are often more helpful than quantitative measurement of air contaminants.
Table 3. Sensitizing agents that can cause occupational asthma
|
Classification |
Sub-groups |
Examples of substances |
Examples of jobs and industries |
|
High-molecular-weight protein antigens |
Animal-derived substances Plant-derived substances |
Laboratory animals, crab/seafood, mites, insects Flour and grain dusts, natural rubber latex gloves, bacterial enzymes, castor bean dust, vegetable gums |
Animal handlers, farming and food processing Bakeries, health care workers, detergent making, food processing |
|
Low-molecular-weight/chemical |
Plasticizers, 2-part paints, adhesives, foams Metals Wood dusts Pharmaceuticals, drugs |
Isocyanates, acid anhydrides, amines Platinum salts, cobalt Cedar (plicatic acid), oak Psyllium, antibiotics |
Auto spray painting, varnishing, woodworking Platinum refineries, metal grinding Sawmill work, carpentry Pharmaceutical manufacturing and packaging |
|
Other chemicals |
Chloramine T, polyvinyl chloride fumes, organophosphate insecticides |
Janitorial work, meat packing |
The clinical history appears to be better for excluding rather than for confirming the diagnosis of OA, and an open-ended history taken by a physician is better than a closed questionnaire. One study compared the results of an open-ended clinical history taken by trained OA specialists with a “gold standard” of specific bronchial challenge testing in 162 patients referred for evaluation of possible OA. The investigators reported that the sensitivity of a clinical history suggestive of OA was 87%, specificity 55%, predictive value positive 63% and predictive value negative 83%. In this group of referred patients, prevalence of asthma and OA were 80% and 46%, respectively (Malo et al. 1991). In other groups of referred patients, predictive values positive of a closed questionnaire ranged from 8 to 52% for a variety of workplace exposures (Bernstein et al. 1993). The applicability of these results to other settings needs to be assessed by the physician.
Physical examination is sometimes helpful, and findings relevant to asthma (e.g., wheezing, nasal polyps, eczematous dermatitis), respiratory irritation or allergy (e.g., rhinitis, conjunctivitis) or other potential causes of symptoms should be noted.
Step 2: Physiological evaluation for reversible airway obstruction and/or non-specific bronchial hyper-responsiveness
If sufficient physiological evidence supporting the diagnosis of asthma (NAEP 1991) is already in the medical record, Step 2 can be skipped. If not, technician-coached spirometry should be performed, preferably post-workshift on a day when the patient is experiencing asthmatic symptoms. If spirometry reveals airway obstruction which reverses with a bronchodilator, this confirms the diagnosis of asthma. In patients without clear evidence of airflow limitation on spirometry, quantitative testing for NBR using methacholine or histamine should be done, the same day if possible. Quantitative testing for NBR in this situation is a key procedure for two reasons. First, it can often identify patients with mild or early stage OA who have the greatest potential for cure but who would be missed if testing stopped with normal spirometry. Second, if NBR is normal in a worker who has ongoing exposure in the workplace environment associated with the symptoms, OA can generally be ruled out without further testing. If abnormal, evaluation can proceed to Step 3 or 4, and the degree of NBR may be useful in monitoring the patient for improvement after diagnostic trial of removal from the suspected causal exposure (Step 5). If spirometry reveals significant airflow limitation that does not improve after inhaled bronchodilator, a re-evaluation after more prolonged trial of therapy, including corticosteroids, should be considered (ATS 1995; NAEP 1991).
Step 3: Immunological assessment, if appropriate
Skin or serological (e.g., RAST) testing can demonstrate immunological sensitization to a specific workplace agent. These immunological tests have been used to confirm the work-relatedness of asthma, and, in some cases, eliminate the need for specific inhalation challenge tests. For example, among psyllium-exposed patients with a clinical history compatible with OA, documented asthma or airway hyper-responsiveness, and evidence of immunological sensitization to psyllium, approximately 80% had OA confirmed on subsequent specific bronchial challenge testing (Malo et al. 1990). In most cases, diagnostic significance of negative immunological tests is less clear. The diagnostic sensitivity of the immunological tests depends critically on whether all the likely causal antigens in the workplace or hapten-protein complexes have been included in the testing. Although the implication of sensitization for an asymptomatic worker is not well defined, analysis of grouped results can be useful in evaluating environmental controls. The utility of immunological evaluation is greatest for agents for which there are standardized in vitro tests or skin-prick reagents, such as platinum salts and detergent enzymes. Unfortunately, most occupational allergens of interest are not currently available commercially. The use of non-commercial solutions in skin-prick testing has on occasions been associated with severe reactions, including anaphylaxis, and thus caution is necessary.
If results of Steps 1 and 2 are compatible with OA, further evaluation should be pursued if possible. The order and extent of further evaluation depends on availability of diagnostic resources, work status of the patient and feasibility of diagnostic trials of removal from and return to work as indicated in Table 7. If further evaluation is not possible, a diagnosis must be based on the information available at this point.
Step 4: Clinical evaluation of asthma at work, or diagnostic trial of return to work
Often the most readily available physiological test of airway obstruction is spirometry. To improve reproducibility, spirometry should be coached by a trained technician. Unfortunately, single-day cross-shift spirometry, performed before and after the workshift, is neither sensitive nor specific in determining work-associated airway obstruction. It is probable that if multiple spirometries are performed each day during and after several workdays, the diagnostic accuracy may be improved, but this has not yet been adequately evaluated.
Due to difficulties with cross-shift spirometry, serial PEF measurement has become an important diagnostic technique for OA. Using an inexpensive portable meter, PEF measurements are recorded every two hours, during waking hours. To improve sensitivity, measurements must be done during a period when the worker is exposed to the suspected causal agents at work and is experiencing a work-related pattern of symptoms. Three repetitions are performed at each time, and measurements are made every day at work and away from work. The measurements should be continued for at least 16 consecutive days (e.g., two five-day work weeks and 3 weekends off) if the patient can safely tolerate continuing to work. PEF measurements are recorded in a diary along with notation of work hours, symptoms, use of bronchodilator medications, and significant exposures. To facilitate interpretation, the diary results should then be plotted graphically. Certain patterns suggest OA, but none are pathognomonic, and interpretation by an experienced reader is often helpful. Advantages of serial PEF testing are low cost and reasonable correlation with results of bronchial challenge testing. Disadvantages include the significant degree of patient cooperation required, inability to definitely confirm that data are accurate, lack of standardized method of interpretation, and the need for some patients to take 1 or 2 consecutive weeks off work to show significant improvement. Portable electronic recording spirometers designed for patient self monitoring, when available, can address some of the disadvantages of serial PEF.
Asthma medications tend to reduce the effect of work exposures on measures of airflow. However, it is not advisable to discontinue medications during airflow monitoring at work. Rather, the patient should be maintained on a constant minimal safe dosage of anti-inflammatory medications throughout the entire diagnostic process, with close monitoring of symptoms and airflow, and the use of short-acting bronchodilators to control symptoms should be noted in the diary.
The failure to observe work-related changes in PEF while a patient is working routine hours does not exclude the diagnosis of OA, since many patients will require more than a two-day weekend to show significant improvement in PEF. In this case, a diagnostic trial of extended removal from work (Step 5) should be considered. If the patient has not yet had quantitative testing for NBR, and does not have a medical contra-indication, it should be done at this time, immediately after at least two weeks of workplace exposure.
Step 5: Clinical evaluation of asthma away from work or diagnostic trial of extended removal from work
This step consists of completion of the serial 2-hourly PEF daily diary for at least 9 consecutive days away from work (e.g., 5 days off work plus weekends before and after). If this record, compared with the serial PEF diary at work, is not sufficient for diagnosing OA, it should be continued for a second consecutive week away from work. After 2 or more weeks away from work, quantitative testing for NBR can be performed and compared to NBR while at work. If serial PEF has not yet been done during at least two weeks at work, then a diagnostic trial of return to work (see Step 4) may be performed, after detailed counselling, and in close contact with the treating physician. Step 5 is often critically important in confirming or excluding the diagnosis of OA, although it may also be the most difficult and expensive step. If an extended removal from work is attempted, it is best to maximize the diagnostic yield and efficiency by including PEF, FEV1, and NBR tests in one comprehensive evaluation. Weekly physician visits for counselling and to review the PEF chart can help to assure complete and accurate results. If, after monitoring the patient for at least two weeks at work and two weeks away from it, the diagnostic evidence is not yet sufficient, Step 6 should be considered next, if available and feasible.
Step 6: Specific bronchial challenge or workplace challenge testing
Specific bronchial challenge testing using an exposure chamber and standardized exposure levels has been labelled the “gold standard” for diagnosis of OA. Advantages include definitive confirmation of OA with ability to identify asthmatic response to sub-irritant levels of specific sensitizing agents, which can then be scrupulously avoided. Of all the diagnostic methods, it is the only one that can reliably distinguish sensitizer-induced asthma from provocation by irritants. Several problems with this approach have included inherent costliness of the procedure, general requirement of close observation or hospitalization for several days, and availability in only very few specialized centres. False negatives may occur if standardized methodology is not available for all suspected agents, if the wrong agents are suspected, or if too long a time has elapsed between last exposure and testing. False positives may result if irritant levels of exposure are inadvertently obtained. For these reasons, specific bronchial challenge testing for OA remains a research procedure in most localities.
Workplace challenge testing involves serial technician-coached spirometry in the workplace, performed at frequent (e.g., hourly) intervals before and during the course of a workday exposure to the suspected causal agents or processes. It may be more sensitive than specific bronchial challenge testing because it involves “real life” exposures, but since airway obstruction may be triggered by irritants as well as sensitizing agents, positive tests do not necessarily indicate sensitization. It also requires cooperation of the employer and much technician time with a mobile spirometer. Both of these procedures carry some risk of precipitating a severe asthmatic attack, and should therefore be done under close supervision of specialists experienced with the procedures.
Treatment and Prevention
Management of OA includes medical and preventive interventions for individual patients, as well as public health measures in workplaces identified as high risk for OA. Medical management is similar to that for non-occupational asthma and is well reviewed elsewhere (NAEP 1991). Medical management alone is rarely adequate to optimally control symptoms, and preventive intervention by control or cessation of exposure is an integral part of the treatment. This process begins with accurate diagnosis and identification of causative exposures and conditions. In sensitizer-induced OA, reducing exposure to the sensitizer does not usually result in complete resolution of symptoms. Severe asthmatic episodes or progressive worsening of the disease may be caused by exposures to very low concentrations of the agent and complete and permanent cessation of exposure is recommended. Timely referral for vocational rehabilitation and job retraining may be a necessary component of treatment for some patients. If complete cessation of exposure is impossible, substantial reduction of exposure accompanied by close medical monitoring and management may be an option, although such reduction in exposure is not always feasible and the long-term safety of this approach has not been tested. As an example, it would be difficult to justify the toxicity of long-term treatment with systemic corticosteroids in order to allow the patient to continue in the same employment. For asthma induced and/or triggered by irritants, dose response may be more predictable, and lowering of irritant exposure levels, accompanied by close medical monitoring, may be less risky and more likely to be effective than for sensitizer-induced OA. If the patient continues to work under modified conditions, medical follow-up should include frequent physician visits with review of the PEF diary, well-planned access to emergency services, and serial spirometry and/or methacholine challenge testing, as appropriate.
Once a particular workplace is suspected to be high risk, due either to occurrence of a sentinel case of OA or use of known asthma-causing agents, public health methods can be very useful. Early recognition and effective treatment and prevention of disability of workers with existing OA, and prevention of new cases, are clear priorities. Identification of specific causal agent(s) and work processes is important. One practical initial approach is a workplace questionnaire survey, evaluating criteria A, B, C, and D1 or D5 in the case definition of OA. This approach can identify individuals for whom further clinical evaluation might be indicated and help identify possible causal agents or circumstances. Evaluation of group results can help decide whether further workplace investigation or intervention is indicated and, if so, provide valuable guidance in targeting future prevention efforts in the most effective and efficient manner. A questionnaire survey is not adequate, however, to establish individual medical diagnoses, since predictive positive values of questionnaires for OA are not high enough. If a greater level of diagnostic certainty is needed, medical screening utilizing diagnostic procedures such as spirometry, quantitative testing for NBR, serial PEF recording, and immunological testing can be considered as well. In known problem workplaces, ongoing surveillance and screening programmes may be helpful. However, differential exclusion of asymptomatic workers with history of atopy or other potential susceptibility factors from workplaces believed to be high risk would result in removal of large numbers of workers to prevent relatively few cases of OA, and is not supported by the current literature.
Control or elimination of causal exposures and avoidance and proper management of spills or episodes of high-level exposures can lead to effective primary prevention of sensitization and OA in co-workers of the sentinel case. The usual exposure control hierarchy of substitution, engineering and administrative controls, and personal protective equipment, as well as education of workers and managers, should be implemented as appropriate. Proactive employers will initiate or participate in some or all of these approaches, but in the event that inadequate preventive action is taken and workers remain at high risk, governmental enforcement agencies may be helpful.
Impairment and Disability
Medical impairment is a functional abnormality resulting from a medical condition. Disability refers to the total effect of the medical impairment on the patient’s life, and is influenced by many non-medical factors such as age and socio-economic status (ATS 1995).
Assessment of medical impairment is done by the physician and may include a calculated impairment index, as well as other clinical considerations. The impairment index is based on (1) degree of airflow limitation after bronchodilator, (2) either degree of reversibility of airflow limitation with bronchodilator or degree of airway hyper-responsiveness on quantitative testing for NBR, and (3) minimum medication required to control asthma. The other major component of the assessment of medical impairment is the physician’s medical judgement of the ability of the patient to work in the workplace environment causing the asthma. For example, a patient with sensitizer-induced OA may have a medical impairment which is highly specific to the agent to which he or she has become sensitized. The worker who experiences symptoms only when exposed to this agent may be able to work in other jobs, but permanently unable to work in the specific job for which she or he has the most training and experience.
Assessment of disability due to asthma (including OA) requires consideration of medical impairment as well as other non-medical factors affecting ability to work and function in everyday life. Disability assessment is initially made by the physician, who should identify all the factors affecting the impact of the impairment on the patient’s life. Many factors such as occupation, educational level, possession of other marketable skills, economic conditions and other social factors may lead to varying levels of disability in individuals with the same level of medical impairment. This information can then be used by administrators to determine disability for purposes of compensation.
Impairment and disability may be classified as temporary or permanent, depending on the likelihood of significant improvement, and whether effective exposure controls are successfully implemented in the workplace. For example, an individual with sensitizer-induced OA is generally considered permanently, totally impaired for any job involving exposure to the causal agent. If the symptoms resolve partially or completely after cessation of exposure, these individuals may be classified with less or no impairment for other jobs. Often this is considered permanent partial impairment/disability, but terminology may vary. An individual with asthma which is triggered in a dose-dependent fashion by irritants in the workplace would be considered to have temporary impairment while symptomatic, and less or no impairment if adequate exposure controls are installed and are effective in reducing or eliminating symptoms. If effective exposure controls are not implemented, the same individual might have to be considered permanently impaired to work in that job, with recommendation for medical removal. If necessary, repeated assessment for long-term impairment/disability may be carried out two years after the exposure is reduced or terminated, when improvement of OA would be expected to have plateaued. If the patient continues to work, medical monitoring should be ongoing and reassessment of impairment/disability should be repeated as needed.
Workers who become disabled by OA or WAA may qualify for financial compensation for medical expenses and/or lost wages. In addition to directly reducing the financial impact of the disability on individual workers and their families, compensation may be necessary to provide proper medical treatment, initiate preventive intervention and obtain vocational rehabilitation. The worker’s and physician’s understanding of specific medico-legal issues may be important to ensuring that the diagnostic evaluation meets local requirements and does not result in compromise of the rights of the affected worker.
Although discussions of cost savings frequently focus on the inadequacy of compensation systems, genuinely reducing the financial and public health burden placed on society by OA and WAA will depend not only on improvements in compensation systems but, more importantly, on effectiveness of the systems deployed to identify and rectify, or prevent entirely, workplace exposures that are causing onset of new cases of asthma.
Conclusions
OA has become the most prevalent occupational respiratory disease in many countries. It is more common than generally recognized, can be severe and disabling, and is generally preventable. Early recognition and effective preventive interventions can substantially reduce the risk of permanent disability and the high human and financial costs associated with chronic asthma. For many reasons, OA merits more widespread attention among clinicians, health and safety specialists, researchers, health policy makers, industrial hygienists, and others interested in prevention of work-related diseases.
More...
Diseases Caused by Organic Dusts
Organic Dust and Disease
Dusts of vegetable, animal and microbial origin have always been part of the human environment. When the first aquatic organisms moved to land some 450 million years ago, they soon developed defence systems against the many noxious substances present in the terrestrial environment, most of them of plant origin. Exposures to this environment usually cause no specific problems, even though plants contain a number of extremely toxic substances, particularly those present in or produced by moulds.
During the development of civilization, climatic conditions in some parts of the world necessitated certain activities to be undertaken indoors. Threshing in the Scandinavian countries was performed indoors during the winter, a practice mentioned by chroniclers in antiquity. The enclosure of dusty processes led to disease among the exposed persons, and one of the first published accounts of this is by the Danish bishop Olaus Magnus (1555, as cited by Rask-Andersen 1988). He described a disease among threshers in Scandinavia as follows:
“In separating the grain from the chaff, care must be taken to choose a time when there is a suitable wind which will sweep away the grain dust, so that it will not damage the vital organs of the threshers. This dust is so fine that it will almost unnoticeably penetrate into the mouth and accumulate in the throat. If this is not quickly dealt with by drinking fresh ale, the thresher may never again or only for a short period eat what he has threshed.”
With the introduction of machine processing of organic materials, treatment of large quantities of materials indoors with poor ventilation led to high levels of airborne dust. The descriptions by bishop Olaus Magnus and later by Ramazzini (1713) were followed by several reports on disease and organic dusts in the nineteenth century, particularly among cotton mill workers (Leach 1863; Prausnitz 1936). Later, the specific pulmonary disease common among farmers handling mouldy materials was also described (Campbell 1932).
During recent decades, a large number of reports on disease among persons exposed to organic dusts have been published. Initially, most of these were based on persons seeking medical help. The names of the diseases, when published, were often related to the particular environment where the disease was first recognized, and a bewildering array of names resulted, such as farmer’s lung, mushroom grower’s lung, brown lung and humidifier fever.
With the advent of modern epidemiology, more reliable figures were obtained for the incidence of occupational respiratory diseases related to organic dust (Rylander, Donham and Peterson 1986; Rylander and Peterson 1990). There was also advancement in the understanding of the pathological mechanisms underlying these diseases, particularly the inflammatory response (Henson and Murphy 1989). This paved the way for a more coherent picture of diseases caused by organic dusts (Rylander and Jacobs 1997).
The following will describe the different organic dust environments where disease has been reported, the disease entities themselves, the classical byssinosis disease and specific preventive measures.
Environments
Organic dusts are airborne particles of vegetable, animal or microbial origin. Table 1 lists examples of environments, work processes and agents involving the risk of exposure to organic dusts.
Table 1. Examples of sources of hazards of exposure to organic dust
Agriculture
Handling of grain, hay or other crops
Sugar-cane processing
Greenhouses
Silos
Animals
Swine/dairy confinement buildings
Poultry houses and processing plants
Laboratory animals, farm animals and pets
Waste-processing
Sewage water and silt
Household garbage
Composting
Industry
Vegetable fibre processing (cotton, flax, hemp, jute, sisal)
Fermentation
Timber and wood processing
Bakeries
Biotechnology processing
Buildings
Contaminated water in humidifiers
Microbial growth on structures or in ventilation ducts
Agents
It is now understood that the specific agents in the dusts are the major reason why disease develops. Organic dusts contain a multitude of agents with potential biological effects. Some of the major agents are found in table 2.
Table 2. Major agents in organic dusts with potential biological activity
Vegetable agents
Tannins
Histamine
Plicatic acid
Alkaloids (e.g., nicotine)
Cytochalasins
Animal agents
Proteins
Enzymes
Microbial agents
Endotoxins
(1→3)–β–D-glucans
Proteases
Mycotoxins
The relative role of each of these agents, alone or in combination with others, for the development of disease, is mostly unknown. Most of the information available relates to bacterial endotoxins which are present in all organic dusts.
Endotoxins are lipopolysaccharide compounds which are attached to the outer cell surface of Gram-negative bacteria. Endotoxin has a wide variety of biological properties. After inhalation it causes an acute inflammation (Snella and Rylander 1982; Brigham and Meyrick 1986). An influx of neutrophils (leukocytes) into the lung and the airways is the hallmark of this reaction. It is accompanied by activation of other cells and secretion of inflammatory mediators. After repeated exposures, the inflammation decreases (adaptation). The reaction is limited to the airway mucosa, and there is no extensive involvement of the lung parenchyma.
Another specific agent in organic dust is (1→3)-β-D-glucan. This is a polyglucose compound present in the cell wall structure of moulds and some bacteria. It enhances the inflammatory response caused by endotoxin and alters the function of inflammatory cells, particularly macrophages and T-cells (Di Luzio 1985; Fogelmark et al. 1992).
Other specific agents present in organic dusts are proteins, tannins, proteases and other enzymes, and toxins from moulds. Very little data are available on the concentrations of these agents in organic dusts. Several of the specific agents in organic dusts, such as proteins and enzymes, are allergens.
Diseases
The diseases caused by organic dusts are shown in table 3 with the corresponding International Classification of Disease (ICD) numbers (Rylander and Jacobs 1994).
Table 3. Diseases induced by organic dusts and their ICD codes
Bronchitis and pneumonitis (ICD J40)
Toxic pneumonitis (inhalation fever, organic dust toxic syndrome)
Airways inflammation (mucous membrane inflammation)
Chronic bronchitis (ICD J42)
Hypersensitivity pneumonitis (allergic alveolitis) (ICD J67)
Asthma (ICD J45)
Rhinitis, conjunctivitis
The primary route of exposure for organic dusts is by inhalation, and consequently the effects on the lung have received the major share of attention in research as well as in clinical work. There is, however, a growing body of evidence from published epidemiological studies and case reports as well as anecdotal reports, that systemic effects also occur. The mechanism involved seems to be a local inflammation at the target site, the lung, and a subsequent release of cytokines either with systemic effects (Dunn 1992; Michel et al. 1991) or an effect on the epithelium in the gut (Axmacher et al. 1991). Non-respiratory clinical effects are fever, joint pains, neurosensory effects, skin problems, intestinal disease, fatigue and headache.
The different disease entities as described in table 3 are easy to diagnose in typical cases, and the underlying pathology is distinctly different. In real life, however, a worker who has a disease due to organic dust exposure, often presents a mixture of the different disease entities. One person may have airways inflammation for a number of years, suddenly develop asthma and in addition have symptoms of toxic pneumonitis during a particularly heavy exposure. Another person may have subclinical hypersensitivity pneumonitis with lymphocytosis in the airways and develop toxic pneumonitis during a particularly heavy exposure.
A good example of the mixture of disease entities that may appear is byssinosis. This disease was first described in the cotton mills, but the individual disease entities are also found in other organic dust environments. An overview of the disease follows.
Byssinosis
The disease
Byssinosis was first described in the 1800s, and a classic report involving clinical as well as experimental work was given by Prausnitz (1936). He described the symptoms among cotton mill workers as follows:
“After working for years without any appreciable trouble except a little cough, cotton mill workers notice either a sudden aggravation of their cough, which becomes dry and exceedingly irritating¼ These attacks usually occur on Mondays ¼ but gradually the symptoms begin to spread over the ensuing days of the week; in time the difference disappears and they suffer continuously.”
The first epidemiological investigations were performed in England in the 1950s (Schilling et al. 1955; Schilling 1956). The initial diagnosis was based on the appearance of a typical Monday morning chest tightness, diagnosed using a questionnaire (Roach and Schilling 1960). A scheme for grading the severity of byssinosis based on the type and periodicity of symptoms was developed (Mekky, Roach and Schilling 1967; Schilling et al. 1955). Duration of exposure was used as a measure of dose and this was related to the severity of the response. Based on clinical interviews of large numbers of workers, this grading scheme was later modified to more accurately reflect the time intervals for the decrease in FEV1 (Berry et al. 1973).
In one study, a difference in the prevalence of byssinosis in mills processing different types of cotton was found (Jones et al. 1979). Mills using high-quality cotton to produce finer yarns had a lower prevalence of byssinosis than mills producing coarse yarns and using a lower quality of cotton. Thus in addition to exposure intensity and duration, both dose-related variables, the type of dust became an important variable for assessing exposure. Later it was demonstrated that the differences in the response of workers exposed to coarse and medium cottons was dependent not only on the type of cotton but on other variables that affect exposure, including: processing variables such as carding speed, environmental variables such as humidification and ventilation, and manufacturing variables such as different yarn treatments (Berry et al. 1973).
The next refinement of the relationship between exposure to cotton dust and a response (either symptoms or objective measures of pulmonary function), was the studies from the United States, comparing those who worked in 100% cotton to workers using the same cotton but in a 50:50 blend with synthetics and workers without exposure to cotton (Merchant et al. 1973). Workers exposed to 100% cotton had the highest prevalence of byssinosis independent of cigarette smoking, one of the confounders of exposure to cotton dust. This semiquantitative relationship between dose and response to cotton dust was further refined in a group of textile workers stratified by sex, smoking, work area and mill type. A relationship was observed in each of these categories between dust concentration in the lower dust ranges and byssinosis prevalence and/or change in forced expiratory volume in one second (FEV1).
In later investigations, the FEV1 decrease over the work shift has been used to assess the effects of exposure, and it is also a part of the US Cotton Dust Standard.
Byssinosis was long regarded as a peculiar disease with a mixture of different symptoms and no knowledge of the specific pathology. Some authors suggested that it was an occupational asthma (Bouhuys 1976). A workgroup meeting in 1987 analysed the symptomatology and pathology of the disease (Rylander et al. 1987). It was agreed that the disease comprised several clinical entities, generally related to organic dust exposure.
Toxic pneumonitis may appear the first time an employee works in the mill, particularly when working in the opening, blowing and carding sections (Trice 1940). Although habituation develops, the symptoms may reappear after an unusually heavy exposure later on.
Airways inflammation is the most widespread disease, and it appears at different degrees of severity from light irritation in the nose and airways to severe dry cough and breathing difficulties. The inflammation causes constriction of airways and a reduced FEV1. Airway responsiveness is increased as measured with a methacholine or histamine challenge test. It has been discussed whether airways inflammation should be accepted as a disease entity by itself or whether it merely represents a symptom. As the clinical findings in terms of severe cough with airways narrowing can lead to a decrease in work ability, it is justified to regard it as an occupational disease.
Continued airways inflammation over several years may develop into chronic bronchitis, particularly among heavily exposed workers in the blowing and carding areas. The clinical picture would be one of chronic obstructive pulmonary disease (COPD).
Occupational asthma develops in a small percentage of the workforce, but is usually not diagnosed in cross-sectional studies as the workers are forced to leave work because of the disease. Hypersensitivity pneumonitis has not been detected in any of the epidemiological studies undertaken, nor have there been case reports relating to cotton dust exposure. The absence of hypersensitivity pneumonitis may be due to the relatively low amount of moulds in cotton, as mouldy cotton is not acceptable for processing.
A subjective feeling of chest tightness, most common on Mondays, is the classical symptom of cotton dust exposure (Schilling et al. 1955). It is not, however, a feature unique to cotton dust exposure as it appears also among persons working with other kinds of organic dusts (Donham et al. 1989). Chest tightness develops slowly over a number of years but it can also be induced in previously unexposed persons, provided that the dose level is high (Haglind and Rylander 1984). The presence of chest tightness is not directly related to a decrease in FEV1.
The pathology behind chest tightness has not been explained. It has been suggested that the symptoms are due to an increased adhesiveness of platelets which accumulate in the lung capillaries and increase the pulmonary artery pressure. It is likely that chest tightness involves some kind of cell sensitization, as it takes repeated exposures for the symptom to develop. This hypothesis is supported by results from studies on blood monocytes from cotton workers (Beijer et al. 1990). A higher ability to produce procoagulant factor, indicative of cell sensitization, was found among cotton workers as compared to controls.
The environment
The disease was originally described among workers in cotton, flax and soft hemp mills. In the first phase of cotton treatment within the mills—bale opening, blowing and carding—more than half of the workers may have symptoms of chest tightness and airways inflammation. The incidence decreases as the cotton is processed, reflecting the successive cleaning of the causative agent from the fibre. Byssinosis has been described in all countries where investigations in cotton mills have been performed. Some countries like Australia have, however, unusually low incidence figures (Gun et al. 1983).
There is now uniform evidence that bacterial endotoxins are the causative agent for toxic pneumonitis and airways inflammation (Castellan et al. 1987; Pernis et al. 1961; Rylander, Haglind and Lundholm 1985; Rylander and Haglind 1986; Herbert et al. 1992; Sigsgaard et al. 1992). Dose-response relationships have been described and the typical symptoms have been induced by inhalation of purified endotoxin (Rylander et al. 1989; Michel et al. 1995). Although this does not exclude the possibility that other agents could contribute to the pathogenesis, endotoxins can serve as markers for disease risk. It is unlikely that endotoxins are related to the development of occupational asthma, but they could act as an adjuvant for potential allergens in cotton dust.
The case
The diagnosis of byssinosis is classically made using questionnaires with the specific question “Does your chest feel tight, and if so, on which day of the week?”. Persons with Monday morning chest tightness are classified as byssinotics according to a scheme suggested by Schilling (1956). Spirometry can be performed, and, according to the different combinations of chest tightness and decrease in FEV1, the diagnostic scheme illustrated in table 4 has evolved.
Table 4. Diagnostic criteria for byssinosis
Grade ½. Chest tightness on the first day of some working weeks
Grade 1. Chest tightness on the first day of every working week
Grade 2. Chest tightness on the first and other days of the working week
Grade 3. Grade 2 symptoms accompanied by evidence of permanent incapacity in the form of diminished effort intolerance and/or reduced ventilatory capacity
Treatment
Treatment in the light stages of byssinosis is symptomatic, and most of the workers learn to live with the slight chest tightness and bronchoconstriction that they experience on Mondays or when cleaning machinery or carrying out similar tasks with a higher than normal exposure. More advanced stages of airways inflammation or regular chest tightness several days of the week require transfer to less dusty operations. The presence of occupational asthma mostly requires work change.
Prevention
Prevention in general is dealt with in detail elsewhere in the Encyclopaedia. The basic principles for prevention in terms of product substitute, exposure limitation, worker protection and screening for disease apply also for cotton dust exposure.
Regarding product substitutes, it has been suggested that cotton with a low level of bacterial contamination be used. An inverse proof of this concept is found in reports from 1863 where the change to dirty cotton provoked an increase in the prevalence of symptoms among the exposed workers (Leach 1863). There is also the possibility of changing to other fibres, particularly synthetic fibres, although this is not always feasible from a product point of view. There is at present no production-applied technique to decrease the endotoxin content of cotton fibres.
Regarding dust reduction, successful programmes have been implemented in the United States and elsewhere (Jacobs 1987). Such programmes are expensive, and the costs for highly efficient dust removal may be prohibitive for developing countries (Corn 1987).
Regarding exposure control, the level of dust is not a sufficiently precise measure of exposure risk. Depending on the degree of contamination with Gram-negative bacteria and thus endotoxin, a given dust level may or may not be associated with a risk. For endotoxins, no official guidelines have been established. It has been suggested that a level of 200 ng/m3 is the threshold for toxic pneumonitis, 100 to 200 ng/m3 for acute airways constriction over the workshift and 10 ng/m3 for airways inflammation (Rylander and Jacobs 1997).
Knowledge about the risk factors and the consequences of exposure are important for prevention. The information basis has expanded rapidly during recent years, but much of it is not yet present in textbooks or other easily available sources. A further problem is that symptoms and findings in respiratory diseases induced by organic dust are non-specific and occur normally in the population. They may thus not be correctly diagnosed in the early stages.
Proper dissemination of knowledge concerning the effects of cotton and other organic dusts requires the establishment of appropriate training programmes. These should be directed not only towards workers with potential exposure but also towards employers and health personnel, particularly occupational health inspectors and engineers. Information must include source identification, symptoms and disease description, and methods of protection. An informed worker can more readily recognize work-related symptoms and communicate more effectively to a health care provider. Regarding health surveillance and screening, questionnaires are a major instrument to be used. Several versions of questionnaires specifically designed for diagnosing diseases induced by organic dust have been reported in the literature (Rylander, Peterson and Donham 1990; Schwartz et al. 1995). Lung function testing is also a useful tool for surveillance and diagnosis. Measurements of airway responsiveness have been found to be useful (Rylander and Bergström 1993; Carvalheiro et al. 1995). Other diagnostic tools such as measurements of inflammatory mediators or cell activity are still in the research phase.
Beryllium Disease
Beryllium disease is a systemic disorder involving multiple organs, with pulmonary manifestations being most prominent and common. It occurs on exposure to beryllium in its alloy form or in one of its various chemical compounds. Route of exposure is by inhalation and the disease can be either acute or chronic. Acute disease is extremely rare currently, and none has been reported since the first widespread industrial use of beryllium in the 1940s after industrial hygiene measures had been implemented to limit high-dose exposures. Chronic beryllium disease continues to be reported.
Beryllium, Alloys and Compounds
Beryllium, an industrial substance suspected of having carcinogenic potential, is notable for its lightness in weight, high tensile strength and corrosion resistance. Table 1 outlines the properties of beryllium and its compounds.
Table 1. Properties of beryllium and its compounds
|
Formula |
Specific |
Melting/boiling point (ºC) |
Solubility |
Description |
|
|
Beryllium (Be) |
9.01 (a.w.) |
1.85 |
1,298±5/2,970 |
— |
Grey to silver metal |
|
Beryllium oxide (BeO) |
25 |
3.02 |
2,530±30/— |
Soluble in acids and alkalis; insoluble in water |
White amorphous powder |
|
Beryllium fluoride1 (BeF2 ) |
47.02 |
1.99 |
Sublimes 800 °C |
Readily soluble in water; sparingly soluble in ethyl alcohol |
Hygroscopic solid |
|
Beryllium chloride2 (BeCl2 ) |
79.9 |
1.90 |
405/520 |
Very soluble in water; soluble in ethyl alcohol, benzene, ethyl ether and carbon disulphide |
White or slightly yellow deliquescent crystals |
|
Beryllium nitrate3 (Be(NO3 )2 ·3H2 O) |
187.08 |
1.56 |
60/142 |
Soluble in water and ethyl alcohol |
White to faintly yellow deliquescent crystals |
|
Beryllium nitride4 (Be3 N2 ) |
55.06 |
— |
2,200±100/— |
— |
Hard, refractory white crystals |
|
Beryllium sulphate |
177.2 |
1.71 |
100/— |
Soluble in water; insoluble in ethyl alcohol |
Colourless crystals |
1 Beryllium fluoride is made by the decompensation at 900–950 ºC of ammonium beryllium fluoride. Its main use is in the production of beryllium metal by reduction with magnesium.
2 Beryllium chloride is manufactured by passing chlorine over a mixture of beryllium oxide and carbon.
3 Beryllium nitrate is produced by the action of nitric acid on beryllium oxide. It is used as a chemical reagent and as a gas mantle hardener.
4 Beryllium nitride is prepared by heating beryllium metal powder in an oxygen-free, nitrogen atmosphere at 700–1,400 ºC. It is used in atomic energy reactions, including the production of the radioactive carbon isotope carbon-14.
5 Beryllium sulphate hydrate is produced by treating the fritted ore with concentrated suphuric acid.It is used in the production of metallic beryllium by the sulphate process.
Sources
Beryl (3BeO·Al2O3·6SiO2) is the chief commercial source of beryllium, the most abundant of the minerals containing high concentrations of beryllium oxide (10 to 13%). Major sources of beryl are to be found in Argentina, Brazil, India, Zimbabwe and the Republic of South Africa. In the United States, beryl is found in Colorado, South Dakota, New Mexico and Utah. Bertrandite, a low-grade ore (0.1 to 3%) with an acid-soluble beryllium content, is now being mined and processed in Utah.
Production
The two most important methods of extracting beryllium from the ore are the sulphate process and the fluoride process.
In the sulphate process, crushed beryl is melted in an arc furnace at 1,65°C and poured through a high-velocity water stream to form a frit. After heat treatment, the frit is ground in a ball mill and mixed with concentrated sulphuric acid to form a slurry, which is sprayed in the form of a jet into a directly heated, rotating sulphating mill. The beryllium, now in a water-soluble form, is leached from the sludge, and ammonium hydroxide is added to the leach liquor, which is then fed to a crystallizer where ammonium alum is crystallized out. Chelating agents are added to the liquor to hold iron and nickel in solution, sodium hydroxide is then added, and the sodium beryllate thus formed is hydrolyzed to precipitate beryllium hydroxide. The latter product may be converted to beryllium fluoride for reduction by magnesium to metallic beryllium, or to beryllium chloride for electrolytic reduction.
In the fluoride process (figure 1) a briquetted mixture of ground ore, sodium silicofluoride and soda ash is sintered in a rotating hearth furnace. The sintered material is crushed, milled and leached. Sodium hydroxide is added to the solution of beryllium fluoride thus obtained and the precipitate of beryllium hydroxide is filtered in a rotary filter. Metallic beryllium is obtained as in the previous process by the magnesium reduction of beryllium fluoride or by electrolysis of beryllium chloride.
Figure 1. Production of beryllium oxide by the fluoride process
Uses
Beryllium is used in alloys with a number of metals including steel, nickel, magnesium, zinc and aluminium, the most widely used alloy being beryllium-copper—properly called “a bronze”—which has a high tensile strength and a capacity for being hardened by heat treatment. Beryllium bronzes are used in non-spark tools, electrical switch parts, watch springs, diaphragms, shims, cams and bushings.
One of the largest uses of the metal is as a moderator of thermal neutrons in nuclear reactors and as a reflector to reduce the leakage of neutrons from the reactor core. A mixed uranium-beryllium source is often used as a neutron source. As a foil, beryllium is used as window material in x-ray tubes. Its lightness, high elastic modulus and heat stability make it an attractive material for the aircraft and aerospace industry.
Beryllium oxide is made by heating beryllium nitrate or hydroxide.
It is used in the manufacture of ceramics, refractory materials and other beryllium compounds. It was used for the manufacture of phosphors for fluorescent lamps until the incidence of beryllium disease in the industry caused its use for this purpose to be abandoned (in 1949 in the United States).
Hazards
Fire and health hazards are associated with processes involving beryllium. Finely divided beryllium powder will burn, the degree of combustibility being a function of particle size. Fires have occurred in dust filtration units and during the welding of ventilation ducting in which finely divided beryllium was present.
Beryllium and its compounds are highly toxic substances. Beryllium can affect all organ systems, although the primary organ involved is the lung. Beryllium causes systemic disease by inhalation and can distribute itself widely throughout the body after absorption from the lungs. Little beryllium is absorbed from the gastro-intestinal tract. Beryllium can cause skin irritation and its traumatic introduction into subcutaneous tissue can cause local irritation and granuloma formation.
Pathogenesis
Beryllium in all its forms, except for beryl ore, has been associated with disease. The route of entry is by inhalation and in the acute disease there is a direct toxic effect on both the nasopharyngeal mucosa and that of the entire tracheobronchial tree as well, causing oedema and inflammation. In the lung it causes an acute chemical pneumonitis. The major form of beryllium toxicity at this point in time is chronic beryllium disease. A beryllium-specific delayed type of hypersensitivity is the major pathway of chronic disease. The entry of beryllium into the system through the lungs leads to proliferation of specific CD+ lymphocytes, with beryllium acting as a specific antigen, either alone or as a hapten through an interleukin-2 (IL2) receptor pathway. Individual susceptibility to beryllium thus can be explained on the basis of the individual CD+ response. Release of lymphokines from the activated lymphocytes then can lead to granuloma formation and macrophage recruitment. Beryllium can be transported to sites outside the lung where it can cause granuloma formation. Beryllium is released slowly from different sites and it is excreted by the kidneys. This slow release can occur over a span of 20 to 30 years. The chronicity and latency of disease can probably be explained on the basis of the slow metabolism and release phenomenon. The immune mechanisms involved in the pathogenesis of beryllium disease also allow for specific approaches to diagnosis, which will be discussed below.
Histopathology
The primary pathological finding in beryllium disease is the formation of non-caseating granulomas in the lungs, lymph nodes and at other sites. Histopathological studies of lungs in patients with acute beryllium disease have shown a non-specific pattern of acute and subacute bronchitis and pneumonitis. In chronic beryllium disease, there are varying degrees of lymphocytic infiltration of the lung interstitium and non-caseating granuloma formation (figure 2).
Figure 2. Lung tissue in a patient with chronic beryllium disease
Both granulomas and round cell infiltration are visible
Many of the granulomas are located in the peribronchiolar areas. In addition, there can be histiocytes, plasma cells and giant cells with calcific inclusion bodies. If it is a case solely of granuloma formation, the long-term prognosis is better. The histology of the lung in chronic beryllium disease is indistinguishable from that of sarcoidosis. Non-caseating granulomas are also found in lymph nodes, liver, spleen, muscle and skin.
Clinical Manifestations
Skin injuries
Acid salts of beryllium cause allergic contact dermatitis. Such lesions may be erythematous, papular or papulovesicular, are commonly pruritic, and are found on exposed parts of the body. There is usually a delay of 2 weeks from first exposure to occurrence of the dermatitis, except in the case of heavy exposures, when an irritant reaction may be immediate. This delay is regarded as the time required to develop the hypersensitive state.
Accidental implantation of beryllium metal or crystals of a soluble beryllium compound in an abrasion, a crack in the skin or under the nail may cause an indurated area with central suppuration. Granulomas can also form at such sites.
Conjunctivitis and dermatitis may occur alone or together. In cases of conjunctivitis, periorbital oedema may be severe.
Acute disease
Beryllium nasopharyngitis is characterized by swollen and hyperaemic mucous membranes, bleeding points, fissures and ulceration. Perforation of the nasal septum has been described. Removal from exposure results in reversal of this inflammatory process within 3 to 6 weeks.
Involvement of the trachea and bronchial tree following exposure to higher levels of beryllium causes non-productive cough, substernal pain and moderate shortness of breath. Rhonchi and/or rales may be audible, and the x ray of the chest may show increased bronchovascular markings. The character and speed of onset and the severity of these signs and symptoms depend on the quality and quantity of exposure. Recovery is to be expected within 1 to 4 weeks if the worker is removed from further exposure.
The use of steroids is quite useful in countering the acute disease. No new cases of acute disease have been reported to the US Beryllium Case Registry in over 30 years. The Registry, which was started by Harriet Hardy in 1952, has almost 1,000 case records, among which are listed 212 acute cases. Almost all of these occurred in the fluorescent lamp manufacturing industry. Forty-four subjects with the acute disease subsequently developed chronic disease.
Chronic beryllium disease
Chronic beryllium disease is a pulmonary and systemic granulomatous disease caused by inhalation of beryllium. The latency of the disease can be from 1 to 30 years, most commonly occurring 10 to 15 years after first exposure. Chronic beryllium disease has a variable course with exacerbations and remissions in its clinical manifestations. However, the disease is usually progressive. There have been a few cases with chest x-ray abnormalities with a stable clinical course and without significant symptoms.
Exertional dyspnoea is the most common symptom of chronic beryllium disease. Other symptoms are cough, fatigue, weight loss, chest pain and arthralgias. Physical findings may be entirely normal or may include bibasilar crackles, lymphadenopathy, skin lesions, hepatosplenomegaly and clubbing. Signs of pulmonary hypertension may be present in severe, long-standing disease.
Renal stones and hyperuricaemia can occur in some patients and there have been rare reports of parotid gland enlargement and central nervous system involvement. The clinical manifestations of chronic beryllium disease are very similar to those of sarcoidosis.
Roentgenologic features
The x-ray pattern in chronic beryllium disease is non-specific and is similar to that which may be observed in sarcoidosis, idiopathic pulmonary fibrosis, tuberculosis, mycoses and dust disease (figure 3). Early in the course of the disease films may show granular, nodular or linear densities. These abnormalities may increase, decrease or remain unchanged, with or without fibrosis. Upper-lobe involvement is common. Hilar adenopathy, seen in approximately one-third of patients, is usually bilateral and accompanied by mottling of the lung fields. The absence of lung changes in the presence of adenopathy is a relative but not an absolute differential consideration in favour of sarcoidosis as opposed to chronic beryllium disease. Unilateral hilar adenopathy has been reported, but is quite rare.
Figure 3. Chest roentgenograph of a patient with chronic beryllium disease, showing diffuse fibronodular infiltrates and prominent hila
The x-ray picture does not correlate well with clinical status and does not reflect particular qualitative or quantitative aspects of the causal exposure.
Pulmonary function tests
Data from the Beryllium Case Registry show that 3 patterns of impairment may be found in chronic beryllium disease. Of 41 patients studied over a period of an average of 23 years after initial beryllium exposure, 20% had a restrictive defect, 36% had an interstitial defect (normal lung volumes and air flow rates but reduced diffusing capacity for carbon monoxide), 39% had an obstructive defect and 5% were normal. The obstructive pattern, which occurred in both smokers and non-smokers, was associated with granulomas in the peribronchial region. This study indicated that the pattern of impairment affects prognosis. Patients with interstitial defect fared best, with the least deterioration over a five-year interval. Patients with obstructive and restrictive defects experienced worsening of their impairment in spite of corticosteroid therapy.
Studies of lung function in beryllium extraction workers who were asymptomatic showed the presence of mild arterial hypoxaemia. This occurred usually within the first 10 years of exposure. In workers exposed to beryllium for 20 years or more there was a reduction in the forced vital capacity (FVC) and the forced expiratory volume in one second (FEV1). These findings suggest that the initial mild hypoxaemia could be due to the early alveolitis and that with further exposure and elapse of time the reduction in FEV1 and FVC could represent fibrosis and granuloma formation.
Other laboratory tests
Non-specific abnormal laboratory tests have been reported in chronic beryllium disease and include elevated sedimentation rate, erythrocytosis, increased gammaglobulin levels, hyperuricaemia and hypercalcaemia.
The Kveim skin test is negative in beryllium disease, whereas it may be positive in sarcoidosis. The angiotensin converting enzyme (ACE) level is usually normal in beryllium disease, but can be increased in 60% or more of patients with active sarcoidosis.
Diagnosis
Diagnosis of chronic beryllium disease for many years was based on the criteria developed through the Beryllium Case Registry, which included:
- a history of significant beryllium exposure
- evidence of lower respiratory tract disease
- abnormal chest x ray with interstitial fibronodular disease
- abnormal lung function tests with decreased carbon monoxide diffusing capacity (DLCO)
- pathological changes consistent with beryllium exposure in lung or thoracic lymph nodes
- the presence of beryllium in tissue.
Four of the six criteria had to be met and should have included either (1) or (6). Since the 1980s, advances in immunology have made it possible to make the diagnosis of beryllium disease without requiring tissue specimens for histological examination or beryllium analysis. The transformation of lymphocytes in blood in response to beryllium exposure (as in the lymphocyte transformation test, LTT) or lymphocytes from bronchoalveolar lavage (BAL) have been proposed by Newman et al. (1989) as useful diagnostic tools in making the diagnosis of beryllium disease in exposed subjects. Their data suggest that a positive blood LTT is indicative of sensitization. However, recent data show that the blood LTT does not correlate well with pulmonary disease. The BAL lymphocyte transformation correlates much better with abnormal pulmonary function and does not correlate well with concurrent abnormalities in the blood LTT. Thus, to make a diagnosis of beryllium disease, one needs a combination of clinical, radiological and lung function abnormalities and a positive LTT in the BAL. A positive blood LTT by itself is not diagnostic. Microprobe analysis of small tissue samples for beryllium is another recent innovation which could help in diagnosis of disease in small lung tissue samples obtained by transbronchial lung biopsy.
Sarcoidosis is the disorder most closely resembling chronic beryllium disease, and the differentiation may be difficult. Thus far, no cystic bone disease or involvement of the eye or tonsil has appeared in chronic beryllium disease. Similarly, the Kveim test is negative in beryllium disease. Skin testing to demonstrate beryllium sensitization is not recommended, in that the test itself is sensitizing, may possibly trigger systemic reactions in sensitized people and does not of itself establish that the presenting disease is necessarily beryllium related.
More sophisticated immunological approaches in differential diagnosis should allow for better differentiation from sarcoidosis in the future.
Prognosis
The prognosis of chronic beryllium disease has altered favourably during the years; it has been suggested that the longer delays in onset observed among beryllium workers may reflect lower exposure or lower beryllium body burden, resulting in a milder clinical course. Clinical evidence is that steroid therapy, if used when measurable disability first appears, in adequate doses for long enough periods, has improved the clinical status of many patients, allowing some of them to return to useful jobs. There is no clear evidence that steroids have cured chronic beryllium poisoning.
Beryllium and cancer
In animals, experimentally administered beryllium is a carcinogen, causing osteogenic sarcoma after intravenous injection in rabbits and lung cancer after inhalation in rats and monkeys. Whether beryllium may be a human carcinogen is a controversial issue. Some epidemiological studies have suggested an association, particularly after acute beryllium disease. This finding has been disputed by others. One can conclude that beryllium is carcinogenic in animals and there may be a link between lung cancer and beryllium in humans, particularly in those with the acute disease.
Safety and Health Measures
Safety and health precautions must cover the fire hazard as well as the much more serious toxicity danger.
Fire prevention
Arrangements must be made to prevent possible sources of ignition, such as the sparking or arcing of electrical apparatus, friction, and so forth, in the vicinity of finely divided beryllium powder. Equipment in which this powder has been present should be emptied and cleaned before acetylene or electrical welding apparatus is used on it. Oxide-free, ultrafine beryllium powder that has been prepared in inert gas is liable to ignite spontaneously on exposure to air.
Suitable dry powder—not water—should be used to extinguish a beryllium fire. Full personal protective equipment, including respiratory protective equipment, should be worn and firefighters should bathe afterwards and arrange for their clothing to be laundered separately.
Health protection
Beryllium processes must be conducted in a carefully controlled manner to protect both the worker and the general population. The main risk takes the form of airborne contamination and the process and plant should be designed to give rise to as little dust or fume as possible. Wet processes should be used instead of dry processes, and the ingredients of beryllium-containing preparations should be unified as aqueous suspensions instead of as dry powders; whenever possible the plant should be designed as groups of separate enclosed units. The permissible concentration of beryllium in the atmosphere is so low that enclosure must be applied even to wet processes, otherwise escaping splashes and spills can dry out and the dust can enter the atmosphere.
Operations from which dust may be evolved should be conducted in areas with maximum degree of enclosure consistent with the needs of manipulation. Some operations are performed in glove boxes, but many more are conducted in enclosures provided with exhaust ventilation similar to that installed in chemical fume cupboards. Machining operations may be ventilated by high-velocity, low-volume local exhaust systems or by hooded enclosures with exhaust ventilation.
To check the effectiveness of these precautionary measures atmosphere monitoring should be done in such a manner that the daily average exposure of workers to respirable beryllium can be calculated. The work area should be cleaned regularly by means of a proper vacuum cleaner or a wet mop. Beryllium processes should be segregated from the other operations in the factory.
Personal protective equipment should be provided for workers engaged in beryllium processes. Where they are fully employed in processes involving the manipulation of beryllium compounds or in processes associated with the extraction of the metal from the ore, provision should be made for a complete change of clothing so that the workers do not go home wearing clothing in which they have been working. Arrangements should be made for the safe laundering of such working clothes, and protective overalls should be provided even to laundry workers to ensure that they too are not exposed to risk. These arrangements should not be left to normal home laundering procedures. Cases of beryllium poisoning in the families of workers have been attributed to workers taking contaminated clothing home or wearing them in the home.
An occupational health standard of 2μg/m3, proposed in 1949 by a committee operating under the auspices of the US Atomic Energy Commission, continues to be widely observed. Existing interpretations generally permit fluctuations to a “ceiling” of 5μg/m3 as long as the time-weighted average is not exceeded. Additionally, an “acceptable maximum peak above the ceiling concentration for an eight-hour shift” of 25μg/m3 for up to 30 min is also permissible. These operational levels are achievable in current industrial practice, and there is no evidence of adverse health experience among persons working in an environment thus controlled. Because of a possible link between beryllium and lung cancer it has been suggested that the allowable limit be reduced to 1μg/m3, but no official action has been taken on this suggestion in the United States.
The population at risk for developing beryllium disease is that which in some manner deals with beryllium in its extraction or subsequent use. However, a few “neighbourhood” cases have been reported from a distance 1 to 2 km from beryllium extraction plants.
Pre-employment and periodical medical examinations of workers exposed to beryllium and its compounds are compulsory in a number of countries. Recommended evaluation includes an annual respiratory questionnaire, a chest x ray and lung function tests. With advances in immunology, the LTT may also become a routine evaluation, although at this time not enough data are available to recommend its use routinely. With evidence of beryllium disease, it is unwise to allow a worker to be exposed to beryllium further, even though the workplace meets the threshold criteria for beryllium concentration in the air.
Treatment
The major step in therapy is avoidance of further exposure to beryllium. Corticosteroids are the primary mode of therapy in chronic beryllium disease. Corticosteroids appear to alter the course of disease favourably but do not “cure” it.
Corticosteroids should be started on a daily basis with a relatively high dose of Prednisone of 0.5 to 1 mg per kg or more, and continued until improvement occurs or no further deterioration in clinical or lung function tests occurs. Usually this takes 4 to 6 weeks. Slow reduction of steroids is recommended, and eventually alternate-day therapy may be possible. Steroid therapy ordinarily becomes a lifelong necessity.
Other supportive measures such as supplemental oxygen, diuretics, digitalis and antibiotics (when infection exists) are indicated as the clinical condition of the patient would dictate. Immunization against influenza and pneumococcus should also be considered, as with any patient with chronic respiratory disease.
Pneumoconioses: Definition
The expression pneumoconiosis, from the Greek pneuma (air, wind) and konis (dust) was coined in Germany by Zenker in 1867 to denote changes in the lungs caused by the retention of inhaled dust. Gradually, the need for distinction between the effects of various types of dust became evident. It was necessary to discriminate among mineral or vegetable dust and their microbiological component. Consequently, the Third International Conference of Experts on Pneumoconiosis, organized by the ILO in Sydney in 1950, adopted the following definition: “Pneumoconiosis is a diagnosable disease of the lungs produced by the inhalation of dust, the term ‘dust’ being understood to refer to particulate matter in the solid phase, but excluding living organisms.”
However, the word disease seems to imply some degree of health impairment which may not be the case with pneumoconioses not connected with the development of lung fibrosis/scarring. In general, the reaction of lung tissue to the presence of dust varies with different dusts. Non-fibrogenic dusts evoke a tissue reaction in lungs characterized by minimal fibrotic reaction and absence of lung function impairment. Such dusts, examples of which are finely divided dusts of kaolinite, titanium dioxide, stannous oxide, barium sulphate and ferric oxide, are frequently referred to as biologically inert.
Fibrogenic dust such as silica or asbestos causes a more pronounced fibrogenic reaction resulting in scars in the lung tissue and obvious disease. The division of dusts into fibrogenic and non-fibrogenic varieties is by no means sharp because there are many minerals, notably silicates, which are intermediate in their ability to produce fibrotic lesions in the lungs. Nevertheless, it proved useful for clinical purposes and is reflected in the classification of pneumoconioses.
A new definition of pneumoconioses was adopted at the Fourth International Conference on Pneumoconiosis, Bucharest, 1971: “Pneumoconiosis is the accumulation of dust in the lungs and the tissue reactions to its presence. For the purpose of this definition, ‘dust’ is meant to be an aerosol composed of solid inanimate particles.”
In order to avoid any misinterpretation, the expression non-neoplastic is sometimes added to the words “tissue reaction”.
The Working Group at the Conference made the following comprehensive statement:
The Definition of Pneumoconiosis
Earlier on, in 1950, a definition of pneumoconiosis was established at the 3rd International Conference of Experts on Pneumoconiosis and this has continued to be used until the present time. In the meantime, the development of new technologies has resulted in more occupational risks, particularly those related to the inhalation of airborne contaminants. Increased knowledge in the field of occupational medicine has enabled new pulmonary diseases of occupational origin to be recognized but has also demonstrated the necessity for a re-examination of the definition of pneumoconiosis established in 1950. The ILO therefore arranged for a Working Group to be convened within the framework of the IVth International Pneumoconiosis Conference in order to examine the question of the definition of pneumoconiosis. The Working Group held a general discussion on the matter and proceeded to examine a number of proposals submitted by its members. It finally adopted a new definition of pneumoconiosis which was prepared together with a commentary. This text is reproduced below.
In recent years a number of countries have included under pneumoconiosis, because of socio-economic reasons, conditions which are manifestly not pneumoconiosis, but are nevertheless occupational pulmonary diseases. Under the term “disease” are included for preventive reasons the earliest manifestations which are not necessarily disabling or life shortening. Therefore the Working Group has undertaken to redefine pneumoconiosis as the accumulation of dust in the lungs and the tissue reactions to its presence. For the purpose of this definition, “dust” is meant to be an aerosol composed of solid inanimate particles. From a pathological point of view pneumoconiosis may be divided for the sake of convenience into collagenous or non-collagenous forms. A non-collagenous pneumoconiosis is caused by a non-fibrogenic dust and has the following characteristics:
- the alveolar architecture remains intact
- the stromal reaction is minimal and consists mainly of reticulin fibres
- the dust reaction is potentially reversible.
Examples of non-collagenous pneumoconiosis are those caused by pure dusts of tin oxide (stannosis) and barium sulphate (barytosis).
Collagenous pneumoconiosis is characterised by:
- permanent alteration or destruction of alveolar architecture
- collagenous stromal reaction of moderate to maximal degree, and
- permanent scarring of lung.
Such collagenous pneumoconiosis may be caused by fibrogenic dusts or by an altered tissue response to a non-fibrogenic dust.
Examples of collagenous pneumoconiosis caused by fibrogenic dusts are silicosis and asbestosis, whereas complicated coalworkers’ pneumoconiosis or progressive massive fibrosis (PMF) is an altered tissue response to a relatively non-fibrogenic dust. In practice, the distinction between collagenous and non-collagenous pneumoconiosis is difficult to establish. Continued exposure to the same dust, such as coal dust, may cause transition from a non-collagenous to a collagenous form. Furthermore, exposure to a single dust is now becoming less common and exposures to mixed dusts having different degrees of fibrogenic potential may result in pneumoconiosis which can range from the non-collagenous to the collagenous forms. There are in addition occupational chronic pulmonary diseases which, although they develop from the inhalation of dust are excluded from the pneumoconiosis because the particles are not known to accumulate in the lungs. The following are examples of potentially disabling occupational chronic pulmonary diseases: byssinosis, berylliosis, farmers’ lung, and related diseases. They have one common denominator, namely the aetiologic component of dust has sensitized the pulmonary or bronchial tissue so that if the lung tissue responds, the inflammation tends to be granulomatous and if the bronchial tissue responds, there is apt to be bronchial constriction. Exposures to noxious inhaled materials in certain industries are associated with an increased risk of mortality from carcinoma of the respiratory tract. Examples of such materials are radioactive ores, asbestos and chromates.
Adopted at the IVth ILO International Conference on Pneumoconiosis. Bucharest, 1971.
ILO International Classification of Radiographs of Pneumoconioses
Despite all the national and international energies devoted to their prevention, pneumoconioses are still very present both in industrialized and developing countries, and are responsible for the disability and impairment of many workers. This is why the International Labour Office (ILO), the World Health Organization (WHO) and many national institutes for occupational health and safety continue their fight against these diseases and to propose sustainable programmes for preventing them. For instance, the ILO, the WHO and the US National Institute for Occupational Safety and Health (NIOSH) have proposed in their programmes to work in cooperation on a global fight against silicosis. Part of this programme is based on medical surveillance which includes the reading of thoracic radiographs to help diagnose this pneumoconiosis. This is one example which explains why the ILO, in cooperation with many experts, has developed and updated on a continuous basis a classification of radiographs of pneumoconioses that provides a means for recording systematically the radiographic abnormalities in the chest provoked by the inhalation of dust. The scheme is designed for classifying the appearances of posterio-anterior chest radiographs.
The object of the classification is to codify the radiographic abnormalities of pneumoconioses in a simple, reproducible manner. The classification does not define pathological entities, nor take into account working capacity. The classification does not imply legal definitions of pneumoconioses for compensation purposes, nor imply a level at which compensation is payable. Nevertheless, the classification has been found to have wider uses than anticipated. It is now extensively used internationally for epidemiological research, for the surveillance of those industry occupations and for clinical purposes. Use of the scheme may lead to better international comparability of pneumoconioses statistics. It is also used for describing and recording, in a systematic way, part of the information needed for assessing compensation.
The most important condition for using this system of classification with full value from a scientific and ethical point of view is to read, at all times, films to be classified by systematically referring to the 22 standard films provided in the ILO International Classification set of standard films. If the reader attempts to classify a film without referring to any of the standard films, then no mention of reading according to the ILO International Classification of Radiographs should be made. The possibility of deviating from the classification by over or under reading is so risky that his or her reading should not be used at least for epidemiological research or international comparability of pneumoconioses statistics.
The first classification was proposed for silicosis at the First International Conference of Experts on Pneumoconioses, held in Johannesburg in 1930. It combined both radiographic appearances and impairment of lung functions. In 1958, a new classification based purely on radiographic changes was established (Geneva classification 1958). Since, it has been revised several times, the last time in 1980, always with the objective of providing improved versions to be extensively used for clinical and epidemiological purposes. Each new version of the classification promoted by the ILO has brought modifications and changes based on international experience gained in the use of earlier classifications.
In order to provide clear instructions for the use of the classification, the ILO issued in 1970 a publication entitled International Classification of Radiographs of Pneumoconioses/1968 in the Occupational Safety and Health Series (No. 22). This publication was revised in 1972 as ILO U/C International Classification of Radiographs of Pneumoconioses/1971 and again in 1980 as Guidelines for the use of ILO International Classification of Radiographs of Pneumoconioses, revised edition 1980. The description of standard radiographs is given in table 1.
Table 1. Description of standard radiographs
| 1980 Standard radiographs showing | Small opacities | Pleural thickening | ||||||||||
| Chest wall | ||||||||||||
| Technical quality | Profusion | Shape- size | Extent | Large opacities | Circum- scribed (plaques) | Diffuse | Diaphragm | Costo- phrenic angle obliteration | Pleural calcification | Symbols | Comments | |
| 0/0 (example 1) | 1 | 0/0 | – | – | No | No | No | No | No | No | None | Vascular pattern is well illustrated |
| 0/0 (example 2) | 1 | 0/0 | – | – | No | No | No | No | No | No | None | Also shows vascular pattern, but not as clearly as example 1 |
| 1/1; p/p | 1 | 1/1 | p/p | R L x x x x x x | A | No | No | No | No | No | rp. | Rheumatoid pneumoconiosis in left lower zone. Small opacities are present in all zones, but the profusion in the right-upper zone is typical of (some would say a little more profuse than) that classifiable as category 1/1 |
| 2/2; p/p | 2 | 2/2 | p/p | R L x x x x x x | No | No | No | No | No | No | pi; tb. | Quality defect: radiograph is too light |
| 3/3; p/p | 1 | 3/3 | p/p | R L x x x x x x | No | No | No | No | Yes R L x – | No | ax. | None |
| 1/1; q/q | 1 | 1/1 | q/q | R L x x x x – – | No | No | No | No | No | No | None | Illustrates profusion 1/1 better than shape or size |
| 2/2; q/q | 1 | 2/2 | q/q | R L x x x x x x | No | No | Yes R L x x width: a a extent: 1 1 | No | Yes R L x x | No | None | None |
| 3/3; q/q | 2 | 3/3 | q/q | R L x x x x x x | No | No | No | No | No | No | pi. | Quality defects: poor definition of pleura and cut basal angles |
| 1/1; r/r | 2 | 1/1 | r/r | R L x x x x – – | No | No | No | No | Yes R L – x | No | None | Quality defect: subject movement. Profusion of small opacities is more marked in right lung |
| 2/2; r/r | 2 | 2/2 | r/r | R L x x x x x x | No | No | No | No | No | No | None | Quality defects: radiograph too light and contrast too high. The heart shadow is slightly displaced to the left |
| 3/3; r/r | 1 | 3/3 | r/r | R L x x x x x x | No | No | No | No | No | No | ax; ih. | None |
| 1/1; s/t | 2 | 1/1 | s/t | R L x – x x x x | No | No | No | No | No | No | kl. | Quality defect: cut bases. Kerley lines in lower right zone |
| 2/2; s/s | 2 | 2/2 | s/s | R L – – x x x x | No | No | No | No | No | No | em. | Quality defect: distortion of bases due to shrinking. Emphysema in upper zones |
| 3/3; s/s | 2 | 3/3 | s/s | R L x x x x x x | No | No | Yes R L x x width: a a extent: 3 3 | No | No | No | ho; ih; pi. | Quality defect: radiograph is too light. Honeycomb lung appearance is not marked |
| 1/1; t/t Costophrenic angle obliteration | 1 | 1/1 | t/t | R L – – x x x x | No | No | Yes R L x x width: a a extent: 2 2 | No | Yes R L x – | Yes R L – x extent: 2 | None | This radiograph defines the lower limit for costophrenic angle obliteration. Note shrinkage in lower lung fields |
| 2/2; t/t | 1 | 2/2 | t/t | R L x x x x x x | No | No | Yes R L x x width: a a extent: 1 1 | No | No | No | ih. | Pleural thickening is present in the apices of the lung |
| 3/3; t/t | 1 | 3/3 | t/t | R L x x x x x x | No | No | No | No | No | No | hi; ho; id; ih; tb. | None |
| 1/1; u/u 2/2; u/u 3/3; u/u | – | – | – | – | – | – | – | – | – | – | – | This composite radiograph illustrates the mid-categories of profusion of small opacities classifiable for shape and size as u/u. |
| A | 2 | 2/2 | p/q | R L x x x x x x | A | No | No | No | No | No | No | Quality defects: radiograph is too light and pleural definition is poor |
| B | 1 | 1/2 | p/q | R L x x x x x x | B | No | No | No | No | No | ax; co. | Definition of pleura is slightly imperfect |
| C | 1 | 2/1 | q/t | R L x x x x x x | C | No | No | No | No | No | bu; di; em; es; hi; ih. | The small opacities are difficult to classify because of the presence of the large opacities. Note the left costophrenic angle obliteration. This is not classifiable because it does not reach the lower limit defined by the standard radiograph 1/1; t/t |
| Pleural thickening (circumscribed) | – | – | – | – | – | Yes | No | No | No | No | The pleural thickening present face on, is of indeterminate width, and extent 2 | |
| Pleural thickening (diffuse) | – | – | – | – | – | No | Yes | No | No | Yes | The pleural thickening present in profile, is of width a, and extent 2. Not associated small calcifications | |
| Pleural thickening (calcification) diaphragm | – | – | – | – | – | No | No | Yes | No | Yes | Circumscribed, calcified pleural thickening of extent 2 | |
| Pleural thickening (calcification) chest wall | – | – | – | – | – | Yes | No | No | No | Yes | Calcified and uncalcified pleural thickening present face on, is of indeterminate width, and extent 2 | |
ILO 1980 Classification
The 1980 revision was carried out by the ILO with the cooperation of the Commission of the European Communities, NIOSH and the American College of Radiology. The summary of the classification is given in table 2. It retained the principle of former classifications (1968 and 1971).
Table 2. ILO 1980 International Classification of Radiographs of Pneumoconioses: Summary of details of classification
| Features | Codes | Definitions | |
| Technical quality | |||
| 1 | Good. | ||
| 2 | Acceptable, with no technical defect likely to impair classification of the radiograph of pneumoconiosis. | ||
| 3 | Poor, with some technical defect but still acceptable for classification purposes. | ||
| 4 | Unacceptable. | ||
| Parenchymal abnormalities | |||
| Small opacities | Profusion | The category of profusion is based on assessment of the concentration of opacities by comparison with the standard radiographs. | |
| 0/- 0/0 0/1 1/0 1/1 1/2 2/1 2/2 2/3 3/2 3/3 3/+ | Category O—small opacities absent or less profuse than the lower limit of category 1. Categories 1, 2 and 3—increasing profusion of small opacities as defined by the corresponding standard radiographs. | ||
| Extent | RU RM RL LU LM LL | The zones in which the opacities are seen are recorded. The right (R) and left (L) thorax are both divided into three zones—upper (U), middle (M) and lower (L). The category of profusion is determined by considering the profusion as a whole over the affected zones of the lung and by comparing this with the standard radiographs. | |
| Shape and Size | |||
| Rounded | p/p q/q r/r | The letters p, q and r denote the presence of small, rounded opacities. Three sizes are defined by the appearances on standard radiographs: p = diameter up to about 1.5 mm q = diameter exceeding about 1.5 mm and up to about 3 mm r = diameter exceeding about 3 mm and up to about 10 mm | |
| Irregular | s/s t/t u/u | The letters s, t and u denote the presence of small, irregular opacities. Three sizes are defined by the appearances on standard radiographs: s = width up to about 1.5 mm t = width exceeding about 1.5 mm and up to about 3 mm u = width exceeding 3 mm and up to about 10 mm | |
| Mixed | p/s p/t p/u p/q p/r q/s q/t q/u q/p q/r r/s r/t r/u r/p r/q s/p s/q s/r s/t s/u t/p t/q t/r t/s t/u u/p u/q u/r u/s u/t | For mixed shapes (or sizes) of small opacities, the predominant shape and size is recorded first. The presence of a significant number of another shape and size is recorded after the oblique stroke. | |
| Large opacities | A B C | The categories are defined in terms of the dimensions of the opacities. Category A – an opacity having a greatest diameter exceeding about 10 mm and up to and including 50 mm, or several opacities each greater than about 10 mm, the sum of whose greatest diameters does not exceed about 50 mm. Category B – one or more opacities larger or more numerous than those in category A whose combined area does not exceed the equivalent of the right upper zone. Category C – one or more opacities whose combined area exceeds the equivalent of the right upper zone. | |
| Pleural abnormalities | |||
| Pleural thickening | |||
| Chest wall | Type | Two types of pleural thickening of the chest wall are recognized: circumscribed (plaques) and diffuse. Both types may occur together | |
| Site | R L | Pleural thickening of the chest wall is recorded separately for the right (R) and left (L) thorax. | |
| Width | a b c | For pleural thickening seen along the lateral chest wall the measurement of maximum width is made from the inner line of the chest wall to the inner margin of the shadow seen most sharply at the parenchymal-pleural boundary. The maximum width usually occurs at the inner margin of the rib shadow at its outermost point. a = maximum width up to abut 5 mm b = maximum width over about 5 mm and up to about 10 mm c = maximum width over about 10 mm | |
| Face on | Y N | The presence of pleural thickening seen face-on is recorded even if it can be seen also in profile. If pleural thickening is seen face-on only, width cannot usually be measured. | |
| Extent | 1 2 3 | Extent of pleural thickening is defined in terms of the maximum length of pleural involvement, or as the sum of maximum lengths, whether seen in profile or face-on. 1 = total length equivalent up to one quarter of the projection of the lateral chest wall 2 = total length exceeding one quarter but not one half of the projection of the lateral chest wall 3 = total length exceeding one half of the projection of the lateral chest wall | |
| Diaphragm | Presence | Y N | A plaque involving the diaphragmatic pleura is recorded as present (Y) or absent (N), separately for the right (R) and left (L) thorax. |
| Site | R L | ||
| Costrophrenic angle obliteration | Presence | Y N | The presence (Y) or absence (N) of costophrenic angle obliteration is recorded separately from thickening over other areas, for the right (R) and left (L) thorax. The lower limit for this obliteration is defined by a standard radiograph |
| Site | R L | If the thickening extends up the chest wall, then both costophrenic angle obliteration and pleural thickening should be recorded. | |
| Pleural calcification | Site | The site and extent of pleural calcification are recorded separately for the two lungs, and the extent defined in terms of dimensions. | |
| Chest wall | R L | ||
| Diaphragm | R L | ||
| Other | R L | “Other” includes calcification of the mediastinal and pericardial pleura. | |
| Extent | 1 2 3 | 1 = an area of calcified pleura with greatest diameter up to about 20 mm, or a number of such areas the sum of whose greatest diameters does not exceed about 20 mm. 2 = an area of calcified pleura with greatest diameter exceeding about 20 mm and up to about 100 mm, or a number of such areas the sum of whose greatest diameters exceeds about 20 mm but does not exceed about 100 mm. 3 = an area of calcified pleura with greatest diameter exceeding about 100 mm, or a number of such areas whose sum of greatest diameters exceeds about 100 mm. | |
| Symbols | |||
| It is to be taken that the definition of each of the symbols is preceded by an appropriate word or phrase such as “suspect”, “changes suggestive of”, or “opacities suggestive of”, etc. | |||
| ax | Coalescence of small pneumoconiotic opacities | ||
| bu | Bulla(e) | ||
| ca | Cancer of lung or pleura | ||
| cn | Calcification in small pneumoconiotic opacities | ||
| co | Abnormality of cardiac size or shape | ||
| cp | Cor pulmonale | ||
| cv | Cavity | ||
| di | Marked distortion of the intrathoracic organs | ||
| ef | Effusion | ||
| em | Definite emphysema | ||
| es | Eggshell calcification of hilar or mediastinal lymph nodes | ||
| fr | Fractured rib(s) | ||
| hi | Enlargement of hilar or mediastinal lymph nodes | ||
| ho | Honeycomb lung | ||
| id | Ill-defined diaphragm | ||
| ih | Ill-defined heart outline | ||
| kl | Septal (Kerley) lines | ||
| od | Other significant abnormality | ||
| pi | Pleural thickening in the interlobar fissure of mediastinum | ||
| px | Pneumothorax | ||
| rp | Rheumatoid pneumoconiosis | ||
| tb | Tuberculosis | ||
| Comments | |||
| Presence | Y N | Comments should be recorded pertaining to the classification of the radiograph, particularly if some other cause is thought to be responsible for a shadow which could be thought by others to have been due to pneumoconiosis; also to identify radiographs for which the technical quality may have affected the reading materially. | |
The Classification is based on a set of standard radiographs, a written text and a set of notes (OHS No. 22). There are no features to be seen in a chest radiograph which are pathognomonic of dust exposure. The essential principle is that all appearances which are consistent with those defined and represented in the standard radiographs and the guideline for the use of the ILO International Classification, are to be classified. If the reader believes that any appearance is probably or definitively not dust related, the radiograph should not be classified but an appropriate comment must be added. The 22 standard radiographs have been selected after international trials, in such a way as to illustrate the mid-categories standards of profusion of small opacities and to give examples of category A, B and C standards for large opacities. Pleural abnormalities (diffuse pleural thickening, plaques and obliteration of costophrenic angle) are also illustrated on different radiographs.
Discussion in particular at the Seventh International Pneumoconioses Conference, held in Pittsburgh in 1988, indicated the need for improvement of some parts of the classification, in particular those concerning pleural changes. A discussion group meeting on the revision of the ILO International Classification of Radiographs of Pneumoconioses was convened in Geneva by the ILO in November 1989. The experts made the suggestion that the short classification is of no advantage and can be deleted. As regards pleural abnormalities, the group agreed that this classification would now be divided into three parts: “Diffuse pleural thickening”; “Pleural plaques”; and “Costophrenic angle obliteration”. Diffuse pleural thickening might be divided into chest wall and diaphragm. They were identified according to the six zones—upper, middle and lower, of both right and left lungs. If a pleural thickening is circumscribed, it could be identified as a plaque. All plaques should be measured in centimetres. The obliteration of the costophrenic angle should be systematically noted (whether it exists or not). It is important to identify whether the costophrenic angle is visible or not. This is because of its special importance in relation to pleural diffuse thickening. Whether plaques are classified or not should be merely indicated by a symbol. The flattening of the diaphragm should be recorded by an additional symbol since it is a very important feature in asbestos exposure. The presence of plaques should be recorded in these boxes using the appropriate symbol “c” (calcified) or “h” (hyaline).
A full description of the classification, including its applications and limitation is found in the publication (ILO 1980). The revision of the classification of radiographs is a continuous ILO process, and a revised guideline should be published in the near future (1997-98) taking into account the recommendations of these experts.
" DISCLAIMER: The ILO does not take responsibility for content presented on this web portal that is presented in any language other than English, which is the language used for the initial production and peer-review of original content. Certain statistics have not been updated since the production of the 4th edition of the Encyclopaedia (1998)."