Children categories

36. Barometric Pressure Increased (2)

36. Barometric Pressure Increased
Chapter Editor: T.J.R. Francis
Table of Contents
Working under Increased Barometric Pressure
Eric Kindwall
Dees F. Gorman
Tables
Click a link below to view table in article context.
1. Instructions for compressed-air workers
2. Decompression illness: Revised classification

37. Barometric Pressure Reduced (4)

37. Barometric Pressure Reduced
Chapter Editor: Walter Dümmer
Table of Contents
Figures and Tables
Ventilatory Acclimatization to High Altitude
John T. Reeves and John V. Weil
Physiological Effects of Reduced Barometric Pressure
Kenneth I. Berger and William N. Rom
Health Considerations for Managing Work at High Altitudes
John B. West
Prevention of Occupational Hazards at High Altitudes
Walter Dümmer
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

38. Biological Hazards (4)

38. Biological Hazards
Chapter Editor: Zuheir Ibrahim Fakhri
Table of Contents
Tables
Workplace Biohazards
Zuheir I. Fakhri
Aquatic Animals
D. Zannini
Terrestrial Venomous Animals
J.A. Rioux and B. Juminer
Clinical Features of Snakebite
David A. Warrell
Tables
Click a link below to view table in article context.
1. Occupational settings with biological agents
2. Viruses, bacteria, fungi & plants in the workplace
3. Animals as a source of occupational hazards

39. Disasters, Natural and Technological (12)

39. Disasters, Natural and Technological
Chapter Editor: Pier Alberto Bertazzi
Table of Contents
Tables and Figures
Disasters and Major Accidents
Pier Alberto Bertazzi
ILO Convention concerning the Prevention of Major Industrial Accidents, 1993 (No. 174)
Disaster Preparedness
Peter J. Baxter
Post-Disaster Activities
Benedetto Terracini and Ursula Ackermann-Liebrich
Weather-Related Problems
Jean French
Avalanches: Hazards and Protective Measures
Gustav Poinstingl
Transportation of Hazardous Material: Chemical and Radioactive
Donald M. Campbell
Radiation Accidents
Pierre Verger and Denis Winter
Case Study: What does dose mean?
Occupational Health and Safety Measures in Agricultural Areas Contaminated by Radionuclides: The Chernobyl Experience
Yuri Kundiev, Leonard Dobrovolsky and V.I. Chernyuk
Case Study: The Kader Toy Factory Fire
Casey Cavanaugh Grant
Impacts of Disasters: Lessons from a Medical Perspective
José Luis Zeballos
Tables
Click a link below to view table in article context.
1. Definitions of disaster types
2. 25-yr average # victims by type & region-natural trigger
3. 25-yr average # victims by type & region-non-natural trigger
4. 25-yr average # victims by type-natural trigger (1969-1993)
5. 25-yr average # victims by type-non-natural trigger (1969-1993)
6. Natural trigger from 1969 to 1993: Events over 25 years
7. Non-natural trigger from 1969 to 1993: Events over 25 years
8. Natural trigger: Number by global region & type in 1994
9. Non-natural trigger: Number by global region & type in 1994
10. Examples of industrial explosions
11. Examples of major fires
12. Examples of major toxic releases
13. Role of major hazard installations management in hazard control
14. Working methods for hazard assessment
15. EC Directive criteria for major hazard installations
16. Priority chemicals used in identifying major hazard installations
17. Weather-related occupational risks
18. Typical radionuclides, with their radioactive half-lives
19. Comparison of different nuclear accidents
20. Contamination in Ukraine, Byelorussia & Russia after Chernobyl
21. Contamination strontium-90 after the Khyshtym accident (Urals 1957)
22. Radioactive sources that involved the general public
23. Main accidents involving industrial irradiators
24. Oak Ridge (US) radiation accident registry (worldwide, 1944-88)
25. Pattern of occupational exposure to ionizing radiation worldwide
26. Deterministic effects: thresholds for selected organs
27. Patients with acute irradiation syndrome (AIS) after Chernobyl
28. Epidemiological cancer studies of high dose external irradiation
29. Thyroid cancers in children in Belarus, Ukraine & Russia, 1981-94
30. International scale of nuclear incidents
31. Generic protective measures for general population
32. Criteria for contamination zones
33. Major disasters in Latin America & the Caribbean, 1970-93
34. Losses due to six natural disasters
35. Hospitals & hospital beds damaged/ destroyed by 3 major disasters
36. Victims in 2 hospitals collapsed by the 1985 earthquake in Mexico
37. Hospital beds lost resulting from the March 1985 Chilean earthquake
38. Risk factors for earthquake damage to hospital infrastructure
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.
Click to return to top of page

40. Electricity (3)

40. Electricity
Chapter Editor: Dominique Folliot
Table of Contents
Figures and Tables
Electricity—Physiological Effects
Dominique Folliot
Static Electricity
Claude Menguy
Prevention And Standards
Renzo Comini
Tables
Click a link below to view table in article context.
1. Estimates of the rate of electrocution-1988
2. Basic relationships in electrostatics-Collection of equations
3. Electron affinities of selected polymers
4. Typical lower flammability limits
5. Specific charge associated with selected industrial operations
6. Examples of equipment sensitive to electrostatic discharges
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

41. Fire (6)

41. Fire
Chapter Editor: Casey C. Grant
Table of Contents
Figures and Tables
Basic Concepts
Dougal Drysdale
Sources of Fire Hazards
Tamás Bánky
Fire Prevention Measures
Peter F. Johnson
Passive Fire Protection Measures
Yngve Anderberg
Active Fire Protection Measures
Gary Taylor
Organizing for Fire Protection
S. Dheri
Tables
Click a link below to view table in article context.
1. Lower & upper flammability limits in air
2. Flashpoints & firepoints of liquid & solid fuels
3. Ignition sources
4. Comparison of concentrations of different gases required for inerting
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

42. Heat and Cold (12)

42. Heat and Cold
Chapter Editor: Jean-Jacques Vogt
Table of Contents
Figures and Tables
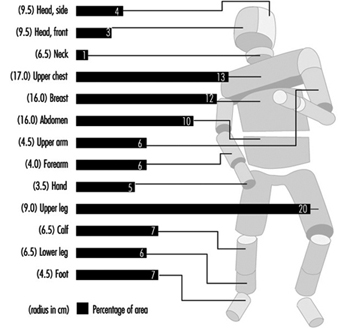
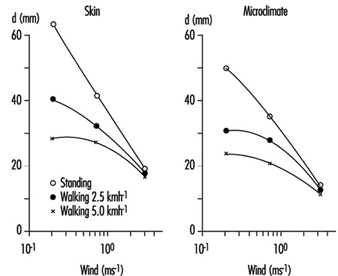
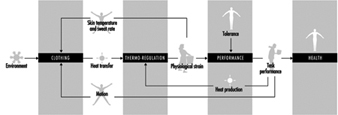
Physiological Responses to the Thermal Environment
W. Larry Kenney
Effects of Heat Stress and Work in the Heat
Bodil Nielsen
Heat Disorders
Tokuo Ogawa
Prevention of Heat Stress
Sarah A. Nunneley
The Physical Basis of Work in Heat
Jacques Malchaire
Assessment of Heat Stress and Heat Stress Indices
Kenneth C. Parsons
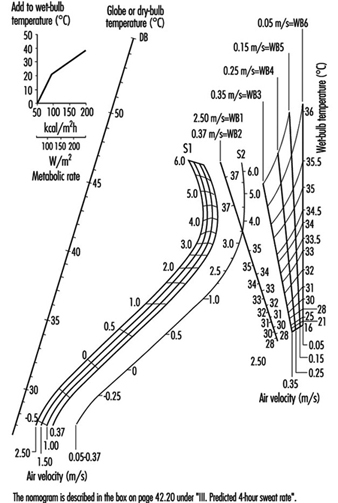
Case Study: Heat Indices: Formulae and Definitions
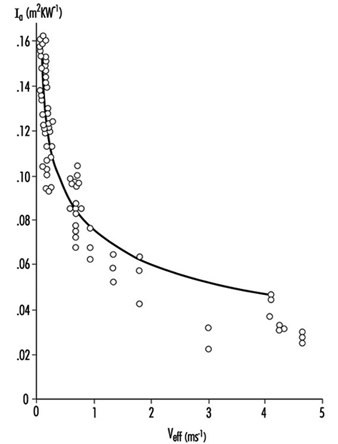
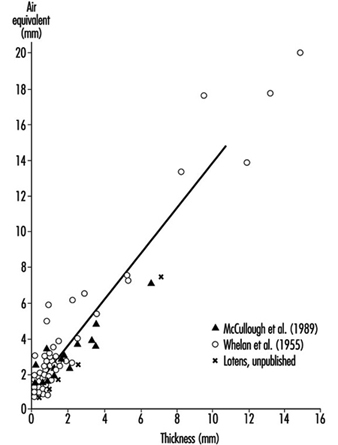
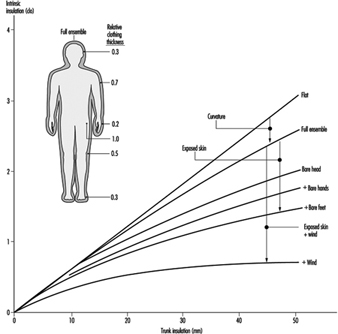
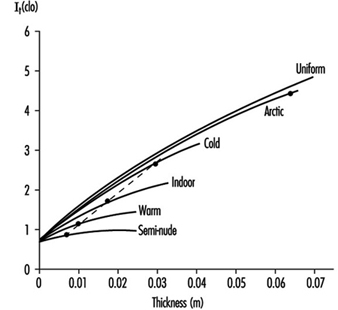
Heat Exchange through Clothing
Wouter A. Lotens
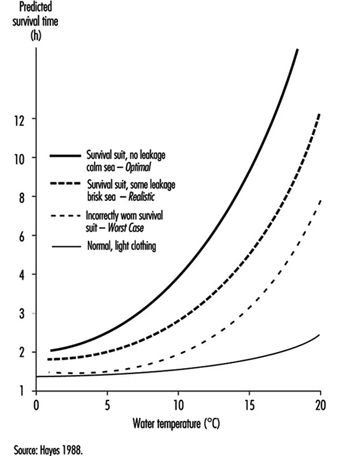
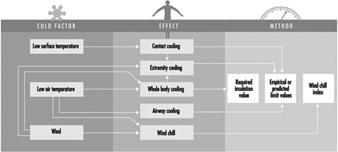
Cold Environments and Cold Work
Ingvar Holmér, Per-Ola Granberg and Goran Dahlstrom
Prevention of Cold Stress in Extreme Outdoor Conditions
Jacques Bittel and Gustave Savourey
Cold Indices and Standards
Ingvar Holmér
Tables
Click a link below to view table in article context.
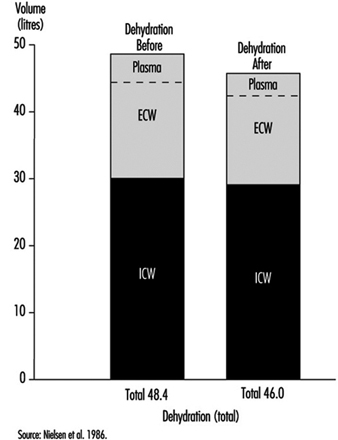
1. Electrolyte concentration in blood plasma & sweat
2. Heat Stress Index & Allowable Exposure Times: calculations
3. Interpretation of Heat Stress Index values
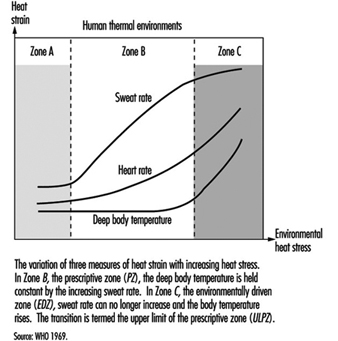
4. Reference values for criteria of thermal stress & strain
5. Model using heart rate to assess heat stress
6. WBGT reference values
7. Working practices for hot environments
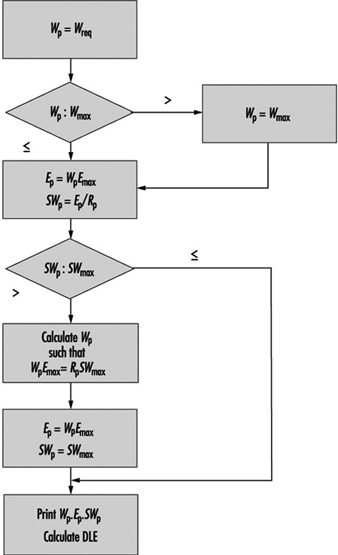
8. Calculation of the SWreq index & assessment method: equations
9. Description of terms used in ISO 7933 (1989b)
10. WBGT values for four work phases
11. Basic data for the analytical assessment using ISO 7933
12. Analytical assessment using ISO 7933
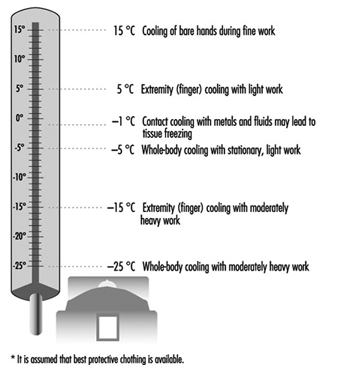
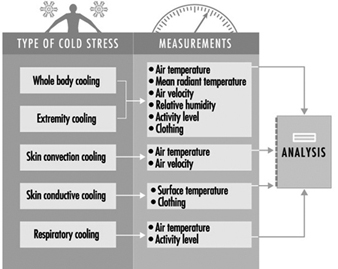
13. Air temperatures of various cold occupational environments
14. Duration of uncompensated cold stress & associated reactions
15. Indication of anticipated effects of mild & severe cold exposure
16. Body tissue temperature & human physical performance
17. Human responses to cooling: Indicative reactions to hypothermia
18. Health recommendations for personnel exposed to cold stress
19. Conditioning programmes for workers exposed to cold
20. Prevention & alleviation of cold stress: strategies
21. Strategies & measures related to specific factors & equipment
22. General adaptational mechanisms to cold
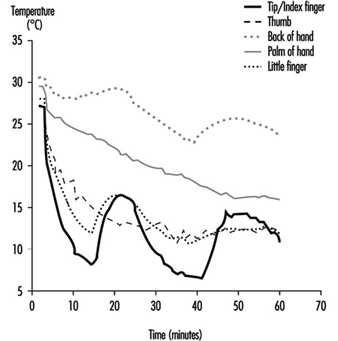
23. Number of days when water temperature is below 15 ºC
24. Air temperatures of various cold occupational environments
25. Schematic classification of cold work
26. Classification of levels of metabolic rate
27. Examples of basic insulation values of clothing
28. Classification of thermal resistance to cooling of handwear
29. Classification of contact thermal resistance of handwear
30. Wind Chill Index, temperature & freezing time of exposed flesh
31. Cooling power of wind on exposed flesh
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

43. Hours of Work (1)

43. Hours of Work
Chapter Editor: Peter Knauth
Table of Contents
Hours of Work
Peter Knauth
Tables
Click a link below to view table in article context.
1. Time intervals from beginning shiftwork until three illnesses
2. Shiftwork & incidence of cardiovascular disorders
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

44. Indoor Air Quality (8)

44. Indoor Air Quality
Chapter Editor: Xavier Guardino Solá
Table of Contents
Figures and Tables
Indoor Air Quality: Introduction
Xavier Guardino Solá
Nature and Sources of Indoor Chemical Contaminants
Derrick Crump
Radon
María José Berenguer
Tobacco Smoke
Dietrich Hoffmann and Ernst L. Wynder
Smoking Regulations
Xavier Guardino Solá
Measuring and Assessing Chemical Pollutants
M. Gracia Rosell Farrás
Biological Contamination
Brian Flannigan
Regulations, Recommendations, Guidelines and Standards
María José Berenguer
Tables
Click a link below to view table in article context.
1. Classification of indoor organic pollutants
2. Formaldehyde emission from a variety of materials
3. Ttl. volatile organic comp’ds concs, wall/floor coverings
4. Consumer prods & other sources of volatile organic comp’ds
5. Major types & concentrations in the urban United Kingdom
6. Field measurements of nitrogen oxides & carbon monoxide
7. Toxic & tumorigenic agents in cigarette sidestream smoke
8. Toxic & tumorigenic agents from tobacco smoke
9. Urinary cotinine in non-smokers
10. Methodology for taking samples
11. Detection methods for gases in indoor air
12. Methods used for the analysis of chemical pollutants
13. Lower detection limits for some gases
14. Types of fungus which can cause rhinitis and/or asthma
15. Micro-organisms and extrinsic allergic alveolitis
16. Micro-organisms in nonindustrial indoor air & dust
17. Standards of air quality established by the US EPA
18. WHO guidelines for non-cancer and non-odour annoyance
19. WHO guideline values based on sensory effects or annoyance
20. Reference values for radon of three organizations
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

45. Indoor Environmental Control (6)

45. Indoor Environmental Control
Chapter Editor: Juan Guasch Farrás
Table of Contents
Figures and Tables
Control of Indoor Environments: General Principles
A. Hernández Calleja
Indoor Air: Methods for Control and Cleaning
E. Adán Liébana and A. Hernández Calleja
Aims and Principles of General and Dilution Ventilation
Emilio Castejón
Ventilation Criteria for Nonindustrial Buildings
A. Hernández Calleja
Heating and Air-Conditioning Systems
F. Ramos Pérez and J. Guasch Farrás
Indoor Air: Ionization
E. Adán Liébana and J. Guasch Farrás
Tables
Click a link below to view table in article context.
1. Most common indoor pollutants & their sources
2. Basic requirements-dilution ventilation system
3. Control measures & their effects
4. Adjustments to working environment & effects
5. Effectiveness of filters (ASHRAE standard 52-76)
6. Reagents used as absorbents for contaminents
7. Levels of quality of indoor air
8. Contamination due to the occupants of a building
9. Degree of occupancy of different buildings
10. Contamination due to the building
11. Quality levels of outside air
12. Proposed norms for environmental factors
13. Temperatures of thermal comfort (based on Fanger)
14. Characteristics of ions
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

46. Lighting (3)

46. Lighting
Chapter Editor: Juan Guasch Farrás
Table of Contents
Figures and Tables
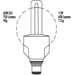
Types of Lamps and Lighting
Richard Forster
Conditions Required for Visual
Fernando Ramos Pérez and Ana Hernández Calleja
General Lighting Conditions
N. Alan Smith
Tables
Click a link below to view table in article context.
1. Improved output & wattage of some 1,500 mm fluorescent tube lamps
2. Typical lamp efficacies
3. International Lamp Coding System (ILCOS) for some lamp types
4. Common colours & shapes of incandescent lamps & ILCOS codes
5. Types of high-pressure sodium lamp
6. Colour contrasts
7. Reflection factors of different colours & materials
8. Recommended levels of maintained illuminance for locations/tasks
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

47. Noise (5)

47. Noise
Chapter Editor: Alice H. Suter
Table of Contents
Figures and Tables
The Nature and Effects of Noise
Alice H. Suter
Noise Measurement and Exposure Evaluation
Eduard I. Denisov and German A. Suvorov
Engineering Noise Control
Dennis P. Driscoll
Hearing Conservation Programmes
Larry H. Royster and Julia Doswell Royster
Standards and Regulations
Alice H. Suter
Tables
Click a link below to view table in article context.
1. Permissible exposure limits (PEL)for noise exposure, by nation
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

48. Radiation: Ionizing (6)

48. Radiation: Ionizing
Chapter Editor: Robert N. Cherry, Jr.
Table of Contents
Introduction
Robert N. Cherry, Jr.
Radiation Biology and Biological Effects
Arthur C. Upton
Sources of Ionizing Radiation
Robert N. Cherry, Jr.
Workplace Design for Radiation Safety
Gordon M. Lodde
Radiation Safety
Robert N. Cherry, Jr.
Planning for and Management of Radiation Accidents
Sydney W. Porter, Jr.

49. Radiation, Non-Ionizing (9)

49. Radiation, Non-Ionizing
Chapter Editor: Bengt Knave
Table of Contents
Tables and Figures
Electric and Magnetic Fields and Health Outcomes
Bengt Knave
The Electromagnetic Spectrum: Basic Physical Characteristics
Kjell Hansson Mild
Ultraviolet Radiation
David H. Sliney
Infrared Radiation
R. Matthes
Light and Infrared Radiation
David H. Sliney
Lasers
David H. Sliney
Radiofrequency Fields and Microwaves
Kjell Hansson Mild
VLF and ELF Electric and Magnetic Fields
Michael H. Repacholi
Static Electric and Magnetic Fields
Martino Grandolfo
Tables
Click a link below to view table in article context.
1. Sources and exposures for IR
2. Retinal thermal hazard function
3. Exposure limits for typical lasers
4. Applications of equipment using range >0 to 30 kHz
5. Occupational sources of exposure to magnetic fields
6. Effects of currents passing through the human body
7. Biological effects of various current density ranges
8. Occupational exposure limits-electric/magnetic fields
9. Studies on animals exposed to static electric fields
10. Major technologies and large static magnetic fields
11. ICNIRP recommendations for static magnetic fields
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

50. Vibration (4)

50. Vibration
Chapter Editor: Michael J. Griffin
Table of Contents
Table and Figures
Vibration
Michael J. Griffin
Whole-body Vibration
Helmut Seidel and Michael J. Griffin
Hand-transmitted Vibration
Massimo Bovenzi
Motion Sickness
Alan J. Benson
Tables
Click a link below to view table in article context.
1. Activities with adverse effects of whole-body vibration
2. Preventive measures for whole-body vibration
3. Hand-transmitted vibration exposures
4. Stages, Stockholm Workshop scale, hand-arm vibration syndrome
5. Raynaud’s phenomenon & hand-arm vibration syndrome
6. Threshold limit values for hand-transmitted vibration
7. European Union Council Directive: Hand-transmitted vibration (1994)
8. Vibration magnitudes for finger blanching
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

51. Violence (1)

51. Violence
Chapter Editor: Leon J. Warshaw
Table of Contents
Violence in the Workplace
Leon J. Warshaw
Tables
Click a link below to view table in article context.
1. Highest rates of occupational homicide, US workplaces, 1980-1989
2. Highest rates of occupational homicide US occupations, 1980-1989
3. Risk factors for workplace homicides
4. Guides for programmes to prevent workplace violence

52. Visual Display Units (11)

52. Visual Display Units
Chapter Editor: Diane Berthelette
Table of Contents
Tables and Figures
Overview
Diane Berthelette
Characteristics of Visual Display Workstations
Ahmet Çakir
Ocular and Visual Problems
Paule Rey and Jean-Jacques Meyer
Reproductive Hazards - Experimental Data
Ulf Bergqvist
Reproductive Effects - Human Evidence
Claire Infante-Rivard
Case Study: A Summary of Studies of Reproductive Outcomes
Musculoskeletal Disorders
Gabriele Bammer
Skin Problems
Mats Berg and Sture Lidén
Psychosocial Aspects of VDU Work
Michael J. Smith and Pascale Carayon
Ergonomic Aspects of Human - Computer Interaction
Jean-Marc Robert
Ergonomics Standards
Tom F.M. Stewart
Tables
Click a link below to view table in article context.
1. Distribution of computers in various regions
2. Frequency & importance of elements of equipment
3. Prevalence of ocular symptoms
4. Teratological studies with rats or mice
5. Teratological studies with rats or mice
6. VDU use as a factor in adverse pregnancy outcomes
7. Analyses to study causes musculoskeletal problems
8. Factors thought to cause musculoskeletal problems
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.
Passive Fire Protection Measures
Confining Fires by Compartmentation
Building and site planning
Fire safety engineering work should begin early in the design phase because the fire safety requirements influence the layout and design of the building considerably. In this way, the designer can incorporate fire safety features into the building much better and more economically. The overall approach includes consideration of both interior building functions and layout, as well as exterior site planning. Prescriptive code requirements are more and more replaced by functionally based requirements, which means there is an increased demand for experts in this field. From the beginning of the construction project, the building designer therefore should contact fire experts to elucidate the following actions:
- to describe the fire problem specific to the building
- to describe different alternatives to obtain the required fire safety level
- to analyse system choice regarding technical solutions and economy
- to create presumptions for technical optimized system choices.
The architect must utilize a given site in designing the building and adapt the functional and engineering considerations to the particular site conditions that are present. In a similar manner, the architect should consider site features in arriving at decisions on fire protection. A particular set of site characteristics may significantly influence the type of active and passive protection suggested by the fire consultant. Design features should consider the local fire-fighting resources that are available and the time to reach the building. The fire service cannot and should not be expected to provide complete protection for building occupants and property; it must be assisted by both active and passive building fire defences, to provide reasonable safety from the effects of fire. Briefly, the operations may be broadly grouped as rescue, fire control and property conservation. The first priority of any fire-fighting operation is to ensure that all occupants are out of the building before critical conditions occur.
Structural design based on classification or calculation
A well-established means of codifying fire protection and fire safety requirements for buildings is to classify them by types of construction, based upon the materials used for the structural elements and the degree of fire resistance afforded by each element. Classification can be based on furnace tests in accordance with ISO 834 (fire exposure is characterized by the standard temperature-time curve), combination of test and calculation or by calculation. These procedures will identify the standard fire resistance (the ability to fulfil required functions during 30, 60, 90 minutes, etc.) of a structural load-bearing and/or separating member. Classification (especially when based on tests) is a simplified and conservative method and is more and more replaced by functionally based calculation methods taking into account the effect of fully developed natural fires. However, fire tests will always be required, but they can be designed in a more optimal way and be combined with computer simulations. In that procedure, the number of tests can be reduced considerably. Usually, in the fire test procedures, load-bearing structural elements are loaded to 100% of the design load, but in real life the load utilization factor is most often less than that. Acceptance criteria are specific for the construction or element tested. Standard fire resistance is the measured time the member can withstand the fire without failure.
Optimum fire engineering design, balanced against anticipated fire severity, is the objective of structural and fire protection requirements in modern performance-based codes. These have opened the way for fire engineering design by calculation with prediction of the temperature and structural effect due to a complete fire process (heating and subsequent cooling is considered) in a compartment. Calculations based on natural fires mean that the structural elements (important for the stability of the building) and the whole structure are not allowed to collapse during the entire fire process, including cool down.
Comprehensive research has been performed during the past 30 years. Various computer models have been developed. These models utilize basic research on mechanical and thermal properties of materials at elevated temperatures. Some computer models are validated against a vast number of experimental data, and a good prediction of structural behaviour in fire is obtained.
Compartmentation
A fire compartment is a space within a building extending over one or several floors which is enclosed by separating members such that the fire spread beyond the compartment is prevented during the relevant fire exposure. Compartmentation is important in preventing the fire to spread into too large spaces or into the whole building. People and property outside the fire compartment can be protected by the fact that the fire is extinguished or burns out by itself or by the delaying effect of the separating members on the spread of fire and smoke until the occupants are rescued to a place of safety.
The fire resistance required by a compartment depends upon its intended purpose and on the expected fire. Either the separating members enclosing the compartment shall resist the maximum expected fire or contain the fire until occupants are evacuated. The load-bearing elements in the compartment must always resist the complete fire process or be classified to a certain resistance measured in terms of periods of time, which is equal or longer than the requirement of the separating members.
Structural integrity during a fire
The requirement for maintaining structural integrity during a fire is the avoidance of structural collapse and the ability of the separating members to prevent ignition and flame spread into adjacent spaces. There are different approaches to provide the design for fire resistance. They are classifications based on standard fire-resistance test as in ISO 834, combination of test and calculation or solely calculation and the performance-based procedure computer prediction based on real fire exposure.
Interior finish
Interior finish is the material that forms the exposed interior surface of walls, ceilings and floor. There are many types of interior finish materials such as plaster, gypsum, wood and plastics. They serve several functions. Some functions of the interior material are acoustical and insulational, as well as protective against wear and abrasion.
Interior finish is related to fire in four different ways. It can affect the rate of fire build-up to flashover conditions, contribute to fire extension by flame spread, increase the heat release by adding fuel and produce smoke and toxic gases. Materials that exhibit high rates of flame spread, contribute fuel to a fire or produce hazardous quantities of smoke and toxic gases would be undesirable.
Smoke movement
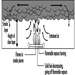
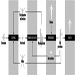
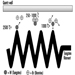
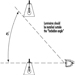
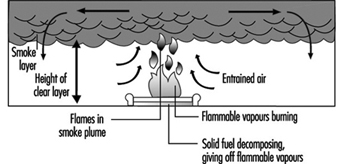
In building fires, smoke often moves to locations remote from the fire space. Stairwells and elevator shafts can become smoke-logged, thereby blocking evacuation and inhibiting fire-fighting. Today, smoke is recognized as the major killer in fire situations (see figure 1).
Figure 1. The production of smoke from a fire.
The driving forces of smoke movement include naturally occurring stack effect, buoyancy of combustion gases, the wind effect, fan-powered ventilation systems and the elevator piston effect.
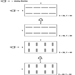
When it is cold outside, there is an upward movement of air within building shafts. Air in the building has a buoyant force because it is warmer and therefore less dense than outside air. The buoyant force causes air to rise within building shafts. This phenomenon is known as the stack effect. The pressure difference from the shaft to the outside, which causes smoke movement, is illustrated below:

where
![]() = the pressure difference from the shaft to the outside
= the pressure difference from the shaft to the outside
g = acceleration of gravity
![]() = absolute atmospheric pressure
= absolute atmospheric pressure
R = gas constant of air
![]() = absolute temperature of outside air
= absolute temperature of outside air
![]() = absolute temperature of air inside the shaft
= absolute temperature of air inside the shaft
z = elevation
High-temperature smoke from a fire has a buoyancy force due to its reduced density. The equation for buoyancy of combustion gases is similar to the equation for the stack effect.
In addition to buoyancy, the energy released by a fire can cause smoke movement due to expansion. Air will flow into the fire compartment, and hot smoke will be distributed in the compartment. Neglecting the added mass of the fuel, the ratio of volumetric flows can simply be expressed as a ratio of absolute temperature.
Wind has a pronounced effect on smoke movement. The elevator piston effect should not be neglected. When an elevator car moves in a shaft, transient pressures are produced.
Heating, ventilating and air conditioning (HVAC) systems transport smoke during building fires. When a fire starts in an unoccupied portion of a building, the HVAC system can transport smoke to another occupied space. The HVAC system should be designed so that either the fans are shut down or the system transfers into a special smoke control mode operation.
Smoke movement can be managed by use of one or more of the following mechanisms: compartmentation, dilution, air flow, pressurization or buoyancy.
Evacuation of Occupants
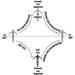
Egress design
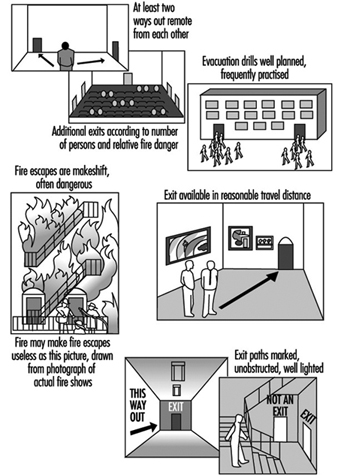
Egress design should be based upon an evaluation of a building’s total fire protection system (see figure 2).
Figure 2. Principles of exit safety.
People evacuating from a burning building are influenced by a number of impressions during their escape. The occupants have to make several decisions during the escape in order to make the right choices in each situation. These reactions can differ widely, depending upon the physical and mental capabilities and conditions of building occupants.
The building will also influence the decisions made by the occupants by its escape routes, guidance signs and other installed safety systems. The spread of fire and smoke will have the strongest impact on how the occupants make their decisions. The smoke will limit the visibility in the building and create a non-tenable environment to the evacuating persons. Radiation from fire and flames creates large spaces that cannot be used for evacuation, which increases the risk.
In designing means of egress one first needs a familiarity with the reaction of people in fire emergencies. Patterns of movement of people must be understood.
The three stages of evacuation time are notification time, reaction time and time to evacuate. The notification time is related to whether there is a fire alarm system in the building or if the occupant is able to understand the situation or how the building is divided into compartments. The reaction time depends on the occupant’s ability to make decisions, the properties of the fire (such as the amount of heat and smoke) and how the building’s egress system is planned. Finally, the time to evacuate depends on where in the building crowds are formed and how people move in various situations.
In specific buildings with mobile occupants, for example, studies have shown certain reproducible flow characteristics from persons exiting the buildings. These predictable flow characteristics have fostered computer simulations and modelling to aid the egress design process.
The evacuation travel distances are related to the fire hazard of the contents. The higher the hazard, the shorter the travel distance to an exit.
A safe exit from a building requires a safe path of escape from the fire environment. Hence, there must be a number of properly designed means of egress of adequate capacity. There should be at least one alternative means of egress considering that fire, smoke and the characteristics of occupants and so on may prevent use of one means of egress. The means of egress must be protected against fire, heat and smoke during the egress time. Thus, it is necessary to have building codes that consider the passive protection, according to evacuation and of course to fire protection. A building must manage the critical situations, which are given in the codes concerning evacuation. For example, in the Swedish Building Codes, the smoke layer must not reach below
1.6 + 0.1H (H is the total compartment height), maximum radiation 10 kW/m2 of short duration, and the temperature in the breathing air must not exceed 80 °C.
An effective evacuation can take place if a fire is discovered early and the occupants are alerted promptly with a detection and alarm system. A proper mark of the means of egress surely facilitates the evacuation. There is also a need for organization and drill of evacuation procedures.
Human behaviour during fires
How one reacts during a fire is related to the role assumed, previous experience, education and personality; the perceived threat of the fire situation; the physical characteristics and means of egress available within the structure; and the actions of others who are sharing the experience. Detailed interviews and studies over 30 years have established that instances of non-adaptive, or panic, behaviour are rare events that occur under specific conditions. Most behaviour in fires is determined by information analysis, resulting in cooperative and altruistic actions.
Human behaviour is found to pass through a number of identified stages, with the possibility of various routes from one stage to the next. In summary, the fire is seen as having three general stages:
- The individual receives initial cues and investigates or misinterprets these initial cues.
- Once the fire is apparent, the individual will try to obtain further information, contact others or leave.
- The individual will thereafter deal with the fire, interact with others or escape.
Pre-fire activity is an important factor. If a person is engaged in a well-known activity, for example eating a meal in a restaurant, the implications for subsequent behaviour are considerable.
Cue reception may be a function of pre-fire activity. There is a tendency for gender differences, with females more likely to be recipient of noises and odours, though the effect is only slight. There are role differences in initial responses to the cue. In domestic fires, if the female receives the cue and investigates, the male, when told, is likely to “have a look” and delay further actions. In larger establishments, the cue may be an alarm warning. Information may come from others and has been found to be inadequate for effective behaviour.
Individuals may or may not have realized that there is a fire. An understanding of their behaviour must take account of whether they have defined their situation correctly.
When the fire has been defined, the “prepare” stage occurs. The particular type of occupancy is likely to have a great influence on exactly how this stage develops. The “prepare” stage includes in chronological order “instruct”, “explore” and “withdraw”.
The “act” stage, which is the final stage, depends upon role, occupancy, and earlier behaviour and experience. It may be possible for early evacuation or effective fire-fighting to occur.
Building transportation systems
Building transportation systems must be considered during the design stage and should be integrated with the whole building’s fire protection system. The hazards associated with these systems must be included in any pre-fire planning and fire protection survey.
Building transportation systems, such as elevators and escalators, make high-rise buildings feasible. Elevator shafts can contribute to the spread of smoke and fire. On the other hand, an elevator is a necessary tool for fire-fighting operations in high-rise buildings.
Transportation systems may contribute to dangerous and complicated fire safety problems because an enclosed elevator shaft acts as a chimney or flue because of the stack effect of hot smoke and gases from fire. This generally results in the movement of smoke and combustion products from lower to upper levels of the building.
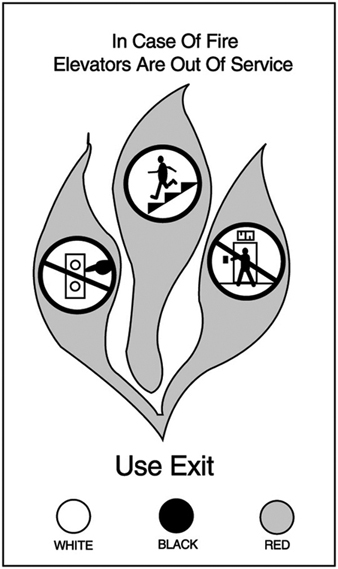
High-rise buildings present new and different problems to fire-suppression forces, including the use of elevators during emergencies. Elevators are unsafe in a fire for several reasons:
- Persons may push a corridor button and have to wait for an elevator that may never respond, losing valuable escape time.
- Elevators do not prioritize car and corridor calls, and one of the calls may be at the fire floor.
- Elevators cannot start until the lift and shaft doors are closed, and panic could lead to overcrowding of an elevator and the blockage of the doors, which would thus prevent closing.
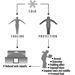
- The power can fail during a fire at any time, thus leading to entrapment. (See figure 3)
Figure 3. An example of a pictographic warning message for elevator use.
Fire drills and occupant training
A proper mark of the means of egress facilitates the evacuation, but it does not ensure life safety during fire. Exit drills are necessary to make an orderly escape. They are specially required in schools, board and care facilities and industries with high hazard. Employee drills are required, for example, in hotel and large business occupancies. Exit drills should be conducted to avoid confusion and ensure the evacuation of all occupants.
All employees should be assigned to check for availability, to count occupants when they are outside the fire area, to search for stragglers and to control re-entry. They should also recognize the evacuation signal and know the exit route they are to follow. Primary and alternative routes should be established, and all employees should be trained to use either route. After each exit drill, a meeting of responsible managers should be held to evaluate the success of the drill and to solve any kind of problem that could have occurred.
Active Fire Protection Measures
Life Safety and Property Protection
As the primary importance of any fire protection measure is to provide an acceptable degree of life safety to inhabitants of a structure, in most countries legal requirements applying to fire protection are based on life safety concerns. Property protection features are intended to limit physical damage. In many cases these objectives are complementary. Where concern exists with the loss of property, its function or contents, an owner may choose to implement measures beyond the required minimum necessary to address life safety concerns.
Fire Detection and Alarm Systems
A fire detection and alarm system provides a means to detect fire automatically and to warn building occupants of the threat of fire. It is the audible or visual alarm provided by a fire detection system that is the signal to begin the evacuation of the occupants from the premises. This is especially important in large or multi-storey buildings where occupants would be unaware that a fire was underway within the structure and where it would be unlikely or impractical for warning to be provided by another inhabitant.
Basic elements of a fire detection and alarm system
A fire detection and alarm system may include all or some of the following:
- a system control unit
- a primary or main electrical power supply
- a secondary (stand-by) power supply, usually supplied from batteries or an emergency generator
- alarm-initiating devices such as automatic fire detectors, manual pull stations and/or sprinkler system flow devices, connected to “initiating circuits” of the system control unit
- alarm-indicating devices, such as bells or lights, connected to “indicating circuits” of the system control unit
- ancillary controls such as ventilation shut-down functions, connected to output circuits of the system control unit
- remote alarm indication to an external response location, such as the fire department
- control circuits to activate a fire protection system or smoke control system.
Smoke Control Systems
To reduce the threat of smoke from entering exit paths during evacuation from a structure, smoke control systems can be used. Generally, mechanical ventilation systems are employed to supply fresh air to the exit path. This method is most often used to pressurize stairways or atrium buildings. This is a feature intended to enhance life safety.
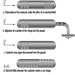
Portable Fire Extinguishers and Hose Reels
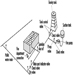
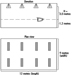
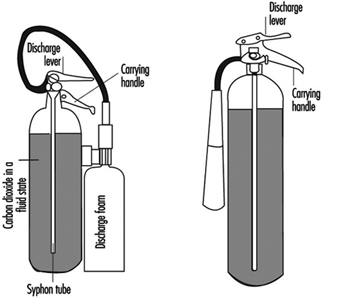
Portable fire extinguishers and water hose reels are often provided for use by building occupants to fight small fires (see figure 1). Building occupants should not be encouraged to use a portable fire extinguisher or hose reel unless they have been trained in their use. In all cases, operators should be very cautious to avoid placing themselves in a position where safe egress is blocked. For any fire, no matter how small, the first action should always be to notify other building occupants of the threat of fire and summon assistance from the professional fire service.
Figure 1. Portable fire extinguishers.
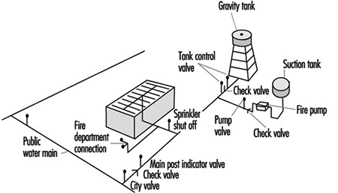
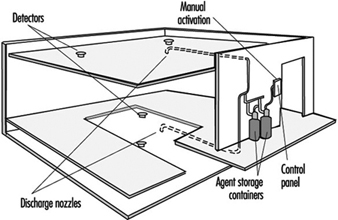
Water Sprinkler Systems
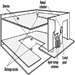
Water sprinkler systems consist of a water supply, distribution valves and piping connected to automatic sprinkler heads (see figure 2). While current sprinkler systems are primarily intended to control the spread of fire, many systems have accomplished complete extinguishment.
Figure 2. A typical sprinkler installation showing all common water supplies, outdoor hydrants and underground piping.
A common misconception is that all automatic sprinkler heads open in the event of a fire. In fact, each sprinkler head is designed to open only when sufficient heat is present to indicate a fire. Water then flows only from the sprinkler head(s) that have opened as the result of fire in their immediate vicinity. This design feature provides efficient use of water for fire-fighting and limits water damage.
Water supply
Water for an automatic sprinkler system must be available in sufficient quantity and at sufficient volume and pressure at all times to ensure reliable operation in the event of fire. Where a municipal water supply cannot meet this requirement, a reservoir or pump arrangement must be provided to provide a secure water supply.
Control valves
Control valves should be maintained in the open position at all times. Often, supervision of the control valves can be accomplished by the automatic fire alarm system by provision of valve tamper switches that will initiate a trouble or supervisory signal at the fire alarm control panel to indicate a closed valve. If this type of monitoring cannot be provided, the valves should be locked in the open position.
Piping
Water flows through a piping network, ordinarily suspended from the ceiling, with the sprinkler heads suspended at intervals along the pipes. Piping used in sprinkler systems should be of a type that can withstand a working pressure of not less than 1,200 kPa. For exposed piping systems, fittings should be of the screwed, flanged, mechanical joint or brazed type.
Sprinkler heads
A sprinkler head consists of an orifice, normally held closed by a temperature-sensitive releasing element, and a spray deflector. The water discharge pattern and spacing requirements for individual sprinkler heads are used by sprinkler designers to ensure complete coverage of the protected risk.
Special Extinguishing Systems
Special extinguishing systems are used in cases where water sprinklers would not provide adequate protection or where the risk of damage from water would be unacceptable. In many cases where water damage is of concern, special extinguishing systems may be used in conjunction with water sprinkler systems, with the special extinguishing system designed to react at an early stage of fire development.
Water and water-additive special extinguishing systems
Water spray systems
Water spray systems increase the effectiveness of water by producing smaller water droplets, and thus a greater surface area of water is exposed to the fire, with a relative increase in heat absorption capability. This type of system is often chosen as a means of keeping large pressure vessels, such as butane spheres, cool when there is a risk of an exposure fire originating in an adjacent area. The system is similar to a sprinkler system; however, all heads are open, and a separate detection system or manual action is used to open control valves. This allows water to flow through the piping network to all spray devices that serve as outlets from the piping system.
Foam systems
In a foam system, a liquid concentrate is injected into the water supply before the control valve. Foam concentrate and air are mixed, either through the mechanical action of discharge or by aspirating air into the discharge device. The air entrained in the foam solution creates an expanded foam. As expanded foam is less dense than most hydrocarbons, the expanded foam forms a blanket on top of the flammable liquid. This foam blanket reduces fuel vapour propagation. Water, which represents as much as 97% of the foam solution, provides a cooling effect to further reduce vapour propagation and to cool hot objects that could serve as a source of re-ignition.
Gaseous extinguishing systems
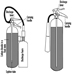
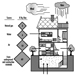
Carbon dioxide systems
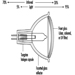
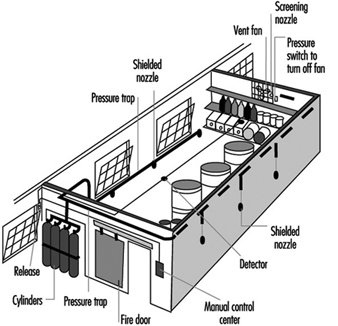
Carbon dioxide systems consist of a supply of carbon dioxide, stored as liquified compressed gas in pressure vessels (see figures 3 and 4). The carbon dioxide is held in the pressure vessel by means of an automatic valve that is opened upon fire by means of a separate detection system or by manual operation. Once released, the carbon dioxide is delivered to the fire by means of a piping and discharge nozzle arrangement. Carbon dioxide extinguishes fire by displacing the oxygen available to the fire. Carbon dioxide systems can be designed for use in open areas such as printing presses or enclosed volumes such as ship machinery spaces. Carbon dioxide, at fire-extinguishing concentrations, is toxic to people, and special measures must be employed to ensure that persons in the protected area are evacuated before discharge occurs. Pre-discharge alarms and other safety measures must be carefully incorporated into the design of the system to ensure adequate safety for people working in the protected area. Carbon dioxide is considered to be a clean extinguishant because it does not cause collateral damage and is electrically non-conductive.
Figure 3. Diagram of a high-pressure carbon dioxide system for total flooding.
Figure 4. A total flooding system installed in a room with a raised floor.
Inert gas systems
Inert gas systems generally use a mixture of nitrogen and argon as an extinguishing medium. In some cases, a small percentage of carbon dioxide is also provided in the gas mixture. The inert gas mixtures extinguish fires by reducing oxygen concentration within a protected volume. They are suitable for use in enclosed spaces only. The unique feature offered by inert gas mixtures is that they reduce the oxygen to a low enough concentration to extinguish many types of fires; however, oxygen levels are not sufficiently lowered to pose an immediate threat to occupants of the protected space. The inert gases are compressed and stored in pressure vessels. System operation is similar to a carbon dioxide system. As the inert gases cannot be liquefied by compression, the number of storage vessels required for protection of a given enclosed protected volume is greater than that for carbon dioxide.
Halon systems
Halons 1301, 1211 and 2402 have been identified as ozone-depleting substances. Production of these extinguishing agents ceased in 1994, as required by the Montreal Protocol, an international agreement to protect the earth’s ozone layer. Halon 1301 was most often used in fixed fire protection systems. Halon 1301 was stored as liquefied, compressed gas in pressure vessels in a similar arrangement to that used for carbon dioxide. The advantage offered by halon 1301 was that storage pressures were lower and that very low concentrations provided effective extinguishing capability. Halon 1301 systems were used successfully for totally enclosed hazards where the extinguishing concentration achieved could be maintained for a sufficient time for extinguishment to occur. For most risks, concentrations used did not pose an immediate threat to occupants. Halon 1301 is still used for several important applications where acceptable alternatives have yet to be developed. Examples include use on-board commercial and military aircraft and for some special cases where inerting concentrations are required to prevent explosions in areas where occupants could be present. The halon in existing halon systems that are no longer required should be made available for use by others with critical applications. This will militate against the need to produce more of these environmentally sensitive extinguishers and help protect the ozone layer.
Halocarbon systems
Halocarbon agents were developed as the result of the environmental concerns associated with halons. These agents differ widely in toxicity, environmental impact, storage weight and volume requirements, cost and availability of approved system hardware. They all can be stored as liquefied compressed gases in pressure vessels. System configuration is similar to a carbon dioxide system.
Design, Installation and Maintenance of Active Fire Protection Systems
Only those skilled in this work are competent to design, install and maintain this equipment. It may be necessary for many of those charged with purchasing, installing, inspecting, testing, approving and maintaining this equipment to consult with an experienced and competent fire protection specialist to discharge their duties effectively.
Further Information
This section of the Encyclopaedia presents a very brief and limited overview of the available choice of active fire protection systems. Readers may often obtain more information by contacting a national fire protection association, their insurer or the fire prevention department of their local fire service.
Organizing for Fire Protection
Private Emergency Organization
Profit is the main objective of any industry. To achieve this objective, an efficient and alert management and continuity of production are essential. Any interruption in production, for any reason, will adversely affect profits. If the interruption is the result of a fire or explosion, it may be long and may cripple the industry.
Very often, a plea is taken that the property is insured and loss due to fire, if any, will be indemnified by the insurance company. It must be appreciated that insurance is only a device to spread the effect of the destruction brought by fire or explosion on as many people as possible. It cannot make good the national loss. Besides, insurance is no guarantee of continuity of production and elimination or minimization of consequential losses.
What is indicated, therefore, is that the management must gather complete information on the fire and explosion hazard, evaluate the loss potential and implement suitable measures to control the hazard, with a view to eliminating or minimizing the incidence of fire and explosion. This involves the setting up of a private emergency organization.
Emergency Planning
Such an organization must, as far as possible, be considered from the planning stage itself, and implemented progressively from the time of selection of site until production has started, and then continued thereafter.
Success of any emergency organization depends to a large extent on the overall participation of all workers and various echelons of the management. This fact must be borne in mind while planning the emergency organization.
The various aspects of emergency planning are mentioned below. For more details, a reference may be made to the US National Fire Protection Association (NFPA) Fire Protection Handbook or any other standard work on the subject (Cote 1991).
Stage 1
Initiate the emergency plan by doing the following:
- Identify and evaluate fire and explosion hazards associated with the transportation, handling and storage of each raw material, intermediate and finished products and each industrial process, as well as work out detailed preventive measures to counteract the hazards with a view to eliminating or minimizing them.
- Work out the requirements of fire protection installations and equipment, and determine the stages at which each is to be provided.
- Prepare specifications for the fire protection installation and equipment.
Stage 2
Determine the following:
- availability of adequate water supply for fire protection in addition to the requirements for processing and domestic use
- susceptibility of site and natural hazards, such as floods, earthquakes, heavy rains, etc.
- environments, i.e., the nature and extent of surrounding property and the exposure hazard involved in the event of a fire or explosion
- existence of private (works) or public fire brigade(s), the distance at which such fire brigade(s) is (are) located and the suitability of the appliances available with them for the risk to be protected and whether they can be called upon to assist in an emergency
- response from the assisting fire brigade(s) with particular reference to impediments, such as railway crossings, ferries, inadequate strength and (or) width of bridges in relation to the fire appliances, difficult traffic, etc.
- socio-political environment , i.e., incidence of crime, and political activities leading to law-and-order problems.
Stage 3
Prepare the layout and building plans, and the specifications of construction material. Carry out the following tasks:
- Limit the floor area of each shop, workplace, etc. by providing fire walls, fire doors, etc.
- Specify the use of fire-resistant materials for construction of building or structure.
- Ensure that steel columns and other structural members are not exposed.
- Ensure adequate separation between building, structures and plant.
- Plan installation of fire hydrants, sprinklers, etc. where necessary.
- Ensure the provision of adequate access roads in the layout plan to enable fire appliances to reach all parts of the premises and all sources of water for fire-fighting.
Stage 4
During construction, do the following:
- Acquaint the contractor and his or her employees with the fire risk management policies, and enforce compliance.
- Thoroughly test all fire protection installations and equipment before acceptance.
Stage 5
If the size of the industry, its hazards or its out-of-the-way location is such that a full-time fire brigade must be available on the premises, then organize, equip and train the required full-time personnel. Also appoint a full-time fire officer.
Stage 6
To ensure full participation of all employees, do the following:
- Train all personnel in the observance of precautionary measures in their day-to-day work and the action required of them upon an outbreak of fire or explosion. The training must include operation of fire-fighting equipment.
- Ensure strict observance of fire precautions by all concerned personnel through periodic reviews.
- Ensure regular inspection and maintenance of all fire protection systems and equipment. All defects must be rectified promptly.
Managing the emergency
To avoid confusion at the time of an actual emergency, it is essential that everyone in the organization knows the precise part that he (she) and others are expected to play during the emergency. A well-thought-out emergency plan must be prepared and promulgated for this purpose, and all concerned personnel must be made fully familiar with it. The plan must clearly and unambiguously lay down the responsibilities of all concerned and also specify a chain of command. As a minimum, the emergency plan should include the following:
1. name of the industry
2. address of the premises, with telephone number and a site plan
3. purpose and objective of the emergency plan and effective date of its coming in force
4. area covered, including a site plan
5. emergency organization, indicating chain of command from the work manager on downwards
6. fire protection systems, mobile appliances and portable equipment, with details
7. details of assistance availability
8. fire alarm and communication facilities
9. action to be taken in an emergency. Include separately and unambiguously the action to be taken by:
- the person discovering the fire
- the private fire brigade on the premises
- head of the section involved in the emergency
- heads of other sections not actually involved in theemergency
- the security organization
- the fire officer, if any
- the works manager
- others
10. chain of command at the scene of incident. Consider all possible situations, and indicate clearly who is to assume command in each case, including the circumstances under which another organization is to be called in to assist.
11. action after a fire. Indicate responsibility for:
- recommissioning or replenishing of all fire protectionsystems, equipment and water sources
- investigating the cause of fire or explosion
- preparation and submission of reports
- initiating remedial measures to prevent re-occurrence of similar emergency.
When a mutual assistance plan is in operation, copies of emergency plan must be supplied to all participating units in return for similar plans of their respective premises.
Evacuation Protocols
A situation necessitating the execution of the emergency plan may develop as a result of either an explosion or a fire.
Explosion may or may not be followed by fire, but in almost all cases, it produces a shattering effect, which may injure or kill personnel present in the vicinity and/or cause physical damage to property, depending upon the circumstances of each case. It may also cause shock and confusion and may necessitate the immediate shut-down of the manufacturing processes or a portion thereof, along with the sudden movement of a large number of people. If the situation is not controlled and guided in an orderly manner immediately, it may lead to panic and further loss of life and property.
Smoke given out by the burning material in a fire may involve other parts of the property and/or trap persons, necessitating an intensive, large-scale rescue operation/evacuation. In certain cases, large-scale evacuation may have to be undertaken when people are likely to get trapped or affected by fire.
In all cases in which large-scale sudden movement of personnel is involved, traffic problems are also created—particularly if public roads, streets or areas have to be used for this movement. If such problems are not anticipated and suitable action is not preplanned, traffic bottlenecks result, which hamper and retard fire extinguishment and rescue efforts.
Evacuation of a large number of persons—particularly from high-rise buildings—may also present problems. For successful evacuation, it is not only necessary that adequate and suitable means of escape are available, but also that the evacuation be effected speedily. Special attention should be given to the evacuation needs of disabled individuals.
Detailed evacuation procedures must, therefore, be included in the emergency plan. These must be frequently tested in the conduct of fire and evacuation drills, which may also involve traffic problems. All participating and concerned organizations and agencies must also be involved in these drills, at least periodically. After each exercise, a debriefing session must be held, during which all mistakes are pointed out and explained. Action must also be taken to prevent repetition of the same mistakes in future exercises and actual incidents by removing all difficulties and reviewing the emergency plan as necessary.
Proper records must be maintained of all exercises and evacuation drills.
Emergency Medical Services
Casualties in a fire or explosion must receive immediate medical aid or be moved speedily to a hospital after being given first aid.
It is essential that management provide one or more first-aid post(s) and, where necessary because of the size and hazardous nature of the industry, one or more mobile paramedical appliances. All first-aid posts and paramedical appliances must be staffed at all times by fully trained paramedics.
Depending upon the size of the industry and the number of workers, one or more ambulance(s) must also be provided and staffed on the premises for removal of casualties to hospitals. In addition, arrangement must be made to ensure that additional ambulance facilities are available at short notice when needed.
Where the size of the industry or workplace so demands, a full-time medical officer should also be made available at all times for any emergency situation.
Prior arrangements must be made with a designated hospital or hospitals at which priority is given to casualties who are removed after a fire or explosion. Such hospitals must be listed in the emergency plan along with their telephone numbers, and the emergency plan must have suitable provisions to ensure that a responsible person shall alert them to receive casualties as soon as an emergency arises.
Facility Restoration
It is important that all fire protection and emergency facilities are restored to a “ready” mode soon after the emergency is over. For this purpose, responsibility must be assigned to a person or section of the industry, and this must be included in the emergency plan. A system of checks to ensure that this is being done must also be introduced.
Public Fire Department Relations
It is not practicable for any management to foresee and provide for all possible contingencies. It is also not economically feasible to do so. In spite of adopting the most up-to-date method of fire risk management, there are always occasions when the fire protection facilities provided on the premises fall short of actual needs. For such occasions, it is desirable to preplan a mutual assistance programme with the public fire department. Good liaison with that department is necessary so that the management knows what assistance that unit can provide during an emergency on its premises. Also, the public fire department must become familiar with the risk and what it could expect during an emergency. Frequent interaction with the public fire department is necessary for this purpose.
Handling of Hazardous Materials
Hazards of the materials used in industry may not be known to fire-fighters during a spill situation, and accidental discharge and improper use or storage of hazardous materials can lead to dangerous situations that can seriously imperil their health or lead to a serious fire or explosion. It is not possible to remember the hazards of all materials. Means of ready identification of hazards have, therefore, been developed whereby the various substances are identified by distinct labels or markings.
Hazardous materials identification
Each country follows its own rules concerning the labelling of hazardous materials for the purpose of storage, handling and transportation, and various departments may be involved. While compliance with local regulations is essential, it is desirable that an internationally recognized system of identification of hazardous materials be evolved for universal application. In the United States, the NFPA has developed a system for this purpose. In this system, distinct labels are conspicuously attached or affixed to containers of hazardous materials. These labels indicate the nature and degree of hazards in respect of health, flammability and the reactive nature of the material. In addition, special possible hazards to fire-fighters can also be indicated on these labels. For an explanation of the degree of hazard, refer to NFPA 704, Standard System for the Identification of the Fire Hazards of Materials (1990a). In this system, the hazards are categorized as health hazards, flammability hazards, and reactivity (instability) hazards.
Health hazards
These include all possibilities of a material causing personal injury from contact with or absorption into the human body. A health hazard may arise out of the inherent properties of the material or from the toxic products of combustion or decomposition of the material. The degree of hazard is assigned on the basis of the greater hazard that may result under fire or other emergency conditions. It indicates to fire-fighters whether they can work safely only with special protective clothing or with suitable respiratory protective equipment or with ordinary clothing.
Degree of health hazard is measured on a scale of 4 to 0, with 4 indicating the most severe hazard and 0 indicating low hazard or no hazard.
Flammability hazards
These indicate the susceptibility of the material to burning. It is recognized that materials behave differently in respect of this property under varying circumstances (e.g., materials that may burn under one set of conditions may not burn if the conditions are altered). The form and inherent properties of the materials influence the degree of hazard, which is assigned on the same basis as for the health hazard.
Reactivity (instability) hazards
Materials capable of releasing energy by itself, (i.e., by self-reaction or polymerization) and substances that can undergo violent eruption or explosive reactions on coming in contact with water, other extinguishing agents or certain other materials are said to possess a reactivity hazard.
The violence of reaction may increase when heat or pressure is applied or when the substance comes in contact with certain other materials to form a fuel-oxidizer combination, or when it comes in contact with incompatible substances, sensitizing contaminants or catalysts.
The degree of reactivity hazard is determined and expressed in terms of the ease, rate and quantity of energy release. Additional information, such as radioactivity hazard or prohibition of water or other extinguishing medium for fire-fighting, can also be given on the same level.
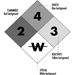
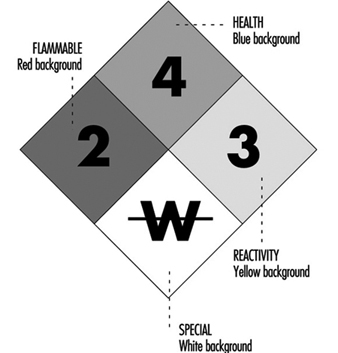
The label warning of a hazardous material is a diagonally placed square with four smaller squares (see figure 1).
Figure 1. The NFPA 704 diamond.
The top square indicates the health hazard, the one on the left indicates the flammability hazard, the one on the right indicates the reactivity hazard, and the bottom square indicates other special hazards, such as radioactivity or unusual reactivity with water.
To supplement the above mentioned arrangement, a colour code may also be used. The colour is used as background or the numeral indicating the hazard may be in coded colour. The codes are health hazard (blue), flammability hazard (red), reactivity hazard (yellow) and special hazard (white background).
Managing hazardous materials response
Depending on the nature of the hazardous material in the industry, it is necessary to provide protective equipment and special fire-extinguishing agents, including the protective equipment required to dispense the special extinguishing agents.
All workers must be trained in the precautions they must take and the procedures they must adopt to deal with each incident in the handling of the various types of hazardous materials. They must also know the meaning of the various identification signs.
All fire-fighters and other workers must be trained in the correct use of any protective clothing, protective respiratory equipment and special fire-fighting techniques. All concerned personnel must be kept alert and prepared to tackle any situation through frequent drills and exercises, of which proper records should be kept.
To deal with serious medical hazards and the effects of these hazards on fire-fighters, a competent medical officer should be available to take immediate precautions when any individual is exposed to unavoidable dangerous contamination. All affected persons must receive immediate medical attention.
Proper arrangements must also be made to set up a decontamination centre on the premises when necessary, and correct decontamination procedures must be laid down and followed.
Waste control
Considerable waste is generated by industry or because of accidents during handling, transportation and storage of goods. Such waste may be flammable, toxic, corrosive, pyrophoric, chemically reactive or radioactive, depending upon the industry in which it is generated or the nature of goods involved. In most cases unless proper care is taken in safe disposal of such waste, it may endanger animal and human life, pollute the environment or cause fire and explosions that may endanger property. A thorough knowledge of the physical and chemical properties of the waste materials and of the merits or limitations of the various methods of their disposal is, therefore, necessary to ensure economy and safety.
Properties of industrial waste are briefly summarized below:
- Most industrial waste is hazardous and can have unexpected significance during and after disposal. The nature and behavioural characteristics of all waste must therefore be carefully examined for their short- and long-term impact and the method of disposal determined accordingly.
- Mixing of two seemingly innocuous discarded substances may create an unexpected hazard because of their chemical or physical interaction.
- Where flammable liquids are involved, their hazards can be assessed by taking into consideration their respective flash points, ignition temperature, flammability limits and the ignition energy required to initiate combustion. In the case of solids, particle size is an additional factor that must be considered.
- Most flammable vapours are heavier than air. Such vapours and heavier-than-air flammable gases that may be accidentally released during collection or disposal or during handling and transportation can travel considerable distances with the wind or towards a lower gradient. On coming in contact with a source of ignition, they flash back to source. Major spills of flammable liquids are particularly hazardous in this respect and may require evacuation to save lives.
- Pyrophoric materials, such as aluminium alkyls, ignite spontaneously when exposed to air. Special care must therefore be taken in handling, transportation, storage and disposal of such materials, preferably carried out under a nitrogen atmosphere.
- Certain materials, such as potassium, sodium and aluminium alkyls, react violently with water or moisture and burn fiercely. Bronze powder generates considerable heat in the presence of moisture.
- The presence of potent oxidants with organic materials can cause rapid combustion or even an explosion. Rags and other materials soaked with vegetable oils or terpenes present a risk of spontaneous combustion due to the oxidation of oils and subsequent build-up of heat to the ignition temperature.
- Several substances are corrosive and may cause severe damage or burns to skin or other living tissues, or may corrode construction materials, especially metals, thereby weakening the structure in which such materials may have been used.
- Some substances are toxic and can poison humans or animals by contact with skin, inhalation or contamination of food or water. Their ability to do so may be short lived or may extend over a long period. Such substances, if disposed of by dumping or burning, can contaminate water sources or come into contact with animals or workers.
- Toxic substances that are spilled during industrial processing, transportation (including accidents), handling or storage, and toxic gases that are released into the atmosphere can affect emergency personnel and others, including the public. The hazard is all the more severe if the spilled substance(s) is vaporized at ambient temperature, because the vapours can be carried over long distances due to wind drift or run-off.
- Certain substances may emit a strong, pungent or unpleasant odour, either by themselves or when they are burnt in the open. In either case, such substances are a public nuisance, even though they may not be toxic, and they must be disposed of by proper incineration, unless it is possible to collect and recycle them. Just as odorous substances are not necessarily toxic, odourless substances and some substances with a pleasant odour may produce harmful physiological effects.
- Certain substances, such as explosives, fireworks, organic peroxides and some other chemicals, are sensitive to heat or shock and may explode with devastating effect if not handled carefully or mixed with other substances. Such substances must, therefore, be carefully segregated and destroyed under proper supervision.
- Waste materials that are contaminated with radioactivity can be as hazardous as the radioactive materials themselves. Their disposal requires specialized knowledge. Proper guidance for disposal of such waste may be obtained from a country’s nuclear energy organization.
Some of the methods that may be employed to dispose of industrial and emergency waste are biodegradation, burial, incineration, landfill, mulching, open burning, pyrolysis and disposal through a contractor. These are briefly explained below.
Biodegradation
Many chemicals are completely destroyed within six to 24 months when they are mixed with the top 15 cm of soil. This phenomenon is known as biodegradation and is due to the action of soil bacteria. Not all substances, however, behave in this way.
Burial
Waste, particularly chemical waste, is often disposed of by burial. This is a dangerous practice in so far as active chemicals are concerned, because, in time, the buried substance may get exposed or leached by rain into water resources. The exposed substance or the contaminated material can have adverse physiological effects when it comes in contact with water that is drunk by humans or animals. Cases are on record in which water was contaminated 40 years after burial of certain harmful chemicals.
Incineration
This is one of the safest and most satisfactory methods of waste disposal if the waste is burned in a properly designed incinerator under controlled conditions. Care must be taken, however, to ensure that the substances contained in the waste are amenable to safe incineration without posing any operating problem or special hazard. Almost all industrial incinerators require the installation of air pollution control equipment, which must be carefully selected and installed after taking into consideration the composition of the stock effluent given out by the incinerator during the burning of industrial waste.
Care must be taken in the operation of the incinerator to ensure that its operative temperature does not rise excessively either because a large amount of volatiles is fed or because of the nature of the waste burned. Structural failure can occur because of excessive temperature, or, over time, because of corrosion. The scrubber must also be periodically inspected for signs of corrosion which can occur because of contact with acids, and the scrubber system must be maintained regularly to ensure proper functioning.
Landfill
Low-lying land or a depression in land is often used as a dump for waste materials until it becomes level with the surrounding land. The waste is then levelled, covered with earth and rolled hard. The land is then used for buildings or other purposes.
For satisfactory landfill operation, the site must be selected with due regard to the proximity of pipelines, sewer lines, power lines, oil and gas wells, mines and other hazards. The waste must then be mixed with earth and evenly spread out in the depression or a wide trench. Each layer must be mechanically compacted before the next layer is added.
A 50 cm layer of earth is typically laid over the waste and compacted, leaving sufficient vents in the soil for the escape of gas that is produced by biological activity in the waste. Attention must also be paid to proper drainage of the landfill area.
Depending on the various constituents of waste material, it may at times ignite within the landfill. Each such area must, therefore, be properly fenced off and continued surveillance maintained until the chances of ignition appear to be remote. Arrangements must also be made for extinguishing any fire that may break out in the waste within the landfill.
Mulching
Some trials have been made for reusing polymers as mulch (loose material for protecting the roots of plants) by chopping the waste into small shreds or granules. When so used, it degrades very slowly. Its effect on the soil is, therefore, purely physical. This method has, however, not been used widely.
Open burning
Open burning of waste causes pollution of the atmosphere and is hazardous in as much as there is a chance of the fire getting out of control and spreading to the surrounding property or areas. Also, there is a chance of explosion from containers, and there is a possibility of harmful physiological effects of radioactive materials that may be contained in the waste. This method of disposal has been banned in some countries. It is not a desirable method and should be discouraged.
Pyrolysis
Recovery of certain compounds, by distillation of the products given out during pyrolysis (decomposition by heating) of polymers and organic substances, is possible, but not yet widely adopted.
Disposal through contractors
This is probably the most convenient method. It is important that only reliable contractors who are knowledgeable and experienced in the disposal of industrial waste and hazardous materials are selected for the job. Hazardous materials must be carefully segregated and disposed of separately.
Specific classes of materials
Specific examples of the types of hazardous materials that are often found in today’s industry include: (1) combustible and reactive metals, such as magnesium, potassium, lithium, sodium, titanium and zirconium; (2) combustible refuse; (3) drying oils; (4) flammable liquids and waste solvents; (5) oxidizing materials (liquids and solids); and (6) radioactive materials. These materials require special handling and precautions that must be carefully studied. For more details on identification of hazardous materials and hazards of industrial materials, the following publications may be consulted: Fire Protection Handbook (Cote 1991) and Sax’s Dangerous Properties of Industrial Materials (Lewis 1979).
Physiological Responses to the Thermal Environment
Humans live their entire lives within a very small, fiercely protected range of internal body temperatures. The maximal tolerance limits for living cells range from about 0ºC (ice crystal formation) to about 45ºC (thermal coagulation of intracellular proteins); however, humans can tolerate internal temperatures below 35ºC or above 41ºC for only very brief periods of time. To maintain internal temperature within these limits, people have developed very effective and in some instances specialized physiological responses to acute thermal stresses. These responses—designed to facilitate the conservation, production or elimination of body heat—involve the finely controlled coordination of several body systems.
Human Thermal Balance
By far, the largest source of heat imparted to the body results from metabolic heat production (M). Even at peak mechanical efficiency, 75 to 80% of the energy involved in muscular work is liberated as heat. At rest, a metabolic rate of 300 ml O2 per minute creates a heat load of approximately 100 Watts. During steady-state work at an oxygen consumption of 1 l/min, approximately 350 W of heat are generated—less any energy associated with external work (W). Even at such a mild to moderate work intensity, body core temperature would rise approximately one degree centigrade every 15 min were it not for an efficient means of heat dissipation. In fact, very fit individuals can produce heat in excess of 1,200 W for 1 to 3 hours without heat injury (Gisolfi and Wenger 1984).
Heat can also be gained from the environment via radiation (R) and convection (C) if the globe temperature (a measure of radiant heat) and air (dry-bulb) temperature, respectively, exceed skin temperature. These avenues of heat gain are typically small relative to M, and actually become avenues of heat loss when the skin-to-air thermal gradient is reversed. The final avenue for heat loss—evaporation (E)—is also typically the most important, since the latent heat of vaporization of sweat is high—approximately 680 W-h/l of sweat evaporated. These relations are discussed elsewhere in this chapter.
Under cool to thermoneutral conditions, heat gain is balanced by heat loss, no heat is stored, and body temperature equilibrates; that is:
M–W ± R ± C–E = 0
However, in more severe exposure to heat:
M–W ± R ± C >E
and heat is stored. In particular, heavy work (high energy expenditure which increases M–W), excessively high air temperatures (which increase R+C), high humidity (which limits E) and the wearing of thick or relatively impermeable clothing (which creates a barrier to effective evaporation of sweat) create such a scenario. Finally, if exercise is prolonged or hydration inadequate, E may be outstripped by the limited ability of the body to secrete sweat (1 to 2 l/h for short periods).
Body Temperature and Its Control
For purposes of describing physiological responses to heat and cold, the body is divided into two components—the “core” and the “shell”. Core temperature (Tc) represents internal or deep body temperature, and can be measured orally, rectally or, in laboratory settings, in the oesophagus or on the tympanic membrane (eardrum). The temperature of the shell is represented by mean skin temperature (Tsk). The average temperature of the body (Tb) at any time is a weighted balance between these temperatures, that is
Tb = k Tc + (1– k) Tsk
where the weighting factor k varies from about 0.67 to 0.90.
When confronted with challenges to thermal neutrality (heat or cold stresses), the body strives to control Tc through physiological adjustments, and Tc provides the major feedback to the brain to coordinate this control. While the local and mean skin temperature are important for providing sensory input, Tsk varies greatly with ambient temperature, averaging about 33 ºC at thermoneutrality and reaching 36 to 37 ºC under conditions of heavy work in the heat. It can drop considerably during whole-body and local exposure to cold; tactile sensitivity occurs between 15 and 20 ºC, whereas the critical temperature for manual dexterity is between 12 and 16 ºC. The upper and lower pain threshold values for Tsk are approximately 43 ºC and 10 ºC, respectively.
Precise mapping studies have localized the site of greatest thermoregulatory control in an area of the brain known as the pre- optic/anterior hypothalamus (POAH). In this region are nerve cells which respond to both heating (warm-sensitive neurons) and cooling (cold-sensitive neurons). This area dominates control of body temperature by receiving afferent sensory information about body temperature and sending efferent signals to the skin, the muscles and other organs involved in temperature regulation, via the autonomic nervous system. Other areas of the central nervous system (posterior hypothalamus, reticular formation, pons, medulla and spinal cord) form ascending and descending connections with the POAH, and serve a variety of facilitory functions.
The body’s control system is analogous to thermostatic control of temperature in a house with both heating and cooling capabilities. When body temperature rises above some theoretical “set point” temperature, effector responses associated with cooling (sweating, increasing skin blood flow) are turned on. When body temperature falls below the set point, heat gain responses (decreasing skin blood flow, shivering) are initiated. Unlike home heating/cooling systems however, the human thermoregulatory control system does not operate as a simple on-off system, but also has proportional control and rate-of-change control characteristics. It should be appreciated that a “set point temperature” exists in theory only, and thus is useful in visualizing these concepts. Much work is yet to be done toward a full understanding of the mechanisms associated with the thermoregulatory set point.
Whatever its basis, the set point is relatively stable and is unaffected by work or ambient temperature. In fact, the only acute perturbation known to shift the set point is the group of endogenous pyrogens involved in the febrile response. The effector responses employed by the body to maintain thermal balance are initiated and controlled in response to a “load error”, that is, a body temperature which is transiently above or below the set point (figure 1). A core temperature below the set point creates a negative load error, resulting in heat gain (shivering, vasoconstriction of the skin) being initiated. A core temperature above the set point creates a positive load error, leading to heat loss effectors (skin vasodilatation, sweating) being turned on. In each case, the resultant heat transfer decreases the load error and helps return the body temperature to a steady state.
Figure 1. A model of thermoregulation in the human body.
Temperature Regulation in the Heat
As mentioned above, humans lose heat to the environment primarily through a combination of dry (radiation and convection) and evaporative means. To facilitate this exchange, two primary effector systems are turned on and regulated—skin vasodilatation and sweating. While skin vasodilatation often results in small increases in dry (radiative and convective) heat loss, it functions primarily to transfer heat from the core to the skin (internal heat transfer), while evaporation of sweat provides an extremely effective means of cooling the blood prior to its return to deep body tissues (external heat transfer).
Skin vasodilatation
The amount of heat transferred from the core to the skin is a function of the skin blood flow (SkBF), the temperature gradient between core and skin, and the specific heat of blood (a little less than 4 kJ/°C per litre of blood). At rest in a thermoneutral environment, the skin gets approximately 200 to 500 ml/min of blood flow, representing only 5 to 10% of the total blood pumped by the heart (cardiac output). Because of the 4ºC gradient between Tc (about 37ºC) and Tsk (about 33ºC under such conditions), the metabolic heat produced by the body to sustain life is constantly convected to the skin for dissipation. By contrast, under conditions of severe hyperthermia such as high-intensity work in hot conditions, the core-to-skin thermal gradient is smaller, and the necessary heat transfer is accomplished by large increases in SkBF. Under maximal heat stress, SkBF can reach 7 to 8 l/min, about one-third of cardiac output (Rowell 1983). This high blood flow is achieved through a poorly understood mechanism unique to humans which has been called the “active vasodilator system”. Active vasodilatation involves sympathetic nerve signals from the hypothalamus to the skin arterioles, but the neurotransmitter has not been determined.
As mentioned above, SkBF is primarily responsive to increases in Tc and, to a lesser extent, Tsk. Tc rises as muscular work is initiated and metabolic heat production begins, and once some threshold Tc is reached, SkBF also begins to increase dramatically. This basic thermoregulatory relationship is also acted upon by non-thermal factors. This second level of control is critical in that it modifies SkBF when overall cardiovascular stability is threatened. The veins in the skin are very compliant, and a significant portion of the circulating volume pools in these vessels. This aids in heat exchange by slowing the capillary circulation to increase transit time; however, this pooling, coupled with fluid losses from sweating, may also decrease the rate of blood return to the heart. Among the non-thermal factors which have been shown to influence SkBF during work are upright posture, dehydration and positive-pressure breathing (respirator use). These act through reflexes which are turned on when cardiac filling pressure is decreased and stretch receptors located in the large veins and right atrium are unloaded, and are therefore most evident during prolonged aerobic work in an upright posture. These reflexes function to maintain arterial pressure and, in the case of work, to maintain adequate blood flow to active muscles. Thus, the level of SkBF at any given point in time represents the aggregate effects of thermoregulatory and non-thermoregulatory reflex responses.
The need to increase blood flow to the skin to aid in temperature regulation greatly impacts on the ability of the cardiovascular system to regulate blood pressure. For this reason, a coordinated response of the entire cardiovascular system to heat stress is necessary. What cardiovascular adjustments occur that allow for this increase in cutaneous flow and volume? During work in cool or thermoneutral conditions, the needed increase in cardiac output is well supported by increasing heart rate (HR), since further increases in stroke volume (SV) are minimal beyond exercise intensities of 40% of maximum. In the heat, HR is higher at any given work intensity as compensation for the reduced central blood volume (CBV) and SV. At higher levels of work, maximal heart rate is reached, and this tachycardia is therefore incapable of sustaining the necessary cardiac output. The second way in which the body supplies a high SkBF is by distributing blood flow away from such areas as the liver, kidneys and intestines (Rowell 1983). This redirection of flow can provide an additional 800 to 1,000 ml of blood flow to the skin, and helps offset the detrimental effects of peripheral pooling of blood.
Sweating
Thermoregulatory sweat in humans is secreted from 2 to 4 million eccrine sweat glands scattered non-uniformly over the body surface. Unlike apocrine sweat glands, which tend to be clustered (on the face and hands and in the axial and genital regions) and which secrete sweat into hair follicles, eccrine glands secrete sweat directly onto the skin surface. This sweat is odourless, colourless and relatively dilute, since it is an ultrafiltrate of plasma. Thus it has a high latent heat of vaporization and is ideally suited for its cooling purpose.
As an example of the effectiveness of this cooling system, a man working at an oxygen cost of 2.3 l/min produces a net metabolic heat (M–W) of about 640 W. Without sweating, body temperature would increase at a rate of about 1°C every 6 to 7 min. With efficient evaporation of about 16 g of sweat per minute (a reasonable rate), the rate of heat loss can match the rate of heat production, and body core temperature can be maintained at a steady state; that is,
M–W±R±C–E = 0
Eccrine glands are simple in structure, consisting of a coiled secretory portion, a duct and a skin pore. The volume of sweat produced by each gland is dependent upon both the structure and the function of the gland, and total sweating rate in turn depends on both the recruitment of glands (active sweat gland density) and sweat gland output. The fact that some people sweat more heavily than others is attributable mainly to differences in sweat gland size (Sato and Sato 1983). Heat acclimation is another major determinant of sweat production. With ageing, lower sweating rates are attributable not to fewer activated eccrine glands, but to a decreased sweat output per gland (Kenney and Fowler 1988). This decline probably relates to a combination of structural and functional alterations which accompany the ageing process.
Like vasomotor signals, nerve impulses to the sweat glands originate in the POAH and descend through the brainstem. The fibres which innervate the glands are sympathetic cholinergic fibres, a rare combination in the human body. While acetylcholine is the primary neurotransmitter, adrenergic transmitters (catecholamines) also stimulate eccrine glands.
In many ways, control of sweating is analogous to control of skin blood flow. Both have similar onset characteristics (threshold) and linear relationships to increasing Tc. The back and chest tend to have earlier onsets of sweating, and the slopes for the relationship of local sweat rate to Tc are steepest for these sites. Like SkBF, sweating is modified by non-thermal factors such as hypohydration and hyperosmolality. Also worth noting is a phenomenon called “hidromeiosis”, which occurs in very humid environments or on skin areas constantly covered with wet clothing. Such areas of skin, due to their continuously wet state, decrease sweat output. This serves as a protective mechanism against continued dehydration, since sweat which stays on the skin rather than evaporating serves no cooling function.
If sweating rate is adequate, evaporative cooling is determined ultimately by the water vapour pressure gradient between the wet skin and the air surrounding it. Thus, high humidity and heavy or impermeable clothing limit evaporative cooling, while dry air, air movement about the body and minimal, porous clothing facilitate evaporation. On the other hand, if work is heavy and sweating profuse, evaporative cooling can likewise be limited by the body’s ability to produce sweat (maximally about 1 to 2 l/h).
Temperature Regulation in the Cold
One important difference in the way humans respond to cold compared to heat is that behaviour plays a much greater role in thermoregulatory response to cold. For example, wearing appropriate clothing and assuming postures which minimize surface area available for heat loss (“huddling”) are far more important in cold ambient conditions than in the heat. A second difference is the greater role played by hormones during cold stress, including the increased secretion of catecholamines (norepinephrine and epinephrine) and thyroid hormones.
Skin vasoconstriction
An effective strategy against heat loss from the body through radiation and convection is to increase the effective insulation provided by the shell. In humans this is accomplished by decreasing blood flow to the skin—that is, by skin vasoconstriction. Constriction of the cutaneous vessels is more pronounced in the extremities than on the trunk. Like active vasodilatation, skin vasoconstriction is also controlled by the sympathetic nervous system, and is influenced by Tc, Tsk and local temperatures.
The effect of skin cooling on the heart rate and blood pressure response varies with the area of the body which is cooled, and whether the cold is severe enough to cause pain. For example, when the hands are immersed in cold water, HR, systolic blood pressure (SBP) and diastolic blood pressure (DBP) all increase. When the face is cooled, SBP and DBP increase due to the generalized sympathetic response; however, HR goes down due to a parasympathetic reflex (LeBlanc 1975). To further confound the complexity of the overall response to cold, there is a wide range of variability in responses from one person to another. If the cold stress is of sufficient magnitude to decrease body core temperature, HR may either increase (due to sympathetic activation) or decrease (due to the increased central blood volume).
A specific case of interest is termed cold-induced vasodilatation (CIVD). When the hands are placed in cold water, SkBF initially decreases to conserve heat. As tissue temperatures drop, SkBF paradoxically increases, decreases again, and repeats this cyclical pattern. It has been suggested that CIVD is beneficial in preventing tissue damage from freezing, but this is unproven. Mechanistically, the transient dilation probably occurs when the direct effects of the cold are severe enough to decrease nerve transmission, which transiently overrides the effect of the cold on the blood vessel sympathetic receptors (mediating the constrictor effect).
Shivering
As body cooling progresses, the second line of defence is shivering. Shivering is the random involuntary contraction of superficial muscle fibres, which does not limit heat loss but rather increases heat production. Since such contractions do not produce any work, heat is generated. A resting person can increase his or her metabolic heat production about three- to fourfold during intense shivering, and can increase Tc by 0.5ºC. The signals to initiate shivering arise principally from the skin, and, in addition to the POAH region of the brain, the posterior hypothalamus is also involved to a large extent.
Although many individual factors contribute to shivering (and cold tolerance in general), one important factor is body fatness. A man with very little subcutaneous fat (2 to 3 mm thickness) starts shivering after 40 min at 15ºC and 20 min at 10ºC, while a man who has more insulating fat (11 mm) may not shiver at all at 15ºC and after 60 min at 10ºC (LeBlanc 1975).
Effect of Heat Stress and Work in the Heat
When a person is exposed to warm environmental conditions the physiological heat loss mechanisms are activated in order to maintain normal body temperature. Heat fluxes between the body and the environment depend on the temperature difference between:
- the surrounding air and objects like walls, windows, the sky, and so on
- the surface temperature of the person
The surface temperature of the person is regulated by physiological mechanisms, such as variations in the blood flow to the skin, and by evaporation of sweat secreted by the sweat glands. Also, the person can change clothing to vary the heat exchange with the environment. The warmer the environmental conditions, the smaller the difference between surrounding temperatures and skin or clothing surface temperature. This means that the “dry heat exchange” by convection and radiation is reduced in warm compared to cool conditions. At environmental temperatures above the surface temperature, heat is gained from the surroundings. In this case this extra heat together with that liberated by the metabolic processes must be lost through evaporation of sweat for the maintenance of body temperature. Thus evaporation of sweat becomes more and more critical with increasing environmental temperature. Given the importance of sweat evaporation it is not surprising that wind velocity and air humidity (water vapour pressure) are critical environmental factors in hot conditions. If the humidity is high, sweat is still produced but evaporation is reduced. Sweat which cannot evaporate has no cooling effect; it drips off and is wasted from a thermoregulatory point of view.
The human body contains approximately 60% water, about 35 to 40 l in an adult person. About one-third of the water in the body, the extracellular fluid, is distributed between the cells and in the vascular system (the blood plasma). The remaining two-thirds of the body water, the intracellular fluid, is located inside the cells. The composition and the volume of the body water compartments is very precisely controlled by hormonal and neural mechanisms. Sweat is secreted from the millions of sweat glands on the skin surface when the thermoregulatory centre is activated by an increase in body temperature. The sweat contains salt (NaCl, sodium chloride) but to a lesser extent than the extracellular fluid. Thus, both water and salt are lost and must be replaced after sweating.
Effects of Sweat Loss
In neutral, comfortable, environmental conditions, small amounts of water are lost by diffusion through the skin. However, during hard work and in hot conditions, large quantities of sweat can be produced by active sweat glands, up to more than 2 l/h for several hours. Even a sweat loss of only 1% of body weight (» 600 to 700 ml) has a measurable effect on the ability to perform work. This is seen by a rise in heart rate (HR) (HR increases about five beats per minute for each per cent loss of body water) and a rise in body core temperature. If work is continued there is a gradual increase in body temperature, which can rise to a value around 40ºC; at this temperature, heat illness may result. This is partly due to the loss of fluid from the vascular system (figure 1). A loss of water from the blood plasma reduces the amount of blood which fills the central veins and the heart. Each heart beat will therefore pump a smaller stroke volume. As a consequence the cardiac output (the amount of blood which is expelled by the heart per minute) tends to fall, and the heart rate must increase in order to maintain the circulation and the blood pressure.
Figure 1. Calculated distributions of water in the extracellular compartment (ECW) and intracellular compartment (ICW) before and after 2 h of exercise dehydration at 30°C room temperature.
A physiological control system called the baroreceptor reflex system maintains the cardiac output and blood pressure close to normal under all conditions. The reflexes involve receptors, sensors in the heart and in the arterial system (aorta and carotid arteries), which monitor the degree of stretching of the heart and vessels by the blood which fills them. Impulses from these travel through nerves to the central nervous system, from which adjustments, in case of dehydration, cause a constriction in the blood vessels and a reduction in blood flow to splanchnic organs (liver, gut, kidneys) and to the skin. In this way the available blood flow is redistributed to favour circulation to the working muscles and to the brain (Rowell 1986).
Severe dehydration may lead to heat exhaustion and circulatory collapse; in this case the person cannot maintain the blood pressure, and fainting is the consequence. In heat exhaustion, symptoms are physical exhaustion, often together with headache, dizziness and nausea. The main cause of heat exhaustion is the circulatory strain induced by water loss from the vascular system. The decline in blood volume leads to reflexes which reduce circulation to the intestines and the skin. The reduction in skin blood flow aggravates the situation, since heat loss from the surface decreases, so the core temperature increases further. The subject may faint due to a fall in blood pressure and the resulting low blood flow to the brain. The lying position improves the blood supply to the heart and brain, and after cooling and having some water to drink the person regains his or her well-being almost immediately.
If the processes causing the heat exhaustion “run wild”, it develops into heat stroke. The gradual reduction in skin circulation makes the temperature rise more and more, and this leads to a reduction, even a stop in sweating and an even faster rise in core temperature, which causes circulatory collapse and may result in death, or irreversible damage to the brain. Changes in the blood (such as high osmolality, low pH, hypoxia, cell adherence of the red blood cells, intravascular coagulation) and damage to the nervous system are findings in heat stroke patients. The reduced blood supply to the gut during heat stress can provoke tissue damage, and substances (endotoxins) may be liberated which induce fever in connection with heat stroke (Hales and Richards 1987). Heat stroke is an acute, life-threatening emergency further discussed in the section on “heat disorders”.
Together with water loss, sweating produces a loss of electrolytes, mainly sodium (Na+) and chloride (Cl–), but also to a lesser degree magnesium (Mg++), potassium (K+) and so on (see table 1). The sweat contains less salt than the body fluid compartments. This means that they become more salty after sweat loss. The increased saltiness seems to have a specific effect on the circulation via effects on vascular smooth muscle, which controls the degree to which the vessels are open. However, it is shown by several investigators to interfere with the ability to sweat, in such a way that it takes a higher body temperature to stimulate the sweat glands—the sensitivity of the sweat glands becomes reduced (Nielsen 1984). If the sweat loss is replaced only by water, this may lead to a situation where the body contains less sodium chloride than in the normal state (hypo-osmotic). This will cause cramps due to the malfunction of nerves and muscles, a condition known in earlier days as “miner’s cramps” or “stoker’s cramps”. It can be prevented by addition of salt to the diet (drinking beer was a suggested preventive measure in the UK in the 1920s!).
Table 1. Electrolyte concentration in blood plasma and in sweat
|
Electrolytes and other |
Blood plasma concen- |
Sweat concentrations |
|
Sodium (Na+) |
3.5 |
0.2–1.5 |
|
Potassium (K+) |
0.15 |
0.15 |
|
Calcium (Ca++) |
0.1 |
small amounts |
|
Magnesium (Mg++) |
0.02 |
small amounts |
|
Chloride (Cl–) |
3.5 |
0.2–1.5 |
|
Bicarbonate (HCO3–) |
1.5 |
small amounts |
|
Proteins |
70 |
0 |
|
Fats, glucose, small ions |
15–20 |
small amounts |
Adapted from Vellar 1969.
The decreased skin circulation and sweat gland activity both affect thermoregulation and heat loss in such a way that core temperature will increase more than in the fully hydrated state.
In many different trades, workers are exposed to external heat stress—for example, workers in steel plants, glass industries, paper mills, bakeries, mining industries. Also chimney sweeps and firefighters are exposed to external heat. People who work in confined spaces in vehicles, ships and aircraft may also suffer from heat. However, it must be noted that persons working in protective suits or doing hard work in waterproof clothes can be victims of heat exhaustion even in moderate and cool environmental temperature conditions. Adverse effects of heat stress occur in conditions where the core temperature is elevated and the sweat loss is high.
Rehydration
The effects of dehydration due to sweat loss may be reversed by drinking enough to replace the sweat. This will usually take place during recovery after work and exercise. However, during prolonged work in hot environments, performance is improved by drinking during activity. The common advice is thus to drink when thirsty.
But, there are some very important problems in this. One is that the urge to drink is not strong enough to replace the simultaneously occurring water loss; and secondly, the time needed to replace a large water deficit is very long, more than 12 hours. Lastly, there is a limit to the rate at which water can pass from the stomach (where it is stored) to the intestine (gut), where the absorption takes place. This rate is lower than observed sweat rates during exercise in hot conditions.
There have been a large number of studies on various beverages to restore body water, electrolytes and carbohydrate stores of athletes during prolonged exercise. The main findings are as follows:
- The amount of the fluid which can be utilized—that is, transported through the stomach to the intestine—is limited by the “gastric emptying rate”, which has a maximum of about 1,000 ml/h.
- If the fluid is “hyperosmotic” (contains ions/molecules in higher concentrations than the blood) the rate is slowed down. On the other hand “iso-osmotic fluids” (containing water and ions/molecules to the same concentration, osmolality, as blood) are passed at the same rate as pure water.
- Addition of small amounts of salt and sugar increases the rate of uptake of water from the gut (Maughan 1991).
With this in mind you can make your own “rehydration fluid” or choose from a large number of commercial products. Normally water and electrolyte balance is regained by drinking in connection with meals. Workers or athletes with large sweat losses should be encouraged to drink more than their urge. Sweat contains about 1 to 3 g of NaCl per litre. This means that sweat losses of above 5 l per day may cause a deficiency in sodium chloride, unless the diet is supplemented.
Workers and athletes are also counselled to control their water balance by weighing themselves regularly—for example, in the morning (at same time and condition)—and try to maintain a constant weight. However, a change in body weight does not necessarily reflect the degree of hypohydration. Water is chemically bound to glycogen, the carbohydrate store in the muscles, and liberated when glycogen is used during exercise. Weight changes of up to about 1 kg may occur, depending on the glycogen content of the body. The body weight “morning to morning” also shows changes due to “biological variations” in water contents—for example, in women in relation to the menstrual cycle up to 1 to 2 kg of water can be retained during the premenstrual phase (“premenstrual tension”).
The control of water and electrolytes
The volume of the body water compartments—that is, the extracellular and intracellular fluid volumes—and their concentrations of electrolytes is held very constant through a regulated balance between intake and loss of fluid and substances.
Water is gained from the intake of food and fluid, and some is liberated by metabolic processes, including combustion of fat and carbohydrates from food. The loss of water takes place from the lungs during breathing, where the inspired air takes up water in the lungs from moist surfaces in the airways before it is exhaled. Water also diffuses through the skin in small amount in comfortable conditions during rest. However, during sweating water can be lost at rates of more than 1 to 2 l/h for several hours. The body water content is controlled. Increased water loss by sweating is compensated for by drinking and by a reduction in urine formation, while excess water is excreted by increased urine production.
This control both of intake and output of water is exerted through the autonomic nervous system, and by hormones. Thirst will increase the water intake, and the water loss by the kidneys is regulated; both the volume and electrolyte composition of urine are under control. The sensors in the control mechanism are in the heart, responding to the “fullness” of the vascular system. If the filling of the heart is reduced—for example, after a sweat loss—the receptors will signal this message to the brain centres responsible for the sensation of thirst, and to areas which induce a liberation of anti-diuretic hormone (ADH) from the posterior pituitary. This hormone acts to reduce the urine volume.
Similarly, physiological mechanisms control the electrolyte composition of the body fluids via processes in the kidneys. The food contains nutrients, minerals, vitamins and electrolytes. In the present context, the intake of sodium chloride is the important issue. The dietary sodium intake varies with eating habits, between 10 and 20 to 30 g per day. This is normally much more than is needed, so the excess is excreted by the kidneys, controlled by the action of multiple hormonal mechanisms (angiotensin, aldosterone, ANF, etc.) which are controlled by stimuli from osmoreceptors in the brain and in the kidneys, responding to the osmolality of primarily Na+ and Cl– in the blood and in the fluid in the kidneys, respectively.
Interindividual and Ethnic Differences
Differences between male and female as well as younger and older persons in reaction to heat might be expected. They differ in certain characteristics which might influence heat transfer, such as surface area, height/weight ratio, thickness of insulating skin fat layers, and in physical ability to produce work and heat (aerobic capacity » maximal oxygen consumption rate). Available data suggest that heat tolerance is reduced in older persons. They start to sweat later than do young individuals, and older people react with a higher blood flow in their skin during heat exposure.
Comparing the sexes it has been observed that women tolerate humid heat better than men do. In this environment the evaporation of sweat is reduced, so the slightly greater surface/mass area in women could be to their advantage. However, aerobic capacity is an important factor to be considered when comparing individuals exposed to heat. In laboratory conditions the physiological responses to heat are similar, if groups of subjects with the same physical work capacity (“maximal oxygen uptake”—VO2 max) are tested—for instance, younger and older males, or males versus females (Pandolf et al. 1988). In this case a certain work task (exercise on a bicycle ergometer) will result in the same load on the circulatory system—that is, the same heart rate and the same rise in core temperature—independent of age and sex.
The same considerations are valid for comparison between ethnic groups. When differences in size and aerobic capacity are taken into account, no significant differences due to race can be pointed out. But in daily life in general, older persons do have, on average, a lower VO2 max than younger persons, and females a lower VO2 max than males in the same age group.
Therefore, when performing a specific task which consists of a certain absolute work rate (measured, e.g., in Watts), the person with a lower aerobic capacity will have a higher heart rate and body temperature and be less able to cope with the extra strain of external heat, than one with a higher VO2 max.
For occupational health and safety purposes a number of heat stress indices have been developed. In these the large interindividual variation in response to heat and work are taken into account, as well as the specific hot environments for which the index is constructed. These are treated elsewhere in this chapter.
Persons exposed repeatedly to heat will tolerate the heat better after even a few days. They become acclimatized. Sweating rate is increased and the resulting increased cooling of the skin leads to a lower core temperature and heart rate during work under the same conditions.
Therefore, artificial acclimation of personnel who are expected to be exposed to extreme heat (firefighters, rescue personnel, military personnel) will probably be of benefit to reduce the strain.
Summing up, the more heat a person produces, the more must be dissipated. In a hot environment the evaporation of sweat is the limiting factor for heat loss. Interindividual differences in the capacity for sweating are considerable. While some persons have no sweat glands at all, in most cases, with physical training and repeated exposure to heat, the amount of sweat produced in a standard heat stress test is increased. Heat stress results in an increase in heart rate and core temperature. Maximal heart rate and/or a core temperature of about 40ºC sets the absolute physiological limit for work performance in a hot environment (Nielsen 1994).
Heat Disorders
High environmental temperature, high humidity, strenuous exercise or impaired heat dissipation may cause a variety of heat disorders. They include heat syncope, heat oedema, heat cramps, heat exhaustion and heat stroke as systemic disorders, and skin lesions as local disorders.
Systemic Disorders
Heat cramps, heat exhaustion and heat stroke are of clinical importance. The mechanisms underlying the development of these systemic disorders are circulatory insufficiency, water and electrolyte imbalance and/or hyperthermia (high body temperature). The most severe of all is heat stroke, which may lead to death unless promptly and properly treated.
Two distinct populations are at risk of developing heat disorders, excluding infants. The first and the larger population is the elderly, especially the poor and those with chronic conditions, such as diabetes mellitus, obesity, malnutrition, congestive heart failure, chronic alcoholism, dementia and the need to use medications that interfere with thermoregulation. The second population at risk of suffering heat disorders comprises healthy individuals who attempt prolonged physical exertion or are exposed to excessive heat stress. Factors predisposing active young people to heat disorders, other than congenital and acquired sweat gland dysfunction, include poor physical fitness, lack of acclimatization, low work efficiency and a reduced ratio of skin area to body mass.
Heat syncope
Syncope is a transient loss of consciousness resulting from a reduction of cerebral blood flow, preceded frequently by pallor, blurring of vision, dizziness and nausea. It may occur in persons suffering from heat stress. The term heat collapse has been used synonymously with heat syncope. The symptoms have been attributed to cutaneous vasodilatation, postural pooling of blood with consequently diminished venous return to the heart, and reduced cardiac output. Mild dehydration, which develops in most persons exposed to heat, contributes to the probability of heat syncope. Individuals who suffer from cardiovascular diseases or who are unacclimatized are predisposed to heat collapse. The victims usually recover consciousness rapidly after they are laid supine.
Heat oedema
Mild dependent oedema—that is, swelling of the hands and feet—may develop in unacclimatized individuals exposed to a hot environment. It typically occurs in women and resolves with acclimatization. It subsides in several hours after the patient has been laid in a cooler place.
Heat cramps
Heat cramps may occur after heavy sweating brought about by prolonged physical work. Painful spasms develop in limb and abdominal muscles subjected to intensive work and fatigue, while body temperature hardly rises. These cramps are caused by the salt depletion that results when the loss of water due to prolonged heavy sweating is replenished with plain water containing no supplementary salt and when the sodium concentration in the blood has fallen below a critical level. Heat cramps themselves are a relatively innocuous condition. The attacks are usually seen in physically fit individuals who are capable of sustained physical exertion, and once were called “miner’s cramps” or “cane-cutter’s cramps” because they would often occur in such labourers.
The treatment of heat cramps consists of cessation of activity, rest in a cool place and replacement of fluid and electrolytes. Heat exposure should be avoided for at least 24 to 48 hours.
Heat exhaustion
Heat exhaustion is the most common heat disorder encountered clinically. It results from severe dehydration after a huge amount of sweat has been lost. It occurs typically in otherwise healthy young individuals who undertake prolonged physical exertion (exertion-induced heat exhaustion), such as marathon runners, outdoor sports players, military recruits, coal miners and construction workers. The basic feature of this disorder is circulatory deficiency due to water and/or salt depletion. It may be considered an incipient stage of heat stroke, and if left untreated, it may eventually progress to heat stroke. It has been conventionally divided into two types: heat exhaustion by water depletion and that by salt depletion; but many cases are a mixture of both types.
Heat exhaustion by water depletion develops as a result of prolonged heavy sweating and insufficient water intake. Since sweat contains sodium ions in a concentration ranging from 30 to 100 milliequivalents per litre, which is lower than that in plasma, a great loss of sweat brings about hypohydration (reduction in body water content) and hypernatraemia (increased sodium concentration in plasma). Heat exhaustion is characterized by thirst, weakness, fatigue, dizziness, anxiety, oliguria (scanty urination), tachycardia (rapid heartbeat) and moderate hyperthermia (39ºC or above). Dehydration also leads to a decline in sweating activity, a rise in skin temperature, and increases in plasma protein and plasma sodium levels and in the haematocrit value (the ratio of blood cell volume to blood volume).
Treatment consists of allowing the victim to rest in a recumbent posture with the knees raised, in a cool environment, wiping the body with a cool towel or sponge and replacing fluid loss by drinking or, if oral ingestion is impossible, by intravenous infusion. The amounts of water and salt replenishment, body temperature and body weight should be monitored carefully. Water ingestion should not be regulated according to the victim’s subjective feeling of thirst, especially when fluid loss is replenished with plain water, because dilution of the blood readily induces disappearance of thirst and dilution diuresis, thus delaying the recovery of body fluid balance. This phenomenon of insufficient water ingestion is called voluntary dehydration. Furthermore, a salt-free water supply may complicate heat disorders, as described below. Dehydration of over 3% of body weight should always be treated by water and electrolyte replacement.
Heat exhaustion by salt depletion results from prolonged heavy sweating and replacement of water and insufficient salt. Its occurrence is promoted by incomplete acclimatization, vomiting and diarrhoea, and so on. This type of heat exhaustion usually develops a few days after the development of water depletion. It is most commonly encountered in sedentary elderly individuals exposed to heat who have drunk a large amount of water in order to quench their thirst. Headache, dizziness, weakness, fatigue, nausea, vomiting, diarrhoea, anorexia, muscle spasms and mental confusion are common symptoms. In blood examinations, decrease in plasma volume, increases in the haematocrit and in plasma protein levels, and hypercalcaemia (excess blood calcium) are noted.
Early detection and prompt management are essential, the latter consisting of letting the patient rest in a recumbent posture in a cool room and providing for replacement of water and electrolytes. The osmolarity or specific gravity of the urine should be monitored, as should urea, sodium and chloride levels in the plasma, and body temperature, body weight, and water and salt intake should also be recorded. If the condition is adequately treated, victims generally feel well within a few hours and recover without sequelae. If not, it may readily proceed to heat stroke.
Heat stroke
Heat stroke is a serious medical emergency which may result in death. It is a complex clinical condition in which uncontrollable hyperthermia causes tissue damage. Such an elevation of body temperature is caused initially by severe heat congestion due to excessive heat load, and the resultant hyperthermia induces dysfunction of the central nervous system, including failure of the normal thermoregulatory mechanism, thus accelerating elevation of the body temperature. Heat stroke occurs basically in two forms: classical heat stroke and exertion-induced heat stroke. The former develops in very young, elderly, obese or unfit individuals undertaking normal activities during prolonged exposure to high environmental temperatures, whereas the latter occurs particularly in young, active adults during physical exertion. In addition, there is a mixed form of heat stoke presenting features consistent with both of the above forms.
Elderly individuals, particularly those who have underlying chronic illness, such as cardiovascular diseases, diabetes mellitus and alcoholism, and those taking certain medications, especially psychotropic drugs, are at a high risk of classical heat stroke. During sustained heat waves, for example, the mortality rate for the population older than 60 years has been recorded as more than ten times greater than that for the population aged 60 and under. A similarly high mortality in the elderly population has also been reported among Muslims during the Mecca pilgrimage, where the mixed form of heat stroke has been found to be prevalent. Factors predisposing the elderly to heat stroke, other than chronic diseases as mentioned above, include reduced thermal perception, sluggish vasomotor and sudomotor (sweating reflex) responses to changes in thermal load, and reduced capacity for acclimatization to heat.
Individuals who work or exercise vigorously in hot, humid environments are at a high risk of exertion-induced heat illness, whether heat exhaustion or heat stroke. Athletes undergoing high physical stress can fall victim to hyperthermia by producing metabolic heat at a high rate, even when the environment is not very hot, and have often suffered heat stress illness as a result. Relatively unfit non-athletes are at a lesser risk in this regard as long as they realize their own capacity and limit their exertions accordingly. However, when they play sports for fun and are highly motivated and enthusiastic, they often try to exert themselves at an intensity beyond that for which they have been trained, and may succumb to heat illness (usually heat exhaustion). Poor acclimatization, inadequate hydration, unsuitable dress, alcohol consumption and skin illness causing anhidrosis (reduction in or lack of sweating), notably prickly heat (see below), all aggravate the symptoms.
Children are more susceptible to heat exhaustion or heat stroke than adults. They produce more metabolic heat per unit mass, and are less able to dissipate heat because of a relatively low capacity to produce sweat.
Clinical features of heat stroke
Heat stroke is defined by three criteria:
- severe hyperthermia with a core (deep body) temperature usually exceeding 42ºC
- disturbances of the central nervous system
- hot, dry skin with cessation of sweating.
The diagnosis of heat stroke is easy to establish when this triad of criteria is met. However, it may be missed when one of those criteria is absent, obscure or overlooked. For example, unless core temperature is measured properly and without delay, severe hyperthermia may not be recognized; or, in a very early stage of exertion-induced heat stroke, sweating may still persist or may even be profuse and the skin may be wet.
The onset of heat stroke is usually abrupt and without precursory symptoms, but some patients with impending heat stroke may have symptoms and signs of disturbances of the central nervous system. They include headache, nausea, dizziness, weakness, drowsiness, confusion, anxiety, disorientation, apathy, aggressiveness and irrational behaviour, tremor, twitching and convulsion. Once heat stroke occurs, disturbances of the central nervous system are present in all cases. The level of consciousness is often depressed, deep coma being most common. Seizures occur in the majority of cases, especially in physically fit individuals. Signs of cerebellar dysfunction are prominent and may persist. Pin-pointed pupils are frequently seen. Cerebellar ataxia (lack of muscular coordination), hemiplegia (paralysis of one side of the body), aphasia and emotional instability may persist in some of survivors.
Vomiting and diarrhoea often occur. Tachypnoea (rapid breathing) is usually present initially and the pulse may be weak and rapid. Hypotension, one of the most common complications, results from marked dehydration, extensive peripheral vasodilatation and eventual depression of cardiac muscle. Acute renal failure may be seen in severe cases, especially in exertion-induced heat stroke.
Haemorrhages occur in all parenchymal organs, in the skin (where they are called petechiae) and in the gastro-intestinal tract in severe cases. Clinical haemorrhagic manifestations include melaena (dark-coloured, tarry faeces), haematemesis (blood vomiting), haematuria (bloody urine), haemoptysis (spitting blood), epistaxis (nosebleed), purpura (purple spots), ecchymosis (black and blue marks) and conjunctival haemorrhage. Intravascular coagulation occurs commonly. Haemorrhagic diathesis (bleeding tendency) is usually associated with disseminated intra-vascular coagulation (DIC). DIC occurs predominantly in exertion-induced heat stroke, where the fibrinolytic (clot-dissolving) activity of plasma is increased. On the other hand, a decrease in platelet count, prolongation of prothrombin time, depletion of coagulation factors and increased level of fibrin degradation products (FDP) are provoked by whole-body hyperthermia. Patients with evidence of DIC and bleeding have higher core temperature, lower blood pressure, lower arterial blood pH and pO2, a higher incidence of oliguria or anuria and of shock, and a higher mortality rate.
Shock is also a common complication. It is attributable to peripheral circulatory failure and is aggravated by DIC, which causes dissemination of clots in the microcirculatory system.
Treatment of heat stroke
Heat stroke is a medical emergency that requires prompt diagnosis and rapid and aggressive treatment to save the patient’s life. Proper measurement of core temperature is mandatory: rectal or oesophageal temperature should be measured by using a thermo-meter which can read up to 45ºC. Measurement of oral and axillary temperatures should be avoided because they can vary significantly from real core temperature.
The objective of treatment measures is to lower body temperature by reducing heat load and promoting heat dissipation from the skin. The treatment includes moving the patient to a safe, cool, shady and well-ventilated place, removing unnecessary clothing, and fanning. Cooling the face and head may promote beneficial brain cooling.
The efficiency of some cooling techniques has been questioned. It has been argued that placing cold packs over major blood vessels in the neck, groin and axillae and immersion of the body in cold water or covering it with iced towels may promote shivering and cutaneous vasoconstriction, thus actually impeding cooling efficiency. Traditionally, immersion in an ice-water bath, combined with vigorous skin massage to minimize cutaneous vasoconstriction, has been recommended as the treatment of choice, once the patient is brought to a medical facility. This method of cooling has several disadvantages: there are the nursing difficulties posed by the need to administer oxygen and fluids and to monitor blood pressure and the electrocardiogram continuously, and there are the hygienic problems of contamination of the bath with the vomitus and diarrhoea of comatose patients. An alternative approach is to spray a cool mist over the patient’s body while fanning to promote evaporation from the skin. This method of cooling can reduce the core temperature by 0.03 to 0.06ºC/min.
Measures to prevent convulsions, seizures and shivering should also be initiated at once. Continuous cardiac monitoring and determination of serum electrolyte levels and arterial and venous blood-gas analysis are essential, and intravenous infusion of electrolyte solutions at a relatively low temperature of approximately 10ºC, together with controlled oxygen therapy, should be commenced in a timely fashion. Tracheal intubation to protect the airway, insertion of a cardiac catheter to estimate central venous pressure, placement of a gastric tube and insertion of a urinary catheter may also be included among additional recommended measures.
Prevention of heat stroke
For the prevention of heat stroke, a wide variety of human factors should be taken into account, such as acclimatization, age, build, general health, water and salt intake, clothing, peculiarities of religious devotion and ignorance of, or liability to neglect, regulations intended to promote public health.
Prior to physical exertion in a hot environment, workers, athletes or pilgrims should be informed of the work load and the level of heat stress they may encounter, and of the risks of heat stroke. A period of acclimatization is recommended before vigorous physical activity and/or severe exposure is risked. The level of activity should be matched to the ambient temperature, and physical exertion should be avoided or at least minimized during the hottest hours of the day. During physical exertion, free access to water is mandatory. Since electrolytes are lost in sweat and the opportunity for voluntary ingestion of water may be limited, thus delaying restitution from thermal dehydration, electrolytes should also be replaced in case of profuse sweating. Proper clothing is also an important measure. Clothes made of fabrics which are both water-absorbent and permeable to air and water vapour facilitate heat dissipation.
Skin Disorders
Miliaria is the most common skin disorder associated with heat load. It occurs when the delivery of sweat onto the skin surface is prevented due to obstruction of the sweat ducts. Sweat retention syndrome ensues when anhidrosis (inability to release sweat) is widespread over the body surface and predisposes the patient to heat stroke.
Miliaria is commonly induced by physical exertion in a hot, humid environment; by febrile diseases; by the application of wet compresses, bandages, plaster casts or adhesive plaster; and by wearing poorly permeable clothes. Miliaria can be classified into three types, according to the depth of sweat retention: miliaria crystallina, miliaria rubra and miliaria profunda.
Miliaria crystallina is caused by retention of sweat within or just beneath the horny layer of the skin, where tiny, clear, non-inflammatory blisters can be seen. They typically appear in “crops” after severe sunburn or during a febrile illness. This type of miliaria is otherwise symptomless, the least distressing, and heals spontaneously in a few days, when the blisters break out to leave scales.
Miliaria rubra occurs when intense heat load causes prolonged and profuse sweating. It is the most common type of miliaria, in which sweat accumulates in the epidermis. Red papules, vesicles or pustules are formed, accompanied by burning and itching sensations (prickly heat). The sweat duct is plugged at the terminal portion. The production of the plug is attributable to the action of resident aerobic bacteria, notably cocci, which increase in population greatly in the horny layer when it is hydrated with sweat. They secrete a toxin which injures the horny epithelial cells of the sweat duct and provokes an inflammatory reaction, precipitating a cast within the lumen of the sweat duct. Infiltration by leukocytes creates an impaction which completely obstructs the passage of sweat for several weeks.
In miliaria profunda, sweat is retained in the dermis, and produces flat, inflammatory papules, nodules and abscesses, with less itching than in miliaria rubra. The occurrence of this type of miliaria is commonly confined to the tropics. It may develop in a progressive sequence from miliaria rubra after repeated bouts of profuse sweating, as the inflammatory reaction extends downwards from the upper skin layers.
Tropical anhidrotic asthenia. The term achieved currency during the Second World War, when troops deployed to tropical theatres suffered from heat rash and heat intolerance. It is a modality of sweat retention syndrome encountered in hot, humid tropical environments. It is characterized by anhidrosis and miliaria-like rashes, accompanied by symptoms of heat congestion, such as palpitation, rapid pulsation, hyperthermia, headache, weakness and gradually to rapidly progressing inability to tolerate physical activity in the heat. It is usually preceded by widespread miliaria rubra.
Treatment. The initial and essential treatment of miliaria and sweat retention syndrome is to transfer the affected person to a cool environment. Cool showers and gentle drying of the skin and the application of calamine lotion may attenuate the patient’s distress. Application of chemical bacteriostats is effective in preventing the expansion of microflora, and is preferable to the use of antibiotics, which may lead these micro-organisms to acquire resistance.
The impactions in the sweat duct slough off after about 3 weeks as a result of epidermal renewal.
Prevention of Heat Stress
Although human beings possess considerable ability to compensate for naturally occurring heat stress, many occupational environments and/or physical activities expose workers to heat loads which are so excessive as to threaten their health and productivity. In this article, a variety of techniques are described which can be used to minimize the incidence of heat disorders and reduce the severity of cases when they do occur. Interventions fall into five categories: maximizing heat tolerance among exposed individuals, assuring timely replacement of lost fluid and electrolytes, altering work practices to reduce exertional heat load, engineering control of climatic conditions, and use of protective clothing.
Factors outside the worksite which may affect thermal tolerance should not be ignored in the evaluation of the extent of exposure and consequently in elaborating preventive strategies. For example, total physiological burden and the potential susceptibility to heat disorders will be much higher if heat stress continues during off-duty hours through work at second jobs, strenuous leisure activities, or living in unremittingly hot quarters. In addition, nutritional status and hydration may reflect patterns of eating and drinking, which may also change with season or religious observances.
Maximizing Individual Heat Tolerance
Candidates for hot trades should be generally healthy and possess suitable physical attributes for the work to be done. Obesity and cardiovascular disease are conditions that add to the risks, and individuals with a history of previous unexplained or repetitive heat illness should not be assigned to tasks involving severe heat stress. Various physical and physiological characteristics which may affect heat tolerance are discussed below and fall into two general categories: inherent characteristics beyond the control of the individual, such as body size, gender, ethnicity and age; and acquired characteristics, which are at least partly subject to control and include physical fitness, heat acclimatization, obesity, medical conditions and self-induced stress.
Workers should be informed of the nature of heat stress and its adverse effects as well as the protective measures provided in the workplace. They should be taught that heat tolerance depends to a large extent upon drinking enough water and eating a balanced diet. In addition, workers should be taught the signs and symptoms of heat disorders, which include dizziness, faintness, breathlessness, palpitations and extreme thirst. They should also learn the basics of first aid and where to call for help when they recognize these signs in themselves or others.
Management should implement a system for reporting heat- related incidents at work. Occurrence of heat disorders in more than one person—or repeatedly in a single individual—is often a warning of serious impending trouble and indicates the need for immediate evaluation of the working environment and review of the adequacy of preventive measures.
Human traits affecting adaptation
Body dimensions. Children and very small adults face two potential disadvantages for work in hot environments. First, externally imposed work represents a greater relative load for a body with a small muscle mass, inducing a greater rise in core body temperature and more rapid onset of fatigue. In addition, the higher surface-to-mass ratio of small people may be a disadvantage under extremely hot conditions. These factors together may explain why men weighing less than 50 kg were found to be at increased risk for heat illness in deep mining activities.
Gender. Early laboratory studies on women seemed to show that they were relatively intolerant to work in heat, compared with men. However, we now recognize that nearly all of the differences can be explained in terms of body size and acquired levels of physical fitness and heat acclimatization. However, there are minor sex differences in heat dissipation mechanisms: higher maximal sweat rates in males may enhance tolerance for extremely hot, dry environments, while females are better able to suppress excess sweating and therefore conserve body water and thus heat in hot, humid environments. Although the menstrual cycle is associated with a shift in basal body temperature and slightly alters thermoregulatory responses in women, these physiological adjustments are too subtle to influence heat tolerance and thermoregulatory efficiency in real work situations.
When allowance is made for individual physique and fitness, men and women are essentially alike in their responses to heat stress and their ability to acclimatize to work under hot conditions. For this reason, selection of workers for hot jobs should be based on individual health and physical capacity, not gender. Very small or sedentary individuals of either sex will show poor tolerance for work in heat.
The effect of pregnancy on women’s heat tolerance is not clear, but altered hormone levels and the increased circulatory demands of the foetus on the mother may increase her susceptibility to fainting. Severe maternal hyperthermia (over-heating) due to illness appears to increase the incidence of foetal malformation, but there is no evidence of a similar effect from occupational heat stress.
Ethnicity. Although various ethnic groups have originated in differing climates, there is little evidence of inherent or genetic differences in response to heat stress. All humans appear to function as tropical animals; their ability to live and work in a range of thermal conditions reflects adaptation through complex behaviour and development of technology. Seeming ethnic differences in response to heat stress probably relate to body size, individual life history and nutritional status rather than to inherent traits.
Age. Industrial populations generally show a gradual decline in heat tolerance after age 50. There is some evidence of an obligatory, age-associated reduction in cutaneous vasodilatation (widening of the cavity of blood vessels of the skin) and maximal sweat rate, but most of the change can be attributed to alterations in lifestyle which reduce physical activity and increase the accumulation of body fat. Age does not appear to impair heat tolerance or ability to acclimatize if the individual maintains a high level of aerobic conditioning. However, ageing populations are subject to increasing incidence of cardiovascular disease or other pathologies which may impair individual heat tolerance.
Physical fitness. Maximal aerobic capacity (VO2 max) is probably the strongest single determinant of an individual’s ability to carry out sustained physical work under hot conditions. As noted above, early findings of group differences in heat tolerance which were attributed to gender, race or age are now viewed as manifestations of aerobic capacity and heat acclimatization.
Induction and maintenance of high work capacity require repetitive challenges to the body’s oxygen transport system through vigorous exercise for at least 30 to 40 min, 3 to 4 days per week. In some cases activity on the job may provide the necessary physical training, but most industrial jobs are less strenuous and require supplementation through a regular exercise programme for optimal fitness.
Loss of aerobic capacity (detraining) is relatively slow, so that weekends or vacations of 1 to 2 weeks cause only minimal changes. Serious declines in aerobic capacity are more likely to occur over weeks to months when injury, chronic illness or other stress causes the individual to change lifestyle.
Heat acclimatization. Acclimatization to work in heat can greatly expand human tolerance for such stress, so that a task which is initially beyond the capability of the unacclimatized person may become easier work after a period of gradual adjustment. Individuals with a high level of physical fitness generally display partial heat acclimatization and are able to complete the process more quickly and with less stress than sedentary persons. Season may also affect the time which must be allowed for acclimatization; workers recruited in summer may already be partly heat acclimatized, while winter hires will require a longer period of adjustment.
In most situations, acclimatization can be induced through gradual introduction of the worker to the hot task. For instance, the new recruit may be assigned to hot work only in the morning or for gradually increasing time periods during the first few days. Such acclimatization on the job should take place under close supervision by experienced personnel; the new worker should have standing permission to withdraw to cooler conditions any time symptoms of intolerance occur. Extreme conditions may warrant a formal protocol of progressive heat exposure such as that used for workers in the South African gold mines.
Maintenance of full heat acclimatization requires exposure to work in heat three to four times per week; lower frequency or passive exposure to heat have a much weaker effect and may allow gradual decay of heat tolerance. However, weekends off work have no measurable effect on acclimatization. Discontinuing exposure for 2 to 3 weeks will cause loss of most acclimatization, although some will be retained in persons exposed to hot weather and/or regular aerobic exercise.
Obesity. High body fat content has little direct effect on thermoregulation, as heat dissipation at the skin involves capillaries and sweat glands which lie closer to the skin surface than the subcutaneous fat layer of skin. However, obese persons are handicapped by their excess body weight because every movement requires greater muscular effort and therefore generates more heat than in a lean person. In addition, obesity often reflects an inactive lifestyle with resulting lower aerobic capacity and absence of heat acclimatization.
Medical conditions and other stresses. A worker’s heat tolerance on a given day may be impaired by a variety of conditions. Examples include febrile illness (higher than normal body temperature), recent immunization, or gastroenteritis with associated disturbance of fluid and electrolyte balance. Skin conditions such as sunburn and rashes may limit ability to secrete sweat. In addition, susceptibility to heat illness may be increased by prescription medications, including sympathomimetics, anticholinergics, diuretics, phenothiazines, cyclic antidepressants, and monoamine-oxidase inhibitors.
Alcohol is a common and serious problem among those who work in heat. Alcohol not only impairs intake of food and water, but also acts as a diuretic (increase in urination) as well as disturbing judgement. The adverse effects of alcohol extend many hours beyond the time of intake. Alcoholics who suffer heat stroke have a far higher mortality rate than non-alcoholic patients.
Oral Replacement of Water and Electrolytes
Hydration. Evaporation of sweat is the main path for dissipating body heat and becomes the only possible cooling mechanism when air temperature exceeds body temperature. Water requirements cannot be reduced by training, but only by lowering the heat load on the worker. Human water loss and rehydration have been extensively studied in recent years, and more information is now available.
A human weighing 70 kg can sweat at a rate of 1.5 to 2.0 l/h indefinitely, and it is possible for a worker to lose several litres or up to 10% of body weight during a day in an extremely hot environment. Such loss would be incapacitating unless at least part of the water were replaced during the work shift. However, since water absorption from the gut peaks at about 1.5 l/h during work, higher sweat rates will produce cumulative dehydration through the day.
Drinking to satisfy thirst is not enough to keep a person well hydrated. Most people do not become aware of thirst until they have lost 1 to 2 l of body water, and persons highly motivated to perform hard work may incur losses of 3 to 4 l before clamorous thirst forces them to stop and drink. Paradoxically, dehydration reduces the capacity to absorb water from the gut. Therefore, workers in hot trades must be educated regarding the importance of drinking enough water during work and continuing generous rehydration during off-duty hours. They should also be taught the value of “prehydration”—consuming a large drink of water immediately before the start of severe heat stress—as heat and exercise prevent the body from eliminating excess water in the urine.
Management must provide ready access to water or other appropriate drinks which encourage rehydration. Any physical or procedural obstacle to drinking will encourage “voluntary” dehydration which predisposes to heat illness. The following details are a vital part of any programme for hydration maintenance:
- Safe, palatable water must be located within a few steps of each worker or brought to the worker every hour—more frequently under the most stressful conditions.
- Sanitary drinking cups should be provided, as it is nearly impossible to rehydrate from a water fountain.
- Water containers must be shaded or cooled to 15 to 20ºC (iced drinks are not ideal because they tend to inhibit intake).
Flavourings may be used to improve the acceptance of water. However, drinks that are popular because they “cut” thirst are not recommended, since they inhibit intake before rehydration is complete. For this reason it is better to offer water or dilute, flavoured beverages and to avoid carbonation, caffeine and drinks with heavy concentrations of sugar or salt.
Nutrition. Although sweat is hypotonic (lower salt content) compared to blood serum, high sweat rates involve a continuous loss of sodium chloride and small amounts of potassium, which must be replaced on a daily basis. In addition, work in heat accelerates the turnover of trace elements including magnesium and zinc. All of these essential elements should normally be obtained from food, so workers in hot trades should be encouraged to eat well-balanced meals and avoid substituting candy bars or snack foods, which lack important nutritional components. Some diets in industrialized nations include high levels of sodium chloride, and workers on such diets are unlikely to develop salt deficits; but other, more traditional diets may not contain adequate salt. Under some conditions it may be necessary for the employer to provide salty snacks or other supplementary foods during the work shift.
Industrialized nations are seeing increased availability of “sports drinks” or “thirst quenchers” which contain sodium chloride, potassium and carbohydrates. The vital component of any beverage is water, but electrolyte drinks may be useful in persons who have already developed significant dehydration (water loss) combined with electrolyte depletion (salt loss). These drinks are generally high in salt content and should be mixed with equal or greater volumes of water before consumption. A much more economical mixture for oral rehydration can be made according to the following recipe: to one litre of water, suitable for drinking, add 40 g of sugar (sucrose) and 6 g of salt (sodium chloride). Workers should not be given salt tablets, as they are easily abused, and overdoses lead to gastro-intestinal problems, increased urine output and greater susceptibility to heat illness.
Modified Work Practices
The common goal of modification to work practices is to lower time-averaged heat stress exposure and to bring it within acceptable limits. This can be accomplished by reducing the physical workload imposed on an individual worker or by scheduling appropriate breaks for thermal recovery. In practice, maximum time-averaged metabolic heat production is effectively limited to about 350 W (5 kcal/min) because harder work induces physical fatigue and a need for commensurate rest breaks.
Individual effort levels can be lowered by reducing external work such as lifting, and by limiting required locomotion and static muscle tension such as that associated with awkward posture. These goals may be reached by optimizing task design according to ergonomic principles, providing mechanical aids or dividing the physical effort among more workers.
The simplest form of schedule modification is to allow individual self-pacing. Industrial workers performing a familiar task in a mild climate will pace themselves at a rate which produces a rectal temperature of about 38°C; imposition of heat stress causes them to voluntarily slow the work rate or take breaks. This ability to voluntarily adjust work rate probably depends on awareness of cardiovascular stress and fatigue. Human beings cannot consciously detect elevations in core body temperature; rather, they rely on skin temperature and skin wettedness to assess thermal discomfort.
An alternative approach to schedule modification is the adoption of prescribed work-rest cycles, where management specifies the duration of each work bout, the length of rest breaks and the number of repetitions expected. Thermal recovery takes much longer than the period required to lower respiratory rate and work-induced heart rate: Lowering core temperature to resting levels requires 30 to 40 min in a cool, dry environment, and takes longer if the person must rest under hot conditions or while wearing protective clothing. If a constant level of production is required, then alternating teams of workers must be assigned sequentially to hot work followed by recovery, the latter involving either rest or sedentary tasks performed in a cool place.
Climate Control
If cost were no object, all heat stress problems could be solved by application of engineering techniques to convert hostile working environments to hospitable ones. A wide variety of techniques may be used depending on the specific conditions of the workplace and available resources. Traditionally, hot industries can be divided into two categories: In hot-dry processes, such as metal smelting and glass production, workers are exposed to very hot air combined with strong radiant heat load, but such processes add little humidity to the air. In contrast, warm-moist industries such as textile mills, paper production and mining involve less extreme heating but create very high humidities due to wet processes and escaped steam.
The most economical techniques of environmental control usually involve reduction of heat transfer from the source to the environment. Hot air may be vented outside the work area and replaced with fresh air. Hot surfaces can be covered with insulation or given reflective coatings to reduce heat emissions, simultaneously conserving heat which is needed for the industrial process. A second line of defence is large-scale ventilation of the work area to provide a strong flow of outside air. The most expensive option is air conditioning to cool and dry the atmosphere in the workplace. Although lowering air temperature does not affect transmission of radiant heat, it does help to reduce the temperature of the walls and other surfaces which may be secondary sources of convective and radiative heating.
When overall environmental control proves impractical or uneconomical, it may be possible to ameliorate thermal conditions in local work areas. Air conditioned enclosures may be provided within the larger work space, or a specific work station may be provided with a flow of cool air (“spot cooling” or “air shower”). Local or even portable reflective shielding may be interposed between the worker and a radiant heat source. Alternatively, modern engineering techniques may allow construction of remote systems to control hot processes so that workers need not suffer routine exposure to highly stressful heat environments.
Where the workplace is ventilated with outside air or there is limited air-conditioning capacity, thermal conditions will reflect climatic changes, and sudden increases in outdoor air temperature and humidity may elevate heat stress to levels which overwhelm workers’ heat tolerance. For instance, a spring heat wave can precipitate an epidemic of heat illness among workers who are not yet heat acclimatized as they would be in summer. Management should therefore implement a system for predicting weather-related changes in heat stress so that timely precautions can be taken.
Protective Clothing
Work in extreme thermal conditions may require personal thermal protection in the form of specialized clothing. Passive protection is provided by insulative and reflective garments; insulation alone can buffer the skin from thermal transients. Reflective aprons may be used to protect personnel who work facing a limited radiant source. Fire-fighters who must deal with extremely hot fuel fires wear suits called “bunkers”, which combine heavy insulation against hot air with an aluminized surface to reflect radiant heat.
Another form of passive protection is the ice vest, which is loaded with slush or frozen packets of ice (or dry ice) and is worn over an undershirt to prevent uncomfortable chilling of the skin. The phase change of the melting ice absorbs part of the metabolic and environmental heat load from the covered area, but the ice must be replaced at regular intervals; the greater the heat load, the more frequently the ice must be replaced. Ice vests have proven most useful in deep mines, ship engine rooms, and other very hot, humid environments where access to freezers can be arranged.
Active thermal protection is provided by air- or liquid-cooled garments which cover the entire body or some portion of it, usually the torso and sometimes the head.
Air cooling. The simplest systems are ventilated with the surrounding, ambient air or with compressed air cooled by expansion or passage through a vortex device. High volumes of air are required; the minimum ventilation rate for a sealed suit is about 450 l/min. Air cooling can theoretically take place through convection (temperature change) or evaporation of sweat (phase change). However, the effectiveness of convection is limited by the low specific heat of air and the difficulty in delivering it at low temperatures in hot surroundings. Most air-cooled garments therefore operate through evaporative cooling. The worker experiences moderate heat stress and attendant dehydration, but is able to thermoregulate through natural control of the sweat rate. Air cooling also enhances comfort through its tendency to dry the underclothing. Disadvantages include (1) the need to connect the subject to the air source, (2) the bulk of air distribution garments and (3) the difficulty of delivering air to the limbs.
Liquid cooling. These systems circulate a water-antifreeze mixture through a network of channels or small tubes and then return the warmed liquid to a heat sink which removes the heat added during passage over the body. Liquid circulation rates are usually on the order of 1 l/min. The heat sink may dissipate thermal energy to the environment through evaporation, melting, refrigeration or thermoelectric processes. Liquid-cooled garments offer far greater cooling potential than air systems. A full-coverage suit linked to an adequate heat sink can remove all metabolic heat and maintain thermal comfort without the need to sweat; such a system is used by astronauts working outside their spacecraft. However, such a powerful cooling mechanism requires some type of comfort control system which usually involves manual setting of a valve which shunts part of the circulating liquid past the heat sink. Liquid-cooled systems can be configured as a back pack to provide continuous cooling during work.
Any cooling device which adds weight and bulk to the human body, of course, may interfere with the work at hand. For instance, the weight of an ice vest significantly increases the metabolic cost of locomotion, and is therefore most useful for light physical work such as watch-standing in hot compartments. Systems which tether the worker to a heat sink are impractical for many types of work. Intermittent cooling may be useful where workers must wear heavy protective clothing (such as chemical protective suits) and cannot carry a heat sink or be tethered while they work. Removing the suit for each rest break is time consuming and involves possible toxic exposure; under these conditions, it is simpler to have the workers wear a cooling garment which is attached to a heat sink only during rest, allowing thermal recovery under otherwise unacceptable conditions.
The Physical Basis of Work in Heat
Thermal Exchanges
The human body exchanges heat with its environment by various pathways: conduction across the surfaces in contact with it, convection and evaporation with the ambient air, and radiation with the neighbouring surfaces.
Conduction
Conduction is the transmission of heat between two solids in contact. Such exchanges are observed between the skin and clothing, footwear, pressure points (seat, handles), tools and so on. In practice, in the mathematical calculation of thermal balance, this heat flow by conduction is approximated indirectly as a quantity equal to the heat flow by convection and radiation which would take place if these surfaces were not in contact with other materials.
Convection
Convection is the transfer of heat between the skin and the air surrounding it. If the skin temperature, tsk, in units of degrees Celsius (°C), is higher than the air temperature (ta), the air in contact with the skin is heated and consequently rises. Air circulation, known as natural convection, is thus established at the surface of the body. This exchange becomes greater if the ambient air passes over the skin at a certain speed: the convection becomes forced. The heat flow exchanged by convection, C, in units of watts per square metre (W/m2), can be estimated by:
C = hc FclC (tsk - ta)
where hc is the coefficient of convection (W/°C m2), which is a function of the difference between tsk and ta in the case of natural convection, and of the air velocity Va (in m/s) in forced convection; FclC is the factor by which clothing reduces convection heat exchange.
Radiation
Every body emits electromagnetic radiation, the intensity of which is a function of the fourth power of its absolute temperature T (in degrees Kelvin—K). The skin, whose temperature may be between 30 and 35°C (303 and 308K), emits such radiation, which is in the infrared zone. Moreover, it receives the radiation emitted by neighbouring surfaces. The thermal flow exchanged by radiation, R (in W/m2), between the body and its surroundings may be described by the following expression:
![]()
where:
s is the universal constant of radiation (5.67 × 10-8 W/m2 K4)
e is the emissivity of the skin, which, for infrared radiation, is equal to 0.97 and independent of the wavelength, and for solar radiation is about 0.5 for the skin of a White subject and 0.85 for the skin of a Black subject
AR/AD is the fraction of the body surface taking part in the ex- changes, which is of the order of 0.66, 0.70 or 0.77, depending upon whether the subject is crouching, seated or standing
FclR is the factor by which clothing reduces radiation heat exchange
Tsk (in K) is the mean skin temperature
Tr (in K) is the mean radiant temperature of the environment —that is, the uniform temperature of a black mat sphere of large diameter that would surround the subject and would exchange with it the same quantity of heat as the real environment.
This expression may be replaced by a simplified equation of the same type as that for exchanges by convection:
R = hr (AR/AD) FclR (tsk - tr)
where hr is the coefficient of exchange by radiation (W/°C m2).
Evaporation
Every wet surface has on it a layer of air saturated with water vapour. If the atmosphere itself is not saturated, the vapour diffuses from this layer towards the atmosphere. The layer then tends to be regenerated by drawing on the heat of evaporation (0.674 Watt hour per gram of water) at the wet surface, which cools. If the skin is entirely covered with sweat, evaporation is maximal (Emax) and depends only on the ambient conditions, according to the following expression:
Emax = he Fpcl (Psk,s - Pa)
where:
he is the coefficient of exchange by evaporation (W/m2kPa)
Psk,s is the saturated pressure of water vapour at the temperature of the skin (expressed in kPa)
Pa is the ambient partial pressure of water vapour (expressed in kPa)
Fpcl is the factor of reduction of exchanges by evaporation due to clothing.
Thermal insulation of clothing
A correction factor operates in the calculation of heat flow by convection, radiation and evaporation so as to take account of clothing. In the case of cotton clothing, the two reduction factors FclC and FclR may be determined by:
Fcl = 1/(1+(hc+hr)Icl)
where:
hc is the coefficient of exchange by convection
hr is the coefficient of exchange by radiation
Icl is the effective thermal isolation (m2/W) of clothing.
As regards the reduction of heat transfer by evaporation, the correction factor Fpcl is given by the following expression:
Fpcl = 1/(1+2.22hc Icl)
The thermal insulation of the clothing Icl is expressed in m2/W or in clo. An insulation of 1 clo corresponds to 0.155 m2/W and is provided, for example, by normal town wear (shirt, tie, trousers, jacket, etc.).
ISO standard 9920 (1994) gives the thermal insulation provided by different combinations of clothing. In the case of special protective clothing that reflects heat or limits permeability to vapour under conditions of heat exposure, or absorbs and insulates under conditions of cold stress, individual correction factors must be used. To date, however, the problem remains poorly understood and the mathematical predictions remain very approximate.
Evaluation of the Basic Parameters of the Work Situation
As seen above, thermal exchanges by convection, radiation and evaporation are a function of four climatic parameters—the air temperature ta in °C, the humidity of the air expressed by its partial vapour pressure Pa in kPa, the mean radiant temperature tr in °C, and the air velocity Va in m/s. The appliances and methods for measuring these physical parameters of the environment are the subject of ISO standard 7726 (1985), which describes the different types of sensor to use, specifies their range of measurement and their accuracy, and recommends certain measurement procedures. This section summarizes part of the data of that standard, with particular reference to the conditions of use of the most common appliances and apparatus.
Air temperature
The air temperature (ta) must be measured independent of any thermal radiation; the accuracy of the measurement should be ±0.2ºC within the range of 10 to 30ºC, and ±0.5 °C outside that range.
There are numerous types of thermometers on the market. Mercury thermometers are the most common. Their advantage is accuracy, provided that they have been correctly calibrated originally. Their main disadvantages are their lengthy response time and lack of automatic recording ability. Electronic thermometers, on the other hand, generally have a very short response time (5 s to 1 min) but may have calibration problems.
Whatever the type of thermometer, the sensor must be protected against radiation. This is generally ensured by a hollow cylinder of shiny aluminium surrounding the sensor. Such protection is ensured by the psychrometer, which will be mentioned in the next section.
Partial pressure of water vapour
The humidity of the air may be characterized in four different ways:
1. the dewpoint temperature: the temperature to which the air must be cooled to become saturated with humidity (td, °C)
2. the partial pressure of water vapour: the fraction of atmospheric pressure due to water vapour (Pa, kPa)
3. the relative humidity (RH), which is given by the expression:
RH = 100·Pa/PS,ta
where PS,ta is the saturated vapour pressure associated with the air temperature
4. the wet bulb temperature (tw), which is the lowest temperature attained by a wet sleeve protected against radiation and ventilated at more than 2 m/s by the ambient air.
All these values are connected mathematically.
The saturated water vapour pressure PS,t at any temperature t is given by:
![]()
while the partial pressure of water vapour is connected to the temperature by:
Pa = PS,tw - (ta - tw)/15
where PS,tw is the saturated vapour pressure at the wet bulb temperature.
The psychrometric diagram (figure 1) allows all these values to be combined. It comprises:
Figure 1. Psychrometric diagram.
- in the y axis, the scale of partial pressure of water vapour Pa, expressed in kPa
- in the x axis, the scale of air temperature
- the curves of constant relative humidity
- the oblique straight lines of constant wet bulb temperature.
- The parameters of humidity most often used in practice are:
- the relative humidity, measured by means of hygrometers or more specialized electronic appliances
- the wet bulb temperature, measured by means of the psychrometer; from this is derived the partial pressure of water vapour, which is the parameter most used in analysing thermal balance
The range of measurement and the accuracy recommended are 0.5 to 6 kPa and ±0.15 kPa. For measurement of the wet bulb temperature, the range extends from 0 to 36ºC, with an accuracy identical with that of the air temperature. As regards hygrometers for measuring relative humidity, the range extends from 0 to 100%, with an accuracy of ±5%.
Mean radiant temperature
The mean radiant temperature (tr) has been defined previously; it can be determined in three different ways:
1. from the temperature measured by the black sphere thermometer
2. from the plane radiant temperatures measured along three perpendicular axes
3. by calculation, integrating the effects of the different sources of radiation.
Only the first technique will be reviewed here.
The black sphere thermometer consists of a thermal probe, the sensitive element of which is placed at the centre of a completely closed sphere, made of a metal that is a good conductor of heat (copper) and painted matt black so as to have a coefficient of absorption in the infrared zone close to 1.0. The sphere is positioned in the workplace and subjected to exchanges by convection and radiation. The temperature of the globe (tg) then depends on the mean radiant temperature, the air temperature and the air velocity.
For a standard black globe 15 cm in diameter, the mean temperature of radiation can be calculated from the temperature of the globe on the basis of the following expression:
![]()
In practice, the need must be stressed to maintain the emissivity of the globe close to 1.0 by carefully repainting it matt black.
The main limitation of this type of globe is its long response time (of the order of 20 to 30 min, depending on the type of globe used and the ambient conditions). The measurement is valid only if the conditions of radiation are constant during this period of time, and this is not always the case in an industrial setting; the measurement is then inaccurate. These response times apply to globes 15 cm in diameter, using ordinary mercury thermometers. They are shorter if sensors of smaller thermal capacity are used or if the diameter of the globe is reduced. The equation above must therefore be modified to take account of this difference in diameter.
The WBGT index makes direct use of the temperature of the black globe. It is then essential to use a globe 15 cm in diameter. On the other hand, other indices make use of the mean radiant temperature. A smaller globe can then be selected to reduce the response time, provided that the equation above is modified to take account of it. ISO standard 7726 (1985) allows for an accuracy of ±2ºC in the measurement of tr between 10 and 40ºC, and ±5ºC outside that range.
Air velocity
The air velocity must be measured disregarding the direction of air flow. Otherwise, the measurement must be undertaken in three perpendicular axes (x, y and z) and the global velocity calculated by vectorial summation:
![]()
The range of measurements recommended by ISO standard 7726 extends from 0.05 to 2 m/s The accuracy required is 5%. It should be measured as a 1- or 3-min average value.
There are two categories of appliances for measuring air velo-city: anemometers with vanes, and thermal anemometers.
Vane anemometers
The measurement is carried out by counting the number of turns made by the vanes during a certain period of time. In this way the mean velocity during that period of time is obtained in a discontinuous manner. These anemometers have two main disadvantages:
- They are very directional and have to be oriented strictly in the direction of the air flow. When this is vague or unknown, measurements have to be taken in three directions at right angles.
- The range of measurement extends from about 0.3 m/s to 10 m/s. This limitation to low velocities is important when, for instance, it is a question of analysing a thermal comfort situation where it is generally recommended that a velocity of 0.25 m/s should not be exceeded. Although the range of measurement can extend beyond 10 m/s, it hardly falls below 0.3 or even 0.5 m/s, which greatly limits the possibilities of use in environments near to comfort, where the maximum permitted velocities are 0.5 or even 0.25 m/s.
Hot-wire anemometers
These appliances are in fact complementary to vane anemometers in the sense that their dynamic range extends essentially from 0 to 1 m/s. They are appliances giving an instantaneous estimate of speed at one point of space: it is therefore necessary to use mean values in time and space. These appliances are also often very directional, and the remarks above also apply. Finally, the measurement is correct only from the moment when the temperature of the appliance has reached that of the environment to be evaluated.
Assessment of Heat Stress and Heat Stress Indices
Heat stress occurs when a person’s environment (air temperature, radiant temperature, humidity and air velocity), clothing and activity interact to produce a tendency for body temperature to rise. The body’s thermoregulatory system then responds in order to increase heat loss. This response can be powerful and effective, but it can also produce a strain on the body which leads to discomfort and eventually to heat illness and even death. It is important therefore to assess hot environments to ensure the health and safety of workers.
Heat stress indices provide tools for assessing hot environments and predicting likely thermal strain on the body. Limit values based upon heat stress indices will indicate when that strain is likely to become unacceptable.
The mechanisms of heat stress are generally understood, and work practices for hot environments are well established. These include knowledge of the warning signs of heat stress, acclimatization programmes and water replacement. There are still many casualties, however, and these lessons seem to have to be relearned.
In 1964, Leithead and Lind described an extensive survey and concluded that heat disorders occur for one or more of the following three reasons:
- the existence of factors such as dehydration or lack of acclimatization
- the lack of proper appreciation of the dangers of heat, either on the part of the supervising authority or of the individuals at risk
- accidental or unforeseeable circumstances leading to exposure to very high heat stress.
They concluded that many deaths can be attributed to neglect and lack of consideration and that even when disorders do occur, much can be done if all the requirements for the correct and prompt remedial treatment are available.
Heat Stress Indices
A heat stress index is a single number which integrates the effects of the six basic parameters in any human thermal environment such that its value will vary with the thermal strain experienced by the person exposed to a hot environment. The index value (measured or calculated) can be used in design or in work practice to establish safe limits. Much research has gone into determining the definitive heat stress index, and there is discussion about which is best. For example, Goldman (1988) presents 32 heat stress indices, and there are probably at least double that number used throughout the world. Many indices do not consider all six basic parameters, although all have to take them into conside ration in application. The use of indices will depend upon individual contexts, hence the production of so many. Some indices are inadequate theoretically but can be justified for specific applications based on experience in a particular industry.
Kerslake (1972) notes that “It is perhaps self evident that the way in which the environmental factors should be combined must depend on the properties of the subject exposed to them, but none of the heat stress indices in current use make formal allowance for this”. The recent surge in standardization (e.g., ISO 7933 (1989b) and ISO 7243 (1989a)) has led to pressure to adopt similar indices worldwide. It will be necessary, however, to gain experience with the use of any new index.
Most heat stress indices consider, directly or indirectly, that the main strain on the body is due to sweating. For example, the more sweating required to maintain heat balance and internal body temperature, the greater the strain on the body. For an index of heat stress to represent the human thermal environment and predict heat strain, a mechanism is required to estimate the capacity of a sweating person to lose heat in the hot environment.
An index related to evaporation of sweat to the environment is useful where persons maintain internal body temperature essentially by sweating. These conditions are generally said to be in the prescriptive zone (WHO 1969). Hence deep body temperature remains relatively constant while heart rate and sweat rate rise with heat stress. At the upper limit of the prescriptive zone (ULPZ), thermoregulation is insufficient to maintain heat balance, and body temperature rises. This is termed the environmentally driven zone (WHO 1969). In this zone heat storage is related to internal body temperature rise and can be used as an index to determine allowable exposure times (e.g., based on a predicted safety limit for “core” temperature of 38 °C; see Figure 1).
Figure 1. Calculated distributions of water in the extracellular compartment (ECW) and intracellular compartment (ICW) before and after 2 h of exercise dehydration at 30°C room temperature.
Heat stress indices can be conveniently categorized as rational, empirical or direct. Rational indices are based upon calculations involving the heat balance equation; empirical indices are based on establishing equations from the physiological responses of human subjects (e.g., sweat loss); and direct indices are based on the measurement (usually temperature) of instruments used to simulate the response of the human body. The most influential and widely used heat stress indices are described below.
Rational indices
The Heat Stress Index (HSI)
The Heat Stress Index is the ratio of evaporation required to maintain heat balance (Ereq) to the maximum evaporation that could be achieved in the environment (Emax), expressed as a percentage (Belding and Hatch 1955). Equations are provided in table 1.
Table 1. Equations used in the calculation of the Heat Stress Index (HSI) and Allowable Exposure Times (AET)
|
|
|
|
Clothed |
Unclothed |
|
(1) Radiation loss (R)
|
|
for |
4.4 |
7.3 |
|
(2) Convection loss (C)
|
|
for |
4.6 |
7.6
|
|
(3) Maximum evaporative loss (
|
|
for |
7.0 |
11.7
|
|
(4) Required evaporation loss (
|
|
|
|
|
|
(5) Heat stress index (HSI) |
|
|
|
|
|
(6) Allowable exposure time (AET) |
|
|
|
|
where: M = metabolic power; ![]() = air temperature;
= air temperature; ![]() = radiant temperature;
= radiant temperature; ![]() = partial vapour pressure; v = air velocity
= partial vapour pressure; v = air velocity
The HSI as an index therefore is related to strain, essentially in terms of body sweating, for values between 0 and 100. At HSI = 100, evaporation required is the maximum that can be achieved, and thus represents the upper limit of the prescriptive zone. For HSI>100, there is body heat storage, and allowable exposure times are calculated based on a 1.8 ºC rise in core temperature (heat storage of 264 kJ). For HSI0 there is mild cold strain—for example, when workers recover from heat strain (see table 2).
Table 2. Interpretation of Heat Stress Index (HSI) values
|
HSI |
Effect of eight hour exposure |
|
–20 |
Mild cold strain (e.g. recovery from heat exposure). |
|
0 |
No thermal strain |
|
10-30 |
Mild to moderate heat strain. Little effect on physical work but possible effect on skilled work |
|
40-60 |
Severe heat strain, involving threat to health unless physically fit. Acclimatization required |
|
70-90 |
Very severe heat strain. Personnel should be selected by medical examination. Ensure adequate water and salt intake |
|
100 |
Maximum strain tolerated daily by fit acclimatized young men |
|
Over 100 |
Exposure time limited by rise in deep body temperature |
An upper limit of 390 W/m2 is assigned to Emax (sweat rate of 1 l/h, taken to be the maximum sweat rate maintained over 8 h). Simple assumptions are made about the effects of clothing (long-sleeved shirt and trousers), and the skin temperature is assumed to be constant at 35ºC.
The Index of Thermal Stress (ITS)
Givoni (1963, 1976) provided the Index of Thermal Stress, which was an improved version of the Heat Stress Index. An important improvement is the recognition that not all sweat evaporates. (See “I. Index of thermal stress” in Case Study: Heat indices.)
Required sweat rate
A further theoretical and practical development of the HSI and ITS was the required sweat rate (SWreq) index (Vogt et al. 1981). This index calculated sweating required for heat balance from an improved heat balance equation but, most importantly, also provided a practical method of interpretation of calculations by comparing what is required with what is physiologically possible and acceptable in humans.
Extensive discussions and laboratory and industrial evaluations (CEC 1988) of this index led to it being accepted as International Standard ISO 7933 (1989b). Differences between observed and predicted responses of workers led to the inclusion of cautionary notes concerning methods of assessing dehydration and evaporative heat transfer through clothing in its adoption as a proposed European Standard (prEN-12515). (See “II. Required sweat rate” in Case Study: Heat indices.)
Interpretation of SWreq
Reference values—in terms of what is acceptable, or what persons can achieve—are used to provide a practical interpretation of calculated values (see table 3).
Table 3. Reference values for criteria of thermal stress and strain (ISO 7933, 1989b)
|
Criteria |
Non-acclimatized subjects |
Acclimatized subjects |
|||
|
Warning |
Danger |
Warning |
Danger |
||
|
Maximum skin wettedness |
|||||
|
wmax |
0.85 |
0.85 |
1.0 |
1.0 |
|
|
Maximum sweat rate |
|||||
|
Rest (M 65 Wm–2 ) |
SWmax Wm–2 gh–1 |
100 |
150 |
200 |
300 |
|
260 |
390 |
520 |
780 |
||
|
Work (M≥65 Wm–2 ) |
SWmax Wm–2 gh–1 |
200 |
250 |
300 |
400 |
|
520 |
650 |
780 |
1,040 |
||
|
Maximum heat storage |
|||||
|
Qmax |
Whm–2 |
50 |
60 |
50 |
60 |
|
Maximum water loss |
|||||
|
Dmax |
Whm–2 g |
1,000 |
1,250 |
1,500 |
2,000 |
|
2,600 |
3,250 |
3,900 |
5,200 |
||
First, a prediction of skin wettedness (Wp), evaporation rate (Ep) and sweat rate (SWp) are made. Essentially, if what is calculated as required can be achieved, then these are predicted values (e.g., SWp = SWreq). If they cannot be achieved, the maximum values can be taken (e.g., SWp=SWmax). More detail is given in a decision flow chart (see figure 2).
Figure 2. Decision flow chart for ![]() (required sweat rate).
(required sweat rate).
If required sweat rate can be achieved by persons and it will not cause unacceptable water loss, then there is no limit due to heat exposure over an 8-hour shift. If not, the duration-limited exposures (DLE) are calculated from the following:
When Ep = Ereq and SWp = Dmax/8, then DLE = 480 mins and SWreq can be used as a heat stress index. If the above are not satisfied, then:
DLE1 = 60Qmax/( Ereq –Ep)
DLE2 = 60Dmax/SWp
DLE is the lower of DLE1 and DLE2. Fuller details are given in ISO 7933 (1989b).
Other rational indices
The SWreq index and ISO 7933 (1989) provide the most sophisticated rational method based on the heat balance equation, and they were major advances. More developments with this approach can be made; however, an alternative approach is to use a thermal model. Essentially, the New Effective Temperature (ET*) and Standard Effective Temperature (SET) provide indices based on the two-node model of human thermoregulation (Nishi and Gagge 1977). Givoni and Goldman (1972, 1973) also provide empirical prediction models for the assessment of heat stress.
Empirical indices
Effective temperature andcorrected effective temperature
The Effective Temperature index (Houghton and Yaglou 1923) was originally established to provide a method for determining the relative effects of air temperature and humidity on comfort. Three subjects judged which of two climatic chambers was warmer by walking between the two. Using different combinations of air temperature and humidity (and later other parameters), lines of equal comfort were determined. Immediate impressions were made so the transient response was recorded. This had the effect of over-emphasizing the effect of humidity at low temperatures and underestimating it at high temperatures (when compared with steady-state responses). Although originally a comfort index, the use of the black globe temperature to replace dry bulb temperature in the ET nomograms provided the Corrected Effective Temperature (CET) (Bedford 1940). Research reported by Macpherson (1960) suggested that the CET predicted physiological effects of increasing mean radiant temperature. ET and CET are now rarely used as comfort indices but have been used as heat stress indices. Bedford (1940) proposed CET as an index of warmth, with upper limits of 34ºC for “reasonable efficiency” and 38.6ºC for tolerance. Further investigation, however, showed that ET had serious disadvantages for use as a heat stress index, which led to the Predicted Four Hour Sweat Rate (P4SR) index.
Predicted Four Hour Sweat Rate
The Predicted Four Hour Sweat Rate (P4SR) index was established in London by McArdle et al. (1947) and evaluated in Singapore in 7 years of work summarized by Macpherson (1960). It is the amount of sweat secreted by fit, acclimatized young men exposed to the environment for 4 hours while loading guns with ammunition during a naval engagement. The single number (index value) which summarizes the effects of the six basic parameters is an amount of sweat from the specific population, but it should be used as an index value and not as an indication of an amount of sweat in an individual group of interest.
It was acknowledged that outside of the prescriptive zone (e.g., P4SR>5 l) sweat rate was not a good indicator of strain. The P4SR nomograms (figure 3) were adjusted to attempt to account for this. The P4SR appears to have been useful under the conditions for which it was derived; however, the effects of clothing are over-simplified and it is most useful as a heat storage index. McArdle et al. (1947) proposed a P4SR of 4.5 l for a limit where no incapacitation of any fit, acclimatized young men occurred.
Figure 3. Nomogram for the prediction of the "predicted 4-hour sweat rate" (P4SR).
Heart rate prediction as an index
Fuller and Brouha (1966) proposed a simple index based on the prediction of heart rate (HR) in beats per minute. The relationship as originally formulated with metabolic rate in BTU/h and partial vapour pressure in mmHg provided a simple prediction of heart rate from (T + p), hence the T + p index.
Givoni and Goldman (1973) also provide equations for changing heart rate with time and also corrections for degree of acclimatization of subjects, which are given in Case Study" Heat Indices under “IV. Heart rate”.
A method of work and recovery heart rate is described by NIOSH (1986) (from Brouha 1960 and Fuller and Smith 1980, 1981). Body temperature and pulse rates are measured during recovery following a work cycle or at specified times during the working day. At the end of a work cycle the worker sits on a stool, oral temperature is taken and the following three pulse rates are recorded:
P1—pulse rate counted from 30 seconds to 1 minute
P2—pulse rate counted from 1.5 to 2 minutes
P3—pulse rate counted from 2.5 to 3 minutes
The ultimate criterion in terms of heat strain is an oral temperature of 37.5 ºC.
If P3≤90 bpm and P3–P1 = 10 bpm, this indicates work level is high but there is little increase in body temperature. If P3>90 bpm and P3–P110 bpm, the stress (heat + work) is too high and action is needed to redesign work.
Vogt et al. (1981) and ISO 9886 (1992) provide a model (table 4) using heart rate for assessing thermal environments:
Table 4. Model using heart rate to assess heat stress
|
Total heart rate |
Activity level |
|
HR0 |
Rest (thermal neutrality) |
|
HR0 + HRM |
Work |
|
HR0 + HRS |
Static exertion |
|
HR0 + HRt |
Thermal strain |
|
HR0 + HRN |
Emotion (psychological) |
|
HR0 + HRe |
Residual |
Based on Vogt et al. (1981) and ISO 9886 (1992).
The component of thermal strain (possible heat stress index) can be calculated from:
HRt = HRr–HR0
where HRr is heart rate after recovery and HR0 is the resting heart rate in a thermally neutral environment.
Direct Heat Stress Indices
The Wet Bulb Globe Temperature index
The Wet Bulb Globe Temperature (WBGT) index is by far the most widely used throughout the world. It was developed in a US Navy investigation into heat casualties during training (Yaglou and Minard 1957) as an approximation to the more cumbersome Corrected Effective Temperature (CET), modified to account for the solar absorptivity of green military clothing.
WBGT limit values were used to indicate when military recruits could train. It was found that heat casualties and time lost due to cessation of training in the heat were both reduced by using the WBGT index instead of air temperature alone. The WBGT index was adopted by NIOSH (1972), ACGIH (1990) and ISO 7243 (1989a) and is still proposed today. ISO 7243 (1989a), based on the WBGT index, provides a method easily used in a hot environment to provide a “fast” diagnosis. The specification of the measuring instruments is provided in the standard, as are WBGT limit values for acclimatized or non- acclimatized persons (see table 5). For example, for a resting acclimatized person in 0.6 clo, the limit value is 33ºC WBGT. The limits provided in ISO 7243 (1989a) and NIOSH 1972 are almost identical. Calculation of the WBGT index is given in section V of the accompanying Case Study: Heat Indices.
Table 5. WBGT reference values from ISO 7243 (1989a)
|
Metabolic rate M (Wm–2 ) |
Reference value of WBGT |
|||
|
Person acclimatized to |
Person not acclimatized to |
|||
|
0. Resting M≤65 |
33 |
32 |
||
|
1. 65M≤130 |
30 |
29 |
||
|
2. 130M≤200 |
28 |
26 |
||
|
No sensible air movement |
Sensible air movement |
No sensible air movement |
Sensible air movement |
|
|
3. 200M260 |
25 |
26 |
22 |
23 |
|
4. M>260 |
23 |
25 |
18 |
20 |
Note: The values given have been established allowing for a maximum rectal temperature of 38°C for the persons concerned.
The simplicity of the index and its use by influential bodies has led to its widespread acceptance. Like all direct indices it has limitations when used to simulate human response, and should be used with caution in practical applications. It is possible to buy portable instruments which determine the WBGT index (e.g., Olesen 1985).
Physiological heat exposure limit (PHEL)
Dasler (1974, 1977) provides WBGT limit values based on a prediction of exceeding any two physiological limits (from experimental data) of impermissible strain. The limits are given by:
PHEL=(17.25×108–12.97M×106+18.61M2 ×103)×WBGT–5.36
This index therefore uses the WBGT direct index in the environmentally driven zone (see Figure 4), where heat storage can occur.
Wet globe temperature (WGT) index
The temperature of a wet black globe of appropriate size can be used as an index of heat stress. The principle is that it is affected by both dry and evaporative heat transfer, as is a sweating man, and the temperature can then be used, with experience, as a heat stress index. Olesen (1985) describes WGT as the temperature of a 2.5 inch (63.5 mm) diameter black globe covered with a damp black cloth. The temperature is read when equilibrium is reached after about 10 to 15 minutes of exposure. NIOSH (1986) describe the Botsball (Botsford 1971) as the simplest and most easily read instrument. It is a 3-inch (76.2 mm) copper sphere covered by a black cloth kept at 100% wettedness from a self-feeding water reservoir. The sensing element of a thermometer is located at the centre of the sphere, and the temperature is read on a (colour coded) dial.
A simple equation relating WGT to WBGT is:
WBGT = WGT + 2 ºC
for conditions of moderate radiant heat and humidity (NIOSH 1986), but of course this relationship cannot hold over a wide range of conditions.
The Oxford Index
Lind (1957) proposed a simple, direct index used for storage- limited heat exposure and based on a weighted summation of aspirated wet bulb temperature (Twb) and dry bulb temperature (Tdb):
WD = 0.85 Twb + 0.15 Tdb
Allowable exposure times for mine rescue teams were based on this index. It is widely applicable but is not appropriate where there is significant thermal radiation.
Working Practices for Hot Environments
NIOSH (1986) provides a comprehensive description of working practices for hot environments, including preventive medical practices. A proposal for medical supervision of individuals exposed to hot or cold environments is provided in ISO CD 12894 (1993). It should always be remembered that it is a basic human right, which was affirmed by the 1985 Declaration of Helsinki, that, when possible, persons can withdraw from any extreme environment without need of explanation. Where exposure does take place, defined working practices will greatly improve safety.
It is a reasonable principle in environmental ergonomics and in industrial hygiene that, where possible, the environmental stressor should be reduced at the source. NIOSH (1986) divides control methods into five types. These are presented in table 6.
Table 6. Working practices for hot environments
|
A. Engineering controls |
Example |
|
1. Reduce heat source |
Move away from workers or reduce temperature. Not always practicable. |
|
2. Convective heat control |
Modify air temperature and air movements. Spot coolers may be useful. |
|
3. Radiant heat control |
Reduce surface temperatures or place reflective shield between radiant source and workers. Change emissivity of surface. Use doors that open only when access required. |
|
4. Evaporative heat control |
Increase air movement, decrease water vapour pressure. Use fans or air conditioning. Wet clothing and blow air across person. |
|
B. Work and hygiene practices |
Example |
|
1. Limiting exposure time and/or |
Perform jobs at cooler times of day and year. Provide cool areas for rest and recovery. Extra personnel, worker freedom to interrupt work, increase water intake. |
|
2. Reduce metabolic heat load |
Mechanization. Redesign job. Reduce work time. Increase workforce. |
|
3. Enhance tolerance time |
Heat acclimatization program. Keep workers physically fit. Ensure water loss is replaced and maintain electrolyte balance if necessary. |
|
4. Health and safety training |
Supervisors trained in recognizing signs of heat illness and in first aid. Basic instruction to all personnel on personal precautions, use of protective equipment and effects of non-occupational factors (e.g. alcohol). Use of a “buddy” system. Contingency plans for treatment should be in place. |
|
5. Screening for heat intolerance |
History of previous heat illness. Physically unfit. |
|
C. Heat alert program |
Example |
|
1. In spring establish heat alert |
Arrange training course. Memos to supervisors to make checks of drinking fountains, etc. Check facilities, practices, readiness, etc. |
|
2. Declare heat alert in predicted |
Postpone non-urgent tasks. Increase workers, increase rest. Remind workers to drink. Improve working practices. |
|
D. Auxiliary body cooling and protective clothing |
|
|
Use if it is not possible to modify worker, work or environment and heat stress is still beyond limits. Individuals should be fully heat acclimatized and well trained in use and practice of wearing the protective clothing. Examples are water-cooled garments, air-cooled garments, ice-packet vests and wetted overgarments. |
|
|
E. Performance degradation |
|
|
It must be remembered that wearing protective clothing that is providing protection from toxic agents will increase heat stress. All clothing will interfere with activities and may reduce performance (e.g. reducing the ability to receive sensory information hence impairing hearing and vision for example). |
|
Source: NIOSH 1986.
There has been a great deal of military research into so-called NBC (nuclear, biological, chemical) protective clothing. In hot environments it is not possible to remove the clothing, and working practices are very important. A similar problem occurs for workers in nuclear power stations. Methods of cooling workers quickly so that they are able to perform again include sponging the outer surface of the clothing with water and blowing dry air over it. Other techniques include active cooling devices and methods for cooling local areas of the body. The transfer of military clothing technology to industrial situations is a new innovation, but much is known, and appropriate working practices can greatly reduce risk.
Table 7. Equations used in the calculation of the index and assessment method of ISO 7933 (1989b)
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]() for natural convection
for natural convection
![]()
![]()
![]()
![]()

![]()
![]()
![]()
![]()

![]()
or ![]() , for an approximation or when values are beyond limits for which the equation was derived.
, for an approximation or when values are beyond limits for which the equation was derived.
____________________________________________________________________________________
Table 8. Description of terms used in ISO 7933 (1989b)
|
Symbol |
Term |
Units |
|
|
fraction of skin surface involved in heat exchange by radiation |
ND |
|
C |
heat exchange on the skin by convection |
Wm−2 |
|
|
respiratory heat loss by convection |
Wm−2 |
|
E |
heat flow by evaporation at skin surface |
Wm−2 |
|
|
maximum evaporative rate which can be achieved with the skin completely wet |
Wm−2 |
|
|
required evaporation for thermal equilibrium |
Wm−2 |
|
|
respiratory heat loss by evaporation |
Wm−2 |
|
|
skin emissivity (0.97) |
ND |
|
|
reduction factor for sensible heat exchange due to clothing |
ND |
|
|
reduction factor for latent heat exchange |
ND |
|
|
ratio of the subject’s clothed to unclothed surface area |
ND |
|
|
convective heat transfer coefficient |
|
|
|
evaporative heat transfer coefficient |
|
|
|
radiative heat transfer coefficient |
|
|
|
basic dry thermal insulation of clothing |
|
|
K |
heat exchange on the skin by conduction |
Wm−2 |
|
M |
metabolic power |
Wm−2 |
|
|
partial vapour pressure |
kPa |
|
|
saturated vapour pressure at skin temperature |
kPa |
|
R |
heat exchange on the skin by radiation |
Wm−2 |
|
|
total evaporative resistance of limiting layer of air and clothing |
|
|
|
evaporative efficiency at required sweat rate |
ND |
|
|
required sweat rate for thermal equilibrium |
Wm−2 |
|
|
Stefan-Boltzman constant, |
|
|
|
air temperature |
|
|
|
mean radiant temperature |
|
|
|
mean skin temperature |
|
|
|
air velocity for a stationary subject |
|
|
|
relative air velocity |
|
|
W |
mechanical power |
Wm−2 |
|
|
skin wettedness |
ND |
|
|
skin wettedness required |
ND |
ND = non-dimensional.
Working Practices for Hot Environments
NIOSH (1986) provides a comprehensive description of working practices for hot environments, including preventive medical practices. A proposal for medical supervision of individuals exposed to hot or cold environments is provided in ISO CD 12894 (1993). It should always be remembered that it is a basic human right, which was affirmed by the 1985Declaration of Helsinki, that, when possible, persons can withdraw from any extreme environment without need of explanation. Where exposure does take place, defined working practices will greatly improve safety.
It is a reasonable principle in environmental ergonomics and in industrial hygiene that, where possible, the environmental stressor should be reduced at the source. NIOSH (1986) divides control methods into five types. These are presented in table 7.There has been a great deal of military research into so-called NBC (nuclear, biological, chemical) protective clothing. In hot environments it is not possible to remove the clothing, and working practices are very important. A similar problem occurs for workers in nuclear power stations. Methods of cooling workers quickly so that they are able to perform again include sponging the outer surface of the clothing with water and blowing dry air over it. Other techniques include active cooling devices and methods for cooling local areas of the body. The transfer of military clothing technology to industrial situations is a new innovation, but much is known, and appropriate working practices can greatly reduce risk.
Assessment of a Hot Environment Using ISO Standards
The following hypothetical example demonstrates how ISO standards can be used in the assessment of hot environments (Parsons 1993):
Workers in a steel mill perform work in four phases. They don clothing and perform light work for 1 hour in a hot radiant environment. They rest for 1 hour, then perform the same light work for an hour shielded from the radiant heat. They then perform work involving a moderate level of physical activity in a hot radiant environment for 30 minutes.
ISO 7243 provides a simple method for monitoring the environment using the WBGT index. If the calculated WBGT levels are less than the WBGT reference values given in the standard, then no further action is required. If the levels exceed the reference values (table 6) then the strain on the workers must be reduced. This can be achieved by engineering controls and working practices. A complementary or alternative action is to conduct an analytical assessment according to ISO 7933.
The WBGT values for the work are presented in table 9 and were measured according to the specifications given in ISO 7243 and ISO 7726. The environmental and personal factors relating to the four phases of the work are presented in table 10.
Table 9. WBGT values (°C) for four work phases
|
Work phase (minutes) |
WBGT = WBGTank + 2 WBGTabd + WBGThd |
WBGT reference |
|
0–60 |
25 |
30 |
|
60–90 |
23 |
33 |
|
90–150 |
23 |
30 |
|
150–180 |
30 |
28 |
Table 10. Basic data for the analytical assessment using ISO 7933
|
Work phase (minutes) |
ta (°C) |
tr (°C) |
Pa (Kpa) |
v (ms–1 ) |
clo (clo) |
Act (Wm–2 ) |
|
0–60 |
30 |
50 |
3 |
0.15 |
0.6 |
100 |
|
60–90 |
30 |
30 |
3 |
0.05 |
0.6 |
58 |
|
90–150 |
30 |
30 |
3 |
0.20 |
0.6 |
100 |
|
150–180 |
30 |
60 |
3 |
0.30 |
1.0 |
150 |
It can be seen that for part of the work the WBGT values exceed those of the reference values. It is concluded that a more detailed analysis is required.
The analytical assessment method presented in ISO 7933 was performed using the data presented in table 10 and the computer program listed in the annex of the standard. The results for acclimatized workers in terms of alarm level are presented in table 11.
Table 11. Analytical assessment using ISO 7933
|
Work phase |
Predicted values |
Duration |
Reason for |
||
|
tsk (°C) |
W (ND) |
SW (gh–1 ) |
|||
|
0–60 |
35.5 |
0.93 |
553 |
423 |
Water loss |
|
60–90 |
34.6 |
0.30 |
83 |
480 |
No limit |
|
90–150 |
34.6 |
0.57 |
213 |
480 |
No limit |
|
150–180 |
35.7 |
1.00 |
566 |
45 |
Body temperature |
|
Overall |
— |
0.82 |
382 |
480 |
No limit |
An overall assessment therefore predicts that unacclimatized workers suitable for the work could carry out an 8-hour shift without undergoing unacceptable (thermal) physiological strain. If greater accuracy is required, or individual workers are to be assessed, then ISO 8996 and ISO 9920 will provide detailed information concerning metabolic heat production and clothing insulation. ISO 9886 describes methods for measuring physiological strain on workers and can be used to design and assess environments for specific workforces. Mean skin temperature, internal body temperature, heart rate and mass loss will be of interest in this example. ISO CD 12894 provides guidance on medical supervision of an investigation.
Heat Exchange Through Clothing
In order to survive and work under colder or hotter conditions, a warm climate at the skin surface must be provided by means of clothing as well as artificial heating or cooling. An understanding of the mechanisms of heat exchange through clothing is necessary to design the most effective clothing ensembles for work at extreme temperatures.
Clothing Heat Transfer Mechanisms
The nature of clothing insulation
Heat transfer through clothing, or conversely the insulation of clothing, depends largely on the air that is trapped in and on the clothing. Clothing consists, as a first approximation, of any sort of material that offers a grip to air layers. This statement is approximate because some material properties are still relevant. These relate to the mechanical construction of the fabrics (for instance wind resistance and the ability of fibres to support thick fabrics), and to intrinsic properties of fibres (for instance, absorption and reflection of heat radiation, absorption of water vapour, wicking of sweat). For not too extreme environmental conditions the merits of various fibre types are often overrated.
Air layers and air motion
The notion that it is air, and in particular still air, that provides insulation, suggests that thick air layers are beneficial for insulation. This is true, but the thickness of air layers is physically limited. Air layers are formed by adhesion of gas molecules to any surface, by cohesion of a second layer of molecules to the first, and so on. However, the binding forces between subsequent layers are less and less, with the consequence that the outer molecules are moved by even tiny external motions of air. In quiet air, air layers may have a thickness up to 12 mm, but with vigorous air motion, as in a storm, the thickness decreases to less than 1 mm. In general there is a square-root relationship between thickness and air motion (see “Formulae and Definitions”). The exact function depends on the size and shape of the surface.
Heat conduction of still and moving air
Still air acts as an insulating layer with a conductivity that is constant, regardless of the shape of the material. Disturbance of air layers leads to loss of effective thickness; this includes disturbances not only due to wind, but also due to the motions of the wearer of the clothing—displacement of the body (a component of wind) and motions of body parts. Natural convection adds to this effect. For a graph showing the effect of air velocity on the insulating ability of a layer of air, see figure 1.
Figure 1. Effect of air velocity on insulating ability of an air layer.
Heat transfer by radiation
Radiation is another important mechanism for heat transfer. Every surface radiates heat, and absorbs heat that is radiated from other surfaces. Radiant heat flow is approximately proportional to the temperature difference between the two exchanging surfaces. A clothing layer between the surfaces will interfere with radiative heat transfer by intercepting the energy flow; the clothing will reach a temperature that is about the average of the temperatures of the two surfaces, cutting the temperature difference between them in two, and therefore the radiant flow is decreased by a factor of two. As the number of intercepting layers is increased, the rate of heat transfer is decreased.
Multiple layers are thus effective in reducing radiant heat transfer. In battings and fibre fleeces radiation is intercepted by distributed fibres, rather than a fabric layer. The density of the fibre material (or rather the total surface of fibre material per volume of fabric) is a critical parameter for radiation transfer inside such fibre fleeces. Fine fibres provide more surface for a given weight than coarse fibres.
Fabric insulation
As a result of the conductivities of enclosed air and radiation transfer, fabric conductivity is effectively a constant for fabrics of various thicknesses and bindings. The heat insulation is therefore proportional to the thickness.
Vapour resistance of air and fabrics
Air layers also create a resistance to the diffusion of evaporated sweat from humid skin to the environment. This resistance is roughly proportional to the thickness of the clothing ensemble. For fabrics, the vapour resistance is dependent on the enclosed air and the density of the construction. In real fabrics, high density and great thickness never go together. Due to this limitation it is possible to estimate the air equivalent of fabrics that do not contain films or coatings (see figure 8). Coated fabrics or fabrics laminated to films may have unpredictable vapour resistance, which should be determined by measurement.
Figure 2. Relationship between thickness and vapour resistance (deq) for fabrics without coatings.
From Fabric and Air Layers to Clothing
Multiple layers of fabric
Some important conclusions from the heat transfer mechanisms are that highly insulating clothing is necessarily thick, that high insulation may be obtained by clothing ensembles with multiple thin layers, that a loose fit provides more insulation than a tight fit, and that insulation has a lower limit, set by the air layer that adheres to the skin.
In cold-weather clothing it is often hard to obtain thickness by using thin fabrics only. A solution is to create thick fabrics, by mounting two thin shell fabrics to a batting. The purpose of the batting is to create the air layer and keep the air inside as still as possible. There is also a drawback to thick fabrics: the more the layers are connected, the stiffer the clothing becomes, thereby restricting motion.
Clothing variety
The insulation of a clothing ensemble depends to a large extent on the design of the clothing. Design parameters which affect insulation are number of layers, apertures, fit, distribution of insulation over the body and exposed skin. Some material properties such as air permeability, reflectivity and coatings are important as well. Furthermore, wind and activity change the insulation. Is it possible to give an adequate description of clothing for the purpose of prediction of comfort and tolerance of the wearer? Various attempts have been made, based on different techniques. Most estimates of complete ensemble insulation have been made for static conditions (no motion, no wind) on indoor ensembles, because the available data were obtained from thermal mannequins (McCullough, Jones and Huck 1985). Measurements on human subjects are laborious, and results vary widely. Since the mid-1980s reliable moving mannequins have been developed and used (Olesen et al. 1982; Nielsen, Olesen and Fanger 1985). Also, improved measurement techniques allowed for more accurate human experiments. A problem that still has not been overcome completely is proper inclusion of sweat evaporation in the evaluation. Sweating mannequins are rare, and none of them has a realistic distribution of sweat rate over the body. Humans sweat realistically, but inconsistently.
Definition of clothing insulation
Clothing insulation (Icl in units of m2K/W) for steady state conditions, without radiation sources or condensation in the clothing, is defined in "Formulae and Definitions." Often I is expressed in the unit clo (not a standard international unit). One clo equals 0.155 m2K/W. The use of the unit clo implicitly means that it relates to the whole body and thus includes heat transfer by exposed body parts.
I is modified by motion and wind, as explained earlier, and after correction the result is called resultant insulation. This is a frequently used but not generally accepted term.
Distribution of clothing over the body
Total heat transfer from the body includes heat that is transferred by exposed skin (usually head and hands) and heat passing through the clothing. Intrinsic insulation (see "Formulae and Definitions") is calculated over the total skin area, not only the covered part. Exposed skin transfers more heat than covered skin and thus has a profound influence on the intrinsic insulation. This effect is enhanced by increasing wind speed. Figure 3 shows how the intrinsic insulation decreases successively due to curvature of body shapes (outer layers less effective than inner), exposed body parts (additional pathway for heat transfer) and increased wind speed (less insulation, in particular for exposed skin) (Lotens 1989). For thick ensembles the reduction in insulation is dramatic.
Figure 3. Intrinsic insulation, as it is influenced by body curvature, bare skin and wind speed.
Typical ensemble thickness and coverage
Apparently both the insulation thickness and the skin coverage are important determinants of heat loss. In real life the two are correlated in the sense that winter clothing is not only thicker, but also covers a larger proportion of the body than summer wear. Figure 4 demonstrates how these effects together result in an almost linear relation between clothing thickness (expressed as volume of insulation material per unit of clothing area) and insulation (Lotens 1989). The lower limit is set by the insulation of the adjacent air and the upper limit by usability of the clothing. Uniform distribution may provide the best insulation in the cold, but it is impractical to have much weight and bulk on the limbs. Therefore the emphasis is often on the trunk, and the sensitivity of local skin to cold is adapted to this practice. Limbs play an important role in controlling human heat balance, and high insulation of the limbs limits the effectiveness of this regulation.
Figure 4. Total insulation resulting from clothing thickness and distribution over the body.
Ventilation of clothing
Trapped air layers in the clothing ensemble are subject to motion and wind, but to a different degree than the adjacent air layer. Wind creates ventilation in the clothing, both as air penetrating the fabric and by passing through apertures, while motion increases internal circulation. Havenith, Heus and Lotens (1990) found that inside clothing, motion is a stronger factor than in the adjacent air layer. This conclusion is dependent on the air permeability of the fabric, however. For highly air-permeable fabrics, ventilation by wind is considerable. Lotens (1993) showed that ventilation can be expressed as a function of effective wind speed and air permeability.
Estimates of Clothing Insulation and Vapour Resistance
Physical estimates of clothing insulation
Thickness of a clothing ensemble provides a first estimate of insulation. Typical conductivity of an ensemble is 0.08 W/mK. At an average thickness of 20 mm, that results in an Icl of 0.25 m2K/W, or 1.6 clo. However, loose-fitting parts, such as trousers or sleeves, have a much higher conductivity, more on the order of 0.15, whereas tightly packed clothing layers have a conductivity of 0.04, the famous 4 clo per inch reported by Burton and Edholm (1955).
Estimates from tables
Other methods use table values for clothing items. These items have been measured previously on a mannequin. An ensemble under investigation has to be separated into its components, and these have to be looked up in the table. Making an incorrect choice of the most similar tabulated clothing item may cause errors. In order to obtain the intrinsic insulation of the ensemble, the single insulation values have to be put in a summation equation (McCullough, Jones and Huck 1985).
Clothing surface area factor
In order to calculate total insulation, fcl has to be estimated (see "Formulae and Definitions"). A practical experimental estimate is to measure the clothing surface area, make corrections for overlapping parts, and divide by total skin area (DuBois and DuBois 1916). Other estimates from various studies show that fcl increases linearly with intrinsic insulation.
Estimate of vapour resistance
For a clothing ensemble, vapour resistance is the sum of resistance of air layers and clothing layers. Usually the number of layers varies over the body, and the best estimate is the area-weighted average, including exposed skin.
Relative vapour resistance
Evaporative resistance is less frequently used than I, because few measurements of Ccl (or Pcl) are available. Woodcock (1962) avoided this problem by defining the water vapour permeability index im as the ratio of I and R, related to the same ratio for a single air layer (this latter ratio is nearly a constant and known as the psychrometric constant S, 0.0165 K/Pa, 2.34 Km3/g or 2.2 K/torr); im= I/(R·S). Typical values for im for non-coated clothing, determined on mannequins, are 0.3 to 0.4 (McCullough, Jones and Tamura 1989). Values for im for fabric composites and their adjacent air can be measured relatively simply on a wet hotplate apparatus, but the value is actually dependent on air flow over the apparatus and the reflectivity of the cabinet in which it is mounted. Extrapolation of the ratio of R and I for clothed humans from measurements on fabrics to clothing ensembles (DIN 7943-2 1992) is sometimes attempted. This is a technically complicated matter. One reason is that R is proportional only to the convective part of I, so that careful corrections have to be made for radiative heat transfer. Another reason is that trapped air between fabric composites and clothing ensembles may be different. In fact, vapour diffusion and heat transfer can be better treated separately.
Estimates by articulated models
More sophisticated models are available to calculate insulation and water vapour resistance than the above-explained methods. These models calculate local insulation on the basis of physical laws for a number of body parts and integrate these to intrinsic insulation for the whole human shape. For this purpose the human shape is approximated by cylinders (figure ). The model by McCullough, Jones and Tamura (1989) requires clothing data for all layers in the ensemble, specified per body segment. The CLOMAN model of Lotens and Havenith (1991) requires fewer input values. These models have similar accuracy, which is better than any of the other methods mentioned, with the exception of experimental determination. Unfortunately and inevitably the models are more complex than would be desirable in a widely accepted standard.
Figure 5. Articulation of human shape in cylinders.
Effect of activity and wind
Lotens and Havenith (1991) also provide modifications, based on literature data, of the insulation and vapour resistance due to activity and wind. Insulation is lower while sitting than standing, and this effect is larger for highly insulating clothing. However, motion decreases insulation more than posture does, depending on the vigour of the movements. During walking both arms and legs move, and the reduction is larger than during cycling, when only the legs move. Also in this case, the reduction is larger for thick clothing ensembles. Wind decreases insulation the most for light clothing and less for heavy clothing. This effect might relate to the air permeability of the shell fabric, which is usually less for cold-weather gear.
Figure 8 shows some typical effects of wind and motion on vapour resistance for rainwear. There is no definite agreement in the literature about the magnitude of motion or wind effects. The importance of this subject is stressed by the fact that some standards, such as ISO 7730 (1994), require resultant insulation as an input when applied for active persons, or persons exposed to significant air motion. This requirement is often overlooked.
Figure 6. Decrease in vapour resistance with wind and walking for various rainwear.
Moisture Management
Effects of moisture absorption
When fabrics can absorb water vapour, as most natural fibres do, clothing works as a buffer for vapour. This changes the heat transfer during transients from one environment to another. As a person in non-absorbing clothing steps from a dry to a humid environment, the evaporation of sweat decreases abruptly. In hygroscopic clothing the fabric absorbs vapour, and the change in evaporation is only gradual. At the same time the absorption process liberates heat in the fabric, increasing its temperature. This reduces the dry heat transfer from the skin. In first approximation, both effects cancel each other, leaving the total heat transfer unchanged. The difference with non-hygroscopic clothing is the more gradual change in evaporation from the skin, with less risk of sweat accumulation.
Vapour absorption capacity
Absorption capacity of fabric depends on the fibre type and the fabric mass. Absorbed mass is roughly proportional to the relative humidity, but is higher above 90%. The absorption capacity (called regain) is expressed as the amount of water vapour that is absorbed in 100 g of dry fibre at the relative humidity of 65%. Fabrics can be classified as follows:
- low absorption—acrylic, polyester (1 to 2 g per 100 g)
- intermediate absorption—nylon, cotton, acetate (6 to 9 g per 100 g)
- high absorption—silk, flax, hemp, rayon, jute, wool (11 to 15 g per 100 g).
Water uptake
Water retention in fabrics, often confused with vapour absorption, obeys different rules. Free water is loosely bound to fabric and spreads well sideways along capillaries. This is known as wicking. Transfer of liquid from one layer to another takes place only for wet fabrics and under pressure. Clothing may be wetted by non-evaporated (superfluous) sweat that is taken up from the skin. The liquid content of fabric may be high and its evaporation at a later moment a threat to the heat balance. This typically happens during rest after hard work and is known as after-chill. The ability of fabrics to hold liquid is more related to fabric construction than to fibre absorption capacity, and for practical purposes is usually sufficient to take up all the superfluous sweat.
Condensation
Clothing may get wet by condensation of evaporated sweat at a particular layer. Condensation occurs if the humidity is higher than the local temperature allows. In cold weather that will often be the case at the inside of the outer fabric, in extreme cold even in deeper layers. Where condensation takes place, moisture accumulates, but the temperature increases, as it does during absorption. The difference between condensation and absorption, however, is that absorption is a temporary process, whereas condensation may continue for extended times. Latent heat transfer during condensation may contribute very significantly to heat loss, which may or may not be desirable. The accumulation of moisture is mostly a drawback, because of discomfort and risk of after-chill. For profuse condensation, the liquid may be transported back to the skin, to evaporate again. This cycle works as a heat pipe and may strongly reduce the insulation of the underclothing.
Dynamic Simulation
Since the early 1900s many standards and indices have been developed to classify clothing and climates. Almost without exception these have dealt with steady states—conditions in which the climate and work were maintained long enough for a person to develop a constant body temperature. This type of work has become rare, due to improved occupational health and work conditions. The emphasis has shifted to short-duration exposure to harsh circumstances, often related to calamity management in protective clothing.
There is thus a need for dynamic simulations involving clothing heat transfer and thermal strain of the wearer (Gagge, Fobelets and Berglund 1986). Such simulations can be carried out by means of dynamic computer models that run through a specified scenario. Among the most sophisticated models to date with respect to clothing is THDYN (Lotens 1993), which allows for a wide range of clothing specifications and has been updated to include individual characteristics of the simulated person (figure 9). More models may be expected. There is a need, however, for extended experimental evaluation, and running such models is the work of experts, rather than the intelligent layperson. Dynamic models based on the physics of heat and mass transfer include all heat transfer mechanisms and their interactions—vapour absorption, heat from radiant sources, condensation, ventilation, moisture accumulation, and so on—for a wide range of clothing ensembles, including civil, work and protective clothing.
Figure 7. General description of a dynamic thermal model.
More...
Cold Environment and Cold Work
A cold environment is defined by conditions that cause greater than normal body heat losses. In this context “normal” refers to what people experience in everyday life under comfortable, often indoor conditions, but this may vary due to social, economic or natural climatic conditions. For the purpose of this article environments with an air temperature below 18 to 20ºC would be considered cold.
Cold work comprises a variety of industrial and occupational activities under different climatic conditions (see table 1). In most countries the food industry requires work under cold conditions—normally 2 to 8ºC for fresh food and below –25ºC for frozen food. In such artificial cold environments, conditions are relatively well defined and the exposure is about the same from day to day.
Table 1. Air temperatures of various cold occupational environments
|
–120 ºC |
Climatic chamber for human cryotherapy |
|
–90 ºC |
Lowest temperature at south polar base Vostock |
|
–55 ºC |
Cold store for fish meat and production of frozen, dried products |
|
–40 ºC |
“Normal” temperature at polar base |
|
–28 ºC |
Cold store for deep-frozen products |
|
+2 to +12 ºC |
Storage, preparation and transportation of fresh, alimentary products |
|
–50 to –20 ºC |
Average January temperature of northern Canada and Siberia |
|
–20 to –10 ºC |
Average January temperature of southern Canada, northern Scandinavia, central Russia |
|
–10 to 0 ºC |
Average January temperature of northern USA, southern Scandinavia, central Europe, parts of middle and far East, central and northern Japan |
Source: Modified from Holmér 1993.
In many countries the seasonal climatic changes imply that outdoor work and work in unheated buildings for shorter or longer periods has to be carried out under cold conditions. The cold exposure may vary considerably between different locations on the earth and type of work (see table 1). Cold water presents another hazard, encountered by people engaged in, for example, offshore work. This article deals with responses to cold stress, and preventive measures. Methods for assessment of cold stress and acceptable temperature limits according to recently adopted international standards are dealt with elsewhere in this chapter.
Cold Stress and Work in the Cold
Cold stress may be present in many different forms, affecting the whole-body heat balance as well as the local heat balance of extremities, skin and lungs. The type and nature of cold stress is extensively described elsewhere in this chapter. The natural means of dealing with cold stress is by behavioural action—in particular, change and adjustment of clothing. Sufficient protection prevents cooling. However, protection itself may cause unwanted, adverse effects. The problem is illustrated in figure 1.
Figure 1. Examples of cold effects.
Cooling of the whole body or parts of the body results in discomfort, impaired sensory and neuro-muscular function and, ultimately, cold injury. Cold discomfort tends to be a strong stimulus to behavioural action, reducing or eliminating the effect. Prevention of cooling by means of donning cold-protective clothing, footwear, gloves and headgear interferes with the mobility and dexterity of the worker. There is a “cost of protection” in the sense that movements and motions become restricted and more exhausting. The continuous need for adjustment of the equipment to maintain a high level of protection requires attention and judgement, and may compromise factors such as vigilance and reaction time. One of the most important objectives of ergonomics research is the improvement of the functionality of clothing while maintaining cold protection.
Accordingly, effects of work in the cold must be divided into:
- effects of tissue cooling
- effects of protective measures (“cost of protection”).
On exposure to cold, behavioural measures reduce the cooling effect and, eventually, allow the maintenance of normal thermal balance and comfort. Insufficient measures evoke thermoregulatory, physiologically compensatory reactions (vasoconstriction and shivering). The combined action of behavioural and physiological adjustments determines the resulting effect of a given cold stress.
In the following sections these effects will be described. They are divided into acute effects (occurring within minutes or hours), long-term effects (days or even years) and other effects (not directly related to cooling reactions per se). Table 2 presents examples of reactions associated with the duration of cold exposure. Naturally, types of responses and their magnitude depend largely upon the stress level. However, long exposures (days and longer) hardly involve the extreme levels that can be attained for a short time.
Table 2. Duration of uncompensated cold stress and associated reactions
|
Time |
Physiological effects |
Psychological effect |
|
Seconds |
Inspiratory gasp |
Skin sensation, discomfort |
|
Minutes |
Tissue cooling |
Performance decrement |
|
Hours |
Impaired physical work capacity |
Impaired mental function |
|
Days/months |
Non-freezing cold injury |
Habituation |
|
Years |
Chronic tissue effects (?) |
Acute effects of cooling
The most obvious and direct effect of cold stress is the immediate cooling of the skin and the upper airways. Thermal receptors respond and a sequence of thermoregulatory reactions is initiated. The type and magnitude of reaction is determined primarily by the type and severity of cooling. As previously mentioned, peripheral vasoconstriction and shivering are the main defence mechanisms. Both contribute to preserving body heat and core temperature, but compromise cardiovascular and neuro-muscular functions.
However, the psychological effects of cold exposure also modify the physiological reactions in a complex and partly unknown way. The cold environment causes distraction in the sense that it requires increased mental effort to handle the new stress factors (avoid cooling, take protective measures, etc.). On the other hand, the cold also causes arousal, in the sense that the increased stress level increases sympathetic nervous activity and, thereby, preparedness for action. In normal conditions people use only minor portions of their capacity, thereby preserving a large buffer capacity for unexpected or demanding conditions.
Cold perception and thermal comfort
Most humans experience a sensation of thermal neutrality at an operative temperature between 20 and 26ºC when engaged in very light, sedentary work (office work at 70 W/m2) in appropriate clothing (insulation values between 0.6 and 1.0 clo). In this state and in the absence of any local thermal imbalances, like draught, people are in thermal comfort. These conditions are well documented and specified in standards such as ISO 7730 (see the chapter Controlling the indoor environment in this Encyclopaedia).
Human perception of cooling is closely related to whole-body heat balance as well as local tissue heat balance. Cold thermal discomfort arises when body heat balance cannot be maintained due to inappropriate matching of activity (metabolic heat production) and clothing. For temperatures between +10 and +30ºC, the magnitude of “cold discomfort” in a population can be predicted by Fanger’s comfort equation, described in ISO 7730.
A simplified and reasonably accurate formula for computation of the thermoneutral temperature (t) for the average person is:
t = 33.5 – 3·Icl – (0.08 + 0.05·Icl)·M
where M is the metabolic heat measured in W/m2 and Icl the insulation value of clothing measured in clo.
The required clothing insulation (clo value) is higher at +10ºC than that calculated with the IREQ method (calculated required insulation value) (ISO TR 11079, 1993). The reason for this discrepancy is the application of different “comfort” criteria in the two methods. ISO 7730 focuses heavily on thermal comfort and allows for considerable sweating, whereas ISO TR 11079 allows only “control” sweating at minimal levels—a necessity in the cold. Figure 2 depicts the relationship between clothing insulation, activity level (heat production) and air temperature according to the equation above and the IREQ method. The filled areas should represent the expected variation in required clothing insulation due to different levels of “comfort”.
Figure 2. Optimal temperature for thermal "comfort" as function of clothing and activity level (![]() ).
).
The information in figure 2 is only a guide for establishing optimal indoor thermal conditions. There is considerable individual variation in perception of thermal comfort and discomfort from cold. This variation originates from differences in clothing and activity patterns, but subjective preferences and habituation also contribute.
In particular, people engaged in very light, sedentary activity become increasingly susceptible to local cooling when air temperature drops below 20 to 22ºC. In such conditions air velocity must be kept low (below 0.2 m/s), and additional insulative clothing must be selected to cover sensitive body parts (e.g., head, neck, back and ankles). Seated work at temperatures below 20ºC requires insulated seat and backrest to reduce local cooling due to compression of clothing.
When ambient temperature falls below 10ºC, the comfort concept becomes more difficult to apply. Thermal asymmetries become “normal” (e.g., cold face and cold air inhalation). Despite an optimal body heat balance, such asymmetries may be felt to be uncomfortable and require extra heat to eliminate. Thermal comfort in the cold, unlike under normal indoor conditions, is likely to coincide with a slight feeling of warmth. This should be remembered when cold stress is assessed using the IREQ index.
Performance
Cold exposure and the associated behavioural and physiological reactions have an impact on human performance at various levels of complexity. Table 3 presents a schematic overview of different types of performance effects that may be anticipated with mild and extreme cold exposure.
Table 3. Indication of anticipated effects of mild and severe cold exposure
|
Performance |
Mild cold exposure |
Severe cold exposure |
|
Manual performance |
0 – |
– – |
|
Muscular performance |
0 |
– |
|
Aerobic performance |
0 |
– |
|
Simple reaction time |
0 |
– |
|
Choice reaction time |
– |
– – |
|
Tracking, vigilance |
0 – |
– |
|
Cognitive, mental tasks |
0 – |
– – |
0 indicates no effect; – indicates impairment; – – indicates strong impairment; 0 – indicates contradictory finding.
Mild exposure in this context implies no or negligible body core cooling and moderate cooling of the skin and extremities. Severe exposure results in negative heat balance, a drop in core temperature and concomitant pronounced lowering of temperature of the extremities.
The physical characteristics of mild and severe cold exposure are very much dependent on the balance between internal body heat production (as a result of physical work) and heat losses. Protective clothing and ambient climatic conditions determine the amount of heat loss.
As previously mentioned, cold exposure causes distraction and cooling (figure 1). Both have an impact on performance, although the magnitude of impact varies with the type of task.
Behaviour and mental function are more susceptible to the distraction effect, whereas physical performance is more affected by cooling. The complex interaction of physiological and psychological responses (distraction, arousal) to cold exposure is not fully understood and requires further research work.
Table 4 indicates reported relationships between physical performance and temperatures of the body. It is assumed that physical performance is highly dependent on tissue temperature and deteriorates when temperature of vital tissue and organ parts drops. Typically, manual dexterity is critically dependent upon finger and hand temperature, as well as muscle temperature of the forehand. Gross muscular activity is little affected by local surface temperature, but very sensitive to muscle temperature. Since some of these temperatures are related to each other (e.g., core and muscle temperature) it is difficult to determine direct relationships.
Table 4. Importance of body tissue temperature for human physical performance
|
Performance |
Hand/finger skin temperature |
Mean skin temperature |
Muscle temperature |
Core temperature |
|
Simple manual |
– |
0 |
– |
0 |
|
Complex manual |
– – |
(–) |
– – |
– |
|
Muscular |
0 |
0 – |
– – |
0 – |
|
Aerobic |
0 |
0 |
– |
– – |
0 indicates no effect; – indicates impairment with lowered temperature; – – indicates strong impairment; 0 – indicates contradictory findings; (–) indicates possible minor effect.
The overview of performance effects in table 3 and 4 is by necessity very schematic. The information should serve as a signal for action, where action means a detailed assessment of conditions or undertaking of preventive measures.
An important factor contributing to performance decrements is exposure time. The longer the cold exposure, the greater the effect upon the deeper tissues and neuro-muscular function. On the other hand, factors such as habituation and experience modify the detrimental effects and restore some of the performance capacity.
Manual performance
Hand function is very susceptible to cold exposure. Due to their small mass and large surface area, hands and fingers lose much heat while maintaining high tissue temperatures (30 to 35ºC). Accordingly, such high temperatures can be maintained only with a high level of internal heat production, allowing for sustained high blood flow to the extremities.
Hand heat loss can be reduced in the cold by wearing appropriate handwear. However, good handwear for cold weather means thickness and volume, and, consequently, impaired dexterity and manual function. Hence, manual performance in the cold cannot be preserved by passive measures. At best, the reduction in performance may be limited as the result of a balanced compromise between the choice of functional handwear, work behaviour and exposure scheme.
Hand and finger function is much dependent on local tissue temperatures (figure 3). Fine, delicate and fast finger movements deteriorate when tissue temperature drops by a few degrees. With more profound cooling and temperature drop, gross hand functions are also impaired. Significant impairment in hand function is found at hand skin temperatures around 15ºC, and severe impairments occur at skin temperatures about 6 to 8ºC due to blocking of function of sensory and thermal skin receptors. Depending on task requirements, it may be necessary to measure skin temperature at several sites on the hand and fingers. Temperature of the fingertip may be more than ten degrees lower than on the back of the hand under certain exposure conditions.
Figure 3. Relation between finger dexterity and finger skin temperature.
Figure 4 indicates critical temperatures for different types of effects on manual function.
Figure 4. Estimated gross effects on manual performance at different levels of hand/finger temperature.
Neuro-muscular performance
It is evident from figures 3 and 4 that there is a pronounced effect of cold on muscular function and performance. Cooling of muscle tissue reduces blood flow and slows down neural processes like transmission of nerve signals and synaptic function. In addition, viscosity of tissues increases, resulting in higher internal friction during motion.
Isometric force output is reduced by 2% per ºC of lowered muscle temperature. Dynamic force output is reduced by 2 to 4% per ºC of lowered muscle temperature. In other words, cooling reduces the force output of muscles and has an even greater effect on dynamic contractions.
Physical work capacity
As previously mentioned, muscular performance deteriorates in the cold. With impaired muscle function there is a general impairment of physical work capacity. A contributing factor to the reduction in aerobic work capacity is the increased peripheral resistance of the systemic circulation. Pronounced vasoconstriction increases central circulation, eventually leading to cold diuresis and elevated blood pressure. Cooling of the core may also have a direct effect on the contractility of the heart muscle.
Work capacity, as measured by maximal aerobic capacity, decreases by 5 to 6% per ºC lowered core temperature. Thus endurance may deteriorate rapidly as the practical consequence of the lowered maximal capacity and with an increased energy requirement of muscular work.
Other cold effects
Body temperatures
As the temperature drops, the surface of the body is most affected (and also most tolerant). Skin temperature may fall below 0ºC in a few seconds when the skin is in contact with very cold metal surfaces. Likewise hand and finger temperatures may decrease by several degrees per minute under conditions of vasoconstriction and poor protection. At normal skin temperature the arms and hands are superperfused due to peripheral arterio-venous shunts. This creates warmth and enhances dexterity. Cooling of the skin shuts these shunts and decreases perfusion in hands and feet to one tenth. The extremities constitute 50% of the body surface and 30% of its volume. The return of blood passes via deep veins concomitant to the arteries, thereby reducing heat loss according to the counter-current principle.
Adrenergic vasoconstriction does not occur in the head-neck region, which must be borne in mind in emergency situations to prevent hypothermia. A bareheaded individual may lose 50% or more of his or her resting heat production at subzero temperatures.
A high and sustained rate of whole-body heat loss is required for the development of hypothermia (drop in core temperature) (Maclean and Emslie-Smith 1977). The balance between heat production and heat loss determines the resultant cooling rate, be it a whole-body cooling or a local cooling of a part of the body. The conditions for heat balance can be analysed and assessed on the basis of the IREQ index. A remarkable response to local cooling of protruding parts of the human body (e.g., fingers, toes and ears) is the hunting phenomenon (Lewis reaction). After an initial drop to a low value, finger temperature increases by several degrees (figure 5). This reaction is repeated in a cyclic manner. The response is very local—more pronounced at the tip of the finger than at the base. It is absent in the hand. The response on the palm of the hand most likely reflects the variation in temperature of the blood flow supplying the fingers. The response can be modified by repeated exposures (amplified), but is more or less abolished in association with whole-body cooling.
Figure 5. Cold-induced vasodilatation of finger vessels causing cyclic rises in tissue temperature.
Progressive cooling of the body results in a number of physio-logical and mental effects. Table 16 indicates some typical responses associated with different levels of core temperature.
Table 5. Human responses to cooling: Indicative reactions to different levels of hypothermia
|
Phase |
Core |
Physiological |
Psychological |
|
Normal |
37 36 |
Normal body temperature Vasoconstriction, cold hands and feet |
Thermoneutral sensation Discomfort |
|
Mild hypothermia |
35 34 33 |
Intense shivering, reduced work capacity Fatigue Fumbling and stumbling |
Impaired judgment, disorientation, apathy Conscious and |
|
Moderate |
32 31 30 29 |
Muscle rigidity Faint breathing No nerve reflexes, heart rate slow and almost unnoticeable |
Progressive Consciousness clouds Stuporous |
|
Severe |
28 27 25 |
Heart dysrhythmias (atrial Pupils non reactive to Death due to ventricular fibrillation or asystole |
Heart and circulation
Cooling of the forehead and head elicit acute elevation of systolic blood pressure and, eventually, elevated heart rate. A similar reaction may be seen when putting bare hands in very cold water. The reaction is of short duration, and normal or slightly elevated values are attained after seconds or minutes.
Excessive body heat loss causes peripheral vasoconstriction. In particular, during the transient phase the increased peripheral resistance results in an elevation of systolic blood pressure and increased heart rate. Cardiac work is greater than it would be for similar activities at normal temperatures, a phenomenon painfully experienced by persons with angina pectoris.
As previously mentioned, deeper tissue cooling generally slows down the physiological processes of cells and organs. Cooling weakens the innervation process and suppresses heart contractions. Contraction power is reduced and, in addition to the increase in peripheral resistance of the blood vessels, cardiac output is reduced. However, with moderate and severe hypothermia, cardiovascular function declines in relation to the general reduction in metabolism.
Lungs and airways
Inhalation of moderate volumes of cold, dry air presents limited problems in healthy persons. Very cold air may cause discomfort, in particular, with nasal breathing. High ventilation volumes of very cold air may also cause micro-inflammation of the mucosal membrane of the upper airways.
With progression of hypothermia, lung function is depressed contemporaneously with the general reduction in body meta-bolism.
Functional aspects (work capacity)
A fundamental requirement for function in cold environments is the provision of sufficient protection against cooling. However, protection itself may seriously interfere with conditions for performance. The hobbling effect of clothing is well-known. Headgear and helmets interfere with speech and vision, and handwear impairs manual function. Whereas protection is necessary for preservation of healthy and comfortable working conditions, the consequences in terms of impaired performance must be fully recognized. Tasks take longer to complete and require greater effort.
Protective clothing against cold may easily weigh 3 to 6 kg including boots and headwear. This weight adds to workload, in particular during ambulatory work. Also, friction between layers in multi-layer clothing yields resistance to motion. The weight of boots should be kept low, since added weight on the legs contributes relatively more to workload.
Work organization, workplace and equipment should be adapted to the specific requirements of a cold work task. More time must be allowed for tasks, and frequent breaks for recovery and warming are needed. The workplace must allow for easy movements, despite bulky clothing. Similarly, equipment must be designed so that it can be operated by a gloved hand or insulated in the case of bare hands.
Cold Injuries
Serious injuries by cold air are in most cases preventable and occur only sporadically in civilian life. On the other hand, these injuries are often of major significance in war and in cataclysms. However, many workers run the risk of getting cold injuries in their routine activities. Outdoor work in harsh climate (as in arctic and subarctic areas—for example, fishing, agriculture, construction, gas and oil exploration and reindeer herding) as well as indoor work carried out in cold environments (as in food or warehousing industries) can all involve danger of cold injury.
Cold injuries may be either systemic or localized. The local injuries, which most often precede systemic hypothermia, constitute two clinically different entities: freezing cold injuries (FCI) and non-freezing cold injuries (NFCI).
Freezing cold injuries
Pathophysiology
This type of local injury occurs when heat loss is sufficient to allow a true freezing of the tissue. Besides a direct cryogenic insult to the cells, vascular damage with decreased perfusion and tissue hypoxia are contributing pathogenic mechanisms.
The vasoconstriction of cutaneous vessels is of great importance in the origin of a frostbite. Due to wide arteriovenous shunts, peripheral structures such as hands, feet, nose and ears are superperfused in a warm environment. Only about one-tenth of the blood flow in the hands, for example, is needed for tissue oxygenation. The rest creates warmth, thereby facilitating dexterity. Even in the absence of any decrease in core temperature, local cooling of the skin occludes these shunts.
In order to protect the viability of the peripheral parts of the extremities during cold exposure, an intermittent cold-induced vasodilatation (CIVD) takes place. This vasodilatation is a result of opening of the arteriovenous anastomoses and occurs every 5 to 10 minutes. The phenomenon is a compromise in the human physiological plan to conserve heat and yet intermittently preserve function of hands and feet. The vasodilatation is perceived by the person as periods of prickling heat. CIVD becomes less pronounced as body temperature decreases. Individual variations in the degree of CIVD might explain different susceptibility to local cold injury. People indigenous to a cold climate present a more pronounced CIVD.
In contrast to cryopreservation of living tissue, where ice crystallization occurs both intra- and extracellularly, the clinical FCI, with a much slower rate of freezing, produces only extra- cellular ice crystals. The process is an exothermic one, liberating heat, and therefore tissue temperature remains at the freezing point until freezing is complete.
As the extracellular ice crystals grow, extracellular solutions are condensed, causing this space to become a hyperosmolar milieu, which leads to passive diffusion of water from the intracellular compartment; that water in turn freezes. This process progresses until all “available” water (not otherwise bound to protein, sugar and other molecules) has been crystallized. Cell dehydration alters protein structures, membrane lipids and cellular pH, leading to destruction incompatible with cell survival. Resistance to FCI varies in different tissues. Skin is more resistant than muscles and nerves, for example, which might be the result of a smaller water content both intra- and intercellularly in the epidermis.
The role of indirect haemorheological factors was earlier interpreted as similar to that found in non-freezing cold injuries. Recent studies in animals have, however, shown that freezing causes lesions in the intima of arterioles, venules and capillaries prior to any evidence of damage to other skin elements. Thus, it is obvious that the rheological part of the pathogenesis of FCI is also a cryobiological effect.
When a frostbite is rewarmed, water begins to rediffuse to the dehydrated cells, leading to intracellular swelling. Thawing induces maximal vascular dilation, creating oedema and blister formation due to the endothelial (internal layer of the skin) cell injury. Disruption of the endothelial cells exposes the basement membrane, which initiates platelet adhesions and starts the coagulation cascade. The following blood stagnation and thrombosis induce anoxia.
As it is the heat loss from the exposed area that determines the risk of getting a frostbite, wind-chill is an important factor in this respect, and this means not only the wind which is blowing but also any movement of air past the body. Running, skiing, skijoring and riding in open vehicles must be considered in this context. However, the exposed flesh will not freeze as long as the ambient temperature is above the freezing point, even at high wind velocities.
Use of alcohol and tobacco products as well as under-nourishment and fatigue are predisposing factors to FCI. A previous cold injury increases the risk of subsequent FCI, due to an abnormal post-traumatic sympathetic response.
Cold metal can rapidly cause a frostbite when grasped with the bare hand. Most people are aware of this, but often don’t realize the risk of handling super-cooled liquids. Petrol cooled down to –30ºC will freeze exposed flesh almost instantly as evaporative heat loss is combined with conductive loss. Such rapid freezing causes extra- as well as intracellular crystallization with destruction of cell membranes primarily on a mechanical basis. A similar type of FCI occurs when liquid propane is spilled directly onto the skin.
Clinical picture
Freezing cold injuries are subdivided into superficial and deep frostbites. The superficial injury is limited to the skin and the immediate underlying subcutaneous tissues. In most cases the injury is localized to nose, earlobes, fingers and toes. Stinging, pricking pain is often the first sign. The affected part of the skin turns pale or wax-white. It is numb, and will indent upon pressure, as the underlying tissues are viable and pliable. When the FCI extends into a deep injury, the skin becomes white and marble-like, feels hard, and adheres when touched.
Treatment
A frostbite should be taken care of immediately in order to prevent a superficial injury from turning into a deep one. Try to take the victim indoors; otherwise protect him or her from the wind by shelter of comrades, a wind sack or other similar means. The frost-bitten area should be thawed by passive transmission of heat from a warmer part of the body. Put the warm hand against the face and the cold hand into the armpit or into the groin. As the frostbitten individual is under cold stress with peripheral vaso-constriction, a warm companion is a much better therapist. Massage and rubbing the frostbitten part with snow or woollen muffler is contraindicated. Such mechanical treatment would only aggravate the injury, as the tissue is filled with ice crystals. Nor should thawing in front of a campfire or a camp stove be considered. Such heat does not penetrate to any depth, and as the area is partly anaesthetized the treatment may even result in a burn injury.
The signals of pain in a frostbitten foot disappear before actual freezing takes place, as nerve conductivity is abolished at around +8ºC. The paradox is that the last sensation one feels is that one does not feel anything at all! Under extreme conditions when evacuation requires travel on foot, thawing should be avoided. Walking on frostbitten feet does not seem to increase the risk of tissue loss, whereas refreezing of a frostbite does so in the highest degree.
The best treatment for a frostbite is thawing in warm water at 40 to 42ºC. The thawing procedure should continue at that water temperature until sensation, colour and tissue softness return. This form of thawing often ends up in not a pink, but rather a burgundy hue due to venous stasis.
Under field conditions one must be aware that treatment requires more than local thawing. The whole individual has to be taken care of, as a frostbite is often the first sign of a creeping hypothermia. Put on more clothes and give warm, nourishing beverages. The victim is most often apathetic and has to be forced to cooperate. Urge the victim to do muscular activity such as buffeting arms against sides. Such manoeuvres open peripheral arteriovenous shunts in the extremities.
A deep frostbite is present when thawing with passive warmth transfer for 20 to 30 minutes is without success. If so, the victim should be sent to the nearest hospital. However, if such transportation can take hours, it is preferable to get the person into the nearest housing and thaw his or her injuries in warm water. After complete thawing, the patient should be put to bed with the injured area elevated, and prompt transportation to the nearest hospital should be arranged.
Rapid rewarming gives moderate to severe pain, and the patient will often need an analgesic. The capillary damage causes leakage of serum with local swelling and blister formation during the first 6 to 18 hours. Blisters should be kept intact in order to prevent infection.
Non-freezing cold injuries
Pathophysiology
Prolonged exposure to cold and wet conditions above the freezing point combined with immobilization causing venous stagnation are the prerequisites for NFCI. Dehydration, inadequate food, stress, inter-current illness or injury, and fatigue are contributory factors. NFCI almost exclusively affects legs and feet. Severe injuries of this type occur with great rarity in civilian life, but in wartime and catastrophes it has been and will always be a serious problem, most often caused by an unawareness of the condition due to the slow and indistinct first appearance of symptoms.
NFCI can occur under any conditions where the environmental temperature is lower than body temperature. As in FCI, sympathetic constrictor fibres, together with the cold itself, induce prolonged vasoconstriction. The initial event is rheological in nature and resembles that observed in ischaemic reperfusion injury. In addition to the duration of the low temperature, the susceptibility of the victim seems to be of importance.
The pathological change due to the ischaemic injury affects many tissues. Muscles degenerate, undergoing necrosis, fibrosis and atrophy; bones show early osteoporosis. Of special interest are the effects on the nerves, as nerve damage accounts for the pain, prolonged dysaesthesia and hyperhidrosis often found as a sequel in these injuries.
Clinical picture
In a non-freezing cold injury the victim realizes too late the threatening danger because the initial symptoms are so vague. The feet become cold and swollen. They feel heavy, woody and numb. The feet are presented as cool, painful, tender, often with wrinkled soles. The first ischaemic phase last for hours up to a few days. It is followed by a hyperaemic phase of 2 to 6 weeks, during which the feet are warm, with bounding pulses and increased oedema. Blistering and ulcerations are not uncommon, and in severe cases gangrene can arise.
Treatment
The treatment is above all supportive. On the worksite, the feet should be dried carefully but kept cool. On the other hand, the whole body should be warmed. Plenty of warm beverages should be given. Contrary to the freezing cold injuries, NFCI should never be actively warmed. Warm water treatment in local cold injuries is only allowed when ice-crystals are present in the tissue. The further treatment should as a rule be conservative. However, fever, signs of disseminated intravascular coagulation, and liquefaction of affected tissues requires surgical intervention, occasionally ending in an amputation.
Non-freezing cold injuries can be prevented. Exposure time should be minimized. Adequate foot care with time to dry the feet is of importance, as well as facilities to change into dry socks. Rest with feet elevated as well as administering hot beverages whenever possible may seem ridiculous but often is of crucial importance.
Hypothermia
Hypothermia means subnormal body temperature. However, from a thermal point of view the body consists of two zones—the shell and the core. The former is superficial and its temperature varies considerably according to the external environment. The core consists of deeper tissues (e.g., brain, heart and lungs, and upper abdomen), and the body strives to maintain a core temperature of 37 ± 2ºC. When thermoregulation is impaired and core temperature starts to decline, the individual suffers cold stress, but not until the central temperature reaches 35ºC is the victim considered to be in a hypothermic state. Between 35 and 32ºC, the hypothermia is classified as mild; between 32 and 28ºC it is moderate and below 28ºC, severe (Table 16).
Physiological effects of lowered core temperature
When core temperature starts to decline, an intense vasoconstriction redirects blood from the shell to the core, thereby preventing heat conduction from the core to the skin. In order to maintain temperature, shivering is induced, often preceded by increased muscular tone. Maximal shivering can increase the metabolic rate four- to sixfold, but as the involuntary contractions oscillate, the net result is often not more than doubled. Heart rate, blood pressure, cardiac output and respiratory rate increase. The centralization of blood volume causes an osmolal diuresis with sodium and chloride as the main constituents.
Atrial irritability in early hypothermia often induces atrial fibrillation. At lower temperatures, ventricular extra systoles are common. Death occurs at or below 28ºC, most often resulting from ventricular fibrillation; asystole may also supervene.
Hypothermia depresses the central nervous system. Lassitude and apathy are early signs of decreasing core temperature. Such effects impair judgement, cause bizarre behaviour and ataxia, and end in lethargy and coma between 30 and 28ºC.
Nerve conduction velocity decreases with lowered temperature. Dysarthria, fumbling and stumbling are clinical manifestations of this phenomena. Cold also affects muscles and joints, impairing manual performance. It slows reaction time and coordination, and increases frequency of mistakes. Muscle rigidity is observed in even mild hypothermia. At a core temperature lower than 30ºC, physical activity is impossible.
Exposure to an abnormally cold environment is the basic prerequisite for hypothermia to occur. Extremes of age are risk factors. Elderly persons with impaired thermoregulatory function, or persons whose muscle mass and insulating fat layer are reduced, run a greater risk of suffering hypothermia.
Classification
From a practical point of view the following subdivision of hypo-thermia is useful (see also Table 16):
- accidental hypothermia
- acute immersion hypothermia
- sub-acute exhaustion hypothermia
- hypothermia in trauma
- sub-clinical chronic hypothermia.
Acute immersion hypothermia occurs when a person falls into cold water. Water has a thermal conductivity approximately 25 times that of air. The cold stress becomes so great that the core temperature is forced down despite a maximal heat production of the body. Hypothermia sets in before the victim becomes exhausted.
Sub-acute exhaustion hypothermia may happen to any worker in a cold environment as well as to skiers, climbers and walkers in the mountains. In this form of hypothermia, muscular activity maintains the body temperature as long as energy sources are available. However, then hypoglycaemia ensures the victim is at risk. Even a relatively mild degree of cold exposure may be sufficient to continue cooling and cause a hazardous situation.
Hypothermia with major trauma is an ominous sign. The injured person is often unable to maintain body temperature, and heat loss may be exacerbated by infusion of cold fluids and by removal of clothing. Patients in shock who become hypothermic have a much higher mortality than normothermic victims.
Sub-clinical chronic hypothermia is often encountered in elderly persons, often in association with malnutrition, inadequate clothing and restricted mobility. Alcoholism, drug abuse and chronic metabolic diseases as well as psychiatric disorders are contributory causes in this type of hypothermia.
Pre-hospital management
The main principle of primary care of a worker suffering from hypothermia is to prevent further heat loss. A conscious victim should be moved indoors, or at least into a shelter. Remove wet clothing and try to insulate the person as much as possible. Keeping the victim in a lying position with the head covered is mandatory.
Patients with acute immersion hypothermia require quite different treatment from that required by those with sub-acute exhaustion hypothermia. The immersion victim is often in a more favourable situation. The decreased core temperature occurs long before the body becomes exhausted, and heat-generating capacity remains unimpaired. Water and electrolyte balance is not deranged. Therefore such an individual may be treated with rapid immersion in a bath. If a tub is not available, put the patient’s feet and hands into warm water. The local heat opens the arterio- venous shunts, rapidly increases the blood circulation in the extremities and enhances the warming process.
In exhaustion hypothermia, on the other hand, the victim is in a much more serious situation. The caloric reserves are consumed, the electrolyte balance is deranged and, above all, the person is dehydrated. The cold diuresis starts immediately after cold exposure; the fight against the cold and wind exaggerates sweating, but this is not perceived in the cold and dry environment; and lastly, the victim does not feel thirsty. A patient suffering from exhaustion hypothermia should never be rapidly rewarmed out in the field due to the risk of inducing hypovolemic shock. As a rule it is better not to actively rewarm the patient out in the field or during transportation to hospital. A prolonged state of not progressing hypothermia is far better than enthusiastic efforts to warm the patient under circumstances where supervening complications cannot be managed. It is mandatory to handle the patient gently to minimize the risk of possible ventricular fibrillation.
Even for trained medical personnel it is often difficult to determine whether a hypothermic individual is alive or not. Apparent cardiovascular collapse may actually be only depressed cardiac output. Palpation or auscultation for at least a minute to detect spontaneous pulses is often necessary.
The decision as to whether or not to administer cardiopulmonary resuscitation (CPR) is difficult out in the field. If there is any sign of life at all, CPR is contra-indicated. Prematurely performed chest compressions may induce ventricular fibrillation. CPR should, however, immediately be initiated following a witnessed cardiac arrest and when the situation allows the procedures to be performed reasonably and continuously.
Health and cold
A healthy person with appropriate clothing and equipment and working in an organization suitable for the task is not in a health risk situation, even if it is very cold. Whether or not long-term cold exposure while living in cold climate areas means health risks is controversial. For individuals with health problems the situation is quite different, and cold exposure could be a problem. In a certain situation cold exposure or exposure to cold-related factors or combinations of cold with other risks can produce health risks, especially in an emergency or accident situation. In remote areas, when communication with a supervisor is difficult or does not exist, the employees themselves must be allowed to decide whether a health risk situation is at hand or not. In these situations they must take necessary precautions to make the situation safe or stop work.
In arctic regions, climate and other factors can be so harsh that other considerations must be taken.
Infectious diseases. Infectious diseases are not related to cold. Endemic diseases occur in arctic and subarctic regions. Acute or chronic infectious disease in an individual dictates cessation of exposure to cold and hard work.
The common cold, without fever or general symptoms, does not make work in the cold harmful. However, for individuals with complicating diseases like asthma, bronchitis or cardiovascular problems, the situation is different and indoor work in warm conditions during the cold season is recommended. This is also valid with a cold with fever, deep cough, muscle pain and impaired general condition.
Asthma and bronchitis are more common in cold regions. Exposure to cold air often worsens the symptoms. Change of medication sometimes reduces the symptoms during the cold season. Some individuals can also be helped by using medicinal inhalers.
People with asthmatic or cardiovascular diseases may respond to cold air inhalation with bronchoconstriction and vasospasm. Athletes training several hours at high intensities in cold climates have been shown to develop asthmatic symptoms. Whether or not extensive cooling of the pulmonary tract is the primary explanation is not yet clear. Special, light masks are now on the market that do provide some kind of heat exchanger function, thereby conserving energy and moisture.
An endemic type of chronic disease is “Eskimo lung”, typical for Eskimo hunters and trappers exposed to extreme cold and hard work for long periods. A progressive pulmonary hyper- tension often ends in a right-sided heart failure.
Cardiovascular disorders. Exposure to cold affects the cardio- vascular system to a higher degree. The noradrenalin released from the sympathetic nerve terminals raises the cardiac output and heart rate. Chest pain due to angina pectoris often worsens in a cold environment. The risk of getting an infarct increases during cold exposure, especially in combination with hard work. Cold raises blood pressure with an increased risk of cerebral haemorrhage. Individuals at risk should therefore be warned and reduce their exposure to hard work in the cold.
Increased mortality during winter season is a frequent observation. One reason could be the previously mentioned increase in heart work, promoting arrhythmia in sensitive persons. Another observation is that the haematocrit is increased during the cold season, causing increased viscosity of blood and increased resistance to flow. A plausible explanation is that cold weather may expose people to sudden, very heavy work loads, such as snow cleaning, walking in deep snow, slipping and so on.
Metabolic disorders. Diabetes mellitus is also found with a higher frequency in the colder areas of the world. Even an uncomplicated diabetes, especially when treated with insulin, can make cold outdoor work impossible in more remote areas. Early peripheral arteriosclerosis makes these individuals more sensitive to cold and increases the risk of local frostbite.
Individuals with impaired thyroid function can easily develop hypothermia due to lack of the thermogenic hormone, while hyperthyroid persons tolerate cold even when lightly dressed.
Patients with these diagnoses should be given extra attention from health professionals and be informed of their problem.
Musculoskeletal problems. Cold itself is not supposed to cause diseases in the musculoskeletal system, not even rheumatism. On the other hand, work in cold conditions is often very demanding for muscles, tendons, joints and spine because of the high load often involved in these kinds of work. The temperature in the joints decreases faster than the temperature of the muscles. Cold joints are stiff joints, because of increasing resistance to movement due to augmented viscosity of the synovial fluid. Cold decreases the power and duration of muscle contraction. In combination with heavy work or local overload, the risk of injury increases. Furthermore, protective clothing may impair the ability to control movement of body parts, hence contributing to the risk.
Arthritis in the hand is a special problem. It is suspected that frequent cold exposure may cause arthritis, but so far the scientific evidence is poor. An existing arthritis of the hand reduces hand function in the cold and causes pain and discomfort.
Cryopathies. Cryopathies are disorders where the individual is hypersensitive to cold. The symptoms vary, including those involving the vascular system, blood, connective tissue, “allergy” and others.
Some individuals suffer from white fingers. White spots on the skin, a sensation of cold, reduced function and pain are symptoms when fingers are exposed to cold. The problems are more common among women, but above all are found in smokers and workers using vibrating tools or driving snowmobiles. Symptoms can be so troublesome that work during even slight cold exposure is impossible. Certain types of medication can also worsen the symptoms.
Cold urticaria, due to sensitized mast cells, appears as an itching erythema of cold-exposed parts of the skin. If exposure is stopped, the symptoms usually disappear within one hour. Rarely the disease is complicated with general and more threatening symptoms. If so, or if the urticaria itself is very troublesome, the individual should avoid exposure to any kind of cold.
Acrocyanosis is manifested by changes in skin colour towards cyanosis after exposure to cold. Other symptoms could be dysfunction of hand and fingers in the acrocyanotic area. The symptoms are very common, and can often be acceptably reduced by reduced cold exposure (e.g., proper clothing) or reduced nicotine use.
Psychological stress. Cold exposure, especially in combination with cold-related factors and remoteness, stresses the individual, not only physiologically but also psychologically. During work in cold climate conditions, in bad weather, over long distances and perhaps in potentially dangerous situations, the psychological stress can disturb or even deteriorate the individual’s psychological function so much that work cannot be safely done.
Smoking and snuffing. The unhealthy long-term effects of smoking and, to some extent, snuffing are well known. Nicotine increases peripheral vasoconstriction, reduces dexterity and raises the risk of cold injury.
Alcohol. Drinking alcohol gives a pleasant feeling of warmth, and it is generally thought that the alcohol inhibits cold-induced vasoconstriction. However, experimental studies on humans during relatively short exposures to cold have shown that alcohol does not interfere with heat balance to any greater extent. However, shivering becomes impaired and, combined with strenuous exercise, the heat loss will become obvious. Alcohol is known to be a dominant cause of death in urban hypothermia. It gives a feeling of bravado and influences judgement, leading to ignoring prophylactic measures.
Pregnancy. During pregnancy women are not more sensitive to cold. To the contrary, they can be less sensitive, due to raised metabolism. Risk factors during pregnancy are combined with the cold-related factors such as accident risks, clumsiness due to clothing, heavy lifting, slipping and extreme working positions. The health care system, the society and the employer should therefore pay extra attention to the pregnant woman in cold work.
Pharmacology and cold
Negative side effects of drugs during cold exposure could be thermoregulatory (general or local), or the effect of the drug can be altered. As long as the worker retains normal body temperature, most prescribed drugs don’t interfere with performance. However, tranquilizers (e.g., barbiturates, benzodiazepines, phentothiazides as well as cyclic antidepressants) may disturb vigilance. In a threatening situation the defence mechanisms against hypothermia may be impaired and the awareness of the hazardous situation is reduced.
Beta-blockers induce peripheral vasoconstriction and decrease the tolerance to cold. If an individual needs medication and has cold exposure in his or her working situation, attention should be paid to negative side effects of these drugs.
On the other hand, no drug or anything else drunk, eaten or otherwise administered to the body has been shown to be able to raise normal heat production, for example in an emergency situation when hypothermia or a cold injury threatens.
Health control programme
Health risks connected to cold stress, cold-related factors and accidents or trauma are known only to a limited extent. There is a large individual variation in capacities and health status, and this requires careful consideration. As previously mentioned, special diseases, medication and some other factors may render a person more susceptible to the effects of cold exposure. A health control programme should be part of the employment procedure, as well as a repeated activity for the staff. Table 6 specifies factors to control for in different types of cold work.
Table 6. Recommended components of health control programs for personnel exposed to cold stress and cold-related factors
|
Factor |
Outdoor work |
Cold store work |
Arctic and subarctic work |
|
Infectious diseases |
** |
** |
*** |
|
Cardio-vascular diseases |
*** |
** |
*** |
|
Metabolic diseases |
** |
* |
*** |
|
Musculoskeletal problems |
*** |
* |
*** |
|
Cryopathies |
** |
** |
** |
|
Psychological stress |
*** |
** |
*** |
|
Smoking and snuffing |
** |
** |
** |
|
Alcohol |
*** |
** |
*** |
|
Pregnancy |
** |
** |
*** |
|
Medication |
** |
* |
*** |
*= routine control, **= important factor to consider, ***= very important factor to consider.
Prevention of Cold Stress
Human adaptation
With repeated exposures to cold conditions, people perceive less discomfort and learn to adjust to and cope with conditions in an individual and more efficient way, than at the onset of exposure. This habituation reduces some of the arousal and distraction effect, and improves judgement and precaution.
Behaviour
The most apparent and natural strategy for prevention and control of cold stress is that of precaution and intentional behaviour. Physiological responses are not very powerful in preventing heat losses. Humans are, therefore, extremely dependent on external measures such as clothing, shelter and external heat supply. The continuous improvement and refinement of clothing and equipment provides one basis for successful and safe exposures to cold. However, it is essential that products be adequately tested in accordance with international standards.
Measures for prevention and control of cold exposure are often the responsibility of the employer or the supervisor. However, the efficiency of protective measures relies to a significant degree upon knowledge, experience, motivation and ability of the individual worker to make the necessary adjustments to his or her requirements, needs and preferences. Hence, education, information and training are important elements in health control programmes.
Acclimatization
There is evidence for different types of acclimatization to long-term cold exposure. Improved hand and finger circulation allows for the maintenance of a higher tissue temperature and produces a stronger cold-induced vasodilatation (see Figure 18). Manual performance is better maintained after repeated cold exposures of the hand.
Repeated whole-body cooling appears to enhance peripheral vasoconstriction, thereby increasing surface tissue insulation. Korean pearl-diving women showed marked increases in skin insulation during the winter season. Recent investigations have revealed that the introduction and use of wet suits reduces the cold stress so much that tissue insulation does not change.
Three types of possible adaptations have been proposed:
- increased tissue insulation (as previously mentioned)
- hypothermic reaction (“controlled” drop in core temperature)
- metabolic reaction (increased metabolism).
The most pronounced adaptations should be found with native people in cold regions. However, modern technology and living habits have reduced most extreme types of cold exposure. Clothing, heated shelters and conscious behaviour allow most people to maintain an almost tropical climate at the skin surface (micro- climate), thereby reducing cold stress. The stimuli to physiological adaptation become weaker.
Probably the most cold-exposed groups today belong to polar expeditions and industrial operations in arctic and subarctic regions. There are several indications that any eventual adaptation found with severe cold exposure (air or cold water) is of the insulative type. In other words, higher core temperatures can be kept with a reduced or unchanged heat loss.
Diet and water balance
In many cases cold work is associated with energy-demanding activities. In addition, protection against cold requires clothing and equipment weighing several kilograms. The hobbling effect of clothing increases muscular effort. Hence, given work tasks require more energy (and more time) under cold conditions. The caloric intake through food must compensate for this. An increase of the percentage of calories provided by fat should be recommended to outdoor workers.
Meals provided during cold operations must provide sufficient energy. Enough carbohydrates must be included to ensure stable and safe blood sugar levels for workers engaged in hard work. Recently, food products have been launched on the market with claims that they stimulate and increase body heat production in the cold. Normally, such products consist merely of carbohydrates, and they have so far failed in tests to perform better than similar products (chocolate), or better than expected from their energy content.
Water loss may be significant during cold exposure. First, tissue cooling causes a redistribution of blood volume, inducing “cold diuresis”. Tasks and clothing must allow for this, since it may develop rapidly and requires urgent execution. The almost dry air at subzero conditions allows a continuous evaporation from skin and respiratory tract that is not readily perceived. Sweating contributes to water loss, and should be carefully controlled and preferably avoided, due to its detrimental effect on insulation when absorbed by clothing. Water is not always readily available at subzero conditions. Outdoors it must be supplied or produced by melting snow or ice. As there is a depression of thirst it is mandatory that workers in the cold drink water frequently to eliminate the gradual development of dehydration. Water deficit may lead to reduced working capacity and increased risk of getting cold injuries.
Conditioning workers for work in the cold
By far the most effective and appropriate measures for adapting humans to cold work, are by conditioning—education, training and practice. As previously mentioned, much of the success of adjustments to cold exposure depends on behavioural action. Experience and knowledge are important elements of this behavioural process.
Persons engaged in cold work should be given a basic introduction to the specific problems of cold. They must receive information about physiological and subjective reactions, health aspects, risk of accidents, and protective measures, including clothing and first aid. They should be gradually trained for the required tasks. Only after a given time (days to weeks) should they work full hours under the extreme conditions. Table 7 provides recommendations as to the contents of conditioning programmes for various types of cold work.
Table 7. Components of conditioning programs for workers exposed to cold
|
Element |
Outdoor work |
Cold store work |
Arctic and subarctic work |
|
Health control |
*** |
** |
*** |
|
Basic introduction |
*** |
** |
*** |
|
Accident prevention |
*** |
** |
*** |
|
Basic first aid |
*** |
*** |
*** |
|
Extended first aid |
** |
* |
*** |
|
Protective measures |
*** |
** |
*** |
|
Survival training |
see text |
* |
*** |
*= routine level, **= important factor to consider, ***= very important factor to consider.
Basic introduction means education and information about the specific cold problems. Registration and analysis of accidents/injuries is the best base for preventive measures. Training in first aid should be given as a basic course for all personnel, and specific groups should get an extended course. Protective measures are natural components of a conditioning programme and are dealt with in the following section. Survival training is important for arctic and subarctic areas, and also for outdoor work in other remote areas.
Technical control
General principles
Due to the many complex factors that influence human heat balance, and the considerable individual variations, it is difficult to define critical temperatures for sustained work. The temperatures given in Figure 6 must be regarded as action levels for improvement of conditions by various measures. At temperatures below those given in figure 6, exposures should be controlled and evaluated. Techniques for assessment of cold stress and recommendations for time-limited exposures are dealt with elsewhere in this chapter. It is assumed that best protection of hands, feet and body (clothing) is available. With inappropriate protection, cooling will be expected at considerably higher temperatures.
Figure 6. Estimated temperatures at which certain thermal imbalances of the body may develop.*
Tables 8 and 9 list different preventive and protective measures that can be applied to most types of cold work. Much effort is saved with careful planning and foresight. Examples given are recommendations. It must be emphasized that the final adjustment of clothing, equipment and work behaviour must be left to the individual. Only with a cautious and intelligent integration of behaviour with the requirements of the real environmental conditions can a safe and efficient exposure be created.
Table 8. Strategies and measures during various phases of work for prevention and alleviation of cold stress
|
Phase/factor |
What to do |
|
Planning phase |
Schedule work for a warmer season (for outdoor work). Check if work can be done indoors (for outdoor work). Allow more time per task with cold work and protective clothing. Analyse suitability of tools and equipment for work. Organize work in suitable work-rest regimens, considering task, load and protection level. Provide heated space or heated shelter for recovery. Provide training for complex work tasks under normal conditions. Check medical records of staff. Ascertain appropriate knowledge and competence of staff. Provide information about risks, problems, symptoms and preventive actions. Separate goods and worker line and keep different temperature zones. Care for low velocity, low humidity and low noise level of the air- Provide extra personnel to shorten exposure. Select adequate protective clothing and other protective equipment. |
|
Before work shift |
Check climatic conditions at onset of work. Schedule adequate work-rest regimens. Allow for individual control of work intensity and clothing. Select adequate clothing and other personal equipment. Check weather and forecast (outdoors). Prepare schedule and control stations (outdoors). Organize communication system (outdoors). |
|
During work shift |
Provide for break and rest periods in heated shelter. Provide for frequent breaks for hot drinks and food. Care for flexibility in terms of intensity and duration of work. Provide for replacement of clothing items (socks, gloves, etc.). Protect from heat loss to cold surfaces. Minimize air velocity in work zones. Keep workplace clear from water, ice and snow. Insulate ground for stationary standing work places. Provide access to extra clothing for warmth. Monitor subjective reactions (buddy system) (outdoors). Report regularly to foreman or base (outdoors). Provide for sufficient recovery time after severe exposures (outdoors). Protect against wind effects and precipitation (outdoors). Monitor climatic conditions and anticipate weather change (outdoors). |
Source: Modified from Holmér 1994.
Table 9. Strategies and measures related to specific factors and equipment
|
Behaviour |
Allow for time to adjust clothing. Prevent sweating and chilling effects by making adjustments of clothing in due time before change in work rate and/or exposure. Adjust work rate (keep sweating minimal). Avoid rapid shifts in work intensity. Allow for adequate intake of hot fluid and hot meals. Allow for time to return to protected areas (shelter, warm room) (outdoors). Prevent wetting of clothing from water or snow. Allow for sufficient recovery in protected area (outdoors). Report on progress of work to foreman or base (outdoors). Report major deviations from plan and schedule (outdoors). |
|
Clothing |
Select clothing you have previous experience with. With new clothing, select tested garments. Select insulation level on the basis of anticipated climate and activity. Care for flexibility in clothing system to allow for great adjustment of insulation. Clothing must be easy to put on and take off. Reduce internal friction between layers by proper selection of fabrics. Select size of outer layers to make room for inner layers. Use multi-layer system: —inner layer for micro climate control —middle layer for insulation control —outer layer for environmental protection. Inner layer should be non-absorbent to water, if sweating cannot be sufficiently controlled. Inner layer may be absorbent, if sweating is anticipated to be none or low. Inner layer may consist of dual-function fabrics, in the sense that fibre in contact with skin is non absorbing and fibres next to the middle layer is absorbing water or moisture. Middle layer should provide loft to allow stagnant air layers. Middle layer should be form-stable and resilient. Middle layer may be protected by vapour barrier layers. Garments should provide sufficient overlap in the waist and back region. Outer layer must be selected according to additional protection requirements, such as wind, water, oil, fire, tear or abrasion. Design of outer garment must allow easy and extensive control of openings at neck, sleeves, wrists etc., to regulate ventilation of interior space. Zippers and other fasteners must function also with snow and windy conditions. Buttons should be avoided. Clothing shall allow operation even with cold, clumsy fingers. Design must allow for bent postures without compression of layers and loss of insulation. Avoid unnecessary constrictions. Carry extra wind proof blankets (NOTE! The aluminized “astronaut blanket” does not protect more than expected from being wind proof. A large polyethylene garbage bag has the same effect). |
|
Education Training |
Provide education and information on the special problems of cold. Provide information and training in first-aid and treatment of cold injuries. Test machinery, tools and equipment in controlled cold conditions. Select tested goods, if available. Train complex operations under controlled cold conditions. Inform about accidents and accident prevention. |
|
Handwear |
Mittens provide the best overall insulation. Mittens should allow fine gloves to be worn underneath. Prolonged exposures requiring fine hand work, must be intercepted by frequent warm-up breaks. Pocket heaters or other external heat sources may prevent or delay hand cooling. Sleeve of clothing must easily accommodate parts of gloves or mittens —underneath or on top. Outer garment must provide easy storage or fixing of handwear when taken off. |
|
Footwear |
Boots shall provide high insulation to the ground (sole). Sole shall be made of a flexible material and have an anti-slippery pattern. Select size of boot so it can accommodate several layers of socks and an insole. Ventilation of most footwear is poor, so moisture should be controlled by frequent replacement of socks and insole. Control moisture by vapour barrier between inner and outer layer. Allow boots to dry completely between shifts. Legs of clothing must easily accommodate parts of boots —underneath or on top. |
|
Headgear |
Flexible headgear comprises an important instrument for control of heat and whole-body heat losses. Headgear should be windproof. Design should allow sufficient protection of ears and neck. Design must accommodate other types of protective equipment (e.g., ear muffs, safety goggles). |
|
Face |
Face mask should be windproof and insulative. No metallic details should contact skin. Significant heating and humidification of inspired air can be achieved by special breathing masks or mouth pieces. Use safety goggles outdoors, especially in sleet and snow. Use eye protection against ultra-violet radiation and glare. |
|
Equipment Tools |
Select tools and equipment intended and tested for cold conditions. Choose design that allows operation by gloved hands. Prewarm tools and equipment. Store tools and equipment in heated space. Insulate handles of tools and equipment. |
|
Machinery |
Select machinery intended for operation in cold environments. Store machinery in protected space. Prewarm machinery before use. Insulate handles and controls. Design handles and controls for operation by gloved hands. Prepare for easy repair and maintenance under adverse conditions. |
|
Workplace |
Keep air velocity as low as possible. Use wind-breaking shields or windproof clothing. Provide insulation to ground with prolonged standing, kneeling or lying work. Provide auxiliary heating with light, stationary work. |
Source: Modified from Holmér 1994.
Some recommendations as to the climatic conditions under which certain measures should be taken have been given by the American Conference of Governmental Industrial Hygienists (ACGIH 1992). The fundamental requirements are that:
- workers be provided with sufficient and appropriate protective clothing
- special precautions should be taken for older workers or workers with circulatory problems.
Further recommendations related to the provision of hand protection, to workplace design and to work practices are presented below.
Hand protection
Fine barehanded operations below 16ºC require provision for heating the hands. Metal handles of tools and bars should be covered by insulating materials at temperatures below –1ºC. Anticontact gloves should be worn when surfaces at –7ºC or lower are within reach. At –17ºC insulative mittens must be used. Evaporative liquids at temperatures below 4 °C should be handled so as to avoid splashes to bare or poorly protected skin areas.
Work practices
Below –12ºC Equivalent Chill Temperature, workers should be under constant supervision (buddy system). Many of the measures given in Table 18 apply. With lowered temperatures it is increasingly important that workers are instructed in safety and health procedures.
Workplace design
Workplaces must be shielded from wind, and air velocities kept below 1 m/s. Wind-protective clothing should be used when appropriate. Eye protection must be supplied for special outdoor conditions with sunshine and snow-covered ground. Medical screening is recommended for persons working routinely in cold below –18ºC. Recommendations as to workplace monitoring include the following:
- Suitable thermometry should be arranged when the temperature is below 16ºC.
- Indoor wind speeds should be monitored at least every 4 hours.
- Outdoor work requires measurement of wind speed and air temperatures below –1ºC.
- The Equivalent Chill Temperature should be determined for combinations of wind and air temperature.
Most of the recommendations in Tables 8 and 9 are pragmatic and straightforward.
Clothing is the most important measure for individual control. The multi-layer approach allows for more flexible solutions than single garments incorporating the function of several layers. In the end, however, the specific needs of the worker should be the ultimate determinant of what would be the most functional system. Clothing protects against cooling. On the other hand overdressing in the cold is a common problem, also reported from the extreme exposures of arctic expeditions. Overdressing may rapidly result in large amounts of sweat, which accumulates in clothing layers. During periods of low activity, the drying of moist clothing increases body heat loss. The obvious preventive measure is to control and reduce sweating by appropriate selection of clothing and early adjustments to changes in work rate and climate conditions. There is no clothing fabric that can absorb large amounts of sweat and also preserve good comfort and insulative properties. Wool remains lofty and apparently dry despite absorption of some water (moisture regain), but large amounts of sweat will condense and cause problems similar to those of other fabrics. The moisture yields some heat liberation and may contribute to the preservation of warmth. However, when the wool garment dries on the body, the process reverses as discussed above, and the person is inevitably cooled.
Modern fibre technology has produced many new materials and fabrics for clothing manufacturing. Garments are now available that combine waterproofness with good water vapour permeability, or high insulation with reduced weight and thickness. It is essential, however, to select garments with guaranteed tested properties and functions. Many products are available that try to mimic the more expensive original products. Some of them represent such poor quality that they may even be hazardous to use.
Protection against cold is determined primarily by the thermal insulation value of the complete clothing ensemble (clo value). However, properties such as air permeability, vapour permeability and waterproofness of the outer layer in particular are essential for cold protection. International standards and test methods are available for measuring and classifying these properties. Similarly, handgear and footwear may be tested for their cold-protective properties using international standards such as European standards EN 511 and EN 344 (CEN 1992, 1993).
Outdoor cold work
Specific problems of outdoor cold work are the aggregate of climatic factors that may result in cold stress. The combination of wind and low air temperature significantly increases the cooling power of the environment, which has to be considered in terms of work organization, workplace shielding and clothing. Precipitation, either in the air as snow or rain, or on the ground, requires adjustments. The variation in weather conditions requires workers to plan for, bring and use additional clothing and equipment.
Much of the problem in outdoor work relates to the sometimes great variations in activity and climate during a work shift. No clothing system is available that can accommodate such large variations. Consequently, clothing must be frequently changed and adjusted. Failure to do so may result in cooling due to insufficient protection, or sweating and overheating caused by too much clothing. In the latter case, most of the sweat condenses or is absorbed by clothing. During periods of rest and low activity, wet clothing represents a potential hazard, since its drying drains the body of heat.
Protective measures for outdoor work include appropriate work-rest regimens with rest pauses taken in heated shelters or cabins. Stationary work tasks can be protected from wind and precipitation by tents with or without additional heating. Spot heating by infrared or gas heaters may be used for certain work tasks. Prefabrication of parts or components may be carried out indoors. Under subzero conditions, workplace conditions including weather should be regularly monitored. Clear rules must exist regarding what procedures to apply when conditions get worse. Temperature levels, eventually corrected for wind (wind chill index), should be agreed upon and linked to an action programme.
Cold storage work
Frozen food requires storage and transportation at low ambient temperatures (–20ºC). Work in cold stores can be found in most parts of the world. This kind of artificial cold exposure is characterized by a constant, controlled climate. Workers may perform continuous work or, most common, intermittent work, shifting between cold and temperate or warm climates outside the storehouse.
As long as work requires some physical effort, heat balance can be achieved by selecting appropriate protective clothing. The special problems of hand and feet often require regular breaks every 1.5 to 2 hours. The break must be long enough to allow rewarming (20 minutes).
Manual handling of frozen goods requires protective gloves with sufficient insulation (in particular, of the palm of the hand). Requirements and test methods for cold-protective gloves are given in the European standard EN 511, which is described in more detail in the article “Cold indices and standards” in this chapter. Local heaters (e.g., infrared radiator), placed in workplaces with stationary work, improve heat balance.
Much work in cold stores is carried out with fork-lifts. Most of these vehicles are open. Driving creates a relative wind speed, which in combination with the low temperature increases body cooling. In addition, the work itself is rather light and the associated metabolic heat production low. Accordingly, the required clothing insulation is quite high (around 4 clo) and cannot be met with most types of overalls in use. The driver gets cold, starting with feet and hands, and exposure has to be time limited. Depending on available protective clothing, appropriate work schedules should be organized in terms of work in cold and work or rest in normal environments. A simple measure to improve heat balance is to install a heated seat in the truck. This may prolong work time in the cold and prevent local cooling of the seat and back. More sophisticated and expensive solutions include the use of heated cabs.
Special problems arise in hot countries, where the cold store worker, usually the truck driver, is intermittently exposed to cold (–30ºC) and heat (30ºC). Brief exposures (1 to 5 min) to each condition make it difficult to adopt suitable clothing—it may be too warm for the outdoor period and too cold for the cold store work. Truck cabs may be one solution, once the problem of condensation upon windows is solved. Appropriate work-rest regimens must be elaborated and based on work tasks and available protection.
Cool workplaces, found for example in the fresh food industry, comprise climatic conditions with air temperatures of +2 to +16ºC, depending on type. Conditions are sometimes characterized by high relative humidities, inducing condensation of water at cold spots and moist or water-covered floors. The risk of slipping is increased in such workplaces. Problems can be solved by good workplace hygiene and cleaning routines, which contribute to reducing the relative humidity.
The local air velocity of work stations is often too high, resulting in complaints of draught. The problems can often be solved by changing or adjusting the inlets for cold air or by rearranging work stations. Buffers of frozen or cold goods close to work stations may contribute to draught sensation due to the increased radiation heat exchange. Clothing must be selected on the basis of an assessment of the requirements. The IREQ method should be used. In addition clothing should be designed to protect from local draught, moisture and water. Special hygienic requirements for food handling put some restrictions on design and type of clothing (i.e., the outer layer). An appropriate clothing system must integrate underwear, insulating middle layers and the outer layer to form a functional and sufficient protective system. Headgear is often required due to hygienic demands. However, existing headgear for this purpose is often a paper cap, which does not offer any protection against cold. Similarly, footwear often comprises clogs or light shoes, with poor insulation properties. Selection of more suitable headgear and footwear should better preserve warmth of these body parts and contribute to an improved general heat balance.
A special problem in many cool workplaces is the preservation of manual dexterity. Hands and fingers cool rapidly when muscular activity is low or moderate. Gloves improve protection but impair dexterity. A delicate balance between the two demands has to be found. Cutting meat often requires a metal glove. A thin textile glove worn underneath may reduce the cooling effect and improve comfort. Thin gloves may be sufficient for many purposes. Additional measures to prevent hand cooling include the provision of insulated handles of tools and equipment or spot heating using, for example, infrared radiators. Electrically heated gloves are on the market, but often suffer from poor ergonomics and insufficient heating or battery capacity.
Cold-water exposure
During immersion of the body in water the potential for large losses of heat in a short time is great and presents an apparent hazard. The heat conductivity of water is more than 25 times higher than that of air, and in many exposure situations the capacity of surrounding water to absorb heat is effectively infinite.
Thermoneutral water temperature is around 32 to 33ºC, and at lower temperatures the body responds by cold vasoconstriction and shivering. Long exposures in water at temperatures between 25 and 30ºC provoke body cooling and progressive development of hypothermia. Naturally, this response becomes stronger and more serious with the lowering of the water temperature.
Exposure to cold water is common in accidents at sea and in conjunction with water sports of various kinds. However, even in occupational activities, workers run the risk of immersion hypo-thermia (e.g., diving, fishing, shipping and other offshore operations).
Victims of shipwrecks may have to enter cold water. Their protection varies from pieces of thin clothing to immersion suits. Lifejackets are mandatory equipment aboard ships. They should be equipped with a collar to reduce heat loss from the head of unconscious victims. The equipment of the ship, the efficiency of the emergency procedures and the behaviour of crew and passengers are important determinants for the success of the operation and the subsequent exposure conditions.
Divers regularly enter cold waters. The temperature of most waters with commercial diving, in particular at some depth, is low—often lower than 10ºC. Any prolonged exposure in such cold water requires thermally insulated diving suits.
Heat loss. Heat exchange in the water may be seen as simply a flow of heat down two temperature gradients—one internal, from core to skin, and one external, from the skin surface to the surrounding water. Body surface heat loss can be simply described by:
Cw = hc·(Tsk–Tw)·AD
where Cw is the rate of convective heat loss (W), hc is the convective heat transfer coefficient (W/°Cm2), Tsk is the average skin temperature (°C), Tw is the water temperature (°C) and AD is the body surface area. The small components of heat loss from respiration and from non-immersed parts (e.g., head) can be neglected (see the section on diving below).
The value of hc is in the range of 100 to 600 W/°Cm2. The lowest value applies to still water. Turbulence, be it caused by swimming movements or flowing water, doubles or triples the convection coefficient. It is easily understood that the unprotected body may suffer a considerable heat loss to the cold water—eventually exceeding what can be produced even with heavy exercise. In fact, a person (dressed or undressed) who falls into cold water in most cases saves more heat by lying still in the water than by swimming.
Heat loss to the water can be significantly reduced by wearing special protective suits.
Diving. Diving operations several hundreds of metres below sea level must protect the diver from the effects of pressure (one ATA or 0.1 MPa/10 m) and cold. Breathing cold air (or a cold gas mixture of helium and oxygen) drains the lung tissues of body heat. This direct heat loss from the body core is large at high pressures and can easily achieve values higher than the resting metabolic heat production of the body. It is poorly sensed by the human organism. Dangerously low internal temperatures may develop without a shivering response if the body surface is warm. Modern offshore work requires the diver to be supplied with extra heat to the suit as well as to the breathing apparatus, to compensate for large convective heat losses. In deep-sea diving, the comfort zone is narrow and warmer than at sea level: 30 to 32ºC at 20 to 30 ATA (2 to 3 MPa) and increasing to 32 to 34ºC up to 50 ATA (5 MPa).
Physiological factors: Cold immersion elicits a strong, acute respiratory drive. The initial responses include an “inspiratory gasp”, hyperventilation, tachycardia, peripheral vasoconstriction and hypertension. An inspiratory apnoea for several seconds is followed by an increased ventilation. The response is almost impossible to control voluntarily. Hence, a person may easily inhale water if the sea is rough and the body becomes submersed. The first seconds of exposure to very cold water, accordingly, are dangerous, and sudden drowning may occur. Slow immersion and proper protection of the body reduce the reaction and allow for better control of respiration. The reaction gradually fades and normal breathing is usually achieved within a few minutes.
The rapid rate of heat loss at the skin surface emphasizes the importance of internal (physiological or constitutional) mechanisms for reducing the core-to-skin heat flow. Vasoconstriction reduces extremity blood flow and preserves central heat. Exercise increases extremity blood flow, and, in conjunction with the increased external convection, it may in fact accelerate heat loss despite the elevated heat production.
After 5 to 10 min in very cold water, extremity temperature drops quickly. Neuromuscular function deteriorates and the ability to coordinate and control muscular performance degrades. Swimming performance may be severely reduced and quickly put the person at risk in open waters.
Body size is another important factor. A tall person has a larger body surface area and loses more heat than a small person at given ambient conditions. However, the relatively larger body mass compensates for this in two ways. Metabolic heat production rate increases in relation to the larger surface area, and the heat content at a given body temperature is greater. The latter factor comprises a larger buffer to heat losses and a slower rate of core temperature decrease. Children are at a greater risk than adults.
By far the most important factor is body fat content—in parti-cular, subcutaneous fat thickness. Adipose tissue is more insulating than other tissues and is bypassed by much of the peripheral circulation. Once vasoconstriction has occurred, the layer of subcutaneous fat acts as an extra layer. The insulative effect is almost linearly related to the layer thickness. Accordingly, women in general have more cutaneous fat than men and lose less heat under the same conditions. In the same way, fat persons are better off than lean persons.
Personal protection. As previously mentioned, prolonged stay in cold and temperate waters requires additional external insulation in the form of diving suits, immersion suits or similar equipment. The wet suit of foamed neoprene provides insulation by the thickness of the material (closed foam cells) and by the relatively controlled “leakage” of water to the skin microclimate. The latter phenomenon results in the warming of this water and the establishment of a higher skin temperature. Suits are available in various thickness, providing more or less insulation. A wet suit compresses at depth and loses thereby much of its insulation.
The dry suit has become standard at temperatures below 10ºC. It allows the maintenance of a higher skin temperature, depending on the amount of extra insulation worn under the suit. It is a fundamental requirement that the suit not leak, as small amounts of water (0.5 to 1 l) seriously reduce the insulative power. Although the dry suit also compresses at depth, dry air is automatically or manually added from the scuba tank to compensate for the reduced volume. Hence, a microclimate air layer of some thickness can be maintained, providing good insulation.
As previously mentioned, deep-sea diving requires auxiliary heating. Breathing gas is prewarmed and the suit is heated by the flushing of warm water from the surface or the diving bell. More recent warming techniques rely upon electrically heated underwear or closed-circuit tubules filled with warm fluid.
Hands are particularly susceptible to cooling and may require extra protection in the form of insulative or heated gloves.
Safe exposures. The rapid development of hypothermia and the imminent danger of death from cold-water exposure necessitates some sort of prediction of safe and unsafe exposure conditions.
Figure 7 depicts predicted survival times for typical North Sea offshore conditions. The applied criterion is a drop in core temperature to 34ºC for the tenth percentile of the population. This level is assumed to be associated with a conscious and manageable person. The proper wearing, use and functioning of a dry suit doubles the predicted survival time. The lower curve refers to the unprotected person immersed in normal clothing. As clothing gets completely soaked with water the effective insulation is very small, resulting in short survival times (modified from Wissler 1988).
Figure 7. Predicted survival times for typical North Sea offshore scenarios.
Work in arctic and subarctic regions
Arctic and subarctic regions of the world comprise additional problems to those of normal cold work. The cold season coincides with darkness. Days with sunlight are short. These regions cover vast, unpopulated or sparsely populated areas, such as Northern Canada, Siberia and Northern Scandinavia. In addition nature is harsh. Transportation takes place over large distances and takes a long time. The combination of cold, darkness and remoteness require special consideration in terms of work organization, preparation and equipment. In particular, training in survival and first aid must be provided and the appropriate equipment supplied and made easily available at work.
For the working population in the arctic regions there are many health-threatening hazards, as mentioned elsewhere. The risks of accident and injury are high, drug abuse is common, cultural patterns produce problems, as does the confrontation between local/native culture and modern western industrial demands. Snowmobile driving is an example of multiple-risk exposure in typical arctic conditions (see below). Cold stress is thought to be one of the risk factors that produces higher frequencies of certain diseases. Geographical isolation is another factor producing different types of genetic defects in some native areas. Endemic diseases—for example, certain infectious diseases—are also of local or regional importance. Settlers and guest workers also run a higher risk for different kinds of psychological stress reactions secondary to new environment, remoteness, harsh climate conditions, isolation and awareness.
Specific measures for this kind of work must be considered. Work must be carried out in groups of three, so that in case of emergency, one person may go for help while one is left taking care of the victim of, for example, an accident. The seasonal variation in daylight and climate must be considered and work tasks planned accordingly. Workers must be checked for health problems. If required, extra equipment for emergency or survival situations must be available. Vehicles such as cars, trucks or snowmobiles must carry special equipment for repair and emergency situations.
A specific work problem in these regions is the snowmobile. Since the sixties the snowmobile has developed from a primitive, low-technology vehicle to one that is fast and technically highly developed. It is most frequently used for leisure activities, but also for work (10 to 20%). Typical professions using the snowmobile are police, military personnel, reindeer herders, lumberjacks, farmers, tourist industry, trappers and search and rescue teams.
The vibration exposure from a snowmobile means a highly increased risk for vibration-induced injuries to the driver. The driver and the passengers are exposed to unpurified exhaust gas. The noise produced by the engine may induce hearing loss. Due to high speed, terrain irregularities and poor protection for the driver and the passengers, the risk of accidents is high.
The musculoskeletal system is exposed to vibrations and extreme working positions and loads, especially when driving in harsh terrain areas or slopes. If you get stuck, handling the heavy engine induces perspiration and often musculoskeletal problems (e.g., lumbago).
Cold injuries are common among snowmobile workers. The speed of the vehicle aggravates the cold exposure. Typical injured parts of the body are especially the face (could in extreme cases include cornea), ears, hands and feet.
Snowmobiles are usually used in remote areas where climate, terrain and other conditions contribute to the risks.
The snowmobile helmet must be developed for the working situation on the snowmobile with attention to the specific exposure risks produced by the vehicle itself, terrain conditions and climate. Clothing must be warm, windproof and flexible. The activity transients experienced during snowmobile riding are difficult to accommodate in one clothing system and require special consideration.
Snowmobile traffic in remote areas also presents a communication problem. Work organization and equipment should ensure safe communication with the home base. Extra equipment must be carried to handle emergency situations and allow protection for a time long enough for the rescue team to function. Such equipment includes, for example, wind sack, extra clothing, first-aid equipment, snow shovel, repair kit and cooking gear.
Prevention of Cold Stress in Extreme Outdoor Conditions
The prevention of the physiopathological effects of exposure to cold must be considered from two points of view: the first concerns the physiopathological effects observed during general exposure to cold (that is, the entire body), and the second concerns those observed during local exposure to cold, mainly affecting the extremities (hands and feet). Preventive measures in this connection aim to reduce the incidence of the two main types of cold stress—accidental hypothermia and frostbite of the extremities. A twofold approach is required: physiological methods (e.g., adequate feeding and hydration, development of adaptational mechanisms) and pharmacological and technological measures (e.g., shelter, clothing). Ultimately all these methods aim to increase tolerance to cold at both the general and local levels. Moreover, it is essential that workers exposed to cold have the information and the understanding of such injury needed to ensure effective prevention.
Physiological Methods for Preventing Cold Injury
Exposure to cold in the human being at rest is accompanied by peripheral vasoconstriction, which limits cutaneous heat loss, and by metabolic heat production (essentially by means of the activity of shivering), which implies the necessity of food intake. The expenditure of energy required by all physical activity in the cold is increased on account of the difficulty of walking in snow or on ice and the frequent need to deal with heavy equipment. Moreover, water loss may be considerable on account of the sweating associated with this physical activity. If this water loss is not compensated for, dehydration may occur, increasing susceptibility to frostbite. The dehydration is often aggravated not only by voluntary restriction of water intake because of the difficulty of taking in adequate fluid (available water may be frozen, or one may have to melt snow) but also by the tendency to avoid adequately frequent micturition (urination), which requires leaving the shelter. The need for water in the cold is difficult to estimate because it depends on the individual’s workload and on the insulation of the clothing. But in any case, fluid intake must be abundant and in the form of hot drinks (5 to 6 l per day in the case of physical activity). Observation of the colour of the urine, which must remain clear, gives a good indication of the course of fluid intake.
As regards caloric intake, it may be assumed that an increase of 25 to 50% in a cold climate, as compared with temperate or hot climates, is necessary. A formula allows the calculation of the caloric intake (in kcal) essential for energy equilibrium in the cold per person and per day: kcal/person per day = 4,151–28.62Ta, where Ta is the ambient temperature in °C (1 kcal = 4.18 joule). Thus, for a Ta of –20ºC, a need for about 4,723 kcal (2.0 x 104 J) must be anticipated. Food intake does not seem to have to be modified qualitatively in order to avoid digestive troubles of the diarrhoea type. For example, the cold weather ration (RCW) of the United States Army consists of 4,568 kcal (1.9 x 104 J), in dehydrated form, per day and per person, and is divided qualitatively as follows: 58% carbohydrate, 11% protein and 31% fat (Edwards, Roberts and Mutter 1992). Dehydrated foods have the advantage of being light and easy to prepare, but they have to be rehydrated before consumption.
As far as possible, meals must be taken hot and divided into breakfast and lunch in normal amounts. A supplement is provided by hot soups, dry biscuits and cereal bars nibbled throughout the day, and by increasing the caloric intake at dinner. This lattermost expedient augments diet-induced thermogenesis and helps the subject to fall asleep. The consumption of alcohol is extremely inadvisable in a cold climate because alcohol induces cutaneous vasodilatation (a source of heat loss) and increases diuresis (a source of water loss), while modifying the sensitivity of the skin and impairing the judgement (which are basic factors involved in recognizing the first signs of cold injury). Excessive consumption of drinks containing caffeine is also harmful because this substance has a peripheral vasoconstrictor effect (increased risk of frostbite) and a diuretic effect.
In addition to adequate food, the development of both general and local adaptational mechanisms can reduce the incidence of cold injury and improve psychological and physical performance by reducing the stress caused by a cold environment. However, it is necessary to define the concepts of adaptation, acclimatization and habituation to cold, the three terms varying in their implications according to the usage of different theorists.
In Eagan’s view (1963), the term adaptation to cold is a generic term. He groups under the concept of adaptation the concepts of genetic adaptation, acclimatization and habituation. Genetic adaptation refers to physiological changes transmitted genetically that favour survival in a hostile environment. Bligh and Johnson (1973) differentiate between genetic adaptation and phenotypic adaptation, defining the concept of adaptation as “changes which reduce the physiological strain produced by a stressful component of the total environment”.
Acclimatization may be defined as functional compensation that is established over a period of several days to several weeks in response either to complex factors of the surroundings such as climatic variations in a natural environment, or to a unique factor in the surroundings, such as in the laboratory (the “artificial acclimatization” or “acclimation” of those writers) (Eagan 1963).
Habituation is the result of a change in physiological responses resulting from a diminution in the responses of the central nervous system to certain stimuli (Eagan 1963). This habituation can be specific or general. Specific habituation is the process involved when a certain part of the body becomes accustomed to a repeated stimulus, while general habituation is that by which the whole body becomes accustomed to a repeated stimulus. Local or general adaptation to cold is generally acquired through habituation.
Both in the laboratory and in natural surroundings, different types of general adaptation to cold have been observed. Hammel (1963) established a classification of these different adaptational types. The metabolic type of adaptation is shown by maintenance of the internal temperature combined with a greater production of metabolic heat, as in the Alacalufs of Tierra del Fuego or the Indians of the Arctic. Adaptation of the insulational type is also shown by maintenance of the internal temperature but with a diminution in the mean cutaneous temperature (aborigines of the tropical coast of Australia). Adaptation of the hypothermal type is shown by a more or less considerable fall in the internal temperature (tribe of the Kalahari Desert, Quechua Indians of Peru). Finally, there is adaptation of mixed isolational and hypothermal type (aborigines of central Australia, Lapps, Amas Korean divers).
In reality, this classification is merely qualitative in character and does not take into account all the components of thermal balance. We have therefore recently proposed a classification that is not only qualitative but also quantitative (see Table 1). Modification in body temperature alone does not necessarily indicate the existence of general adaptation to cold. Indeed, a change in the delay in starting to shiver is a good indication of the sensitivity of the thermoregulatory system. Bittel (1987) has also proposed reduction in the thermal debt as an indicator of adaptation to cold. In addition, this author demonstrated the importance of the caloric intake in the development of adaptational mechanisms. We have confirmed this observation in our laboratory: subjects acclimatized to cold in the laboratory at 1 °C for 1 month in a discontinuous manner developed an adaptation of the hypothermal type (Savourey et al. 1994, 1996). The hypothermia is directly related to the reduction in the percentage of the body’s fat mass. The level of aerobic physical aptitude (VO2max) does not seem to be involved in the development of this type of adaptation to cold (Bittel et al. 1988; Savourey, Vallerand and Bittel 1992). Adaptation of the hypothermal type appears to be the most advantageous because it maintains the energy reserves by delaying the onset of shivering but without the hypothermia’s being dangerous (Bittel et al. 1989). Recent work in the laboratory has shown that it is possible to induce this type of adaptation by subjecting people to intermittent localized immersion of the lower limbs in iced water. Moreover, this type of acclimatization has developed a “polar tri-iodothyronine syndrome” described by Reed and co-workers in 1990 in subjects who had spent long periods in the polar region. This complex syndrome remains imperfectly understood and is evidenced mainly by a diminution in the pool of total tri-iodothyronine both when the environment is thermally neutral and during acute exposure to cold. The relationship between this syndrome and adaptation of the hypo-thermal type has yet to be defined, however (Savourey et al. 1996).
Table 1. General adaptational mechanisms to cold studied during a standard cold test carried out before and after a period of acclimatization.
|
Measure |
Use of measure as indicator |
Change in |
Type of adaptation |
|
Rectal |
Difference between tre at the end of the cold test and tre at thermal neutrality after acclimatization |
+ or = |
normothermal |
|
|
|
|
|
|
|
|
|
|
Local adaptation of the extremities is well documented (LeBlanc 1975). It has been studied both in native tribes or professional groups naturally exposed to cold in the extremities (Eskimos, Lapps, fishermen on the island of Gaspé, English fish carvers, letter carriers in Quebec) and in subjects artificially adapted in the laboratory. All these studies have shown that this adaptation is evidenced by higher skin temperatures, less pain and earlier paradoxical vasodilatation that occurs at higher skin temperatures, thus permitting the prevention of frostbite. These changes are basically connected with an increase in peripheral skin blood flow and not with local production of heat at the muscular level, as we have recently shown (Savourey, Vallerand and Bittel 1992). Immersion of the extremities several times a day in cold water (5ºC) over several weeks is sufficient to induce the establishment of these local adaptational mechanisms. On the other hand, there are few scientific data on the persistence of these different types of adaptation.
Pharmacological Methods for Preventing Cold Injury
The use of drugs to enhance tolerance to cold has been the subject of a number of studies. General tolerance to cold can be enhanced by favouring thermogenesis with drugs. Indeed, it has been shown in human subjects that the activity of shivering is accompanied notably by an increase in the oxidation of carbohydrates, combined with an increased consumption of muscular glycogen (Martineau and Jacob 1988). Methylxanthinic compounds exert their effects by stimulating the sympathetic system, exactly like cold, thereby increasing the oxidation of carbohydrates. However, Wang, Man and Bel Castro (1987) have shown that theophylline was ineffective in preventing the fall in body temperature in resting human subjects in the cold. On the other hand, the combination of caffeine with ephedrine permits a better maintenance of body temperature under the same conditions (Vallerand, Jacob and Kavanagh 1989), while the ingestion of caffeine alone modifies neither the body temperature nor the metabolic response (Kenneth et al. 1990). The pharmacological prevention of the effects of cold at the general level is still a matter for research. At the local level, few studies have been carried out on the pharmacological prevention of frostbite. Using an animal model for frostbite, a certain number of drugs were tested. Platelet anti-aggregants, corticoids and also various other substances had a protective effect provided that they were administered before the rewarming period. To our knowledge, no study has been carried out in humans on this subject.
Technical Methods for PreventingCold Injury
These methods are a basic element in the prevention of cold injury, and without their use human beings would be incapable of living in cold climatic zones. The construction of shelters, the use of a source of heat and also the use of clothing permit people to live in very cold regions by creating a favourable ambient microclimate. However, the advantages provided by civilization are sometimes not available (in the case of civil and military expeditions, shipwrecked persons, injured persons, vagrants, victims of avalanches, etc.). These groups are therefore particularly liable to cold injury.
Precautions for Work in the Cold
The problem of conditioning for work in the cold relates mainly to people who are not accustomed to work in the cold and/or who come from temperate climatic zones. Information on injury that can be caused by cold is of basic importance, but it is also necessary to acquire information about a certain number of types of behaviour too. Every worker in a cold zone must be familiar with the first signs of injury, especially local injury (skin colour, pain). Behaviour as regards clothing is vital: several layers of clothing permit the wearer to adjust the insulation given by clothing to current levels of energy expenditure and external stress. Wet garments (rain, sweat) must be dried. Every attention must be given to the protection of the hands and feet (no tight bandages, attention to adequate covering, timely changing of socks—say twice or three times a day—because of sweating). Direct contact with all cold metallic objects must be avoided (risk of immediate frostbite). The clothing must be guaranteed against cold and tested before any exposure to cold. Feeding rules should be remembered (with attention to caloric intake and hydration needs). Abuse of alcohol, caffeine and nicotine must be forbidden. Accessory equipment (shelter, tents, sleeping bags) must be checked. Condensation in tents and sleeping bags must be removed in order to avoid ice formation. Workers must not blow into their gloves to warm them or this will also cause the formation of ice. Finally, recommendations should be made for improving physical fitness. Indeed, a good level of aerobic physical fitness allows greater thermogenesis in severe cold (Bittel et al. 1988) but also ensures better physical endurance, a favourable factor because of the extra energy loss from physical activity in the cold.
Middle-aged persons must be kept under careful surveillance because they are more susceptible to cold injury than younger people on account of their more limited vascular response. Excessive fatigue and a sedentary occupation increase the risk of injury. Persons with certain medical conditions (cold urticaria, Raynaud’s syndrome, angina pectoris, prior frostbite) must avoid exposure to intense cold. Certain additional advice may be useful: protect exposed skin against solar radiation, protect the lips with special creams and protect the eyes with sunglasses against ultraviolet radiation.
When a problem does occur, workers in a cold zone must keep calm, must not separate themselves from the group, and must maintain their body heat by digging holes and huddling together. Careful attention must be paid to the provision of food and means of calling for help (radio, distress rockets, signal mirrors, etc.). Where there is a risk of immersion in cold water, lifeboats must be provided as well as equipment that is watertight and gives good thermal insulation. In case of shipwreck without a lifeboat, the individual must try to limit heat loss to the maximum by hanging on to floating materials, curling up and swimming in moderation with the chest out of the water if possible, because the convection created by swimming considerably increases heat loss. Drinking sea-water is harmful because of its high salt level.
Modification of Tasks in the Cold
In a cold zone, work tasks are considerably modified. The weight of the clothing, the carrying of loads (tents, food, etc.) and the need to traverse difficult terrain increase the energy expended by physical activity. Moreover, movement, coordination and manual dexterity are hindered by clothing. The field of vision is often reduced by the wearing of sunglasses. Further, perception of the background is altered and reduced to 6 m when the temperature of dry air is below –18ºC or when there is a wind. Visibility may be nil in a snowfall or in fog. The presence of gloves makes difficult certain tasks requiring fine work. Because of condensation, tools are often coated with ice, and grasping them with bare hands carries a certain risk of frostbite. The physical structure of clothing is altered in extreme cold, and the ice that may form as a result of freezing combined with condensation often blocks zip-fasteners. Finally, fuels must be protected against freezing by the use of antifreeze.
Thus, for the optimal performance of tasks in a cold climate there must be several layers of clothing; adequate protection of the extremities; measures against condensation in clothing, on tools and in tents; and regular warming in a heated shelter. Work tasks must be undertaken as a sequence of simple tasks, if possible carried out by two work teams, one working while the other is warming itself. Inactivity in the cold must be avoided, as must solitary work, away from used paths. A competent person may be designated to be responsible for protection and accident prevention.
In conclusion, it appears that a good knowledge of cold injury, a knowledge of the surroundings, good preparation (physical fitness, feeding, induction of adaptational mechanisms), appropriate clothing and suitable distribution of tasks can prevent cold injury. Where injury does occur, the worst can be avoided by means of rapid assistance and immediate treatment.
Protective Clothing: Waterproof Garments
Wearing waterproof garments has the object of protecting against the consequences of accidental immersion and therefore concerns not only all workers likely to suffer such accidents (sailors, air pilots) but also those working in cold water (professional divers). Table 2, extracted from the Oceanographic Atlas of the North American Ocean, shows that even in the western Mediterranean the water temperature rarely exceeds 15ºC. Under conditions of immersion, the survival time for a clothed individual with a lifebelt but without anti-immersion equipment has been estimated at 1.5 hours in the Baltic and 6 hours in the Mediterranean in January, whereas in August it is 12 hours in the Baltic and is limited only by exhaustion in the Mediterranean. Wearing protective equipment is therefore a necessity for workers at sea, particularly those liable to be immersed without immediate assistance.
Table 2. Monthly and annual mean of the number of days when water temperature is below 15 °C.
|
Month |
Western Baltic |
German Gulf |
Atlantic Ocean |
Western Mediterranean |
|
January |
31 |
31 |
31 |
31 |
|
February |
28 |
28 |
28 |
28 |
|
March |
31 |
31 |
31 |
31 |
|
April |
30 |
30 |
30 |
26 to 30 |
|
May |
31 |
31 |
31 |
8 |
|
June |
25 |
25 |
25 |
sometimes |
|
July |
4 |
6 |
sometimes |
sometimes |
|
August |
4 |
sometimes |
sometimes |
0 |
|
September |
19 |
3 |
sometimes |
sometimes |
|
October |
31 |
22 |
20 |
2 |
|
November |
30 |
30 |
30 |
30 |
|
December |
31 |
31 |
31 |
31 |
|
Total |
295 |
268 |
257 |
187 |
The difficulties of producing such equipment are complex, because account has to be taken of multiple, often conflicting, requirements. These constraints include: (1) the fact that the thermal protection must be effective in both air and water without impeding evaporation of sweat (2) the need to keep the subject at the surface of the water and (3) the tasks to be carried out. The equipment must furthermore be designed in accordance with the risk involved. This requires exact definition of the anticipated needs: thermal environment (temperature of water, air, wind), time before help arrives, and presence or absence of a lifeboat, for example. The insulation characteristics of the clothing depend on the materials used, the contours of the body, the compressibility of the protective fabric (which determines the thickness of the layer of air imprisoned in the clothing on account of the pressure exerted by the water), and the humidity that may be present in the clothing. The presence of humidity in this type of clothing depends mainly on how watertight it is. Evaluation of such equipment must take into account the effectiveness of the thermal protection provided not only in the water but also in cold air, and involve estimates of both probable survival time in terms of the water and air temperatures, and the anticipated thermal stress and the possible mechanical hindrance of the clothing (Boutelier 1979). Finally, tests of watertightness carried out on a moving subject will allow possible deficiencies in this respect to be detected. Ultimately, anti-immersion equipment must meet three requirements:
- It must provide effective thermal protection in both water and air.
- It must be comfortable.
- It must be neither too restrictive nor too heavy.
To meet these requirements, two principles have been adopted: either to use a material that is not watertight but maintains its insulating properties in the water (as is the case of so-called “wet” suiting) or to ensure total watertightness with materials that are in addition insulating (“dry” suiting). At present, the principle of the wet garment is being applied less and less, especially in aviation. During the last decade, the International Maritime Organization has recommended the use of an anti-immersion or survival suit meeting the criteria of the International Convention for the safety of human life at sea (SOLAS) adopted in 1974. These criteria concern in particular insulation, minimum infiltration of water into the suit, the size of the suit, ergonomics, compatibility with aids for floating, and testing procedures. However, the application of these criteria poses a certain number of problems (notably, those to do with the definition of the tests to be applied).
Although they have been known for a very long time, since the Eskimos used sealskin or seal intestines sewn together, anti- immersion suits are difficult to perfect and the criteria for standardization will probably be reviewed in future years.
Cold Indices and Standards
Cold stress is defined as a thermal load on the body under which greater than normal heat losses are anticipated and compensatory thermoregulatory actions are required to maintain the body thermally neutral. Normal heat losses, hence, refer to what people normally experience during indoor living conditions (air temperature 20 to 25ºC).
In contrast to conditions in the heat, clothing and activity are positive factors in the sense that more clothing reduces heat loss and more activity means higher internal heat production and a greater potential for balancing heat loss. Accordingly, assessment methods focus on the determination of required protection (clothing) at given activity levels, required activity levels for given protection or “temperature” values for given combinations of the two (Burton and Edholm 1955; Holmér 1988; Parsons 1993).
It is important to recognize, however, that there are limits as to how much clothing can be worn and how high a level of activity can be sustained for extended time periods. Cold-protective clothing tends to be bulky and hobbling. More space is required for motion and movements. Activity level may be determined by paced work but should, preferably, be controlled by the individual. For each individual there is a certain highest energy production rate, depending on physical work capacity, that can be sustained for prolonged time periods. Thus, high physical work capacity may be advantageous for prolonged, extreme exposures.
This article deals with methods for assessment and control of cold stress. Problems related to organizational, psychological, medical and ergonomic aspects are dealt with elsewhere.
Cold Work
Cold work encompasses a variety of conditions under natural as well as artificial conditions. The most extreme cold exposure is associated with missions in outer space. However, cold working conditions on the surface of the earth cover a temperature range of more than 100ºC (table 1). Naturally, the magnitude and severity of cold stress will be expected to increase with lowered ambient temperature.
Table 1. Air temperatures of various cold occupational environments
|
–120 ºC |
Climatic chamber for human cryotherapy |
|
–90 ºC |
Lowest temperature at south polar base Vostock |
|
–55 ºC |
Cold store for fish meat and production of frozen, dried products |
|
–40 ºC |
“Normal” temperature at polar base |
|
–28 ºC |
Cold store for deep-frozen products |
|
+2 to +12 ºC |
Storage, preparation and transportation of fresh, alimentary products |
|
–50 to –20 ºC |
Average January temperature of northern Canada and Siberia |
|
–20 to –10 ºC |
Average January temperature of southern Canada, northern Scandinavia, central Russia |
|
–10 to 0 ºC |
Average January temperature of northern USA, southern Scandinavia, central Europe, parts of middle and far East, central and northern Japan |
Source: Modified from Holmér 1993.
It is clear from 1 table that large populations of outdoor workers in many countries experience more or less severe cold stress. In addition cold store work occurs in all parts of the world. Surveys in Scandinavian countries reveal that approximately 10% of the total worker population regard cold as a major annoyance factor in the workplace.
Types of Cold Stress
The following types of cold stress can be defined:
- whole-body cooling
- local cooling, including extremity cooling, convective skin cooling (wind chill), conductive skin cooling (contact cooling) and cooling of respiratory tract.
Most likely, several if not all of these may be present at the same time.
The assessment of cold stress involves the ascertainment of a risk of one or more of the mentioned effects. Typically, table 2 may be used as a first rough classification. In general cold stress increases, the lower the level of physical activity and the less protection available.
Table 2. Schematic classification of cold work
|
Temperature |
Type of work |
Type of cold stress |
|
10 to 20 ºC |
Sedentary, light work, fine manual work |
Whole-body cooling, extremity cooling |
|
0 to 10 ºC |
Sedentary and stationary, light work |
Whole-body cooling, extremity cooling |
|
–10 to 0 ºC |
Light physical work, handling tools and materials |
Whole-body cooling, extremity cooling, contact cooling |
|
–20 to –10 ºC |
Moderate activity, handling metals and fluids (petrol etc.), windy conditions |
Whole-body cooling, extremity cooling, contact cooling, convective cooling |
|
Below –20 ºC |
All types of work |
All types of cold stress |
Information given in the table should be interpreted as a signal to action. In other words, the particular type of cold stress should be evaluated and controlled, if required. At moderate temperatures problems associated with discomfort and losses of function due to local cooling prevail. At lower temperatures the imminent risk of a cold injury as a sequel to the other effects is the important factor. For many of the effects discrete relationships between stress level and effect do not yet exist. It cannot be excluded that a particular cold problem may persist also outside the range of temperatures denoted by the table.
Assessment Methods
Methods for assessment of cold stress are presented in ISO Technical Report 11079 (ISO TR 11079, 1993). Other standards concerning determination of metabolic heat production (ISO 8996, 1988), estimation of clothing thermal characteristics (ISO 9920, 1993), and physiological measurements (ISO DIS 9886, 1989c) provide complementary information useful for the evaluation of cold stress.
Figure 1 outlines the relationships between climate factors, anticipated cooling effect and recommended method for assessment. Further details about methods and data collection are given below.
Figure 1. Assessment of cold stress in relation to climatic factors and cooling effects.
Whole-Body Cooling
The risk of whole-body cooling is determined by analysing the conditions for body heat balance. The clothing insulation level required for heat balance at defined levels of physiological strain, is calculated with a mathematical heat balance equation. The calculated required insulation value, IREQ, can be regarded as a cold stress index. The value indicates a protection level (expressed in clo). The higher the value, the greater the risk of body heat imbalance. The two levels of strain correspond to a low level (neutral or “comfort” sensation) and a high level (slightly cold to cold sensation).
Using IREQ comprises three evaluation steps:
- determination of IREQ for given exposure conditions
- comparison of IREQ with protection level provided by clothing
- determination of exposure time if protection level is of lesser value than IREQ
Figure 2 shows IREQ values for low physiological strain (neutral thermal sensation). Values are given for different activity levels.
Figure 2. IREQ values needed to maintain low-level physiological strain (neutral thermal sensation) at varying temperature.
Methods to estimate activity levels are described in ISO 7243 (table 3).
Table 3. Classification of levels of metabolic rate
|
Class |
Metabolic rate range, M |
Value to be used for calculation of mean metabolic rate |
Examples |
||
|
Related to |
For a mean skin surface area |
|
|
||
|
0 |
M≤65 |
M≥117 |
65 |
117 |
Resting |
|
1 |
65M≤130 |
117M≤234 |
100 |
180 |
Sitting at ease: light manual work (writing, typing, drawing, sewing, book-keeping); hand and arm work (small bench tools, inspection, assembly or sorting of light material); arm and leg work (driving vehicle in normal conditions, operating foot switch or pedals). Standing: drill (small parts); milling machine (small parts); coil winding; small armature winding; machining with low power tools; casual walking (speed up to 3.5 km/h). |
|
2 |
130M≤200 |
234M≤360 |
165 |
297 |
Sustained hand and arm work (hammering in nails, filling); arm and leg work (off-road operation of lorries, tractors or construction equipment); arm and trunk work (work with pneumatic hammer, tractor assembly, plastering, intermittent handling of moderately heavy material, weeding, hoeing, picking fruit or vegetables); pushing or pulling light weight carts or wheelbarrows; walking at a speed of 3.5 km/h; forging. |
|
3 |
200M≤260 |
360M≤468 |
230 |
414 |
Intense arm and trunk work: carrying heavy material; shoveling; sledge hammer work; sawing, planning or chiselling hard wood; hand mowing; digging; walking at a speed of 5.5 km/h to 7 km/h. Pushing or pulling heavily loaded handcarts or wheelbarrows; chipping castings; concrete block laying. |
|
4 |
M>260 |
M>468 |
290 |
522 |
Very intensive activity at fast to maximum pace; working with an axe; intense shovelling or digging; climbing stairs, ramp or ladder; walking quickly with small steps, running, walking at a speed greater than 7 km/h. |
Source: ISO 7243 1989a
Once IREQ is determined for given conditions, the value is compared with the protection level offered by clothing. Protection level of a clothing ensemble is determined by its resultant insulation value (“clo-value”). This property is measured according to the draft European standard prEN-342 (1992). It can also be derived from basic insulation values provided in tables (ISO 9920).
Table 4. provides examples of basic insulation values for typical ensembles. Values must be corrected for presumed reduction caused by body motion and ventilation. Typically, no correction is made for resting level. Values are reduced by 10% for light work and by 20% for higher activity levels.
Table 4. Examples of basic insulation values (Icl) of clothing*
|
Clothing ensemble |
Icl (m2 ºC/W) |
Icl (clo) |
|
Briefs, short-sleeve shirt, fitted trousers, calf-length socks, shoes |
0.08 |
0.5 |
|
Underpants, shirt, fitted, trousers, socks, shoes |
0.10 |
0.6 |
|
Underpants, coverall, socks, shoes |
0.11 |
0.7 |
|
Underpants, shirt, coverall, socks, shoes |
0.13 |
0.8 |
|
Underpants, shirt, trousers, smock, socks, shoes |
0.14 |
0.9 |
|
Briefs, undershirt, underpants, shirt, overalls, calf-length socks, shoes |
0.16 |
1.0 |
|
Underpants, undershirt, shirt, trousers, jacket, vest, socks, shoes |
0.17 |
1.1 |
|
Underpants, shirt, trousers, jacket, coverall, socks, shoes |
0.19 |
1.3 |
|
Undershirt, underpants, insulated trousers, insulated jacket, socks, shoes |
0.22 |
1.4 |
|
Briefs, T-shirt, shirt, fitted trousers, insulated coveralls, calf-length socks, shoes |
0.23 |
1.5 |
|
Underpants, undershirt, shirt, trousers, jacket, overjacket, hat, gloves, socks, shoes |
0.25 |
1.6 |
|
Underpants, undershirt, shirt, trousers, jacket, overjacket, overtrousers, socks, shoes |
0.29 |
1.9 |
|
Underpants, undershirt, shirt, trousers, jacket, overjacket, overtrousers, socks, shoes, hat, gloves |
0.31 |
2.0 |
|
Undershirt, underpants, insulated trousers, insulated jacket, overtrousers, overjacket, socks, shoes |
0.34 |
2.2 |
|
Undershirt, underpants, insulated trousers, insulated jacket, overtrousers, socks, shoes, hat, gloves |
0.40 |
2.6 |
|
Undershirt, underpants, insulated trousers, insulated jacket, overtrousers and parka with lining, socks, shoes, hat, mittens |
0.40–0.52 |
2.6–3.4 |
|
Arctic clothing systems |
0.46–0.70 |
3–4.5 |
|
Sleeping bags |
0.46–1.1 |
3–8 |
*Nominal protection level applies only to static, windstill conditions (resting). Values must be reduced with increased activity level.
Source: Modified from ISO/TR-11079 1993.
The protection level offered by the best available clothing systems corresponds to 3 to 4 clo. When the available clothing system does not provide sufficient insulation, a time limit is calculated for the actual conditions. This time limit depends on the difference between required clothing insulation and that of the available clothing. Since, full protection against cooling is no longer achieved, the time limit is calculated on the basis of an anticipated reduction of body heat content. Similarly, a recovery time can be calculated to restore the same amount of heat.
Figure 3 shows examples of time limits for light and moderate work with two insulation levels of clothing. Time limits for other combinations may be estimated by interpolation. Figure 4 can be used as a guideline for assessment of exposure time, when the best cold protective clothing is available.
Figure 3. Time limits for light and moderate work with two insulation levels of clothing.
Figure 4. Time-weighted IREQ values for intermittent and continuous exposure to cold.
Intermittent exposures typically comprise work periods interrupted by warm-up breaks or by work periods in a warmer environment. In most conditions, little or no replacement of clothing takes place (mostly for practical reasons). IREQ may then be determined for the combined exposure as a time-weighted average. Averaging period must not be longer than one to two hours. Time-weighted IREQ values for some types of intermittent exposure are given in figure 4.
IREQ values and time limits should be indicative rather than normative. They refer to the average person. The individual variation in terms of characteristics, requirements and preferences is large. Much of this variation must be handled by selecting clothing ensembles with great flexibility in terms of, for example, adjustment of the protection level.
Extremity Cooling
The extremities—in particular, fingers and toes—are susceptible to cooling. Unless sufficient heat input by warm blood can be maintained, tissue temperature progressively falls. Extremity blood flow is determined by energetic (required for muscles activity) as well as thermoregulatory needs. When whole-body thermal balance is challenged, peripheral vasoconstriction helps to reduce core heat losses at the expense of peripheral tissues. With high activity more heat is available and extremity blood flow can more easily be maintained.
The protection offered by handwear and footwear in terms of reducing heat losses is limited. When heat input to the extremity is low (e.g., with resting or low activity), the insulation required to keep hands and feet warm is very large (van Dilla, Day and Siple 1949). The protection offered by gloves and mittens only provides retardation of cooling rate and, correspondingly, longer times to reach a critical temperature. With higher activity levels, improved protection allows warm hands and feet at lower ambient temperatures.
No standard method is available for assessment of extremity cooling. However, ISO TR 11079 recommends 24ºC and 15ºC as critical hand temperatures for levels of low and high stress, respectively. Fingertip temperature may easily be 5 to 10 °C lower than the average hand skin temperature or simply the temperature of the back of the hand.
The information given in figure 5 is useful when determining acceptable exposure times and required protection. The two curves refer to conditions with and without vasoconstriction (high and low activity level). Furthermore, it is assumed that finger insulation is high (two clo) and adequate clothing is used.
Figure 5. Finger protection.
A similar set of curves should apply to toes. However, more clo may be available for protection of feet, resulting in longer exposure times. Nevertheless, it follows from figures 3 and 5 that extremity cooling most likely is more critical for exposure time than whole-body-cooling.
Protection provided by handwear is evaluated by using methods described in the European standard EN-511 (1993). Thermal insulation of the whole handwear is measured with an electrically heated hand model. A wind speed of 4 m/s is used to simulate realistic wear conditions. Performance is given in four classes (table 5).
Table 5. Classification of thermal resistance (I) to convective cooling of handwear
|
Class |
I (m2 ºC/W) |
|
1 |
0.10 ≤ I 0.15 |
|
2 |
0.15 ≤ I 0.22 |
|
3 |
0.22 ≤ I 0.30 |
|
4 |
I ≤ 0.30 |
Source: Based on EN 511 (1993).
Contact Cold
Contact between bare hand and cold surfaces may quickly reduce skin temperature and cause freezing injury. Problems may arise with surface temperatures as high as 15ºC. In particular, metal surfaces provide excellent conductive properties and may quickly cool contacting skin areas.
At present no standard method exists for general assessment of contact cooling. The following recommendations can be given (ACGIH 1990; Chen, Nilsson and Holmér 1994; Enander 1987):
- Prolonged contact with metal surfaces below 15ºC may impair dexterity.
- Prolonged contact with metal surfaces below 7ºC may induce numbness.
- Prolonged contact with metal surfaces below 0ºC may induce frostnip or frostbite.
- Brief contact with metal surfaces below –7ºC may induce frostnip or frostbite.
- Any contact with liquids at subzero temperature must be avoided.
Other materials present a similar sequence of hazards, but temperatures are lower with less conducting material (plastics, wood, foam).
Protection against contact cooling provided by handwear can be determined using the European standard EN 511. Four performance classes are given (table 6).
Table 6. Classification of contact thermal resistance of handwear (I)
|
Class |
I (m2 ºC/W) |
|
1 |
0.025 ≤ I 0.05 |
|
2 |
0.05 ≤ I 0.10 |
|
3 |
0.10 ≤ I 0.15 |
|
4 |
I ≤ 0.15 |
Source: Based on EN 511 (1993).
Convective Skin Cooling
The Wind Chill Index (WCI) represents a simple, empirical method for assessment of cooling of unprotected skin (face) (ISO TR 11079). The method predicts tissue heat loss on the basis of air temperature and wind speed.
Responses associated with different values of WCI are denoted in table 7.
Table 7. Wind Chill Index (WCI), equivalent cooling temperature (Teq ) and freezing time of exposed flesh
|
WCI (W/m2) |
Teq (ºC) |
Effect |
|
1,200 |
–14 |
Very cold |
|
1,400 |
–22 |
Bitterly cold |
|
1,600 |
–30 |
Exposed flesh freezes |
|
1,800 |
–38 |
within 1 hour |
|
2,000 |
–45 |
Exposed flesh freezes |
|
2,200 |
–53 |
within 1 minute |
|
2,400 |
–61 |
Exposed flesh freezes |
|
2,600 |
–69 |
within 30 seconds |
A frequently used interpretation of WCI is the equivalent cooling temperature. This temperature under calm conditions (1.8 m/s) represents the same WCI value as the actual combination of temperature and wind. Table 8 provides equivalent cooling temperatures for combinations of air temperature and wind speed. The table applies to active, well-dressed persons. A risk is present when equivalent temperature drops below –30ºC, and skin may freeze within 1 to 2 min below –60ºC.
Table 8. Cooling power of wind on exposed flesh expressed as an equivalent cooling temperature under almost calm conditions (wind speed 1.8 m/s)
|
Wind speed (m/s) |
Actual thermometer reading (ºC) |
||||||||||
|
0 |
–5 |
–10 |
–15 |
–20 |
–25 |
–30 |
–35 |
–40 |
–45 |
–50 |
|
|
Equivalent cooling temperature (ºC) |
|||||||||||
|
1.8 |
0 |
–5 |
–10 |
–15 |
–20 |
–25 |
–30 |
–35 |
–40 |
–45 |
–50 |
|
2 |
–1 |
–6 |
–11 |
–16 |
–21 |
–27 |
–32 |
–37 |
–42 |
–47 |
–52 |
|
3 |
–4 |
–10 |
–15 |
–21 |
–27 |
–32 |
–38 |
–44 |
–49 |
–55 |
–60 |
|
5 |
–9 |
–15 |
–21 |
–28 |
–34 |
–40 |
–47 |
–53 |
–59 |
–66 |
–72 |
|
8 |
–13 |
–20 |
–27 |
–34 |
–41 |
–48 |
–55 |
–62 |
–69 |
–76 |
–83 |
|
11 |
–16 |
–23 |
–31 |
–38 |
–46 |
–53 |
–60 |
–68 |
–75 |
–83 |
–90 |
|
15 |
–18 |
–26 |
–34 |
–42 |
–49 |
–57 |
–65 |
–73 |
–80 |
–88 |
–96 |
|
20 |
–20 |
–28 |
–36 |
–44 |
–52 |
–60 |
–68 |
–76 |
–84 |
–92 |
–100 |
Underlined values represent a risk for frostnip or frostbite.
Cooling of Respiratory Tract
Inhaling cold, dry air may cause problems for sensitive persons at +10 to 15ºC. Healthy persons performing light to moderate work require no particular protection of the respiratory tract down to –30ºC. Very heavy work during prolonged exposures (e.g., athletic endurance events) should not take place at temperatures below –20ºC.
Similar recommendations apply to cooling of the eye. In practice, the great discomfort and visual impairment associated with eye cooling normally require the use of goggles or other protection long before the exposure becomes hazardous.
Measurements
Depending on type of expected risk, different sets of measurements are required (figure 6). Procedures for data collection and accuracy of measurements depend on the purpose of the measurements. Pertinent information must be obtained regarding variation in time of the climatic parameters, as well as of activity level and/or clothing. Simple time-weighting procedures should be adopted (ISO 7726).
Figure 6. The relationship of expected cold stress risk to required measurement procedures.
Preventive Measures for Alleviation of Cold Stress
Actions and measures for the control and reduction of cold stress imply a number of considerations during the planning and preparatory phases of work shifts, as well as during work, which are dealt with elsewhere in this chapter and this Encyclopaedia.
Case Study: Heat Indices: Formulae and Definitions
I. Index of thermal stress (ITS)
The improved heat balance equation is:
![]()
where is the evaporation required to maintain heat balance, is the solar load, and metabolic heat production H is used instead of metabolic rate to account for external work. An important improvement is the recognition that not all sweat evaporates (e.g., some drips) hence required sweat rate is related to required evaporation rate by:
![]()
where nsc is the efficiency of sweating.
Used indoors, sensible heat transfer is calculated from:
![]()
For outdoor conditions with solar load, is replaced with and allowance made for solar load (RS ) by:
![]()
The equations used are fits to experimental data and are not strictly rational.
Maximum evaporation heat loss is:
![]()
and efficiency of sweating is given by:
![]()
but
nsc = 1, если
and
nsc = 0.29, если
The index of thermal stress (ITS) in g/h is given by:
![]()
where is the required evaporation rate , 0.37 converts into g/h andnsc is the efficiency of sweating (McIntyre 1980).
II. Required sweat rate
Similar to the other rational indices, is derived from the six basic parameters (air temperature (), radiant temperature ( ), relative humidity air velocity (v), clothing insulation ( ), metabolic rate (M) and external work (W)). Effective radiation area values for posture (sitting = 0.72, standing = 0.77) are also required. From this the evaporation required is calculated from:
![]()
Equations are provided for each component (see table 8 and table 9). Mean skin temperature is calculated from a multiple linear regression equation or a value of 36°C is assumed.
From the required evaporation (Ereg) and maximum evaporation (Emax) and sweating efficiency (r), the following are calculated:
Required skin wettedness ![]()
Required sweat rate ![]()
III. Predicted 4-hour sweat rate (P4SR)
Steps taken to obtain the P4SR index value are summarized by McIntyre (1980) as follows:
If ![]() , increase wet bulb temperature by
, increase wet bulb temperature by ![]() .
.
If the metabolic rate M > 63 ![]() , increase wet bulb temperature by the amount indicated in the chart (see figure 6).
, increase wet bulb temperature by the amount indicated in the chart (see figure 6).
If the men are clothed, increase the wet bulb temperature by ![]() .
.
The modifications are additive.
The (P4SR) is determined from figure 6. The P4SR is then:
![]()
IV. Heart rate
![]()
where M is metabolic rate![]() , is air temperature in °C and Pa is vapour pressure in Mb.
, is air temperature in °C and Pa is vapour pressure in Mb.
Givoni and Goldman (1973) provide equations for predicting heart rate of persons (soldiers) in hot environments. They define an index for heart rate (IHR) from a modification of predicted equilibrium rectal temperature,
![]()
IHR is then:
![]()
where M = metabolic rate (watts), ![]() = mechanical work (watts), clo = thermal insulation of clothing,
= mechanical work (watts), clo = thermal insulation of clothing, ![]() = air temperature
= air temperature![]() ,
, ![]() = total metabolic and environmental heat load (watts), = evaporative cooling capacity for clothing and environment (watts).
= total metabolic and environmental heat load (watts), = evaporative cooling capacity for clothing and environment (watts).
The equilibrium heart rate (![]() in beats per minute) is then given by:
in beats per minute) is then given by:
![]() for IHR
for IHR ![]() 225
225
that is, a linear relationship (between rectal temperature and heart rate) for heart rates up to about 150 beats per minute. For IHR >225:
![]()
that is, an exponential relationship as heart rate approaches maximum, where:
![]() = equilibrium heart rate (bpm),
= equilibrium heart rate (bpm),
65 = assumed resting heart rate in comfortable conditions (bpm), and t = time in hours.
V. Wet bulb globe temperature index (WBGT)
Wet bulb globe temperature is given by:
![]()
for conditions with solar radiation, and:
![]()
for indoor conditions with no solar radiation, where Tnwb= temperature of a naturally ventilated wet bulb thermometer, Ta = air temperature, and Tg = temperature of a 150 mm diameter black globe thermometer.
" DISCLAIMER: The ILO does not take responsibility for content presented on this web portal that is presented in any language other than English, which is the language used for the initial production and peer-review of original content. Certain statistics have not been updated since the production of the 4th edition of the Encyclopaedia (1998)."