Children categories

36. Barometric Pressure Increased (2)

36. Barometric Pressure Increased
Chapter Editor: T.J.R. Francis
Table of Contents
Working under Increased Barometric Pressure
Eric Kindwall
Dees F. Gorman
Tables
Click a link below to view table in article context.
1. Instructions for compressed-air workers
2. Decompression illness: Revised classification

37. Barometric Pressure Reduced (4)

37. Barometric Pressure Reduced
Chapter Editor: Walter Dümmer
Table of Contents
Figures and Tables
Ventilatory Acclimatization to High Altitude
John T. Reeves and John V. Weil
Physiological Effects of Reduced Barometric Pressure
Kenneth I. Berger and William N. Rom
Health Considerations for Managing Work at High Altitudes
John B. West
Prevention of Occupational Hazards at High Altitudes
Walter Dümmer
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

38. Biological Hazards (4)

38. Biological Hazards
Chapter Editor: Zuheir Ibrahim Fakhri
Table of Contents
Tables
Workplace Biohazards
Zuheir I. Fakhri
Aquatic Animals
D. Zannini
Terrestrial Venomous Animals
J.A. Rioux and B. Juminer
Clinical Features of Snakebite
David A. Warrell
Tables
Click a link below to view table in article context.
1. Occupational settings with biological agents
2. Viruses, bacteria, fungi & plants in the workplace
3. Animals as a source of occupational hazards

39. Disasters, Natural and Technological (12)

39. Disasters, Natural and Technological
Chapter Editor: Pier Alberto Bertazzi
Table of Contents
Tables and Figures
Disasters and Major Accidents
Pier Alberto Bertazzi
ILO Convention concerning the Prevention of Major Industrial Accidents, 1993 (No. 174)
Disaster Preparedness
Peter J. Baxter
Post-Disaster Activities
Benedetto Terracini and Ursula Ackermann-Liebrich
Weather-Related Problems
Jean French
Avalanches: Hazards and Protective Measures
Gustav Poinstingl
Transportation of Hazardous Material: Chemical and Radioactive
Donald M. Campbell
Radiation Accidents
Pierre Verger and Denis Winter
Case Study: What does dose mean?
Occupational Health and Safety Measures in Agricultural Areas Contaminated by Radionuclides: The Chernobyl Experience
Yuri Kundiev, Leonard Dobrovolsky and V.I. Chernyuk
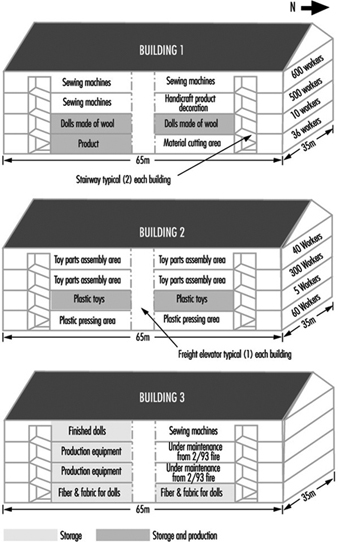
Case Study: The Kader Toy Factory Fire
Casey Cavanaugh Grant
Impacts of Disasters: Lessons from a Medical Perspective
José Luis Zeballos
Tables
Click a link below to view table in article context.
1. Definitions of disaster types
2. 25-yr average # victims by type & region-natural trigger
3. 25-yr average # victims by type & region-non-natural trigger
4. 25-yr average # victims by type-natural trigger (1969-1993)
5. 25-yr average # victims by type-non-natural trigger (1969-1993)
6. Natural trigger from 1969 to 1993: Events over 25 years
7. Non-natural trigger from 1969 to 1993: Events over 25 years
8. Natural trigger: Number by global region & type in 1994
9. Non-natural trigger: Number by global region & type in 1994
10. Examples of industrial explosions
11. Examples of major fires
12. Examples of major toxic releases
13. Role of major hazard installations management in hazard control
14. Working methods for hazard assessment
15. EC Directive criteria for major hazard installations
16. Priority chemicals used in identifying major hazard installations
17. Weather-related occupational risks
18. Typical radionuclides, with their radioactive half-lives
19. Comparison of different nuclear accidents
20. Contamination in Ukraine, Byelorussia & Russia after Chernobyl
21. Contamination strontium-90 after the Khyshtym accident (Urals 1957)
22. Radioactive sources that involved the general public
23. Main accidents involving industrial irradiators
24. Oak Ridge (US) radiation accident registry (worldwide, 1944-88)
25. Pattern of occupational exposure to ionizing radiation worldwide
26. Deterministic effects: thresholds for selected organs
27. Patients with acute irradiation syndrome (AIS) after Chernobyl
28. Epidemiological cancer studies of high dose external irradiation
29. Thyroid cancers in children in Belarus, Ukraine & Russia, 1981-94
30. International scale of nuclear incidents
31. Generic protective measures for general population
32. Criteria for contamination zones
33. Major disasters in Latin America & the Caribbean, 1970-93
34. Losses due to six natural disasters
35. Hospitals & hospital beds damaged/ destroyed by 3 major disasters
36. Victims in 2 hospitals collapsed by the 1985 earthquake in Mexico
37. Hospital beds lost resulting from the March 1985 Chilean earthquake
38. Risk factors for earthquake damage to hospital infrastructure
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.
Click to return to top of page

40. Electricity (3)

40. Electricity
Chapter Editor: Dominique Folliot
Table of Contents
Figures and Tables
Electricity—Physiological Effects
Dominique Folliot
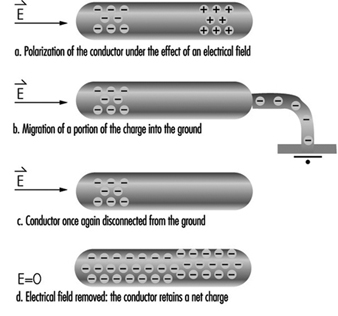
Static Electricity
Claude Menguy
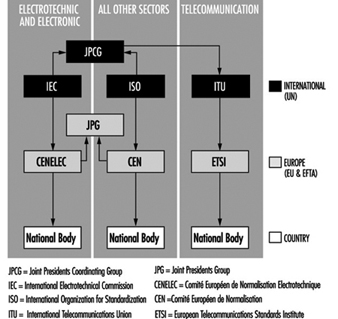
Prevention And Standards
Renzo Comini
Tables
Click a link below to view table in article context.
1. Estimates of the rate of electrocution-1988
2. Basic relationships in electrostatics-Collection of equations
3. Electron affinities of selected polymers
4. Typical lower flammability limits
5. Specific charge associated with selected industrial operations
6. Examples of equipment sensitive to electrostatic discharges
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

41. Fire (6)

41. Fire
Chapter Editor: Casey C. Grant
Table of Contents
Figures and Tables
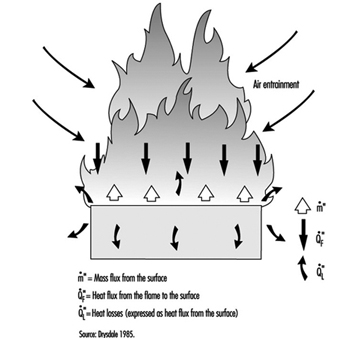
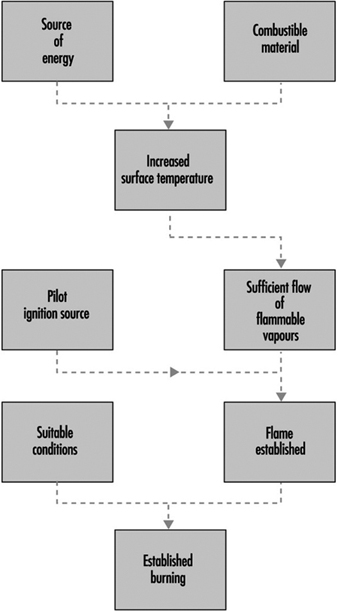
Basic Concepts
Dougal Drysdale
Sources of Fire Hazards
Tamás Bánky
Fire Prevention Measures
Peter F. Johnson
Passive Fire Protection Measures
Yngve Anderberg
Active Fire Protection Measures
Gary Taylor
Organizing for Fire Protection
S. Dheri
Tables
Click a link below to view table in article context.
1. Lower & upper flammability limits in air
2. Flashpoints & firepoints of liquid & solid fuels
3. Ignition sources
4. Comparison of concentrations of different gases required for inerting
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

42. Heat and Cold (12)

42. Heat and Cold
Chapter Editor: Jean-Jacques Vogt
Table of Contents
Figures and Tables
Physiological Responses to the Thermal Environment
W. Larry Kenney
Effects of Heat Stress and Work in the Heat
Bodil Nielsen
Heat Disorders
Tokuo Ogawa
Prevention of Heat Stress
Sarah A. Nunneley
The Physical Basis of Work in Heat
Jacques Malchaire
Assessment of Heat Stress and Heat Stress Indices
Kenneth C. Parsons
Case Study: Heat Indices: Formulae and Definitions
Heat Exchange through Clothing
Wouter A. Lotens
Cold Environments and Cold Work
Ingvar Holmér, Per-Ola Granberg and Goran Dahlstrom
Prevention of Cold Stress in Extreme Outdoor Conditions
Jacques Bittel and Gustave Savourey
Cold Indices and Standards
Ingvar Holmér
Tables
Click a link below to view table in article context.
1. Electrolyte concentration in blood plasma & sweat
2. Heat Stress Index & Allowable Exposure Times: calculations
3. Interpretation of Heat Stress Index values
4. Reference values for criteria of thermal stress & strain
5. Model using heart rate to assess heat stress
6. WBGT reference values
7. Working practices for hot environments
8. Calculation of the SWreq index & assessment method: equations
9. Description of terms used in ISO 7933 (1989b)
10. WBGT values for four work phases
11. Basic data for the analytical assessment using ISO 7933
12. Analytical assessment using ISO 7933
13. Air temperatures of various cold occupational environments
14. Duration of uncompensated cold stress & associated reactions
15. Indication of anticipated effects of mild & severe cold exposure
16. Body tissue temperature & human physical performance
17. Human responses to cooling: Indicative reactions to hypothermia
18. Health recommendations for personnel exposed to cold stress
19. Conditioning programmes for workers exposed to cold
20. Prevention & alleviation of cold stress: strategies
21. Strategies & measures related to specific factors & equipment
22. General adaptational mechanisms to cold
23. Number of days when water temperature is below 15 ºC
24. Air temperatures of various cold occupational environments
25. Schematic classification of cold work
26. Classification of levels of metabolic rate
27. Examples of basic insulation values of clothing
28. Classification of thermal resistance to cooling of handwear
29. Classification of contact thermal resistance of handwear
30. Wind Chill Index, temperature & freezing time of exposed flesh
31. Cooling power of wind on exposed flesh
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

43. Hours of Work (1)

43. Hours of Work
Chapter Editor: Peter Knauth
Table of Contents
Hours of Work
Peter Knauth
Tables
Click a link below to view table in article context.
1. Time intervals from beginning shiftwork until three illnesses
2. Shiftwork & incidence of cardiovascular disorders
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

44. Indoor Air Quality (8)

44. Indoor Air Quality
Chapter Editor: Xavier Guardino Solá
Table of Contents
Figures and Tables
Indoor Air Quality: Introduction
Xavier Guardino Solá
Nature and Sources of Indoor Chemical Contaminants
Derrick Crump
Radon
María José Berenguer
Tobacco Smoke
Dietrich Hoffmann and Ernst L. Wynder
Smoking Regulations
Xavier Guardino Solá
Measuring and Assessing Chemical Pollutants
M. Gracia Rosell Farrás
Biological Contamination
Brian Flannigan
Regulations, Recommendations, Guidelines and Standards
María José Berenguer
Tables
Click a link below to view table in article context.
1. Classification of indoor organic pollutants
2. Formaldehyde emission from a variety of materials
3. Ttl. volatile organic comp’ds concs, wall/floor coverings
4. Consumer prods & other sources of volatile organic comp’ds
5. Major types & concentrations in the urban United Kingdom
6. Field measurements of nitrogen oxides & carbon monoxide
7. Toxic & tumorigenic agents in cigarette sidestream smoke
8. Toxic & tumorigenic agents from tobacco smoke
9. Urinary cotinine in non-smokers
10. Methodology for taking samples
11. Detection methods for gases in indoor air
12. Methods used for the analysis of chemical pollutants
13. Lower detection limits for some gases
14. Types of fungus which can cause rhinitis and/or asthma
15. Micro-organisms and extrinsic allergic alveolitis
16. Micro-organisms in nonindustrial indoor air & dust
17. Standards of air quality established by the US EPA
18. WHO guidelines for non-cancer and non-odour annoyance
19. WHO guideline values based on sensory effects or annoyance
20. Reference values for radon of three organizations
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

45. Indoor Environmental Control (6)

45. Indoor Environmental Control
Chapter Editor: Juan Guasch Farrás
Table of Contents
Figures and Tables
Control of Indoor Environments: General Principles
A. Hernández Calleja
Indoor Air: Methods for Control and Cleaning
E. Adán Liébana and A. Hernández Calleja
Aims and Principles of General and Dilution Ventilation
Emilio Castejón
Ventilation Criteria for Nonindustrial Buildings
A. Hernández Calleja
Heating and Air-Conditioning Systems
F. Ramos Pérez and J. Guasch Farrás
Indoor Air: Ionization
E. Adán Liébana and J. Guasch Farrás
Tables
Click a link below to view table in article context.
1. Most common indoor pollutants & their sources
2. Basic requirements-dilution ventilation system
3. Control measures & their effects
4. Adjustments to working environment & effects
5. Effectiveness of filters (ASHRAE standard 52-76)
6. Reagents used as absorbents for contaminents
7. Levels of quality of indoor air
8. Contamination due to the occupants of a building
9. Degree of occupancy of different buildings
10. Contamination due to the building
11. Quality levels of outside air
12. Proposed norms for environmental factors
13. Temperatures of thermal comfort (based on Fanger)
14. Characteristics of ions
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

46. Lighting (3)

46. Lighting
Chapter Editor: Juan Guasch Farrás
Table of Contents
Figures and Tables
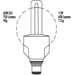
Types of Lamps and Lighting
Richard Forster
Conditions Required for Visual
Fernando Ramos Pérez and Ana Hernández Calleja
General Lighting Conditions
N. Alan Smith
Tables
Click a link below to view table in article context.
1. Improved output & wattage of some 1,500 mm fluorescent tube lamps
2. Typical lamp efficacies
3. International Lamp Coding System (ILCOS) for some lamp types
4. Common colours & shapes of incandescent lamps & ILCOS codes
5. Types of high-pressure sodium lamp
6. Colour contrasts
7. Reflection factors of different colours & materials
8. Recommended levels of maintained illuminance for locations/tasks
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

47. Noise (5)

47. Noise
Chapter Editor: Alice H. Suter
Table of Contents
Figures and Tables
The Nature and Effects of Noise
Alice H. Suter
Noise Measurement and Exposure Evaluation
Eduard I. Denisov and German A. Suvorov
Engineering Noise Control
Dennis P. Driscoll
Hearing Conservation Programmes
Larry H. Royster and Julia Doswell Royster
Standards and Regulations
Alice H. Suter
Tables
Click a link below to view table in article context.
1. Permissible exposure limits (PEL)for noise exposure, by nation
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

48. Radiation: Ionizing (6)

48. Radiation: Ionizing
Chapter Editor: Robert N. Cherry, Jr.
Table of Contents
Introduction
Robert N. Cherry, Jr.
Radiation Biology and Biological Effects
Arthur C. Upton
Sources of Ionizing Radiation
Robert N. Cherry, Jr.
Workplace Design for Radiation Safety
Gordon M. Lodde
Radiation Safety
Robert N. Cherry, Jr.
Planning for and Management of Radiation Accidents
Sydney W. Porter, Jr.

49. Radiation, Non-Ionizing (9)

49. Radiation, Non-Ionizing
Chapter Editor: Bengt Knave
Table of Contents
Tables and Figures
Electric and Magnetic Fields and Health Outcomes
Bengt Knave
The Electromagnetic Spectrum: Basic Physical Characteristics
Kjell Hansson Mild
Ultraviolet Radiation
David H. Sliney
Infrared Radiation
R. Matthes
Light and Infrared Radiation
David H. Sliney
Lasers
David H. Sliney
Radiofrequency Fields and Microwaves
Kjell Hansson Mild
VLF and ELF Electric and Magnetic Fields
Michael H. Repacholi
Static Electric and Magnetic Fields
Martino Grandolfo
Tables
Click a link below to view table in article context.
1. Sources and exposures for IR
2. Retinal thermal hazard function
3. Exposure limits for typical lasers
4. Applications of equipment using range >0 to 30 kHz
5. Occupational sources of exposure to magnetic fields
6. Effects of currents passing through the human body
7. Biological effects of various current density ranges
8. Occupational exposure limits-electric/magnetic fields
9. Studies on animals exposed to static electric fields
10. Major technologies and large static magnetic fields
11. ICNIRP recommendations for static magnetic fields
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

50. Vibration (4)

50. Vibration
Chapter Editor: Michael J. Griffin
Table of Contents
Table and Figures
Vibration
Michael J. Griffin
Whole-body Vibration
Helmut Seidel and Michael J. Griffin
Hand-transmitted Vibration
Massimo Bovenzi
Motion Sickness
Alan J. Benson
Tables
Click a link below to view table in article context.
1. Activities with adverse effects of whole-body vibration
2. Preventive measures for whole-body vibration
3. Hand-transmitted vibration exposures
4. Stages, Stockholm Workshop scale, hand-arm vibration syndrome
5. Raynaud’s phenomenon & hand-arm vibration syndrome
6. Threshold limit values for hand-transmitted vibration
7. European Union Council Directive: Hand-transmitted vibration (1994)
8. Vibration magnitudes for finger blanching
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

51. Violence (1)

51. Violence
Chapter Editor: Leon J. Warshaw
Table of Contents
Violence in the Workplace
Leon J. Warshaw
Tables
Click a link below to view table in article context.
1. Highest rates of occupational homicide, US workplaces, 1980-1989
2. Highest rates of occupational homicide US occupations, 1980-1989
3. Risk factors for workplace homicides
4. Guides for programmes to prevent workplace violence

52. Visual Display Units (11)

52. Visual Display Units
Chapter Editor: Diane Berthelette
Table of Contents
Tables and Figures
Overview
Diane Berthelette
Characteristics of Visual Display Workstations
Ahmet Çakir
Ocular and Visual Problems
Paule Rey and Jean-Jacques Meyer
Reproductive Hazards - Experimental Data
Ulf Bergqvist
Reproductive Effects - Human Evidence
Claire Infante-Rivard
Case Study: A Summary of Studies of Reproductive Outcomes
Musculoskeletal Disorders
Gabriele Bammer
Skin Problems
Mats Berg and Sture Lidén
Psychosocial Aspects of VDU Work
Michael J. Smith and Pascale Carayon
Ergonomic Aspects of Human - Computer Interaction
Jean-Marc Robert
Ergonomics Standards
Tom F.M. Stewart
Tables
Click a link below to view table in article context.
1. Distribution of computers in various regions
2. Frequency & importance of elements of equipment
3. Prevalence of ocular symptoms
4. Teratological studies with rats or mice
5. Teratological studies with rats or mice
6. VDU use as a factor in adverse pregnancy outcomes
7. Analyses to study causes musculoskeletal problems
8. Factors thought to cause musculoskeletal problems
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.
Avalanches: Hazards and Protective Measures
Ever since people began to settle in mountainous regions, they have been exposed to the specific hazards associated with mountain living. Among the most treacherous hazards are avalanches and landslides, which have taken their toll of victims even up to the present day.
When the mountains are covered with several feet of snow in winter, under certain conditions, a mass of snow lying like a thick blanket on the steep slopes or mountain tops can become detached from the ground underneath and slide downhill under its own weight. This can result in huge quantities of snow hurtling down the most direct route and settling into the valleys below. The kinetic energy thus released produces dangerous avalanches, which sweep away, crush or bury everything in their path.
Avalanches can be divided into two categories according to the type and condition of the snow involved: dry snow or “dust” avalanches, and wet snow or “ground” avalanches. The former are dangerous because of the shock waves they set off, and the latter because of their sheer volume, due to the added moisture in the wet snow, flattening everything as the avalanche rolls downhill, often at high speeds, and sometimes carrying away sections of the subsoil.
Particularly dangerous situations can arise when the snow on large, exposed slopes on the windward side of the mountain is compacted by the wind. Then it often forms a cover, held together only on the surface, like a curtain suspended from above, and resting on a base that can produce the effect of ball-bearings. If a “cut” is made in such a cover (e.g., if a skier leaves a track across the slope), or if for any reason, this very thin cover is torn apart (e.g., by its own weight), then the whole expanse of snow can slide downhill like a board, usually developing into an avalanche as it progresses.
In the interior of the avalanche, enormous pressure can build up, which can carry off, smash or crush locomotives or entire buildings as though they were toys. That human beings have very little chance of surviving in such an inferno is obvious, bearing in mind that anyone who is not crushed to death is likely to die from suffocation or exposure. It is not surprising, therefore, in cases where people have been buried in avalanches, that, even if they are found immediately, about 20% of them are already dead.
The topography and vegetation of the area will cause the masses of snow to follow set routes as they come down to the valley. People living in the region know this from observation and tradition, and therefore keep away from these danger zones in the winter.
In earlier times, the only way to escape such dangers was to avoid exposing oneself to them. Farmhouses and settlements were built in places where topographical conditions were such that avalanches could not occur, or which years of experience had shown to be far removed from any known avalanche paths. People even avoided the mountain areas altogether during the danger period.
Forests on the upper slopes also afford considerable protection against such natural disasters, as they support the masses of snow in the threatened areas and can curb, halt or divert avalanches that have already started, provided they have not built up too much momentum.
Nevertheless, the history of mountainous countries is punctuated by repeated disasters caused by avalanches, which have taken, and still take, a heavy toll of life and property. On the one hand, the speed and momentum of the avalanche is often underestimated. On the other hand, avalanches will sometimes follow paths which, on the basis of centuries of experience, have not previously been considered to be avalanche paths. Certain unfavourable weather conditions, in conjunction with a particular quality of snow and the state of the ground underneath (e.g., damaged vegetation or erosion or loosening of the soil as a result of heavy rains) produce circumstances that can lead to one of those “disasters of the century”.
Whether an area is particularly exposed to the threat of an avalanche depends not only on prevailing weather conditions, but to an even greater extent on the stability of the snow cover, and on whether the area in question is situated in one of the usual avalanche paths or outlets. There are special maps showing areas where avalanches are known to have occurred or are likely to occur as a result of topographical features, especially the paths and outlets of frequently occurring avalanches. Building is prohibited in high-risk areas.
However, these precautionary measures are no longer sufficient today, as, despite the prohibition of building in particular areas, and all the information available on the dangers, increasing numbers of people are still attracted to picturesque mountain regions, causing more and more building even in areas known to be dangerous. In addition to this disregard or circumvention of building bans, one of the manifestations of the modern leisure society is that thousands of tourists go to the mountains for sport and recreation in winter, and to the very areas where avalanches are virtually pre-programmed. The ideal ski slope is steep, free of obstacles and should have a sufficiently thick carpet of snow—ideal conditions for the skier, but also for the snow to sweep down into the valley.
If, however, risks cannot be avoided or are to a certain extent consciously accepted as an unwelcome “side-effect” of the enjoyment gained from the sport, then it becomes necessary to develop ways and means of coping with these dangers in another manner.
To improve the chances of survival for people buried in avalanches, it is essential to provide well-organized rescue services, emergency telephones near the localities at risk and up-to-date information for the authorities and for tourists on the prevailing situation in dangerous areas. Early warning systems and excellent organization of rescue services with the best possible equipment can considerably increase chances of survival for people buried in avalanches, as well as reducing the extent of the damage.
Protective Measures
Various methods of protection against avalanches have been developed and tested all over the world, such as cross-frontier warning services, barriers and even the artificial triggering-off of avalanches by blasting or firing guns over the snow fields.
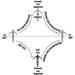
The stability of the snow cover is basically determined by the ratio of mechanical stress to density. This stability can vary considerably according to the type of stress (e.g., pressure, tension, shearing strain) within a geographical region (e.g., that part of the snow field where an avalanche might start). Contours, sunshine, winds, temperature and local disturbances in the structure of the snow cover—resulting from rocks, skiers, snowploughs or other vehicles—can also affect stability. Stability can therefore be reduced by deliberate local intervention such as blasting, or increased by the installation of additional supports or barriers. These measures, which can be of a permanent or temporary nature, are the two main methods used for protection against avalanches.
Permanent measures include effective and durable structures, support barriers in the areas where the avalanche might start, diversionary or braking barriers on the avalanche path, and blocking barriers in the avalanche outlet area. The object of temporary protective measures is to secure and stabilize the areas where an avalanche might start by deliberately triggering off smaller, limited avalanches to remove the dangerous quantities of snow in sections.
Support barriers artificially increase the stability of the snow cover in potential avalanche areas. Drift barriers, which prevent additional snow from being carried by the wind to the avalanche area, can reinforce the effect of support barriers. Diversionary and braking barriers on the avalanche path and blocking barriers in the avalanche outlet area can divert or slow down the descending mass of snow and shorten the outflow distance in front of the area to be protected. Support barriers are structures fixed in the ground, more or less perpendicular to the slope, which put up sufficient resistance to the descending mass of snow. They must form supports reaching up to the surface of the snow. Support barriers are usually arranged in several rows and must cover all parts of the terrain from which avalanches could, under various possible weather conditions, threaten the locality to be protected. Years of observation and snow measurement in the area are required in order to establish correct positioning, structure and dimensions.
The barriers must have a certain permeability to let minor avalanches and surface landslides flow through a number of barrier rows without getting larger or causing damage. If permeability is not sufficient, there is the danger that the snow will pile up behind the barriers, and subsequent avalanches will slide over them unimpeded, carrying further masses of snow with them.
Temporary measures, unlike the barriers, can also make it possible to reduce the danger for a certain length of time. These measures are based on the idea of setting off avalanches by artificial means. The threatening masses of snow are removed from the potential avalanche area by a number of small avalanches deliberately triggered off under supervision at selected, predetermined times. This considerably increases the stability of the snow cover remaining on the avalanche site, by at least reducing the risk of further and more dangerous avalanches for a limited period of time when the threat of avalanches is acute.
However, the size of these artificially produced avalanches cannot be determined in advance with any great degree of accuracy. Therefore, in order to keep the risk of accidents as low as possible, while these temporary measures are being carried out, the entire area to be affected by the artificial avalanche, from its starting point to where it finally comes to a halt, must be evacuated, closed off and checked beforehand.
The possible applications of the two methods of reducing hazards are fundamentally different. In general, it is better to use permanent methods to protect areas that are impossible or difficult to evacuate or close off, or where settlements or forests could be endangered even by controlled avalanches. On the other hand, roads, ski runs and ski slopes, which are easy to close off for short periods, are typical examples of areas in which temporary protective measures can be applied.
The various methods of artificially setting off avalanches involve a number of operations which also entail certain risks and, above all, require additional protective measures for persons assigned to carry out this work. The essential thing is to cause initial breaks by setting off artificial tremors (blasts). These will sufficiently reduce the stability of the snow cover to produce a snow-slip.
Blasting is especially suitable for releasing avalanches on steep slopes. It is usually possible to detach small sections of snow at intervals and thus avoid major avalanches, which take a long distance to run their course and can be extremely destructive. However, it is essential that the blasting operations be carried out at any time of day and in all types of weather, and this is not always possible. Methods of artificially producing avalanches by blasting differ considerably according to the means used to reach the area where the blasting is to take place.
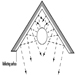
Areas where avalanches are likely to start can be bombarded with grenades or rockets from safe positions, but this is successful (i.e., produces the avalanche) in only 20 to 30% of cases, as it is virtually impossible to determine and to hit the most effective target point with any accuracy from a distance, and also because the snow cover absorbs the shock of the explosion. Besides, shells may fail to go off.
Blasting with commercial explosives directly into the area where avalanches are likely to start is generally more successful. The most successful methods are those whereby the explosive is carried on stakes or cables over the part of the snow field where the avalanche is to start, and detonated at a height of 1.5 to 3 m above the snow cover.
Apart from the shelling of the slopes, three different methods have been developed for getting the explosive for the artificial production of avalanches to the actual location where the avalanche is to start:
- dynamite cableways
- blasting by hand
- throwing or lowering the explosive charge from helicopters.
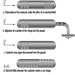
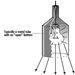
The cableway is the surest and at the same time the safest method. With the help of a special small cableway, the dynamite cableway, the explosive charge is carried on a winding rope over the blasting location in the area of snow cover in which the avalanche is to start. With proper rope control and with the help of signals and markings, it is possible to steer accurately towards what are known from experience to be the most effective locations, and to get the charge to explode directly above them. The best results with respect to triggering off avalanches are achieved when the charge is detonated at the correct height above the snow cover. Since the cableway runs at a greater height above the ground, this requires the use of lowering devices. The explosive charge hangs from a string wound around the lowering device. The charge is lowered to the correct height above the site selected for the explosion with the help of a motor which unwinds the string. The use of dynamite cableways makes it possible to carry out the blasting from a safe position, even with poor visibility, by day or night.
Because of the good results obtained and the relatively low production costs, this method of setting off avalanches is used extensively in the entire Alpine region, a licence being required to operate dynamite cableways in most Alpine countries. In 1988, an intensive exchange of experience in this field took place between manufacturers, users and government representatives from the Austrian, Bavarian and Swiss Alpine areas. The information gained from this exchange of experience has been summarized in leaflets and legally binding regulations. These documents basically contain the technical safety standards for equipment and installations, and instructions on carrying out these operations safely. When preparing the explosive charge and operating the equipment, the blasting crew must be able to move as freely as possible around the various cableway controls and appliances. There must be safe and easily accessible footpaths to enable the crew to leave the site quickly in case of emergency. There must be safe access routes up to cableway supports and stations. In order to avoid failure to explode, two fuses and two detonators must be used for every charge.
In the case of blasting by hand, a second method for artificially producing avalanches, which was frequently done in earlier times, the dynamiter has to climb to the part of the snow cover where the avalanche is to be set off. The explosive charge can be placed on stakes planted in the snow, but more generally thrown down the slope towards a target point known from experience to be particularly effective. It is usually imperative for helpers to secure the dynamiter with a rope throughout the entire operation. Nonetheless, however carefully the blasting team proceeds, the danger of falling or of encountering avalanches on the way to the blasting site cannot be eliminated, as these activities often involve long ascents, sometimes under unfavourable weather conditions. Because of these hazards, this method, which is also subject to safety regulations, is rarely used today.
Using helicopters, a third method, has been practised for many years in the Alpine and other regions for operations to set off avalanches. In view of the dangerous risks for persons on board, this procedure is used in most Alpine and other mountainous countries only when it is urgently needed to avert an acute danger, when other procedures cannot be used or would involve even greater risk. In view of the special legal situation arising from the use of aircraft for such purposes and the risks involved, specific guidelines on setting off avalanches from helicopters have been drawn up in the Alpine countries, with the collaboration of the aviation authorities, the institutions and authorities responsible for occupational health and safety, and experts in the field. These guidelines deal not only with matters concerning the laws and regulations on explosives and safety provisions, but also are concerned with the physical and technical qualifications required of persons entrusted with such operations.
Avalanches are set off from helicopters either by lowering the charge on a rope and detonating it above the snow cover or by dropping a charge with its fuse already lit. The helicopters used must be specially adapted and licensed for such operations. With regard to safely carrying out the operations on board, there must be a strict division of responsibilities between the pilot and the blasting technician. The charge must be correctly prepared and the length of fuse selected according to whether it is to be lowered or dropped. In the interests of safety, two detonators and two fuses must be used, as in the case of the other methods. As a rule, the individual charges contain between 5 and 10 kg of explosive. Several charges can be lowered or dropped one after the other during one operational flight. The detonations must be visually observed in order to check that none has failed to go off.
All these blasting processes require the use of special explosives, effective in cold conditions and not sensitive to mechanical influences. Persons assigned to carry out these operations must be specially qualified and have the relevant experience.
Temporary and permanent protective measures against avalanches were originally designed for distinctly different areas of application. The costly permanent barriers were mainly constructed to protect villages and buildings especially against major avalanches. The temporary protective measures were originally limited almost exclusively to protecting roads, ski resorts and amenities which could be easily closed off. Nowadays, the tendency is to apply a combination of the two methods. To work out the most effective safety programme for a given area, it is necessary to analyse the prevailing situation in detail in order to determine the method that will provide the best possible protection.
Transportation of Hazardous Material: Chemical and Radioactive
The industries and economies of nations depend, in part, on the large numbers of hazardous materials transported from the supplier to the user and, ultimately, to the waste disposer. Hazardous materials are transported by road, rail, water, air and pipeline. The vast majority reach their destination safely and without incident. The size and scope of the problem is illustrated by the petroleum industry. In the United Kingdom it distributes around 100 million tons of product every year by pipeline, rail, road and water. Approximately 10% of those employed by the UK chemical industry are involved in distribution (i.e., transport and warehousing).
A hazardous material can be defined as “a substance or material determined to be capable of posing an unreasonable risk to health, safety or property when transported”. “Unreasonable risk” covers a broad spectrum of health, fire and environmental considerations. These substances include explosives, flammable gases, toxic gases, highly flammable liquids, flammable liquids, flammable solids, substances which become dangerous when wet, oxidizing substances and toxic liquids.
The risks arise directly from a release, ignition, and so on, of the dangerous substance(s) being transported. Road and rail threats are those which could give rise to major accidents “which could affect both employees and members of the public”. These dangers can occur when materials are being loaded or unloaded or are en route. The population at risk is people living near the road or railway and the people in other road vehicles or trains who might become involved in a major accident. Areas of risk include temporary stopover points such as railway marshalling yards and lorry parking areas at motorway service points. Marine risks are those linked to ships entering or leaving ports and loading or discharging cargoes there; risks also arise from coastal and straits traffic and inland waterways.
The range of incidents which can occur in association with transport both while in transit and at fixed installations include chemical overheating, spillage, leakage, escape of vapour or gas, fire and explosion. Two of the principal events causing incidents are collision and fire. For road tankers other causes of release may be leaks from valves and from overfilling. Generally, for both road and rail vehicles, non-crash fires are much more frequent than crash fires. These transport-associated incidents can occur in rural, urban industrial and urban residential areas, and can involve both attended and unattended vehicles or trains. Only in the minority of cases is an accident the primary cause of the incident.
Emergency personnel should be aware of the possibility of human exposure and contamination by a hazardous substance in accidents involving railways and rail yards, roads and freight terminals, vessels (both ocean and inland based) and associated waterfront warehouses. Pipelines (both long distance and local utility distribution systems) can be a hazard if damage or leakage occurs, either in isolation or in association with other incidents. Transportation incidents are often more dangerous than those at fixed facilities. The materials involved may be unknown, warning signs may be obscured by rollover, smoke or debris, and knowledgeable operatives may be absent or casualties of the event. The number of people exposed depends on population density, both by day and night, on the proportions indoors and outdoors, and on the proportion who may be considered particularly vulnerable. In addition to the population who are normally in the area, personnel of the emergency services who attend the accident are also at risk. It is not uncommon in an incident involving transport of hazardous materials that a significant proportion of the casualties include such personnel.
In the 20-year period 1971 through 1990, about 15 people were killed on the roads of the United Kingdom because of dangerous chemicals, compared with the annual average of 5,000 persons every year in motor accidents. However, small quantities of dangerous goods can cause significant damage. International examples include:
- A plane crashed near Boston, USA, because of leaking nitric acid.
- Over 200 people were killed when a road tanker of propylene exploded over a campsite in Spain.
- In a rail accident involving 22 rail cars of chemicals in Mississauga, Canada, a tanker containing 90 tonnes of chlorine was ruptured and there was an explosion and a large fire. There were no fatalities, but 250,000 persons were evacuated.
- A rail collision alongside the motorway in Eccles, United Kingdom, resulted in three deaths and 68 injuries from the collision, but none from the resulting serious fire of the petroleum products being transported.
- A petrol tanker went out of control in Herrborn, Germany, burning down a large part of the town.
- In Peterborough, United Kingdom, a vehicle carrying explosives killed one person and almost destroyed an industrial centre.
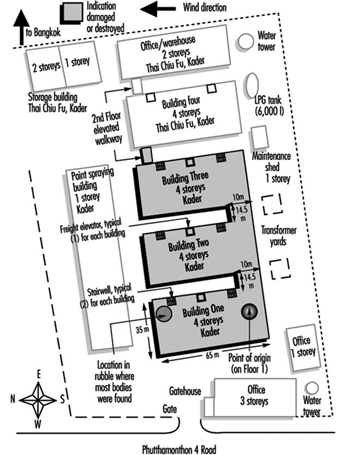
- A petrol tanker exploded in Bangkok, Thailand, killing a large number of people.
The largest number of serious incidents have arisen with flammable gas or liquids (partially related to the volumes moved), with some incidents from toxic gases and toxic fumes (including products of combustion).
Studies in the UK have shown the following for road transport:
- frequency of accident while conveying hazardous materials: 0.12 x 10–6/km
- frequency of release while conveying hazardous materials: 0.027 x 10–6/km
- probability of a release given a traffic accident: 3.3%.
These events are not synonymous with hazardous material incidents involving vehicles, and may constitute only a small proportion of the latter. There is also the individuality of accidents involving the road transport of hazardous materials.
International agreements covering the transport of potentially hazardous materials include:
Regulations for the Safe Transport of Radioactive Material 1985 (as amended 1990): International Atomic Energy Agency, Vienna, 1990 (STI/PUB/866). Their purpose is to establish standards of safety which provide an acceptable level of control of the radiation hazards to persons, property and the environment that are associated with the transport of radioactive material.
The International Convention for the Safety of Life at Sea 1974 (SOLAS 74). This sets basic safety standards for all passenger and cargo ships, including ships carrying hazardous bulk cargoes.
The International Convention for the Prevention of Pollution from Ships 1973, as modified by the Protocol of 1978 (MARPOL 73/78). This provides regulations for the prevention of pollution by oil, noxious liquid substances in bulk, pollutants in packaged form or in freight containers, portable tanks or road and rail wagons, sewage and garbage. Regulation requirements are amplified in the International Maritime Dangerous Goods Code.
There is a substantial body of international regulation of the transportation of harmful substances by air, rail, road and sea (converted into national legislation in many countries). Most are based on standards sponsored by the United Nations, and cover the principles of identification, labelling, prevention and mitigation. The United Nations Committee of Experts on the Transport of Dangerous Goods has produced Recommendations on the Transport of Dangerous Goods. They are addressed to governments and international organizations concerned with the regulation of the transport of dangerous goods. Among other aspects, the recommendations cover principles of classification and definitions of classes, listing of the content of dangerous goods, general packing requirements, testing procedures, making, labelling or placarding, and transport documents. These recommendations—the “Orange Book”—do not have the force of law, but form the basis of all the international regulations. These regulations are generated by various organizations:
- the International Civil Aviation Organization: Technical Instructions for Safe Transport of Dangerous Goods by Air (Tis)
- the International Maritime Organization: International Maritime Dangerous Goods Code (IMDG Code)
- the European Economic Community: The European Agreement Concerning the International Carriage of Dangerous Goods by Road (ADR)
- the Office of International Rail Transport: Regulations Concerning the International Carriage of Dangerous Goods by Rail (RID).
The preparation of major emergency plans to deal with and mitigate the effects of a major accident involving dangerous substances is as much needed in the transportation field as for fixed installations. The planning task is made more difficult in that the location of an incident will not be known in advance, thus requiring flexible planning. The substances involved in a transport accident cannot be foreseen. Because of the nature of the incident a number of products may be mixed together at the scene, causing considerable problems to the emergency services. The incident may occur in an area which is highly urbanized, remote and rural, heavily industrialized, or commercialized. An added factor is the transient population who may be unknowingly involved in an event because the accident has caused a backlog of vehicles either on the public highway or where passenger trains are stopped in response to a rail incident.
There is therefore a necessity for the development of local and national plans to respond to such events. These must be simple, flexible and easily understood. As major transport accidents can occur in a multiplicity of locations the plan must be appropriate to all potential scenes. For the plan to work effectively at all times, and in both remote rural and heavily populated urban locales, all organizations contributing to the response must have the ability to maintain flexibility while conforming to the basic principles of the overall strategy.
The initial responders should obtain as much information as possible to try to identify the hazard involved. Whether the incident is a spillage, a fire, a toxic release, or a combination of these will determine responses. The national and international marking systems used to identify vehicles transporting hazardous substances and carrying hazardous packaged goods should be known to the emergency services, who should have access to one of the several national and international databases which can help to identify the hazard and the problems associated with it.
Rapid control of the incident is vital. The chain of command must be identified clearly. This may change during the course of the event from the emergency services through the police to the civil government of the affected area. The plan must be able to recognize the effect on the population, both those working in or resident in the potentially affected area and those who may be transients. Sources of expertise on public health matters should be mobilized to advise on both the immediate management of the incident and on the potential for longer-term direct health effects and indirect ones through the food chain. Contact points for obtaining advice on environmental pollution to water courses and so on, and the effect of weather conditions on the movement of gas clouds must be identified. Plans must identify the possibility of evacuation as one of the response measures.
However, the proposals must be flexible, as there may be a range of costs and benefits, both in incident management and in public health terms, which will have to be considered. The arrangements must outline clearly the policy with respect to keeping the media fully informed and the action being taken to mitigate the effects. The information must be accurate and timely, with the spokesperson being knowledgeable as to the overall response and having access to experts to respond to specialized queries. Poor media relations can disrupt the management of the event and lead to unfavourable and sometimes unjustified comments on the overall handling of the episode. Any plan must include adequate mock disaster drills. These enable the responders to and managers of an incident to learn each other’s personal and organizational strengths and weaknesses. Both table-top and physical exercises are required.
Although the literature dealing with chemical spills is extensive, only a minor part describes the ecological consequences. Most concern case studies. The descriptions of actual spills have focused on human health and safety problems, with ecological consequences described only in general terms. The chemicals enter the environment predominantly through the liquid phase. In only a few cases did accidents having ecological consequences also affect humans immediately, and the effects on the environment were not caused by identical chemicals or by identical release routes.
Controls to prevent risk to human health and life from the transport of hazardous materials include quantities carried, direction and control of means of transport, routing, as well as authority over interchange and concentration points and developments near such areas. Further research is required into risk criteria, quantification of risk, and risk equivalence. The United Kingdom Health and Safety Executive has developed a Major Incident Data Service (MHIDAS) as a database of major chemical incidents worldwide. It currently holds information on over 6,000 incidents.
Case Study: Transport of Hazardous Materials
An articulated road tanker carrying about 22,000 litres of toluene was travelling on a main arterial road which runs through Cleveland, UK. A car pulled into the path of the vehicle, and, as the truckdriver took evasive action, the tanker overturned. The manlids of all five compartments sprang open and toluene spilled on the roadway and ignited, resulting in a pool fire. Five cars travelling on the opposite carriageway were involved in the fire but all occupants escaped.
The fire brigade arrived within five minutes of being called. Burning liquid had entered the drains, and drain fires were evident approximately 400m from the main incident. The County Emergency Plan was put into action, with social services and public transport put on alert in case evacuation was needed. Initial action by the fire brigade concentrated on extinguishing car fires and searching for occupants. The next task was identifying an adequate water supply. A member of the chemical company’s safety team arrived to coordinate with the police and fire commanders. Also in attendance were staff from the ambulance service and the environmental health and water boards. Following consultation it was decided to permit the leaking toluene to burn rather than extinguish the fire and have the chemical emitting vapours. Police put out warnings over a four-hour period utilizing national and local radio, advising people to stay indoors and close their windows. The road was closed for eight hours. When the toluene fell below the level of the manlids, the fire was extinguished and the remaining toluene removed from the tanker. The incident was concluded approximately 13 hours after the accident.
Potential harm to humans existed from thermal radiation; to the environment, from air, soil and water pollution; and to the economy, from traffic disruption. The company plan which existed for such a transportation incident was activated within 15 minutes, with five persons in attendance. A county offsite plan existed and was instigated with a control centre coming into being involving police and the fire brigade. Concentration measurement but not dispersion prediction was performed. The fire brigade response involved over 50 persons and ten appliances, whose major actions were fire-fighting, washing down and spillage retention. Over 40 police officers were committed in traffic direction, warning the public, security and press control. The health service response encompassed two ambulances and two onsite medical staff. Local government reaction involved environmental health, transport and social services. The public were informed of the incident by loudspeakers, radio and word of mouth. The information focused on what to do, especially on sheltering indoors.
The outcome to humans was two admissions to a single hospital, a member of the public and a company employee, both injured in the crash. There was noticeable air pollution but only slight soil and water contamination. From an economic perspective there was major damage to the road and extensive traffic delays, but no loss of crops, livestock or production. Lessons learned included the value of rapid retrieval of information from the Chemdata system and the presence of a company technical expert enabling correct immediate action to be taken. The importance of joint press statements from responders was highlighted. Consideration needs to be given to the environmental impact of fire-fighting. If the fire had been fought in the initial stages, a considerable amount of contaminated liquid (firewater and toluene) potentially could have entered the drains, water supplies and soil.
Radiation Accidents
Description, Sources, Mechanisms
Apart from the transportation of radioactive materials, there are three settings in which radiation accidents can occur:
- use of nuclear reactions to produce energy or arms, or for research purposes
- industrial applications of radiation (gamma radiography, irradiation)
- research and nuclear medicine (diagnosis or therapy).
Radiation accidents may be classified into two groups on the basis of whether or not there is environmental emission or dispersion of radionuclides; each of these types of accident affects different populations.
The magnitude and duration of the exposure risk for the general population depends on the quantity and the characteristics (half-life, physical and chemical properties) of the radionuclides emitted into the environment (table 1). This type of contamination occurs when there is rupture of the containment barriers at nuclear power plants or industrial or medical sites which separate radioactive materials from the environment. In the absence of environmental emissions, only workers present onsite or handling radioactive equipment or materials are exposed.
Table 1. Typical radionuclides, with their radioactive half-lives
|
Radionuclide |
Symbol |
Radiation emitted |
Physical half-life* |
Biological half-life |
|
Barium-133 |
Ba-133 |
γ |
10.7 y |
65 d |
|
Cerium-144 |
Ce-144 |
β,γ |
284 d |
263 d |
|
Caesium-137 |
Cs-137 |
β,γ |
30 y |
109 d |
|
Cobalt-60 |
Co-60 |
β,γ |
5.3 y |
1.6 y |
|
Iodine-131 |
I-131 |
β,γ |
8 d |
7.5 d |
|
Plutonium-239 |
Pu-239 |
α,γ |
24,065 y |
50 y |
|
Polonium-210 |
Po-210 |
α |
138 d |
27 d |
|
Strontium-90 |
Sr-90 |
β |
29.1 y |
18 y |
|
Tritium |
H-3 |
β |
12.3 y |
10 d |
* y = years; d = days.
Exposure to ionizing radiation may occur through three routes, regardless of whether the target population is composed of workers or the general public: external irradiation, internal irradiation, and contamination of skin and wounds.
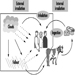
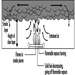
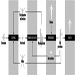
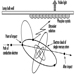
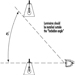
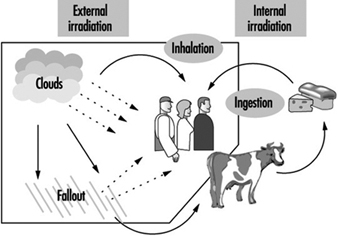
External irradiation occurs when individuals are exposed to an extracorporeal radiation source, either point (radiotherapy, irradiators) or diffuse (radioactive clouds and fallout from accidents, figure 1). Irradiation may be local, involving only a portion of the body, or whole body.
Figure 1. Exposure pathways to ionizing radiation after an accidental release of radioactivity in the environment
Internal radiation occurs following incorporation of radioactive substances into the body (figure 1) through either inhalation of airborne radioactive particles (e.g., caesium-137 and iodine-131, present in the Chernobyl cloud) or ingestion of radioactive materials in the food chain (e.g., iodine-131 in milk). Internal irradiation may affect the whole body or only certain organs, depending on the characteristics of the radionuclides: caesium-137 distributes itself homogeneously throughout the body, while iodine-131 and strontium-90 concentrate in the thyroid and the bones, respectively.
Finally, exposure may also occur through direct contact of radioactive materials with skin and wounds.
Accidents involving nuclear power plants
Sites included in this category include power-generating stations, experimental reactors, facilities for the production and processing or reprocessing of nuclear fuel and research laboratories. Military sites include plutonium breeder reactors and reactors located aboard ships and submarines.
Nuclear power plants
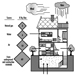
The capture of heat energy emitted by atomic fission is the basis for the production of electricity from nuclear energy. Schematically, nuclear power plants can be thought of as comprising: (1) a core, containing the fissile material (for pressurized-water reactors, 80 to 120 tonnes of uranium oxide); (2) heat-transfer equipment incorporating heat-transfer fluids; (3) equipment capable of transforming heat energy into electricity, similar to that found in power plants that are not nuclear.
Strong, sudden power surges capable of causing core meltdown with emission of radioactive products are the primary hazards at these installations. Three accidents involving reactor-core meltdown have occurred: at Three Mile Island (1979, Pennsylvania, United States), Chernobyl (1986, Ukraine), and Fukushima (2011, Japan) [Edited, 2011].
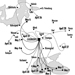
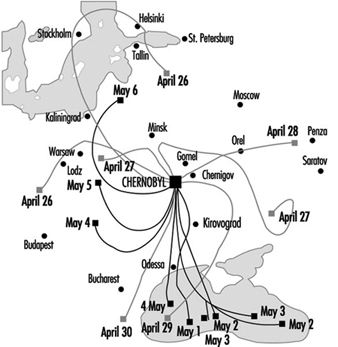
The Chernobyl accident was what is known as a criticality accident—that is, a sudden (within the space of a few seconds) increase in fission leading to a loss of process control. In this case, the reactor core was completely destroyed and massive amounts of radioactive materials were emitted (table 2). The emissions reached a height of 2 km, favouring their dispersion over long distances (for all intents and purposes, the entire Northern hemisphere). The behaviour of the radioactive cloud has proven difficult to analyse, due to meteorological changes during the emission period (figure 2) (IAEA 1991).
Table 2. Comparison of different nuclear accidents
|
Accident |
Type of facility |
Accident |
Total emitted |
Duration |
Main emitted |
Collective |
|
Khyshtym 1957 |
Storage of high- |
Chemical explosion |
740x106 |
Almost |
Strontium-90 |
2,500 |
|
Windscale 1957 |
Plutonium- |
Fire |
7.4x106 |
Approximately |
Iodine-131, polonium-210, |
2,000 |
|
Three Mile Island |
PWR industrial |
Coolant failure |
555 |
? |
Iodine-131 |
16–50 |
|
Chernobyl 1986 |
RBMK industrial |
Critically |
3,700x106 |
More than 10 days |
Iodine-131, iodine-132, |
600,000 |
|
Fukushima 2011
|
The final report of the Fukushima Assessment Task Force will be submitted in 2013. |
|
|
|
|
|
Source: UNSCEAR 1993.
Figure 2. Trajectory of emissions from the Chernobyl accident, 26 April-6 May 1986
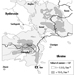
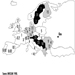
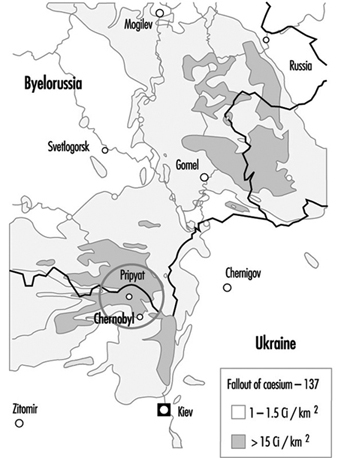
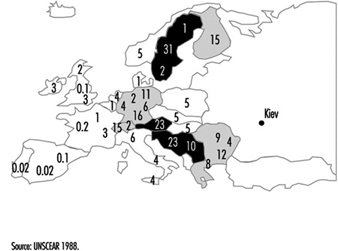
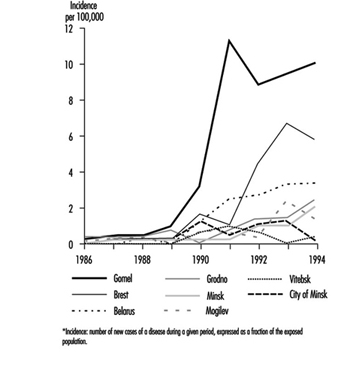
Contamination maps were drawn up on the basis of environmental measurements of caesium-137, one of the main radioactive emission products (table 1 and table 2). Areas of Ukraine, Byelorussia (Belarus) and Russia were heavily contaminated, while fallout in the rest of Europe was less significant (figure 3 and figure 4 (UNSCEAR 1988). Table 3 presents data on the area of the contaminated zones, characteristics of the exposed populations and routes of exposure.
FIgure 3. Caesium-137 deposition in Byelorussia, Russia and Ukraine following the Chernobyl accident.
Figure 4. Caesium-137 fallout (kBq/km2) in Europe following the Chernobyl accident
Table 3. Area of contaminated zones, types of populations exposed and modes of exposure in Ukraine, Byelorussia and Russia following the Chernobyl accident
|
Type of population |
Surface area ( km2 ) |
Population size (000) |
Main modes of exposure |
|
Occupationally exposed populations: |
|||
|
Employees onsite at |
≈0.44 |
External irradiation, |
|
|
General public: |
|||
|
Evacuated from the |
|
115 |
External irradiation by |
* Individuals participating in clean-up within 30 km of the site. These include fire-fighters, military personnel, technicians and engineers who intervened during the first weeks, as well as physicians and researchers active at a later date.
** Caesium-137 contamination.
Source: UNSCEAR 1988; IAEA 1991.
The Three Mile Island accident is classified as a thermal accident with no reactor runaway, and was the result of a reactor-core coolant failure lasting several hours. The containment shell ensured that only a limited quantity of radioactive material was emitted into the environment, despite the partial destruction of the reactor core (table 2). Although no evacuation order was issued, 200,000 residents voluntarily evacuated the area.
Finally, an accident involving a plutonium production reactor occurred on the west coast of England in 1957 (Windscale, table 2). This accident was caused by a fire in the reactor core and resulted in environmental emissions from a chimney 120 metres high.
Fuel-processing facilities
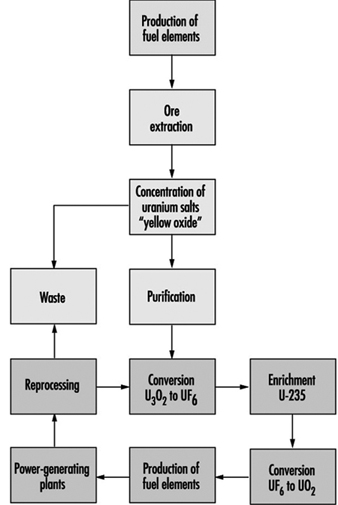
Fuel production facilities are located “upstream” from nuclear reactors and are the site of ore extraction and the physical and chemical transformation of uranium into fissile material suitable for use in reactors (figure 5). The primary accident hazards present in these facilities are chemical in nature and related to the presence of uranium hexafluoride (UF6), a gaseous uranium compound which may decompose upon contact with air to produce hydrofluoric acid (HF), a very corrosive gas.
Figure 5. Nuclear fuel processing cycle.
“Downstream” facilities include fuel storage and reprocessing plants. Four criticality accidents have occurred during chemical reprocessing of enriched uranium or plutonium (Rodrigues 1987). In contrast to accidents occurring at nuclear power plants, these accidents involved small quantities of radioactive materials—tens of kilograms at most—and resulted in negligible mechanical effects and no environmental emission of radioactivity. Exposure was limited to very high dose, very short term (of the order of minutes) external gamma ray and neutron irradiation of workers.
In 1957, a tank containing highly radioactive waste exploded at Russia’s first military-grade plutonium production facility, located in Khyshtym, in the south Ural Mountains. Over 16,000 km2 were contaminated and 740 PBq (20 MCi) were emitted into the atmosphere (table 2 and table 4).
Table 4. Surface area of the contaminated zones and size of population exposed after the Khyshtym accident (Urals 1957), by strontium-90 contamination
|
Contamination ( kBq/m2 ) |
( Ci/km2 ) |
Area ( km2 ) |
Population |
|
≥ 37,000 |
≥ 1,000 |
20 |
1,240 |
|
≥ 3,700 |
≥100 |
120 |
1,500 |
|
≥ 74 |
≥ 2 |
1,000 |
10,000 |
|
≥ 3.7 |
≥ 0.1 |
15,000 |
270,000 |
Research reactors
Hazards at these facilities are similar to those present at nuclear power plants, but are less serious, given the lower power generation. Several criticality accidents involving significant irradiation of personnel have occurred (Rodrigues 1987).
Accidents related to the use of radioactive sources in industry and medicine (excluding nuclear plants) (Zerbib 1993)
The most common accident of this type is the loss of radioactive sources from industrial gamma radiography, used, for example, for the radiographic inspection of joints and welds. However, radioactive sources may also be lost from medical sources (table 5). In either case, two scenarios are possible: the source may be picked up and kept by a person for several hours (e.g., in a pocket), then reported and restored, or it may be collected and carried home. While the first scenario causes local burns, the second may result in long-term irradiation of several members of the general public.
Table 5. Accidents involving the loss of radioactive sources and which resulted in exposure of the general public
|
Country (year) |
Number of |
Number of |
Number of deaths** |
Radioactive material involved |
|
Mexico (1962) |
? |
5 |
4 |
Cobalt-60 |
|
China (1963) |
? |
6 |
2 |
Cobalt 60 |
|
Algeria (1978) |
22 |
5 |
1 |
Iridium-192 |
|
Morocco (1984) |
? |
11 |
8 |
Iridium-192 |
|
Mexico |
≈4,000 |
5 |
0 |
Cobalt-60 |
|
Brazil |
249 |
50 |
4 |
Caesium-137 |
|
China |
≈90 |
12 |
3 |
Cobalt-60 |
|
United States |
≈90 |
1 |
1 |
Iridium-192 |
* Individuals exposed to doses capable of causing acute or long-term effects or death.
** Among individuals receiving high doses.
Source: Nénot 1993.
The recovery of radioactive sources from radiotherapy equipment has resulted in several accidents involving the exposure of scrap workers. In two cases—the Juarez and Goiânia accidents—the general public was also exposed (see table 5 and box below).
The Goiвnia Accident, 1987
Between 21 September and 28 September 1987, several people suffering from vomiting, diarrhoea, vertigo and skin lesions at various parts of the body were admitted to the hospital specializing in tropical diseases in Goiânia, a city of one million inhabitants in the Brazilian state of Goias. These problems were attributed to a parasitic disease common in Brazil. On 28 September, the physician responsible for health surveillance in the city saw a woman who presented him with a bag containing debris from a device collected from an abandoned clinic, and a powder which emitted, according to the woman “a blue light”. Thinking that the device was probably x-ray equipment, the physician contacted his colleagues at the hospital for tropical diseases. The Goias Department of the Environment was notified, and the next day a physicist took measurements in the hygiene department’s yard, where the bag was stored overnight. Very high radioactivity levels were found. In subsequent investigations the source of radioactivity was identified as a caesium-137 source (total activity: approximately 50 TBq (1,375 Ci)) which had been contained within radiotherapy equipment used in a clinic abandoned since 1985. The protective housing surrounding the caesium had been disassembled on 10 September 1987 by two scrapyard workers and the caesium source, in powder form, removed. Both the caesium and the fragments of the contaminated housing were gradually dispersed throughout the city. Several people who had transported or handled the material, or who had simply come to see it (including parents, friends and neighbours) were contaminated. In all, over 100,000 people were examined, of whom 129 were very seriously contaminated; 50 were hospitalized (14 for medullary failure), and 4, including a 6-year-old girl, died. The accident had dramatic economic and social consequences for the entire city of Goiânia and the state of Goias: 1/1000 of the city’s surface area was contaminated, and the price of agricultural produce, rents, real estate, and land all fell. The inhabitants of the entire state suffered real discrimination.
Source: IAEA 1989a
The Juarez accident was discovered serendipitously (IAEA 1989b). On 16 January 1984, a truck entering the Los Alamos (New Mexico, United States) scientific laboratory loaded with steel bars triggered a radiation detector. Investigation revealed the presence of cobalt-60 in the bars and traced the cobalt-60 to a Mexican foundry. On January 21, a heavily contaminated scrapyard in Juarez was identified as the source of the radioactive material. Systematic monitoring of roads and highways by detectors resulted in the identification of a heavily contaminated truck. The ultimate radiation source was determined to be a radiotherapy device stored in a medical centre until December 1983, at which time it was disassembled and transported to the scrapyard. At the scrapyard, the protective housing surrounding the cobalt-60 was broken, freeing the cobalt pellets. Some of the pellets fell into the truck used to transport scrap, and others were dispersed throughout the scrapyard during subsequent operations, mixing with the other scrap.
Accidents involving the entry of workers into active industrial irradiators (e.g., those used to preserve food, sterilize medical products, or polymerize chemicals) have occurred. In all cases, these have been due to failure to follow safety procedures or to disconnected or defective safety systems and alarms. The dose levels of external irradiation to which workers in these accidents were exposed were high enough to cause death. Doses were received within a few seconds or minutes (table 6).
Table 6. Main accidents involving industrial irradiators
|
Site, date |
Equipment* |
Number of |
Exposure level |
Affected organs |
Dose received (Gy), |
Medical effects |
|
Forbach, August 1991 |
EA |
2 |
several deciGy/ |
Hands, head, trunk |
40, skin |
Burns affecting 25–60% of |
|
Maryland, December 1991 |
EA |
1 |
? |
Hands |
55, hands |
Bilateral finger amputation |
|
Viet nam, November 1992 |
EA |
1 |
1,000 Gy/minute |
Hands |
1.5, whole body |
Amputation of the right hand and a finger of the left hand |
|
Italy, May 1975 |
CI |
1 |
Several minutes |
Head, whole body |
8, bone marrow |
Death |
|
San Salvador, February 1989 |
CI |
3 |
? |
Whole body, legs, |
3–8, whole body |
2 leg amputations, 1 death |
|
Israel, June 1990 |
CI |
1 |
1 minute |
Head, whole body |
10–20 |
Death |
|
Belarus, October 1991 |
CI |
1 |
Several minutes |
Whole body |
10 |
Death |
* EA: electron accelerator CI: cobalt-60 irradiator.
Source: Zerbib 1993; Nénot 1993.
Finally, medical and scientific personnel preparing or handling radioactive sources may be exposed through skin and wound contamination or inhalation or ingestion of radioactive materials. It should be noted that this type of accident is also possible in nuclear power plants.
Public Health Aspects of the Problem
Temporal patterns
The United States Radiation Accident Registry (Oak Ridge, United States) is a worldwide registry of radiation accidents involving humans since 1944. To be included in the registry, an accident must have been the subject of a published report and have resulted in whole-body exposure exceeding 0.25 Sievert (Sv), or skin exposure exceeding 6 Sv or exposure of other tissues and organs exceeding 0.75 Sv (see "Case Study: What does dose mean?" for a definition of dose). Accidents that are of interest from the point of view of public health but which resulted in lower exposures are thus excluded (see below for a discussion of the consequences of exposure).
Analysis of the registry data from 1944 to 1988 reveals a clear increase in both the frequency of radiation accidents and the number of exposed individuals starting in 1980 (table 7). The increase in the number of exposed individuals is probably accounted for by the Chernobyl accident, particularly the approximately 135,000 individuals initially residing in the prohibited area within 30 km of the accident site. The Goiânia (Brazil) and Juarez (Mexico) accidents also occurred during this period and involved significant exposure of many people (table 5).
Table 7. Radiation accidents listed in the Oak Ridge (United States) accident registry (worldwide, 1944-88)
|
1944–79 |
1980–88 |
1944–88 |
|
|
Total number of accidents |
98 |
198 |
296 |
|
Number of individuals involved |
562 |
136,053 |
136,615 |
|
Number of individuals exposed to doses exceeding |
306 |
24,547 |
24,853 |
|
Number of deaths (acute effects) |
16 |
53 |
69 |
* 0.25 Sv for whole-body exposure, 6 Sv for skin exposure, 0.75 Sv for other tissues and organs.
Potentially exposed populations
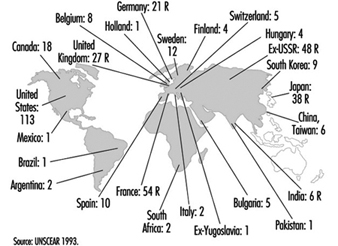
From the point of view of exposure to ionizing radiation, there are two populations of interest: occupationally exposed populations and the general public. United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR 1993) estimates that 4 million workers worldwide were occupationally exposed to ionizing radiation in the period 1985-1989; of these, approximately 20% were employed in the production, use and processing of nuclear fuel (table 8). IAEA member countries were estimated to possess 760 irradiators in 1992, of which 600 were electron accelerators and 160 gamma irradiators.
Table 8. Temporal pattern of occupational exposure to ionizing radiation worldwide (in thousands)
|
Activity |
1975–79 |
1980–84 |
1985–89 |
|
Nuclear fuel processing* |
560 |
800 |
880 |
|
Military applications** |
310 |
350 |
380 |
|
Industrial applications |
530 |
690 |
560 |
|
Medical applications |
1,280 |
1,890 |
2,220 |
|
Total |
2,680 |
3,730 |
4,040 |
* Production and reprocessing of fuel: 40,000; reactor operation: 430,000.
** including 190,000 shipboard personnel.
Source: UNSCEAR 1993.
The number of nuclear sites per country is a good indicator of the potential for exposure of the general public (figure 6).
Figure 6. Distribution of power-generating reactors and fuel reprocessing plants in the world, 1989-90
Health Effects
Direct health effects of ionizing radiation
In general, the health effects of ionizing radiation are well known and depend on the dose level received and the dose rate (received dose per unit of time (see "Case Study: What does dose mean?").
Deterministic effects
These occur when the dose exceeds a given threshold and the dose rate is high. The severity of the effects is proportional to the dose, although the dose threshold is organ specific (table 9).
Table 9. Deterministic effects: thresholds for selected organs
|
Tissue or effect |
Equivalent single dose |
|
Testicles: |
|
|
Temporary sterility |
0.15 |
|
Permanent sterility |
3.5–6.0 |
|
Ovaries: |
|
|
Sterility |
2.5–6.0 |
|
Crystalline lens: |
|
|
Detectable opacities |
0.5–2.0 |
|
Impaired vision (cataracts) |
5.0 |
|
Bone marrow: |
|
|
Depression of haemopoiesis |
0.5 |
Source: ICRP 1991.
In the accidents such as those discussed above, deterministic effects may be caused by local intense irradiation, such as that caused by external irradiation, direct contact with a source (e.g., a misplaced source picked up and pocketed) or skin contamination. All these result in radiological burns. If the local dose is of the order of 20 to 25 Gy (table 6, "Case Study: What does dose mean?") tissue necrosis may ensue. A syndrome known as acute irradiation syndrome, characterized by digestive disorders (nausea, vomiting, diarrhoea) and bone marrow aplasia of variable severity, may be induced when the average whole-body irradiation dose exceeds 0.5 Gy. It should be recalled that whole-body and local irradiation may occur simultaneously.
Nine of 60 workers exposed during criticality accidents at nuclear fuel processing plants or research reactors died (Rodrigues 1987). Decedents received 3 to 45 Gy, while survivors received 0.1 to 7 Gy. The following effects were observed in survivors: acute irradiation syndrome (gastro-intestinal and haematological effects), bilateral cataracts and necrosis of limbs, requiring amputation.
At Chernobyl, power plant personnel, as well as emergency response personnel not using special protective equipment, suffered high beta and gamma radiation exposure in the initial hours or days following the accident. Five hundred people required hospitalization; 237 individuals who received whole-body irradiation exhibited acute irradiation syndrome, and 28 individuals died despite treatment (table 10) (UNSCEAR 1988). Others received local irradiation of the limbs, in some cases affecting over 50% of the body surface and continue to suffer, many years later, multiple skin disorders (Peter, Braun-Falco and Birioukov 1994).
Table 10. Distribution of patients exhibiting acute irradiation syndrome (AIS) after the Chernobyl accident, by severity of condition
|
Severity of AIS |
Equivalent dose |
Number of |
Number of |
Average survival |
|
I |
1–2 |
140 |
– |
– |
|
II |
2–4 |
55 |
1 (1.8) |
96 |
|
III |
4–6 |
21 |
7 (33.3) |
29.7 |
|
IV |
>6 |
21 |
20 (95.2) |
26.6 |
Source: UNSCEAR 1988.
Stochastic effects
These are probabilistic in nature (i.e., their frequency increases with received dose), but their severity is independent of dose. The main stochastic effects are:
- Mutation. This has been observed in animal experiments but has been difficult to document in humans.
- Cancer. The effect of irradiation on the risk of developing cancer has been studied in patients receiving radiation therapy and in survivors of the Hiroshima and Nagasaki bombings. UNSCEAR (1988, 1994) regularly summarizes the results of these epidemiological studies. The duration of the latency period is typically 5 to 15 years from the date of exposure depending on organ and tissue. Table 11 lists the cancers for which an association with ionizing radiation has been established. Significant cancer excesses have been demonstrated among survivors of the Hiroshima and Nagasaki bombings with exposures above 0.2 Sv.
- Selected benign tumours. Benign thyroid adenomas.
Table 11. Results of epidemiological studies of the effect of high dose rate of external irradiation on cancer
|
Cancer site |
Hiroshima/Nagasaki |
Other studies |
|
|
Mortality |
Incidence |
||
|
Haematopoietic system |
|||
|
Leukaemia |
+* |
+* |
6/11 |
|
Lymphoma (not specified) |
+ |
0/3 |
|
|
Non-Hodgkin lymphoma |
+* |
1/1 |
|
|
Myeloma |
+ |
+ |
1/4 |
|
Oral cavity |
+ |
+ |
0/1 |
|
Salivary glands |
+* |
1/3 |
|
|
Digestive system |
|||
|
Oesophagus |
+* |
+ |
2/3 |
|
Stomach |
+* |
+* |
2/4 |
|
Small intestine |
1/2 |
||
|
Colon |
+* |
+* |
0/4 |
|
Rectum |
+ |
+ |
3/4 |
|
Liver |
+* |
+* |
0/3 |
|
Gall bladder |
0/2 |
||
|
Pancreas |
3/4 |
||
|
Respiratory system |
|||
|
Larynx |
0/1 |
||
|
Trachea, bronchi, lungs |
+* |
+* |
1/3 |
|
Skin |
|||
|
Not specified |
1/3 |
||
|
Melanoma |
0/1 |
||
|
Other cancers |
+* |
0/1 |
|
|
Breast (women) |
+* |
+* |
9/14 |
|
Reproductive system |
|||
|
Uterus (non-specific) |
+ |
+ |
2/3 |
|
Uterine body |
1/1 |
||
|
Ovaries |
+* |
+* |
2/3 |
|
Other (women) |
2/3 |
||
|
Prostate |
+ |
+ |
2/2 |
|
Urinary system |
|||
|
Bladder |
+* |
+* |
3/4 |
|
Kidneys |
0/3 |
||
|
Other |
0/1 |
||
|
Central nervous system |
+ |
+ |
2/4 |
|
Thyroid |
+* |
4/7 |
|
|
Bone |
2/6 |
||
|
Connective tissue |
0/4 |
||
|
All cancers, excluding leukaemias |
1/2 |
||
+ Cancer sites studied in the Hiroshima and Nagasaki survivors.
* Positive association with ionizing radiation.
1 Cohort (incidence or mortality) or case-control studies.
Source: UNSCEAR 1994.
Two important points concerning the effects of ionizing radiation remain controversial.
Firstly, what are the effects of low-dose irradiation (below 0.2 Sv) and low dose rates? Most epidemiological studies have examined survivors of the Hiroshima and Nagasaki bombings or patients receiving radiation therapy—populations exposed over very short periods to relatively high doses—and estimates of the risk of developing cancer as a result of exposure to low doses and dose rates depends essentially on extrapolations from these populations. Several studies of nuclear power plant workers, exposed to low doses over several years, have reported cancer risks for leukaemia and other cancers that are compatible with extrapolations from high-exposure groups, but these results remain unconfirmed (UNSCEAR 1994; Cardis, Gilbert and Carpenter 1995).
Secondly, is there a threshold dose (i.e., a dose below which there is no effect)? This is currently unknown. Experimental studies have demonstrated that damage to genetic material (DNA) caused by spontaneous errors or environmental factors are constantly repaired. However, this repair is not always effective, and may result in malignant transformation of cells (UNSCEAR 1994).
Other effects
Finally, the possibility of teratogenic effects due to irradiation during pregnancy should be noted. Microcephaly and mental retardation have been observed in children born to female survivors of the Hiroshima and Nagasaki bombings who received irradiation of at least 0.1 Gy during the first trimester (Otake, Schull and Yoshimura 1989; Otake and Schull 1992). It is unknown whether these effects are deterministic or stochastic, although the data do suggest the existence of a threshold.
Effects observed following the Chernobyl accident
The Chernobyl accident is the most serious nuclear accident to have occurred to date. However, even now, ten years after the fact, not all the health effects on the most highly exposed populations have been accurately evaluated. There are several reasons for this:
- Some effects appear only many years after the date of exposure: for example, solid-tissue cancers typically take 10 to 15 years to appear.
- As some time elapsed between the accident and the commencement of epidemiological studies, some effects occurring in the initial period following the accident may not have been detected.
- Useful data for the quantification of the cancer risk were not always gathered in a timely fashion. This is particularly true for data necessary to estimate the exposure of the thyroid gland to radioactive iodides emitted during the incident (tellurium-132, iodine-133) (Williams et al. 1993).
- Finally, many initially exposed individuals subsequently left the contaminated zones and were probably lost for follow-up.
Workers. Currently, comprehensive information is unavailable for all the workers who were strongly irradiated in the first few days following the accident. Studies on the risk to clean-up and relief workers of developing leukaemia and solid-tissue cancers are in progress (see table 3). These studies face many obstacles. Regular follow-up of the health status of clean-up and relief workers is greatly hindered by the fact that many of them came from different parts of the ex-USSR and were redispatched after working on the Chernobyl site. Further, received dose must be estimated retrospectively, as there are no reliable data for this period.
General population. The only effect plausibly associated with ionizing radiation in this population to date is an increase, starting in 1989, of the incidence of thyroid cancer in children younger than 15 years. This was detected in Byelorussia (Belarus) in 1989, only three years after the incident, and has been confirmed by several expert groups (Williams et al. 1993). The increase was particularly noteworthy in the most heavily contaminated areas of Belarus, especially the Gomel region. While thyroid cancer was normally rare in children younger than 15 years, (annual incidence rate of 1 to 3 per million), its incidence increased tenfold on a national basis and twentyfold in the Gomel area (table 12, figure 7), (Stsjazhko et al. 1995). A tenfold increase of the incidence of thyroid cancer was subsequently reported in the five most heavily contaminated areas of Ukraine, and an increase in thyroid cancer was also reported in the Bryansk (Russia) region (table 12). An increase among adults is suspected but has not been confirmed. Systematic screening programmes undertaken in the contaminated regions allowed latent cancers present prior to the accident to be detected; ultrasonographic programmes capable of detecting thyroid cancers as small as a few millimetres were particularly helpful in this regard. The magnitude of the increase in incidence in children, taken together with the aggressiveness of the tumours and their rapid development, suggests that the observed increases in thyroid gland cancer are partially due to the accident.
Table 12. Temporal pattern of the incidence and total number of thyroid cancers in children in Belarus, Ukraine & Russia, 1981-94
|
Incidence* (/100,000) |
Number of cases |
|||
|
1981–85 |
1991–94 |
1981–85 |
1991–94 |
|
|
Belarus |
||||
|
Entire country |
0.3 |
3.06 |
3 |
333 |
|
Gomel area |
0.5 |
9.64 |
1 |
164 |
|
Ukraine |
||||
|
Entire country |
0.05 |
0.34 |
25 |
209 |
|
Five most heavily |
0.01 |
1.15 |
1 |
118 |
|
Russia |
||||
|
Entire country |
? |
? |
? |
? |
|
Bryansk and |
0 |
1.00 |
0 |
20 |
* Incidence: the ratio of the number of new cases of a disease during a given period to the size of the population studied in the same period.
Source: Stsjazhko et al. 1995.
Figure 7. Incidence of cancer of the thyroid in children younger than 15 years in Belarus
In the most heavily contaminated zones (e.g., the Gomel region), the thyroid doses were high, particularly among children (Williams et al. 1993). This is consistent with the significant iodine emissions associated with the accident and the fact that radioactive iodine will, in the absence of preventive measures, concentrate preferentially in the thyroid gland.
Exposure to radiation is a well-documented risk factor for thyroid cancer. Clear increases in the incidence of thyroid cancer have been observed in a dozen studies of children receiving radiation therapy to the head and neck. In most cases, the increase was clear ten to 15 years after exposure, but was detectable in some cases within three to seven years. On the other hand, the effects in children of internal irradiation by iodine-131 and by short half-life iodine isotopes are not well established (Shore 1992).
The precise magnitude and pattern of the increase in the coming years of the incidence of thyroid cancer in the most highly exposed populations should be studied. Epidemiological studies currently under way should help to quantify the association between the dose received by the thyroid gland and the risk of developing thyroid cancer, and to identify the role of other genetic and environmental risk factors. It should be noted that iodine deficiency is widespread in the affected regions.
An increase in the incidence of leukaemia, particularly juvenile leukaemia (since children are more sensitive to the effects of ionizing radiation), is to be expected among the most highly exposed members of the population within five to ten years of the accident. Although no such increase has yet been observed, the methodological weaknesses of the studies conducted to date prevent any definitive conclusions from being drawn.
Psychosocial effects
The occurrence of more or less severe chronic psychological problems following psychological trauma is well established and has been studied primarily in populations faced with environmental disasters such as floods, volcanic eruptions and earthquakes. Post-traumatic stress is a severe, long-lasting and crippling condition (APA 1994).
Most of our knowledge on the effect of radiation accidents on psychological problems and stress is drawn from studies conducted in the wake of the Three Mile Island accident. In the year following the accident, immediate psychological effects were observed in the exposed population, and mothers of young children in particular exhibited increased sensitivity, anxiety and depression (Bromet et al. 1982). Further, an increase in depression and anxiety-related problems was observed in power-plant workers, compared to workers in another power plant (Bromet et al. 1982). In the following years (i.e., after the reopening of the power plant), approximately one-quarter of the surveyed population exhibited relatively significant psychological problems. There was no difference in the frequency of psychological problems in the rest of the survey population, compared to control populations (Dew and Bromet 1993). Psychological problems were more frequent among individuals living close to the power plant who were without a social support network, had a history of psychiatric problems, or who had evacuated their home at the time of the accident (Baum, Cohen and Hall 1993).
Studies are also under way among populations exposed during the Chernobyl accident and for whom stress appears to be an important public health issue (e.g., clean-up and relief workers and individuals living in a contaminated zone). For the moment, however, there are no reliable data on the nature, severity, frequency and distribution of psychological problems in the target populations. The factors that must be taken into account when evaluating the psychological and social consequences of the accident on residents of the contaminated zones include the harsh social and economic situation, the diversity of the available compensation systems, the effects of evacuation and resettlement (approximately 100,000 additional people were resettled in the years following the accident), and the effects of lifestyle limitations (e.g., modification of nutrition).
Principles of Prevention and Guidelines
Safety principles and guidelines
Industrial and medical use of radioactive sources
While it is true that the major radiation accidents reported have all occurred at nuclear power plants, the use of radioactive sources in other settings has nevertheless resulted in accidents with serious consequences for workers or the general public. The prevention of accidents such as these is essential, especially in light of the disappointing prognosis in cases of high-dose exposure. Prevention depends on proper worker training and on the maintenance of a comprehensive life-cycle inventory of radioactive sources which includes information on both the sources’ nature and location. The IAEA has established a series of safety guidelines and recommendations for the use of radioactive sources in industry, medicine and research (Safety Series No. 102). The principles in question are similar to those presented below for nuclear power plants.
Safety in nuclear power plants (IAEA Safety Series No. 75, INSAG-3)
The goal here is to protect both humans and the environment from the emission of radioactive materials under any circumstance. To this end, it is necessary to apply a variety of measures throughout the design, construction, operation and decommissioning of nuclear power plants.
The safety of nuclear power plants is fundamentally dependent on the “defence in depth” principle—that is, the redundancy of systems and devices designed to compensate for technical or human errors and deficiencies. Concretely, radioactive materials are separated from the environment by a series of successive barriers. In nuclear power production reactors, the last of these barriers is the containment structure (absent on the Chernobyl site but present at Three Mile Island). To avoid the breakdown of these barriers and to limit the consequences of breakdowns, the following three safety measures should be practised throughout the power plant’s operational life: control of the nuclear reaction, cooling of fuel, and containment of radioactive material.
Another essential safety principle is “operating experience analysis”—that is, using information gleaned from events, even minor ones, occurring at other sites to increase the safety of an existing site. Thus, analysis of the Three Mile Island and Chernobyl accidents has resulted in the implementation of modifications designed to ensure that similar accidents do not occur elsewhere.
Finally, it should be noted that significant efforts have been expended to promote a culture of safety, that is, a culture that is continually responsive to safety concerns related to the plant’s organization, activities and practices, as well as to individual behaviour. To increase the visibility of incidents and accidents involving nuclear power plants, an international scale of nuclear events (INES), identical in principle to scales used to measure the severity of natural phenomena such as earthquakes and wind, has been developed (table 12). This scale is not however suitable for the evaluation of a site’s safety or for performing international comparisons.
Table 13. International scale of nuclear incidents
|
Level |
Offsite |
Onsite |
Protective structure |
|
7—Major accident |
Major emission, |
||
|
6—Serious accident |
Significant emission, |
||
|
5—Accident |
Limited emission, |
Serious damage to |
|
|
4—Accident |
Low emission, public |
Damage to reactors |
|
|
3—Serious incident |
Very low emission, |
Serious |
Accident barely avoided |
|
2—Incident |
Serious contamination |
Serious failures of safety measures |
|
|
1—Abnormality |
Abnormality beyond |
||
|
0—Disparity |
No significance from |
Principles of the protection of the general public from exposure to radiation
In cases involving the potential exposure of the general public, it may be necessary to apply protective measures designed to prevent or limit exposure to ionizing radiation; this is particularly important if deterministic effects are to be avoided. The first measures which should be applied in emergency are evacuation, sheltering and administration of stable iodine. Stable iodine should be distributed to exposed populations, since this will saturate the thyroid and inhibit its uptake of radioactive iodine. To be effective, however, thyroid saturation must occur before or soon after the start of exposure. Finally, temporary or permanent resettlement, decontamination, and control of agriculture and food may eventually be necessary.
Each of these countermeasures has its own “action level” (table 14), not to be confused with the ICRP dose limits for workers and the general public, developed to ensure adequate protection in cases of non-accidental exposure (ICRP 1991).
Table 14. Examples of generic intervention levels for protective measures for general population
|
Protective measure |
Intervention level (averted dose) |
|
Emergency |
|
|
Containment |
10 mSv |
|
Evacuation |
50 mSv |
|
Distribution of stable iodine |
100 mGy |
|
Delayed |
|
|
Temporary resettlement |
30 mSv in 30 days; 10 mSv in the next 30 days |
|
Permanent resettlement |
1 Sv lifetime |
Source: IAEA 1994.
Research Needs and Future Trends
Current safety research concentrates on improving the design of nuclear power-generating reactors—more specifically, on the reduction of the risk and effects of core meltdown.
The experience gained from previous accidents should lead to improvements in the therapeutic management of seriously irradiated individuals. Currently, the use of bone marrow cell growth factors (haematopoietic growth factors) in the treatment of radiation-induced medullary aplasia (developmental failure) is being investigated (Thierry et al. 1995).
The effects of low doses and dose rates of ionizing radiation remains unclear and needs to be clarified, both from a purely scientific point of view and for the purposes of establishing dose limits for the general public and for workers. Biological research is necessary to elucidate the carcinogenic mechanisms involved. The results of large-scale epidemiological studies, especially those currently under way on workers at nuclear power plants, should prove useful in improving the accuracy of cancer risk estimates for populations exposed to low doses or dose rates. Studies on populations which are or have been exposed to ionizing radiation due to accidents should help further our understanding of the effects of higher doses, often delivered at low dose rates.
The infrastructure (organization, equipment and tools) necessary for the timely collection of data essential for the evaluation of the health effects of radiation accidents must be in place well in advance of the accident.
Finally, extensive research is necessary to clarify the psychological and social effects of radiation accidents (e.g., the nature and frequency of, and risk factors for, pathological and non-pathological post-traumatic psychological reactions). This research is essential if the management of both occupationally and non-occupationally exposed populations is to be improved.
Occupational Health and Safety Measures in Agricultural Areas Contaminated by Radionuclides: The Chernobyl Experience
Massive contamination of agricultural lands by radionuclides occurs, as a rule, due to large accidents at the enterprises of nuclear industry or nuclear power stations. Such accidents occurred at Windscale (England) and South Ural (Russia). The largest accident happened in April 1986 at the Chernobyl nuclear power station. The latter entailed intensive contamination of soils over several thousands of square kilometres.
The major factors contributing to radiation effects in agricultural areas are as follows:
- whether radiation is from a single or a long-term exposure
- total quantity of radioactive substances entering the environment
- ratio of radionuclides in the fallout
- distance from the source of radiation to agricultural lands and settlements
- hydrogeological and soil characteristics of agricultural lands and the purpose of their use
- peculiarities of work of the rural population; diet, water supply
- time since the radiological accident.
As a result of the Chernobyl accident more than 50 million Curies (Ci) of mostly volatile radionuclides entered the environment. At the first stage, which covered 2.5 months (the “iodine period”), iodine-131 produced the greatest biological hazard, with significant doses of high-energy gamma radiation.
Work on agricultural lands during the iodine period should be strictly regulated. Iodine-131 accumulates in the thyroid gland and damages it. After the Chernobyl accident, a zone of very high radiation intensity, where no one was permitted to live or work, was defined by a 30 km radius around the station.
Outside this prohibited zone, four zones with various rates of gamma radiation on the soils were distinguished according to which types of agricultural work could be performed; during the iodine period, the four zones had the following radiation levels measured in roentgen (R):
- zone 1—less than 0.1 mR/h
- zone 2—0.1 to 1 mR/h
- zone 3—1.0 to 5 mR/h
- zone 4—5 mR/h and more.
Actually, due to the “spot” contamination by radionuclides over the iodine period, agricultural work in these zones was performed at levels of gamma irradiation from 0.2 to 25 mR/h. Apart from uneven contamination, variation in gamma radiation levels was caused by different concentrations of radionuclides in different crops. Forage crops in particular are exposed to high levels of gamma emitters during harvesting, transportation, ensilage and when they are used as fodder.
After the decay of iodine-131, the major hazard for agricultural workers is presented by the long-lived nuclides caesium-137 and strontium-90. Caesium-137, a gamma emitter, is a chemical analogue of potassium; its intake by humans or animals results in uniform distribution throughout the body and it is relatively quickly excreted with urine and faeces. Thus, the manure in the contaminated areas is an additional source of radiation and it must be removed as quickly as possible from stock farms and stored in special sites.
Strontium-90, a beta emitter, is a chemical analogue of calcium; it is deposited in bone marrow in humans and animals. Strontium-90 and caesium-137 can enter the human body through contaminated milk, meat or vegetables.
The division of agricultural lands into zones after the decay of short-lived radionuclides is carried out according to a different principle. Here, it is not the level of gamma radiation, but the amount of soil contamination by caesium-137, strontium-90 and plutonium-239 that are taken into account.
In the case of particularly severe contamination, the population is evacuated from such areas and farm work is performed on a 2-week rotation schedule. The criteria for zone demarcation in the contaminated areas are given in table 1.
Table 1. Criteria for contamination zones
|
Contamination zones |
Soil contamination limits |
Dosage limits |
Type of action |
|
1. 30 km zone |
– |
– |
Residing of |
|
2. Unconditional |
15 (Ci)/km2 |
0.5 cSv/year |
Agricultural work is performed with 2-week rotation schedule under strict radiological control. |
|
3. Voluntary |
5–15 Ci/km2 |
0.01–0.5 |
Measures are undertaken to reduce |
|
4. Radio- ecological |
1–5 Ci/km2 |
0.01 cSv/year |
Agricultural work is |
When people work on agricultural lands contaminated by radionuclides, the intake of radionuclides by the body through respiration and contact with soil and vegetable dusts may occur. Here, both beta emitters (strontium-90) and alpha emitters are extremely dangerous.
As a result of accidents at nuclear power stations, part of radioactive materials entering the environment are low-dispersed, highly active particles of the reactor fuel—“hot particles”.
Considerable amounts of dust containing hot particles are generated during agricultural work and in windy periods. This was confirmed by the results of investigations of tractor air filters taken from machines which were operated on the contaminated lands.
The assessment of dose loads on the lungs of agricultural workers exposed to hot particles revealed that outside the 30 km zone the doses amounted to several millisieverts (Loshchilov et al. 1993).
According to the data of Bruk et al. (1989) the total activity of caesium-137 and caesium-134 in the inspired dust in machine operators amounted to 0.005 to 1.5 nCi/m3. According to their calculations, over the total period of field work the effective dose to lungs ranged from 2 to
70 cSv.
The relation between the amount of soil contamination by caesium-137 and radioactivity of work zone air was established. According to the data of the Kiev Institute for Occupational Health it was found that when the soil contamination by caesium-137 amounted to 7.0 to 30.0 Ci/km2 the radioactivity of the breathing zone air reached 13.0 Bq/m3. In the control area, where the density of contamination amounted to 0.23 to 0.61 Ci/km3, the radioactivity of work zone air ranged from 0.1 to 1.0 Bq/m3 (Krasnyuk, Chernyuk and Stezhka 1993).
The medical examinations of agricultural machine operators in the “clear” and contaminated zones revealed an increase in cardiovascular diseases in workers in the contaminated zones, in the form of ischaemic heart disease and neurocirculatory dystonia. Among other disorders dysplasia of the thyroid gland and an increased level of monocytes in the blood were registered more frequently.
Hygienic Requirements
Work schedules
After large accidents at nuclear power stations, temporary regulations for the population are usually adopted. After the Chernobyl accident temporary regulations for a period of one year were adopted, with the TLV of 10 cSv. It is assumed that workers receive 50% of their dose due to external radiation during work. Here, the threshold of intensity of radiation dose over the eight-hour work day should not exceed 2.1 mR/h.
During agricultural work, the radiation levels at workplaces can fluctuate significantly, depending on the concentrations of radioactive substances in soils and plants; they also fluctuate during technological processing (siloing, preparation of dry fodder and so on). In order to reduce dosages to workers, regulations of time limits for agricultural work are introduced. Figure 1 shows regulations which were introduced after the Chernobyl accident.
Figure 1. Time limits for agricultural work depending on intensity of gamma-ray radiation at workplaces.
Agrotechnologies
When carrying out agricultural work in conditions of high contamination of soils and plants, it is necessary to strictly observe measures directed at prevention of dust contamination. The loading and unloading of dry and dusty substances should be mechanized; the neck of the conveyer tube should be covered with fabric. Measures directed at the decrease of dust release must be undertaken for all types of field work.
Work using agricultural machinery should be carried out taking due account of cabin pressurization and the choice of the proper direction of operation, with the wind at the side being preferable. If possible it is desirable to first water the areas being cultivated. The wide use of industrial technologies is recommended so as to eliminate manual work on the fields as much as possible.
It is appropriate to apply substances to the soils which can promote absorption and fixation of radionuclides, changing them into insoluble compounds and thus preventing the transfer of radionuclides into plants.
Agricultural machinery
One of the major hazards for the workers is agricultural machinery contaminated by radionuclides. The allowable work time on the machines depends on the intensity of gamma radiation emitted from the cabin surfaces. Not only is the thorough pressurization of cabins required, but due control over ventilation and air conditioning systems as well. After work, wet cleaning of cabins and replacement of filters should be carried out.
When maintaining and repairing the machines after decontamination procedures, the intensity of gamma radiation at the outer surfaces should not exceed 0.3 mR/h.
Buildings
Routine wet cleaning should be done inside and outside buildings. Buildings should be equipped with showers. When preparing fodder which contains dust components, it is necessary to adhere to procedures aimed at prevention of dust intake by the workers, as well as to keep the dust off the floor, equipment and so on.
Pressurization of the equipment should be under control. Workplaces should be equipped with effective general ventilation.
Use of pesticides and mineral fertilizers
The application of dust and granular pesticides and mineral fertilizers, as well as spraying from aeroplanes, should be restricted. Machine spraying and application of granular chemicals as well as liquid mixed fertilizers are preferable. The dust mineral fertilizers should be stored and transported only in tightly closed containers.
Loading and unloading work, preparation of pesticide solutions and other activities should be performed using maximum individual protective equipment (overalls, helmets, goggles, respirators, rubber gauntlets and boots).
Water supply and diet
There should be special closed premises or motor vans without draughts where workers can take their meals. Before taking meals workers should clean their clothes and thoroughly wash their hands and faces with soap and running water. During summer periods field workers should be supplied with drinking water. The water should be kept in closed containers. Dust must not enter containers when filling them with water.
Preventive medical examinations of workers
Periodic medical examinations should be carried out by a physician; laboratory analysis of blood, ECG and tests of respiratory function are compulsory. Where radiation levels do not exceed permissible limits, the frequency of medical examinations should be not less than once every 12 months. Where there are higher levels of ionizing radiation the examinations should be carried out more frequently (after sowing, harvesting and so on) with due account of radiation intensity at workplaces and the total absorbed dose.
Organization of Radiological Control over Agricultural Areas
The major indices characterizing the radiological situation after fallout are gamma radiation intensity in the area, contamination of agricultural lands by the selected radionuclides and content of radionuclides in agricultural products.
The determination of gamma radiation levels in the areas allows the drawing of the borders of severely contaminated areas, estimation of doses of external radiation to people engaged in agricultural work and the establishing of corresponding schedules providing for radiological safety.
The functions of radiological monitoring in agriculture are usually the responsibility of radiological laboratories of the sanitary service as well as veterinary and agrochemical radiological laboratories. The training and education of the personnel engaged in dosimetric control and consultations for the rural population are carried out by these laboratories.
Case Study: The Kader Toy Factory Fire
A tragic industrial fire in Thailand has focused worldwide attention on the need to adopt and enforce state-of-the-art codes and standards in industrial occupancies.
On May 10, 1993, a major fire at the Kader Industrial (Thailand) Co. Ltd. factory located in the Nakhon Pathom Province of Thailand killed 188 workers (Grant and Klem 1994). This disaster stands as the world’s worst accidental loss-of-life fire in an industrial building in recent history, a distinction held for 82 years by the Triangle Shirtwaist factory fire that killed 146 workers in New York City (Grant 1993). Despite the years between these two disasters, they share striking similarities.
Various domestic and international agencies have focused on this incident following its occurrence. With respect to fire protection concerns, the National Fire Protection Association (NFPA) cooperated with the International Labour Organization (ILO) and with the Bangkok Police Fire Brigade in documenting this fire.
Questions for a Global Economy
In Thailand, the Kader fire has created a great deal of interest about the country’s fire safety measures, particularly its building code design requirements and enforcement policies. Thai Prime Minister Chuan Leekpai, who travelled to the scene on the evening of the fire, has pledged that the government will address fire safety issues. According to the Wall Street Journal (1993), Leekpai has called for tough action against those who violate the safety laws. Thai Industry Minister Sanan Kachornprasart is quoted as saying that “Those factories without fire prevention systems will be ordered to install one, or we will shut them down”.
The Wall Street Journal goes on to state that labour leaders, safety experts and officials say that the Kader fire may help tighten building codes and safety regulations, but they fear that lasting progress is still far off as employers flout rules and governments allow economic growth to take priority over worker safety.
Because the majority of the shares of Kader Industrial (Thailand) Co. Ltd. are owned by foreign interests, the fire has also fuelled international debate about foreign investors’ responsibilities for ensuring the safety of the workers in their sponsoring country. Twenty per cent of the Kader shareholders are from Taiwan, and 79.96% are from Hong Kong. A mere 0.04% of Kader is owned by Thai nationals.
Moving into a global economy implies that products are manufactured at one location and used at other locations throughout the world. Desire for competitiveness in this new market should not lead to compromise in fundamental industrial fire safety provisions. There is a moral obligation to provide workers with an adequate level of fire protection, no matter where they are located.
The Facility
The Kader facility, which manufactured stuffed toys and plastic dolls primarily intended for export to the United States and other developed countries, is located in the Sam Phran District of Nakhon Pathom Province. This is not quite halfway between Bangkok and the nearby city of Kanchanaburi, the site of the infamous Second World War railroad bridge over the River Kwai.
The structures that were destroyed in the blaze were all owned and operated directly by Kader, which owns the site. Kader has two sister companies that also operate at the location on a lease arrangement.
The Kader Industrial (Thailand) Co. Ltd. was first registered on 27 January 1989, but the company’s licence was suspended on 21 November 1989, after a fire on 16 August 1989 destroyed the new plant. This fire was attributed to the ignition of polyester fabric used in the manufacture of dolls in a spinning machine. After the plant was rebuilt, the Ministry of Industry allowed it to reopen on 4 July 1990.
Between the time the factory reopened and the May 1993 fire, the facility experienced several other, smaller fires. One of them, which occurred in February 1993, did considerable damage to Building Three, which was still being repaired at the time of the fire in May 1993. The February fire occurred late at night in a storage area and involved polyester and cotton materials. Several days after this blaze a labour inspector visited the site and issued a warning that pointed out the plant’s need for safety officers, safety equipment and an emergency plan.
Initial reports following the May 1993 fire noted that there were four buildings on the Kader site, three of which were destroyed by the fire. In a sense this is true, but the three buildings were actually a single E-shaped structure (see figure 1), the three primary portions of which were designated Buildings One, Two and Three. Nearby was a one-storey workshop and another four-storey structure referred to as Building Four.
Figure 1. Site plan of the Kader toy factory
The E-shaped building was a four-storey structure composed of concrete slabs supported by a structural steel frame. There were windows around the perimeter of each floor and the roof was a gently sloped, peaked arrangement. Each portion of the building had a freight elevator and two stairwells that were each 1.5 metres (3.3 feet) wide. The freight elevators were caged assemblies.
Each building at the plant was equipped with a fire alarm system. None of the buildings had automatic sprinklers, but portable extinguishers and hose stations were installed on outside walls and in the stairwells of each building. None of the structural steel in the building was fireproofed.
There is conflicting information about the total number of workers at the site. The Federation of Thai Industries had pledged to help 2,500 plant employees displaced by the fire, but it is unclear how many employees were at the site at any one time. When the fire occurred, it was reported that there were 1,146 workers in Building One. Thirty-six were on the first floor, 10 were on the second, 500 were on the third, and 600 were on the fourth. There were 405 workers in Building Two. Sixty of them were on the first floor, 5 were on the second, 300 were on the third and 40 were on the fourth. It is not clear how many workers were in Building Three since a portion of it was still being refurbished. Most of the workers at the plant were women.
The Fire
Monday, May 10, was a normal workday at the Kader facility. At approximately 4:00 p.m., as the end of the day shift approached, someone discovered a small fire on the first floor near the south end of Building One. This portion of the building was used to package and store the finished products, so it contained a considerable fuel load (see figure 2). Each building at the facility had a fuel load composed of fabric, plastics and materials used for stuffing, as well as other normal workplace materials.
Figure 2. Internal layout of buildings one, two and three
Security guards in the vicinity of the fire tried unsuccessfully to extinguish the flames before they called the local police fire brigade at 4:21 p.m. Authorities received two more calls, at 4:30 p.m. and 4:31 p.m. The Kader facility is just beyond the jurisdictional boundaries of Bangkok, but fire apparatus from Bangkok, as well as apparatus from Nakhon Pathom Province, responded.
As the workers and security guards tried in vain to extinguish the fire, the building began filling with smoke and other products of combustion. Survivors reported that the fire alarm never sounded in Building One, but many workers grew concerned when they saw smoke on the upper floors. Despite the smoke, security guards reportedly told some workers to stay at their stations because it was a small fire that would soon be under control.
The fire spread rapidly throughout Building One, and the upper floors soon became untenable. The blaze blocked the stairwell at the south end of the building, so most of the workers rushed to the north stairwell. This meant that approximately 1,100 people were trying to leave the third and fourth floors through a single stairwell.
The first fire apparatus arrived at 4:40 p.m., their response time having been extended because of the relatively remote location of the facility and the gridlock conditions typical of Bangkok traffic. Arriving fire-fighters found Building One heavily involved in flames and already beginning to collapse, with people jumping from the third and fourth floors.
Despite the fire-fighters’ efforts, Building One collapsed completely at approximately 5:14 p.m. Fanned by strong winds blowing toward the north, the blaze spread quickly into Buildings Two and Three before the fire brigade could effectively defend them. Building Two reportedly collapsed at 5:30 p.m., and Building Three at 6:05 p.m. The fire brigade successfully kept the fire from entering Building Four and the smaller, one-storey workshop nearby, and the fire-fighters had the blaze under control by 7:45 p.m. Approximately 50 pieces of fire apparatus were involved in the battle.
The fire alarms in Buildings Two and Three reportedly functioned properly, and all the workers in those two buildings escaped. The workers in Building One were not so fortunate. A large number of them jumped from the upper floors. In all, 469 workers were taken to the hospital, where 20 died. The other dead were found during the post-fire search of what had been the north stairwell of the building. Many of them apparently succumbed to lethal products of combustion before or during the building’s collapse. According to the latest information available, 188 people, most of them female, have died as a result of this fire.
Even with the help of six large hydraulic cranes that were moved to the site to facilitate the search for victims, it was several days before all the bodies could be removed from the rubble. There were no fatalities among the fire-fighters, although there was one injury.
Traffic in the vicinity, which is normally congested, made transporting the victims to hospitals difficult. Nearly 300 injured workers were taken to the nearby Sriwichai II Hospital, although many of them were transferred to alternate medical facilities when the number of victims exceeded the hospital’s capacity to treat them.
The day after the fire, Sriwichai II Hospital reported that it had kept 111 fire victims. The Kasemrat Hospital received 120; Sriwichai Pattanana received 60; Sriwichai I received 50; Ratanathibet I received 36; Siriraj received 22; and Bang Phai received 17. The remaining 53 injured workers were sent to various other medical facilities in the area. In all, 22 hospitals throughout Bangkok and Nakhon Pathom Province participated in treating victims of the disaster.
Sriwichai II Hospital reported that 80% of their 111 victims suffered serious injuries and that 30% required surgery. Half of the patients suffered only from smoke inhalation, while the remainder also suffered burns and fractures that ranged from broken ankles to fractured skulls. At least 10% of the injured Kader workers admitted to Sriwichai II Hospital risk permanent paralysis.
Determining the cause of this fire became a challenge because the portion of the facility in which it began was totally destroyed and the survivors have provided conflicting information. Since the fire started near a large electrical control panel, investigators first thought that problems with the electrical system might have been the cause. They also considered arson. At this time, however, Thai authorities feel that a carelessly discarded cigarette may have been the source of ignition.
Analysing the Fire
For 82 years, the world has recognized the 1911 Triangle Shirtwaist factory fire in New York City as the worst accidental loss-of-life industrial fire in which the fatalities were limited to the building of fire origin. With 188 fatalities, however, the Kader factory fire now replaces the Triangle fire in the record books.
When analysing the Kader fire, a direct comparison with the Triangle fire provides a useful benchmark. The two buildings were similar in a number of ways. The arrangement of the exits was poor, the fixed fire protection systems were insufficient or ineffective, the initial fuel package was readily combustible, and the horizontal and vertical fire separations were inadequate. In addition, neither company had provided its workers with adequate fire safety training. However, there is one distinct difference between these two fires: the Triangle Shirtwaist factory building did not collapse and the Kader buildings did.
Inadequate exit arrangements were perhaps the most significant factor in the high loss of life at both the Kader and the Triangle fires. Had the exiting provisions of NFPA 101, the Life Safety Code, which was established as a direct result of the Triangle fire, been applied at the Kader facility, substantially fewer lives would have been lost (NFPA 101, 1994).
Several fundamental requirements of the Life Safety Code pertain directly to the Kader fire. For example, the Code requires that every building or structure be constructed, arranged and operated in such a way that its occupants are not placed in any undue danger by fire, smoke, fumes or the panic that may occur during an evacuation or during the time it takes to defend the occupants in place.
The Code also requires that every building have enough exits and other safeguards of the proper size and at the proper locations to provide an escape route for every occupant of a building. These exits should be appropriate to the individual building or structure, taking into account the character of the occupancy, the capabilities of the occupants, the number of occupants, the fire protection available, the height and type of building construction and any other factor necessary to provide all the occupants with a reasonable degree of safety. This was obviously not the case in the Kader facility, where the blaze blocked one of Building One’s two stairwells, forcing approximately 1,100 people to flee the third and fourth floors through a single stairwell.
In addition, the exits should be arranged and maintained so that they provide free and unobstructed egress from all parts of a building whenever it is occupied. Each of these exits should be clearly visible, or the route to every exit should be marked in such a way that every occupant of the building who is physically and mentally able readily knows the direction of escape from any point.
Every vertical exit or opening between the floors of a building should be enclosed or protected as necessary to keep the occupants reasonably safe while they exit and to prevent fire, smoke and fumes from spreading from floor to floor before the occupants have had a chance to use the exits.
The outcomes of both the Triangle and the Kader fires were significantly affected by the lack of adequate horizontal and vertical fire separations. The two facilities were arranged and built in such a way that a fire on a lower floor could spread rapidly to the upper floors, thus trapping a large number of workers.
Large, open work spaces are typical of industrial facilities, and fire-rated floors and walls must be installed and maintained to slow the spread of fire from one area to another. Fire also must be kept from spreading externally from the windows on one floor to those on another floor, as it did during the Triangle fire.
The most effective way to limit vertical fire spread is to enclose stairwells, elevators, and other vertical openings between floors. Reports of features such as caged freight elevators at the Kader factory raise significant questions about the ability of the buildings’ passive fire protection features to prevent vertical spread of fire and smoke.
Fire Safety Training and Other Factors
Another factor that contributed to the large loss of life in both the Triangle and Kader fires was the lack of adequate fire safety training, and the rigid security procedures of both companies.
After the fire at the Kader facility, survivors reported that fire drills and fire safety training were minimal, although the security guards had apparently had some incipient fire training. The Triangle Shirtwaist factory had no evacuation plan, and fire drills were not implemented. Furthermore, post-fire reports from Triangle survivors indicate that they were routinely stopped as they left the building at the end of the work day for security purposes. Various post-fire accusations by Kader survivors also imply that security arrangements slowed their exit, although these accusations are still being investigated. In any case, the lack of a well-understood evacuation plan seems to have been an important factor in the high loss of life sustained in the Kader fire. Chapter 31 of the Life Safety Code addresses fire drills and evacuation training.
The absence of fixed automatic fire protection systems also affected the outcome of both the Triangle and the Kader fires. Neither facility was equipped with automatic sprinklers, although the Kader buildings did have a fire alarm system. According to the Life Safety Code, fire alarms should be provided in buildings whose size, arrangement or occupancy make it unlikely that the occupants themselves will notice a fire immediately. Unfortunately, the alarms reportedly never operated in Building One, which resulted in a significant delay in evacuation. There were no fatalities in Buildings Two and Three, where the fire alarm system functioned as intended.
Fire alarm systems should be designed, installed and maintained in accordance with documents like NFPA 72, the National Fire Alarm Code (NFPA 72, 1993). Sprinkler systems should be designed and installed in accordance with documents like NFPA 13, Installation of Sprinkler Systems, and maintained in accordance with NFPA 25, Inspection, Testing, and Maintenance of Water-Based Fire Protection Systems (NFPA 13, 1994; NFPA 25, 1995).
The initial fuel packages in both the Triangle and Kader fires were similar. The Triangle fire started in rag bins and quickly spread to combustible clothing and garments before involving wood furnishings, some of which were impregnated with machine oil. The initial fuel package at the Kader plant consisted of polyester and cotton fabrics, various plastics, and other materials used to manufacture stuffed toys, plastic dolls, and other related products. These are materials that can typically be ignited easily, can contribute to rapid fire growth and spread, and have a high heat release rate.
Industry will probably always handle materials that have challenging fire protection characteristics, but manufacturers should recognize these characteristics and take the necessary precautions to minimize associated hazards.
The Building’s Structural Integrity
Probably the most notable difference between the Triangle and Kader fires is the effect they had on the structural integrity of the buildings involved. Even though the Triangle fire gutted the top three floors of the ten-storey factory building, the building remained structurally intact. The Kader buildings, on the other hand, collapsed relatively early in the fire because their structural steel supports lacked the fireproofing that would have allowed them to maintain their strength when exposed to high temperatures. A post-fire review of the debris at the Kader site showed no indication that any of the steel members had been fireproofed.
Obviously, building collapse during a fire presents a great threat to both the building’s occupants and to the fire-fighters involved in controlling the blaze. However, it is unclear whether the collapse of the Kader building had any direct effect on the number of fatalities, since the victims may have already succumbed to the effects of heat and products of combustion by the time the building collapsed. If the workers on the upper floors of Building One had been shielded from the products of combustion and heat while they were trying to escape, the building’s collapse would have been a more direct factor in the loss of life.
Fire Focused Attention on Fire Protection Principles
Among the fire protection principles on which the Kader fire has focused attention are exit design, occupant fire safety training, automatic detection and suppression systems, fire separations and structural integrity. These lessons are not new. They were first taught more than 80 years ago at the Triangle Shirtwaist fire and again, more recently, in a number of other fatal workplace fires, including those at the chicken-processing plant in Hamlet, North Carolina, USA, that killed 25 workers; at a doll factory in Kuiyong, China, that killed 81 workers; and at the electrical power plant in Newark, New Jersey, USA, that killed all 3 workers in the plant (Grant and Klem 1994; Klem 1992; Klem and Grant 1993).
The fires in North Carolina and New Jersey, in particular, demonstrate that the mere availability of state-of-the-art codes and standards, such as NFPA’s Life Safety Code, cannot prevent tragic losses. These codes and standards must also be adopted and rigorously enforced if they are to have any effect.
National, state and local public authorities should examine the way they enforce their building and fire codes to determine whether new codes are needed or existing codes need to be updated. This review should also determine whether a building plan review and inspection process is in place to ensure that the appropriate codes are followed. Finally, provisions must be made for periodic follow-up inspections of existing buildings to ensure that the highest levels of fire protection are maintained throughout the life of the building.
Building owners and operators must also be aware that they are responsible for ensuring that their employees’ working environment is safe. At the very least, the state-of-the-art fire protection design reflected in fire codes and standards must be in place to minimize the possibility of a catastrophic fire.
Had the Kader buildings been equipped with sprinklers and working fire alarms, the loss of life might not have been so high. Had Building One’s exits been better designed, hundreds of people might not have been injured jumping from the third and fourth floors. Had vertical and horizontal separations been in place, the fire might not have spread so quickly throughout the building. Had the buildings’ structural steel members been fireproofed, the buildings might not have collapsed.
Philosopher George Santayana has written: “Those who forget the past are condemned to repeat it.” The Kader Fire of 1993 was unfortunately, in many ways, a repeat of the Triangle Shirtwaist Fire of 1911. As we look to the future, we need to recognize all that we need to do, as a global society, to prevent history from repeating itself.
Impacts of Disasters: Lessons From a Medical Perspective
This article was adapted, with permission, from Zeballos 1993b.
Latin America and the Caribbean have not been spared their share of natural disasters. Almost every year catastrophic events cause deaths, injuries and enormous economic damage. Overall, it is estimated that the major natural disasters of the last two decades in this region caused property losses affecting nearly 8 million people, some 500,000 injuries and 150,000 deaths. These figures rely heavily on official sources. (It is quite difficult to obtain accurate information in sudden-onset disasters, because there are multiple information sources and no standardized information system.) The Economic Commission for Latin America and the Caribbean (ECLAC) estimates that during an average year, disasters in Latin America and the Caribbean cost US$1.5 billion and take 6,000 lives (Jovel 1991).
Table 1 lists major natural disasters that struck countries of the region in the 1970-93 period. It should be noted that slow- onset disasters, such as droughts and floods, are not included.
Table 1. Major disasters in Latin America and the Caribbean, 1970-93
|
Year |
Country |
Type of |
No.of deaths |
Est. no. of |
|
1970 |
Peru |
Earthquake |
66,679 |
3,139,000 |
|
1972 |
Nicaragua |
Earthquake |
10,000 |
400,000 |
|
1976 |
Guatemala |
Earthquake |
23,000 |
1,200,000 |
|
1980 |
Haiti |
Hurricane (Allen) |
220 |
330,000 |
|
1982 |
Mexico |
Volcanic eruption |
3,000 |
60,000 |
|
1985 |
Mexico |
Earthquake |
10,000 |
60,000 |
|
1985 |
Colombia |
Volcanic eruption |
23,000 |
200,000 |
|
1986 |
El Salvador |
Earthquake |
1,100 |
500,000 |
|
1988 |
Jamaica |
Hurricane (Gilbert) |
45 |
500,000 |
|
1988 |
Mexico |
Hurricane (Gilbert) |
250 |
200,000 |
|
1988 |
Nicaragua |
Hurricane (Joan) |
116 |
185,000 |
|
1989 |
Montserrat, |
Hurricane (Hugo) |
56 |
220,000 |
|
1990 |
Peru |
Earthquake |
21 |
130,000 |
|
1991 |
Costa Rica |
Earthquake |
51 |
19,700 |
|
1992 |
Nicaragua |
Tsunami |
116 |
13,500 |
|
1993 |
Honduras |
Tropical storm |
103 |
11,000 |
Source: PAHO 1989; OFDA (USAID),1989; UNDRO 1990.
Economic Impact
In recent decades, ECLAC has carried out extensive research on the social and economic impacts of disasters. This has clearly demonstrated that disasters have negative repercussions on social and economic development in developing countries. Indeed, the monetary losses caused by a major disaster often exceed the total annual gross income of the affected country. Not surprisingly, such events can paralyze affected countries and foster widespread political and social turmoil.
In essence, disasters have three kinds of economic impacts:
- direct impacts on the affected population’s property
- indirect impacts caused by lost economic production and services
- secondary impacts that become apparent after the disaster—such as reduced national income, increased inflation, foreign trade problems, heightened financial expenses, a resulting fiscal deficit, decreased monetary reserves and so on (Jovel 1991).
Table 2 shows the estimated losses caused by six major natural disasters. While such losses might not seem particularly devastating for developed countries with strong economies, they can have a serious and lasting impact on the weak and vulnerable economies of developing countries (PAHO 1989).
Table 2. Losses due to six natural disasters
|
Disaster |
Location |
Year(s) |
Total losses |
|
Earthquake |
Mexico |
1985 |
4,337 |
|
Earthquake |
El Salvador |
1986 |
937 |
|
Earthquake |
Ecuador |
1987 |
1,001 |
|
Volcanic eruption (Nevado del Ruiz) |
Colombia |
1985 |
224 |
|
Floods, drought (“El Niño”) |
Peru, Ecuador, Bolivia |
1982-83 |
3,970 |
|
Hurricane (Joan) |
Nicaragua |
1988 |
870 |
Source: PAHO 1989; ECLAC.
The Health Infrastructure
In any major disaster-related emergency, the first priority is to save lives and provide immediate emergency care for the injured. Among the emergency medical services mobilized for these purposes, hospitals play a key role. Indeed, in countries with a standardized emergency response system (one where the concept of “emergency medical services” encompasses provision of emergency care through the coordination of independent subsystems involving paramedics, fire-fighters and rescue teams) hospitals constitute the major component of that system (PAHO 1989).
Hospitals and other health care facilities are densely occupied. They house patients, personnel and visitors, and they operate 24 hours a day. Patients may be surrounded by special equipment or connected to life-support systems dependent on power supplies. According to project documents available from the Inter-American Development Bank (IDB) (personal communication, Tomas Engler, IDB), the estimated cost of one hospital bed in a specialized hospital varies from country to country, but the average runs from US$60,000 to US$80,000 and is greater for highly specialized facilities.
In the United States, particularly California, with its extensive experience in seismic-resistant engineering, the cost of one hospital bed can exceed US$110,000. In sum, modern hospitals are highly complex facilities combining the functions of hotels, offices, laboratories and warehouses (Peisert et al. 1984; FEMA 1990).
These health care facilities are highly vulnerable to hurricanes and earthquakes. This has been amply demonstrated by past experience in Latin America and the Caribbean. For example, as table 3 shows, just three disasters of the 1980s damaged 39 hospitals and destroyed some 11,332 hospital beds in El Salvador, Jamaica and Mexico. Besides damage to these physical plants at critical times, the loss of human life (including the death of highly qualified local professionals with promising futures) needs to be considered (see table 4 and table 5).
Table 3. Number of hospitals and hospital beds damaged or destroyed by three major natural disasters
|
Type of disaster |
No. of hospitals |
No. of beds lost |
|
Earthquake, Mexico (Federal District, September 1985) |
13 |
4,387 |
|
Earthquake, El Salvador (San Salvador, October 1986) |
4 |
1,860 |
|
Hurricane Gilbert (Jamaica, September 1988) |
23 |
5,085 |
|
Total |
40 |
11,332 |
Source: PAHO 1989; OFDA(USAID) 1989; ECLAC.
Table 4. Victims in two hospitals collapsed by the 1985 earthquake in Mexico
|
Collapsed hospitals |
||||
|
General hospital |
Juarez hospital |
|||
|
Number |
% |
Number |
% |
|
|
Fatalities |
295 |
62.6 |
561 |
75.8 |
|
Rescued |
129 |
27.4 |
179 |
24.2 |
|
Missing |
47 |
10.0 |
– |
– |
|
Total |
471 |
100.0 |
740 |
100.0 |
Source: PAHO 1987.
Table 5. Hospital beds lost as a result of the March 1985 Chilean earthquake
|
Region |
No. of existing hospitals |
No. of beds |
Beds lost in region |
|
|
No. |
% |
|||
|
Metropolitan Area |
26 |
11,464 |
2,373 |
20.7 |
|
Region 5 (Viña del Mar, Valparaíso, |
23 |
4,573 |
622 |
13.6 |
|
Region 6 (Rancagua) |
15 |
1,413 |
212 |
15.0 |
|
Region 7 (Ralca, Meula) |
15 |
2,286 |
64 |
2.8 |
|
Total |
79 |
19,736 |
3,271 |
16.6 |
Source: Wyllie and Durkin 1986.
At present the ability of many Latin American hospitals to survive earthquake disasters is uncertain. Many such hospitals are housed in old structures, some dating from Spanish colonial times; and while many others occupy contemporary buildings of appealing architectural design, lax application of building codes makes their ability to resist earthquakes questionable.
Risk Factors in Earthquakes
Of the various types of sudden natural disasters, earthquakes are by far the most damaging to hospitals. Of course, each earthquake has its own characteristics relating to its epicentre, type of seismic waves, geological nature of the soil through which the waves travel and so on. Nevertheless, studies have revealed certain common factors that tend to cause death and injuries and certain others that tend to prevent them. These factors include structural characteristics related to building failure, various factors related to human behaviour and certain characteristics of nonstructural equipment, furnishings and other items inside buildings.
In recent years, scholars and planners have been paying special attention to identification of risk factors affecting hospitals, in hopes of framing better recommendations and norms to govern the building and organization of hospitals in highly vulnerable zones. A brief listing of relevant risk factors is shown in table 6. These risk factors, particularly those related to the structural aspects, were observed to influence patterns of destruction during a December 1988 earthquake in Armenia that killed some 25,000 people, affected 1,100,000 and destroyed or severely damaged 377 schools, 560 health facilities and 324 community and cultural centres (USAID 1989).
Table 6. Risk factors associated with earthquake damage to hospital infrastructure
|
Structural |
Non-structural |
Behavioural |
|
Design |
Medical equipment |
Public information |
|
Quality of construction |
Laboratory equipment |
Motivation |
|
|
Office equipment |
Plans |
|
Materials |
Cabinets, shelves |
Educational programmes |
|
Soil conditions |
Stoves, refrigerators, heaters |
Health care staff training |
|
Seismic characteristics |
X-ray machines |
|
|
Time of the event |
Reactive materials |
|
|
Population density |
|
|
Damage on a similar scale occurred in June 1990, when an earthquake in Iran killed about 40,000 people, injured 60,000 others, left 500,000 homeless, and collapsed 60 to 90% of buildings in affected zones (UNDRO 1990).
To address these and like calamities, an international seminar was held in Lima, Peru, in 1989 on the planning, design, repair and management of hospitals in earthquake-prone areas. The seminar, sponsored by PAHO, Peru’s National University of Engineering and the Peruvian-Japanese Center for Seismic Research (CISMID), brought together architects, engineers and hospital administrators to study issues related to health facilities located in these areas. The seminar approved a core of technical recommendations and commitments directed at carrying out vulnerability analyses of hospital infrastructures, improving the design of new facilities and establishing safety measures for existing hospitals, with emphasis on those located in high-risk earthquake areas (CISMID 1989).
Recommendations on Hospital Preparedness
As the foregoing suggests, hospital disaster preparedness constitutes an important component of PAHO’s Office of Emergency Preparedness and Disaster Relief. Over the last ten years, member countries have been encouraged to pursue activities directed toward this end, including the following:
- classifying hospitals according to their risk factors and vulnerabilities
- developing internal and external hospital response plans and training personnel
- developing contingency plans and establishing safety measures for the professional and technical hospital staffs
- strengthening lifeline backup systems that help hospitals to function during emergency situations.
More broadly, a principal aim of the current International Decade for Natural Disaster Reduction (IDNDR) is to attract, motivate and commit national health authorities and policy-makers around the world, thereby encouraging them to strengthen the health services directed at coping with disasters and to reduce the vulnerability of those services in the developing world.
Issues Concerning Technological Accidents
During the last two decades, developing countries have entered into intense competition to achieve industrial development. The main reasons for this competition are as follows:
- to attract capital investment and to generate jobs
- to satisfy domestic demand for products at a lower cost and to alleviate dependency on the international market
- to compete with international and subregional markets
- to establish foundations for development.
Unfortunately, efforts made have not always resulted in obtaining the intended objectives. In effect, flexibility in attracting capital investment, lack of sound regulation with respect to industrial safety and environmental protection, negligence in the operation of industrial plants, use of obsolete technology, and other aspects have contributed to increasing the risk of technological accidents in certain areas.
In addition, the lack of regulation regarding the establishment of human settlements near or around industrial plants is an additional risk factor. In major Latin American cities it is common to see human settlements practically surrounding industrial complexes, and the inhabitants of these settlements are ignorant of the potential risks (Zeballos 1993a).
In order to avoid accidents such as those that occurred in Guadalajara (Mexico) in 1992, the following guidelines are suggested for the establishment of chemical industries, to protect industrial workers and the population at large:
- selection of appropriate technology and study of alternatives
- appropriate location of industrial plants
- regulation of human settlements in the neighbourhood of industrial plants
- security considerations for technology transfer
- routine inspection of industrial plants by local authorities
- expertise provided by specialized agencies
- role of workers in compliance with security rules
- rigid legislation
- classification of toxic materials and close supervision of their use
- public education and training of workers
- establishment of response mechanisms in case of emergency
- training of health workers in emergency plans for technological accidents.
ILO Convention concerning the Prevention of Major Industrial Accidents, 1993 (No. 74)
ILO 80th Session, 2nd June 1993
ILO 80th Session, 2nd June 1993
PART I. SCOPE AND DEFINITIONS
Article 1
1. The purpose of this Convention is the prevention of major accidents involving hazardous substances and the limitation of the consequences of such accidents.…
Article 3
For the purposes of this Convention:
(a) the term “hazardous substance” means a substance or mixture of substances which by virtue of chemical, physical or toxicological properties, either singly or in combination, constitutes a hazard;
(b) the term “threshold quantity” means for a given hazardous substance or category of substances that quantity, prescribed in national laws and regulations by reference to specific conditions, which if exceeded identifies a major hazard installation;
(c) the term “major hazard installation” means one which produces, processes, handles, uses, disposes of or stores, either permanently or temporarily, one or more hazardous substances or categories of substances in quantities which exceed the threshold quantity;
(d) the term “major accident” means a sudden occurrence—such as a major emission, fire or explosion—in the course of an activity within a major hazard installation, involving one or more hazardous substances and leading to a serious danger to workers, the public or the environment, whether immediate or delayed;
(e) the term “safety report“ means a written presentation of the technical, management and operational information covering the hazards and risks of a major hazard installation and their control and providing justification for the measures taken for the safety of the installation;
(f) the term “near miss” means any sudden event involving one or more hazardous substances which, but for mitigating effects, actions or systems, could have escalated to a major accident.
PART II. GENERAL PRINCIPLES
Article 4
1. In the light of national laws and regulations, conditions and practices, and in consultation with the most representative organizations of employers and workers and with other interested parties who may be affected, each Member shall formulate, implement and periodically review a coherent national policy concerning the protection of workers, the public and the environment against the risk of major accidents.
2. This policy shall be implemented through preventive and protective measures for major hazard installations and, where practicable, shall promote the use of the best available safety technologies.
Article 5
1. The competent authority, or a body approved or recognized by the competent authority, shall, after consulting the most representative organizations of employers and workers and other interested parties who may be affected, establish a system for the identification of major hazard installations as defined in Article 3(c), based on a list of hazardous substances or of categories of hazardous substances or of both, together with their respective threshold quantities, in accordance with national laws and regulations or international standards.
2. The system mentioned in paragraph 1 above shall be regularly reviewed and updated.
Article 6
The competent authority, after consulting the representative organizations of employers and workers concerned, shall make special provision to protect confidential information transmitted or made available to it in accordance with Articles 8, 12, 13 or 14, whose disclosure would be liable to cause harm to an employer’s business, so long as this provision does not lead to serious risk to the workers, the public or the environment.
PART III. RESPONSIBILITIES OF EMPLOYERS IDENTIFICATION
Article 7
Employers shall identify any major hazard installation within their control on the basis of the system referred to in Article 5.
NOTIFICATION
Article 8
1. Employers shall notify the competent authority of any major hazard installation which they have identified:
(a) within a fixed time-frame for an existing installation;
(b) before it is put into operation in the case of a new installation.
2. Employers shall also notify the competent authority before any permanent closure of a major hazard installation.
Article 9
In respect of each major hazard installation employers shall establish and maintain a documented system of major hazard control which includes provision for:
(a) the identification and analysis of hazards and the assessment of risks including consideration of possible interactions between substances;
(b) technical measures, including design, safety systems, construction, choice of chemicals, operation, maintenance and systematic inspection of the installation;
(c) organizational measures, including training and instruction of personnel, the provision of equipment in order to ensure their safety, staffing levels, hours of work, definition of responsibilities, and controls on outside contractors and temporary workers on the site of the installation;
(d) emergency plans and procedures, including:
(i) the preparation of effective site emergency plans and procedures, including
emergency medical procedures, to be applied in case of major accidents or threat
thereof, with periodic testing and evaluation of their effectiveness and revision as
necessary;
(ii) the provision of information on potential accidents and site emergency plans to
authorities and bodies responsible for the preparation of emergency plans and
procedures for the protection of the public and the environment outside the site of
the installation;
(iii) any necessary consultation with such authorities and bodies;
(e) measures to limit the consequences of a major accident;
(f) consultation with workers and their representatives;
(g) improvement of the system, including measures for gathering information and analysing accidents and near misses. The lessons so learnt shall be discussed with the workers and their representatives and shall be recorded in accordance with national law and practice.…
* * *
PART IV. RESPONSIBILITIES OF COMPETENT AUTHORITIES
OFF-SITE EMERGENCY PREPAREDNESS
Article 15
Taking into account the information provided by the employer, the competent authority shall ensure that emergency plans and procedures containing provisions for the protection of the public and the environment outside the site of each major hazard installation are established, updated at appropriate intervals and coordinated with the relevant authorities and bodies.
Article 16
The competent authority shall ensure that:
(a) information on safety measures and the correct behaviour to adopt in the case of a major accident is disseminated to members of the public liable to be affected by a major accident without their having to request it and that such information is updated and redisseminated at appropriate intervals;
(b) warning is given as soon as possible in the case of a major accident;
(c) where a major accident could have transboundary effects, the information required in (a) and (b) above is provided to the States concerned, to assist in cooperation and coordination arrangements.
Article 17
The competent authority shall establish a comprehensive siting policy arranging for the appropriate separation of proposed major hazard installations from working and residential areas and public facilities, and appropriate measures for existing installations. Such a policy shall reflect the General Principles set out in Part II of the Convention.
INSPECTION
Article 18
1. The competent authority shall have properly qualified and trained staff with the appropriate skills, and sufficient technical and professional support, to inspect, investigate, assess, and advise on the matters dealt with in this Convention and to ensure compliance with national laws and regulations.
2. Representatives of the employer and representatives of the workers of a major hazard installation shall have the opportunity to accompany inspectors supervising the application of the measures prescribed in pursuance of this Convention, unless the inspectors consider, in the light of the general instructions of the competent authority, that this may be prejudicial to the performance of their duties.
Article 19
The competent authority shall have the right to suspend any operation which poses an imminent threat of a major accident.
PART V. RIGHTS AND DUTIES OF WORKERS AND THEIR REPRESENTATIVES
Article 20
The workers and their representatives at a major hazard installation shall be consulted through appropriate cooperative mechanisms in order to ensure a safe system of work. In particular, the workers and their representatives shall:
(a) be adequately and suitably informed of the hazards associated with the major hazard installation and their likely consequences;
(b) be informed of any orders, instructions or recommendations made by the competent authority;
(c) be consulted in the preparation of, and have access to, the following documents:
(i) the safety report;
(ii) emergency plans and procedures;
(iii) accident reports;
(d) be regularly instructed and trained in the practices and procedures for the prevention of major accidents and the control of developments likely to lead to a major accident and in the emergency procedures to be followed in the event of a major accident;
(e) within the scope of their job, and without being placed at any disadvantage, take corrective action and if necessary interrupt the activity where, on the basis of their training and experience, they have reasonable justification to believe that there is an imminent danger of a major accident, and notify their supervisor or raise the alarm, as appropriate, before or as soon as possible after taking such action;
(f) discuss with the employer any potential hazards they consider capable of generating a major accident and have the right to notify the competent authority of those hazards.
Article 21
Workers employed at the site of a major hazard installation shall:
(a) comply with all practices and procedures relating to the prevention of major accidents and the control of developments likely to lead to a major accident within the major hazard installation;
(b) comply with all emergency procedures should a major accident occur.
PART VI. RESPONSIBILITY OF EXPORTING STATES
Article 22
When, in an exporting member State, the use of hazardous substances, technologies or processes is prohibited as a potential source of a major accident, the information on this prohibition and the reasons for it shall be made available by the exporting member State to any importing country.
Source: Excerpts, Convention No. 174 (ILO 1993).
Case Study: What does dose mean?
There are several ways to define a dose of ionizing radiation, each appropriate for different purposes.
Absorbed dose
Absorbed dose resembles pharmacological dose the most closely. While pharmacological dose is the quantity of substance administered to a subject per unit weight or surface, radiological absorbed dose is the amount of energy transmitted by ionizing radiation per unit mass. Absorbed dose is measured in Grays (1 Gray = 1 joule/kg).
When individuals are exposed homogeneously—for example, by external irradiation by cosmic and terrestrial rays or by internal irradiation by potassium-40 present in the body—all organs and tissues receive the same dose. Under these circumstances, it is appropriate to speak of whole-body dose. It is, however, possible for exposure to be non-homogenous, in which case some organs and tissues will receive significantly higher doses than others. In this case, it is more relevant to think in terms of organ dose. For example, inhalation of radon daughters results in exposure of essentially only the lungs, and incorporation of radioactive iodine results in irradiation of the thyroid gland. In these cases, we may speak of lung dose and thyroid dose.
However, other units of dose that take into account differences in the effects of different types of radiation and the different radiation sensitivities of tissues and organs, have also been developed.
Equivalent dose
The development of biological effects (e.g., inhibition of cell growth, cell death, azoospermia) depends not only on the absorbed dose, but also on the specific type of radiation. Alpha radiation has a greater ionizing potential than beta or gamma radiation. Equivalent dose takes this difference into account by applying radiation-specific weighting factors. The weighting factor for gamma and beta radiation (low ionizing potential), is equal to 1, while that for alpha particles (high ionizing potential) is 20 (ICRP 60). Equivalent dose is measured in Sieverts (Sv).
Effective dose
In cases involving non-homogenous irradiation (e.g., the exposure of various organs to different radionuclides), it may be useful to calculate a global dose that integrates the doses received by all organs and tissues. This requires taking into account the radiation sensitivity of each tissue and organ, calculated from the results of epidemiological studies of radiation-induced cancers. Effective dose is measured in Sieverts (Sv) (ICRP 1991). Effective dose was developed for the purposes of radiation protection (i.e., risk management) and is thus inappropriate for use in epidemiological studies of the effects of ionizing radiation.
Collective dose
Collective dose reflects the exposure of a group or population and not of an individual, and is useful for evaluating the consequences of exposure to ionizing radiation at the population or group level. It is calculated by summing the individual received doses, or by multiplying the average individual dose by the number of exposed individuals in the groups or populations in question. Collective dose is measured in man-Sieverts (man Sv).
Electricity-Physiological Effects
The study of the hazards, electrophysiology and prevention of electrical accidents requires an understanding of several technical and medical concepts.
The following definitions of electrobiological terms are taken from chapter 891 of the International Electrotechnical Vocabulary (Electrobiology) (International Electrotechnical Commission) (IEC) (1979).
An electrical shock is the physiopathological effect resulting from the direct or indirect passage of an external electrical current through the body. It includes direct and indirect contacts and both unipolar and bipolar currents.
Individuals—living or deceased—having suffered electrical shocks are said to have suffered electrification; the term electrocution should be reserved for cases in which death ensues. Lightning strikes are fatal electrical shocks resulting from lightning (Gourbiere et al. 1994).
International statistics on electrical accidents have been compiled by the International Labour Office (ILO), the European Union (EU), the Union internationale des producteurs et distributeurs d’énergie électrique (UNIPEDE), the International Social Security Association (ISSA) and the TC64 Committee of the International Electrotechnical Commission. Interpretation of these statistics is hampered by variations in data collection techniques, insurance policies and definitions of fatal accidents from country to country. Nevertheless, the following estimates of the rate of electrocution are possible (table 1).
Table 1. Estimates of the rate of electrocution - 1988
|
Electrocutions |
Total |
|
|
United States* |
2.9 |
714 |
|
France |
2.0 |
115 |
|
Germany |
1.6 |
99 |
|
Austria |
0.9 |
11 |
|
Japan |
0.9 |
112 |
|
Sweden |
0.6 |
13 |
* According to the National Fire Protection Association (Massachusetts, US) these US statistics are more reflective of extensive data collection and legal reporting requirements than of a more dangerous environment. US statistics include deaths from exposure to public utility transmission systems and electrocutions caused by consumer products. In 1988, 290 deaths were caused by consumer products (1.2 deaths per million inhabitants). In 1993, the rate of death by electrocution from all causes dropped to 550 (2.1 deaths per million inhabitants); 38% were consumer product-related (0.8 deaths per million inhabitants).
The number of electrocutions is slowly decreasing, both in absolute terms and, even more strikingly, as a function of the total consumption of electricity. Approximately half of electrical accidents are occupational in origin, with the other half occurring at home and during leisure activities. In France, the average number of fatalities between 1968 and 1991 was 151 deaths per year, according to the Institut national de la santé et de la recherche médicale (INSERM).
Physical and Physiopathological Basis of Electrification
Electrical specialists divide electrical contacts into two groups: direct contacts, involving contact with live components, and indirect contacts, involving grounded contacts. Each of these requires fundamentally different preventive measures.
From a medical point of view, the current’s path through the body is the key prognostic and therapeutic determinant. For example, bipolar contact of a child’s mouth with an extension cord plug causes extremely serious burns to the mouth—but not death if the child is well insulated from the ground.
In occupational settings, where high voltages are common, arcing between an active component carrying a high voltage and workers who approach too closely is also possible. Specific work situations can also affect the consequences of electrical accidents: for example, workers may fall or act inappropriately when surprised by an otherwise relatively harmless electrical shock.
Electrical accidents may be caused by the entire range of voltages present in workplaces. Every industrial sector has its own set of conditions capable of causing direct, indirect, unipolar, bipolar, arcing, or induced contact, and, ultimately, accidents. While it is of course beyond the scope of this article to describe all human activities which involve electricity, it is useful to remind the reader of the following major types of electrical work, which have been the object of international preventive guidelines described in the chapter on prevention:
- activities involving work on live wires (the application of extremely rigorous protocols has succeeded in reducing the number of electrifications during this type of work)
- activities involving work on unpowered wires, and
- activities performed in the vicinity of live wires (these activities require the most attention, as they are often performed by personnel who are not electricians).
Physiopathology
All the variables of Joule’s law of direct current—
W=V x I x t = RI2t
(the heat produced by an electric current is proportional to the resistance and the square of the current)—are closely interrelated. In the case of alternating current, the effect of frequency must also be taken into account (Folliot 1982).
Living organisms are electrical conductors. Electrification occurs when there is a potential difference between two points in the organism. It is important to emphasize that the danger of electrical accidents arises not from mere contact with a live conductor, but rather from simultaneous contact with a live conductor and another body at a different potential.
The tissues and organs along the current path may undergo functional motor excitation, in some cases irreversible, or may suffer temporary or permanent injury, generally as a result of burns. The extent of these injuries is a function of the energy released or the quantity of electricity passing through them. The transit time of the electric current is therefore critical in determining the degree of injury. (For example, electric eels and rays produce extremely unpleasant discharges, capable of inducing a loss of consciousness. However, despite a voltage of 600V, a current of approximately 1A and a subject resistance of approximately 600 ohms, these fish are incapable of inducing a lethal shock, since the discharge duration is too brief, of the order of tens of microseconds.) Thus, at high voltages (>1,000V), death is often due to the extent of the burns. At lower voltages, death is a function of the amount of electricity (Q=I x t), reaching the heart, determined by the type, location and area of the contact points.
The following sections discuss the mechanism of death due to electrical accidents, the most effective immediate therapies and the factors determining the severity of injury—namely, resistance, intensity, voltage, frequency and wave-form.
Causes of Death in Electrical Accidents in Industry
In rare cases, asphyxia may be the cause of death. This may result from prolonged tetanus of the diaphragm, inhibition of the respiratory centres in cases of contact with the head, or very high current densities, for example as a result of lightning strikes (Gourbiere et al. 1994). If care can be provided within three minutes, the victim may be revived with a few puffs of mouth-to-mouth resuscitation.
On the other hand, peripheral circulatory collapse secondary to ventricular fibrillation remains the main cause of death. This invariably develops in the absence of cardiac massage applied simultaneously with mouth-to-mouth resuscitation. These interventions, which should be taught to all electricians, should be maintained until the arrival of emergency medical aid, which almost always takes more than three minutes. A great many electropathologists and engineers around the world have studied the causes of ventricular fibrillation, in order to design better passive or active protective measures (International Electrotechnical Commission 1987; 1994). Random desynchronization of the myocardium requires a sustained electric current of a specific frequency, intensity and transit time. Most importantly, the electrical signal must arrive at the myocardium during the so-called vulnerable phase of the cardiac cycle, corresponding to the start of the T-wave of the electrocardiogram.
The International Electrotechnical Commission (1987; 1994) has produced curves describing the effect of current intensity and transit time on the probability (expressed as percentages) of fibrillation and the hand-foot current path in a 70-kg male in good health. These tools are appropriate for industrial currents in the frequency range of 15 to 100 Hz, with higher frequencies currently under study. For transit times of less than 10 ms, the area under the electrical signal curve is a reasonable approximation of the electrical energy.
Role of Various Electrical Parameters
Each of the electrical parameters (current, voltage, resistance, time, frequency) and wave-form are important determinants of injury, both in their own right and by virtue of their interaction.
Current thresholds have been established for alternating current, as well as for other conditions defined above. The current intensity during electrification is unknown, since it is a function of tissue resistance at the moment of contact (I = V/R), but is generally perceptible at levels of approximately 1 mA. Relatively low currents can cause muscular contractions that may prevent a victim from letting go of an energized object. The threshold of this current is a function of condensity, contact area, contact pressure and individual variations. Virtually all men and almost all women and children can let go at currents up to 6 mA. At 10 mA it has been observed that 98.5% of men and 60% of women and 7.5% of children can let go. Only 7.5% of men and no women or children can let go at 20mA. No one can let go at 30mA and greater.
Currents of approximately 25 mA may cause tetanus of the diaphragm, the most powerful respiratory muscle. If contact is maintained for three minutes, cardiac arrest may also ensue.
Ventricular fibrillation becomes a danger at levels of approximately 45 mA, with a probability in adults of 5% after a 5-second contact. During heart surgery, admittedly a special condition, a current of 20 to 100 × 10–6A applied directly to the myocardium is sufficient to induce fibrillation. This myocardial sensitivity is the reason for strict standards applied to electromedical devices.
All other things (V, R, frequency) being equal, current thresholds also depend on the wave-form, animal species, weight, current direction in the heart, ratio of the current transit time to the cardiac cycle, point in the cardiac cycle at which the current arrives, and individual factors.
The voltage involved in accidents is generally known. In cases of direct contact, ventricular fibrillation and the severity of burns are directly proportional to voltage, since
V = RI and W = V x I x t
Burns arising from high-voltage electric shock are associated with many complications, only some of which are predictable. Accordingly accident victims must be cared for by knowledgeable specialists. Heat release occurs primarily in the muscles and neurovascular bundles. Plasma leakage following tissue damage causes shock, in some cases rapid and intense. For a given surface area, electrothermic burns—burns caused by an electrical current—are always more severe than other types of burn. Electrothermic burns are both external and internal and, although this may not be initially apparent, can induce vascular damage with serious secondary effects. These include internal stenoses and thrombi which, by virtue of the necrosis they induce, often necessitate amputation.
Tissue destruction is also responsible for the release of chromoproteins such as myoglobin. Such release is also observed in victims of crush injuries, although the extent of release is remarkable in victims of high-voltage burns. Myoglobin precipitation in renal tubules, secondary to acidosis brought on by anoxia and hyperkalaemia, is thought to be the cause of anuria. This theory, experimentally confirmed but not universally accepted, is the basis for recommendations for immediate alkalization therapy. Intravenous alkalization, which also corrects hypovolaemia and acidosis secondary to cell death, is the recommended practice.
In the case of indirect contacts, the contact voltage (V) and conventional voltage limit must also be taken into account.
The contact voltage is the voltage to which a person is subjected on simultaneously touching two conductors between which a voltage differential exists due to defective insulation. The intensity of the resultant current flow depends on the resistances of the human body and the external circuit. This current should not be allowed to rise above safe levels, which is to say that it must conform to safe time-current curves. The highest contact voltage that can be tolerated indefinitely without inducing electropathological effects is termed the conventional voltage limit or, more intuitively, the safety voltage.
The actual value of the resistance during electrical accidents is unknown. Variations in in-series resistances—for example, clothes and shoes—explain much of the variation observed in the effects of ostensibly similar electrical accidents, but exert little influence on the outcome of accidents involving bipolar contacts and high-voltage electrifications. In cases involving alternating current, the effect of capacitive and inductive phenomena must be added to the standard calculation based on voltage and current (R=V/I).
The resistance of the human body is the sum of the skin resistance (R) at the two points of contact and the body’s internal resistance (R). Skin resistance varies with environmental factors and, as noted by Biegelmeir (International Electrotechnical Commission 1987; 1994), is partially a function of the contact voltage. Other factors such as pressure, contact area, the state of the skin at the point of contact, and individual factors also influence resistance. It is thus unrealistic to attempt to base preventive measures on estimates of skin resistance. Prevention should instead be based on the adaptation of equipment and procedures to humans, rather than the reverse. In order to simplify matters, the IEC has defined four types of environment—dry, humid, wet and immersion—and has defined parameters useful for the planning of prevention activities in each case.
The frequency of the electrical signal responsible for electrical accidents is generally known. In Europe, it is almost always 50 Hz and in the Americas, it is generally 60 Hz. In rare cases involving railways in countries such as Germany, Austria and Switzerland, it may be 16 2/3 Hz, a frequency which theoretically represents a greater risk of tetanization and of ventricular fibrillation. It should be recalled that fibrillation is not a muscle reaction but is caused by repetitive stimulation, with a maximum sensitivity at approximately 10 Hz. This explains why, for a given voltage, extremely low-frequency alternating current is considered to be three to five times more dangerous than direct current with regard to effects other than burns.
The thresholds described previously are directly proportional to the frequency of the current. Thus, at 10 kHz, the detection threshold is ten times higher. The IEC is studying revised fibrillation hazard curves for frequencies above 1,000 Hz (International Electrotechnical Commission 1994).
Above a certain frequency, the physical laws governing penetration of current into the body change completely. Thermal effects related to the amount of energy released become the main effect, as capacitive and inductive phenomena start to predominate.
The wave-form of the electrical signal responsible for an electrical accident is usually known. It may be an important determinant of injury in accidents involving contact with capacitors or semiconductors.
Clinical Study of Electric Shock
Classically, electrifications have been divided into low- (50 to 1,000 V) and high- (>1,000 V) voltage incidents.
Low voltage is a familiar, indeed omnipresent, hazard, and shocks due to it are encountered in domestic, leisure, agricultural and hospital settings as well as in industry.
In reviewing the range of low-voltage electric shocks, from the most trivial to the most serious, we must start with uncomplicated electrical shock. In these cases, victims are able to remove themselves from harm on their own, retain consciousness and maintain normal ventilation. Cardiac effects are limited to simple sinus tachycardia with or without minor electrocardiographic abnormalities. Despite the relatively minor consequences of such accidents, electrocardiography remains an appropriate medical and medico-legal precaution. Technical investigation of these potentially serious incidents is indicated as a complement to clinical examination (Gilet and Choquet 1990).
Victims of shock involving somewhat stronger and longer-lasting electrical contact shocks may suffer from perturbations or loss of consciousness, but completely recover more or less rapidly; treatment accelerates recovery. Examination generally reveals neuromuscular hypertonias, hyper-reflective ventilation problems and congestion, the last of which is often secondary to oropharyngeal obstruction. Cardiovascular disorders are secondary to hypoxia or anoxia, or may take the form of tachycardia, hypertension and, in some cases, even infarction. Patients with these conditions require hospital care.
The occasional victims who lose consciousness within a few seconds of contact appear pale or cyanotic, stop breathing, have barely perceptible pulses and exhibit mydriasis indicative of acute cerebral injury. Although usually due to ventricular fibrillation, the precise pathogenesis of this apparent death is, however, irrelevant. The important point is the rapid commencement of well-defined therapy, since it has been known for some time that this clinical state never leads to actual death. The prognosis in these cases of electric shock—from which total recovery is possible— depends on the rapidity and quality of first aid. Statistically, this is most likely to be administered by non-medical personnel, and the training of all electricians in the basic interventions likely to ensure survival is therefore indicated.
In cases of apparent death, emergency treatment must take priority. In other cases, however, attention must be paid to multiple traumas resulting from violent tetanus, falls or the projection of the victim through the air. Once the immediate life-threatening danger has been resolved, trauma and burns, including those caused by low-voltage contacts, should be attended to.
Accidents involving high voltages result in significant burns as well as the effects described for low-voltage accidents. The conversion of electrical energy to heat occurs both internally and externally. In a study of electrical accidents in France made by the medical department of the power utility, EDF-GDF, almost 80% of the victims suffered burns. These can be classified into four groups:
- arc burns, usually involving exposed skin and complicated in some cases by burns from burning clothing
- multiple, extensive and deep electrothermic burns, caused by high-voltage contacts
- classical burns, caused by burning clothing and the projection of burning matter, and
- mixed burns, caused by arcing, burning and current flow.
Follow-up and complementary examinations are performed as required, depending on the particulars of the accident. The strategy used to establish a prognosis or for medico-legal purposes is of course determined by the nature of observed or expected complications. In high-voltage electrifications (Folliot 1982) and lightning strikes (Gourbiere et al. 1994), enzymology and the analysis of chromoproteins and blood clotting parameters are obligatory.
The course of recovery from electrical trauma may well be compromised by early or late complications, especially those involving the cardiovascular, nervous and renal systems. These complications in their own right are sufficient reason to hospitalize victims of high-voltage electrifications. Some complications may leave functional or cosmetic sequelae.
If the current path is such that significant current reaches the heart, cardiovascular complications will be present. The most frequently observed and most benign of these are functional disorders, in the presence or absence of clinical correlates. Arrhythmias—sinus tachycardia, extrasystole, flutter and atrial fibrillation (in that order)—are the most common electrocardiographic abnormalities, and may leave permanent sequelae. Conduction disorders are rarer, and are difficult to relate to electrical accidents in the absence of a previous electrocardiogram.
More serious disorders such as cardiac failure, valve injury and myocardial burns have also been reported, but are rare, even in victims of high-voltage accidents. Clear-cut cases of angina and even infarction have also been reported.
Peripheral vascular injury may be observed in the week following high-voltage electrification. Several pathogenic mechanisms have been proposed: arterial spasm, the action of electrical current on the media and muscular layers of the vessels and modification of the blood clotting parameters.
A wide variety of neurological complications is possible. The earliest to appear is stroke, regardless of whether the victim initially experienced loss of consciousness. The physiopathology of these complications involves cranial trauma (whose presence should be ascertained), the direct effect of current on the head, or the modification of cerebral blood flow and the induction of a delayed cerebral oedema. In addition, medullary and secondary peripheral complications may be caused by trauma or the direct action of electric current.
Sensory disorders involve the eye and the audiovestibular or cochlear systems. It is important to examine the cornea, crystalline lens and fundus of the eye as soon as possible, and to follow up victims of arcing and direct head contact for delayed effects. Cataracts may develop after an intervening symptom-free period of several months. Vestibular disorders and hearing loss are primarily due to blast effects and, in victims of lightning strike transmitted over telephone lines, to electrical trauma (Gourbiere et al. 1994).
Improvements in mobile emergency practices have greatly reduced the frequency of renal complications, especially oligo-anuria, in victims of high-voltage electrifications. Early and careful rehydration and intravenous alkalinization is the treatment of choice in victims of serious burns. A few cases of albuminuria and persistent microscopic haematuria have been reported.
Clinical Portraits and Diagnostic Problems
The clinical portrait of electric shock is complicated by the variety of industrial applications of electricity and the increasing frequency and variety of medical applications of electricity. For a long time, however, electrical accidents were caused solely by lightning strikes (Gourbiere et al. 1994). Lightning strikes may involve quite remarkable quantities of electricity: one out of every three victims of lightning strikes dies. The effects of a lightning strike—burns and apparent death—are comparable to those resulting from industrial electricity and are attributable to electrical shock, the transformation of electrical energy into heat, blast effects and the electrical properties of lightning.
Lightning strikes are three times as prevalent in men as in women. This reflects patterns of work with differing risks for exposure to lightning.
Burns resulting from contact with grounded metallic surfaces of electric scalpels are the most common effects observed in victims of iatrogenic electrification. The magnitude of acceptable leakage currents in electromedical devices varies from one device to another. At the very least, manufacturers’ specifications and usage recommendations should be followed.
To conclude this section, we would like to discuss the special case of electric shock involving pregnant women. This may cause the death of the woman, the foetus or both. In one remarkable case, a live foetus was successfully delivered by Caesarian section 15 minutes after its mother had died as a result of electrocution by a 220 V shock (Folliot 1982).
The pathophysiological mechanisms of abortion caused by electric shock requires further study. Is it caused by conduction disorders in the embryonic cardiac tube subjected to a voltage gradient, or by a tearing of the placenta secondary to vasoconstriction?
The occurrence of electrical accidents such as this happily rare one is yet another reason to require notification of all cases of injuries arising from electricity.
Positive and Medico-Legal Diagnosis
The circumstances under which electric shock occurs are generally sufficiently clear to allow unequivocal aetiological diagnosis. However, this is not invariably the case, even in industrial settings.
The diagnosis of circulatory failure following electric shock is extremely important, since it requires bystanders to commence immediate and basic first aid once the current has been shut off. Respiratory arrest in the absence of a pulse is an absolute indication for the commencement of cardiac massage and mouth-to-mouth resuscitation. Previously, these were only performed when mydriasis (dilation of the pupils), a diagnostic sign of acute cerebral injury, was present. Current practice is, however, to begin these interventions as soon as the pulse is no longer detectable.
Since loss of consciousness due to ventricular fibrillation may take a few seconds to develop, victims may be able to distance themselves from the equipment responsible for the accident. This may be of some medico-legal importance—for example, when an accident victim is found several metres from an electrical cabinet or other source of voltage with no traces of electrical injury.
It cannot be overemphasized that the absence of electrical burns does not exclude the possibility of electrocution. If autopsy of subjects found in electrical environments or near equipment capable of developing dangerous voltages reveals no visible Jelinek lesions and no apparent sign of death, electrocution should be considered.
If the body is found outdoors, a diagnosis of lightning strike is arrived at by the process of elimination. Signs of lightning strike should be searched for within a 50-metre radius of the body. The Museum of Electropathology of Vienna offers an arresting exhibition of such signs, including carbonized vegetation and vitrified sand. Metal objects worn by the victim may be melted.
Although suicide by electrical means remains thankfully rare in industry, death due to contributory negligence remains a sad reality. This is particularly true at non-standard sites, especially those involving the installation and operation of provisional electrical facilities under demanding conditions.
Electrical accidents should by all rights no longer occur, given the availability of effective preventive measures described in the article “Prevention and Standards”.
Static Electricity
All materials differ in the degree to which electric charges can pass through them. Conductors allow charges to flow, while insulators hinder the motion of charges. Electrostatics is the field devoted to studying charges, or charged bodies at rest. Static electricity results when electric charges which do not move are built up on objects. If the charges flow, then a current results and the electricity is no longer static. The current that results from moving charges is commonly referred to by laypeople as electricity, and is discussed in the other articles in this chapter. Static electrification is the term used to designate any process resulting in the separation of positive and negative electrical charges. Conduction is measured with a property called conductance, while an insulator is characterized by its resistivity. Charge separation which leads to electrification can occur as the result of mechanical processes—for example, contact between objects and friction, or the collision of two surfaces. The surfaces can be two solids or a solid and a liquid. The mechanical process can, less commonly, be the rupture or separation of solid or liquid surfaces. This article focuses on contact and friction.
Electrification Processes
The phenomenon of generation of static electricity by friction (triboelectrification) has been known for thousands of years. Contact between two materials is sufficient to induce electrification. Friction is simply a type of interaction which increases the area of contact and generates heat—friction is the general term to describe the movement of two objects in contact; the pressure exerted, its shear velocity and the heat generated are the prime determinants of the charge generated by friction. Sometimes friction will lead to the tearing away of solid particles as well.
When the two solids in contact are metals (metal-metal contact), electrons migrate from one to the other. Every metal is characterized by a different initial potential (Fermi potential), and nature always moves towards equilibrium—that is, natural phenomena work to eliminate the differences in potential. This migration of electrons results in the generation of a contact potential. Because the charges in a metal are very mobile (metals are excellent conductors), the charges will even recombine at the last point of contact before the two metals are separated. It is therefore impossible to induce electrification by bringing together two metals and then separating them; the charges will always flow to eliminate the potential difference.
When a metal and an insulator come into nearly friction-free contact in a vacuum, the energy level of electrons in the metal approaches that of the insulator. Surface or bulk impurities cause this to occur and also prevent arcing (the discharge of electricity between the two charged bodies—the electrodes) upon separation. The charge transferred to the insulator is proportional to the electron affinity of the metal, and every insulator also has an electron affinity, or attraction for electrons, associated with it. Thus, transfer of positive or negative ions from the insulator to the metal is also possible. The charge on the surface following contact and separation is described by equation 1 in table 1.
Table 1. Basic relationships in electrostatics - Collection of equations
Equation 1: Charging by contact of a metal and an insulator
In general, the surface charge density (![]() ) following contact and separation
) following contact and separation
can be expressed by:
![]()
where
e is the charge of an electron
NE is the energy state density at the insulator’s surface
fi is the electron affinity of the insulator, and
fm is the electron affinity of the metal
Equation 2: Charging following contact between two insulators
The following general form of equation 1 applies to the charge transfer
between two insulators with different energy states (perfectly clean surfaces only):
![]()
where NE1 and NE2 are the energy state densities at the surface of the two insulators,
and Ø1 and Ø 2 are the electron affinities of the two insulators.
Equation 3: Maximum surface charge density
The dielectric strength (EG) of the surrounding gas imposes an upper limit on the charge it is
possible to generate on a flat insulating surface. In air, EG is approximately 3 MV/m.
The maximum surface charge density is given by:
![]()
Equation 4: Maximum charge on a spherical particle
When nominally spherical particles are charged by the corona effect, the maximum
charge which each particle can acquire is given by Pauthenier’s limit:
![]()
where
qmax is the maximum charge
a is the particle radius
eI is the relative permittivity and
![]()
Equation 5: Discharges from conductors
The potential of an insulated conductor carrying charge Q is given by V = Q/C and
the stored energy by:
![]()
Equation 6: Time course of potential of charged conductor
In a conductor charged by a constant current (IG), the time course of the
potential is described by:
![]()
where Rf is the conductor’s leak resistance
Equation 7: Final potential of charged conductor
For long time course, t >Rf C, this reduces to:
![]()
and the stored energy is given by:
Equation 8: Stored energy of charged conductor
![]()
When two insulators come into contact, charge transfer occurs because of the different states of their surface energy (equation 2, table 1). Charges transferred to the surface of an insulator can migrate deeper within the material. Humidity and surface contamination can greatly modify the behaviour of charges. Surface humidity in particular increases surface energy state densities by increasing surface conduction, which favours charge recombination, and facilitates ionic mobility. Most people will recognize this from their daily life experiences by the fact that they tend to be subjected to static electricity during dry conditions. The water content of some polymers (plastics) will change as they are being charged. The increase or decrease in water content may even reverse the direction of the charge flow (its polarity).
The polarity (relative positivity and negativity) of two insulators in contact with each other depends on each material’s electron affinity. Insulators can be ranked by their electron affinities, and some illustrative values are listed in table 2. The electron affinity of an insulator is an important consideration for prevention programmes, which are discussed later in this article.
Table 2. Electron affinities of selected polymers*
|
Charge |
Material |
Electron affinity (EV) |
|
– |
PVC (polyvinyl chloride) |
4.85 |
|
Polyamide |
4.36 |
|
|
Polycarbonate |
4.26 |
|
|
PTFE (polytetrafluoroethylene) |
4.26 |
|
|
PETP (polyethylene terephthalate) |
4.25 |
|
|
Polystyrene |
4.22 |
|
|
+ |
Polyamide |
4.08 |
* A material acquires a positive charge when it comes into contact with a material listed above it, and a negative charge when it comes into contact with a material listed below it. The electron affinity of an insulator is multifactorial, however.
Although there have been attempts to establish a triboelectric series which would rank materials so that those which acquire a positive charge upon contact with materials would appear higher in the series than those that acquire a negative charge upon contact, no universally recognized series has been established.
When a solid and a liquid meet (to form a solid-liquid interface), charge transfer occurs due to the migration of ions that are present in the liquid. These ions arise from the dissociation of impurities which may be present or by electrochemical oxidation-reduction reactions. Since, in practice, perfectly pure liquids do not exist, there will always be at least some positive and negative ions in the liquid available to bind to the liquid-solid interface. There are many types of mechanisms by which this binding may occur (e.g., electrostatic adherence to metal surfaces, chemical absorption, electrolytic injection, dissociation of polar groups and, if the vessel wall is insulating, liquid-solid reactions.)
Since substances which dissolve (dissociate) are electrically neutral to begin with, they will generate equal numbers of positive and negative charges. Electrification occurs only if either the positive or the negative charges preferentially adhere to the solid’s surface. If this occurs, a very compact layer, known as the Helmholtz layer is formed. Because the Helmholtz layer is charged, it will attract ions of the opposite polarity to it. These ions will cluster into a more diffuse layer, known as the Gouy layer, which rests on top of the surface of the compact Helmholtz layer. The thickness of the Gouy layer increases with the resistivity of the liquid. Conducting liquids form very thin Gouy layers.
This double layer will separate if the liquid flows, with the Helmholtz layer remaining bound to the interface and the Gouy layer becoming entrained by the flowing liquid. The movement of these charged layers produces a difference in potential (the zeta potential), and the current induced by the moving charges is known as the streaming current. The amount of charge that accumulates in the liquid depends on the rate at which the ions diffuse towards the interface and on the liquid’s resistivity (r). The streaming current is, however, constant over time.
Neither highly insulating nor conducting liquids will become charged—the first because very few ions are present, and the second because in liquids which conduct electricity very well, the ions will recombine very rapidly. In practice, electrification occurs only in liquids with resistivity greater than 107Ωm or less than 1011Ωm, with the highest values observed for r 109 to 1011 Ωm.
Flowing liquids will induce charge accumulation in insulating surfaces over which they flow. The extent to which the surface charge density will build up is limited by (1) how quickly the ions in the liquid recombine at the liquid-solid interface, (2) how quickly the ions in the liquid are conducted through the insulator, or (3) whether surface or bulk arcing through the insulator occurs and the charge is thus discharged. Turbulent flow and flow over rough surfaces favour electrification.
When a high voltage—say several kilovolts—is applied to a charged body (an electrode) which has a small radius (e.g., a wire), the electrical field in the immediate vicinity of the charged body is high, but it decreases rapidly with distance. If there is a discharge of the stored charges, the discharge will be limited to the region in which the electrical field is stronger than the dielectric strength of the surrounding atmosphere, a phenomenon known as the corona effect, because the arcing also emits light. (People may actually have seen small sparks formed when they have personally experienced a shock from static electricity.)
The charge density on an insulating surface can also be changed by the moving electrons that are generated by a high-intensity electrical field. These electrons will generate ions from any gas molecules in the atmosphere with which they come into contact. When the electric charge on the body is positive, the charged body will repel any positive ions which have been created. Electrons created by negatively charged objects will lose energy as they recede from the electrode, and they will attach themselves to gas molecules in the atmosphere, thus forming negative ions which continue to recede away from the charge points. These positive and negative ions can come to rest on any insulating surface and will modify the surface’s charge density. This type of charge is much easier to control and more uniform than the charges created by friction. There are limits to the extent of the charges it is possible to generate in this way. The limit is described mathematically in equation 3 in table 1.
To generate higher charges, the dielectric strength of the environment must be increased, either by creating a vacuum or by metallizing the other surface of the insulating film. The latter stratagem draws the electrical field into the insulator and consequently reduces the field strength in the surrounding gas.
When a conductor in an electrical field (E) is grounded (see figure 1), charges can be produced by induction. Under these conditions, the electrical field induces polarization—the separation of the centres of gravity of the negative and positive ions of the conductor. A conductor temporarily grounded at only one point will carry a net charge when disconnected from the ground, due to the migration of charges in the vicinity of the point. This explains why conducting particles located in a uniform field oscillate between electrodes, charging and discharging at each contact.
Figure 1. Mechanism of charging a conductor by induction
Hazards Associated with Static Electricity
The ill effects caused by the accumulation of static electricity range from the discomfort one experiences when touching a charged object, such as a door handle, to the very serious injuries, even fatalities, which can occur from an explosion induced by static electricity. The physiological effect of electrostatic discharges on humans ranges from uncomfortable prickling to violent reflex actions. These effects are produced by the discharge current and, especially, by the current density on the skin.
In this article we will describe some practical ways in which surfaces and objects can become charged (electrification). When the electrical field induced exceeds the ability of the surrounding environment to withstand the charge (that is, exceeds the dielectric strength of the environment), a discharge occurs. (In air, the dielectric strength is described by Paschen’s curve and is a function of the product of the pressure and the distance between the charged bodies.)
Disruptive discharges can take the following forms:
- sparks or arcs which bridge two charged bodies (two metal electrodes)
- partial, or brush, discharges which bridge a metal electrode and an insulator, or even two insulators; these discharges are termed partial because the conducting path does not totally short-circuit two metal electrodes, but is usually multiple and brushlike
- corona discharges, also known as point effects, which arise in the strong electric field around small-radius charged bodies or electrodes.
Insulated conductors have a net capacitance C relative to ground. This relationship between charge and potential is expressed in equation 5 in table 1.
A person wearing insulating shoes is a common example of an insulated conductor. The human body is an electrostatic conductor, with a typical capacitance relative to ground of approximately 150 pF and a potential of up to 30 kV. Because people can be insulating conductors, they can experience electrostatic discharges, such as the more or less painful sensation sometimes produced when a hand approaches a door handle or other metal object. When the potential reaches approximately 2 kV, the equivalent to an energy of 0.3 mJ will be experienced, although this threshold varies from person to person. Stronger discharges may cause uncontrollable movements resulting in falls. In the case of workers using tools, the involuntary reflex motions may lead to injuries to the victim and others who may be working nearby. Equations 6 to 8 in table 1 describe the time course of the potential.
Actual arcing will occur when the strength of the induced electrical field exceeds the dielectric strength of air. Because of the rapid migration of charges in conductors, essentially all the charges flow to the discharge point, releasing all the stored energy into a spark. This can have serious implications when working with flammable or explosive substances or in flammable conditions.
The approach of a grounded electrode to a charged insulating surface modifies the electric field and induces a charge in the electrode. As the surfaces approach each other, the field strength increases, eventually leading to a partial discharge from the charged insulated surface. Because charges on insulating surfaces are not very mobile, only a small proportion of the surface participates in the discharge, and the energy released by this type of discharge is therefore much lower than in arcs.
The charge and transferred energy appear to be directly proportional to the diameter of the metal electrode, up to approximately 20 mm. The initial polarity of the insulator also influences charge and transferred energy. Partial discharges from positively charged surfaces are less energetic than those from negatively charged ones. It is impossible to determine, a priori, the energy transferred by a discharge from an insulating surface, in contrast to the situation involving conducting surfaces. In fact, because the insulating surface is not equipotential, it is not even possible to define the capacitances involved.
Creeping Discharge
We saw in equation 3 (table 1) that the surface charge density of an insulating surface in air cannot exceed 2,660 pC/cm2.
If we consider an insulating plate or a film of thickness a, resting on a metal electrode or having one metal face, it is easy to demonstrate that the electrical field is drawn into the insulator by the induced charge on the electrode as charges are deposited on the non-metallic face. As a result, the electric field in the air is very weak, and lower than it would be if one of the faces were not metal. In this case, the dielectric strength of air does not limit charge accumulation on the insulating surface, and it is possible to reach very high surface charge densities (>2,660 pC/cm2). This charge accumulation increases the surface conductivity of the insulator.
When an electrode approaches an insulating surface, a creeping discharge involving a large proportion of the charged surface which has become conducting occurs. Because of the large surface areas involved, this type of discharge releases large amounts of energy. In the case of films, the air field is very weak, and the distance between the electrode and the film must be no more than the film thickness for a discharge to occur. A creeping discharge may also occur when a charged insulator is separated from its metallic undercoating. Under these circumstances, the air field increases abruptly and the entire surface of the insulator discharges to re-establish equilibrium.
Electrostatic Discharges and Fire and Explosion Hazards
In explosive atmospheres, violent exothermic oxidation reactions, involving energy transfer to the atmosphere, may be triggered by:
- open flames
- electric sparks
- radio-frequency sparks near a strong radio source
- sparks produced by collisions (e.g., between metal and concrete)
- electrostatic discharges.
We are interested here only in the last case. The flash points (the temperature at which liquid vapours ignite on contact with a naked flame) of various liquids, and the auto-ignition temperature of various vapours are given in the Chemical Section of this Encyclopaedia. The fire hazard associated with electrostatic discharges can be assessed by reference to the lower flammability limit of gases, vapours and solid or liquid aerosols. This limit may vary considerably, as table 3 illustrates.
Table 3. Typical lower flammability limits
|
Discharge |
Limit |
|
Some powders |
Several joules |
|
Very fine sulphur and aluminium aerosols |
Several millijoules |
|
Vapours of hydrocarbons and other organic liquids |
200 microjoules |
|
Hydrogen and acetylene |
20 microjoules |
|
Explosives |
1 microjoule |
A mixture of air and a flammable gas or vapour can explode only when the concentration of the flammable substance is between its upper and lower explosive limits. Within this range, the minimal ignition energy (MIE)—the energy which an electrostatic discharge must possess to ignite the mixture—is highly concentration dependent. The minimal ignition energy has been consistently shown to depend on the speed of energy release and, by extension, on discharge duration. Electrode radius is also a factor:
- Small-diameter electrodes (of the order of several millimetres) result in corona discharges rather than sparks.
- With larger-diameter electrodes (of the order of several centimetres), the electrode mass serves to cool the sparks.
In general, the lowest MIEs are obtained with electrodes that are just big enough to prevent corona discharges.
The MIE also depends on the interelectrode distance, and is lowest at the quenching distance (“distance de pincement”), the distance at which the energy produced in the reaction zone exceeds the thermal losses at the electrodes. It has been experimentally demonstrated that each flammable substance has a maximum safe distance, corresponding to the minimum interelectrode distance at which an explosion can occur. For hydrocarbons, this is less than 1 mm.
The probability of powder explosions is concentration dependent, with the highest probability associated with concentrations of the order of 200 to 500 g/m3. The MIE is also dependent on particle size, with finer powders exploding more easily. For both gases and aerosols, the MIE decreases with temperature.
Industrial Examples
Many processes routinely used for handling and transporting chemicals generate electrostatic charges. These include:
- pouring powders from sacks
- screening
- transport in pipework
- liquid agitation, especially in the presence of multiple phases, suspended solids or droplets of non-miscible liquids
- liquid spraying or misting.
The consequences of electrostatic charge generation include mechanical problems, an electrostatic discharge hazard for operators and, if products containing inflammable solvents or vapours are used, even explosion (see table 4).
Table 4. Specific charge associated with selected industrial operations
|
Operation |
Specific charge |
|
Screening |
10-8 –10-11 |
|
Silo filling or emptying |
10-7 –10-9 |
|
Transport by worm conveyor |
10-6 –10-8 |
|
Grinding |
10-6 –10-7 |
|
Micronization |
10-4 –10-7 |
|
Pneumatic transport |
10-4 –10-6 |
Liquid hydrocarbons, such as oil, kerosene and many common solvents, have two characteristics which render them particularly sensitive to problems of static electricity:
- high resistivity, which allows them to accumulate high levels of charges
- flammable vapours, which increase the risk of low-energy discharges triggering fires and explosions.
Charges may be generated during transport flow (e.g., through pipework, pumps or valves). Passage through fine filters, such as those used during the filling of aeroplane tanks, may result in the generation of charge densities of several hundred microcoulombs per cubic metre. Particle sedimentation and the generation of charged mists or foams during flow-filling of tanks may also generate charges.
Between 1953 and 1971, static electricity was responsible for 35 fires and explosions during or following the filling of kerosene tanks, and even more accidents occurred during the filling of truck tanks. The presence of filters or splashing during filling (due to the generation of foams or mists) were the most commonly identified risk factors. Accidents have also occurred on board oil tankers, especially during tank cleaning.
Principles of Static Electricity Prevention
All problems related to static electricity derive from the:
- generation of electric charges
- accumulation of these charges on insulators or insulated conductors
- electric field produced by these charges, which in turn results in a force or a disruptive discharge.
Preventive measures seek to avoid the accumulation of electrostatic charges, and the strategy of choice is to avoid generating the electric charges in the first place. If this is not possible, measures designed to ground the charges should be implemented. Finally, if discharges are unavoidable, sensitive objects should be protected from the effects of the discharges.
Suppression or reduction of the electrostatic charge generation
This is the first approach to electrostatic prevention that should be undertaken, because it is the only preventive measure that eliminates the problem at its source. However, as discussed earlier, charges are generated whenever two materials, at least one of which is insulating, come into contact and are subsequently separated. In practice, charge generation can occur even on contact and separation of a material with itself. In fact, charge generation involves the surface layers of materials. Because the slightest difference in surface humidity or surface contamination results in the generation of static charges, it is impossible to avoid charge generation completely.
To reduce the quantity of charges generated by surfaces coming into contact:
- Avoid having materials come into contact with one another if they have very different electron affinities—that is, if they are very far apart in the triboelectric series. For example, avoid contact between glass and Teflon (PTFE), or between PVC and polyamide (nylon) (see table 2).
- Reduce the rate of flow between materials. This reduces the shear velocity between solid materials. For example, one can reduce the flow rate of the extrusion of plastic films, of the movement of crushed materials on a conveyor, or of liquids in a pipeline.
No definitive safety limits for flow rates have been established. The British standard BS-5958-Part 2 Code of Practice for Control of Undesirable Static Electricity recommends that the product of the velocity (in metres per second) and the pipe diameter (in metres) be less than 0.38 for liquids with conductivities of less than 5 pS/m (in pico-siemens per metre) and less than 0.5 for liquids with conductivities above 5 pS/m. This criterion is valid only for single-phase liquids transported at speeds no greater than 7 m/s.
It should be noted that reducing shear or flow velocity not only reduces charge generation but also helps dissipate any charges that are generated. This is because lower flow velocities result in residence times that are higher than those associated with relaxation zones, where flow rates are reduced by strategies such as increasing pipe diameter. This, in turn, increases grounding.
Grounding of static electricity
The basic rule of electrostatic prevention is to eliminate the potential differences between objects. This can be done by connecting them or by grounding (earthing) them. Insulated conductors, however, can accumulate charges and thus may become charged by induction, a phenomenon which is unique to them. Discharges from conductors may take the form of high-energy—and dangerous—sparks.
This rule is consistent with recommendations regarding the prevention of electric shocks, which also require all accessible metal parts of electrical equipment to be grounded as in the French standard Low voltage electrical installations (NFC 15-100). For maximum electrostatic safety, our concern here, this rule should be generalized to all conducting elements. This includes metal table frames, door handles, electronic components, tanks used in the chemical industries, and the chassis of vehicles used to transport hydrocarbons.
From the point of view of electrostatic safety, the ideal world would be one in which everything would be a conductor and would be permanently grounded, thus transferring all charges into the earth. Under these circumstances, everything would be permanently equipotential, and the electric field—and the discharge risk—would consequently be zero. However, it is almost never possible to attain this ideal, for the following reasons:
- Not all products which have to be handled are conductors, and many cannot be made conductive by the use of additives. Agricultural and pharmaceutical products, and high-purity liquids, are examples of these.
- Desirable end-product properties, such as optical transparency or low thermal conductivity, may preclude the use of conductive materials.
- It is impossible to permanently ground mobile equipment such as metal carts, cordless electronic tools, vehicles and even human operators.
Protection against electrostatic discharges
It should be borne in mind that this section is concerned only with the protection of electrostatically sensitive equipment from unavoidable discharges, the reduction of charge generation and the elimination of charges. The ability to protect equipment does not eliminate the fundamental necessity of preventing electrostatic charge accumulation in the first place.
As figure 2 illustrates, all electrostatic problems involve a source of electrostatic discharge (the initially charged object), a target which receives the discharge, and the environment through which the discharge travels (dielectric discharge). It should be noted that either the target or the environment can be electrostatically sensitive. Some examples of sensitive elements are listed in table 5.
Figure 2. Schematic of electrostatic discharge problem
Table 6. Examples of equipment sensitive to electrostatic discharges
|
Sensitive element |
Examples |
|
Source |
An operator touching a door handle or the chassis of a car A |
|
Target |
Electronic components or materials touching a charged operator |
|
Environment |
An explosive mixture ignited by an electrostatic discharge |
Protection of workers
Workers who have reason to believe that they have become electrically charged (for example, when dismounting from a vehicle in dry weather or walking with certain types of shoes), can apply a number of protective measures, such as the following:
- Reduce the current density at the skin level by touching a grounded conductor with a piece of metal such as a key or tool.
- Reduce the peak value of the current by discharging to a dissipating object, if one is available (a table top or special device such as a protective wrist strap with serial resistance).
Protection in explosive atmospheres
In explosive atmospheres, it is the environment itself that is sensitive to electrostatic discharges, and discharges may result in ignition or explosion. Protection in these cases consists of replacing the air, either with a gas mixture whose oxygen content is less than the lower explosive limit, or with an inert gas, such as nitrogen. Inert gas has been used in silos and in reaction vessels in the chemical and pharmaceutical industries. In this case, adequate precautions to assure that workers receive an adequate air supply are needed.
More...
Prevention and Standards
Hazards and Preventive Measures at Electrical Facilities
The many components making up electrical installations exhibit varying degrees of robustness. Regardless of their inherent fragility, however, they must all operate reliably under rigorous conditions. Unfortunately, even under the best circumstances, electrical equipment is subject to failures that may result in human injury or material damage.
Safe operation of electrical installations is the result of good initial design, not the mere retrofitting of safety systems. This is a corollary of the fact that while current flows at the speed of light, all electromechanical and electronic systems exhibit reaction latencies, caused primarily by thermal inertia, mechanical inertia and maintenance conditions. These latencies, whatever their origins, are sufficiently lengthy to allow humans to be injured and equipment damaged (Lee, Capelli-Schellpfeffer and Kelly 1994; Lee, Cravalho and Burke 1992; Kane and Sternheim 1978).
It is essential that equipment be installed and maintained by qualified personnel. Technical measures, it should be emphasized, are necessary both to ensure the safe operation of installations and to protect humans and equipment.
Introduction to electrical hazards
Proper operation of electrical installations requires that machinery, equipment, and electrical circuits and lines be protected from hazards caused by both internal (i.e., arising within the installation) and external factors (Andreoni and Castagna 1983).
Internal causes include:
- overvoltages
- short circuits
- modification of the current’s wave-form
- induction
- interference
- overcurrents
- corrosion, leading to electrical current leakages to ground
- heating of conducting and insulating materials, which may result in operator burns, emissions of toxic gases, component fires and, in flammable atmospheres, explosions
- leaks of insulating fluids, such as oil
- generation of hydrogen or other gases which may lead to the formation of explosive mixtures.
Each hazard-equipment combination requires specific protective measures, some of which are mandated by law or internal technical regulations. Manufacturers have a responsibility to be aware of specific technical strategies capable of reducing risks.
External causes include:
- mechanical factors (falls, bumps, vibration)
- physical and chemical factors (natural or artificial radiation, extreme temperatures, oils, corrosive liquids, humidity)
- wind, ice, lightning
- vegetation (trees and roots, both dry and wet)
- animals (in both urban and rural settings); these may damage the power-line insulation, and so cause short circuits or false contacts
and, last but not least,
- adults and children who are careless, reckless or ignorant of risks and operating procedures.
Other external causes include electromagnetic interference by sources such as high-voltage lines, radio receivers, welding machines (capable of generating transient overvoltages) and solenoids.
The most frequently encountered causes of problems arise from malfunctioning or non-standard:
- mechanical, thermal or chemical protective equipment
- ventilation systems, machine cooling systems, equipment, lines or circuits
- coordination of insulators used in different parts of the plant
- coordination of fuses and automatic circuit-breakers.
A single fuse or automatic circuit-breaker is incapable of providing adequate protection against overcurrent on two different circuits. Fuses or automatic circuit breakers can provide protection against phase-neutral failures, but protection against phase-ground failures requires automatic residual-current circuit-breakers.
- use of voltage relays and dischargers to coordinate protective systems
- sensors and mechanical or electrical components in the installation’s protective systems
- separation of circuits at different voltages (adequate air gaps must be maintained between conductors; connections should be insulated; transformers should be equipped with grounded shields and suitable protection against overvoltage, and have fully segregated primary and secondary coils)
- colour codes or other suitable provisions to avoid misidentification of wires
- mistaking the active phase for a neutral conductor results in electrification of the equipment’s external metallic components
- protective equipment against electromagnetic interference.
These are particularly important for instrumentation and lines used for data transmission or the exchange of protection and/or controlling signals. Adequate gaps must be maintained between lines, or filters and shields used. Fibre-optic cables are sometimes used for the most critical cases.
The risk associated with electrical installations increases when the equipment is subjected to severe operating conditions, most commonly as a result of electrical hazards in humid or wet environments.
The thin liquid conductive layers that form on metallic and insulating surfaces in humid or wet environments create new, irregular and dangerous current pathways. Water infiltration reduces the efficiency of insulation, and, should water penetrate the insulation, it can cause current leakages and short circuits. These effects not only damage electrical installations but greatly increase human risks. This fact justifies the need for special standards for work in harsh environments such as open-air sites, agricultural installations, construction sites, bathrooms, mines and cellars, and some industrial settings.
Equipment providing protection against rain, side-splashes or full immersion is available. Ideally, the equipment should be enclosed, insulated and corrosion proof. Metallic enclosures must be grounded. The mechanism of failure in these wet environments is the same as that observed in humid atmospheres, but the effects may be more severe.
Electrical hazards in dusty atmospheres
Fine dusts that enter machines and electrical equipment cause abrasion, particularly of mobile parts. Conducting dusts may also cause short circuits, while insulating dusts may interrupt current flow and increase contact resistance. Accumulations of fine or coarse dusts around equipment cases are potential humidity and water reservoirs. Dry dust is a thermal insulator, reducing heat dispersion and increasing local temperature; this may damage electrical circuits and cause fires or explosions.
Water- and explosion-proof systems must be installed in industrial or agricultural sites where dusty processes are carried out.
Electrical hazards in explosive atmospheres or at sites containing explosive materials
Explosions, including those of atmospheres containing explosive gases and dusts, may be triggered by opening and closing live electrical circuits, or by any other transient process capable of generating sparks of sufficient energy.
This hazard is present in sites such as:
- mines and underground sites where gases, especially methane, may accumulate
- chemical industries
- lead-battery storage rooms, where hydrogen may accumulate
- the food industry, where natural organic powders may be generated
- the synthetic materials industry
- metallurgy, especially that involving aluminium and magnesium.
Where this hazard is present, the number of electrical circuits and equipment should be minimized—for example, by removing electrical motors and transformers or replacing them with pneumatic equipment. Electrical equipment which cannot be removed must be enclosed, to avoid any contact of flammable gases and dusts with sparks, and a positive-pressure inert-gas atmosphere maintained within the enclosure. Explosion-proof enclosures and fireproof electrical cables must be used where there is the possibility of explosion. A full range of explosion-proof equipment has been developed for some high-risk industries (e.g., the oil and chemical industries).
Because of the high cost of explosion-proof equipment, plants are commonly divided into electrical hazard zones. In this approach, special equipment is used in high-risk zones, while a certain amount of risk is accepted in others. Various industry-specific criteria and technical solutions have been developed; these usually involve some combination of grounding, component segregation and the installation of zoning barriers.
Equipotential Bonding
If all the conductors, including the earth, that can be touched simultaneously were at the same potential, there would be no danger to humans. Equipotential bonding systems are an attempt to achieve this ideal condition (Andreoni and Castagna 1983; Lee, Cravalho and Burke 1992).
In equipotential bonding, every exposed conductor of non-transmission electrical equipment and every accessible extraneous conductor in the same site are connected to a protective grounded conductor. It should be recalled that while the conductors of non-transmission equipment are dead during normal operation, they may become live following insulation failure. By decreasing the contact voltage, equipotential bonding prevents metallic components from reaching voltages that are hazardous to both humans and equipment.
In practice, it may prove necessary to connect the same machine to the equipotential bonding grid at more than one point. Areas of poor contact, due, for example, to the presence of insulators such as lubricants and paint, should be carefully identified. Similarly, it is good practice to connect all the local and external service piping (e.g., water, gas and heating) to the equipotential bonding grid.
Grounding
In most cases, it is necessary to minimize the voltage drop between the installation’s conductors and the earth. This is accomplished by connecting the conductors to a grounded protective conductor.
There are two types of ground connections:
- functional grounds—for example, grounding the neutral conductor of a three-phase system, or the midpoint of a transformer’s secondary coil
- protective grounds—for example, grounding every conductor on a piece of equipment. The object of this type of grounding is to minimize conductor voltages by creating a preferential path for fault currents, especially those currents likely to affect humans.
Under normal operating conditions, no current flows through ground connections. In the event of accidental activation of the circuit, however, the current flow through the low-resistance grounding connection is high enough to melt the fuse or the ungrounded conductors.
The maximum fault voltage in equipotential grids allowed by most standards is 50 V for dry environments, 25 V for wet or humid environments and 12 V for medical laboratories and other high-risk environments. Although these values are only guidelines, the necessity of ensuring adequate grounding in workplaces, public spaces and especially residences, should be emphasized.
The efficiency of grounding depends primarily on the existence of high and stable ground leakage currents, but also on adequate galvanic coupling of the equipotential grid, and the diameter of the conductors leading to the grid. Because of the importance of ground leakage, it must be evaluated with great accuracy.
Ground connections must be as reliable as equipotential grids, and their proper operation must be verified on a regular basis.
As the earth resistance increases, the potential of both the grounding conductor and the earth around the conductor approaches that of the electrical circuit; in the case of the earth around the conductor, the potential generated is inversely proportional to the distance from the conductor. In order to avoid dangerous step voltages, ground conductors must be properly shielded and set in the ground at adequate depths.
As an alternative to equipment grounding, standards allow for the use of double-insulated equipment. This equipment, recommended for use in residential settings, minimizes the chance of insulation failure by providing two separate insulation systems. Double-insulated equipment cannot be relied upon to adequately protect against interface failures such as those associated with loose but live plugs, since some countries’ plug and wall-socket standards do not address the use of such plugs.
Circuit-breakers
The surest method of reducing electrical hazards to humans and equipment is to minimize the duration of the fault current and voltage increase, ideally before the electrical energy has even begun to increase. Protective systems in electrical equipment usually incorporate three relays: a residual-current relay to protect against failure towards ground, a magnetic relay and a thermal relay to protect against overloads and short circuits.
In residual-current circuit-breakers, the conductors in the circuit are wound around a ring which detects the vector sum of the currents entering and exiting the equipment to be protected. The vector sum is equal to zero during normal operation, but equals the leakage current in cases of failure. When the leakage current reaches the breaker’s threshold, the breaker is tripped. Residual-current circuit-breakers can be tripped by currents as low as 30 mA, with latencies as low as 30 ms.
The maximum current that can be safely carried by a conductor is a function of its cross-sectional area, insulation and installation. Overheating will result if the maximum safe load is exceeded or if heat dissipation is limited. Overcurrent devices such as fuses and magneto-thermal circuit-breakers automatically break the circuit if excessive current flow, ground faults, overloading or short circuits occur. Overcurrent devices should interrupt the current flow when it exceeds the conductor’s capacity.
Selection of protective equipment capable of protecting both personnel and equipment is one of the most important issues in the management of electrical installations and must take into account not only the current-carrying capacity of conductors but also the characteristics of the circuits and the equipment connected to them.
Special high-capacity fuses or circuit-breakers must be used on circuits carrying very high current loads.
Fuses
Several types of fuse are available, each designed for a specific application. Use of the wrong type of fuse or of a fuse of the wrong capacity may cause injury and damage equipment. Overfusing frequently results in overheated wiring or equipment, which in turn may cause fires.
Before replacing fuses, lock out, tag and test the circuit, to verify that the circuit is dead. Testing can save lives. Next, identify the cause of any short circuits or overloads, and replace blown fuses with fuses of the same type and capacity. Never insert fuses in a live circuit.
Circuit-breakers
Although circuit-breakers have long been used in high-voltage circuits with large current capacities, they are increasingly used in many other kinds of circuits. Many types are available, offering a choice of immediate and delayed onset and manual or automatic operation.
Circuit-breakers fall into two general categories: thermal and magnetic.
Thermal circuit-breakers react solely to a rise of temperature. Variations in the circuit-breaker’s ambient temperature will therefore affect the point at which the breaker is tripped.
Magnetic circuit-breakers, on the other hand, react solely to the amount of current passing through the circuit. This type of breaker is preferable where wide temperature fluctuations would require overrating the circuit-breaker, or where the breaker is frequently tripped.
In the case of contact with lines carrying high current loads, protective circuits cannot prevent personal injury or equipment damage, as they are designed only to protect power-lines and systems from excess current flow caused by faults.
Because of the resistance of the contact with the earth, the current passing through an object simultaneously contacting the line and the earth will usually be less than the tripping current. Fault currents flowing through humans may be further reduced by body resistance to the point where they do not trip the breaker, and are therefore extremely dangerous. It is virtually impossible to design a power system that would prevent injury or damage to any object that faults the power lines while remaining a useful energy transmission system, as the trip thresholds for the relevant circuit protection devices are well above the human hazard level.
Standards and Regulations
The framework of international standards and regulations is illustrated in figure 1 (Winckler 1994). The rows correspond to the geographic scope of the standards, either worldwide (international), continental (regional) or national, while the columns correspond to the standards’ fields of application. The IEC and the International Organization for Standardization (ISO) both share an umbrella structure, the Joint Presidents Coordinating Group (JPCG); the European equivalent is the Joint Presidents Group (JPG).
Figure 1. The framework of international standards and regulations
Each standardization body holds regular international meetings. The composition of the various bodies reflects the development of standardization.
The Comité européen de normalisation électrotechnique (CENELEC) was created by the electrical engineering committees of the countries signing the 1957 Rome Treaty establishing the European Economic Community. The six founding members were later joined by the members of the European Free Trade Association (EFTA), and CENELEC in its present form dates from 13 February, 1972.
In contrast to the International Electrotechnical Commission (IEC), CENELEC focuses on the implementation of international standards in member countries rather than on the creation of new standards. It is particularly important to recall that while the adoption of IEC standards by member countries is voluntary, adoption of CENELEC standards and regulations is obligatory in the European Union. Over 90% of CENELEC standards are derived from IEC standards, and over 70% of them are identical. CENELEC’s influence has also attracted the interest of Eastern European countries, most of which became affiliated members in 1991.
The International Association for Testing and Materials, the forerunner of the ISO, as it is known today, was founded in 1886 and was active until The First World War, after which it ceased to function as an international association. Some national organizations, like the American Society for Testing and Materials (ASTM), survived. In 1926, the International Standards Association (ISA) was founded in New York and was active until The Second World War. The ISA was replaced in 1946 by the ISO, which is responsible for all fields except electrical engineering and telecommunications. The Comité européen de normalisation (CEN) is the European equivalent of the ISO and has the same function as CENELEC, although only 40% of CEN standards are derived from ISO standards.
The current wave of international economic consolidation creates a need for common technical databases in the field of standardization. This process is presently under way in several parts of the world, and it is likely that new standardization bodies will evolve outside of Europe. CANENA is a regional standardization body created by the North American Free Trade Agreement (NAFTA) countries (Canada, Mexico and the United States). Wiring of premises in the US is governed by the National Electrical Code, ANSI/NFPA 70-1996. This Code is also in use in several other countries in North and South America. It provides installation requirements for premises wiring installations beyond the point of connection to the electric utility system. It covers installation of electric conductors and equipment within or on public and private buildings, including mobil homes, recreational vehicles, and floating buildings, stock yards, carnivals, parking and other lots, and industrial substations. It does not cover installations in ships or watercraft other than floating buildings—railway rolling stop, aircraft, or automotive vehicles. The National Electric Code also does not apply to other areas that are normally regulated by the National Electrical Safety Code, such as installations of communications utility equipment and electric utility installations.
European and American Standards for the Operation of Electrical Installations
The European Standard EN 50110-1, Operation of Electrical Installations (1994a) prepared by CENELEC Task Force 63-3, is the basic document that applies to the operation of and work activities on, with or near electrical installations. The standard sets the minimum requirements for all CENELEC countries; additional national standards are described in separate subparts of the standard (EN 50110-2).
The standard applies to installations designed for the generation, transmission, conversion, distribution and use of electrical power, and operating at commonly encountered voltage levels. Although typical installations operate at low voltages, the standard also applies to extra-low and high-voltage installations. Installations may be either permanent and fixed (e.g., distribution installations in factories or office complexes) or mobile.
Safe operation and maintenance procedures for work on or near electrical installations are set out in the standard. Applicable work activities include non-electrical work such as construction near overhead lines or underground cables, in addition to all types of electrical work. Certain electrical installations, such as those on board aircraft and ships, are not subject to the standard.
The equivalent standard in the United States is the National Electrical Safety Code (NESC), American National Standards Institute (1990). The NESC applies to utility facilities and functions from the point of generation of electricity and communication signals, through the transmission grid, to the point of delivery to a customer’s facilities. Certain installations, including those in mines and ships, are not subject to the NESC. NESC guidelines are designed to ensure the safety of workers engaged in the installation, operation or maintenance of electric supply and communication lines and associated equipment. These guidelines constitute the minimum acceptable standard for occupational and public safety under the specified conditions. The code is not intended as a design specification or an instruction manual. Formally, the NESC must be regarded as a national safety code applicable to the United States.
The extensive rules of both the European and American standards provide for the safe performance of work on electrical installations.
The European Standard (1994a)
Definitions
The standard provides definitions only for the most common terms; further information is available in the International Electrotechnical Commission (1979). For the purposes of this standard, electrical installation refers to all equipment involved in the generation, transmission, conversion, distribution and use of electrical energy. This includes all energy sources, including batteries and capacitors (ENEL 1994; EDF-GDF 1991).
Basic principles
Safe operation: The basic principle of safe work on, with or near an electrical installation is the need to assess the electrical risk before commencing work.
Personnel: The best rules and procedures for work on, with or near electrical installations are of no value if workers are not thoroughly conversant with them and do not comply strictly with them. All personnel involved in work on, with or near an electrical installation shall be instructed in the safety requirements, safety rules and company policies applicable to their work. Where the work is long or complex, this instruction shall be repeated. Workers shall be required to comply with these requirements, rules and instructions.
Organization: Each electrical installation shall be placed under the responsibility of the designated person in control of the electrical installation. In cases of undertakings involving more than one installation, it is essential that the designated persons in control of each installation cooperate with each other.
Each work activity shall be the responsibility of the designated person in control of the work. Where the work comprises sub-tasks, persons responsible for the safety of each sub-task will be designated, each reporting to the coordinator. The same person can act as the designated person in control of the work and the designated person in control of the electrical installation.
Communication: This includes all means of information transmission between persons, i.e., spoken word (including telephones, radio and speech), writing (including fax) and visual means (including instrument panels, video, signals and lights).
Formal notification of all information necessary for the safe operation of the electrical installation, e.g., network arrangements, switchgear status and the position of safety devices, shall be given.
Worksite: Adequate working space, access and lighting shall be provided at electrical installations on, with or near which any work is to be carried out.
Tools, equipment and procedures: Tools, equipment and procedures shall comply with the requirements of relevant European, national and international standards, where these exist.
Drawings and reports: The installation’s drawings and reports shall be up to date and readily available.
Signage: Adequate signage drawing attention to specific hazards shall be displayed as needed when the installation is operating and during any work.
Standard operating procedures
Operating activities: Operating activities are designed to change the electrical state of an electrical installation. There are two types:
- operations intended to modify the electrical state of an electrical installation, e.g., in order to use equipment, connect, disconnect, start or stop an installation or section of an installation to carry out work. These activities may be carried out locally or by remote control.
- disconnecting before or reconnecting after dead-working, to be carried out by qualified or trained workers.
Functional checks: This includes measurement, testing and inspection procedures.
Measurement is defined as the entire range of activities used to collect physical data in electrical installations. Measurement shall be carried out by qualified professionals.
Testing includes all activities designed to verify the operation or electrical, mechanical or thermal condition of an electrical installation. Testing shall be carried out by qualified workers.
Inspection is verification that an electrical installation conforms to applicable specified technical and safety regulations.
Work procedures
General: The designated person in control of the electrical installation and the designated person in control of the work shall both ensure that workers receive specific and detailed instructions before starting the work, and on its completion.
Before the start of work, the designated person in control of the work shall notify the designated person in control of the electrical installation of the nature, site and consequences to the electrical installation of the intended work. This notification shall be given preferably in writing, especially when the work is complex.
Work activities can be divided into three categories: dead-working, live-working and work in the vicinity of live installations. Measures designed to protect against electrical shocks, short circuits and arcing have been developed for each type of work.
Induction: The following precautions shall be taken when working on electrical lines subject to current induction:
- grounding at appropriate intervals; this reduces the potential between conductors and earth to a safe level
- equipotential bonding of the worksite; this prevents workers from introducing themselves into the induction loop.
Weather conditions: When lightning is seen or thunder heard, no work shall be started or continued on outdoor installations or on indoor installations directly connected to overhead lines.
Dead-working
The following basic work practices will ensure that the electrical installations at the worksite remain dead for the duration of the work. Unless there are clear contraindications, the practices should be applied in the order listed.
Complete disconnection: The section of the installation in which the work is to be carried out shall be isolated from all sources of current supply, and secured against reconnection.
Securing against reconnection: All circuit-breaking devices used to isolate the electrical installation for the work shall be locked out, preferably by locking the operating mechanism.
Verification that the installation is dead: The absence of current shall be verified at all poles of the electrical installation at or as near as practicable to the worksite.
Grounding and short-circuiting: At all high- and some low-voltage worksites, all parts to be worked on shall be grounded and short-circuited after they have been disconnected. Grounding and short-circuiting systems shall be connected to the earth first; the components to be grounded must be connected to the system only after it has been earthed. As far as practical, the grounding and short-circuiting systems shall be visible from the worksite. Low- and high-voltage installations have their own specific requirements. At these types of installation, all sides of the worksites and all conductors entering the site must be grounded and short-circuited.
Protecting against adjacent live parts: Additional protective measures are necessary if parts of an electrical installation in the vicinity of the worksite cannot be made dead. Workers shall not commence work before receiving permission to do so from the designated person in control of the work, who in turn must receive authorization from the designated person in control of the electrical installation. Once the work has been completed, workers shall leave the worksite, tools and equipment shall be stored, and grounding and short-circuiting systems removed. The designated person in control of the work shall then notify the designated person in control of the electrical installation that the installation is available for reconnection.
Live-working
General: Live-working is work carried out within a zone in which there is current flow. Guidance for the dimensions of the live-working zone can be found in standard EN 50179. Protective measures designed to prevent electric shocks, arcing and short circuits shall be applied.
Training and qualification: Specific training programmes shall be established to develop and maintain the ability of qualified or trained workers to perform live-working. After completing the programme, workers will receive a qualification rating and authorization to perform specific live-work on specific voltages.
Maintenance of qualifications: The ability to carry out live-working shall be maintained by either practice or new training.
Work techniques: Currently, there are three recognized techniques, distinguished by their applicability to different types of live parts and the equipment required to prevent electric shocks, arcing and short circuits:
- hot-stick working
- insulating-glove working
- bare-hand working.
Each technique requires different preparation, equipment and tools, and selection of the most appropriate technique will depend on the characteristics of the work in question.
Tools and equipment: The characteristics, storage, maintenance, transportation and inspection of tools, equipment and systems shall be specified.
Weather conditions: Restrictions apply to live-working in adverse weather conditions, since insulating properties, visibility and worker mobility are all reduced.
Work organization: The work shall be adequately prepared; written preparation shall be submitted in advance for complex work. The installation in general, and the section where the work is to be carried out in particular, shall be maintained in a condition consistent with the preparation required. The designated person in control of the work shall inform the designated person in control of the electrical installation of the nature of the work, the site in the installation at which the work will be performed, and the estimated duration of the work. Before work begins, workers shall have the nature of the work, the relevant safety measures, the role of each worker, and the tools and equipment to be used explained to them.
Specific practices exist for extra-low-voltage, low-voltage, and high-voltage installations.
Work in the vicinity of live parts
General: Work in the vicinity of live parts with nominal voltages above 50 VAC or 120 VDC shall be performed only when safety measures have been applied to ensure that live parts cannot be touched or that the live zone cannot be entered. Screens, barriers, enclosures or insulating coverings may be used for this purpose.
Before the work starts, the designated person in control of the work shall instruct the workers, particularly those unfamiliar with work in the vicinity of live parts, on the safety distances to be observed on the worksite, the principal safety practices to follow, and the need for behaviour that ensures the safety of the entire work crew. Worksite boundaries shall be precisely defined and marked and attention drawn to unusual working conditions. This information shall be repeated as needed, particularly after changes in working conditions.
Workers shall ensure that no part of their body nor any object enters the live zone. Particular care shall be taken when handling long objects, for example, tools, cable ends, pipes and ladders.
Protection by screens, barriers, enclosures or insulating coverings: The selection and installation of these protective devices shall ensure sufficient protection against predictable electrical and mechanical stressors. The equipment shall be suitably maintained and kept secured during the work.
Maintenance
General: The purpose of maintenance is to maintain the electrical installation in the required condition. Maintenance may be preventive (i.e., carried out on a regular basis to prevent breakdowns and keep equipment in working order) or corrective (i.e., carried out to replace defective parts).
Maintenance work can be divided into two risk categories:
- work involving the risk of electrical shock, where procedures applicable to live-working and work in the vicinity of live parts must be followed
- work where equipment design allows some maintenance work to be performed in the absence of full live-working procedures
Personnel: Personnel who are to carry out the work shall be adequately qualified or trained and shall be provided with appropriate measuring and testing tools and devices.
Repair work: Repair work consists of the following steps: fault location; fault rectification and/or replacement of components; recommissioning of the repaired section of the installation. Each of these steps may require specific procedures.
Replacement work: In general, fuse replacement in high-voltage installations shall be performed as dead-work. Fuse replacement shall be performed by qualified workers following appropriate work procedures. The replacement of lamps and removable parts such as starters shall be carried out as dead-work. In high-voltage installations, repair procedures shall also apply to replacement work.
Training of Personnel about Electrical Hazards
Effective work organization and safety training is a key element in every successful organization, prevention programme and occupational health and safety programme. Workers must have proper training to do their jobs safely and efficiently.
The responsibility for implementing employee training rests with management. Management must recognize that employees must perform at a certain level before the organization can achieve its objectives. In order to achieve these levels, worker training policies and, by extension, concrete training programmes must be established. Programmes should include training and qualification phases.
Live-working programmes should include the following elements:
Training: In some countries, programmes and training facilities must be formally approved by a live-working committee or similar body. Programmes are based primarily on practical experience, complemented by technical instruction. Training takes the form of practical work on indoor or outdoor model installations similar to those on which actual work is to be performed.
Qualifications: Live-working procedures are very demanding, and it is essential to use the right person at the right place. This is most easily achieved if qualified personnel of different skill levels are available. The designated person in control of the work should be a qualified worker. Where supervision is necessary, it too should be carried out by a qualified person. Workers should work only on installations whose voltage and complexity corresponds to their level of qualification or training. In some countries, qualification is regulated by national standards.
Finally, workers should be instructed and trained in essential life-saving techniques. The reader is referred to the chapter on first-aid for further information.
Basic Concepts
The Chemistry and Physics of Fire
Fire is a manifestation of uncontrolled combustion. It involves combustible materials which are found around us in the buildings in which we live, work and play, as well as a wide range of gases, liquids and solids which are encountered in industry and commerce. They are commonly carbon-based, and may be referred to collectively as fuels in the context of this discussion. Despite the wide variety of these fuels in both their chemical and physical states, in fire they share features that are common to them all. Differences are encountered in the ease with which fire can be initiated (ignition), the rate with which fire can develop (flame spread), and the power that can be generated (rate of heat release), but as our understanding of the science of fire improves, we become better able to quantify and predict fire behaviour and apply our knowledge to fire safety in general. The purpose of this section is to review some of the underlying principles and provide guidance to an understanding of fire processes.
Basic Concepts
Combustible materials are all around us. Given the appropriate circumstances, they can be made to burn by subjecting them to an ignition source which is capable of initiating a self-sustaining reaction. In this process, the “fuel” reacts with oxygen from the air to release energy (heat), while being converted to products of combustion, some of which may be harmful. The mechanisms of ignition and burning need to be clearly understood.
Most everyday fires involve solid materials (e.g., wood, wood products and synthetic polymers), although gaseous and liquid fuels are not uncommon. A brief review of the combustion of gases and liquids is desirable before some of the basic concepts are discussed.
Diffusion and premixed flames
A flammable gas (e.g., propane, C3H8) can be burned in two ways: a stream or jet of gas from a pipe (cf. the simple Bunsen burner with the air inlet closed) can be ignited and will burn as a diffusion flame in which burning occurs in those regions where gaseous fuel and air mix by diffusive processes. Such a flame has a characteristic yellow luminosity, indicating the presence of minute soot particles formed as a result of incomplete combustion. Some of these will burn in the flame, but others will emerge from the flame tip to form smoke.
If the gas and air are intimately mixed before ignition, then premixed combustion will occur, provided that the gas/air mixture lies within a range of concentrations bounded by the lower and upper flammability limits (see table 1). Outside these limits, the mixture is non-flammable. (Note that a premixed flame is stabilized at the mouth of a Bunsen burner when the air inlet is open.) If a mixture is flammable, then it can be ignited by a small ignition source, such as an electrical spark. The stoichiometric mixture is the most readily ignited, in which the amount of oxygen present is in the correct proportion to burn all the fuel to carbon dioxide and water (see accompanying equation, below, in which nitrogen can be seen to be present in the same proportion as in air but does not take part in the reaction). Propane (C3H8) is the combustible material in this reaction:
C3H8 + 5O2 + 18.8N2 = 3CO2 + 4H2O + 18.8N2
An electrical discharge as small as 0.3 mJ is sufficient to ignite a stoichiometric propane/air mixture in the reaction illustrated. This represents a barely perceptible static spark, as experienced by someone who has walked across a synthetic carpet and touched a grounded object. Even smaller amounts of energy are required for certain reactive gases such as hydrogen, ethylene and ethyne. In pure oxygen (as in the reaction above, but with no nitrogen present as a diluent), even lower energies are sufficient.
Table 1. Lower and upper flammability limits in air
|
Lower flammability |
Upper flammability |
|
|
Carbon monoxide |
12.5 |
74 |
|
Methane |
5.0 |
15 |
|
Propane |
2.1 |
9.5 |
|
n-Hexane |
1.2 |
7.4 |
|
n-Decane |
0.75 |
5.6 |
|
Methanol |
6.7 |
36 |
|
Ethanol |
3.3 |
19 |
|
Acetone |
2.6 |
13 |
|
Benzene |
1.3 |
7.9 |
The diffusion flame associated with a flow of gaseous fuel exemplifies the mode of burning that is observed when a liquid or solid fuel is undergoing flaming combustion. However, in this case, the flame is fed by fuel vapours generated at the surface of the condensed phase. The rate of supply of these vapours is coupled to their rate of burning in the diffusion flame. Energy is transferred from the flame to the surface, thus providing the energy necessary to produce the vapours. This is a simple evaporative process for liquid fuels, but for solids, enough energy must be provided to cause chemical decomposition of the fuel, breaking large polymeric molecules into smaller fragments which can vaporize and escape from the surface. This thermal feedback is essential to maintain the flow of vapours, and hence support the diffusion flame (figure 1). Flames can be extinguished by interfering with this process in a number of ways (see below).
Figure 1. Schematic representation of a burning surface showing the heat and mass transfer processes.
Heat transfer
An understanding of heat (or energy) transfer is the key to an understanding of fire behaviour and fire processes. The subject deserves careful study. There are many excellent texts to which one may turn (Welty, Wilson and Wicks 1976; DiNenno 1988), but for the present purposes it is necessary only to draw attention to the three mechanisms: conduction, convection and radiation. The basic equations for steady-state heat transfer () are:
Conduction: ![]()
Convection: ![]()
Radiation: ![]()
Conduction is relevant to heat transfer through solids; (k is a material property known as thermal conductivity (kW/mK ) and l is the distance (m) over which the temperature falls from T1 to T2 (in degrees Kelvin). Convection in this context refers to the transfer of heat from a fluid (in this case, air, flames or fire products) to a surface (solid or liquid); h is the convective heat transfer coefficient kW/m2K) and depends on the configuration of the surface and nature of the flow of fluid past that surface. Radiation is similar to visible light (but with a longer wavelength) and requires no intervening medium (it can traverse a vacuum); e is the emissivity (efficiency by which a surface can radiate), s is the Stefan-Boltzman constant (). Thermal radiation travels at the speed of light (3 x 108 m/s) and an intervening solid object will cast a shadow.
Rate of burning and rate of heat release
Heat transfer from flames to the surface of condensed fuels (liquids and solids) involves a mixture of convection and radiation, although the latter dominates when the effective diameter of the fire exceeds 1 m. The rate of burning (, (g/s)) can be expressed by the formula:
![]()
![]() is the heat flux from the flame to the surface (kW/m2);
is the heat flux from the flame to the surface (kW/m2); ![]() is the heat loss from the surface (e.g., by radiation, and by conduction through the solid) expressed as a flux (kW/m2); Afuel is the surface area of the fuel (m2); and Lv is the heat of gasification (equivalent to the latent heat of evaporation for a liquid) (kJ/g). If a fire develops in a confined space, the hot smoky gases rising from the fire (driven by buoyancy) are deflected beneath the ceiling, heating the upper surfaces. The resulting smoke layer and the hot surfaces radiate down to the lower part of the enclosure, in particular to the fuel surface, thus increasing the rate of burning:
is the heat loss from the surface (e.g., by radiation, and by conduction through the solid) expressed as a flux (kW/m2); Afuel is the surface area of the fuel (m2); and Lv is the heat of gasification (equivalent to the latent heat of evaporation for a liquid) (kJ/g). If a fire develops in a confined space, the hot smoky gases rising from the fire (driven by buoyancy) are deflected beneath the ceiling, heating the upper surfaces. The resulting smoke layer and the hot surfaces radiate down to the lower part of the enclosure, in particular to the fuel surface, thus increasing the rate of burning:
![]()
where ![]() is the extra heat supplied by radiation from the upper part of the enclosure (kW/m2). This additional feedback leads to greatly enhanced rates of burning and to the phenomenon of flashover in enclosed spaces where there is an adequate supply of air and sufficient fuel to sustain the fire (Drysdale 1985).
is the extra heat supplied by radiation from the upper part of the enclosure (kW/m2). This additional feedback leads to greatly enhanced rates of burning and to the phenomenon of flashover in enclosed spaces where there is an adequate supply of air and sufficient fuel to sustain the fire (Drysdale 1985).
The rate of burning is moderated by the magnitude of the value of Lv, the heat of gasification. This tends to be low for liquids and relatively high for solids. Consequently, solids tend to burn much more slowly than liquids.
It has been argued that the most important single parameter which determines the fire behaviour of a material (or assembly of materials) is the rate of heat release (RHR) which is coupled to the rate of burning through the equation:
![]()
whereis the effective heat of combustion of the fuel (kJ/g). New techniques are now available for measuring the RHR at different heat fluxes (e.g., the Cone Calorimeter), and it is now possible to measure the RHR of large items, such as upholstered furniture and wall linings in large-scale calorimeters which use oxygen consumption measurements to determine the rate of heat release (Babrauskas and Grayson 1992).
It should be noted that as a fire grows in size, not only does the rate of heat release increase, but the rate of production of “fire products” also increases. These contain toxic and noxious species as well as particulate smoke, the yields of which will increase when a fire developing in a building enclosure becomes underventilated.
Ignition
Ignition of a liquid or solid involves raising the surface temperature until vapours are being evolved at a rate sufficient to support a flame after the vapours have been ignited. Liquid fuels can be classified according to their flashpoints, the lowest temperature at which there is a flammable vapour/air mixture at the surface (i.e., the vapour pressure corresponds to the lower flammability limit). These can be measured using a standard apparatus, and typical examples are given in table 2. A slightly higher temperature is required to produce a sufficient flow of vapours to support a diffusion flame. This is known as the firepoint. For combustible solids, the same concepts are valid, but higher temperatures are required as chemical decomposition is involved. The firepoint is typically in excess of 300 °C, depending on the fuel. In general, flame-retarded materials have significantly higher firepoints (see Table 2).
Table 2. Flashpoints and firepoints of liquid and solid fuels
|
Closed cup flashpoint1 (°C) |
Firepoint2 (°C) |
|
|
Gasoline (100 Octane) (l) |
–38 |
– |
|
n-Decane (l) |
46 |
61.5 |
|
n-Dodecane (l) |
74 |
103 |
|
Polymethylmethacrylate (s) |
– |
310 |
|
FR polymethylmethacrylate (s) |
– |
377 |
|
Polypropylene (s) |
– |
330 |
|
FR polypropylene (s) |
– |
397 |
|
Polystyrene (s) |
– |
367 |
|
FR polystyrene (s) |
– |
445 |
l = liquid; s = solid.
1 By Pensky-Martens closed cup apparatus.
2 Liquids: by Cleveland open cup apparatus. Solids: Drysdale and Thomson (1994).
(Note that the results for the flame-retarded species refer to a heat flux of 37 kW/m2).
Ease of ignition of a solid material is therefore dependent on the ease with which its surface temperature can be raised to the firepoint, e.g., by exposure to radiant heat or to a flow of hot gases. This is less dependent on the chemistry of the decomposition process than on the thickness and physical properties of the solid, namely, its thermal conductivity (k), density (r) and heat capacity (c). Thin solids, such as wood shavings (and all thin sections), can be ignited very easily because they have a low thermal mass, that is, relatively little heat is required to raise the temperature to the firepoint. However, when heat is transferred to the surface of a thick solid, some will be conducted from the surface into the body of the solid, thus moderating the temperature rise of the surface. It can be shown theoretically that the rate of rise of the surface temperature is determined by the thermal inertia of the material, that is, the product krc. This is borne out in practice, since thick materials with a high thermal inertia (e.g., oak, solid polyurethane) will take a long time to ignite under a given heat flux, whereas under identical conditions thick materials with a low thermal inertia (e.g., fibre insulating board, polyurethane foam) will ignite quickly (Drysdale 1985).
Ignition sources
Ignition is illustrated schematically in figure 2 (piloted ignition). For successful ignition, an ignition source must be capable not only of raising the surface temperature to the firepoint, or above, but it must also cause the vapours to ignite. An impinging flame will act in both capacities, but an imposed radiative flux from a remote source may lead to the evolution of vapours at a temperature above the firepoint, without the vapours igniting. However, if the evolved vapours are hot enough (which requires the surface temperature to be much higher than the firepoint), they may ignite spontaneously as they mix with air. This process is known as spontaneous ignition.
Figure 2. The scenario for piloted ignition.
A large number of ignition sources can be identified, but they have one thing in common, which is that they are the result of some form of carelessness or inaction. A typical list would include naked flames, “smokers’ materials”, frictional heating, electrical devices (heaters, irons, cookers, etc.) and so on. An excellent survey may be found in Cote (1991). Some of these are summarized in table 3.
Table 3. Ignition sources
|
|
Examples
|
|
Electrically powered equipment |
Electric heaters, hair dryers, electric blankets, etc. |
|
Open flame source |
Match, cigarette lighter, blow torch, etc. |
|
Gas-fuelled equipment |
Gas fire, space heater, cooker, etc. |
|
Other fuelled equipment |
Wood stove, etc. |
|
Lighted tobacco product |
Cigar, pipe, etc. |
|
Hot object |
Hot pipes, mechanical sparks, etc. |
|
Exposure to heating |
Adjacent fire, etc. |
|
Spontaneous heating |
Linseed oil-soaked rags, coal piles, etc. |
|
Chemical reaction |
Rare-e.g., potassium permanganate with glycerol |
It should be noted that smouldering cigarettes cannot initiate flaming combustion directly (even in common gaseous fuels), but can cause smouldering in materials which have the propensity to undergo this type of combustion. This is observed only with materials which char on heating. Smouldering involves the surface oxidation of the char, which generates enough heat locally to produce fresh char from adjacent unburnt fuel. It is a very slow process, but may eventually undergo a transition to flaming. Thereafter, the fire will develop very rapidly.
Materials which have the propensity to smoulder can also exhibit the phenomenon of self-heating (Bowes 1984). This arises when such a material is stored in large quantities and in such a way that heat generated by slow surface oxidation cannot escape, leading to a rise in temperature within the mass. If the conditions are right, this can lead to a runaway process ultimately developing into a smouldering reaction at depth within the material.
Flame spread
A major component in the growth of any fire is the rate at which flame will spread over adjacent combustible surfaces. Flame spread can be modelled as an advancing ignition front in which the leading edge of the flame acts as an ignition source for the fuel that is not yet burning. The rate of spread is determined partly by the same material properties that control the ease of ignition and partly by the interaction between the existing flame and the surface ahead of the front. Upward, vertical spread is the most rapid as buoyancy ensures that the flames flow upwards, exposing the surface above the burning area to direct heat transfer from the flames. This should be contrasted with spread over a horizontal surface when the flames from the burning area rise vertically, away from the surface. Indeed, it is common experience that vertical spread is the most hazardous (e.g., flame spread on curtains and drapes and on loose clothing such as dresses and nightgowns).
The rate of spread is also affected by an imposed radiant heat flux. In the development of a fire in a room, the area of the fire will grow more rapidly under the increasing level of radiation that builds up as the fire progresses. This will contribute to the acceleration of fire growth that is characteristic of flashover.
Theory of Fire Extinguishment
Fire extinction and suppression can be examined in terms of the above outline of the theory of fire. The gas phase combustion processes (i.e., the flame reactions) are very sensitive to chemical inhibitors. Some of the flame retardants used to improve the “fire properties” of materials rely on the fact that small amounts of inhibitor released with the fuel vapours will suppress the establishment of flame. The presence of a flame retardant cannot render a combustible material non-combustible, but it can make ignition more difficult—perhaps preventing ignition altogether provided that the ignition source is small. However, if a flame-retarded material becomes involved in an existing fire, it will burn as the high heat fluxes overwhelm the effect of the retardant.
Extinction of a fire may be achieved in a number of ways:
1. stopping the supply of fuel vapours
2. quenching the flame by chemical extinguishers (inhibiting)
3. removing the supply of air (oxygen) to the fire (smothering)
4. “blow-out”.
Controlling the flow of fuel vapours
The first method, stopping the supply of fuel vapours, is clearly applicable to a gas-jet fire in which the supply of the fuel can simply be turned off. However, it is also the most common and safest method of extinguishing a fire involving condensed fuels. In the case of a fire involving a solid, this requires the fuel surface to be cooled below the firepoint, when the flow of vapours becomes too small to support a flame. This is achieved most effectively by the application of water, either manually or by means of an automatic system (sprinklers, water spray, etc.). In general, liquid fires cannot be dealt with in this manner: liquid fuels with low firepoints simply cannot be cooled sufficiently, while in the case of a high-firepoint fuel, vigorous vaporization of water when it comes into contact with the hot liquid at the surface can lead to burning fuel being ejected from the container. This can have very serious consequences for those fighting the fire. (There are some special cases in which an automatic high-pressure water-spray system may be designed to deal with the latter type of fire, but this is not common.)
Liquid fires are commonly extinguished by the use of fire-fighting foams (Cote 1991). This is produced by aspirating a foam concentrate into a stream of water which is then directed at the fire through a special nozzle which permits air to be entrained into the flow. This produces a foam which floats on top of the liquid, reducing the rate of supply of fuel vapours by a blockage effect and by shielding the surface from heat transfer from the flames. The foam has to be applied carefully to form a “raft” which gradually increases in size to cover the liquid surface. The flames will decrease in size as the raft grows, and at the same time the foam will gradually break down, releasing water which will aid the cooling of the surface. The mechanism is in fact complex, although the net result is to control the flow of vapours.
There are a number of foam concentrates available, and it is important to choose one that is compatible with the liquids that are to be protected. The original “protein foams” were developed for hydrocarbon liquid fires, but break down rapidly if brought into contact with liquid fuels that are water soluble. A range of “synthetic foams” have been developed to tackle the entire range of liquid fires that may be encountered. One of these, aqueous film-forming foam (AFFF), is an all-purpose foam which also produces a film of water on the surface of the liquid fuel, thus increasing its effectiveness.
Quenching the flame
This method makes use of chemical suppressants to extinguish the flame. The reactions which occur in the flame involve free radicals, a highly reactive species which have only a fleeting existence but are continuously regenerated by a branched chain process that maintains high enough concentrations to allow the overall reaction (e.g., an R1 type reaction) to proceed at a fast rate. Chemical suppressants applied in sufficient quantity will cause a dramatic fall in the concentration of these radicals, effectively quenching the flame. The most common agents that operate in this way are the halons and dry powders.
Halons react in the flame to generate other intermediate species with which the flame radicals react preferentially. Relatively small amounts of the halons are required to extinguish a fire, and for this reason they were traditionally considered highly desirable; extinguishing concentrations are “breathable” (although the products generated while passing through the flame are noxious). Dry powders act in a similar fashion, but under certain circumstances are much more effective. Fine particles are dispersed into the flame and cause termination of the radical chains. It is important that the particles are small and numerous. This is achieved by the manufacturers of many proprietary brands of dry powders by selecting a powder that “decrepitates”, that is, the particles fragment into smaller particles when they are exposed to the high temperatures of the flame.
For a person whose clothing has caught fire, a dry powder extinguisher is recognized as the best method to control flames and to protect that individual. Rapid intervention gives rapid “knockdown”, thus minimizing injury. However, the flame must be completely extinguished because the particles quickly fall to the ground and any residual flaming will quickly regain hold. Similarly, halons will only remain effective if the local concentrations are maintained. If it is applied out of doors, the halon vapour rapidly disperses, and once again the fire will rapidly re-establish itself if there is any residual flame. More significantly, the loss of the suppressant will be followed by re-ignition of the fuel if the surface temperatures are high enough. Neither halons nor dry powders have any significant cooling effect on the fuel surface.
Removing the supply of air
The following description is an oversimplification of the process. While “removing the supply of air” will certainly cause the fire to extinguish, to do this it is only necessary to reduce the oxygen concentration below a critical level. The well-known “oxygen index test” classifies combustible materials according to the minimum oxygen concentration in an oxygen/nitrogen mixture that will just support flaming. Many common materials will burn at oxygen concentrations down to approximately 14% at ambient temperatures (ca. 20°C) and in the absence of any imposed heat transfer. The critical concentration is temperature dependent, decreasing as the temperature is increased. Thus, a fire that has been burning for some time will be capable of supporting flames at concentrations perhaps as low as 7%. A fire in a room may be held in check and may even self-extinguish if the supply of oxygen is limited by keeping doors and windows closed. Flaming may cease, but smouldering will continue at very much lower oxygen concentrations. Admission of air by opening a door or breaking a window before the room has cooled sufficiently can lead to a vigorous eruption of the fire, known as backdraught, or backdraft.
“Removal of air” is difficult to achieve. However, an atmosphere may be rendered “inert” by total flooding by means of a gas which will not support combustion, such as nitrogen, carbon dioxide or gases from a combustion process (e.g., a ship’s engines) which are low in oxygen and high in carbon dioxide. This technique can only be used in enclosed spaces as it is necessary to maintain the required concentration of the “inert gas” until either the fire has extinguished completely or fire-fighting operations can begin. Total flooding has special applications, such as for ships’ holds and rare book collections in libraries. The required minimum concentrations of the inert gases are shown in Table 4. These are based on the assumption that the fire is detected at an early stage and that the flooding is carried out before too much heat has accumulated in the space.
Table 4: Comparison of concentrations of different gases required for inerting
|
Agent |
Minimum concentration (% volume) |
|
Halon 1301 |
8.0 |
|
Halon 1211 |
8.1 |
|
Nitrogen |
|
|
Carbon dioxide |
|
“Removal of air” can be effected in the immediate vicinity of a small fire by local application of a suppressant from an extinguisher. Carbon dioxide is the only gas that is used in this way. However, as this gas quickly disperses, it is essential to extinguish all flaming during the attack on the fire; otherwise, flaming will re-establish itself. Re-ignition is also possible because carbon dioxide has little if any cooling effect. It is worth noting that a fine water spray entrained into a flame can cause extinction as the combined result of evaporation of the droplets (which cools the burning zone) and reduction of the oxygen concentration by dilution by water vapour (which acts in the same way as carbon dioxide). Fine water sprays and mists are being considered as possible replacements for halons.
It is appropriate to mention here that it is inadvisable to extinguish a gas flame unless the gas flow can be stopped immediately thereafter. Otherwise, a substantial volume of flammable gas may build up and subsequently ignite, with potentially serious consequences.
Blow-out
This method is included here for completeness. A match flame can easily be blown out by increasing the air velocity above a critical value in the vicinity of the flame. The mechanism operates by destabilizing the flame in the vicinity of the fuel. In principle, larger fires can be controlled in the same way, but explosive charges are normally required to generate sufficient velocities. Oil well fires can be extinguished in this manner.
Finally, a common feature that needs to be emphasized is that the ease with which a fire can be extinguished decreases rapidly as the fire increases in size. Early detection permits extinction with minimal quantities of suppressant, with reduced losses. In choosing a suppressant system, one should take into account the potential rate of fire development and what type of detection system is available.
Explosions
An explosion is characterized by the sudden release of energy, producing a shock wave, or blast wave, that may be capable of causing remote damage. There are two distinct types of sources, namely, the high explosive and the pressure burst. The high explosive is typified by compounds such as trinitrotoluene (TNT) and cyclotrimethylenetrinitramine (RDX). These compounds are highly exothermic species, decomposing to release substantial quantities of energy. Although thermally stable (although some are less so and require desensitization to make them safe to handle), they can be induced to detonate, with decomposition, propagating at the velocity of sound through the solid. If the amount of energy released is high enough, a blast wave will propagate from the source with the potential to do significant damage at a distance.
By assessing remote damage, one can estimate the size of the explosion in terms of “TNT equivalent” (normally in metric tons). This technique relies on the large amount of data that has been gathered on the damage potential of TNT (much of it during wartime), and uses empirical scaling laws which have been developed from studies of the damage caused by known quantities of TNT.
In peacetime, high explosives are used in a variety of activities, including mining, quarrying and major civil engineering works. Their presence on a site represents a particular hazard that requires specific management. However, the other source of “explosions” can be equally devastating, particularly if the hazard has not been recognized. Overpressures leading to pressure bursts can be the result of chemical processes within plants or from purely physical effects, as will occur if a vessel is heated externally, leading to overpressurization. The term BLEVE (boiling liquid expanding vapour explosion) has its origins here, referring originally to the failure of steam boilers. It is now also commonly used to describe the event in which a pressure vessel containing a liquefied gas such as LPG (liquefied petroleum gas) fails in a fire, releasing the flammable contents, which then ignite to produce a “fireball”.
On the other hand, the overpressure may be caused internally by a chemical process. In the process industries, self-heating can lead to a runaway reaction, generating high temperatures and pressures capable of causing a pressure burst. However, the most common type of explosion is caused by the ignition of a flammable gas/air mixture which is confined within an item of a plant or indeed within any confining structure or enclosure. The prerequisite is the formation of a flammable mixture, an occurrence which should be avoided by good design and management. In the event of an accidental release, a flammable atmosphere will exist wherever the concentration of the gas (or vapour) lies between the lower and upper flammability limits (Table 1). If an ignition source is introduced to one of these regions, a premixed flame will propagate rapidly from the source, converting the fuel/air mixture into combustion products at an elevated temperature. This can be as high as 2,100 K, indicating that in a completely closed system initially at 300 K, an overpressure as high as 7 bars is possible. Only specially designed pressure vessels are capable of containing such overpressures. Ordinary buildings will fall unless protected by pressure relief panels or bursting discs or by an explosion suppression system. Should a flammable mixture form within a building, the subsequent explosion can cause significant structural damage—perhaps total destruction—unless the explosion can vent to the outside through openings (e.g., the failure of windows) created during the early stages of the explosion.
Explosions of this type are also associated with the ignition of dust suspensions in air (Palmer 1973). These are encountered when there is a substantial accumulation of “explosible” dust which is dislodged from shelves, rafters and ledges within a building to form a cloud, which is then exposed to an ignition source (e.g., in flour mills, grain elevators, etc.). The dust must (obviously) be combustible, but not all combustible dusts are explosible at ambient temperatures. Standard tests have been designed to determine whether a dust is explosible. These can also be used to illustrate that explosible dusts exhibit “explosibility limits”, similar in concept to the “flammability limits” of gases and vapours. In general, a dust explosion has the potential to do a great deal of damage because the initial event may cause more dust to be dislodged, forming an even greater dust cloud which will inevitably ignite, to produce an even greater explosion.
Explosion venting, or explosion relief, will only operate successfully if the rate of development of the explosion is relatively slow, such as associated with the propagation of a premixed flame through a stationary flammable mixture or an explosible dust cloud. Explosion venting is of no use if detonation is involved. The reason for this is that the pressure relief openings have to be created at an early stage of the event when the pressure is still relatively low. If a detonation occurs, the pressure rises too rapidly for relief to be effective, and the enclosing vessel or item of a plant experiences very high internal pressures which will lead to massive destruction. Detonation of a flammable gas mixture can occur if the mixture is contained within a long pipe or duct. Under certain conditions, propagation of the premixed flame will push the unburnt gas ahead of the flame front at a rate that will increase turbulence, which in turn will increase the rate of propagation. This provides a feedback loop which will cause the flame to accelerate until a shock wave is formed. This, combined with the combustion process, is a detonation wave which can propagate at velocities well in excess of 1,000 m/s. This may be compared with the fundamental burning velocity of a stoichiometric propane/air mixture of 0.45 m/s. (This is the rate at which a flame will propagate through a quiescent (i.e., non-turbulent) propane/air mixture.)
The importance of turbulence on the development of this type of explosion cannot be underestimated. The successful operation of an explosion protection system relies on early venting or early suppression. If the rate of development of the explosion is too fast, then the protection system will not be effective, and unacceptable overpressures can be produced.
An alternative to explosion relief is explosion suppression. This type of protection requires that the explosion is detected at a very early stage, as close to ignition as possible. The detector is used to initiate the rapid release of a suppressant into the path of the propagating flame, effectively arresting the explosion before the pressure has increased to an extent at which the integrity of the enclosing boundaries is threatened. The halons have been commonly used for this purpose, but as these are being phased out, attention is now being paid to the use of high-pressure water-spray systems. This type of protection is very expensive and has limited application as it can only be used in relatively small volumes within which the suppressant can be distributed quickly and uniformly (e.g., ducts carrying flammable vapour or explosible dusts).
Information Analysis for Fire Protection
In general terms, fire science has only recently been developed to a stage at which it is capable of providing the knowledge base on which rational decisions regarding engineering design, including safety issues, can be based. Traditionally, fire safety has developed on an ad hoc basis, effectively responding to incidents by imposing regulations or other restrictions to ensure that there will be no re-occurrence. Many examples could be quoted. For example, the Great Fire of London in 1666 led in due course to the establishment of the first building regulations (or codes) and the development of fire insurance. More recent incidents, such as the high-rise office block fires in São Paulo, Brazil, in 1972 and 1974, initiated changes to the building codes, framed in such a way as to prevent similar multiple-fatality fires in the future. Other problems have been addressed in a similar fashion. In California in the United States, the hazard associated with certain types of modern upholstered furniture (particularly those containing standard polyurethane foam) was recognized, and eventually strict regulations were introduced to control its availability.
These are simple cases in which observations of the consequences of fire have led to the imposition of a set of rules intended to improve the safety of the individual and the community in the event of fire. The decision for action on any issue has to be justified on the basis of an analysis of our knowledge of fire incidents. It is necessary to show that the problem is real. In some cases—such as the São Paulo fires—this exercise is academic, but in others, such as “proving” that modern furnishings are a problem, it is necessary to ensure that the associated costs are wisely spent. This requires a reliable database on fire incidents which over a number of years is capable of showing trends in the number of fires, the number of fatalities, the incidence of a particular type of ignition, etc. Statistical techniques can then be used to examine whether a trend, or a change, is significant, and appropriate measures taken.
In a number of countries, the fire brigade is required to submit a report on each fire attended. In the United Kingdom and the United States, the officer in charge completes a report form which is then submitted to a central organization (the Home Office in the United Kingdom, the National Fire Protection Association, NFPA, in the United States) which then codes and processes the data in a prescribed fashion. The data are then available for inspection by government bodies and other interested parties. These databases are invaluable in highlighting (for example) the principal sources of ignition and the items first ignited. An examination of the incidence of fatalities and their relationship to sources of ignition, etc. has shown that the number of people who die in fires started by smokers’ materials is significantly out of proportion with the number of fires which originate in this way.
The reliability of these databases depends on the skill with which the fire officers carry out the fire investigation. Fire investigation is not an easy task, and requires considerable ability and knowledge—in particular, a knowledge of fire science. The Fire Service in the United Kingdom has a statutory duty to submit a fire report form for every fire attended, which places a considerable responsibility on the officer in charge. The construction of the form is crucial, as it must elicit the required information in sufficient detail. The “Basic Incident Report Form” recommended by the NFPA is shown in the Fire Protection Handbook (Cote 1991).
The data can be used in two ways, either to identify a fire problem or to provide the rational argument necessary to justify a particular course of action that may require public or private expenditure. A long-established database can be used to show the effects of actions taken. The following ten points have been gleaned from NFPA statistics over the period 1980 to 1989 (Cote 1991):
1. Home smoke detectors are widely used and very effective (but significant gaps in the detector strategy remain).
2. Automatic sprinklers produce large reductions in loss of life and property. Increased use of portable and area heating equipment sharply increased home fires involving heating equipment.
3. Incendiary and suspicious fires continued to decline from the 1970’s peak, but associated property damage stopped declining.
4. A large share of fire-fighter fatalities are attributed to heart attacks and activities away from the fireground.
5. Rural areas have the highest fire death rates.
6. Smoking materials igniting upholstered furniture, mattresses or bedding produce the most deadly residential fire scenarios.
7. US and Canadian fire death rates are amongst the highest of all the developed countries.
8. The states of the Old South in the United States have the highest fire death rates.
9. Older adults are at particularly high risk of death in fire.
Such conclusions are, of course, country-specific, although there are some common trends. Careful use of such data can provide the means of formulating sound policies regarding fire safety in the community. However, it must be remembered that these are inevitably “reactive”, rather than “proactive”. Proactive measures can only be introduced following a detailed fire hazard assessment. Such a course of action has been introduced progressively, starting in the nuclear industry and moving into the chemical, petrochemical and offshore industries where the risks are much more easily defined than in other industries. Their application to hotels and public buildings generally is much more difficult and requires the application of fire modelling techniques to predict the course of a fire and how the fire products will spread through the building to affect the occupants. Major advances have been made in this type of modelling, although it must be said that there is a long way to go before these techniques can be used with confidence. Fire safety engineering is still in need of much basic research in fire safety science before reliable fire hazard assessment tools can be made widely available.
Sources of Fire Hazards
Fire and combustion have been defined in various ways. For our purposes, the most important statements in connection with combustion, as a phenomenon, are as follows:
- Combustion represents a self-sustaining run of reactions consisting of physical and chemical transformations.
- The materials involved enter into reaction with the oxidizing agent in their surroundings, which in most cases is with the oxygen in the air.
- Ignition requires favourable starting conditions, which are generally a sufficient heating up of the system that covers the initial energy demand of the chain reaction of burning.
- The resultant of the reactions are often exothermic, which means that during burning, heat is released and this phenomenon is often accompanied by visibly observable flaming.
Ignition may be considered the first step of the self-sustaining process of combustion. It may occur as piloted ignition (or forced ignition) if the phenomenon is caused by any outer ignition source, or it may occur as auto ignition (or self ignition) if the phenomenon is the result of reactions taking place in the combustible material itself and coupled with heat release.
The inclination to ignition is characterized by an empirical parameter, the ignition temperature (i.e., the lowest temperature, to be determined by test, to which the material has to be heated to for ignition). Depending upon whether or not this parameter is determined—with special test methods—by the use of any ignition source, we distinguish between the piloted ignition temperature and the auto ignition temperature.
In the case of piloted ignition, the energy required for the activation of the materials involved in the burning reaction is supplied by ignition sources. However, there is no direct relationship between the heat quantity needed for ignition and the ignition temperature, because although the chemical composition of the components in the combustible system is an essential parameter of ignition temperature, it is considerably influenced by the sizes and shapes of materials, the pressure of the environment, conditions of air flow, parameters of ignition source, the geometrical features of the testing device, etc. This is the reason for which the data published in literature for autoignition temperature and piloted ignition temperature can be significantly different.
The ignition mechanism of materials in different states may be simply illustrated. This involves examining materials as either solids, liquids or gases.
Most solid materials take up energy from any outer ignition source either by conduction, convection or radiation (mostly by their combination), or are heated up as a result of the heat-producing processes taking place internally that start decomposition on their surfaces.
For ignition to occur with liquids, these must have the formation of a vapour space above their surface that is capable of burning. The vapours released and the gaseous decomposition products mix with the air above the surface of liquid or solid material.
The turbulent flows that arise in the mixture and/or the diffusion help the oxygen to reach the molecules, atoms and free radicals on and above the surface, which are already suitable for reaction. The particles induced enter into interaction, resulting in the release of heat. The process steadily accelerates, and as the chain reaction starts, the material comes to ignition and burns.
The combustion in the layer under the surface of solid combustible materials is called smouldering, and the burning reaction taking place on the interface of solid materials and gas is called glowing. Burning with flames (or flaming) is the process in the course of which the exothermic reaction of burning runs in the gas phase. This is typical for the combustion of both liquid and solid materials.
Combustible gases burn naturally in the gas phase. It is an important empirical statement that the mixtures of gases and air are capable of ignition in a certain range of concentration only. This is valid also for the vapours of liquids. The lower and upper flammable limits of gases and vapours depend on the temperature and pressure of the mixture, the ignition source and the concentration of the inert gases in the mixture.
Ignition Sources
The phenomena supplying heat energy may be grouped into four fundamental categories as to their origin (Sax 1979):
1. heat energy generated during chemical reactions (heat of oxidation, heat of combustion, heat of solution, spontaneous heating, heat of decomposition, etc.)
2. electrical heat energy (resistance heating, induction heating, heat from arcing, electric sparks, electrostatical discharges, heat generated by lightning stroke, etc.)
3. mechanical heat energy (frictional heat, friction sparks)
4. heat generated by nuclear decomposition.
The following discussion addresses the most frequently encountered sources of ignition.
Open flames
Open flames may be the simplest and most frequently used ignition source. A large number of tools in general use and various types of technological equipment operate with open flames, or enable the formation of open flames. Burners, matches, furnaces, heating equipment, flames of welding torches, broken gas and oil pipes, etc. may practically be considered potential ignition sources. Because with an open flame the primary ignition source itself represents an existing self-sustaining combustion, the ignition mechanism means in essence the spreading of burning to another system. Provided that the ignition source with open flame possesses sufficient energy for initiating ignition, burning will start.
Spontaneous ignition
The chemical reactions generating heat spontaneously imply the risk of ignition and burning as “internal ignition sources”. The materials inclined to spontaneous heating and spontaneous ignition may, however, become secondary ignition sources and give rise to ignition of the combustible materials in the surroundings.
Although some gases (e.g., hydrogen phosphide, boron hydride, silicon hydride) and liquids (e.g., metal carbonyls, organometallic compositions) are inclined to spontaneous ignition, most spontaneous ignitions occur as surface reactions of solid materials. Spontaneous ignition, like all ignitions, depends on the chemical structure of the material, but its occurrence is determined by the grade of dispersity. The large specific surface enables the local accumulation of reaction heat and contributes to the increase of temperature of material above spontaneous ignition temperature.
Spontaneous ignition of liquids is also promoted if they come into contact with air on solid materials of large specific surface area. Fats and especially unsaturated oils containing double bonds, when absorbed by fibrous materials and their products, and when impregnated into textiles of plant or animal origin, are inclined to spontaneous ignition under normal atmospheric conditions. Spontaneous ignition of glass-wool and mineral-wool products produced from non-combustible fibres or inorganic materials covering large specific surfaces and contaminated by oil have caused very severe fire accidents.
Spontaneous ignition has been observed mainly with dusts of solid materials. For metals with good heat conductivity, local heat accumulation needed for ignition necessitates very fine crushing of metal. As the particle size decreases, the likelihood of spontaneous ignition increases, and with some metal dusts (for example, iron) pyrophorosity ensues. When storing and handling coal dust, soot of fine distribution, dusts of lacquers and synthetic resins, as well as during the technological operations carried out with them, special attention should be given to the preventive measures against fire to reduce the hazard of spontaneous ignition.
Materials inclined to spontaneous decomposition show special ability to ignite spontaneously. Hydrazine, when set on any material with a large surface area, bursts into flames immediately. The peroxides, which are widely used by the plastics industry, easily decompose spontaneously, and as a consequence of decomposition, they become dangerous ignition sources, occasionally initiating explosive burning.
The violent exothermic reaction that occurs when certain chemicals come into contact with each other may be considered a special case of spontaneous ignition. Examples of such cases are contact of concentrated sulphuric acid with all the organic combustible materials, chlorates with sulphur or ammonium salts or acids, the organic halogen compounds with alkali metals, etc. The feature of these materials to be “unable to bear each other” (incompatible materials) requires special attention particularly when storing and co-storing them and elaborating the regulations of fire-fighting.
It is worth mentioning that such hazardously high spontaneous heating may, in some cases, be due to the wrong technological conditions (insufficient ventilation, low cooling capacity, discrepancies of maintenance and cleaning, overheating of reaction, etc.), or promoted by them.
Certain agricultural products, such as fibrous feedstuffs, oily seeds, germinating cereals, final products of the processing industry (dried beetroot slices, fertilizers, etc.), show an inclination for spontaneous ignition. The spontaneous heating of these materials has a special feature: the dangerous temperature conditions of the systems are exacerbated by some exothermic biological processes that cannot be controlled easily.
Electric ignition sources
Power machines, instruments and heating devices operated by electric energy, as well as the equipment for power transformation and lighting, typically do not present any fire hazard to their surroundings, provided that they have been installed in compliance with the relevant regulations of safety and requirements of standards and that the associated technological instructions have been observed during their operation. Regular maintenance and periodic supervision considerably diminish the probability of fires and explosions. The most frequent causes of fires in electric devices and wiring are overloading, short circuits, electric sparks and high contact resistances.
Overloading exists when the wiring and electrical appliances are exposed to higher current than that for which they are designed. The overcurrent passing through the wiring, devices and equipment might lead to such an overheating that the overheated components of the electrical system become damaged or broken, grow old or carbonize, resulting in cord and cable coatings melting down, metal parts glowing and the combustible structural units coming to ignition and, depending on the conditions, also spreading fire to the environment. The most frequent cause of overloading is that the number of consumers connected is higher than permitted or their capacity exceeds the value stipulated.
The working safety of electrical systems is most frequently endangered by short circuits. They are always the consequences of any damage and occur when the parts of the electrical wiring or the equipment at the same potential level or various potential levels, insulated from each other and the earth, come into contact with each other or with the earth. This contact may arise directly as metal-metal contact or indirectly, through electric arc. In cases of short circuits, when some units of the electric system come in contact with each other, the resistance will be considerably lower, and as a consequence, the intensity of current will be extremely high, perhaps several orders of magnitude lower. The heat energy released during overcurrents with large short circuits might result in a fire in the device affected by the short circuit, with the materials and equipment in the surrounding area coming to ignition and with the fire spreading to the building.
Electric sparks are heat energy sources of a small nature, but as shown by experience, act frequently as ignition sources. Under normal working conditions, most electrical appliances do not release sparks, but the operation of certain devices is normally accompanied by sparks.
Sparking introduces a hazard foremost at places where, in the zone of their generation, explosive concentrations of gas, vapour or dust might arise. Consequently, equipment normally releasing sparks during operation is permitted to be set up only at places where the sparks cannot give rise to fire. On its own, the energy content of sparks is insufficient for the ignition of the materials in the environment or to initiate an explosion.
If an electrical system has no perfect metallic contact between the structural units through which the current flows, high contact resistance will occur at this spot. This phenomenon is in most cases due to the faulty construction of joints or to unworkmanlike installations. The disengagement of joints during operation and natural wear may also be cause for high contact resistance. A large portion of the current flowing through places with increased resistance will transform to heat energy. If this energy cannot be dissipated sufficiently (and the reason cannot be eliminated), the extremely large increase of temperature might lead to a fire condition that endangers the surrounding.
If the devices work on the basis of the induction concept (engines, dynamos, transformers, relays, etc.) and are not properly calculated, eddy currents may arise during operation. Due to the eddy currents, the structural units (coils and their iron cores) might warm up, which might lead to the ignition of insulating materials and the burning of the equipment. Eddy currents might arise—with these harmful consequences—also in the metal structural units around high-voltage equipment.
Electrostatic sparks
Electrostatic charging is a process in the course of which any material, originally with electric neutrality (and independent of any electric circuit) becomes charged positively or negatively. This may occur in one of three ways:
1. charging with separation, such that charges of subtractive polarity accumulate on two bodies simultaneously
2. charging with passing, such that the charges passing away leave charges of opposed polarity signs behind
3. charging by taking up, such that the body receives charges from outside.
These three ways of charging may arise from various physical processes, including separation after contact, splitting, cutting, pulverizing, moving, rubbing, flowing of powders and fluids in pipe, hitting, change of pressure, change of state, photoionization, heat ionization, electrostatical distribution or high-voltage discharge.
Electrostatic charging may occur both on conducting bodies and insulating bodies as a result of any of the processes mentioned above, but in most cases the mechanical processes are responsible for the accumulation of the unwanted charges.
From the large number of the harmful effects and risks due to electrostatic charging and the spark discharge resulting from it, two risks can be mentioned in particular: endangering of electronic equipment (for example, computer for process control) and the hazard of fire and explosion.
Electronic equipment is endangered first of all if the discharge energy from the charging is sufficiently high to cause destruction of the input of any semi-conductive part. The development of electronic units in the last decade has been followed by the rapid increase of this risk.
The development of fire or explosion risk necessitates the coincidence in space and time of two conditions: the presence of any combustible medium and the discharge with ability for ignition. This hazard occurs mainly in the chemical industry. It may be estimated on the basis of the so-called spark sensitivity of hazardous materials (minimum ignition energy) and depends on the extent of charging.
It is an essential task to reduce these risks, namely, the large variety of consequences that extend from technological troubles to catastrophes with fatal accidents. There are two means of protecting against the consequences of electrostatic charging:
1. preventing the initiation of the charging process (it is evident, but usually very difficult to realize)
2. restricting the accumulation of charges to prevent the occurrence of dangerous discharges (or any other risk).
Lightning is an atmospherical electric phenomenon in nature and may be considered an ignition source. The static charging produced in the clouds is equalized towards the earth (lightning stroke) and is accompanied by a high-energy discharge. The combustible materials at the place of lightning stroke and its surroundings might ignite and burn off. At some strokes of lightning, very strong impulses are generated, and the energy is equalized in several steps. In other cases, long-lasting currents start to flow, sometimes reaching the order of magnitude of 10 A.
Mechanical heat energy
Technical practice is steadily coupled with friction. During mechanical operation, frictional heat is developed, and if heat loss is restricted to such an extent that heat accumulates in the system, its temperature may increase to a value that is dangerous for the environment, and fire may occur.
Friction sparks normally occur at metal technological operations because of heavy friction (grinding, chipping, cutting, hitting) or because of metal objects or tools dropping or falling on to a hard floor or during grinding operations because of metal contaminations within the material under grinding impact. The temperature of the spark generated is normally higher than the ignition temperature of the conventional combustible materials (such as for sparks from steel, 1,400-1,500 °C; sparks from copper-nickel alloys, 300-400 °C); however, the ignition ability depends on the whole heat content and the lowest ignition energy of the material and substance to be ignited, respectively. It has been proven in practice that friction sparks mean real fire risk in air spaces where combustible gases, vapours and dusts are present in dangerous concentrations. Thus, under these circumstances the use of materials that easily produce sparks, as well as processes with mechanical sparking, should be avoided. In these cases, safety is provided by tools that do not spark, i.e., made from wood, leather or plastic materials, or by using tools of copper and bronze alloys that produce sparks of low energy.
Hot surfaces
In practice, the surfaces of equipment and devices may warm up to a dangerous extent either normally or due to malfunction. Ovens, furnaces, drying devices, waste-gas outlets, vapour pipes, etc. often cause fires in explosive air spaces. Furthermore, their hot surfaces may ignite combustible materials coming close to them or by coming in contact. For prevention, safe distances should be observed, and regular supervision and maintenance will reduce the probability of the occurrence of dangerous overheating.
Fire Hazards of Materials and Products
The presence of combustible material in combustible systems represents an obvious condition of burning. Burning phenomena and the phases of the burning process fundamentally depend on the physical and chemical properties of the material involved. Therefore, it seems reasonable to make a survey of the flammability of the various materials and products with respect to their character and properties. For this section, the ordering principle for the grouping of materials is governed by technical aspects rather than by theoretical conceptions (NFPA 1991).
Wood and wood-based products
Wood is one of the most common materials in the human milieu. Houses, building structures, furniture and consumer goods are made of wood, and it is also widely used for products such as paper as well as in the chemical industry.
Wood and wood products are combustible, and when in contact with high-temperature surfaces and exposed to heat radiation, open flames or any other ignition source, will carbonize, glow, ignite or burn, depending upon the condition of combustion. To widen the field of their application, the improvement of their combustion properties is required. In order to make structural units produced from wood less combustible, they are typically treated with fire-retardant agents (e.g., saturated, impregnated, provided with surface coating).
The most essential characteristic of combustibility of the various kinds of wood is the ignition temperature. Its value strongly depends on some of the properties of wood and the test conditions of determination, namely, the wood sample’s density, humidity, size and shape, as well as the ignition source, time of exposure, intensity of exposure and the atmosphere during testing. It is interesting to note that the ignition temperature as determined by various test methods differs. Experience has shown that the inclination of clean and dry wood products to ignition is extremely low, but several fire cases caused by spontaneous ignition have been known to occur from storing dusty and oily waste wood in rooms with imperfect ventilation. It has been proven empirically that higher moisture content increases the ignition temperature and reduces the burning speed of wood. The thermal decomposition of wood is a complicated process, but its phases may clearly be observed as follows:
- The thermal decomposition with mass loss starts already in the range 120-200 °C; moisture content releases and the non-combustible degradates occur in the combustion space.
- At 200-280 °C, mainly endothermic reactions occur while the heat energy of ignition source is taken up.
- At 280-500 °C, the exothermic reactions of decomposition products are steadily accelerating as the primary process, while carbonization phenomena may be observed. In this temperature range, sustaining combustion has already developed. After ignition, burning is not steady in time because of the good heat-insulating ability of its carbonized layers. Consequently, the warming up of the deeper layers is limited and time consuming. When the surfacing of the combustible decomposition products is accelerated, burning will be complete.
- At temperatures exceeding 500 °C, the wood char forms residues. During its additional glowing, ash containing solid, inorganic materials is produced, and the process has come to an end.
Fibres and textiles
The majority of the textiles produced from fibrous materials that are found in the close surrounding of people is combustible. Clothing, furniture and the built environment partly or totally consists of textiles. The hazard which they present exists during their production, processing and storing as well as during their wearing.
The basic materials of textiles are both natural and artificial; synthetic fibres are used either alone or mixed with natural fibres. The chemical composition of the natural fibres of plant origin (cotton, hemp, jute, flax) is cellulose, which is combustible, and these fibres have a relatively high ignition temperature (<<400°C). It is an advantageous feature of their burning that when brought to high temperature they carbonize but do not melt. This is especially advantageous for the medical treatments of burn casualties.
The fire hazardous properties of fibres of protein base of animal origin (wool, silk, hair) are even more favourable than those of fibres of plant origin, because a higher temperature is required for their ignition (500-600 °C), and under the same conditions, their burning is less intensive.
The plastics industry, utilizing several extremely good mechanical properties of polymer products, has also gained prominence in the textile industry. Among the properties of acrylic, polyester and the thermoplastic synthetic fibres (nylon, polypropylene, polyethylene), those associated with burning are the least advantageous. Most of them, in spite of their high ignition temperature (<<400-600 °C), melt when exposed to heat, easily ignite, burn intensively, drop or melt when burning and release considerably high quantities of smoke and toxic gases. These burning properties may be improved by addition of natural fibres, producing so-called textiles with mixed fibres. Further treatment is accomplished with flame-retardant agents. For the manufacture of textiles for industrial purposes and heat-protective clothing, inorganic, non-combustible fibre products (including glass and metal fibres) are already used in large quantities.
The most important fire hazard characteristics of textiles are the properties connected with ignitability, flame spread, heat generation and the toxic combustion products. Special testing methods have been developed for their determination. The test results obtained influence the fields of application for these products (tents and flats, furniture, vehicle upholstery, clothes, carpets, curtains, special protective clothing against heat and weather), as well as the stipulations to restrict the risks in their use. An essential task of industrial researchers is to develop textiles that sustain high temperature, treated with fire-retardant agents, (heavily combustible, with long ignition time, low flame spread rate, low speed of heat release) and produce small amounts of toxic combustion products, as well as to improve the unfavourable effect of fire accidents due to the burning of such materials.
Combustible and flammable liquids
In the presence of ignition sources, combustible and flammable liquids are potential sources of risk. First, the closed or open vapour space above such liquids provides a fire and explosion hazard. Combustion, and more frequently explosion, might occur if the material is present in the vapour-air mixture in suitable concentration. From this it follows that burning and explosion in the zone of combustible and flammable liquids may be prevented if:
- the ignition sources, air, and oxygen are excluded; or
- instead of oxygen, inert gas is present in the surrounding; or
- the liquid is stored in a closed vessel or system (see Figure 1); or
- by proper ventilation, the development of the dangerous vapour concentration is prevented.
Figure 1. Common types of tanks for storage of flammable and combustible liquids.
In practice, a large number of material characteristics are known in connection with the dangerous nature of combustible and flammable liquids. These are closed-cup and open-cup flash points, boiling point, ignition temperature, rate of evaporation, upper and lower limits of the concentration for combustibility (flammable or explosive limits), the relative density of vapours compared to air and energy required for the ignition of vapours. These factors provide full information about the sensitivity for ignition of various liquids.
Nearly all over the world the flash point, a parameter determined by standard test under atmospherical conditions, is used as the basis to group the liquids (and materials behaving as liquids at relatively low temperatures) into categories of risk. The safety requirements for storage of liquids, their handling, the technological processes, and the electrical equipment to be set up in their zone should be elaborated for each category of flammability and combustibility. The zones of risk around the technological equipment should also to be identified for each category. Experience has shown that fire and explosion might occur—depending on the temperature and pressure of the system—within the range of concentration between the two flammable limits.
Gases
Although all materials—under a specific temperature and pressure—may become gases, the materials considered gaseous in practice are those that are in a gas state at normal temperature (~20 °C) and normal atmospheric pressure (~100 kPa).
In respect to fire and explosion hazards, gases may be ranked in two main groups: combustible and non-combustible gases. According to the definition accepted in practice, combustible gases are those that burn in air with normal oxygen concentration, provided that the conditions required for burning exist. Ignition only occurs above a certain temperature, with the necessary ignition temperature, and within a given range of concentration.
Non-combustible gases are those that do not burn either in oxygen or in air with any concentration of air. A portion of these gases support combustion (e.g., oxygen), while the other portion inhibit burning. The non-combustible gases not supporting burning are called inert gases (nitrogen, noble gases, carbon dioxide, etc.).
In order to achieve economic efficiency, the gases stored and transported in containers or transporting vessels are typically in compressed, liquefied, or cooled-condensated (cryogenic) state. Basically, there are two hazardous situations in connection with gases: when they are in containers and when they are released from their containers.
For compressed gases in storage containers, external heat might considerably increase the pressure within the container, and the extreme overpressure might lead to explosion. Gaseous storage containers will typically include a vapour phase and a liquid phase. Because of changes in pressure and temperature, the extension of the liquid phase gives rise to the further compression of vapour space, while the vapour pressure of the liquid increases in proportion with the increase of temperature. As a result of these processes, critically dangerous pressure may be produced. Storage containers are generally required to contain the application of overpressure relief devices. These are capable of mitigating a hazardous situation due to higher temperatures.
If the storage vessels are insufficiently sealed or damaged, the gas will flow out to the free air space, mix with air and depending on its quantity and the way of its flowing, may cause the formation of a large, explosive air space. The air around a leaking storage vessel can be unsuitable for breathing and may be dangerous for people nearby, partly due to the toxic effect of some gases and partly due to the diluted concentration of oxygen.
Bearing in mind the potential fire hazard due to gases and the need for safe operation, one must get detailed knowledge of the following features of gases either stored or used, especially for industrial consumers: the chemical and physical properties of gases, ignition temperature, the lower and upper limits of concentration for flammability, the hazardous parameters of the gas in the container, the risk factors of the hazardous situation caused by the gases released into the open air, the extent of the necessary safety zones and the special measures to be taken in case of a possible emergency situation connected with fire-fighting.
Chemicals
Knowledge of the hazardous parameters of chemicals is one of the basic conditions of safe working. The preventive measures and requirements for protection against fire may be elaborated only if the physical and chemical properties connected with fire hazard are taken into consideration. Of these properties, the most important ones are the following: combustibility; ignitability; ability to react with other materials, water or air; inclination to corrosion; toxicity; and radioactivity.
Information on the properties of chemicals can be obtained from the technical data sheets issued by manufacturers and from the manuals and handbooks containing the data of hazardous chemicals. These provide users with information not only about the general technical features of materials, but also about the actual values of hazard parameters (decomposition temperature, ignition temperature, limit concentrations of combustion, etc.), their special behaviour, requirements for storage and fire-fighting, as well as recommendations for first aid and medical therapy.
The toxicity of chemicals, as potential fire hazard, may act in two ways. First, the high toxicity of certain chemicals themselves, may be hazardous in a fire. Second, their presence within the fire zone may effectively restrict fire-fighting operations.
The oxidizing agents (nitrates, chlorates, inorganic peroxides, permanganates, etc.), even if they themselves are non-combustible, largely contribute to the ignition of combustible materials and to their intensive, occasionally explosive burning.
The group of unstable materials includes the chemicals (acetaldehyde, ethylene oxide, organic peroxides, hydrogen cyanide, vinyl chloride) which polymerize or decompose in violent exothermic reactions spontaneously or very easily.
The materials sensitive to water and air are extremely dangerous. These materials (oxides, hydroxides, hydrides, anhydrides, alkali metals, phosphorus, etc.) interact with the water and air that are always present in the normal atmosphere, and start reactions accompanied by very high heat generation. If they are combustible materials, they will come to spontaneous ignition. However, the combustible components that initiate the burning may possibly explode and spread to the combustible materials in the surrounding area.
The majority of corrosive materials (inorganic acids—sulphuric acid, nitric acid, perchloric acid, etc.—and halogens —fluorine, chlorine, bromine, iodine) are strong oxidizing agents, but at the same time they have very strong destructive effects on living tissues, and therefore special measures have to be taken for fire-fighting.
The dangerous characteristic of radioactive elements and compounds is increased by the fact that the radiation emitted by them may be harmful in several ways, besides that such materials may be fire hazards themselves. If in a fire the structural containment of the radioactive objects involved becomes damaged, λ-radiating materials might be released. They can have a very strong ionizing effect, and are capable of the fatal destruction of living organisms. Nuclear accidents can be accompanied by fires, the decomposition products of which bind radioactive (α-and β-radiating) contaminants by adsorption. These may cause permanent injuries to the persons taking part in rescue operations if they penetrate into their bodies. Such materials are extremely dangerous, because the persons affected do not perceive any radiation by their sensing organs, and their general state of health does not seem to be any worse. It is obvious that if radioactive materials burn, the radioactivity of the site, the decomposition products and the water used for fire-fighting should be kept under constant observation by means of radioactive signalling devices. The knowledge of these factors has to be taken into account for the strategy of intervention and all additional operations. The buildings for handling and storing radioactive materials as well as for their technological use need to be built of non-combustible materials of high fire resistance. At the same time, high-quality, automatic equipment for detecting, signalling and extinguishing a fire should be provided.
Explosives and blasting agents
Explosive materials are used for many military and industrial purposes. These are chemicals and mixtures which, when affected by strong mechanical force (hitting, shock, friction) or starting ignition, suddenly transform to gases of large volume through an extremely rapid oxidizing reaction (e.g., 1,000-10,000 m/s). The volume of these gases is the multiple of the volume of the explosive material already exploded, and they will exert very high pressure on the surroundings. During an explosion, high temperatures can arise (2,500-4,000 °C) that promote the ignition of the combustible materials in the zone of explosion.
Manufacture, transport and storage of the various explosive materials are governed by rigorous requirements. An example is NFPA 495, Explosive Materials Code.
Besides the explosive materials used for military and industrial purposes, the inductive blasting materials and pyrotechnical products are also treated as hazards. In general, mixtures of explosive materials are often used (picric acid, nitroglycerin, hexogene, etc.), but mixtures of materials capable of explosion are also in use (black powder, dynamite, ammonium nitrate, etc.). In the course of acts of terrorism, plastic materials have become well-known, and are, in essence, mixtures of brisant and plasticizing materials (various waxes, Vaseline, etc.).
For explosive materials, the most effective method of protection against fire is the exclusion of ignition sources from the surroundings. Several explosive materials are sensitive to water or various organic materials with an ability to oxidate. For these materials, the requirements for the conditions of storage and the rules for storing in the same place together with other materials should be carefully considered.
Metals
It is known from practice that nearly all the metals, under certain conditions, are capable of burning in atmospheric air. Steel and aluminium in large structural thickness, on the basis of their behaviour in fire, are clearly evaluated as non-combustible. However, the dusts of aluminium, iron in fine distribution and metal cottons from thin metal fibres can easily be ignited and thus burn intensively. The alkali metals (lithium, sodium, potassium), the alkaline-earth metals (calcium, magnesium, zinc), zirconium, hafnium, titanium, etc. ignite extremely easily in the form of a powder, filings or thin bands. Some metals have such a high sensitivity that they are stored separately from air, in inert gas atmospheres or under a liquid that is neutral for the metals.
The combustible metals and those that are conditioned to burn produce extremely violent burning reactions that are high-speed oxidation processes releasing considerably higher quantities of heat than observed from the burning of combustible and flammable liquids. The burning of metal dust in the case of settled powder, following the preliminary phase of glowing-ignition, might grow to rapid burning. With stirred-up dusts and clouds of dusts that might result, severe explosions can occur. The burning activity and affinity for oxygen of some metals (such as magnesium) are so high that after being ignited they will continue to burn in certain media (e.g., nitrogen, carbon dioxide, steam atmosphere) that are used for extinguishing fires derived from combustible solid materials and liquids.
Extinguishing metal fires presents a special task for fire-fighters. The choice of the proper extinguishing agent and the process in which it is applied are of great importance.
Fires of metals may be controlled with very early detection, the rapid and appropriate action of fire-fighters using the most effective method and, if possible, removal of metals and any other combustible materials from the zone of burning or at least a reduction of their quantities.
Special attention should be given to the protection against radiation when radioactive metals (plutonium, uranium) burn. Preventive measures have to be taken to avoid the penetration of toxic decomposition products into living organisms. For example, alkali metals, because of their ability to react violently with water may be extinguished with dry fire-extinguishing powders only. Burning of magnesium cannot be extinguished with water, carbon dioxide, halons or nitrogen with good success, and more important, if these agents are used in fire-fighting, the hazardous situation will become even more severe. The only agents that can be applied successfully are the noble gases or in some cases boron trifluoride.
Plastics and rubber
Plastics are macromolecular organic compounds produced synthetically or by modification of natural materials. The structure and shape of these macromolecular materials, produced by polymerizational, polyadditional or polycondensational reactions, will strongly influence their properties. The chain molecules of thermoplastics (polyamides, polycarbonates, polyesters, polystyrene, polyvinyl chloride, polymethyl-metacrylate, etc.) are linear or branched, the elastomers (neoprene, polysulphides, isoprene, etc.) are lightly cross-linked, while thermosetting plastics (duroplastics: polyalkydes, epoxy resins, polyurethanes, etc.) are densely cross-linked.
Natural caoutchouc is used as raw material by the rubber industry, and after being vulcanized, rubber is produced. The artificial caoutchoucs, the structure of which is similar to that of natural chaoutchouc, are polymers and co-polymers of butadiene.
The range of products from plastics and rubber used in nearly all fields of everyday life is steadily widening. Use of the large variety and excellent technical properties of this group of materials results in items such as various building structures, furniture, clothes, commodities, parts for vehicles and machines.
Typically, as organic materials, plastics and rubber also are considered to be combustible materials. For the description of their fire behaviour, a number of parameters are used that can be tested by special methods. With the knowledge of these parameters, one can allocate the fields of their application (determined, pointed out, set), and the fire safety provisions can be elaborated. These parameters are combustibility, ignitability, ability to develop smoke, inclination to produce toxic gases and burning dripping.
In many cases the ignition temperature of plastics is higher than that of wood or any other materials, but in most cases they ignite more easily, and their burning takes place more rapidly and with higher intensity. Fires of plastics are often accompanied by the unpleasant phenomena of large quantities of dense smoke being released that can strongly restrict visibility and develop various toxic gases (hydrochloric acid, phosgene, carbon monoxide, hydrogen cyanide, nitrous gases, etc.). Thermoplastic materials melt during burning, then flow and depending on their location (if mounted in or on a ceiling) produce drops which remain in the burning area and might ignite the combustible materials underneath.
The improvement of burning properties represents a complex problem and a “key issue” of plastics chemistry. Fire-retardant agents inhibit combustibility, ignition will be slower, the rate of combustion will fall, and flame propagation will slow down. At the same time, the quantity and optical density of smoke will be higher and the gas mixture produced will be more toxic.
Dusts
With regard to physical state, dusts belong to the solid materials, but their physical and chemical properties differ from those of those same materials in compact form. It is known that industrial accidents and catastrophes are caused by dust explosions. Materials that are non-combustible in their usual form, such as metals, may initiate an explosion in the form of dust mixed with air when affected by any ignition source, even of low energy. The hazard of an explosion also exists with dusts of combustible materials.
Dust can be an explosion hazard not only when floating in the air, but also when settled. In layers of dust, heat may accumulate, and slow burning may develop in the inside as a result of the increased ability of particles to react and their lower thermal conductivity. Then the dust may be stirred up by flashes, and the possibility of dust explosion will grow.
Floating particles in fine distribution present a more severe hazard. Similar to the explosion properties of combustible gases and vapours, dusts also have a special range of air-dust concentration in which an explosion may occur. The lower and upper limit values of explosion concentration and the width of concentration range depend on the size and distribution of particles. If the dust concentration exceeds the highest concentration leading to an explosion, a portion of the dust is not destroyed by fire and absorbs heat, and as a consequence the explosion pressure developed remains below the maximum. The moisture content of air also influences the occurrence of an explosion. At higher humidity, the ignition temperature of the cloud of dust will increase in proportion with the heat quantity necessary for the evaporation of humidity. If an inert foreign dust is mixed in a cloud of dust, the explosivity of the dust-air mixture will be reduced. The effect will be the same if inert gases are mixed in the mixture of air and dust, because the oxygen concentration necessary for burning will be lower.
Experience has shown that all the ignition sources, even of minimum ignition energy, are capable of igniting dust clouds (open flames, electric arc, mechanical or electrostatic spark, hot surfaces, etc.). According to test results obtained in laboratory, the energy demand for ignition of dust clouds is 20 to 40 times higher than in the case of mixtures of combustible vapour and air.
The factors that influence the explosion hazard for settled dusts are the physical and thermal engineering properties of the dust layer, the glowing temperature of the dust and the ignition properties of the decomposition products released by the dust layer.
Fire Prevention Measures
History tells us that fires were useful for heating and cooking but caused major damage in many cities. Many houses, major buildings and sometimes whole cities were destroyed by fire.
One of the first fire prevention measures was a requirement to extinguish all fires before nightfall. For example, in 872 in Oxford, England, authorities ordered a curfew bell to be rung at sunset to remind citizens to extinguish all indoor fires for the night (Bugbee 1978). Indeed, the word curfew is derived from the French couvre feu which literally means “cover fire”.
The cause of fires is often a result of human action bringing fuel and an ignition source together (e.g., waste paper stored next to heating equipment or volatile flammable liquids being used near open flames).
Fires require fuel, an ignition source and some mechanism to bring the fuel and ignition source together in the presence of air or some other oxidizer. If strategies can be developed to reduce fuel loads, eliminate ignition sources or prevent the fuel/ignition interaction, then fire loss and human death and injury can be reduced.
In recent years, there has been increasing emphasis on fire prevention as one of the most cost-effective measures in dealing with the fire problem. It is often easier (and cheaper) to prevent fires starting than to control or extinguish them once they have started.
This is illustrated in the Fire Safety Concepts Tree (NFPA 1991; 1995a) developed by the NFPA in the United States. This systematic approach to fire safety problems shows that objectives, such as reducing fire deaths in the workplace, can be achieved by preventing fire ignition or managing the impact of fire.
Fire prevention inevitably means changing human behaviour. This requires fire safety education, supported by management, using the latest training manuals, standards and other educational materials. In many countries such strategies are reinforced by law, requiring companies to meet legislated fire prevention objectives as part of their occupational health and safety commitment to their workers.
Fire safety education will be discussed in the next section. However, there is now clear evidence in commerce and industry of the important role of fire prevention. Great use is being made internationally of the following sources: Lees, Loss Prevention in the Process Industries, Volumes 1 and 2 (1980); NFPA 1—Fire Prevention Code (1992); The Management of Health and Safety at Work Regulations (ECD 1992); and Fire Protection Handbook of the NFPA (Cote 1991). These are supplemented by many regulations, standards and training materials developed by national governments, businesses and insurance companies to minimize losses of life and property.
Fire Safety Education and Practices
For a fire safety education programme to be effective, there must be a major corporate policy commitment to safety and the development of an effective plan that has the following steps: (a) Planning phase—establishment of goals and objectives; (b) Design and implementation phase; and (c) Program evaluation phase—monitoring effectiveness.
Goals and objectives
Gratton (1991), in an important article on fire safety education, defined the differences between goals, objectives and implementation practices or strategies. Goals are general statements of intent that in the workplace may be said “to reduce the number of fires and thus reduce death and injury among workers, and the financial impact on companies”.
The people and financial parts of the overall goal are not incompatible. Modern risk management practice has demonstrated that improvements in safety for workers through effective loss control practices can be financially rewarding to the company and have a community benefit.
These goals need to be translated into specific fire safety objectives for particular companies and their workforce. These objectives, which must be measurable, usually include statements such as:
- reduce industrial accidents and resulting fires
- reduce fire deaths and injuries
- reduce company property damage.
For many companies, there may be additional objectives such as reduction in business interruption costs or minimization of legal liability exposure.
The tendency among some companies is to assume that compliance with local building codes and standards is sufficient to ensure that their fire safety objectives are met. However, such codes tend to concentrate on life safety, assuming fires will occur.
Modern fire safety management understands that absolute safety is not a realistic goal but sets measurable performance objectives to:
- minimize fire incidents through effective fire prevention
- provide effective means of limiting the size and consequence of fire incidents through effective emergency equipment and procedures
- use insurance to safeguard against large, unforeseen fires, particularly those arising from natural hazards such as earthquakes and bushfires.
Design and implementation
The design and implementation of fire safety education programmes for fire prevention are critically dependent upon development of well-planned strategies and effective management and motivation of people. There must be strong and absolute corporate support for full implementation of a fire safety programme for it to be successful.
The range of strategies have been identified by Koffel (1993) and in NFPA’s Industrial Fire Hazards Handbook (Linville 1990). They include:
- promoting the company policy and strategies on fire safety to all company employees
- identifying all potential fire scenarios and implementing appropriate risk reduction actions
- monitoring all local codes and standards that define the standard of care in a particular industry
- operating a loss administration programme to measure all losses for comparison with performance objectives
- training of all employees in proper fire prevention and emergency response techniques.
- Some international examples of implementation strategies include:
- courses operated by the Fire Protection Association (FPA) in the United Kingdom that lead to the European Diploma in Fire Prevention (Welch 1993)
- the creation of SweRisk, a subsidiary company of the Swedish Fire Protection Association, to assist companies in undertaking risk assessments and in developing fire prevention programmes (Jernberg 1993)
- massive citizen and worker involvement in fire prevention in Japan to standards developed by the Japan Fire Defence Agency (Hunter 1991)
- fire safety training in the United States through the use of the Firesafety Educator’s Handbook (NFPA 1983) and the Public Fire Education Manual (Osterhoust 1990).
It is critically important to measure the effectiveness of fire safety education programmes. This measurement provides the motivation for further programme financing, development and adjustment where necessary.
The best example of monitoring and success of fire safety education is probably in the United States. The Learn Not to BurnÒ programme, aimed at educating the young people in America on the dangers of fire, has been coordinated by the Public Education Division of the NFPA. Monitoring and analysis in 1990 identified a total of 194 lives saved as a result of proper life safety actions learned in fire safety education programmes. Some 30% of these lives saved can be directly attributed to the Learn Not to BurnÒ programme.
The introduction of residential smoke detectors and fire safety education programmes in the United States have also been suggested as the primary reasons for the reduction in home fire deaths in that country, from 6,015 in 1978 to 4,050 in 1990 (NFPA 1991).
Industrial housekeeping practices
In the industrial field, Lees (1980) is an international authority. He indicated that in many industries today, the potential for very large loss of life, serious injuries or property damage is far greater than in the past. Large fires, explosions and toxic releases can result, particularly in the petrochemical and nuclear industries.
Fire prevention is therefore the key to minimizing fire ignition. Modern industrial plants can achieve good fire safety records through well-managed programmes of:
- housekeeping and safety inspections
- employee fire prevention training
- equipment maintenance and repair
- security and arson prevention (Blye and Bacon 1991).
A useful guide, on the importance of housekeeping for fire prevention in commercial and industrial premises is given by Higgins (1991) in the NFPA’s Fire Protection Handbook.
The value of good housekeeping in minimizing combustible loads and in preventing exposure of ignition sources is recognized in modern computer tools used for assessing fire risks in industrial premises. The FREM (Fire Risk Evaluation Method) software in Australia identifies housekeeping as a key fire safety factor (Keith 1994).
Heat Utilization Equipment
Heat utilization equipment in commerce and industry includes ovens, furnaces, kilns, dehydrators, dryers and quench tanks.
In the NFPA’s Industrial Fire Hazards Handbook, Simmons (1990) identified the fire problems with heating equipment to be:
- the possibility of igniting combustible materials stored nearby
- fuel hazards resulting from unburned fuel or incomplete combustion
- overheating leading to equipment failure
- ignition of combustible solvents, solid materials or other products being processed.
These fire problems can be overcome through a combination of good housekeeping, proper controls and interlocks, operator training and testing, and cleaning and maintenance in an effective fire prevention programme.
Detailed recommendations for the various categories of heat utilization equipment are set out in the NFPA’s Fire Protection Handbook (Cote 1991).These are summarized below.
Ovens and furnaces
Fires and explosions in ovens and furnaces typically result from the fuel used, from volatile substances provided by the material in the oven or by a combination of both. Many of these ovens or furnaces operate at 500 to 1,000 °C, which is well above the ignition temperature of most materials.
Ovens and furnaces require a range of controls and interlocks to ensure that unburned fuel gases or products of incomplete combustion cannot accumulate and be ignited. Typically, these hazards develop while firing up or during shut-down operations. Therefore, special training is required to ensure that operators always follow safety procedures.
Non-combustible building construction, separation of other equipment and combustible materials and some form of automatic fire suppression are usually essential elements of a fire safety system to prevent spread should a fire start.
Kilns
Kilns are used to dry timber (Lataille 1990) and to process or “fire” clay products (Hrbacek 1984).
Again, this high-temperature equipment represents a hazard to its surroundings. Proper separation design and good housekeeping are essential to prevent fire.
Lumber kilns used for drying timber are additionally hazardous because the timber itself is a high fire load and is often heated close to its ignition temperature. It is essential that kilns be cleaned regularly to prevent a build-up of small pieces of wood and sawdust so that this does not come in contact with the heating equipment. Kilns made of fire-resistive construction material, fitted with automatic sprinklers and provided with high-quality ventilation/air circulation systems are preferred.
Dehydrators and dryers
This equipment is used to reduce the moisture content of agricultural products such as milk, eggs, grains, seeds and hay. The dryers may be direct-fired, in which case the productions of combustion contact the material being dried, or they may be indirect-fired. In each case, controls are required to shut off the heat supply in the event of excessive temperature or fire in the dryer, exhaust system or conveyor system or failure of air circulation fans. Again, adequate cleaning to prevent build-up of products that could ignite is required.
Quench tanks
The general principles of fire safety of quench tanks are identified by Ostrowski (1991) and Watts (1990).
The process of quenching, or controlled cooling, occurs when a heated metal item is immersed in a tank of quenching oil. The process is undertaken to harden or temper the material through metallurgical change.
Most quenching oils are mineral oils which are combustible. They must be chosen carefully for each application to ensure that the ignition temperature of the oil is above the operating temperature of the tank as the hot metal pieces are immersed.
It is critical that the oil does not overflow the sides of the tank. Therefore, liquid level controls and appropriate drains are essential.
Partial immersion of hot items is the most common cause of quench tank fires. This can be prevented by appropriate material transfer or conveyor arrangements.
Likewise, appropriate controls must be provided to avoid excessive oil temperatures and entry of water into the tank that can result in boil-over and major fire in and around the tank.
Specific automatic fire extinguishing systems such as carbon dioxide or dry chemical are often used to protect the tank surface. Overhead, automatic sprinkler protection of the building is desirable. In some cases, special protection of operators who need to work close to the tank is also required. Often, water spray systems are provided for exposure protection for workers.
Above all, proper training of workers in emergency response, including use of portable fire extinguishers, is essential.
Chemical Process Equipment
Operations to chemically change the nature of materials have often been the source of major catastrophes, causing severe plant damage and death and injury to workers and surrounding communities. Risks to life and property from incidents in chemical process plants may come from fires, explosions or toxic chemical releases. The energy of destruction often comes from uncontrolled chemical reaction of process materials, combustion of fuels leading to pressure waves or high levels of radiation and flying missiles that can cause damage at large distances.
Plant operations and equipment
The first stage of design is to understand the chemical processes involved and their potential for energy release. Lees (1980) in his Loss Prevention in the Process Industries sets out in detail the steps required to be undertaken, which include:
- proper process design
- study of failure mechanisms and reliability
- hazard identification and safety audits
- hazard assessment—cause/consequences.
- The assessment of the degrees of hazard must examine:
- potential emission and dispersal of chemicals, particularly toxic and contaminating substances
- effects of fire radiation and dispersal of combustion products
- results of explosions, particularly pressure shock waves that can destroy other plants and buildings.
More details of process hazards and their control are given in Plant guidelines for technical management of chemical process safety (AIChE 1993); Sax’s Dangerous Properties of Industrial Materials (Lewis 1979); and the NFPA’s Industrial Fire Hazards Handbook (Linville 1990).
Siting and exposure protection
Once the hazards and consequences of fire, explosion and toxic releases have been identified, siting of chemical process plants can be undertaken.
Again, Lees (1980) and Bradford (1991) provided guidelines on plant siting. Plants must be separated from surrounding communities sufficiently to ensure that those communities cannot be affected by an industrial accident. The technique of quantitative risk assessment (QRA) to determine separation distances is widely used and legislated for in the design of chemical process plants.
The disaster in Bhopal, India, in 1984 demonstrated the consequences of locating a chemical plant too close to a community: over 1,000 people were killed by toxic chemicals in an industrial accident.
Provision of separating space around chemical plants also allows ready access for fire-fighting from all sides, regardless of wind direction.
Chemical plants must provide exposure protection in the form of explosion-resistant control rooms, worker refuges and fire-fighting equipment to ensure that workers are protected and that effective fire-fighting can be undertaken after an incident.
Spill control
Spills of flammable or hazardous materials should be kept small by appropriate process design, fail-safe valves and appropriate detection/control equipment. However, if large spills occur, they should be confined to areas surrounded by walls, sometimes of earth, where they can burn harmlessly if ignited.
Fires in drainage systems are common, and special attention must be paid to drains and sewerage systems.
Heat transfer hazards
Equipment that transfers heat from a hot fluid to a cooler one can be a source of fire in chemical plants. Excessive localized temperatures can cause decomposition and burn out of many materials. This may sometimes cause rupture of the heat-transfer equipment and transfer of one fluid into another, causing an unwanted violent reaction.
High levels of inspection and maintenance, including cleaning of heat transfer equipment, is essential to safe operation.
Reactors
Reactors are the vessels in which the desired chemical processes are undertaken. They can be of a continuous or batch type but require special design attention. Vessels must be designed to withstand pressures that might result from explosions or uncontrolled reactions or alternatively must be provided with appropriate pressure-relief devices and sometimes emergency venting.
Safety measures for chemical reactors include:
- appropriate instrumentation and controls to detect potential incidents, including redundant circuitry
- high quality cleaning, inspection and maintenance of the equipment and the safety controls
- adequate training of operators in control and emergency response
- appropriate fire suppression equipment and fire-fighting personnel.
Welding and Cutting
The Factory Mutual Engineering Corporation’s (FM) Loss Prevention Data Sheet (1977) shows that nearly 10% of losses in industrial properties are due to incidents involving cutting and welding of materials, generally metals. It is clear that the high temperatures required to melt the metals during these operations can start fires, as can the sparks generated in many of these processes.
The FM Data Sheet (1977) indicates that the materials most frequently involved in fires due to welding and cutting are flammable liquids, oily deposits, combustible dusts and wood. The types of industrial areas where accidents are most likely are storage areas, building construction sites, facilities undergoing repair or alteration and waste disposal systems.
Sparks from cutting and welding can often travel up to 10 m and lodge in combustible materials where smouldering and later flaming fires can occur.
Electrical processes
Arc welding and arc cutting are examples of processes involving electricity to provide the arc that is the heat source for melting and joining metals. Flashes of sparks are common, and protection of workers from electrocution, spark flashes and intense arc radiation is required.
Oxy-fuel gas processes
This process uses the heat of combustion of the fuel gas and oxygen to generate flames of high temperature that melt the metals being joined or cut. Manz (1991) indicated that acetylene is the most widely used fuel gas because of its high flame temperature of about 3,000 °C.
The presence of a fuel and oxygen at high pressure makes for an increased hazard, as is leakage of these gases from their storage cylinders. It is important to remember that many materials that do not burn, or only burn slowly in air, burn violently in pure oxygen.
Safeguards and precautions
Good safety practices are identified by Manz (1991) in the NFPA Fire Protection Handbook.
These safeguards and precautions include:
- proper design, installation and maintenance of welding and cutting equipment, particularly storage and leak testing of fuel and oxygen cylinders
- proper preparation of work areas to remove all chance of accidental ignition of surrounding combustibles
- strict management control over all welding and cutting processes
- training of all operators in safe practices
- proper fire-resistant clothing and eye protection for operators and nearby workers
- adequate ventilation to prevent exposure of operators or nearby workers to noxious gases and fumes.
Special precautions are required when welding or cutting tanks or other vessels that have held flammable materials. A useful guide is the American Welding Society’s Recommended Safe Practices for the Preparation for Welding and Cutting of Containers that have held Hazardous Substances (1988).
For building works and alterations, a UK publication, the Loss Prevention Council’s Fire Prevention on Construction Sites (1992) is useful. It contains a sample hot-work permit to control cutting and welding operations. This would be useful for management in any plant or industrial site. A similar sample permit is provided in the FM Data Sheet on cutting and welding (1977).
Lightning Protection
Lightning is a frequent cause of fires and deaths of people in many countries in the world. For example, each year some 240 US citizens die as a result of lightning.
Lightning is a form of electrical discharge between charged clouds and the earth. The FM Data Sheet (1984) on lightning indicates that lightning strikes may range from 2,000 to 200,000 A as a result of a potential difference of 5 to 50 million V between clouds and the earth.
The frequency of lightning varies between countries and areas depending on the number of thunderstorm-days per year for the locality. The damage that lightning can cause depends very much on the ground condition, with more damage occurring in areas of high earth resistivity.
Protective measures—buildings
The NFPA 780 Standard for the Installation of Lightning Protection Systems (1995b) sets out the design requirements for protection of buildings. While the exact theory of lightning discharges is still being investigated, the basic principle of protection is to provide a means by which a lightning discharge may enter or leave the earth without damaging the building being protected.
Lightning systems, therefore, have two functions:
- to intercept the lightning discharge before it strikes the building
- provide a harmless discharge path to earth.
- This requires buildings to be fitted with:
- lightning rods or masts
- down conductors
- good ground connections, typically 10 ohms or less.
More details for the design of lightning protection for buildings is provided by Davis (1991) in the NFPA Fire Protection Handbook (Cote 1991) and in the British Standards Institute’s Code of Practice (1992).
Overhead transmission lines, transformers, outdoor substations and other electrical installations can be damaged by direct lightning strikes. Electrical transmission equipment can also pick up induced voltage and current surges that can enter buildings. Fires, damage to equipment and serious interruption to operations may result. Surge arresters are required to divert these voltage peaks to ground through effective earthing.
The increased use of sensitive computer equipment in commerce and industry has made operations more sensitive to transient over-voltages induced in power and communication cables in many buildings. Appropriate transient protection is required and special guidance is provided in the British Standards Institute BS 6651:1992, The Protection of Structures Against Lightning.
Maintenance
Proper maintenance of lightning systems is essential for effective protection. Special attention has to be paid to ground connections. If they are not effective, lightning protection systems will be ineffective.
" DISCLAIMER: The ILO does not take responsibility for content presented on this web portal that is presented in any language other than English, which is the language used for the initial production and peer-review of original content. Certain statistics have not been updated since the production of the 4th edition of the Encyclopaedia (1998)."