Children categories

36. Barometric Pressure Increased (2)

36. Barometric Pressure Increased
Chapter Editor: T.J.R. Francis
Table of Contents
Working under Increased Barometric Pressure
Eric Kindwall
Dees F. Gorman
Tables
Click a link below to view table in article context.
1. Instructions for compressed-air workers
2. Decompression illness: Revised classification

37. Barometric Pressure Reduced (4)

37. Barometric Pressure Reduced
Chapter Editor: Walter Dümmer
Table of Contents
Figures and Tables
Ventilatory Acclimatization to High Altitude
John T. Reeves and John V. Weil
Physiological Effects of Reduced Barometric Pressure
Kenneth I. Berger and William N. Rom
Health Considerations for Managing Work at High Altitudes
John B. West
Prevention of Occupational Hazards at High Altitudes
Walter Dümmer
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

38. Biological Hazards (4)

38. Biological Hazards
Chapter Editor: Zuheir Ibrahim Fakhri
Table of Contents
Tables
Workplace Biohazards
Zuheir I. Fakhri
Aquatic Animals
D. Zannini
Terrestrial Venomous Animals
J.A. Rioux and B. Juminer
Clinical Features of Snakebite
David A. Warrell
Tables
Click a link below to view table in article context.
1. Occupational settings with biological agents
2. Viruses, bacteria, fungi & plants in the workplace
3. Animals as a source of occupational hazards

39. Disasters, Natural and Technological (12)

39. Disasters, Natural and Technological
Chapter Editor: Pier Alberto Bertazzi
Table of Contents
Tables and Figures
Disasters and Major Accidents
Pier Alberto Bertazzi
ILO Convention concerning the Prevention of Major Industrial Accidents, 1993 (No. 174)
Disaster Preparedness
Peter J. Baxter
Post-Disaster Activities
Benedetto Terracini and Ursula Ackermann-Liebrich
Weather-Related Problems
Jean French
Avalanches: Hazards and Protective Measures
Gustav Poinstingl
Transportation of Hazardous Material: Chemical and Radioactive
Donald M. Campbell
Radiation Accidents
Pierre Verger and Denis Winter
Case Study: What does dose mean?
Occupational Health and Safety Measures in Agricultural Areas Contaminated by Radionuclides: The Chernobyl Experience
Yuri Kundiev, Leonard Dobrovolsky and V.I. Chernyuk
Case Study: The Kader Toy Factory Fire
Casey Cavanaugh Grant
Impacts of Disasters: Lessons from a Medical Perspective
José Luis Zeballos
Tables
Click a link below to view table in article context.
1. Definitions of disaster types
2. 25-yr average # victims by type & region-natural trigger
3. 25-yr average # victims by type & region-non-natural trigger
4. 25-yr average # victims by type-natural trigger (1969-1993)
5. 25-yr average # victims by type-non-natural trigger (1969-1993)
6. Natural trigger from 1969 to 1993: Events over 25 years
7. Non-natural trigger from 1969 to 1993: Events over 25 years
8. Natural trigger: Number by global region & type in 1994
9. Non-natural trigger: Number by global region & type in 1994
10. Examples of industrial explosions
11. Examples of major fires
12. Examples of major toxic releases
13. Role of major hazard installations management in hazard control
14. Working methods for hazard assessment
15. EC Directive criteria for major hazard installations
16. Priority chemicals used in identifying major hazard installations
17. Weather-related occupational risks
18. Typical radionuclides, with their radioactive half-lives
19. Comparison of different nuclear accidents
20. Contamination in Ukraine, Byelorussia & Russia after Chernobyl
21. Contamination strontium-90 after the Khyshtym accident (Urals 1957)
22. Radioactive sources that involved the general public
23. Main accidents involving industrial irradiators
24. Oak Ridge (US) radiation accident registry (worldwide, 1944-88)
25. Pattern of occupational exposure to ionizing radiation worldwide
26. Deterministic effects: thresholds for selected organs
27. Patients with acute irradiation syndrome (AIS) after Chernobyl
28. Epidemiological cancer studies of high dose external irradiation
29. Thyroid cancers in children in Belarus, Ukraine & Russia, 1981-94
30. International scale of nuclear incidents
31. Generic protective measures for general population
32. Criteria for contamination zones
33. Major disasters in Latin America & the Caribbean, 1970-93
34. Losses due to six natural disasters
35. Hospitals & hospital beds damaged/ destroyed by 3 major disasters
36. Victims in 2 hospitals collapsed by the 1985 earthquake in Mexico
37. Hospital beds lost resulting from the March 1985 Chilean earthquake
38. Risk factors for earthquake damage to hospital infrastructure
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.
Click to return to top of page

40. Electricity (3)

40. Electricity
Chapter Editor: Dominique Folliot
Table of Contents
Figures and Tables
Electricity—Physiological Effects
Dominique Folliot
Static Electricity
Claude Menguy
Prevention And Standards
Renzo Comini
Tables
Click a link below to view table in article context.
1. Estimates of the rate of electrocution-1988
2. Basic relationships in electrostatics-Collection of equations
3. Electron affinities of selected polymers
4. Typical lower flammability limits
5. Specific charge associated with selected industrial operations
6. Examples of equipment sensitive to electrostatic discharges
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

41. Fire (6)

41. Fire
Chapter Editor: Casey C. Grant
Table of Contents
Figures and Tables
Basic Concepts
Dougal Drysdale
Sources of Fire Hazards
Tamás Bánky
Fire Prevention Measures
Peter F. Johnson
Passive Fire Protection Measures
Yngve Anderberg
Active Fire Protection Measures
Gary Taylor
Organizing for Fire Protection
S. Dheri
Tables
Click a link below to view table in article context.
1. Lower & upper flammability limits in air
2. Flashpoints & firepoints of liquid & solid fuels
3. Ignition sources
4. Comparison of concentrations of different gases required for inerting
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

42. Heat and Cold (12)

42. Heat and Cold
Chapter Editor: Jean-Jacques Vogt
Table of Contents
Figures and Tables
Physiological Responses to the Thermal Environment
W. Larry Kenney
Effects of Heat Stress and Work in the Heat
Bodil Nielsen
Heat Disorders
Tokuo Ogawa
Prevention of Heat Stress
Sarah A. Nunneley
The Physical Basis of Work in Heat
Jacques Malchaire
Assessment of Heat Stress and Heat Stress Indices
Kenneth C. Parsons
Case Study: Heat Indices: Formulae and Definitions
Heat Exchange through Clothing
Wouter A. Lotens
Cold Environments and Cold Work
Ingvar Holmér, Per-Ola Granberg and Goran Dahlstrom
Prevention of Cold Stress in Extreme Outdoor Conditions
Jacques Bittel and Gustave Savourey
Cold Indices and Standards
Ingvar Holmér
Tables
Click a link below to view table in article context.
1. Electrolyte concentration in blood plasma & sweat
2. Heat Stress Index & Allowable Exposure Times: calculations
3. Interpretation of Heat Stress Index values
4. Reference values for criteria of thermal stress & strain
5. Model using heart rate to assess heat stress
6. WBGT reference values
7. Working practices for hot environments
8. Calculation of the SWreq index & assessment method: equations
9. Description of terms used in ISO 7933 (1989b)
10. WBGT values for four work phases
11. Basic data for the analytical assessment using ISO 7933
12. Analytical assessment using ISO 7933
13. Air temperatures of various cold occupational environments
14. Duration of uncompensated cold stress & associated reactions
15. Indication of anticipated effects of mild & severe cold exposure
16. Body tissue temperature & human physical performance
17. Human responses to cooling: Indicative reactions to hypothermia
18. Health recommendations for personnel exposed to cold stress
19. Conditioning programmes for workers exposed to cold
20. Prevention & alleviation of cold stress: strategies
21. Strategies & measures related to specific factors & equipment
22. General adaptational mechanisms to cold
23. Number of days when water temperature is below 15 ºC
24. Air temperatures of various cold occupational environments
25. Schematic classification of cold work
26. Classification of levels of metabolic rate
27. Examples of basic insulation values of clothing
28. Classification of thermal resistance to cooling of handwear
29. Classification of contact thermal resistance of handwear
30. Wind Chill Index, temperature & freezing time of exposed flesh
31. Cooling power of wind on exposed flesh
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

43. Hours of Work (1)

43. Hours of Work
Chapter Editor: Peter Knauth
Table of Contents
Hours of Work
Peter Knauth
Tables
Click a link below to view table in article context.
1. Time intervals from beginning shiftwork until three illnesses
2. Shiftwork & incidence of cardiovascular disorders
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

44. Indoor Air Quality (8)

44. Indoor Air Quality
Chapter Editor: Xavier Guardino Solá
Table of Contents
Figures and Tables
Indoor Air Quality: Introduction
Xavier Guardino Solá
Nature and Sources of Indoor Chemical Contaminants
Derrick Crump
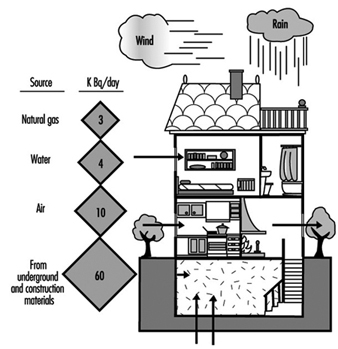
Radon
María José Berenguer
Tobacco Smoke
Dietrich Hoffmann and Ernst L. Wynder
Smoking Regulations
Xavier Guardino Solá
Measuring and Assessing Chemical Pollutants
M. Gracia Rosell Farrás
Biological Contamination
Brian Flannigan
Regulations, Recommendations, Guidelines and Standards
María José Berenguer
Tables
Click a link below to view table in article context.
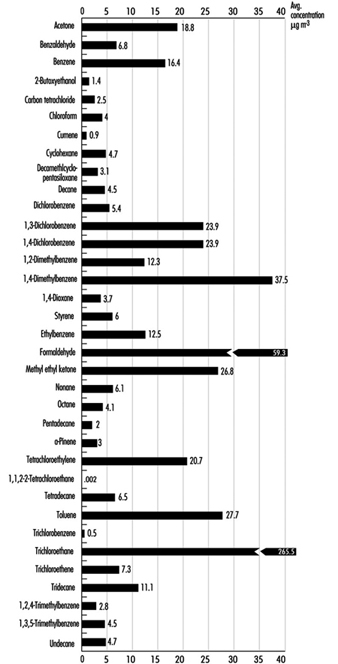
1. Classification of indoor organic pollutants
2. Formaldehyde emission from a variety of materials
3. Ttl. volatile organic comp’ds concs, wall/floor coverings
4. Consumer prods & other sources of volatile organic comp’ds
5. Major types & concentrations in the urban United Kingdom
6. Field measurements of nitrogen oxides & carbon monoxide
7. Toxic & tumorigenic agents in cigarette sidestream smoke
8. Toxic & tumorigenic agents from tobacco smoke
9. Urinary cotinine in non-smokers
10. Methodology for taking samples
11. Detection methods for gases in indoor air
12. Methods used for the analysis of chemical pollutants
13. Lower detection limits for some gases
14. Types of fungus which can cause rhinitis and/or asthma
15. Micro-organisms and extrinsic allergic alveolitis
16. Micro-organisms in nonindustrial indoor air & dust
17. Standards of air quality established by the US EPA
18. WHO guidelines for non-cancer and non-odour annoyance
19. WHO guideline values based on sensory effects or annoyance
20. Reference values for radon of three organizations
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

45. Indoor Environmental Control (6)

45. Indoor Environmental Control
Chapter Editor: Juan Guasch Farrás
Table of Contents
Figures and Tables
Control of Indoor Environments: General Principles
A. Hernández Calleja
Indoor Air: Methods for Control and Cleaning
E. Adán Liébana and A. Hernández Calleja
Aims and Principles of General and Dilution Ventilation
Emilio Castejón
Ventilation Criteria for Nonindustrial Buildings
A. Hernández Calleja
Heating and Air-Conditioning Systems
F. Ramos Pérez and J. Guasch Farrás
Indoor Air: Ionization
E. Adán Liébana and J. Guasch Farrás
Tables
Click a link below to view table in article context.
1. Most common indoor pollutants & their sources
2. Basic requirements-dilution ventilation system
3. Control measures & their effects
4. Adjustments to working environment & effects
5. Effectiveness of filters (ASHRAE standard 52-76)
6. Reagents used as absorbents for contaminents
7. Levels of quality of indoor air
8. Contamination due to the occupants of a building
9. Degree of occupancy of different buildings
10. Contamination due to the building
11. Quality levels of outside air
12. Proposed norms for environmental factors
13. Temperatures of thermal comfort (based on Fanger)
14. Characteristics of ions
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

46. Lighting (3)

46. Lighting
Chapter Editor: Juan Guasch Farrás
Table of Contents
Figures and Tables
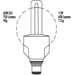
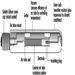
Types of Lamps and Lighting
Richard Forster
Conditions Required for Visual
Fernando Ramos Pérez and Ana Hernández Calleja
General Lighting Conditions
N. Alan Smith
Tables
Click a link below to view table in article context.
1. Improved output & wattage of some 1,500 mm fluorescent tube lamps
2. Typical lamp efficacies
3. International Lamp Coding System (ILCOS) for some lamp types
4. Common colours & shapes of incandescent lamps & ILCOS codes
5. Types of high-pressure sodium lamp
6. Colour contrasts
7. Reflection factors of different colours & materials
8. Recommended levels of maintained illuminance for locations/tasks
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

47. Noise (5)

47. Noise
Chapter Editor: Alice H. Suter
Table of Contents
Figures and Tables
The Nature and Effects of Noise
Alice H. Suter
Noise Measurement and Exposure Evaluation
Eduard I. Denisov and German A. Suvorov
Engineering Noise Control
Dennis P. Driscoll
Hearing Conservation Programmes
Larry H. Royster and Julia Doswell Royster
Standards and Regulations
Alice H. Suter
Tables
Click a link below to view table in article context.
1. Permissible exposure limits (PEL)for noise exposure, by nation
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

48. Radiation: Ionizing (6)

48. Radiation: Ionizing
Chapter Editor: Robert N. Cherry, Jr.
Table of Contents
Introduction
Robert N. Cherry, Jr.
Radiation Biology and Biological Effects
Arthur C. Upton
Sources of Ionizing Radiation
Robert N. Cherry, Jr.
Workplace Design for Radiation Safety
Gordon M. Lodde
Radiation Safety
Robert N. Cherry, Jr.
Planning for and Management of Radiation Accidents
Sydney W. Porter, Jr.

49. Radiation, Non-Ionizing (9)

49. Radiation, Non-Ionizing
Chapter Editor: Bengt Knave
Table of Contents
Tables and Figures
Electric and Magnetic Fields and Health Outcomes
Bengt Knave
The Electromagnetic Spectrum: Basic Physical Characteristics
Kjell Hansson Mild
Ultraviolet Radiation
David H. Sliney
Infrared Radiation
R. Matthes
Light and Infrared Radiation
David H. Sliney
Lasers
David H. Sliney
Radiofrequency Fields and Microwaves
Kjell Hansson Mild
VLF and ELF Electric and Magnetic Fields
Michael H. Repacholi
Static Electric and Magnetic Fields
Martino Grandolfo
Tables
Click a link below to view table in article context.
1. Sources and exposures for IR
2. Retinal thermal hazard function
3. Exposure limits for typical lasers
4. Applications of equipment using range >0 to 30 kHz
5. Occupational sources of exposure to magnetic fields
6. Effects of currents passing through the human body
7. Biological effects of various current density ranges
8. Occupational exposure limits-electric/magnetic fields
9. Studies on animals exposed to static electric fields
10. Major technologies and large static magnetic fields
11. ICNIRP recommendations for static magnetic fields
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

50. Vibration (4)

50. Vibration
Chapter Editor: Michael J. Griffin
Table of Contents
Table and Figures
Vibration
Michael J. Griffin
Whole-body Vibration
Helmut Seidel and Michael J. Griffin
Hand-transmitted Vibration
Massimo Bovenzi
Motion Sickness
Alan J. Benson
Tables
Click a link below to view table in article context.
1. Activities with adverse effects of whole-body vibration
2. Preventive measures for whole-body vibration
3. Hand-transmitted vibration exposures
4. Stages, Stockholm Workshop scale, hand-arm vibration syndrome
5. Raynaud’s phenomenon & hand-arm vibration syndrome
6. Threshold limit values for hand-transmitted vibration
7. European Union Council Directive: Hand-transmitted vibration (1994)
8. Vibration magnitudes for finger blanching
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.

51. Violence (1)

51. Violence
Chapter Editor: Leon J. Warshaw
Table of Contents
Violence in the Workplace
Leon J. Warshaw
Tables
Click a link below to view table in article context.
1. Highest rates of occupational homicide, US workplaces, 1980-1989
2. Highest rates of occupational homicide US occupations, 1980-1989
3. Risk factors for workplace homicides
4. Guides for programmes to prevent workplace violence

52. Visual Display Units (11)

52. Visual Display Units
Chapter Editor: Diane Berthelette
Table of Contents
Tables and Figures
Overview
Diane Berthelette
Characteristics of Visual Display Workstations
Ahmet Çakir
Ocular and Visual Problems
Paule Rey and Jean-Jacques Meyer
Reproductive Hazards - Experimental Data
Ulf Bergqvist
Reproductive Effects - Human Evidence
Claire Infante-Rivard
Case Study: A Summary of Studies of Reproductive Outcomes
Musculoskeletal Disorders
Gabriele Bammer
Skin Problems
Mats Berg and Sture Lidén
Psychosocial Aspects of VDU Work
Michael J. Smith and Pascale Carayon
Ergonomic Aspects of Human - Computer Interaction
Jean-Marc Robert
Ergonomics Standards
Tom F.M. Stewart
Tables
Click a link below to view table in article context.
1. Distribution of computers in various regions
2. Frequency & importance of elements of equipment
3. Prevalence of ocular symptoms
4. Teratological studies with rats or mice
5. Teratological studies with rats or mice
6. VDU use as a factor in adverse pregnancy outcomes
7. Analyses to study causes musculoskeletal problems
8. Factors thought to cause musculoskeletal problems
Figures
Point to a thumbnail to see figure caption, click to see figure in article context.
Formulae and Definitions
In general there is a square root relationship between thickness d of a static air layer and air velocity v. The exact function depends on the size and shape of the surface, but for the human body a useful approximation is:
![]()
Still air acts as an insulating layer with a conductivity ![]() (a material constant, regardless of the shape of the material) of .026 W/mK, which has a heat transfer coefficient h (units of
(a material constant, regardless of the shape of the material) of .026 W/mK, which has a heat transfer coefficient h (units of ![]() ) (the conductive property of a slab of material) of:
) (the conductive property of a slab of material) of:
![]() (Kerslake 1972).
(Kerslake 1972).
Radiant heat flow (![]() ) between two surfaces is approximately proportional to their temperature difference:
) between two surfaces is approximately proportional to their temperature difference:
![]()
where T is the average absolute temperature (in Kelvin) of the two surfaces, ![]() is the absorption coefficient and
is the absorption coefficient and ![]() is the Stefan-Boltzmann constant (
is the Stefan-Boltzmann constant (![]()
![]() ). The amount of radiation exchange is inversely related to the number of intercepting layers (n):
). The amount of radiation exchange is inversely related to the number of intercepting layers (n):
![]()
Clothing insulation (![]() ) is defined by the following equations:
) is defined by the following equations:
![]()
![]()
![]()
where ![]() is intrinsic insulation,
is intrinsic insulation, ![]() is (adjacent) air insulation,
is (adjacent) air insulation, ![]() is total insulation,
is total insulation, ![]() is average skin temperature,
is average skin temperature, ![]() is the average temperature of the outer surface of the clothing,
is the average temperature of the outer surface of the clothing, ![]() is air temperature,
is air temperature, ![]() is the dry heat flow (convective and radiant heat) per unit of skin area and
is the dry heat flow (convective and radiant heat) per unit of skin area and ![]() is the clothing area factor. This coefficient has been underestimated in older studies, but more recent studies converge to the expression
is the clothing area factor. This coefficient has been underestimated in older studies, but more recent studies converge to the expression ![]()
Often I is expressed in the unit clo; one clo equals ![]() .
.
McCullough et al. (1985) deduced a regression equation from data on a mix of clothing ensembles, using thickness of the textile (![]() , in mm) and percentage covered body area (
, in mm) and percentage covered body area (![]() ) as determinants. Their formula for the insulation of single clothing items (
) as determinants. Their formula for the insulation of single clothing items (![]() ) is:
) is:
![]()
The evaporative resistance R (units of s/m) can be defined as:
![]()
(or sometimes ![]() , in
, in ![]() )
)
![]()
For fabric layers, the air equivalent (![]() ) is the thickness of air that provides the same resistance to diffusion as the fabric does. The associated vapour
) is the thickness of air that provides the same resistance to diffusion as the fabric does. The associated vapour ![]() and latent heat (
and latent heat (![]() ) flows are:
) flows are:
![]()
![]()
where D is the diffusion coefficient (![]() ), C the vapour concentration (
), C the vapour concentration (![]() ) and
) and ![]() the heat of evaporation (2430 J/g).
the heat of evaporation (2430 J/g).
![]()
(from Lotens 1993). ![]() is related to R by:
is related to R by:
![]()
where:
D is the diffusion coefficient for water vapour in air, ![]() .
.
Hours of Work
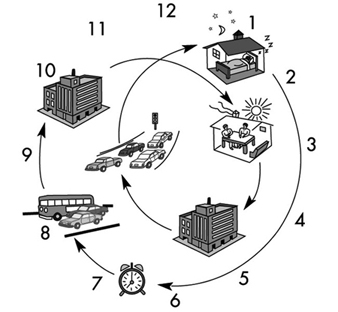
Shiftwork is work scheduled, either permanently or frequently, outside normal daytime working hours. Shiftwork can be e.g., permanent work at night, permanent work during the evening, or work hours can have changing assignment patterns. Each type of shift system has its advantages and disadvantages, and each is associated with differing effects on well-being, health, social life and work performance.
In the traditional slowly rotating shift systems, shifts change weekly; that is, a week of night shifts is followed by a week of evening shifts and then a week of morning shifts. In a quickly rotating shift system only one, two or a maximum of three consecutive days are spent on each shift. In some countries, like the United States, shifts longer than 8 hours, in particular 12 hours, are gaining in popularity (Rosa et al. 1990).
Human beings have evolved as essentially diurnal; that is, the body is mainly “programmed” towards daytime work performance and for night-time recreation and rest. Internal mechanisms (sometimes called the body or biological clock) control the physiology and biochemistry of the body to fit in with a 24-hour environment. These cycles are called circadian rhythms. The disruption of circadian variations in physiological function caused by having to be awake and at work at biologically unusual hours, as well as to sleep during the daytime, is one of the major stresses associated with shiftwork.
Despite the widespread assumption that disturbances of the circadian system may result, over the long run, in harmful effects, the actual cause-effect relation has been difficult to establish. Despite this lack of absolute proof, it is widely accepted that it is prudent to adopt shift systems at the workplace that minimize long-lasting disruption of circadian rhythms.
Combined Effects of Workplace Factors
Some shiftworkers are also exposed to other workplace hazards, such as toxic agents, or to jobs with high mental loads or physical demands. Only a few studies, however, have addressed the problems caused by the combination of shiftwork and unfavourable working, organizational and environmental conditions where the negative effects of shiftwork could be caused not only by the phase difference between circadian rhythms and living conditions, but also by the adverse negative working conditions that may be combined with shiftwork.
A variety of workplace hazards, such as noise, unfavourable climatic conditions, unfavourable lighting conditions, vibration and combinations of these, can sometimes occur more often in three-shift systems, irregular systems and night-shift systems than in two-shift systems or daywork.
Intervening Variables
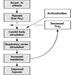
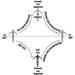
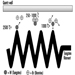
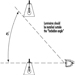
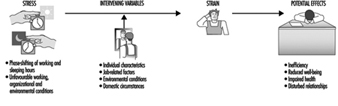
People vary widely in their tolerance of shiftwork, according to Härmä (1993), which may be explained by the influence of many intervening variables. Some individual differences which may modify the strain of shiftworkers are: differences in the phase and amplitude of the circadian cycle, age, gender, pregnancy, physical fitness and flexibility in sleeping habits, and the ability to overcome drowsiness, as illustrated by figure 1.
Figure 1. Model of stress and strain of shiftworkers.
Although some authors found a correlation between a larger amplitude of circadian rhythms and fewer medical complaints (Andlauer et al. 1979; Reinberg et al. 1988; Costa et al. 1989; Knauth and Härmä 1992), others have found that it does not predict adjustment to shiftwork (Costa et al. 1989; Minors and Waterhouse 1981) even after three years of work (Vidacek et al. 1987).
There appear to be two main dimensions of personality related to the circadian phase: “morningness”/“eveningness” and intro-version/extroversion (Kerkhof 1985). Morningness/eveningness can be assessed by questionnaire (Horne and Östberg 1976; Folkard et al. 1979; Torsval and Åkerstedt 1980; Moog 1981) or by measuring body temperature (Breithaupt et al. 1978). Morning types, “larks”, having an advanced phase position of the circadian body temperature, go to bed earlier and rise earlier than the average population, whereas evening types, “owls,” have a delayed circadian phase position and go to bed and rise later. To be a “lark” would appear to be an advantage for morning shifts and an “owl” for night shifts. However, some authors report that a disproportionally large number of those who give up shiftwork were morning types (Åkerstedt and Fröberg 1976; Hauke et al. 1979; Torsvall and Åkerstedt 1979). A relation between morningness and decreased tolerance to shiftwork has been found by Bohle and Tilley (1989) and Vidacek et al. (1987). Other researchers, however, have found opposite results (Costa et al. 1989), and it should be noted that most studies have involved only extreme “larks” and “owls”, where each represents only 5% of the population.
In many questionnaire studies, more adverse health effects of shiftwork have been found with increasing age, the critical age being 40 to 50 years on average (Foret et al. 1981; Koller 1983; Åkerstedt and Torsvall 1981). With increasing age, sleep during the day becomes progressively more difficult (Åkerstedt and Torsvall 1981). There are also some indications of slower circadian adjustment to shiftwork in middle-aged shiftworkers compared to younger ones (Härmä et al. 1990; Matsumoto and Morita 1987).
Gender and pregnancy are two intervening variables which have often been discussed but not yet adequately investigated in longitudinal studies. Based on a review of the literature, Rutenfranz et al. (1987) conclude that the circadian rhythms of men and women react in the same way to the phase shifting of work and sleep in connection with night work. However, two aspects—the menstrual cycle and the additional load of child care and household duties—have to be taken into consideration.
Although some authors have found more frequent menstrual problems in groups of women shiftworkers compared to women in day work (Tasto et al. 1978; Uehata and Sasakawa 1982), the comparability of these shift and day-work groups was question-able. Pokorski et al. (1990) studied perception of discomfort among female triple-shift workers during three phases of the menstrual cycle (praemenstruum, menstruation and postmen-struum). Phase-related differences were more pronounced than differences between morning, evening and night shifts.
Child care at home reduced the duration of sleep and of leisure time in female shiftworking nurses. Estryn-Behar questioned 120 women on permanent night shift and found that the average duration of sleep after night shifts was 6 h 31 min for women without children, 5 h 30 min for women with older children, and 4 h 55 min for women with very young children (Estryn-Behar et al. 1978). Nevertheless, a study of policewomen found that those with children were more favourable towards shiftwork than were women without children (Beermann et al. 1990).
Physical fitness appeared to be a factor in increasing tolerance to shiftwork in a study by Härmä et al. (1988a, b). In a follow-up study with matched pair design, the group of participants who exercised regularly on a four-month programme reported a significant decrease in general fatigue, particularly when on the night shift, as well as a decrease in musculoskeletal symptoms and an increase in sleep length.
The “flexibility of sleeping habits” and “ability to overcome drowsiness”, as assessed by a questionnaire developed by Folkard et al. (1979; 1982) were related, in some studies, to a better tolerance to shiftwork (Wynne et al. 1986; Costa et al. 1989; Vidacek et al. 1987). In other studies, however, this relationship was not confirmed (e.g., Bohle and Tilley 1989).
Other intervening variables that may be important for shiftwork tolerance are the “commitment to night work” as the way in which people schedule their lives (Folkard et al. 1979; Minors and Waterhouse 1981) or the coping style of shiftworkers (Olsson et al. 1987; Olsson and Kandolin 1990).
Besides individual characteristics, situational factors seem to be of importance for explaining the extent of problems reported by shiftworkers. Küpper et al. (1980) and Knauth (1983) found that shiftworkers who tried to sleep during the day and were often or always disturbed by noise, complained more frequently about nervous and gastrointestinal symptoms than did shiftworkers with undisturbed or rarely disturbed sleep.
Health Effects of Shiftwork
Most of the health complaints of shiftworkers can be related to the quality of the day sleep after night shifts and, to a lesser extent, to the sleep before morning shifts. As circadian rhythms generally function such that the body is programmed for daytime performance and for night-time sleep, after the night shift the body is, in general, not completely adjusted for going to sleep. Other factors may also intervene. Daylight may disturb sleep. Noise during the day is in general louder than during the night. Most nightworkers complain about the noise of children and of traffic. Some nightworkers interrupt their day sleep in order to partake of a joint meal with the family, and some reduce their sleep because of their household duties and child care responsibilities. In one study of shiftworkers, the duration of night sleep was found to be reduced to 6 hours (Knauth 1983). Although there are large interindividual differences in sleep needs, 6 or less hours of sleep per day is inadequate for many human beings (Williams et al. 1974). In particular, after many consecutive night shifts an accumulation of sleep deficits has to be expected, with its accompanying effects in both social life and productivity (Naitoh et al. 1990) as well as the possibility of an increased accident rate. Several electroencephalographic studies have also shown that the quality of day sleep is also lower (Knauth 1983).
Sleep deficits may occur in both a week of night shifts and in a week of morning shifts. The prolonged sleep duration at the weekend after a week of morning shifts seems to indicate that there is an increased need for sleep.
Hak and Kampmann (1981) studied sleep and fatigue in train drivers. The earlier the morning shift started, the shorter was the preceding night-shift sleep and the more fatigued the train drivers during the morning shift. The reduction of sleep in connection with an earlier start of the morning shift has also been confirmed by studies of Moors (1990) as well as Folkard and Barton (1993). Such findings may be partially explained by the social pressure of the family not to go to bed too early, or by the body clock, which according to Lavie (1986) causes a “forbidden zone” for sleep, during which sleep propensity is greatly reduced. The latter explanation means that even if the shiftworkers go to bed earlier—because of the early start of the following morning shift—they might find it difficult to fall asleep.
Gastrointestinal disturbances. Night work leads to a change in the sequence and timing of meals. During the night, the stomach cannot cope with the composition and the quantity of a typical daytime meal. It is then understandable that nightworkers often suffer more from disturbances of appetite than do dayworkers or shiftworkers not on night shift, as Rutenfranz et al. (1981) have concluded from a review of the literature.
In the long run, irregular food intake can lead to gastrointestinal complaints or even to disorders. However, the reasons for the complex gastrointestinal symptoms are surely manifold. An analysis of existing studies, such as that of Costa (1996), is difficult, because of methodological differences. Most results are based on cross-sectional studies—that is, on workers currently engaged in shiftwork. Thus, if individuals have left shiftwork because of problems or diseases, we are left with a more or less self-selected population (the “healthy worker” effect). Therefore the health status of a group of shiftworkers may be better than a group of dayworkers, simply because shiftworkers with poorer health or social problems have changed to day work and those that remain may be better able to cope.
In longitudinal studies, which have been almost exclusively retrospective, the problems with self-selection and loss to follow-up are well known. For example, for the sample in the study of Leuliet (1963), the study population was almost halved in size during the study period of 12 years. As with cross-sectional studies, it is often former shiftworkers, who have transferred to day work out of shifts because of medical problems, who show the most serious effects. Thiis-Evensen (1958) found that peptic ulcers were twice as frequent among former shiftworkers as among dayworkers. Aanonsen (1964) and Angersbach et al. (1980) observed, respectively, two and three-and-a-half times as many cases of peptic ulcers among former regular shiftworkers, with a subsequent significant decrease in gastrointestinal disease after the transfer out of the shiftwork pattern.
Costa et al. (1981) computed the time interval between beginning shiftwork and when illnesses were diagnosed (table 1). Comparing groups with different working time arrangements, Costa et al. found the shortest mean intervals (4.7 years) for the appearance of gastroduodenitis in permanent nightworkers. In groups with night work (i.e., three-shift workers and permanent nightworkers), within an interval of about 5 years peptic ulcers developed. In his review Costa (1996) concludes that “there is sufficient evidence to consider shiftwork as a risk factor for gastrointestinal disorders and diseases—in particular peptic ulcer” (table 1).
Table 1. Time intervals from the beginning of shiftwork to the moment when the three illnesses were diagnosed (mean and standard deviation in years).
|
Work schedule |
Gastroduodenitis |
Peptic ulcer |
Neurotic disorders |
|
Day work |
12.6 ± 10.9 |
12.2 ± 9.9 |
9.7 ± 6.8 |
|
Two shifts |
7.8 ± 6.6 |
14.4 ± 8.2 |
9.0 ± 7.5 |
|
Three shifts |
7.4 ± 6.5 |
5.0 ± 3.9 |
6.8 ± 5.2 |
|
Night work |
4.7 ± 4.3 |
5.6 ± 2.8 |
3.6 ± 3.3 |
Source: Costa et al. 1981
Cardiovascular disorders. Kristensen (1989) has analysed the relevant studies on the incidence of cardiovascular disorders in shiftworkers for methodological and analytical factors, as shown in Table 2. Papers published after 1978 were more likely to report an increase in cardiovascular disorders, particularly among those who transferred away from shiftwork. Waterhouse et al. (1992) conclude that it is not possible simply to dismiss the relationship as had been generally accepted (Harrington 1978).
Table 2. Relationship between shiftwork and incidence of cardiovascular disorders
|
Reference |
Publication years |
Conclusion |
Methodological comments/ratings |
|
Thiis-Evenson (1949); Aanonsen (1964) |
1949-1964 |
0 |
2 |
|
Taylor and Pocock (1972) |
1972 |
0 |
? correct choice for controls |
|
Rutenfranz et el. (1977); Carpentier et al. (1977) |
1977 |
0, review articles |
|
|
Angersbach et al. (1980); |
1980-1983 |
+, particularly dropouts; |
2-3 |
|
Michel-Briand et al. (1981) |
1981 |
+, in retired workers |
1 |
|
Alfredsson et al. (1982; 1983; 1985); |
1982-1986 |
+, in men and women; |
3-4 |
|
Åkerstedt et al. (1984) |
1984-1986 |
+, review article |
|
|
Orth-Gomer (1985) |
1985 |
+, review article |
|
|
Andersen (1985) |
1985 |
+, occupations involving shiftwork |
|
|
Frese and Semmer (1986) |
1986 |
+, in dropouts |
Source: Waterhouse et al. 1992. Based on Kristensen 1989. Ratings on conclusions used by Kristensen: +, increased incidence; 0, no difference.
Methodological ratings, 1-4 from lowest to highest quality methodology.
Neurological disorders. Although there is a lack of standardization of the symptoms and disorders in studies of neurological disorders of shiftworkers (Waterhouse et al. 1992; Costa 1996), according to Waterhouse (1992), however, “there is now evidence for a greater tendency towards general malaise—including anxiety and depression elements—in shiftworkers than in day-working colleagues”. Costa (1996) comes to a similar but more cautious conclusion: “there is sufficient evidence to suggest that morbidity for psychoneurotic disorders can be influenced by shiftwork to a greater or lesser extent in relation to other individual and social factors.”
Mortality. There is only one very careful epidemiological study on the mortality of shiftworkers. Taylor and Pocock (1972) compared mortality rates in shiftworkers and dayworkers over a 13-year period in a sample of over 8,000 persons. There were no differences in rates between current shiftworkers and dayworkers. However, the standardized mortality ratio for former shiftworkers was 118.9, compared to 101.5 for current shiftworkers, which “might imply a selecting-out of less fit men” (Harrington 1978).
Social Problems of Shiftworkers
Shiftwork may have negative effects on family life, participation in institutional life and social contacts. The extent of problems which may exist is dependent on many factors, such as the type of shift system, gender, age, marital status, composition of family of the shiftworker, as well as how common shiftwork is in a particular region.
During a week of evening shifts, regular contacts between a shiftworker and his or her school-age children, or partner who may work in morning or day shifts, are dramatically reduced. This is an important problem for shiftworkers who work so-called permanent afternoon shifts (Mott et al. 1965). In the traditional discontinuous two-shift system, a week of morning shifts and evening shifts alternates such that every second week the contacts are disturbed. The traditional weekly rotating three-shift system has evening shifts every third week. In quickly rotating shift systems, contacts within the family are never impaired during a whole week. Researchers have obtained contradicting results. Mott et al. (1965) found that many consecutive evening or night shifts could impair the marital happiness of shiftworkers, while Maasen (1981) did not observe this. Shiftwork—in particular when both parents are shiftworkers—may have negative effects on the school performance of children (Maasen 1981; Diekmann et al. 1981).
Studies concerning the subjective value of free time during different hours of the week showed that weekends were rated higher than weekdays, and evenings higher than time off during the day (Wedderburn 1981; Hornberger and Knauth 1993). The contacts with friends, relatives, clubs, political parties, churches and so on are mainly impoverished by weekend work, evening shifts and night shifts (Mott et al. 1965), as has been reviewed by Bunnage (1981); Walker (1985); and Colligan and Rosa (1990).
Only with respect to hobbies and activities of a solitary or near-solitary nature are shiftworkers at an advantage compared to dayworkers, since gardening, walking, fishing or “do it yourself” projects are comparatively flexible activities which are possible any time, not only in the evening or on weekends.
Some studies have dealt with the burden of shiftworkers’ spouses (Banks 1956; Ulich 1957; Downie 1963; Sergean 1971), who have to alter their lifestyle (for example mealtimes) in order to fit in with the shift system of their mates. They may be forced to postpone noisy household duties and to keep children quiet when the shiftworker is asleep after the night shift. Furthermore, they are alone during evening, night and weekend shifts and have to cope with an irritable spouse. After a change from a weekly to a quickly rotating continuous shift system, 87% of shiftworkers’ spouses voted in favour of the new shift system. They argued that in the old shift system the spouse was very tired after the end of the period of night shifts, needed several days to recover and was not in the mood for joint leisure activities. However, in the new shift system with only two or three consecutive night shifts, the worker was less tired and they enjoyed more joint leisure activities.
Women on shiftwork may have more problems with domestic duties and sleep since household responsibility is not equally shared by the marriage partners. Nevertheless some permanent night nurses have specifically chosen to work at night for domestic reasons (Barton et al. 1993). However, as Walker (1985) concludes in his review, “to say that fixed night shifts for mothers is compatible with their child-rearing responsibilities ignores the ‘costs’”. Constant tiredness because of reduced sleep may be the cost.
Worker Performance
In addition to possible effects of shiftwork on worker health, worker performance can also be affected. Harrington’s (1978) generalized conclusions about performance were reached through consideration of productivity and accidents. They are still valid and have been reformulated by Waterhouse et al. (1992):
- Errors and general performance often showed rhythmic changes, with the night shift being worst.
- The nocturnal decrement in performance could be lessened or prevented if breaks in the work were feasible, if the work were interesting, or if motivation could be maintained.
- The performance worsened (generally with the night shift being more adversely affected than others) if boring, repetitive tasks were involved, if sleep loss had occurred, or if the amount of time spent on duty were increased.
Differences between individuals were often the largest variable in performance.
One problem in comparing productivity and accidents in morning, afternoon and night shifts is methodological. Working, environmental and organizational conditions at night and in the daytime in general are not completely comparable (Colquhoun 1976; Carter and Corlett 1982; Waterhouse et al. 1992). Therefore it is difficult to control all the variables. It is not astonishing that in a review of 24 studies there were almost as many studies with a higher frequency of accidents at night as studies with a higher frequency of accidents in the daytime (Knauth 1983). In some studies the workload in the daytime and during night-time were comparable and measures were available for all 24 hours. In most of these studies the authors found a degraded night-shift performance (e.g., Browne 1949; Bjerner et al. 1955; Hildebrandt et al. 1974; Harris 1977; Hamelin 1981). However, as Monk (1990) has concluded, it is possible that circadian effects can “show through” only when workers are under pressure. In the absence of pressure, workers may be able to equate day-shift and night-shift performance, because both are considerably suboptimal.
The Design of Shift Systems
The most important recommendations for the design of shift systems are summarized in figure 2.
Figure 2. Recommendations for the design of shift systems.
Permanent night work
The night shift is the most disruptive of all shifts in terms of physiological adjustment, sleep and well-being. The circadian physiological rhythms of most shiftworkers may require more than one week for complete adjustment to night work. Any partial adjustment will be lost following days off from night shift. Thus, the body rhythms of permanent nightworkers are constantly in a state of disruption. In one study (Alfredsson et al. 1991) the permanent night security guards had a 2-to-3-times higher occurrence of sleep disturbances and fatigue than the national sample of the working population.
Some authors have suggested various ways in which to match employee tolerance for shiftwork and certain external stimuli for helping workers to adjust. According to Hildebrandt et al. (1987) persons with a late phase position (evening types) are able to adjust to night work. Moog (1988) posited that they should work in very long periods of night shifts—that is, much more than 10 nights in a row. To profit from an adjustment to night work, Folkard (1990) even suggested the creation of a “nocturnal subsociety”, which in addition to working permanently at night, would continue to be active at night and to sleep during the day, even when not at work. Although performance at night might in the long run be increased (Wilkinson 1992), such a proposal causes an accumulation of sleep deficits and social isolation, which seems to be unacceptable for most persons (Smith and Folkard 1993).
There are an increasing number of studies dealing with the influence of bright light on re-entrainment of circadian rhythms (some examples are Wever et al. 1983; special session at the IXth International Symposium on Night and Shift Work; Costa et al. 1990a; Rosa et al. 1990; Czeisler et al. 1990). However, “much work is necessary to determine the optimal light-work-sleep schedules for shift workers in terms of their ability to shift circadian rhythms, improve sleep, reduce fatigue, as well as in terms of their social feasibility”, according to Eastman (1990).
In comparison with other shift systems, fixed night shifts have more negative effects on families who must adapt their lifestyles to this schedule, on sexual relations and on workers’ ability to fulfil familial roles (Stein 1963; Mott et al. 1965; Tasto et al. 1978; Gadbois 1981). However, in some studies of permanent night shift, nurses reported fewer complaints than rotating nurses or dayshift nurses (Verhaegen et al. 1987; Barton et al. 1993). Barton et al. propose that one possible explanation for these results may be that the freedom to choose either day- or night-work may greatly influence the degree to which subsequent problems are experienced. The notion that this represents “freedom” is, however, questionable when many female nurses prefer permanent night work because this represents the only way of better arranging domestic responsibilities and employment outside the home (Gadbois 1981).
Permanent night work also has some advantages. Nightworkers report a greater feeling of independence and less supervision at night (Brown 1990; Hoff and Ebbing 1991). Furthermore, because it is less easy to obtain work relief for night-shift staff, apparently more “team spirit” (esprit de corps) develops. However, in most cases night work is chosen because of the increase in income due to the night-shift allowance (Hoff and Ebbing 1991).
Although we have insufficient knowledge about the long-term health effects of permanent night work and about optimal bright-light work-sleep schedules, it is known that the night shift is the most disruptive of all shifts in terms of physiological adjustment, sleep and well-being, and until results from further research are available, we will assume for the moment that permanent night work is not recommendable for the majority of shiftworkers.
Quickly rotating versus slowly rotating shift systems
More rapidly rotating schedules are more advantageous compared to weekly shift rotation. A fast rotation keeps the circadian rhythm in a daytime orientation and it is not in a constant state of disruption from partial adjustment to different day and night orientations. Consecutive night shifts may cause an accumulation of sleep deficits—that is, a chronic sleep deprivation (Tepas and Mahan 1989; Folkard et al. 1990). In the long run this could lead to long-term biological “costs” or even medical disorders. However, no well-controlled epidemiological study is available that compares the effects of permanent, slowly and quickly rotating shift systems. In most published studies the groups are not comparable with regard to the age structure, job content, degree of self-selection (e.g., Tasto et al. 1978; Costa et al. 1981) or because the employees working on fixed morning, afternoon and night shifts were combined to form a single category (Jamal and Jamal 1982). In several longitudinal field studies, the effects of a change from weekly to quicker rotating shift systems have been investigated (Williamson and Sanderson 1986; Knauth and Kiesswetter 1987; Knauth and Schönfelder 1990; Hornberger and Knauth 1995; Knauth 1996). In all 27 studied groups of shiftworkers, the majority of the shiftworkers voted in favour of the quicker rotating shifts after a trial period. Summing up, quickly rotating shift systems are preferable to slowly rotating ones. Åkerstedt (1988), however, does not agree, because the maximum sleepiness usually occurs on the first night shift because of extended prior waking. He recommends slow rotation.
Another argument for a quickly rotating shift system is that shiftworkers have free evenings in every week and thus more regular contact with friends and colleagues is possible than with weekly rotating shifts. Based on analyses of the periodic components of work and leisure time, Hedden et al. (1990) conclude that rotations that allow for a shorter but more frequent synchronization of work life with social life result in less impairment than rotations that lead to longer but infrequent synchronization.
Duration of shifts
There are many contradictory results of the effects of extended workdays, and thus a general recommendation for extended workdays cannot be made (Kelly and Schneider 1982; Tepas 1985). An extended workday of 9 to 12 hours should be contemplated only in the following cases (Knauth and Rutenfranz 1982; Wallace 1989; Tsaneva et al. 1990; Ong and Kogi 1990):
- The nature of work and the workload are suitable for extended working hours.
- The shift system is designed to minimize the accumulation of fatigue.
- There are adequate arrangements for cover of absentees.
- Overtime is not added.
- Toxic exposure is limited.
- It is likely that a complete recovery after work and a high acceptance of the working time arrangements are possible (e.g., housing, family problems, commuting, climate, no moonlighting).
Physiological requirements must be taken into account. According to Bonjer (1971), the acceptable oxygen rate consumption during an 8-hour shift should be about 30% or less of the maximum oxygen consumption. During a 12-hour shift it should be about 23% or less of the maximum oxygen consumption. Since the amount of oxygen consumption increases with the physical demands of the job, it would seem that 12-hour shifts are acceptable only for physically light work. However, even in this case, if the mental or emotional stress caused by the job is too high, extended working hours are not advisable. Before the introduction of extended working hours, the stress and strain at the specific workplace must be accurately evaluated by experts.
One of the potential disadvantages of 12-hour shifts, in particular 12-hour night shifts, is increased fatigue. Therefore the shift system should be designed to minimize the accumulation of fatigue—that is, there should not be many 12-hour shifts in a row and the day shift should not start too early. Koller et al. (1991) recommend single night shifts or a maximum of two night shifts. This recommendation is supported by favourable results of studies in shift systems with single 12-hour night shifts (Nachreiner et al. 1975; Nedeltcheva et al. 1990). In a Belgian study, the length of the shift was extended to 9 hours by starting one hour earlier in the morning (Moors 1990). The day shift started at 0630 instead of 0730 and the morning shift in a two-shift system started at 0500 instead of 0600. In a 5-day week these working time arrangements led to an accumulation of sleep deficits and complaints of tiredness. The author recommends that the shifts start as in the old working time arrangements and that the shift be extended by one hour in the evening.
Our knowledge is very limited concerning another problem: toxic exposure and toxic clearance during the time off work in connection with extended working hours (Bolt and Rutenfranz 1988). In general, exposure limits are based on 8 hours exposure, and one cannot simply extrapolate them to cover a 12-hour shift. Some authors have proposed mathematical procedures for adjusting these exposures for working times that deviate from the usual 8-hour shift, but no method has been uniformly adopted (e.g., Hickey and Reist 1977; OSHA 1978; Brief and Scala 1986; Koller et al. 1991).
Designers of shift systems must consider the workload, the working environment and the conditions outside the place of work. Ong and Kogi (1990) report that “the hot, tropical climate and noisy dwelling units of Singapore were not conducive to sound sleep for shiftworkers, who needed to sleep in the daytime”. Such circumstances increased fatigue and affected productivity on the 12-hour shift worked the next day. Another concern that relates to workers’ well-being is the way shiftworkers use their large blocks of leisure time. In some studies it appears that they may have second jobs (moonlighting), thus increasing their total workload (Angersbach et al. 1980; Wallace 1989; Ong and Kogi 1990). Many other social factors, like commuting, individual differences, social support or events in life must also be considered in the 12-hour shift systems (e.g., Tsaneva et al. 1990).
Timing of shifts
Although there is no optimal solution for the timing of shifts, there is much evidence in the literature that an early start for the morning shift should be avoided. An early start often reduces total sleep because the majority of shiftworkers go to bed at the usual time (Knauth et al. 1980; Åkerstedt et al. 1990; Costa et al. 1990b; Moors 1990; Folkard and Barton 1993). An increase in fatigue during the morning shift has also been observed (Reinberg et. al. 1975; Hak and Kampman 1981; Moors 1990), as well as an increase in the risk of errors and accidents in the morning shift (Wild and Theis 1967; Hildebrandt et al. 1974; Pokorny et al. 1981; Folkard and Totterdell 1991).
Assuming a constant shift length of 8 hours, a late start for the morning shift also means a late start for the night shift (e.g., shift change times at 0700/1500/2300 or 0800/1600/2400). A late start for the night shift means also a late end for the evening shift. In both cases there might be transport problems because buses, trams and trains run less frequently.
The decision in favour of a specific shift change time may also be dependent on the job content. In hospitals, in general, it is the night shift that wakes up, washes and prepares patients (Gadbois 1991).
Arguments in favour of an earlier start have also been made. Some studies have shown that the later the day sleep begins after a night shift, the shorter it will be (Foret and Lantin 1972; Åkerstedt and Gillberg 1981; Knauth and Rutenfranz 1981). Day sleep may be disturbed and a very early start of sleep after night shifts might avoid these problems. Debry et al. (1967) have proposed shift change times at 0400, 1200 and 2000 in order to facilitate workers having as many meals with the family as possible. According to Gadbois (1991) an early start for the night shift improves the contact between staff and patients in hospitals.
Flexible working time arrangements are also possible even in three-shift systems, where employees can choose their working hours (McEwan 1978; Knauth et al. 1981b; 1984; Knauth and Schönfelder 1988). However, in contrast to flexitime in dayworkers, shiftworkers must make pre-arrangements with co-workers.
Distribution of leisure time within the shift system
The distribution of leisure time between consecutive shifts has important implications for sleep, fatigue and well-being, as well as social and family life and the overall satisfaction of the shiftworker with the shift system. If there are only 8 hours between the end of one shift and the start of the next, there will be a reduction of sleep between the shifts and increased fatigue in the second shift (Knauth and Rutenfranz 1972; Saito and Kogi 1978; Knauth et al. 1983; Totterdell and Folkard 1990).
Too many working days in succession can lead to an accumulation of fatigue and sometimes overexposure to toxic substances (Bolt and Rutenfranz 1988). It is not easy to define a limit for the maximum number of consecutive working days, because the workload, the organization of breaks, and exposure to unfavourable environmental conditions vary. However, Koller et al. (1991) recommend limiting the number of consecutive working days to between 5 and 7.
Free weekends are of particular social importance. Pátkei and Dahlgren (1981) studied satisfaction with different types of rapidly rotating shift systems. The satisfaction with a 7-day shift system with 3 to 5 regular days free was significantly higher than in a system with only 2 free days. The authors concluded that “the length of the break might be an important factor in determining the attractivity of rapidly rotating shifts”. On the other hand, free days in the first shift system were counterbalanced by additional periods of holidays during the year.
Direction of rotation. The direction of rotation is another important consideration (Tsaneva et al. 1987; Totterdell and Folkard 1990). A shift system which first moves from morning shift to evening shift, and then to night shift, has a forward rotation (phase delay, clockwise rotation). An anticlockwise, or backward, rotation has a phase advance which moves from night to evening to morning shifts. The forward rotation appears to correspond more closely to the endogenous circadian rhythm, which has a period of more than 24 hours, but only two longitudinal field studies on the effects of different directions of rotation exist (Landen et al. 1981; Czeisler et al. 1982). The majority of the shiftworkers in these studies seem to prefer the forward rotation, but the studies are not definitive. Barton and Folkard (1993) found that an anticlockwise system led to higher levels of fatigue and more sleep disturbances between shifts. “Hybrid” systems were not better. Clockwise rotation was associated with the fewest problems. Turek (1986) proposes, however, that the sleep disturbance of both systems would be comparable.
Shiftworkers on a discontinuous shift system with backward rotation were found to like the long period off work between the end of the last morning shift and the start of the first night shift, in particular if this period includes a weekend.
Although the evidence is limited and further research is needed, forward rotation seems to be recommendable at least in continuous shift systems.
Optimizing shift systems
There is no “optimal” shift system. Each enterprise, its managers and shiftworkers should seek the best compromise between the demands of the enterprise and the needs of the workers. Furthermore, the decision should be founded on scientific recommendations for the design of shift systems. The implementation strategy is of particular importance for the acceptance of a new shift system. Many manuals and guidelines for the implementation of new working time arrangements have been published (ILO 1990). Too often shiftworkers are not sufficiently involved in the analysis, planning and design stage of the shifts.
A continuous shift system that has a rapid forward rotation pattern, with 8 hours of work per shift, some free weekends, at least two successive full days off and no quick changeovers, appears to be the system to be recommended. Such a basic shift system has an average of 33.6 hours per week, which may not be universally acceptable. If additional shifts are required, acceptance is higher when the additional shifts are planned on a long-term basis, such as at the beginning of the year so workers can plan holidays. Some employers do not require older shiftworkers to work additional shifts.
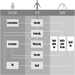
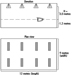
Figures 3 and 4 show schemes for continuous and discontinuous shift systems which accommodate these rules. Figure 5 shows a shift system for a less flexible workplace. It covers 128 operational hours per week, with an average individual workweek of 37 hours. This system has a maximum of three night shifts and two longer free weekends (third week: Thursday to Sunday; fifth/sixth week: Saturday to Monday). It is irregular and does not rotate in a forward direction, which is less optimizing. For shift systems with an operational time of 120 hours per week, gradually rotating shift systems cannot be used, such as from Monday 0600 to Saturday 0600, and an average working time of 40 hours per week.
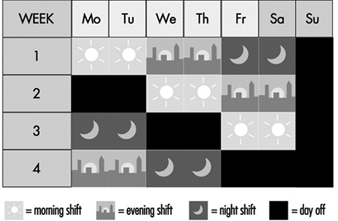
Figure 3. Rotating continuous shift system.
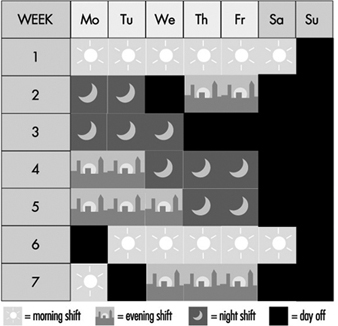
Figure 4. Rotating discontinuous shift system.
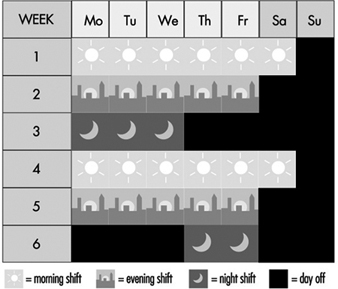
Figure 5. Rotating discontinuous shift system with seven teams.
When the crew can be thinned out during the night, a shift system as shown in Figure 6 may be possible. From Monday to Friday, each day two subgroups work in morning shifts, two in evening shifts but only one subgroup works in night shifts. Therefore, the number of night shifts per person would be reduced, compared with the traditional three-shift system.
Figure 6. Discontinuous shift system with a 50% reduced staffing of night shifts.
Rest Periods
In connection with the arrangement of hours of work, adequate periods of rest, such as breaks during working hours, breaks for meals, daily or nightly rest and weekly rest are also important for the workers’ well-being, health and safety.
There are various reasons for the introduction of rest periods.
Recuperation
When a worker performs heavy physical work, fatigue develops and it is necessary for the worker to stop and rest at intervals. During the breaks the symptoms of reversible functional changes of the organism disappear. When, for instance, heart rate is increased by physical work, it will return to the initial value before work during an adequate rest period. The efficiency of a rest period decreases exponentially with the increasing length of the break. As short breaks have a high efficiency, the rule has been deduced that many short breaks are better than a few long breaks.
Prevention of fatigue
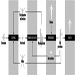
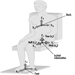
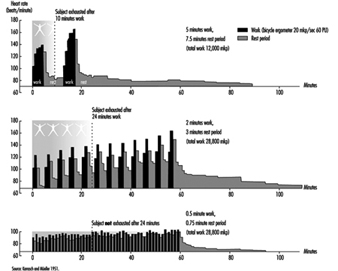
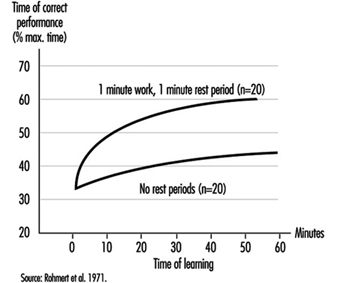
During heavy physical work, many rest periods may not only reduce, but under certain circumstances, also prevent fatigue. This is illustrated by the classic studies of Karrasch and Müller (1951). In the laboratory, subjects had to exercise on bicycle ergonometers (Figure 7). This heavy physical work (10 mkp/s) was organized in the following way: after each period of work (100%) a longer rest period (150%) followed. The three experiments each had a different arrangement of work and rest periods. In the first experiment the subject worked 5 min, rested for 7.5 min, then worked again for 5 min and broke off the experiment when exhausted. The heart rate reached about 140 beats/minute in the first work period and more than 160 beats/minute in the second work period. Even one hour after the end of the experiment the heart rate had not returned to the initial value before the experiment. The second experiment shown in the figure involved shorter work and shorter rest periods (2 min and 3 min). Although the workload was identical to the first experiment, the subject in the second experiment was able to work longer before complete exhaustion set in. An extreme arrangement of 0.5 min work and 0.75 min rest period was set up in the third experiment. The heart rate remained at the steady-state level. The experiment was stopped, not because the subject was exhausted but for technical reasons. This extreme organization of work and of rest periods of course cannot be implemented in industry, but it illustrates that extreme fatigue may be prevented if rest periods are split up.
This phenomenon has also been demonstrated in other studies with other indicators such as blood lactic acid (Åstrand and Rodahl 1970).
Figure 7. Heart rate during and after heavy physical work with different lengths of work and rest periods but a constant work/rest ratio of 2:3.
In a study on foundry workers, the comparison of an arrangement of 20 min of work followed always by a 10-min break with an arrangement of 10 min of work and a 5-min break showed the superiority of the second approach (Scholz 1963), because the average heart rate over 8 hours was lower in the second case.
The prevention of fatigue has also been demonstrated with help of heart rate measurements in experiments with learning of sensorimotoric performances (Rutenfranz et al. 1971). Moreover, the progress in learning was clearly greater in experiments with regular rest periods compared with experiments without rest periods, as shown in figure 8.
Figure 8. Effect of rest periods on the learning of simple sensumotoric performance.
Increase in performance
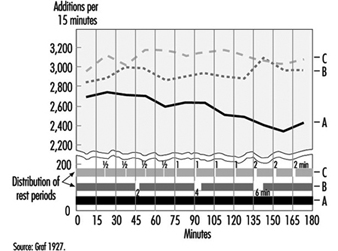
In general, rest periods are considered simply as unproductive interruptions of working time. However, Graf (1922; 1927) showed that rest periods may be, so-to-speak, “rewarding”. We know from sports that athletes running 100 metres start at a high speed, whereas athletes running 5,000 metres start at a “throttled down” speed. Analogue findings on mental work have been published by Graf (figure 9). Three experimental groups were asked to perform calculations. Wages were dependent on the performance. Without being aware of this fact, group A (having the first rest period after 3 hours) started with a reduced speed compared to group B (expecting the first rest period after 45 minutes of work). The highest initial speed and subsequent performance was found in the group C (with rest periods after each 15 minutes of work).
Figure 9. Effects of short rest periods on mental performance.
Maintaining an adequate level of vigilance
In some monotonous monitoring or watchkeeping tasks and in highly simplified tasks with short cycle times, it is difficult to remain alert over longer periods. The reduction of alertness may be overcome by rest periods (or work-structuring measures).
Food intake
The recuperative value of meal breaks is often limited, in particular when the worker has to go a long distance to the canteen, line up for food, eat quickly and hurry back to the working place.
Compensatory physical exercise
If workers, such as visual display unit operators, have to work in constrained postures, it is recommended that they do some compensatory physical exercises during rest periods. Of course the better solution would be to improve the design of the workplace according to ergonomic principles. Physical exercises at the workplace seem to be more accepted in Asian countries than in many other places.
Communication
The social aspect of rest periods, referring to private communication between the workers, should not be neglected. There is a contradiction between the physiologically based recommendation of very short breaks in connection with heavy physical work and the wish of the workers to come together in rest areas and talk with colleagues. Therefore a compromise has to be found.
Hettinger (1993) has published the following rules for the optimal design of rest periods:
- The initial parts of a rest period have the highest recuperative value, which is what results in the effectiveness of short breaks (i.e., many short breaks are more favourable than a few longer breaks with regard to the recuperative value).
- Exceptions to this rule: The cooling-down rest period after work in a hot climate should last at least 10 min in a room with a neutral climate. The warming-up rest period after work in a cold climate (–15 to –30°C) should last for at least 30 min in a room with a neutral climate. The rest period after working in a very loud working environment should be relatively long in a room with less than 70 dB(A). These rest periods are problematic, if one bears in mind that, if the time of exposure is halved, merely a reduction of about 3 dB(A) is achieved in the noise dose.
- The frequency and duration of the rest period is dependent on the degree of difficulty of the physical or mental work. Concerning physical work, it may be specified that physical work with an energy consumption above the acceptable endurance limit but less than 40 KJ/min permits the rest period to be arranged within the shiftworking time. Physical work with an energy consumption of greater than 40 KJ/min demands a rest period immediately after stopping the heavy work, because of the exponential increase of fatigue.
- The efficiency of a rest period should not be reduced by “pseudo-activities” (disguised breaks). Workers and superiors should be informed accordingly.
Rest periods for food intake should last at least 15 minutes.
For further information concerning rest periods after muscular work, see Laurig (1981); and for rest periods after mental work, see Luczak (1982).
Reduction of Sleep Problems
There are no magic formulae to help shiftworkers fall asleep quickly or sleep well. What works for one person may not work for another.
Some useful proposals, mainly for day sleep following night shifts, include:
- Use individual headphones for TV and radio for the other members of the family, and a silent telephone answering machine. Switch off the door-bell.
- Let one’s family know about the work schedule and avoid household noise during sleep times.
- Reduce outside light and noise by using heavy, dark curtains, soundproofed doors and windows, and an air conditioner.
- Ear plugs, a sleeping mask and not drinking any caffeinated drinks within 5 hours of your expected bedtime can also help.
- If the living quarters are noisy, workers should consider moving to quieter living quarters.
Workers should avoid using alcohol to assist in going to sleep and should give themselves time to slow down after work (Community Health Network 1984; Monk 1988; Wedderburn 1991).
For cases where safety is at stake, some authors recommend “maintenance naps” during the night shift as a bridge over the night-time low point in circadian alertness (Andlauer et al. 1982). Many Japanese 24-hour industries allow napping practices on night shifts (Kogi 1981).
Diet
Although there is no evidence that diet assists in coping with night work (Rosa et al. 1990), the following prudent recommendations have been made:
- During night shift, the main meal should be eaten at or before 0100 and should be rich in protein rather than carbohydrates, and have a low fat content.
- Have a snack of fresh fruit or milk products at about 0400–0415.
- Eating meals at the same time each day is recommended.
- A heavy meal just before bedtime should be avoided. Workers should learn to listen to their bodies, to judge stomach comfort and energy levels (Community Health Network 1984; Wedderburn 1991; Knauth et al. 1991).
Occupational Health Measures
Some authors recommend pre-employment screening and medical surveillance of shiftworkers (e.g., Rutenfranz et al. 1985; Scott and LaDou 1990). Workers should be counselled against night work if they have or are:
- a history of digestive tract disorders (e.g., recurrent peptic ulcer disease, irritable bowel syndrome, if symptoms are severe)
- insulin-dependent diabetes mellitus
- thyrotoxicosis
- coronary artery disease, especially if there is unstable angina or a history of myocardial infarction
- narcoleptics and others suffering from chronic sleep disturbances
- epileptics
- severe psychiatric disorders, in particular chronic depression
- asthma requiring medication, especially if the patient is steroid dependent
- active and extensive tuberculosis
- alcoholics and drug addicts
- marked visual impairment or hemeralopia (day blindness) that is too severe for effective correction.
In addition, Scott and LaDou (1990) also mention some “relative contra-indications” most appropriately used for counselling prospective employees, such as extreme “morningness”, sleep rigidity. They may wish to consider their age and the extent of their family responsibilities.
Hermann (1982) has proposed the following intervals for regular health checks: there should be a second health check not later than 12 months after starting night work, and regular health checks at least every 2 years for those under 25, every 5 years for those between 25 and 50, every 2 to 3 years for those between 50 and 60, and every 1 to 2 years for those above 60.
Individual Behavioural Techniques
There are only a few studies analysing shiftworkers’ ability to cope with stress (Olsson et al. 1987; Olsson and Kandolin 1990; Kandolin 1993, Spelten et al. 1993). An active coping strategy—for example, discussing the problems with others—appears to reduce stress better than passive strategies, such as the use of alcohol (Kandolin 1993). However, longitudinal studies are necessary to study the relationship between coping style or behavioural techniques and stress.
Money Payments
Although many compensation plans exist whereby a worker is compensated more for shiftwork (shift bonus), money payments are not an appropriate trade-off for possible negative health effects and disruption of social life.
The best way, of course, to solve problems is to eliminate or reduce the causes. However, since complete elimination of shiftwork is not possible, an alternative strategy worth considering is such as the following: a reduction of unusual working hours for the individual; reduction of night shifts; reduce the unnecessary part of the night work (sometimes activities may be shifted to the morning or evening shift by reorganization of work); implement mixed shift systems with, for example, at least one month per year without shiftwork; insertion of additional shift crews, such as by changing from a 3-shift system to a 4-shift system or from a 4-shift system to a 5-shift system, or by reduction of overtime. Reduction of working time for shiftworkers is another possibility, with shorter weekly working hours for shiftworkers than for dayworkers, with paid breaks and longer holiday periods. Extra days off and stepwise or early retirement are other possible remedies.
All these proposals have already been implemented in some companies in industry or the services sector (e.g., Knauth et al. 1990).
Other Measures
Many other measures such as physical exercise (Härmä et al. 1988a, b), pharmacological aids (Rosa et al. 1990), family counselling (Rosa et al. 1990), improvement of environmental conditions at work (Knauth et al. 1989), better communication between shiftworkers and unions or shiftworkers and their congressperson (Monk 1988; Knauth et al. 1989), or a “Shift Work Awareness Programme” within the company (Monk 1988) have been proposed to reduce the problems of shiftworkers. As there is not one best way to reduce the problems of shiftworkers many creative solutions should be tried (Colquhoun et al. 1996).
Indoor Air Quality: Introduction
The connection between the use of a building either as a workplace or as a dwelling and the appearance, in certain cases, of discomfort and symptoms that may be the very definition of an illness is a fact that can no longer be disputed. The main culprit is contamination of various kinds within the building, and this contamination is usually referred to as “poor quality of indoor air”. The adverse effects due to poor air quality in closed spaces affect a considerable number of people, since it has been shown that urban dwellers spend between 58 and 78% of their time in an indoor environment which is contaminated to a greater or lesser degree. These problems have increased with the construction of buildings that are designed to be more airtight and that recycle air with a smaller proportion of new air from the outside in order to be more energy efficient. The fact that buildings that do not offer natural ventilation present risks of exposure to contaminants is now generally accepted.
The term indoor air is usually applied to nonindustrial indoor environments: office buildings, public buildings (schools, hospitals, theatres, restaurants, etc.) and private dwellings. Concentrations of contaminants in the indoor air of these structures are usually of the same order as those commonly found in outdoor air, and are much lower than those found in air in industrial premises, where relatively well-known standards are applied in order to assess air quality. Even so, many building occupants complain of the quality of the air they breathe and there is therefore a need to investigate the situation. Indoor air quality began to be referred to as a problem at the end of the 1960s, although the first studies did not appear until some ten years later.
Although it would seem logical to think that good air quality is based on the presence in the air of the necessary components in suitable proportions, in reality it is the user, through respiration, who is the best judge of its quality. This is because inhaled air is perceived perfectly through the senses, as human beings are sensitive to the olfactory and irritant effects of about half a million chemical compounds. Consequently, if the occupants of a building are as a whole satisfied with the air, it is said to be of high quality; if they are unsatisfied, it is of poor quality. Does this mean that it is possible to predict on the basis of its composition how the air will be perceived? Yes, but only in part. This method works well in industrial environments, where specific chemical compounds related to production are known, and their concentrations in the air are measured and compared with threshold limit values. But in nonindustrial buildings where there may be thousands of chemical substances in the air but in such low concentrations that they are, perhaps, thousands of times less than the limits set for industrial environments, the situation is different. In most of these cases information about the chemical composition of indoor air does not allow us to predict how the air will be perceived, since the combined effect of thousands of these contaminants, together with temperature and humidity, can produce air that is perceived as irritating, foul, or stale—that is, of poor quality. The situation is comparable to what happens with the detailed composition of an item of food and its taste: chemical analysis is inadequate to predict whether the food will taste good or bad. For this reason, when a ventilation system and its regular maintenance are being planned, an exhaustive chemical analysis of indoor air is rarely called for.
Another point of view is that people are considered the only sources of contamination in indoor air. This would certainly be true if we were dealing with building materials, furniture and ventilation systems as they were used 50 years ago, when bricks, wood and steel predominated. But with modern materials the situation has changed. All materials contaminate, some a little and others much, and together they contribute to a deterioration in the quality of indoor air.
The changes in a person’s health due to poor indoor air quality can show up as a wide array of acute and chronic symptoms and in the form of a number of specific illnesses. These are illustrated in figure 1. Although poor indoor air quality results in fully developed illness in only a few cases, it can give rise to malaise, stress, absenteeism and loss of productivity (with concomitant increases in production costs); and allegations about problems related to the building can develop rapidly into conflict between the occupants, their employers and the owners of the buildings.
Figure 1. Symptoms and illnesses related to the quality of indoor air.
Normally it is difficult to establish precisely to what extent poor indoor air quality can harm health, since not enough information is available concerning the relationship between exposure and effect at the concentrations in which the contaminants are usually found. Hence, there is a need to take information obtained at high doses—as with exposures in industrial settings—and extrapolate to much lower doses with a corresponding margin of error. In addition, for many contaminants present in the air, the effects of acute exposure are well known, whereas there are considerable gaps in the data regarding both long-term exposures at low concentrations and mixtures of different contaminants. The concepts of no-effect-level (NOEL), harmful effect and tolerable effect, already confusing even in the sphere of industrial toxicology, are here even more difficult to define. There are few conclusive studies on the subject, whether relating to public buildings and offices or private dwellings.
Series of standards for outdoor air quality exist and are relied on to protect the general population. They have been obtained by measuring adverse effects on health resulting from exposure to contaminants in the environment. These standards are therefore useful as general guidelines for an acceptable quality of indoor air, as is the case with those proposed by the World Health Organization. Technical criteria such as the threshold limit value of the American Conference of Governmental Industrial Hygienists (ACGIH) in the United States and the limit values legally established for industrial environments in different countries have been set for the working, adult population and for specific lengths of exposure, and cannot therefore be applied directly to the general population. The American Society of Heating, Refrigeration and Air Conditioning Engineers (ASHRAE) in the United States has developed a series of standards and recommendations that are widely used in assessing indoor air quality.
Another aspect that should be considered as part of the quality of indoor air is its smell, because smell is often the parameter that ends up being the defining factor. The combination of a certain smell with the slight irritating effect of a compound in indoor air can lead us to define its quality as “fresh” and “clean” or as “stale” and “polluted”. Smell is therefore very important when defining the quality of indoor air. While odours objectively depend on the presence of compounds in quantities above their olfactory thresholds, they are very often evaluated from a strictly subjective point of view. It should also be kept in mind that the perception of an odour may result from the smells of many different compounds and that temperature and humidity may also affect its characteristics. From the standpoint of perception there are four characteristics that allow us to define and measure odours: intensity, quality, tolerability and threshold. When considering indoor air, however, it is very difficult to “measure” odours from a chemical standpoint. For that reason the tendency is to eliminate odours that are “bad” and to use, in their place, those considered good in order to give air a pleasant quality. The attempt to mask bad odours with good ones usually ends in failure, because odours of very different qualities can be recognized separately and lead to unforeseeable results.
A phenomenon known as sick building syndrome occurs when more than 20% of the occupants of a building complain about air quality or have definite symptoms. It is evidenced by a variety of physical and environmental problems associated with non-industrial indoor environments. The most common features seen in cases of sick building syndrome are the following: those affected complain of non-specific symptoms similar to the common cold or respiratory illnesses; the buildings are efficient as regards energy conservation and are of modern design and construction or recently remodelled with new materials; and the occupants cannot control the temperature, humidity and illumination of the workplace. The estimated percentage distribution of the most common causes of sick building syndrome are inadequate ventilation due to lack of maintenance; poor distribution and insufficient intake of fresh air (50 to 52%); contamination generated indoors, including from office machines, tobacco smoke and cleaning products (17 to 19%); contamination from the outside of the building due to inadequate placement of air intake and exhaust vents (11%); microbiological contamination from stagnant water in the ducts of the ventilation system, humidifiers and refrigeration towers (5%); and formaldehyde and other organic compounds emitted by building and decoration materials (3 to 4%). Thus, ventilation is cited as an important contributory factor in the majority of cases.
Another question of a different nature is that of building-related illnesses, which are less frequent, but often more serious, and are accompanied by very definite clinical signs and clear laboratory findings. Examples of building-related illnesses are hypersensitivity pneumonitis, humidifier fever, legionellosis and Pontiac fever. A fairly general opinion among investigators is that these conditions should be considered separately from sick building syndrome.
Studies have been done to ascertain both the causes of air quality problems and their possible solutions. In recent years, knowledge of the contaminants present in indoor air and the factors contributing to a decline in indoor air quality has increased considerably, although there is a long way to go. Studies carried out in the last 20 years have shown that the presence of contaminants in many indoor environments is higher than anticipated, and moreover, different contaminants have been identified from those that exist in outside air. This contradicts the assumption that indoor environments without industrial activity are relatively free of contaminants and that in the worst of cases they may reflect the composition of outside air. Contaminants such as radon and formaldehyde are identified almost exclusively in the indoor environment.
Indoor air quality, including that of dwellings, has become a question of environmental health in the same way as has happened with control of outdoor air quality and exposure at work. Although, as already mentioned, an urban person spends 58 to 78% of his or her time indoors, it should be remembered that the most susceptible persons, namely the elderly, small children and the sick, are the ones who spend most of their time indoors. This subject began to be particularly topical from around 1973 onwards, when, because of the energy crisis, efforts directed at energy conservation concentrated on reducing the entry of outside air into indoor spaces as much as possible in order to minimize the cost of heating and cooling buildings. Although not all the problems relating to indoor air quality are the result of actions aimed at saving energy, it is a fact that as this policy spread, complaints about indoor air quality began to increase, and all the problems appeared.
Another item requiring attention is the presence of micro-organisms in indoor air which can cause problems of both an infectious and an allergic nature. It should not be forgotten that micro-organisms are a normal and essential component of ecosystems. For example, saprophytic bacteria and fungi, which obtain their nutrition from dead organic material in the environment, are found normally in the soil and atmosphere, and their presence can also be detected indoors. In recent years problems of biological contamination in indoor environments have received considerable attention.
The outbreak of Legionnaire’s disease in 1976 is the most discussed case of an illness caused by a micro-organism in the indoor environment. Other infectious agents, such as viruses that can cause acute respiratory illness, are detectable in indoor environments, especially if the occupation density is high and much recirculation of air is taking place. In fact, the extent to which micro-organisms or their components are implicated in the outbreak of building-associated conditions is not known. Protocols for demonstrating and analysing many types of microbial agents have been developed only to a limited degree, and in those cases where they are available, the interpretation of the results is sometimes inconsistent.
Aspects of the Ventilation System
Indoor air quality in a building is a function of a series of variables which include the quality of the outdoor air, the design of the ventilation and air-conditioning system, the conditions in which this system operates and is serviced, the compartmentalization of the building and the presence of indoor sources of contaminants and their magnitude. (See figure 2) By way of summary it may be noted that the most common defects are the result of inadequate ventilation, contamination generated indoors and contamination coming from outside.
Figure 2. Diagram of building showing sources of indoor and outdoor pollutants.
Regarding the first of these problems, causes of inadequate ventilation can include: an insufficient supply of fresh air due to a high level of recirculation of the air or a low volume of intake; incorrect placement and orientation in the building of intake points for outside air; poor distribution and consequently incomplete mixing with the air of the premises, which can produce stratification, unventilated zones, unforeseen pressure differences giving rise to unwanted air currents and continuous changes in the thermohygrometric characteristics noticeable as one moves about the building—and incorrect filtration of the air because of lack of maintenance or inadequate design of the filtering system—a deficiency which is particularly serious where the outdoor air is of poor quality or where there is a high level of recirculation.
Origins of Contaminants
Indoor contamination has different origins: the occupants themselves; inadequate materials or materials with technical defects used in the construction of the building; the work performed within; excessive or improper use of normal products (pesticides, disinfectants, products used for cleaning and polishing); combustion gases (from smoking, kitchens, cafeterias and laboratories); and cross-contamination coming from other poorly ventilated zones which then diffuses towards neighbouring areas and affects them. It should be borne in mind that substances emitted in indoor air have much less opportunity of being diluted than those emitted in outdoor air, given the difference in the volumes of air available. As regards biological contamination, its origin is most frequently due to the presence of stagnant water, materials impregnated with water, exhausts and so on, and to defective maintenance of humidifiers and refrigeration towers.
Finally, contamination coming from outside must also be considered. As regards human activity, three main sources may be mentioned: combustion in stationary sources (power stations); combustion in moving sources (vehicles); and industrial processes. The five main contaminants emitted by these sources are carbon monoxide, oxides of sulphur, oxides of nitrogen, volatile organic compounds (including hydrocarbons), polycyclic aromatic hydrocarbons and particles. Internal combustion in vehicles is the principal source of carbon monoxide and hydrocarbons and is an important source of oxides of nitrogen. Combustion in stationary sources is the main origin of oxides of sulphur. Industrial processes and stationary sources of combustion generate more than half of the particles emitted into the air by human activity, and industrial processes can be a source of volatile organic compounds. There are also contaminants generated naturally that are propelled through the air, such as particles of volcanic dust, soil and sea salt, and spores and micro-organisms. The composition of outdoor air varies from place to place, depending both on the presence and the nature of the sources of contamination in the vicinity and on the direction of the prevailing wind. If there are no sources generating contaminants, the concentration of certain contaminants that will typically be found in “clean” outdoor air are as follows: carbon dioxide, 320 ppm; ozone, 0.02 ppm: carbon monoxide, 0.12 ppm; nitric oxide, 0.003 ppm; and nitrogen dioxide, 0.001 ppm. However, urban air always contains much higher concentrations of these contaminants.
Apart from the presence of the contaminants originating from outside, it sometimes happens that contaminated air from the building itself is expelled to the exterior and then returns inside again through the intakes of the air-conditioning system. Another possible way by which contaminants may enter from the exterior is by infiltration through the foundations of the building (e.g., radon, fuel vapors, sewer effluvia, fertilizers, insecticides and disinfectants). It has been shown that when the concentration of a contaminant in the outdoor air increases, its concentration in the air inside the building also increases, although more slowly (a corresponding relationship obtains when the concentration decreases); it is therefore said that buildings exert a shielding effect against external contaminants. However, the indoor environment is not, of course, an exact reflection of the conditions outside.
Contaminants present in indoor air are diluted in the outdoor air that enters the building and they accompany it when it leaves. When the concentration of a contaminant is less in the outdoor air than the indoor air, the interchange of indoor and outdoor air will result in a reduction in the concentration of the contaminant in the air inside the building. If a contaminant originates from outside and not inside, this interchange will result in a rise in its indoor concentration, as mentioned above.
Models for the balance of amounts of contaminants in indoor air are based on the calculation of their accumulation, in units of mass versus time, from the difference between the quantity that enters plus what is generated indoors, and what leaves with the air plus what is eliminated by other means. If appropriate values are available for each of the factors in the equation, the indoor concentration can be estimated for a wide range of conditions. Use of this technique makes possible the comparison of different alternatives for controlling an indoor contamination problem.
Buildings with low interchange rates with outdoor air are classified as sealed or energy-efficient. They are energy-efficient because less cold air enters in winter, reducing the energy required to heat the air to the ambient temperature, thus cutting the cost of heating. When the weather is hot, less energy is also used to cool the air. If the building does not have this property, it is ventilated through open doors and windows by a process of natural ventilation. Although they may be closed, differences of pressure, resulting both from the wind and from the thermal gradient existing between the interior and the exterior, force the air to enter through crevices and cracks, window and door joints, chimneys and other apertures, giving rise to what is called ventilation by infiltration.
The ventilation of a building is measured in renewals per hour. One renewal per hour means that a volume of air equal to the volume of the building enters from outside every hour; in the same way, an equal volume of indoor air is expelled to the exterior every hour. If there is no forced ventilation (with a ventilator) this value is difficult to determine, although it is considered to vary between 0.2 and 2.0 renewals per hour. If the other parameters are assumed to be unchanged, the concentration of contaminants generated indoors will be less in buildings with high renewal values, although a high renewal value is not a complete guarantee of indoor air quality. Except in areas with marked atmospheric pollution, buildings that are more open will have a lower concentration of contaminants in the indoor air than those constructed in a more closed manner. However, buildings that are more open are less energy-efficient. The conflict between energy efficiency and air quality is of great importance.
Much action undertaken to reduce energy costs affects indoor air quality to a greater or lesser extent. In addition to reducing the speed with which the air circulates within the building, efforts to increase the insulation and waterproofing of the building involve the installation of materials that may be sources of indoor contamination. Other action, such as supplementing old and frequently inefficient central heating systems with secondary sources that heat or consume the indoor air can also raise contaminant levels in indoor air.
Contaminants whose presence in indoor air is most frequently mentioned, apart from those coming from outside, include metals, asbestos and other fibrous materials, formaldehyde, ozone, pesticides and organic compounds in general, radon, house dust and biological aerosols. Together with these, a wide variety of types of micro-organisms can be found, such as fungi, bacteria, viruses and protozoa. Of these, the saprophytic fungi and bacteria are relatively well known, probably because a technology is available for measuring them in air. The same is not true of agents such as viruses, rickettsiae, chlamydias, protozoa and many pathogenic fungi and bacteria, for the demonstration and counting of which no methodology is as yet available. Among the infectious agents, special mention should be made of: Legionella pneumophila, Mycobacterium avium, viruses, Coxiella burnetii and Histoplasma capsulatum; and among the allergens: Cladosporium, Penicillium and Cytophaga.
Investigating Indoor Air Quality
Experience so far suggests that the traditional techniques used in industrial hygiene and heating, ventilation and air-conditioning do not always provide satisfactory results at present for solving the ever more common problems of indoor air quality, although basic knowledge of these techniques permits good approximations for dealing with or reducing problems rapidly and inexpensively. The solution to problems of indoor air quality often requires, in addition to one or more experts in heating, ventilation and air-conditioning and industrial hygiene, specialists in indoor air quality control, analytical chemistry, toxicology, environmental medicine, microbiology, and also epidemiology and psychology.
When a study is carried out on indoor air quality, the objectives set for it will profoundly affect its design and the activities directed at sampling and evaluation, since in some cases procedures giving a rapid response will be required, while in others overall values will be of interest. The duration of the programme will be dictated by the time required to obtain representative samples, and will also depend on the season and on meteorological conditions. If the aim is to carry out an exposure-effect study, in addition to long-term and short-term samples for evaluating peaks, personal samples will be required for ascertaining the direct exposure of individuals.
For some contaminants, well-validated and widely used methods are available, but for the majority this is not the case. Techniques for measuring levels of many contaminants found indoors are normally derived from applications in industrial hygiene but, given that the concentrations of interest in indoor air are usually much lower than those occurring in industrial environments, these methods are frequently inappropriate. As for the measurement methods used in atmospheric contamination, they operate with margins of similar concentrations, but are available for relatively few contaminants and present difficulties in indoor use, such as would arise, for example, with a high-volume sampler for determining particulate matter, which on the one hand would be too noisy and on the other could modify the quality of the indoor air itself.
The determination of contaminants in indoor air is usually carried out by using different procedures: with continuous monitors, whole-time active samplers, whole-time passive samplers, direct sampling and personal samplers. Adequate procedures exist at present for measuring levels of formaldehyde, oxides of carbon and nitrogen, volatile organic compounds and radon, among others. Biological contaminants are measured using techniques of sedimentation on open culture plates or, more frequently nowadays, by using active systems that cause the air to impact on plates containing nutrient, which are subsequently cultured, the quantity of micro-organisms present being expressed in colony-forming units per cubic meter.
When a problem of indoor air quality is being investigated, it is usual to design beforehand a practical strategy consisting of an approximation in phases. This approximation begins with a first phase, the initial investigation, which can be carried out using industrial hygiene techniques. It must be structured so that the investigator does not need to be a specialist in the field of indoor air quality in order to carry out his work. A general inspection of the building is undertaken and its installations are checked, particularly as regards the regulation and adequate functioning of the heating, ventilation and air-conditioning system, according to the standards set at the time of its installation. It is important in this respect to consider whether the persons affected are able to modify the conditions of their surroundings. If the building does not have systems of forced ventilation, the degree of effectiveness of the existing natural ventilation must be studied. If after revision—and adjustment if necessary—the operational conditions of the ventilation systems are adequate for the standards, and if despite this the complaints continue, a technical investigation of a general kind will have to ensue to determine the degree and nature of the problem. This initial investigation should also allow an assessment to be made as to whether the problems can be considered solely from the functional point of view of the building, or whether the intervention of specialists in hygiene, psychology or other disciplines will be necessary.
If the problem is not identified and resolved in this first phase, other phases can follow involving more specialized investigations concentrating on potential problems identified in the first phase. The subsequent investigations may include a more detailed analysis of the heating, ventilation and air-conditioning system of the building, a more extensive evaluation of the presence of materials suspected of emitting gases and particles, a detailed chemical analysis of the ambient air in the building and medical or epidemiological assessments to detect signs of disease.
As regards the heating, ventilation and air-conditioning system, the refrigeration equipment should be checked in order to ensure that there is no microbial growth in them or accumulation of water in their drip trays, the ventilation units must be checked to see that they are functioning correctly, the air intake and return systems must be examined at various points to see that they are watertight, and the interior of a representative number of ducts must be checked to confirm the absence of micro-organisms. This last consideration is particularly important when humidifiers are used. These units require particularly careful programmes of maintenance, operation and inspection in order to prevent the growth of micro-organisms, which can propagate themselves throughout the air-conditioning system.
The options generally considered for improving indoor air quality in a building are the elimination of the source; its insulation or independent ventilation; separating the source from those who may be affected; general cleaning of the building; and increased checking and improvement of the heating, ventilation and air-conditioning system. This may require anything from modifications at particular points to a new design. The process is frequently of a repetitive nature, so that the study has to be started again several times, using more sophisticated techniques on each occasion. A more detailed description of control techniques will be found elsewhere in this Encyclopaedia.
Finally, it should be emphasized that, even with the most complete investigations of indoor air quality, it may be impossible to establish a clear relationship between the characteristics and composition of the indoor air and the health and comfort of the occupants of the building under study. Only the accumulation of experience on the one hand, and the rational design of ventilation, occupation and compartmentalization of buildings on the other, are possible guarantees from the outset of obtaining indoor air quality that is adequate for the majority of the occupants of a building.
Nature and Sources of Indoor Chemical Contaminants
Characteristic Chemical Pollutants
Chemical contaminants of the indoor air can occur as gases and vapors (inorganic and organic) and particulates. Their presence in the indoor environment is the result of entry into the building from the outdoor environment or their generation within the building. The relative importance of these indoor and outdoor origins differs for different pollutants and may vary over time.
The major chemical pollutants commonly found in the indoor air are the following:
- carbon dioxide (CO2), which is a metabolic product and often used as an indicator of the general level of air pollution related to the presence of humans indoors
- carbon monoxide (CO), nitrogen oxides (NOx) and sulphur dioxide (SO2), which are inorganic combustion gases formed predominantly during the combustion of fuels and ozone (O3), which is a product of photochemical reactions in polluted atmospheres but may also be released by some indoor sources
- organic compounds that originate from a variety of indoor sources and outdoors. Hundreds of organic chemicals occur in indoor air although most are present at very low concentrations. These can be grouped according to their boiling points and one widely used classification, shown in Table 1, identifies four groups of organic compounds: (1) very volatile organic compounds (VVOC); (2) volatile (VOC); (3) semi-volatile (SVOC); and (4) organic compounds associated with particulate matter (POM). Particle-phase organics are dissolved in or adsorbed on particulate matter. They may occur in both the vapor and particle phase depending on their volatility. For example, polyaromatic hydrocarbons (PAHs) consisting of two fused benzene rings (e.g., naphthalene) are found principally in the vapor phase and those consisting of five rings (e.g., benz[a]pyrene) are found predominantly in the particle phase.
Table 1. Classification of indoor organic pollutants
|
Category |
Description |
Abbreviation |
Boiling range (ºC) |
Sampling methods typically used in field studies |
|
1 |
Very volatile (gaseous) organic compounds |
VVOC |
0 to 50-100 |
Batch sampling; adsorption on charcoal |
|
2 |
Volatile organic compounds |
VOC |
50-100 to 240-260 |
Adsorption on Tenax, carbon molecular black or charcoal |
|
3 |
Semivolatile organic compounds |
SVOC |
240-260 to 380-400 |
Adsorption on polyurethane foam or XAD-2 |
|
4 |
Organic compounds associated with particulate matter or particulate organic matter |
|
|
|
An important characteristic of indoor air contaminants is that their concentrations vary both spatially and temporally to a greater extent than is common outdoors. This is due to the large variety of sources, the intermittent operation of some of the sources and the various sinks present.
Concentrations of contaminants that arise principally from combustion sources are subject to very large temporal variation and are intermittent. Episodic releases of volatile organic compounds due to human activities such as painting also lead to large variations in emission with time. Other emissions, such as formaldehyde release from wood-based products may vary with temperature and humidity fluctuations in the building, but the emission is continuous. The emission of organic chemicals from other materials may be less dependent upon temperature and humidity conditions but their concentrations in indoor air will be greatly influenced by ventilation conditions.
Spatial variations within a room tend to be less pronounced than temporal variations. Within a building there may be large differences in the case of localized sources, for example, photocopiers in a central office, gas cookers in the restaurant kitchen and tobacco smoking restricted to a designated area.
Sources within the Building
Elevated levels of pollutants generated by combustion, particularly nitrogen dioxide and carbon monoxide in indoor spaces, usually result from unvented, improperly vented or poorly maintained combustion appliances and the smoking of tobacco products. Unvented kerosene and gas space heaters emit significant quantities of CO, CO2, NOx, SO2, particulates and formaldehyde. Gas cooking stoves and ovens also release these products directly into the indoor air. Under normal operating conditions, vented gas-fired forced air heaters and water heaters should not release combustion products into the indoor air. However flue gas spillage and backdrafting can occur with faulty appliances when the room is depressurized by competing exhaust systems and under certain meteorological conditions.
Environmental tobacco smoke
Indoor air contamination from tobacco smoke results from sidestream and exhaled mainstream smoke, usually referred to as environmental tobacco smoke (ETS). Several thousand different constituents have been identified in tobacco smoke and the total quantities of individual components varies depending upon the type of cigarette and the conditions of smoke generation. The main chemicals associated with ETS are nicotine, nitrosamines, PAHs, CO, CO2, NOx, acrolein, formaldehyde and hydrogen cyanide.
Building materials and furnishings
The materials which have received greatest attention as sources of indoor air pollution have been wood-based boards containing urea formaldehyde (UF) resin and UF cavity wall insulation (UFFI). Emission of formaldehyde from these products results in elevated levels of formaldehyde in buildings and this has been associated with many complaints of poor indoor air quality in developed countries, particularly during the late 1970s and early 1980s. Table 2 gives examples of materials that release formaldehyde in buildings. These show that the highest emission rates may be associated with the wood-based products and UFFI which are products often used extensively in buildings. Particleboard is manufactured from fine (about 1 mm) wood particles which are mixed with UF resins (6 to 8 weight%) and pressed into wood panels. It is widely used for flooring, wall panelling, shelving and components of cabinets and furniture. The plies of hardwood are bonded with UF resin and are commonly used for decorative wall panelling and components of furniture. Medium-density fibreboard (MDF) contains finer wood particles than those used in particleboard and these are also bound with UF resin. MDF is most often used for furniture. The primary source of formaldehyde in all these products is the residual formaldehyde trapped in the resin as a result of its presence in excess needed for the reaction with urea during the manufacture of the resin. Release is therefore highest when the product is new, and declines at a rate dependent upon product thickness, initial emission strength, presence of other formaldehyde sources, local climate and occupant behaviour. The initial decline rate of emissions may be 50% over the first eight to nine months, followed by a much slower rate of decline. Secondary emission can occur due to hydrolysis of the UF resin and hence emission rates increase during periods of elevated temperature and humidity. Considerable efforts by manufacturers have led to the development of lower-emitting materials by use of lower ratios (i.e. closer to 1:1) of urea to formaldehyde for resin production and the use of formaldehyde scavengers. Regulation and consumer demand have resulted in widespread use of these products in some countries.
Table 2. Formaldehyde emission rates from a variety of construction material furnishings and consumer products
|
Range of formaldehyde emission rates (mg/m2/day) |
|
|
Medium-density fiberboard |
17,600-55,000 |
|
Hardwood plywood panelling |
1,500-34,000 |
|
Particleboard |
2,000-25,000 |
|
Urea-formaldehyde foam insulation |
1,200-19,200 |
|
Softwood plywood |
240-720 |
|
Paper products |
260-680 |
|
Fiberglass products |
400-470 |
|
Clothing |
35-570 |
|
Resilient flooring |
240 |
|
Carpeting |
0-65 |
|
Upholstery fabric |
0-7 |
Building materials and furnishings release a wide range of other VOCs which have been the subject of increasing concern during the 1980s and 1990s. The emission can be a complex mixture of individual compounds, though a few may be dominant. A study of 42 building materials identified 62 different chemical species. These VOCs were primarily aliphatic and aromatic hydrocarbons, their oxygen derivatives and terpenes. The compounds with the highest steady-state emission concentrations, in decreasing order, were toluene, m-xylene, terpene, n-butylacetate, n-butanol, n-hexane, p-xylene, ethoxyethylacetate, n-heptane and o-xylene. The complexity of emission has resulted in emissions and concentrations in air often being reported as the total volatile organic compound (TVOC) concentration or release. Table 3 gives examples of rates of TVOC emission for a range of building products. These show that significant differences in emissions exist between products, which means that if adequate data were available materials could be selected at the planning stage to minimize the VOC release in newly constructed buildings.
Table 3. Total volatile organic compound (TVOC) concentrations and emission rates associated with various floor and wall coverings and coatings
|
Type of material |
Concentrations (mg/m3) |
Emission rate |
|
Wallpaper |
||
|
Vinyl and paper |
0.95 |
0.04 |
|
Vinyl and glass fibres |
7.18 |
0.30 |
|
Printed paper |
0.74 |
0.03 |
|
Wall covering |
||
|
Hessian |
0.09 |
0.005 |
|
PVCa |
2.43 |
0.10 |
|
Textile |
39.60 |
1.60 |
|
Textile |
1.98 |
0.08 |
|
Floor covering |
||
|
Linoleum |
5.19 |
0.22 |
|
Synthetic fibres |
1.62 |
0.12 |
|
Rubber |
28.40 |
1.40 |
|
Soft plastic |
3.84 |
0.59 |
|
Homogeneous PVC |
54.80 |
2.30 |
|
Coatings |
||
|
Acrylic latex |
2.00 |
0.43 |
|
Varnish, clear epoxy |
5.45 |
1.30 |
|
Varnish, polyurethane, |
28.90 |
4.70 |
|
Varnish, acid-hardened |
3.50 |
0.83 |
a PVC, polyvinyl chloride.
Wood preservatives have been shown to be a source of pentachlorophenol and lindane in the air and in dust within buildings. They are used primarily for timber protection for outdoor exposure and are also used in biocides applied for treatment of dry rot and insect control.
Consumer products and other indoor sources
The variety and number of consumer and household products change constantly, and their chemical emissions depend on use patterns. Products that may contribute to indoor VOC levels include aerosol products, personal hygiene products, solvents, adhesives and paints. Table 4 illustrates major chemical components in a range of consumer products.
Table 4. Components and emissions from consumer products and other sources of volatile organic compounds (VOC)
|
Source |
Compound |
Emission rate |
|
Cleaning agents and |
Chloroform |
15 μg/m2.h |
|
Moth cake |
p-Dichlorobenzene |
14,000 μg/m2.h |
|
Dry-cleaned clothes |
Tetrachloroethylene |
0.5-1 mg/m2.h |
|
Liquid floor wax |
TVOC (trimethylpentene and |
96 g/m2.h |
|
Paste leather wax |
TVOC (pinene and 2-methyl- |
3.3 g/m2.h |
|
Detergent |
TVOC (limonene, pinene and |
240 mg/m2.h |
|
Human emissions |
Acetone |
50.7 mg/day |
|
Copy paper |
Formaldehyde |
0.4 μg/form |
|
Steam humidifier |
Diethylaminoethanol, |
— |
|
Wet copy machine |
2,2,4-Trimethylheptane |
— |
|
Household solvents |
Toluene, ethyl benzene |
— |
|
Paint removers |
Dichloromethane, methanol |
— |
|
Paint removers |
Dichloromethane, toluene, |
— |
|
Fabric protector |
1,1,1-Trichloroethane, pro- |
— |
|
Latex paint |
2-Propanol, butanone, ethyl- |
— |
|
Room freshener |
Nonane, decane, ethyl- |
— |
|
Shower water |
Chloroform, trichloroethylene |
— |
Other VOCs have been associated with other sources. Chloroform is introduced into the indoor air chiefly as a result of dispensing or heating tap water. Liquid process copiers release isodecanes into the air. Insecticides used to control cockroaches, termites, fleas, flies, ants and mites are widely used as sprays, fogging devices, powders, impregnated strips, bait and pet collars. Compounds include diazinon, paradichlorobenzene, pentachlorophenol, chlordane, malathion, naphthalene and aldrin.
Other sources include occupants (carbon dioxide and odours), office equipment (VOCs and ozone), mould growth (VOCs, ammonia, carbon dioxide), contaminated land (methane, VOCs) and electronic air cleaners and negative ion generators (ozone).
Contribution from the external environment
Table 5 shows typical indoor-outdoor ratios for the major types of pollutant that occur in indoor air and average concentrations measured in outdoor air of urban areas in the United Kingdom. Sulphur dioxide in the indoor air is normally of outdoor origin and results from both natural and anthropogenic sources. Combustion of fossil fuels containing sulphur and smelting of sulphide ores are major sources of sulphur dioxide in the troposphere. Background levels are very low (1 ppb) but in urban areas maximum hourly concentrations may be 0.1 to 0.5 ppm. Sulphur dioxide can enter a building in air used for ventilation and can infiltrate through small gaps in the building structure. This depends upon the airtightness of the building, meteorological conditions and internal temperatures. Once inside, the incoming air will mix and be diluted by the indoor air. Sulphur dioxide that comes into contact with building and furnishing materials is adsorbed and this can significantly reduce the indoor concentration with respect to the outdoors, particularly when outdoor sulphur dioxide levels are high.
Table 5. Major types of chemical indoor air contaminant and their concentrations in the urban United Kingdom
|
Substance/group of |
Ratio of concentrations |
Typical urban con- |
|
Sulphur dioxide |
~0.5 |
10-20 ppb |
|
Nitrogen dioxide |
≤5-12 (indoor sources) |
10-45 ppb |
|
Ozone |
0.1-0.3 |
15-60 ppb |
|
Carbon dioxide |
1-10 |
350 ppm |
|
Carbon monoxide |
≤5-11 (indoor source) |
0.2-10 ppm |
|
Formaldehyde |
≤10 |
0.003 mg/m3 |
|
Other organic compounds |
1-50 |
|
|
Suspended particles |
0.5-1 (excluding ETSa) |
50-150 μg/m3 |
a ETS, environmental tobacco smoke.
Nitrogen oxides are a product of combustion, and major sources include automobile exhaust, fossil fuel-fired electric generating stations and home space heaters. Nitric oxide (NO) is relatively non-toxic but can be oxidized to nitrogen dioxide (NO2), particularly during episodes of photochemical pollution. Background concentrations of nitrogen dioxide are about 1 ppb but may reach 0.5 ppm in urban areas. The outdoors is the major source of nitrogen dioxide in buildings without unvented fuel appliances. As with sulphur dioxide, adsorption by internal surfaces reduces the concentration indoors compared with that outdoors.
Ozone is produced in the troposphere by photochemical reactions in polluted atmospheres, and its generation is a function of intensity of sunlight and concentration of nitrogen oxides, reactive hydrocarbons and carbon monoxide. At remote sites, background ozone concentrations are 10 to 20 ppb and can exceed 120 ppb in urban areas in summer months. Indoor concentrations are significantly lower due to reaction with indoor surfaces and the lack of strong sources.
Carbon monoxide release as a result of anthropogenic activities is estimated to account for 30% of that present in the atmosphere of the northern hemisphere. Background levels are approximately 0.19 ppm and in urban areas a diurnal pattern of concentrations is related to use of the motor vehicle with peak hourly levels ranging from 3 ppm to 50 to 60 ppm. It is a relatively unreactive substance and so is not depleted by reaction or adsorption on indoor surfaces. Indoor sources such as unvented fuel appliances therefore add to the background level otherwise due to the outdoor air.
The indoor-outdoor relationship of organic compounds is compound-specific and may vary over time. For compounds with strong indoor sources such as formaldehyde, indoor concentrations are usually dominant. For formaldehyde outdoor concentrations are typically below 0.005 mg/m3 and indoor concentrations are ten times higher than outdoor values. Other compounds such as benzene have strong outdoor sources, petrol-driven vehicles being of particular importance. Indoor sources of benzene include ETS and these result in mean concentrations in buildings in the United Kingdom being 1.3 times higher than those outdoors. The indoor environment appears not to be a significant sink for this compound and it is therefore not protective against benzene from outdoors.
Typical Concentrations in Buildings
Carbon monoxide concentrations in indoor environments commonly range from 1 to 5 ppm. Table 6 summarizes results reported in 25 studies. Concentrations are higher in the presence of environmental tobacco smoke, though it is exceptional for concentrations to exceed 15 ppm.
Table 6. Summary of field measurements of nitrogen oxides (NOx) and carbon monoxide (CO)
|
Site |
NOx values (ppb) |
CO mean values |
|
Offices |
||
|
Smoking |
42-51 |
1.0-2.8 |
|
Other workplaces |
||
|
Smoking |
NDa-82 |
1.4-4.2 |
|
Transportation |
||
|
Smoking |
150-330 |
1.6-33 |
|
Restaurants and cafeterias |
||
|
Smoking |
5-120 |
1.2-9.9 |
|
Bars and taverns |
||
|
Smoking |
195 |
3-17 |
a ND = not detected.
Nitrogen dioxide concentrations indoors are typically 29 to 46 ppb. If particular sources such as gas stoves are present, concentrations may be significantly higher, and smoking can have a measurable effect (see table 6).
Many VOCs are present in the indoor environment at concentrations ranging from approximately 2 to 20 mg/m3. A US database containing 52,000 records on 71 chemicals in homes, public buildings and offices is summarized in Figure 3. Environments where heavy smoking and/or poor ventilation create high concentrations of ETS can produce VOC concentrations of 50 to 200 mg/m3. Building materials make a significant contribution to indoor concentrations and new homes are likely to have a greater number of compounds exceeding 100 mg/m3. Renovation and painting contribute to significantly higher VOC levels. Concentrations of compounds such as ethyl acetate, 1,1,1-trichloroethane and limonene can exceed 20 mg/m3 during occupant activities, and during residents’ absence the concentration of a range of VOCs may decrease by about 50%. Specific cases of elevated concentrations of contaminants due to materials and furnishings being associated with occupant complaints have been described. These include white spirit from injected damp-proof courses, naphthalene from products containing coal tar, ethylhexanol from vinyl flooring and formaldehyde from wood-based products.
Figure 1. Daily indoor concentrations of selected compounds for indoor sites.
The large number of individual VOCs occurring in buildings makes it difficult to detail concentrations for more than selected compounds. The concept of TVOC has been used as a measure of the mixture of compounds present. There is no widely used definition as to the range of compounds that the TVOC represents, but some investigators have proposed that limiting concentrations to below 300 mg/m3 should minimize complaints by occupants about indoor air quality.
Pesticides used indoors are of relatively low volatility and concentrations occur in the low microgram-per-cubic-meter range. The volatilized compounds can contaminate dust and all indoor surfaces because of their low vapor pressures and tendency to be adsorbed by indoor materials. PAH concentrations in air are also strongly influenced by their distribution between the gas and aerosol phases. Smoking by occupants can have a strong effect on indoor air concentrations. Concentrations of PAHs range typically range from 0.1 to 99 ng/m3.
Radon
Most of the radiation that a human being will be exposed to during a lifetime comes from natural sources in outer space or from materials present in the earth’s crust. Radioactive materials may affect the organism from without or, if inhaled or ingested with food, from within. The dose received may be very variable because it depends, on the one hand, on the amount of radioactive minerals present in the area of the world where the person lives—which is related to the amount of radioactive nuclides in the air and the amount found both in food and especially in drinking water—and, on the other, on the use of certain construction materials and the use of gas or coal for fuel, as well as the type of construction employed and the traditional habits of people in the given locality.
Today, radon is considered the most prevalent source of natural radiation. Together with its “daughters," or radionuclides formed by its disintegration, radon constitutes approximately three fourths of the effective equivalent dose to which humans are exposed due to natural terrestrial sources. The presence of radon is associated with an increase in the occurrence of lung cancer due to the deposition of radioactive substances in the bronchial region.
Radon is a colourless, odourless and tasteless gas seven times as heavy as air. Two isotopes occur most frequently. One is radon-222, a radionuclide present in the radioactive series from the disintegration of uranium-238; its main source in the environment is the rocks and the soil in which its predecessor, radium-226, occurs. The other is radon-220 from the thorium radioactive series, which has a lower incidence than radon-222.
Uranium occurs extensively in the earth’s crust. The median concentration of radium in soil is in the order of 25 Bq/kg. A Becquerel (Bq) is the unit of the international system and it represents a unit of radionuclide activity equivalent to one disintegration per second. The average concentration of radon gas in the atmosphere at the surface of the earth is 3 Bq/m3, with a range of 0.1 (over the oceans) to 10 Bq/m3. The level depends on the porousness of the soil, the local concentration of radium-226 and the atmospheric pressure. Given that the half-life of radon-222 is 3.823 days, most of the dosage is not caused by the gas but by radon daughters.
Radon is found in existing materials and flows from the earth everywhere. Because of its characteristics it disperses easily outdoors, but it has a tendency to become concentrated in enclosed spaces, notably in caves and buildings, and especially in lower spaces where its elimination is difficult without proper ventilation. In temperate regions, the concentrations of radon indoors are estimated to be in the order of eight times higher than the concentrations outdoors.
Exposure to radon by most of the population, therefore, occurs for the most part within buildings. The median concentrations of radon depend, basically, on the geological characteristics of the soil, on the construction materials used for the building and on the amount of ventilation it receives.
The main source of radon in indoor spaces is the radium present in the soil on which the building rests or the materials employed in its construction. Other significant sources—even though their relative influence is much less—are outside air, water and natural gas. Figure 1 shows the contribution that each source makes to the total.
Figure 1. Sources of radon in the indoor environment.
The most common construction materials, such as wood, bricks and cinder blocks, emit relatively little radon, in contrast to granite and pumice-stone. However, the main problems are caused by the use of natural materials such as alum slate in the production of construction materials. Another source of problems has been the use of by-products from the treatment of phosphate minerals, the use of by-products from the production of aluminium, the use of dross or slag from the treatment of iron ore in blast furnaces, and the use of ashes from the combustion of coal. In addition, in some instances, residues derived from uranium mining were also used in construction.
Radon can enter water and natural gas in the subsoil. The water used to supply a building, especially if it is from deep wells, may contain significant amounts of radon. If this water is used for cooking, boiling can free a large part of the radon it contains. If the water is consumed cold, the body eliminates the gas readily, so that drinking this water does not generally pose a significant risk. Burning natural gas in stoves without chimneys, in heaters and in other home appliances can also lead to an increase of radon in indoor spaces, especially dwellings. Sometimes the problem is more acute in bathrooms, because radon in water and in the natural gas used for the water heater accumulates if there is not enough ventilation.
Given that the possible effects of radon on the population at large were unknown just a few years ago, the data available on concentrations found in indoor spaces are limited to those countries which, because of their characteristics or special circumstances, are more sensitized to this problem. What is known for a fact is that it is possible to find concentrations in indoor spaces that are far above the concentrations found outdoors in the same region. In Helsinki (Finland), for instance, concentrations of radon in indoor air have been found that are five thousand times higher than the concentrations normally found outdoors. This may be due in large part to energy-saving measures that can noticeably favour the concentration of radon in indoor spaces, especially if they are heavily insulated. Buildings studied so far in different countries and regions show that the concentrations of radon found within them present a distribution that approximates the normal log. It is worth noting that a small number of the buildings in each region show concentrations ten times above the median. The reference values for radon in indoor spaces, and the remedial recommendations of various organizations are given in “Regulations, recommendations, guidelines and standards” in this chapter.
In conclusion, the main way to prevent exposures to radon is based on avoiding construction in areas that by their nature emit a greater amount of radon into the air. Where that is not possible, floors and walls should be properly sealed, and construction materials should not be used if they contain radioactive matter. Interior spaces, especially basements, should have an adequate amount of ventilation.
Tobacco Smoke
In 1985 the Surgeon General of the US Public Health Service reviewed the health consequences of smoking with regard to cancer and chronic lung disease in the workplace. It was concluded that for most US workers, cigarette smoking represents a greater cause of death and disability than their workplace environment. However, the control of smoking and a reduction of the exposure to hazardous agents at the workplace are essential, since these factors often act synergistically with smoking in the induction and development of respiratory diseases. Several occupational exposures are known to induce chronic bronchitis in workers. These include exposures to dust from coal, cement and grain, to silica aerosols, to vapors generated during welding, and to sulphur dioxide. Chronic bronchitis among workers in these occupations is often aggravated by cigarette smoking (US Surgeon General 1985).
Epidemiological data have clearly documented that uranium miners and asbestos workers who smoke cigarettes carry significantly higher risks of cancer of the respiratory tract than non-smokers in these occupations. The carcinogenic effect of uranium and asbestos and cigarette smoking is not merely additive, but synergistic in inducing squamous cell carcinoma of the lung (US Surgeon General 1985; Hoffmann and Wynder 1976; Saccomanno, Huth and Auerbach 1988; Hilt et al. 1985). The carcinogenic effects of exposure to nickel, arsenicals, chromate, chloromethyl ethers, and those of cigarette smoking are at least additive (US Surgeon General 1985; Hoffmann and Wynder 1976; IARC 1987a, Pershagen et al. 1981). One would assume that coke-oven workers who smoke have a higher risk of lung and kidney cancer than non-smoking coke-oven workers; however, we lack epidemiological data that substantiate this concept (IARC 1987c).
It is the aim of this overview to evaluate the toxic effects of the exposure of men and women to environmental tobacco smoke (ETS) at the workplace. Certainly, curtailing smoking at the workplace will benefit active smokers by reducing their consumption of cigarettes during the workday, thereby increasing the possibility that they become ex-smokers; but smoking cessation will also be of benefit to those non-smokers who are allergic to tobacco smoke or who have pre-existing lung or heart ailments.
Physico-Chemical Nature of EnvironmentalTobacco Smoke
Mainstream and sidestream smoke
ETS is defined as the material in indoor air that originates from tobacco smoke. Although pipe and cigar smoking contribute to ETS, cigarette smoke is generally the major source. ETS is a composite aerosol that is emitted primarily from the burning cone of a tobacco product between puffs. This emission is called sidestream smoke (SS). To a minor extent, ETS consists also of mainstream smoke (MS) constituents, that is, those that are exhaled by the smoker. Table 7 lists the ratios of major toxic and carcinogenic agents in the smoke that is inhaled, the mainstream smoke, and in the sidestream smoke (Hoffmann and Hecht 1990; Brunnemann and Hoffmann 1991; Guerin et al. 1992; Luceri et al. 1993). Under “Type of toxicity”, smoke components marked “C” represent animal carcinogens that are recognized by the International Agency for Research on Cancer (IARC). Among these are benzene,β-naphthylamine, 4-aminobiphenyl and polonium-210, which are also established human carcinogens (IARC 1987a; IARC 1988). When filter cigarettes are being smoked, certain volatile and semi-volatile components are selectively removed from the MS by filter tips (Hoffmann and Hecht 1990). However, these compounds occur in far higher amounts in undiluted SS than in MS. Furthermore, those smoke components that are favoured to be formed during smouldering in the reducing atmosphere of the burning cone, are released into SS to a far greater extent than into MS. This includes groups of carcinogens like the volatile nitrosamines, tobacco-specific nitrosamines (TSNA) and aromatic amines.
Table 1. Some toxic and tumorigenic agents in undiluted cigarette sidestream smoke
|
Compound |
Type of |
Amount in |
Ratio of side- |
|
Vapour phase |
|||
|
Carbon monoxide |
T |
26.80-61 mg |
2.5-14.9 |
|
Carbonyl sulphide |
T |
2-3 μg |
0.03-0.13 |
|
1,3-Butadiene |
C |
200-250 μg |
3.8-10.8 |
|
Benzene |
C |
240-490 μg |
8-10 |
|
Formaldehyde |
C |
300-1,500 μg |
10-50 |
|
Acrolein |
T |
40-100 μg |
8-22 |
|
3-Vinylpyridine |
T |
330-450 μg |
24-34 |
|
Hydrogen cyanide |
T |
14-110 μg |
0.06-0.4 |
|
Hydrazine |
C |
90 ng |
3 |
|
Nitrogen oxides (NOx) |
T |
500-2,000 μg |
3.7-12.8 |
|
N-Nitrosodimethylamine |
C |
200-1,040 ng |
12-440 |
|
N-Nitrosodiethylamine |
C |
NDb-1,000 ng |
<40 |
|
N-Nitrosopyrrolidine |
C |
7-700 ng |
4-120 |
|
Particulate phase |
|||
|
Tar |
C |
14-30 mg |
1.1-15.7 |
|
Nicotine |
T |
2.1-46 mg |
1.3-21 |
|
Phenol |
TP |
70-250 μg |
1.3-3.0 |
|
Catechol |
CoC |
58-290 μg |
0.67-12.8 |
|
2-Toluidine |
C |
2.0-3.9 μg |
18-70 |
|
β-Naphthylamine |
C |
19-70 ng |
8.0-39 |
|
4-Aminobiphenyl |
C |
3.5-6.9 ng |
7.0-30 |
|
Benz(a)anthracene |
C |
40-200 ng |
2-4 |
|
Benzo(a)pyrene |
C |
40-70 ng |
2.5-20 |
|
Quinoline |
C |
15-20 μg |
8-11 |
|
NNNc |
C |
0.15-1.7 μg |
0.5-5.0 |
|
NNKd |
C |
0.2-1.4 μg |
1.0-22 |
|
N-Nitrosodiethanolamine |
C |
43 ng |
1.2 |
|
Cadmium |
C |
0.72 μg |
7.2 |
|
Nickel |
C |
0.2-2.5 μg |
13-30 |
|
Zinc |
T |
6.0 ng |
6.7 |
|
Polonium-210 |
C |
0.5-1.6 pCi |
1.06-3.7 |
a C=Carcinogenic; CoC=co-carcinogenic; T=toxic; TP=tumor promoter.
b ND=not detected.
c NNN=N‘-nitrosonornicotine.
d NNK=4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone.
ETS in indoor air
Although undiluted SS contains higher amounts of toxic and carcinogenic components than MS, the SS inhaled by non-smokers is highly diluted by air and its properties are altered because of the decay of certain reactive species. Table 8 lists reported data for toxic and carcinogenic agents in indoor air samples of various degrees of tobacco smoke pollution (Hoffmann and Hecht 1990; Brunnemann and Hoffmann 1991; Luceri et al. 1993). The air dilution of SS has a significant impact on the physical characteristics of this aerosol. In general, the distribution of various agents between vapor phase and particulate phase is changed in favour of the former. The particles in ETS are smaller (<0.2 μ) than those in MS (~0.3 μ) and the pH levels of SS (pH 6.8 - 8.0) and of ETS are higher than the pH of MS (5.8 - 6.2; Brunnemann and Hoffmann 1974). Consequently, 90 to 95% of nicotine is present in the vapor phase of ETS (Eudy et al. 1986). Similarly, other basic components such as the minor Nicotiana alkaloids, as well as amines and ammonia, are present mostly in the vapor phase of ETS (Hoffmann and Hecht 1990; Guerin et al. 1992).
Table 2. Some toxic and tumorigenic agents in indoor environments polluted by tobacco smoke
|
Pollutant |
Location |
Concentration/m3 |
|
Nitric oxide |
Workrooms |
50-440 μg |
|
Nitrogen dioxide |
Workrooms |
68-410 μg |
|
Hydrogen cyanide |
Living-rooms |
8-122 μg |
|
1,3-Butadiene |
Bars |
2.7-4.5 μg |
|
Benzene |
Public places |
20-317 μg |
|
Formaldehyde |
Living-rooms |
2.3-5.0 μg |
|
Acrolein |
Public places |
30-120 μg |
|
Acetone |
Coffee houses |
910-1,400 μg |
|
Phenols (volatile) |
Coffee houses |
7.4-11.5 ng |
|
N-Nitrosodimethylamine |
Bars, restaurants, offices |
<10-240 ng |
|
N-Nitrosodiethylamine |
Restaurants |
<10-30 ng |
|
Nicotine |
Residences |
0.5-21 μg |
|
2-Toluidine |
Offices |
3.0-12.8 ng |
|
b-Naphthylamine |
Offices |
0.27-0.34 ng |
|
4-Aminobiphenyl |
Offices |
0.1 ng |
|
Benz(a)anthracene |
Restaurants |
1.8-9.3 ng |
|
Benzo(a)pyrene |
Restaurants |
2.8-760 μg |
|
NNNa |
Bars |
4.3-22.8 ng |
|
NNKc |
Bars |
9.6-23.8 ng |
a NNN=N‘-nitrosonornicotine.
b ND=not detected.
c NNK=4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone.
Biomarkers of the Uptake of ETS by Non-Smokers
Although a significant number of non-smoking workers are exposed to ETS at the workplace, in restaurants, in their own homes or in other indoor places, it is hardly possible to estimate the actual uptake of ETS by an individual. ETS exposure can be more precisely determined by measuring specific smoke constituents or their metabolites in physiological fluids or in exhaled air. Although several parameters have been explored, such as CO in exhaled air, carboxyhaemoglobin in blood, thiocyanate (a metabolite of hydrogen cyanide) in saliva or urine, or hydroxyproline and N-nitrosoproline in urine, only three measures are actually helpful for estimating the uptake of ETS by non-smokers. They allow us to distinguish passive smoke exposure from that of active smokers and from non-smokers who have absolutely no exposure to tobacco smoke.
The most widely used biomarker for ETS exposure of non-smokers is cotinine, a major nicotine metabolite. It is determined by gas chromatography, or by radioimmunoassay in blood or preferably urine, and reflects the absorption of nicotine through the lung and oral cavity. A few millilitres of urine from passive smokers is sufficient to determine cotinine by either of the two methods. In general, a passive smoker has cotinine levels of 5 to 10 ng/ml of urine; however, higher values have occasionally been measured for non-smokers who were exposed to heavy ETS over a longer period. A dose response has been established between duration of ETS exposure and urinary cotinine excretion (table 3, Wald et al. 1984). In most field studies, cotinine in the urine of passive smokers amounted to between 0.1 and 0.3% of the mean concentrations found in the urine of smokers; however, upon prolonged exposure to high concentrations of ETS, cotinine levels have corresponded to as much as 1% of the levels measured in the urine of active smokers (US National Research Council 1986; IARC 1987b; US Environmental Protection Agency 1992).
Table 3. Urinary cotinine in non-smokers according to the number of reported hours of exposure to other people’s tobacco smoke within the previous seven days
|
Duration of exposure |
|||
|
Quintile |
Limits (hrs) |
Number |
Urinary cotinine (mean ± SD) |
|
1st |
0.0-1.5 |
43 |
2.8±3.0 |
|
2nd |
1.5-4.5 |
47 |
3.4±2.7 |
|
3rd |
4.5-8.6 |
43 |
5.3±4.3 |
|
4th |
8.6-20.0 |
43 |
14.7±19.5 |
|
5th |
20.0-80.0 |
45 |
29.6±73.7 |
|
All |
0.0-80.0 |
221 |
11.2±35.6 |
a Trend with increasing exposure was significant (p<0.001).
Source: Based on Wald et al. 1984.
The human bladder carcinogen 4-aminobiphenyl, which transfers from tobacco smoke into ETS, has been detected as a haemoglobin adduct in passive smokers in concentrations up to 10% of the mean adduct level found in smokers (Hammond et al. 1993). Up to 1% of the mean levels of a metabolite of the nicotine-derived carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), which occurs in the urine of cigarette smokers, has been measured in the urine of non-smokers who had been exposed to high concentrations of SS in a test laboratory (Hecht et al. 1993). Although the latter biomarker method has not as yet been applied in field studies, it holds promise as a suitable indicator of the exposure of non-smokers to a tobacco-specific lung carcinogen.
Environmental Tobacco Smoke and Human Health
Disorders other than cancer
Prenatal exposure to MS and/or ETS and early postnatal exposure to ETS increase the probability of complications during viral respiratory infections in children during the first year of life.
The scientific literature contains several dozens of clinical reports from various countries, reporting that children of parents who smoke, especially children under the age of two years, show an excess of acute respiratory illness (US Environmental Protection Agency 1992; US Surgeon General 1986; Medina et al. 1988; Riedel et al. 1989). Several studies also described an increase of middle ear infections in children who had exposure to parental cigarette smoke. The increased prevalence of middle ear effusion attributable to ETS led to increased hospitalization of young children for surgical intervention (US Environmental Protection Agency 1992; US Surgeon General 1986).
In recent years, sufficient clinical evidence has led to the conclusion that passive smoking is associated with increased severity of asthma in those children who already have the disease, and that it most likely leads to new cases of asthma in children (US Environmental Protection Agency 1992).
In 1992, the US Environmental Protection Agency (1992) critically reviewed the studies on respiratory symptoms and lung functions in adult non-smokers exposed to ETS, concluding that passive smoking has subtle but statistically significant effects on the respiratory health of non-smoking adults.
A search of the literature on the effect of passive smoking on respiratory or coronary diseases in workers revealed only a few studies. Men and women who were exposed to ETS at the workplace (offices, banks, academic institutions, etc.) for ten or more years had impaired pulmonary function (White and Froeb 1980; Masi et al. 1988).
Lung cancer
In 1985, the International Agency for Research on Cancer (IARC) reviewed the association of passive tobacco smoke exposure with lung cancer in non-smokers. Although in some studies, each non-smoker with lung cancer who had reported ETS exposure was personally interviewed and had supplied detailed information on exposure (US National Research Council 1986; US EPA 1992; US Surgeon General 1986; Kabat and Wynder 1984), the IARC concluded:
The observations on non-smokers that have been made so far, are compatible with either an increased risk from ‘passive’ smoking, or an absence of risk. Knowledge of the nature of sidestream and mainstream smoke, of the materials absorbed during ‘passive’ smoking and of the quantitative relationship between dose and effect that are commonly observed from exposure to carcinogens, however, leads to the conclusion that passive smoking gives rise to some risk of cancer (IARC 1986).
Thus, there is an apparent dichotomy between experimental data which support the concept that ETS gives rise to some risk of cancer, and epidemiological data, which are not conclusive with respect to ETS exposure and cancer. Experimental data, including biomarker studies, have further strengthened the concept that ETS is carcinogenic, as was discussed earlier. We will now discuss how far the epidemiological studies that have been completed since the cited IARC report have contributed to a clarification of the ETS lung cancer issue.
According to the earlier epidemiological studies, and in about 30 studies reported after 1985, ETS exposure of non-smokers constituted a risk factor for lung cancer of less than 2.0, relative to the risk of a non-smoker without significant ETS exposure (US Environmental Protection Agency 1992; Kabat and Wynder 1984; IARC 1986; Brownson et al. 1992; Brownson et al. 1993). Few, if any, of these epidemiological studies meet the criteria of causality in the association between an environmental or occupational factor and lung cancer. Criteria that fulfil these requirements are:
- a well-established degree of association (risk factor≥3)
- reproducibility of the observation by a number of studies
- agreement between duration of exposure and effect
- biological plausibility.
One of the major uncertainties about the epidemiological data lies in the limited reliability of the answers obtained by questioning cases and/or their next-of-kin with regard to the smoking habits of the cases. It appears that there is generally an accord between parental and spousal smoking histories provided by cases and controls; however, there are low agreement rates for duration and intensity of smoking (Brownson et al. 1993; McLaughlin et al. 1987; McLaughlin et al. 1990). Some investigators have challenged the reliability of the information derived from individuals about their smoking status. This is exemplified by a large-scale investigation carried out in southern Germany. A randomly selected study population consisted of more than 3,000 men and women, ranging in age from 25 to 64 years. These same people were questioned three times in 1984-1985, in 1987-1988 and again in 1989-1990 as to their smoking habits, while each time urine was collected from each proband and was analysed for cotinine. Those volunteers who were found to have more than 20 ng of cotinine per ml of urine were considered to be smokers. Among 800 ex-smokers who claimed to be non-smokers, 6.3%, 6.5% and 5.2% had cotinine levels above 20 ng/ml during the three time periods tested. The self-proclaimed never-smokers, who were identified as actual smokers according to cotinine analyses, constituted 0.5%, 1.0% and 0.9%, respectively (Heller et al. 1993).
The limited reliability of the data obtained by questionnaire, and the relatively limited number of non-smokers with lung cancer who were not exposed to carcinogens at their workplaces, point to the need for a prospective epidemiological study with assessment of biomarkers (e.g., cotinine, metabolites of polynuclear aromatic hydrocarbons, and/or metabolites of NNK in urine) to bring about a conclusive evaluation of the question on causality between involuntary smoking and lung cancer. While such prospective studies with biomarkers represent a major task, they are essential in order to answer the questions on exposure which have major public health implications.
Environmental Tobacco Smoke and the Occupational Environment
Although epidemiological studies have thus far not demonstrated a causal association between ETS exposure and lung cancer, it is nevertheless highly desirable to protect workers at the site of employment from exposure to environmental tobacco smoke. This concept is supported by the observation that long-term exposure of non-smokers to ETS at the workplace can lead to reduced pulmonary function. Furthermore, in occupational environments with exposure to carcinogens, involuntary smoking may increase the risk of cancer. In the United States, the Environmental Protection Agency has classified ETS as a Group A (known human) carcinogen; therefore, the law in the United States requires that employees be protected against exposure to ETS.
Several measures can be taken to protect the non-smoker from exposure to ETS: prohibiting smoking at the worksite, or at least separating smokers from non-smokers where possible, and assuring that the smokers’ rooms have a separate exhaust system. The most rewarding and by far the most promising approach is to assist employees who are cigarette smokers in cessation efforts.
The worksite can offer excellent opportunities for implementing smoking cessation programmes; in fact, numerous studies have shown that worksite programmes are more successful than clinic-based programmes, because employer-sponsored programmes are more intense in nature and they offer economic and/or other incentives (US Surgeon General 1985). It is also indicated that the elimination of occupationally related chronic lung diseases and cancer frequently cannot proceed without efforts to convert the workers into ex-smokers. Furthermore, worksite interventions, including smoking cessation programmes, can produce lasting changes in reducing some cardiovascular risk factors for the employees (Gomel et al. 1993).
We greatly appreciate the editorial assistance of Ilse Hoffmann and the preparation of this manuscript by Jennifer Johnting. These studies are supported by USPHS Grants CA-29580 and CA-32617 from the National Cancer Institute.
Smoking Regulations
In regard to taking action to reduce the use of tobacco, governments should keep in mind that while people decide on their own whether they should stop smoking, it is a government’s responsibility to take all the necessary measures to encourage them to stop. The steps taken by legislators and governments of many countries have been indecisive, because while the reduction in the use of tobacco is an undisputed improvement in public health—with attendant savings in public health expenditures—there would be a series of economic losses and dislocations in many sectors, at least of a temporary nature. The pressure that international health and environmental organizations and agencies can exert in this regard is very important, because many countries may water down measures against the use of tobacco because of economic problems—especially if tobacco is an important source of income.
This article briefly describes regulatory measures that can be adopted to reduce smoking in a country.
Warnings on Cigarette Packs
One of the first measures adopted in many countries is to require that cigarette packs prominently display the warning that smoking seriously injures the smoker’s health. This warning, whose aim is not so much to exert an immediate effect on the smoker, but rather to show that the government is concerned about the problem, is creating a psychological climate that will favour the adoption of later measures that otherwise would be considered aggressive by the smoking population.
Some experts advocate the inclusion of these warnings on cigars and pipe tobacco. But the more general opinion is that those warnings are unnecessary, because people who use that type of tobacco do not normally inhale the smoke, and extending these warnings would lead more likely to a disregard of the messages as a whole. This is why the prevalent opinion is that the warnings should be applied only to cigarette packs. A reference to second-hand smoke has not, for the moment, been considered, but it is not an option that should be discarded.
Smoking Restrictions in Public Spaces
Forbidding smoking in public spaces is one of the most effective regulatory instruments. These prohibitions can significantly reduce the number of people exposed to second-hand smoke and, in addition, can reduce smokers’ daily cigarette consumption. The common complaints by owners of public spaces, such as hotels, restaurants, recreational facilities, dance halls, theatres and so forth, are based on the argument that these measures will result in a loss of customers. However, if governments implement these measures across the board, the negative impact of a loss of clientele will occur only in the first phase, because people will eventually adapt to the new situation.
Another possibility is the design of specific spaces for smokers. The separation of smokers from non-smokers should be effective in order to obtain the desired benefits, creating barriers that prevent non-smokers from inhaling tobacco smoke. Separation must thus be physical and, if the air-conditioning system uses recycled air, the air from smoking areas should not be mixed with that from non-smoking areas. Creating spaces for smokers therefore implies construction and compartmentalization expenses, but may be a solution for those who want to serve the smoking public.
Aside from locations where smoking is obviously forbidden for security reasons because of possible explosion or fire, there should also be areas—such as health care and sports facilities, schools and day-care centres—where smoking is not permitted even though there are no safety risks of that kind.
Smoking Restrictions at Work
Smoking restrictions in the workplace may also be considered in light of the above. Governments and business owners, together with trade unions, can establish programmes to reduce the use of tobacco at work. Campaigns to curtail smoking at work are generally successful.
Whenever possible, creating non-smoking areas to establish a policy against tobacco use and to support people who defend the right not to be second-hand smokers is recommended. In case of a conflict between a smoker and a non-smoker, regulations should always allow the non-smoker to prevail, and whenever they cannot be separated, the smoker should be pressured to abstain from smoking at the workstation.
In addition to places where for health or safety reasons smoking should be forbidden, the possibility of synergism between the effects of chemical pollution in the workplace and tobacco smoke should not be ignored in other areas either. The weight of such considerations will result, without a doubt, in a broad extension of smoking restrictions, especially in industrial workplaces.
Greater Economic Pressure against Tobacco
Another regulatory tool governments rely on to curb the use of tobacco is levying higher taxes, chiefly on cigarettes. This policy is intended to lead to lower tobacco consumption, which would justify the inverse relation between the price of tobacco and its consumption and which can be measured when comparing the situation in different countries. It is considered effective where the population is forewarned of the dangers of tobacco use and advised of the need to stop consuming it. An increase in the price of tobacco can be a motivation to quit smoking. This policy, however, has many opponents, who base their criticisms on arguments briefly mentioned below.
In the first place, according to many specialists, the increase in the price of tobacco for fiscal reasons is followed by a temporary reduction in the use of tobacco, followed by a gradual return to the previous consumption levels as the smokers get used to the new price. In other words, smokers assimilate a rise in the price of tobacco much in the same way that people get used to other taxes or to the rise in the cost of living.
In the second place, a shift in the habits of smokers has also been observed. When prices go up they tend to seek out cheaper brands of lower quality that probably also pose a greater risk to their health (because they lack filters or have higher amounts of tar and nicotine). This shift may go so far as to induce smokers to adopt the practice of making home-made cigarettes, which would completely eliminate any possibility of controlling the problem.
In the third place, many experts are of the opinion that measures of this kind tend to bolster the belief that the government accepts tobacco and its consumption as yet another means to collect taxes, leading to the contradictory belief that what the government really wants is that people smoke so that it can collect more money with the special tax on tobacco.
Limiting Publicity
Another weapon used by governments to reduce tobacco consumption is to restrict or simply forbid any publicity for the product. Governments and many international organizations have a policy of forbidding publicity for tobacco in certain spheres, such as sports (at least some sports), health care, the environment, and education. This policy has unquestionable benefits, which are especially effective when it eliminates publicity in those environments that affect young people at a time when they are likely to take up the smoking habit.
Public Programmes that Encourage People to QuitSmoking
The use of anti-smoking campaigns as a normal practice, adequately funded and organized as a rule of conduct in certain spheres, such as the world of work, has been shown to be highly successful.
Campaigns to Educate Smokers
Complementing what was said above, educating smokers so that they will smoke “better” and cut down on their consumption of cigarettes is another avenue available to governments to reduce the adverse health effects of tobacco use on the population. These efforts should be directed at reducing the daily consumption of cigarettes, at inhibiting the inhalation of smoke as much as possible, at not smoking the butts of cigarettes (the toxicity of smoke increases towards the end of the cigarette), at not keeping the cigarette steadily at the lips, and at adopting preferences for brands with lower tar and nicotine.
Measures of this type evidently do not reduce the number of smokers, but they do reduce how much smokers are harmed by their habit. There are arguments against this type of remedy because it may give the impression that smoking is not intrinsically a bad habit, since smokers are told how best to smoke.
Concluding Remarks
Regulatory and legislative action by different governments is slow and not sufficiently effective, especially given what would be required due to the problems caused by tobacco use. Often this is the case because of legal hurdles against implementing such measures, arguments against unfair competition, or even the protection of the individual’s right to smoke. Progress in the use of regulations has been slow but it is nonetheless steady. On the other hand, the difference between active smokers and second-hand or passive smokers should be kept in mind. All the measures that would help someone to stop smoking, or at least to reduce daily consumption effectively, should be directed at the smoker; all the weight of regulations should be brought to bear against this habit. The passive smoker should be given every possible argument to support his or her right not to inhale tobacco smoke, and to defend the right to enjoy the use of smoke-free environments at home, at work and at play.
Measuring and Assessing Chemical Pollutants
From the standpoint of pollution, indoor air in non-industrial situations displays several characteristics that differentiate it from outside, or atmospheric, air and from the air in industrial workplaces. Besides contaminants found in atmospheric air, indoor air also includes contaminants generated by building materials and by the activities that take place within the building. The concentrations of contaminants in indoor air tend to be the same or less than concentrations found in outside air, depending on ventilation; contaminants generated by building materials are usually different from those found in outside air and can be found in high concentrations, while those generated by activities inside the building depend on the nature of such activities and may be the same as those found in outside air, as in the case of CO and CO2.
For this reason, the number of contaminants found in non-industrial inside air is large and varied and the levels of concentration are low (except for instances where there is an important generating source); they vary according to atmospheric/climatologic conditions, the type or characteristics of the building, its ventilation and the activities carried out within it.
Analysis
Much of the methodology used to gauge the quality of indoor air stems from industrial hygiene and from measurements of immission of outdoor air. There are few analytic methods validated specifically for this type of testing, although some organizations, such as the World Health Organization and the Environmental Protection Agency in the United States are conducting research in this field. An additional obstacle is the paucity of information on the exposure-effect relationship when dealing with long-term exposures to low concentrations of pollutants.
The analytical methods used for industrial hygiene are designed to measure high concentrations and have not been defined for many pollutants, while the number of contaminants in indoor air can be large and varied and the levels of concentration can be low, except in certain cases. Most methods used in industrial hygiene are based on the taking of samples and their analysis; many of these methods can be applied to indoor air if several factors are taken into account: adjusting the methods to the typical concentrations; increasing their sensitivity without detriment to precision (for example, increasing the volume of air tested); and validating their specificity.
The analytical methods used to measure concentrations of pollutants in outdoor air are similar to those used for indoor air, and therefore some can be used directly for indoor air while others can be easily adapted. However, it is important to keep in mind that some methods are designed for a direct reading of one sample, while others require bulky and sometimes noisy instrumentation and use large volumes of sampled air which can distort the reading.
Planning the Readings
The traditional procedure in the field of workplace environmental control can be used to improve the quality of indoor air. It consists of identifying and quantifying a problem, proposing corrective measures, making sure that these measures are implemented, and then assessing their effectiveness after a period of time. This common procedure is not always the most adequate because often such an exhaustive evaluation, including the taking of many samples, is not necessary. Exploratory measures, which can range from a visual inspection to assaying of ambient air by direct reading methods, and which can provide an approximate concentration of pollutants, are sufficient for solving many of the existing problems. Once corrective measures have been taken, the results can be evaluated with a second measurement, and only when there is no clear evidence of an improvement a more thorough inspection (with in-depth measurements) or a complete analytical study can be undertaken (Swedish Work Environment Fund 1988).
The main advantages of such an exploratory procedure over the more traditional one are economy, speed and effectiveness. It requires competent and experienced personnel and the use of suitable equipment. Figure 1 summarizes the goals of the different stages of this procedure.
Figure 1. Planning the readings for exploratory evaluation.
Sampling Strategy
Analytical control of the quality of indoor air should be considered as a last resort only after the exploratory measurement has not given positive results, or if further evaluation or control of the initial tests is needed.
Assuming some previous knowledge of the sources of pollution and of the types of contaminants, the samples, even when limited in number, should be representative of the various spaces studied. Sampling should be planned to answer the questions What? How? Where? and When?
What
The pollutants in question must be identified in advance and, keeping in mind the different types of information that can be obtained, one should decide whether to make emission or immission measurements.
Emission measurements for indoor air quality can determine the influence of different sources of pollution, of climatic conditions, of the building’s characteristics, and of human intervention, which allow us to control or reduce the sources of emissions and improve the quality of indoor air. There are different techniques for taking this type of measurement: placing a collection system adjacent to the source of the emission, defining a limited work area and studying emissions as if they represented general working conditions, or working in simulated conditions applying monitoring systems that rely on head space measures.
Immission measurements allow us to determine the level of indoor air pollution in the different compartmentalized areas of the building, making it possible to produce a map of pollution for the entire structure. Using these measurements and identifying the different areas where people have carried out their activities and calculating the time they have spent at each task, it will be possible to determine the levels of exposure. Another way of doing this is by having individual workers wear monitoring devices while working.
It may be more practical, if the number of pollutants is large and varied, to select a few representative substances so that the reading is representative and not too expensive.
How
Selecting the type of reading to be made will depend on the available method (direct reading or sample-taking and analysis) and on the measuring technique: emission or immission.
Where
The location selected should be the most appropriate and representative for obtaining samples. This requires knowledge of the building being studied: its orientation relative to the sun, the number of hours it receives direct sunlight, the number of floors, the type of compartmentalization, if ventilation is natural or forced air, if its windows can be opened, and so on. Knowing the source of the complaints and the problems is also necessary, for example, whether they occur in the upper or the lower floors, or in the areas close to or far from the windows, or in the areas that have poor ventilation or illumination, among other locations. Selecting the best sites to draw the samples will be based on all of the available information regarding the above-mentioned criteria.
When
Deciding when to take the readings will depend on how concentrations of air pollutants change relative to time. Pollution may be detected first thing in the morning, during the workday or at the end of the day; it may be detected at the beginning or the end of the week; during the winter or the summer; when air-conditioning is on or off; as well as at other times.
To address these questions properly, the dynamics of the given indoor environment must be known. It is also necessary to know the goals of the measurements taken, which will be based on the types of pollutant that are being investigated. The dynamics of indoor environments are influenced by the diversity of the sources of pollution, the physical differences in the spaces involved, the type of compartmentalization, the type of ventilation and climate control used, outside atmospheric conditions (wind, temperature, season, etc.), and the building’s characteristics (number of windows, their orientation, etc.).
The goals of the measurements will determine if sampling will be carried out for short or long intervals. If the health effects of the given contaminants are thought to be long-term, it follows that average concentrations should be measured over long periods of time. For substances that have acute but not cumulative effects, measurements over short periods are sufficient. If intense emissions of short duration are suspected, frequent sampling over short periods is called for in order to detect the time of the emission. Not to be overlooked, however, is the fact that in many cases the possible choices in the type of sampling methods used may be determined by the analytical methods available or required.
If after considering all these questions it is not sufficiently clear what the source of the problem is, or when the problem occurs with greatest frequency, the decision as to where and when to take samples must be made at random, calculating the number of samples as a function of the expected reliability and cost.
Measuring techniques
The methods available for taking samples of indoor air and for their analysis can be grouped into two types: methods that involve a direct reading and those that involve taking samples for later analysis.
Methods based on a direct reading are those by which taking the sample and measuring the concentration of pollutants is done simultaneously; they are fast and the measurement is instantaneous, allowing for precise data at a relatively low cost. This group includes colorimetric tubes and specific monitors.
The use of colorimetric tubes is based on the change in the colour of a specific reactant when it comes in contact with a given pollutant. The most commonly used are tubes that contain a solid reactant and air is drawn through them using a manual pump. Assessing the quality of indoor air with colorimetric tubes is useful only for exploratory measurements and for measuring sporadic emissions since their sensitivity is generally low, except for some pollutants such as CO and CO2 that can be found at high concentrations in indoor air. It is important to keep in mind that the precision of this method is low and interference from unlooked-for contaminants is often a factor.
In the case of specific monitors, detection of pollutants is based on physical, electric, thermal, electromagnetic and chemoelectromagnetic principles. Most monitors of this type can be used to make measurements of short or long duration and gain a profile of contamination at a given site. Their precision is determined by their respective manufacturers and proper use demands periodic calibrations by means of controlled atmospheres or certified gas mixtures. Monitors are becoming increasingly precise and their sensitivity more refined. Many have built-in memory to store the readings, which can then be downloaded to computers for the creation of databases and the easy organization and retrieval of the results.
Sampling methods and analyses can be classified into active (or dynamic) and passive, depending on the technique.
With active systems, this pollution can be collected by forcing air through collecting devices in which the pollutant is captured, concentrating the sample. This is accomplished with filters, adsorbent solids, and absorbent or reactive solutions which are placed in bubblers or are impregnated onto porous material. Air is then forced through and the contaminant, or the products of its reaction, are analysed. For the analysis of air sampled with active systems the requirements are a fixative, a pump to move the air and a system to measure the volume of sampled air, either directly or by using flow and duration data.
The flow and the volume of sampled air are specified in the reference manuals or should be determined by previous tests and will depend on the quantity and type of absorbent or adsorbent used, the pollutants that are being measured, the type of measurement (emission or immission) and the condition of the ambient air during the taking of the sample (humidity, temperature, pressure). The efficacy of the collection increases by reducing the rate of intake or by increasing the amount of fixative used, directly or in tandem.
Another type of active sampling is the direct capture of air in a bag or any other inert and impermeable container. This type of sample gathering is used for some gases (CO, CO2, H2S, O2) and is useful as an exploratory measure when the type of pollutant is unknown. The drawback is that without concentrating the sample there may be insufficient sensitivity and further laboratory processing may be necessary to increase the concentration.
Passive systems capture pollutants by diffusion or permeation onto a base that may be a solid adsorbent, either alone or impregnated with a specific reactant. These systems are more convenient and easy to use than active systems. They do not require pumps to capture the sample nor highly trained personnel. But capturing the sample may take a long time and the results tend to furnish only medium concentration levels. This method cannot be used to measure peak concentrations; in those instances active systems should be used instead. To use passive systems correctly it is important to know the speed at which each pollutant is captured, which will depend on the diffusion coefficient of the gas or vapor and the design of the monitor.
Table 1 shows the salient characteristics of each sampling method and table 2 outlines the various methods used to gather and analyse the samples for the most significant indoor air pollutants.
Table 1. Methodology for taking samples
|
Characteristics |
Active |
Passive |
Direct reading |
|
Timed interval measurements |
+ |
+ |
|
|
Long-term measurements |
+ |
+ |
|
|
Monitoring |
+ |
||
|
Concentration of sample |
+ |
+ |
|
|
Immission measurement |
+ |
+ |
+ |
|
Emission measurement |
+ |
+ |
+ |
|
Immediate response |
+ |
+ Means that the given method is suitable to the method of measurement or desired measurement criteria.
Table 2. Detection methods for gases in indoor air
|
Pollutant |
Direct reading |
Methods |
Analysis |
||
|
Capture by diffusion |
Capture by concentration |
Direct capture |
|||
|
Carbon monoxide |
Electrochemical cell |
Bag or inert container |
GCa |
||
|
Ozone |
Chemiluminescence |
Bubbler |
UV-Visb |
||
|
Sulphur dioxide |
Electrochemical cell |
Bubbler |
UV-Vis |
||
|
Nitrogen dioxide |
Chemiluminescence |
Filter impregnated with a |
Bubbler |
UV-Vis |
|
|
Carbon dioxide |
Infrared spectroscopy |
Bag or inert container |
GC |
||
|
Formaldehyde |
— |
Filter impregnated with a |
Bubbler |
HPLCc |
|
|
VOCs |
Portable GC |
Adsorbent solids |
Adsorbent solids |
Bag or inert container |
GC (ECDd-FIDe-NPDf-PIDg) |
|
Pesticides |
— |
Adsorbent solids |
GC (ECD-FPD-NPD) |
||
|
Particulate matter |
— |
Optical sensor |
Filter |
Impactor |
Gravimetry |
— = Method unsuitable for pollutant.
a GC = gas chromatography.
b UV-Vis = visible ultraviolet spectrophotometry.
c HPLC = high precision liquid chromatography.
d CD = electron capture detector.
e FID = flame, ionization detector.
f NPD = nitrogen/phosphorous detector.
g PID = photoionization detector.
h MS = mass spectrometry.
Selecting the method
To select the best sampling method, one should first determine that validated methods for the pollutants being studied exist and see to it that the proper instruments and materials are available to gather and analyse the pollutant. One usually needs to know what their cost will be, and the sensitivity required for the job, as well as things that can interfere with the measurement, given the method chosen.
An estimate of the minimum concentrations of what one hopes to measure is very useful when evaluating the method used to analyse the sample. The minimum concentration required is directly related to the amount of pollutant that can be gathered given the conditions specified by the method used (i.e., the type of system used to capture the pollutant or the duration of sample taking and volume of air sampled). This minimum amount is what determines the sensitivity required of the method used for analysis; it can be calculated from reference data found in the literature for a particular pollutant or group of pollutants, if they were arrived at by a similar method to the one that will be used. For example, if it is found that hydrocarbon concentrations of 30 (mg/m3) are commonly found in the area under study, the analytical method used should allow the measurement of those concentrations easily. If the sample is obtained with a tube of active carbon in four hours and with a flow of 0.5 litres per minute, the amount of hydrocarbons gathered in the sample is calculated by multiplying the flow rate of the substance by the period of time monitored. In the given example this equals:
![]() of hydrocarbons
of hydrocarbons ![]()
Any method for detecting hydrocarbons that requires the amount in the sample to be under 3.6 μg can be used for this application.
Another estimate could be calculated from the maximum limit established as the allowable limit for indoor air for the pollutant being measured. If these figures don’t exist and the usual concentrations found in indoor air are not known, nor the rate at which the pollutant is being discharged into the space, approximations can be used based on the potential levels of the pollutant that can negatively affect health. The method chosen should be capable of measuring 10% of the established limit or of the minimal concentration that could affect health. Even if the method of analysis chosen has an acceptable degree of sensitivity, it is possible to find concentrations of pollutants that are below the lower limit of detection of the chosen method. This should be kept in mind when calculating average concentrations. For example, if out of ten readings taken three are below the detection limit, two averages should be calculated, one assigning these three readings the value of zero and another giving them the lowest detection limit, which renders a minimum average and a maximum average. The true measured average will be found between the two.
Analytical Procedures
The number of indoor air pollutants is great and they are found in small concentrations. The methodology that has been available is based on adapting methods used to monitor the quality of outdoor, atmospheric, air and air found in industrial situations. Adapting these methods for the analysis of indoor air implies changing the range of the concentration sought, when the method allows, using longer sampling times and greater amounts of absorbents or adsorbents. All these changes are appropriate when they do not lead to a loss in reliability or precision. Measuring a mixture of contaminants is usually expensive and the results obtained imprecise. In many cases all that will be ascertained will be a pollution profile that will indicate the level of contamination during sampling intervals, compared to clean air, to outside air, or to other indoor spaces. Direct reading monitors are used to monitor the pollution profile and may not be suitable if they are too noisy or too large. Ever smaller and quieter monitors, that afford greater precision and sensitivity, are being designed. Table 3 shows in outline the current state of the methods used to measure the different types of contaminants.
Table 3. Methods used for the analysis of chemical pollutants
|
Pollutant |
Direct-reading monitora |
Sampling and analysis |
|
Carbon monoxide |
+ |
+ |
|
Carbon dioxide |
+ |
+ |
|
Nitrogen dioxide |
+ |
+ |
|
Formaldehyde |
– |
+ |
|
Sulphur dioxide |
+ |
+ |
|
Ozone |
+ |
+ |
|
VOCs |
+ |
+ |
|
Pesticides |
– |
+ |
|
Particulates |
+ |
+ |
a ++ = most commonly used; + = less commonly used; – = not applicable.
Analysis of gases
Active methods are the most common for the analysis of gases, and are carried out using absorbent solutions or adsorbent solids, or by directly taking a sample of air with a bag or some other inert and airtight container. In order to prevent loss of part of the sample and increase the accuracy of the reading, the volume of the sample must be lower and the amount of absorbent or adsorbent used should be more than for other types of pollution. Care should also be taken in transporting and storing the sample (keeping it at low temperature) and minimizing the time before the sample is tested. Direct reading methods are widely used for measuring gases because of the considerable improvement in the capabilities of modern monitors, which are more sensitive and more precise than before. Because of their ease of use and the level and type of information that they furnish, they are increasingly replacing traditional methods of analysis. Table 4 shows the minimum detection levels for the various gases studied given the method of sampling and analysis used.
Table 4. Lower detection limits for some gases by monitors used to assess indoor air quality
|
Pollutant |
Direct-reading monitora |
Sample-taking and |
|
Carbon monoxide |
1.0 ppm |
0.05 ppm |
|
Nitrogen dioxide |
2 ppb |
1.5 ppb (1 week)b |
|
Ozone |
4 ppb |
5.0 ppb |
|
Formaldehyde |
5.0 ppb (1 week)b |
a Carbon dioxide monitors that use infrared spectroscopy are always sensitive enough.
b Passive monitors (length of exposure).
These gases are common pollutants in indoor air. They are measured by using monitors that detect them directly by electrochemical or infrared means, even though infrared detectors are not very sensitive. They can also be measured by taking air samples directly with inert bags and analysing the sample by gas chromatography with a flame ionization detector, transforming the gases into methane first by means of a catalytic reaction. Thermal conduction detectors are usually sensitive enough to measure normal concentrations of CO2.
Nitrogen dioxide
Methods have been developed to detect nitrogen dioxide, NO2, in indoor air by using passive monitors and taking samples for later analysis, but these methods have presented sensitivity problems that will hopefully be overcome in the future. The best known method is the Palmes tube, which has a detection limit of 300 ppb. For non-industrial situations, sampling should be for a minimum of five days in order to obtain a detection limit of 1.5 ppb, which is three times the value of the blank for a one-week exposure. Portable monitors that measure in real time have also been developed based on the chemiluminescence reaction between NO2 and the reactant luminol, but the results obtained by this method can be affected by temperature and their linearity and sensitivity depend on the characteristics of the solution of luminol used. Monitors that have electrochemical sensors have improved sensitivity but are subject to interference from compounds that contain sulphur (Freixa 1993).
Sulphur dioxide
A spectrophotometric method is used to measure sulphur dioxide, SO2, in an indoor environment. The air sample is bubbled through a solution of potassium tetrachloromercuriate to form a stable complex which is in turn measured spectrophotometrically after reacting with pararosaniline. Other methods are based on flame photometry and pulsating ultraviolet fluorescence, and there are also methods based on deriving the measurement before the spectroscopic analysis. This type of detection, which has been used for outside air monitors, is not suited for indoor air analysis because of a lack of specificity and because many of these monitors require a venting system to eliminate the gases that they generate. Because emissions of SO2 have been greatly reduced and it is not considered an important pollutant of indoor air, the development of monitors for its detection have not advanced very much. However, there are portable instruments available on the market that can detect SO2 based on the detection of pararosaniline (Freixa 1993).
Ozone
Ozone, O3, can only be found in indoor environments in special situations in which it is generated continuously, since it decays rapidly. It is measured by direct reading methods, by colorimetric tubes and by chemiluminescence methods. It can also be detected by methods used in industrial hygiene that can be easily adapted for indoor air. The sample is obtained with an absorbent solution of potassium iodide in a neutral medium and then subjected to spectrophotometric analysis.
Formaldehyde
Formaldehyde is an important pollutant of indoor air, and because of its chemical and toxic characteristics an individualized evaluation is recommended. There are different methods for detecting formaldehyde in air, all of them based on taking samples for later analysis, with active fixing or by diffusion. The most appropriate capturing method will be determined by the type of sample (emission or immission) used and the sensitivity of the analytical method. The traditional methods are based on obtaining a sample by bubbling air through distilled water or a solution of 1% sodium bisulphate at 5°C, and then analysing it with spectrofluorometric methods. While the sample is stored, it should also be kept at 5°C. SO2 and the components of tobacco smoke can create interference. Active systems or methods that capture pollutants by diffusion with solid adsorbents are used more and more frequently in indoor air analysis; they all consist of a base that can be a filter or a solid saturated with a reactant, such as sodium bisulphate or 2,4-diphenylhydrazine. Methods that capture the pollutant by diffusion, in addition to general advantages of that method, are more sensitive than active methods because the time required to obtain the sample is longer (Freixa 1993).
Detection of volatile organic compounds (VOCs)
The methods used to measure or monitor organic vapors in indoor air must meet a series of criteria: they should have a sensitivity in the order of parts per billion (ppb) to parts per trillion (ppt), the instruments used to take the sample or make a direct reading must be portable and easy to handle in the field, and the results obtained must be precise and capable of being duplicated. There are a great many methods that meet these criteria, but the ones most frequently used to analyse indoor air are based on sample taking and analysis. Direct detection methods exist that consist of portable gas chromatographs with different detection methods. These instruments are expensive, their handling is sophisticated and they can be operated only by trained personnel. For polar and nonpolar organic compounds that have a boiling point between 0°C and 300°C, the most widely used adsorbent both for active and passive sampling systems has been activated carbon. Porous polymers and polymer resins, such as Tenax GC, XAD-2 and Ambersorb are also used. The most widely used of these is Tenax. The samples obtained with activated carbon are extracted with carbon disulphide and they are analysed by gas chromatography with flame ionization, electron-capture, or mass spectrometry detectors, followed by qualitative and quantitative analysis. Samples obtained with Tenax are usually extracted by thermal desorption with helium and are condensed in a nitrogen cold trap before being fed to the chromatograph. Another common method consists in obtaining samples directly, using bags or inert containers, feeding the air directly to the gas chromatograph, or concentrating the sample first with an adsorbent and a cold trap. The detection limits of these methods depend on the compound analysed, the volume of the sample taken, the background pollution and the detection limits of the instrument used. Because quantifying each and every one of the compounds present is impossible, quantification is normally done by families, by using as a reference compounds that are characteristic of each family of compounds. In detecting VOCs in indoor air, the purity of the solvents used is very important. If thermal desorption is used, the purity of the gases is also important.
Detection of pesticides
To detect pesticides in indoor air, the methods commonly employed consist of taking samples with solid adsorbents, although the use of bubblers and mixed systems is not ruled out. The solid adsorbent most commonly used has been porous polymer Chromosorb 102, although polyurethane foams (PUFs) that can capture a wider number of pesticides are being used more and more. The methods of analysis vary according to the sampling method and the pesticide. Usually they are analysed by using gas chromatography with different specific detectors, from electron capture to mass spectrometry. The potential of the latter for identifying compounds is considerable. The analysis of these compounds presents certain problems, which include the contamination of glass parts in the sample-taking systems with traces of polychlorinated biphenyls (PCBs), phthalates or pesticides.
Detection of environmental dust or particles
For the capture and analysis of particles and fibres in air a great variety of techniques and equipment are available and suited for assessing indoor air quality. Monitors that permit a direct reading of the concentration of particles in the air use diffuse light detectors, and methods that employ sample taking and analysis use weighting and analysis with a microscope. This type of analysis requires a separator, such as a cyclone or an impactor, to sift out larger particles before a filter can be used. Methods that employ a cyclone can handle small volumes, which results in long sessions of sample taking. Passive monitors offer excellent precision, but they are affected by ambient temperature and tend to give readings with higher values when the particles are small.
Biological Contamination
Characteristics and Origins of Biological Indoor Air Contamination
Although there is a diverse range of particles of biological origin (bioparticles) in indoor air, in most indoor work environments micro-organisms (microbes) are of the greatest significance for health. As well as micro-organisms, which include viruses, bacteria, fungi and protozoa, indoor air can also contain pollen grains, animal dander and fragments of insects and mites and their excretory products (Wanner et al. 1993). In addition to bioaerosols of these particles, there may also be volatile organic compounds which emanate from living organisms such as indoor plants and micro-organisms.
Pollen
Pollen grains contain substances (allergens) which may cause in susceptible, or atopic, individuals allergic responses usually manifested as “hay fever”, or rhinitis. Such allergy is associated primarily with the outdoor environment; in indoor air, pollen concentrations are usually considerably lower than in outdoor air. The difference in pollen concentration between outdoor and indoor air is greatest for buildings where heating, ventilation and air-conditioning (HVAC) systems have efficient filtration at the intake of external air. Window air-conditioning units also give lower indoor pollen levels than those found in naturally ventilated buildings. The air of some indoor work environments may be expected to have high pollen counts, for example, in premises where large numbers of flowering plants are present for aesthetic reasons, or in commercial glasshouses.
Dander
Dander consists of fine skin and hair/feather particles (and associated dried saliva and urine) and is a source of potent allergens which can cause bouts of rhinitis or asthma in susceptible individuals. The main sources of dander in indoor environments are usually cats and dogs, but rats and mice (whether as pets, experimental animals or vermin), hamsters, gerbils (a species of desert-rat), guinea pigs and cage-birds may be additional sources. Dander from these and from farm and recreational animals (e.g., horses) can be brought in on clothes, but in work environments the greatest exposure to dander is likely to be in animal-rearing facilities and laboratories or in vermin-infested buildings.
Insects
These organisms and their excretory products may also cause respiratory and other allergies, but do not appear to contribute significantly to the airborne bioburden in most situations. Particles from cockroaches (especially Blatella germanica and Periplaneta americana) may be significant in unsanitary, hot and humid work environments. Exposures to particles from cockroaches and other insects, including locusts, weevils, flour beetles and fruit flies, can be the cause of ill health among employees in rearing facilities and laboratories.
Mites
These arachnids are associated particularly with dust, but fragments of these microscopic relatives of spiders and their excretory products (faeces) may be present in indoor air. The house dust mite, Dermatophagoides pteronyssinus, is the most important species. With its close relatives, it is a major cause of respiratory allergy. It is associated primarily with homes, being particularly abundant in bedding but also present in upholstered furniture. There is limited evidence indicating that such furniture may provide a niche in offices. Storage mites associated with stored foods and animal feedstuffs, for example, Acarus, Glyciphagus and Tyrophagus, may also contribute allergenic fragments to indoor air. Although they are most likely to affect farmers and workers handling bulk food commodities, like D. pteronyssinus, storage mites can exist in dust in buildings, particularly under warm humid conditions.
Viruses
Viruses are very important micro-organisms in terms of the total amount of ill health they cause, but they cannot lead an independent existence outside living cells and tissues. Although there is evidence indicating that some are spread in recirculating air of HVAC systems, the principal means of transmission is by person-to-person contact. Inhalation at short range of aerosols generated by coughing or sneezing, for example, common cold and influenza viruses, is also important. Rates of infection are therefore likely to be higher in crowded premises. There are no obvious changes in building design or management which can alter this state of affairs.
Bacteria
These micro-organisms are divided into two major categories according to their Gram’s stain reaction. The most common Gram-positive types originate from the mouth, nose, nasopharynx and skin, namely, Staphylococcus epidermidis, S. aureus and species of Aerococcus, Micrococcus and Streptococcus. Gram-negative bacteria are generally not abundant, but occasionally Actinetobacter, Aeromonas, Flavobacterium and especially Pseudomonas species may be prominent. The cause of Legionnaire’s disease, Legionella pneumophila, may be present in hot water supplies and air-conditioning humidifiers, as well as in respiratory therapy equipment, jacuzzis, spas and shower stalls. It is spread from such installations in aqueous aerosols, but also may enter buildings in air from nearby cooling towers. The survival time for L. pneumophila in indoor air appears to be no greater than 15 minutes.
In addition to the unicellular bacteria mentioned above, there are also filamentous types which produce aerially dispersed spores, that is, the Actinomycetes. They appear to be associated with damp structural materials, and may give off a characteristic earthy odour. Two of these bacteria that are able to grow at 60°C, Faenia rectivirgula (formerly Micropolyspora faeni) and Thermoactinomyces vulgaris, may be found in humidifiers and other HVAC equipment.
Fungi
Fungi comprise two groups: first, the microscopic yeasts and moulds known as microfungi, and, second, plaster and wood-rotting fungi, which are referred to as macrofungi as they produce macroscopic sporing bodies visible to the naked eye. Apart from unicellular yeasts, fungi colonize substrates as a network (mycelium) of filaments (hyphae). These filamentous fungi produce numerous aerially dispersed spores, from microscopic sporing structures in moulds and from large sporing structures in macrofungi.
There are spores of many different moulds in the air of houses and nonindustrial workplaces, but the most common are likely to be species of Cladosporium, Penicillium, Aspergillus and Eurotium. Some moulds in indoor air, such as Cladosporium spp., are abundant on leaf surfaces and other plant parts outdoors, particularly in summer. However, although spores in indoor air may originate outdoors, Cladosporium is also able to grow and produce spores on damp surfaces indoors and thus add to the indoor air bioburden. The various species of Penicillium are generally regarded as originating indoors, as are Aspergillus and Eurotium. Yeasts are found in most indoor air samples, and occasionally may be present in large numbers. The pink yeasts Rhodotorula or Sporobolomyces are prominent in the airborne flora and can also be isolated from mould-affected surfaces.
Buildings provide a broad range of niches in which the dead organic material which serves as nutriment that can be utilized by most fungi and bacteria for growth and spore production is present. The nutrients are present in materials such as: wood; paper, paint and other surface coatings; soft furnishings such as carpets and upholstered furniture; soil in plant pots; dust; skin scales and secretions of human beings and other animals; and cooked foods and their raw ingredients. Whether any growth occurs or not depends on moisture availability. Bacteria are able to grow only on saturated surfaces, or in water in HVAC drain pans, reservoirs and the like. Some moulds also require conditions of near saturation, but others are less demanding and may proliferate on materials that are damp rather than fully saturated. Dust can be a repository and, also, if it is sufficiently moist, an amplifier for moulds. It is therefore an important source of spores which become airborne when dust is disturbed.
Protozoa
Protozoa such as Acanthamoeba and Naegleri are microscopic unicellular animals which feed on bacteria and other organic particles in humidifiers, reservoirs and drain pans in HVAC systems. Particles of these protozoa may be aerosolized and have been cited as possible causes of humidifier fever.
Microbial volatile organic compounds
Microbial volatile organic compounds (MVOCs) vary considerably in chemical composition and odour. Some are produced by a wide range of micro-organisms, but others are associated with particular species. The so-called mushroom alcohol, 1-octen-3-ol (which has a smell of fresh mushrooms) is among those produced by many different moulds. Other less common mould volatiles include 3,5-dimethyl-1,2,4-trithiolone (described as “foetid”); geosmin, or 1,10-dimethyl-trans-9-decalol (“earthy”); and 6-pentyl-α-pyrone (“coconut”, “musty”). Among bacteria, species of Pseudomonas produce pyrazines with a “musty potato” odour. The odour of any individual micro-organism is the product of a complex mixture of MVOCs.
History of Microbiological Indoor Air Quality Problems
Microbiological investigations of air in homes, schools and other buildings have been made for over a century. Early investigations were sometimes concerned with the relative microbiological “purity” of the air in different types of building and any relation it might have to the death rate among occupants. Allied to a long-time interest in the spread of pathogens in hospitals, the development of modern volumetric microbiological air samplers in the 1940s and 1950s led to systematic investigations of airborne micro-organisms in hospitals, and subsequently of known allergenic moulds in air in homes and public buildings and outdoors. Other work was directed in the 1950s and 1960s to investigation of occupational respiratory diseases like farmer’s lung, malt worker’s lung and byssinosis (among cotton workers). Although influenza-like humidifier fever in a group of workers was first described in 1959, it was another ten to fifteen years before other cases were reported. However, even now, the specific cause is not known, although micro-organisms have been implicated. They have also been invoked as a possible cause of “sick building syndrome”, but as yet the evidence for such a link is very limited.
Although the allergic properties of fungi are well recognized, the first report of ill health due to inhalation of fungal toxins in a non-industrial workplace, a Quebec hospital, did not appear until 1988 (Mainville et al. 1988). Symptoms of extreme fatigue among staff were attributed to trichothecene mycotoxins in spores of Stachybotrys atra and Trichoderma viride, and since then “chronic fatigue syndrome” caused by exposure to mycotoxic dust has been recorded among teachers and other employees at a college. The first has been the cause of illness in office workers, with some health effects being of an allergic nature and others of a type more often associated with a toxicosis (Johanning et al. 1993). Elsewhere, epidemiological research has indicated that there may be some non-allergic factor or factors associated with fungi affecting respiratory health. Mycotoxins produced by individual species of mould may have an important role here, but there is also the possibility that some more general attribute of inhaled fungi is detrimental to respiratory well-being.
Micro-organisms Associated with Poor Indoor Air Quality and their Health Effects
Although pathogens are relatively uncommon in indoor air, there have been numerous reports linking airborne micro-organisms with a number of allergic conditions, including: (1) atopic allergic dermatitis; (2) rhinitis; (3) asthma; (4) humidifier fever; and (5) extrinsic allergic alveolitis (EAA), also known as hypersensitivity pneumonitis (HP).
Fungi are perceived as being more important than bacteria as components of bioaerosols in indoor air. Because they grow on damp surfaces as obvious mould patches, fungi often give a clear visible indication of moisture problems and potential health hazards in a building. Mould growth contributes both numbers and species to the indoor air mould flora which would otherwise not be present. Like Gram-negative bacteria and Actinomycetales, hydrophilic (“moisture-loving”) fungi are indicators of extremely wet sites of amplification (visible or hidden), and therefore of poor indoor air quality. They include Fusarium, Phoma, Stachybotrys, Trichoderma, Ulocladium, yeasts and more rarely the opportunistic pathogens Aspergillus fumigatus and Exophiala jeanselmei. High levels of moulds which show varying degrees of xerophily (“love of dryness”), in having a lower requirement for water, can indicate the existence of amplification sites which are less wet, but nevertheless significant for growth. Moulds are also abundant in house dust, so that large numbers can also be a marker of a dusty atmosphere. They range from slightly xerophilic (able to withstand dry conditions) Cladosporium species to moderately xerophilic Aspergillus versicolor, Penicillium (for example, P. aurantiogriseum and P. chrysogenum) and the extremely xerophilic Aspergillus penicillioides, Eurotium and Wallemia.
Fungal pathogens are rarely abundant in indoor air, but A. fumigatus and some other opportunistic aspergilli which can invade human tissue may grow in the soil of potted plants. Exophiala jeanselmei is able to grow in drains. Although the spores of these and other opportunistic pathogens such as Fusarium solani and Pseudallescheria boydii are unlikely to be hazardous to the healthy, they may be so to immunologically compromised individuals.
Airborne fungi are much more important than bacteria as causes of allergic disease, although it appears that, at least in Europe, fungal allergens are less important than those of pollen, house dust mites and animal dander. Many types of fungus have been shown to be allergenic. Some of the fungi in indoor air which are most commonly cited as causes of rhinitis and asthma are given in table 1. Species of Eurotium and other extremely xerophilic moulds in house dust are probably more important as causes of rhinitis and asthma than has been previously recognized. Allergic dermatitis due to fungi is much less common than rhinitis/asthma, with Alternaria, Aspergillus and Cladosporium being implicated. Cases of EAA, which are relatively rare, have been attributed to a range of different fungi, from the yeast Sporobolomyces to the wood-rotting macrofungus Serpula (table 2). It is generally considered that development of symptoms of EAA in an individual requires exposure to at least one million and more, probably one hundred million or so allergen-containing spores per cubic meter of air. Such levels of contamination are only likely to occur where there is profuse fungal growth in a building.
Table 1. Examples of types of fungus in indoor air, which can cause rhinitis and/or asthma
|
Alternaria |
Geotrichum |
Serpula |
|
Aspergillus |
Mucor |
Stachybotrys |
|
Cladosporium |
Penicillium |
Stemphylium/Ulocladium |
|
Eurotium |
Rhizopus |
Wallemia |
|
Fusarium |
Rhodotorula/Sporobolomyces |
|
Table 2. Micro-organisms in indoor air reported as causes of building-related extrinsic allergic alveolitis
|
Type |
Micro-organis |
Source
|
|
Bacteria |
Bacillus subtilis |
Decayed wood |
|
|
Faenia rectivirgula |
Humidifier |
|
|
Pseudomonas aeruginosa |
Humidifier
|
|
|
Thermoactinomyces vulgaris |
Air conditioner
|
|
Fungi |
Aureobasidium pullulans |
Sauna; room wall |
|
|
Cephalosporium sp. |
Basement; humidifier |
|
|
Cladosporium sp. |
Unventilated bathroom |
|
|
Mucor sp. |
Pulsed air heating system |
|
|
Penicillium sp. |
Pulsed air heating system humidifier |
|
|
P. casei |
Room wall |
|
|
P. chrysogenum / P. cyclopium |
Flooring |
|
|
Serpula lacrimans |
Dry rot affected timber |
|
|
Sporobolomyces |
Room wall; ceiling |
|
|
Trichosporon cutaneum |
Wood; matting |
As indicated earlier, inhalation of spores of toxicogenic species presents a potential hazard (Sorenson 1989; Miller 1993). It is not just the spores of Stachybotrys which contain high concentrations of mycotoxins. Although the spores of this mould, which grows on wallpaper and other cellulosic substrates in damp buildings and is also allergenic, contain extremely potent mycotoxins, other toxicogenic moulds which are more often present in indoor air include Aspergillus (especially A. versicolor) and Penicillium (for example, P. aurantiogriseum and P. viridicatum) and Trichoderma. Experimental evidence indicates that a range of mycotoxins in the spores of these moulds are immunosuppressive and strongly inhibit scavenging and other functions of the pulmonary macrophage cells essential to respiratory health (Sorenson 1989).
Little is known about the health effects of the MVOCs produced during the growth and sporulation of moulds, or of their bacterial counterparts. Although many MVOCs appear to have relatively low toxicity (Sorenson 1989), anecdotal evidence indicates that they can provoke headache, discomfort and perhaps acute respiratory responses in humans.
Bacteria in indoor air do not generally present a health hazard as the flora is usually dominated by the Gram-positive inhabitants of the skin and upper respiratory passages. However, high counts of these bacteria indicate overcrowding and poor ventilation. The presence of large numbers of Gram-negative types and/or Actinomycetales in air indicate that there are very wet surfaces or materials, drains or particularly humidifiers in HVAC systems in which they are proliferating. Some Gram-negative bacteria (or endotoxin extracted from their walls) have been shown to provoke symptoms of humidifier fever. Occasionally, growth in humidifiers has been great enough for aerosols to be generated which contained sufficient allergenic cells to have caused the acute pneumonia-like symptoms of EAA (see Table 15).
On rare occasions, pathogenic bacteria such as Mycobacterium tuberculosis in droplet nuclei from infected individuals can be dispersed by recirculation systems to all parts of an enclosed environment. Although the pathogen, Legionella pneumophila, has been isolated from humidifiers and air-conditioners, most outbreaks of Legionellosis have been associated with aerosols from cooling towers or showers.
Influence of Changes in Building Design
Over the years, the increase in the size of buildings concomitantly with the development of air-handling systems which have culminated in modern HVAC systems has resulted in quantitative and qualitative changes in the bioburden of air in indoor work environments. In the last two decades, the move to the design of buildings with minimum energy usage has led to the development of buildings with greatly reduced infiltration and exfiltration of air, which allows a build-up of airborne micro-organisms and other contaminants. In such “tight” buildings, water vapor, which would previously have been vented to the outdoors, condenses on cool surfaces, creating conditions for microbial growth. In addition, HVAC systems designed only for economic efficiency often promote microbial growth and pose a health risk to occupants of large buildings. For example, humidifiers which utilize recirculated water rapidly become contaminated and act as generators of micro-organisms, humidification water-sprays aerosolize micro-organisms, and siting of filters upstream and not downstream of such areas of microbial generation and aerosolization allows onward transmission of microbial aerosols to the workplace. Siting of air intakes close to cooling towers or other sources of micro-organisms, and difficulty of access to the HVAC system for maintenance and cleaning/disinfection, are also among the design, operation and maintenance defects which may endanger health. They do so by exposing occupants to high counts of particular airborne micro-organisms, rather than to the low counts of a mixture of species reflective of outdoor air that should be the norm.
Methods of Evaluating Indoor Air Quality
Air sampling of micro-organisms
In investigating the microbial flora of air in a building, for example, in order to try to establish the cause of ill health among its occupants, the need is to gather objective data which are both detailed and reliable. As the general perception is that the microbiological status of indoor air should reflect that of outdoor air (ACGIH 1989), organisms must be accurately identified and compared with those in outdoor air at that time.
Air samplers
Sampling methods which allow, directly or indirectly, the culture of viable airborne bacteria and fungi on nutritive agar gel offer the best chance of identification of species, and are therefore most frequently used. The agar medium is incubated until colonies develop from the trapped bioparticles and can be counted and identified, or are subcultured onto other media for further examination. The agar media needed for bacteria are different from those for fungi, and some bacteria, for example, Legionella pneumophila, can be isolated only on special selective media. For fungi, the use of two media is recommended: a general-purpose medium as well as a medium that is more selective for isolation of xerophilic fungi. Identification is based on the gross characteristics of the colonies, and/or their microscopical or biochemical characteristics, and requires considerable skill and experience.
The range of sampling methods available has been adequately reviewed (e.g., Flannigan 1992; Wanner et al. 1993), and only the most commonly used systems are mentioned here. It is possible to make a rough-and-ready assessment by passively collecting micro-organisms gravitating out of the air into open Petri dishes containing agar medium. The results obtained using these settlement plates are non-volumetric, are strongly affected by atmospheric turbulence and favour collection of large (heavy) spores or clumps of spores/cells. It is therefore preferable to use a volumetric air sampler. Impaction samplers in which the airborne particles impact on an agar surface are widely used. Air is either drawn through a slit above a rotating agar plate (slit-type impaction sampler) or through a perforated disc above the agar plate (sieve-type impaction sampler). Although single-stage sieve samplers are widely used, the six-stage Andersen sampler is preferred by some investigators. As air cascades through successively finer holes in its six stacked aluminium sections, the particles are sorted out onto different agar plates according to their aerodynamic size. The sampler therefore reveals the size of particles from which colonies develop when the agar plates are subsequently incubated, and indicates where in the respiratory system the different organisms would most likely be deposited. A popular sampler which works on a different principle is the Reuter centrifugal sampler. Centrifugal acceleration of air drawn in by an impeller fan causes particles to impact at high velocity onto agar in a plastic strip lining the sampling cylinder.
Another approach to sampling is to collect micro-organisms on a membrane filter in a filter cassette connected to a low-volume rechargeable pump. The whole assembly can be clipped to a belt or harness and used to collect a personal sample over a normal working day. After sampling, small portions of washings from the filter and dilutions of the washings can then be spread out on a range of agar media, incubated and counts of viable micro-organisms made. An alternative to the filter sampler is the liquid impinger, in which particles in air drawn in through capillary jets impinge on and collect in liquid. Portions of the collection liquid and dilutions prepared from it are treated in the same way as those from filter samplers.
A serious deficiency in these “viable” sampling methods is that what they assess is only organisms which are actually culturable, and these may only be one or two per cent of the total air spora. However, total counts (viable plus non-viable) can be made using impaction samplers in which particles are collected on the sticky surfaces of rotating rods (rotating-arm impaction sampler) or on the plastic tape or glass microscope slide of different models of slit-type impaction sampler. The counts are made under the microscope, but only relatively few fungi can be identified in this way, namely, those that have distinctive spores. Filtration sampling has been mentioned in relation to the assessment of viable micro-organisms, but it is also a means of obtaining a total count. A portion of the same washings that are plated out on agar medium can be stained and the micro-organisms counted under a microscope. Total counts can be also made in the same way from the collection fluid in liquid impingers.
Choice of air sampler and sampling strategy
Which sampler is used is largely determined by the experience of the investigator, but the choice is important for both quantitative and qualitative reasons. For example, the agar plates of single-stage impaction samplers are much more easily “overloaded” with spores during sampling than those of a six-stage sampler, resulting in overgrowth of the incubated plates and serious quantitative and qualitative errors in assessment of the airborne population. The way in which different samplers operate, their sampling times and the efficiency with which they remove different sizes of particle from the ambient air, extract them from the airstream and collect them on a surface or in liquid all differ considerably. Because of these differences, it is not possible to make valid comparisons between data obtained using one type of sampler in one investigation with those from another type of sampler in a different investigation.
The sampling strategy as well as the choice of sampler, is very important. No general sampling strategy can be set down; each case demands its own approach (Wanner et al. 1993). A major problem is that the distribution of micro-organisms in indoor air is not uniform, either in space or time. It is profoundly affected by the degree of activity in a room, particularly any cleaning or construction work which throws up settled dust. Consequently, there are considerable fluctuations in numbers over relatively short time intervals. Apart from filter samplers and liquid impingers, which are used for several hours, most air samplers are used to obtain a “grab” sample over only a few minutes. Samples should therefore be taken under all conditions of occupation and usage, including both times when HVAC systems are functioning and when not. Although extensive sampling may reveal the range of concentrations of viable spores encountered in an indoor environment, it is not possible to assess satisfactorily the exposure of individuals to micro-organisms in the environment. Even samples taken over a working day with a personal filter sampler do not give an adequate picture, as they give only an average value and do not reveal peak exposures.
In addition to the clearly recognized effects of particular allergens, epidemiological research indicates that there may be some non-allergic factor associated with fungi which affects respiratory health. Mycotoxins produced by individual species of mould may have an important role, but there is also the possibility that some more general factor is involved. In the future, the overall approach to investigating the fungal burden in indoor air is therefore likely to be: (1) to assess which allergenic and toxicogenic species are present by sampling for viable fungi; and (2) to obtain a measure of the total amount of fungal material to which individuals are exposed in a work environment. As noted above, to obtain the latter information, total counts could be taken over a working day. However, in the near future, methods which have recently been developed for the assay of 1,3-β-glucan or ergosterol (Miller 1993) may be more widely adopted. Both substances are structural components of fungi, and therefore give a measure of the amount of fungal material (i.e., its biomass). A link has been reported between levels of 1,3-β-glucan in indoor air and symptoms of sick building syndrome (Miller 1993).
Standards and Guidelines
While some organizations have categorized levels of contamination of indoor air and dust (table 3), because of air sampling problems there has been a justifiable reluctance to set numerical standards or guideline values. It has been noted that the airborne microbial load in air-conditioned buildings should be markedly lower than in outdoor air, with the differential between naturally ventilated buildings and outdoor air being less. The ACGIH (1989) recommends that the rank order of fungal species in indoor and outdoor air be used in interpreting air sampling data. The presence or preponderance of some moulds in indoor air, but not outdoors, may identify a problem inside a building. For example, abundance in indoor air of such hydrophilic moulds as Stachybotrys atra almost invariably indicates a very damp amplification site within a building.
Table 3. Observed levels of micro-organisms in air and dust of nonindustrial indoor environments
|
Category of |
CFUa per meter of air |
Fungi as CFU/g |
|
|
Bacteria |
Fungi |
||
|
Very low |
<50 |
<25 |
<10,000 |
|
Low |
<100 |
<100 |
<20,000 |
|
Intermediate |
<500 |
<500 |
<50,000 |
|
High |
<2,000 |
<2,000 |
<120,000 |
|
Very high |
>2,000 |
>2,000 |
>120,000 |
a CFU, colony-forming units.
Source: adapted from Wanner et al. 1993.
Although influential bodies such as the ACGIH Bioaerosols Committee have not established numerical guidelines, a Canadian guide on office buildings (Nathanson 1993), based on some five years of investigation of around 50 air-conditioned federal government buildings, includes some guidance on numbers. The following are among the main points made:
- The “normal” air flora should be quantitatively lower than, but qualitatively similar to, that of outdoor air.
- The presence of one or more fungal species at significant levels in indoor but not outdoor samples is evidence of an indoor amplifier.
- Pathogenic fungi such as Aspergillus fumigatus, Histoplasma and Cryptococcus should not be present in significant numbers.
- The persistence of toxicogenic moulds such as Stachybotrys atra and Aspergillus versicolor in significant numbers requires investigation and action.
- More than 50 colony-forming units per cubic meter (CFU/m3) may be of concern if there is only one species present (other than certain common outdoor leaf-inhabiting fungi); up to 150 CFU/m3 is acceptable if the species present reflect the flora outdoors; up to 500 CFU/m3 is acceptable in summer if outdoor leaf-inhabiting fungi are the main components.
These numerical values are based on four-minute air samples collected with a Reuter centrifugal sampler. It must be emphasized that they cannot be translated to other sampling procedures, other types of building or other climatic/geographical regions. What is the norm or is acceptable can only be based on extensive investigations of a range of buildings in a particular region using well-defined procedures. No threshold limit values can be set for exposure to moulds in general or to particular species.
Control of Micro-organisms in Indoor Environments
The key determinant of microbial growth and production of cells and spores which can become aerosolized in indoor environments is water, and by reducing moisture availability, rather than by using biocides, control should be achieved. Control involves proper maintenance and repair of a building, including prompt drying and elimination of causes of leakage/flood damage (Morey 1993a). Although maintaining the relative humidity of rooms at a level less than 70% is often cited as a control measure, this is effective only if the temperature of the walls and other surfaces are close to that of the air temperature. At the surface of poorly insulated walls, the temperature may be below the dew point, with the result that condensation develops and hydrophilic fungi, and even bacteria, grow (Flannigan 1993). A similar situation can arise in humid tropical or subtropical climates where the moisture in the air permeating the building envelope of an air-conditioned building condenses at the cooler inner surface (Morey 1993b). In such cases, control lies in the design and correct use of insulation and vapor barriers. In conjunction with rigorous moisture control measures, maintenance and cleaning programmes should ensure removal of dust and other detritus that supply nutrients for growth, and also act as reservoirs of micro-organisms.
In HVAC systems (Nathanson 1993), accumulation of stagnant water should be prevented, for example, in drain pans or under cooling coils. Where sprays, wicks or heated water tanks are integral to humidification in HVAC systems, regular cleaning and disinfection are necessary to limit microbial growth. Humidification by dry steam is likely to reduce greatly the risk of microbial growth. As filters can accumulate dirt and moisture and therefore provide amplification sites for microbial growth, they should be replaced regularly. Micro-organisms can also grow in porous acoustical insulation used to line ducts if it becomes moist. The solution to this problem is to apply such insulation to the exterior rather than the interior; internal surfaces should be smooth and should not provide an environment conducive to growth. Such general control measures will control growth of Legionella in HVAC systems, but additional features, such as the installation of a high-efficiency particulate air (HEPA) filter at the intake have been recommended (Feeley 1988). In addition, water systems should ensure that hot water is heated uniformly to 60°C, that there are no areas in which water stagnates and that no fittings contain materials that promote growth of Legionella.
Where controls have been inadequate and mould growth occurs, remedial action is necessary. It is essential to remove and discard all porous organic materials, such as carpets and other soft furnishings, ceiling tiles and insulation, on and in which there is growth. Smooth surfaces should be washed down with sodium hypochlorite bleach or suitable disinfectant. Biocides which can be aerosolized should not be used in operating HVAC systems.
During remediation, care must always be taken that micro-organisms on or in contaminated materials are not aerosolized. In cases where large areas of mould growth (ten square meters or more) are being dealt with it may be necessary to contain the potential hazard, maintaining negative pressure in the containment area during remediation and having air locks/decontamination areas between the contained area and the remainder of the building (Morey 1993a, 1993b; New York City Department of Health 1993). Dusts present before or generated during removal of contaminated material into sealed containers should be collected using a vacuum cleaner with a HEPA filter. Throughout operations, the specialist remediation personnel must wear full-face HEPA respiratory protection and disposable protective clothing, footwear and gloves (New York City Department of Health 1993). Where smaller areas of mould growth are being dealt with, regular maintenance staff may be employed after appropriate training. In such cases, containment is not considered necessary, but the staff must wear full respiratory protection and gloves. In all cases, both regular occupants and personnel to be employed in remediation should be made aware of the hazard. The latter should not have pre-existing asthma, allergy or immunosuppressive disorders (New York City Department of Health 1993).
Regulations, Recommendations, Guidelines and Standards
Criteria for Establishment
The setting of specific guides and standards for indoor air is the product of proactive policies in this field on the part of the bodies responsible for their establishment and for maintaining the quality of indoor air at acceptable levels. In practice, the tasks are divided and shared among many entities responsible for controlling pollution, maintaining health, ensuring the safety of products, watching over occupational hygiene and regulating building and construction.
The establishment of a regulation is intended to limit or reduce the levels of pollution in indoor air. This goal can be achieved by controlling the existing sources of pollution, diluting indoor air with outside air and checking the quality of available air. This requires the establishment of specific maximum limits for the pollutants found in indoor air.
The concentration of any given pollutant in indoor air follows a model of balanced mass expressed in the following equation:

where:
Ci = the concentration of the pollutant in indoor air (mg/m3);
Q = the emission rate (mg/h);
V = the volume of indoor space (m3);
Co = the concentration of the pollutant in outdoor air (mg/m3);
n = the ventilation rate per hour;
a = the pollutant decay rate per hour.
It is generally observed that—in static conditions—the concentration of pollutants present will depend in part on the amount of the compound released into the air from the source of contamination and its concentration in outdoor air, and on the different mechanisms by which the pollutant is removed. The elimination mechanisms include the dilution of the pollutant and its “disappearance” with time. All regulations, recommendations, guidelines and standards that may be set in order to reduce pollution must take stock of these possibilities.
Control of the Sources of Pollution
One of the most effective ways to reduce the levels of concentration of a pollutant in indoor air is to control the sources of contamination within the building. This includes the materials used for construction and decoration, the activities within the building and the occupants themselves.
If it is deemed necessary to regulate emissions that are due to the construction materials used, there are standards that limit directly the content in these materials of compounds for which harmful effects to health have been demonstrated. Some of these compounds are considered carcinogenic, like formaldehyde, benzene, some pesticides, asbestos, fibreglass and others. Another avenue is to regulate emissions by the establishment of emission standards.
This possibility presents many practical difficulties, chief among them being the lack of agreement on how to go about measuring these emissions, a lack of knowledge about their effects on the health and comfort of the occupants of the building, and the inherent difficulties of identifying and quantifying the hundreds of compounds emitted by the materials in question. One way to go about establishing emission standards is to start out from an acceptable level of concentration of the pollutant and to calculate a rate of emission that takes into account the environmental conditions—temperature, relative humidity, air exchange rate, loading factor and so forth—that are representative of the way in which the product is actually used. The main criticism levelled against this methodology is that more than one product may generate the same polluting compound. Emission standards are obtained from readings taken in controlled atmospheres where conditions are perfectly defined. There are published guides for Europe (COST 613 1989 and 1991) and for the United States (ASTM 1989). The criticisms usually directed against them are based on: (1) the fact that it is difficult to get comparative data and (2) the problems that surface when an indoor space has intermittent sources of pollution.
As for the activities that may take place in a building, the greatest focus is placed on building maintenance. In these activities the control can be established in the form of regulations about the performance of certain duties—like recommendations relating to the application of pesticides or the reduction of exposure to lead or asbestos when a building is being renovated or demolished.
Because tobacco smoke—attributable to the occupants of a building—is so often a cause of indoor air pollution, it deserves separate treatment. Many countries have laws, at the state level, that prohibit smoking in certain types of public space such as restaurants and theatres, but other arrangements are very common whereby smoking is permitted in certain specially designated parts of a given building.
When the use of certain products or materials is prohibited, these prohibitions are made based on their alleged detrimental health effects, which are more or less well documented for levels normally present in indoor air. Another difficulty that arises is that often there is not enough information or knowledge about the properties of the products that could be used in their stead.
Elimination of the Pollutant
There are times when it is not possible to avoid the emissions of certain sources of pollution, as is the case, for example, when the emissions are due to the occupants of the building. These emissions include carbon dioxide and bioeffluents, the presence of materials with properties that are not controlled in any way, or the carrying out of everyday tasks. In these cases one way to reduce the levels of contamination is with ventilation systems and other means used to clean indoor air.
Ventilation is one of the options most heavily relied on to reduce the concentration of pollutants in indoor spaces. However, the need to also save energy requires that the intake of outside air to renew indoor air be as sparing as possible. There are standards in this regard that specify minimum ventilation rates, based on the renewal of the volume of indoor air per hour with outdoor air, or that set a minimum contribution of air per occupant or unit of space, or that take into account the concentration of carbon dioxide considering the differences between spaces with smokers and without smokers. In the case of buildings with natural ventilation, minimum requirements have also been set for different parts of a building, such as windows.
Among the references most often cited by a majority of the existing standards, both national and international—even though it is not legally binding—are the norms published by the American Society of Heating, Refrigerating and Air Conditioning Engineers (ASHRAE). They were formulated to aid air-conditioning professionals in the design of their installations. In ASHRAE Standard 62-1989 (ASHRAE 1989), the minimum amounts of air needed to ventilate a building are specified, as well as the acceptable quality of indoor air required for its occupants in order to prevent adverse health effects. For carbon dioxide (a compound most authors do not consider a pollutant given its human origin, but that is used as an indicator of the quality of indoor air in order to establish the proper functioning of ventilation systems) this standard recommends a limit 1,000 ppm in order to satisfy criteria of comfort (odour). This standard also specifies the quality of outdoor air required for the renewal of indoor air.
In cases where the source of contamination—be it interior or exterior—is not easy to control and where equipment must be used to eliminate it from the environment, there are standards to guarantee their efficacy, such as those that state specific methods to check the performance of a certain type of filter.
Extrapolation from Standards of Occupational Hygiene to Standards of Indoor Air Quality
It is possible to establish different types of reference value that are applicable to indoor air as a function of the type of population that needs to be protected. These values can be based on quality standards for ambient air, on specific values for given pollutants (like carbon dioxide, carbon monoxide, formaldehyde, volatile organic compounds, radon and so on), or they can be based on standards usually employed in occupational hygiene. The latter are values formulated exclusively for applications in industrial environments. They are designed, first of all, to protect workers from the acute effects of pollutants—like irritation of mucous membranes or of the upper respiratory tract—or to prevent poisoning with systemic effects. Because of this possibility, many authors, when they are dealing with indoor environment, use as a reference the limit values of exposure for industrial environments established by the American Conference of Governmental Industrial Hygienists (ACGIH) of the United States. These limits are called threshold limit values (TLVs), and they include limit values for workdays of eight hours and work weeks of 40 hours.
Numerical ratios are applied in order to adapt TLVs to the conditions of the indoor environment of a building, and the values are commonly reduced by a factor of two, ten, or even one hundred, depending on the kind of health effects involved and the type of population affected. Reasons given for reducing the values of TLVs when they are applied to exposures of this kind include the fact that in non-industrial environments personnel are exposed simultaneously to low concentrations of several, normally unknown chemical substances which are capable of acting synergistically in a way that cannot be easily controlled. It is generally accepted, on the other hand, that in industrial environments the number of dangerous substances that need to be controlled is known, and is often limited, even though concentrations are usually much higher.
Moreover, in many countries, industrial situations are monitored in order to secure compliance with the established reference values, something that is not done in non-industrial environments. It is therefore possible that in non-industrial environments, the occasional use of some products can produce high concentrations of one or several compounds, without any environmental monitoring and with no way of revealing the levels of exposure that have occurred. On the other hand, the risks inherent in an industrial activity are known or should be known and, therefore, measures for their reduction or monitoring are in place. The affected workers are informed and have the means to reduce the risk and protect themselves. Moreover, workers in industry are usually adults in good health and in acceptable physical condition, while the population of indoor environments presents, in general, a wider range of health statuses. The normal work in an office, for example, may be done by people with physical limitations or people susceptible to allergic reactions who would be unable to work in certain industrial environments. An extreme case of this line of reasoning would apply to the use of a building as a family dwelling. Finally, as noted above, TLVs, just like other occupational standards, are based on exposures of eight hours a day, 40 hours a week. This represents less than one fourth of the time a person would be exposed if he or she remained continually in the same environment or were exposed to some substance for the entire 168 hours of a week. In addition, the reference values are based on studies that include weekly exposures and that take into account times of non-exposure (between exposures) of 16 hours a day and 64 hours on weekends, which makes it is very hard to make extrapolations on the strength of these data.
The conclusion most authors arrive at is that in order to use the standards for industrial hygiene for indoor air, the reference values must include a very ample margin of error. Therefore, the ASHRAE Standard 62-1989 suggests a concentration of one tenth of the TLV value recommended by the ACGIH for industrial environments for those chemical contaminants which do not have their own established reference values.
Regarding biological contaminants, technical criteria for their evaluation which could be applicable to industrial environments or indoor spaces do not exist, as is the case with the TLVs of the ACGIH for chemical contaminants. This could be due to the nature of biological contaminants, which exhibit a wide variability of characteristics that make it difficult to establish criteria for their evaluation that are generalized and validated for any given situation. These characteristics include the reproductive capacity of the organism in question, the fact that the same microbial species may have varying degrees of pathogenicity or the fact that alterations in environmental factors like temperature and humidity may have an effect upon their presence in any given environment. Nonetheless, in spite of these difficulties, the Bioaerosol Committee of the ACGIH has developed guidelines to evaluate these biological agents in indoor environments: Guidelines for the Assessment of Bioaerosols in the Indoor Environment (1989). The standard protocols that are recommended in these guidelines set sampling systems and strategies, analytical procedures, data interpretation and recommendations for corrective measures. They can be used when medical or clinical information points to the existence of illnesses like humidifier fever, hypersensitivity pneumonitis or allergies related to biological contaminants. These guidelines can be applied when sampling is needed in order to document the relative contribution of the sources of bioaerosols already identified or to validate a medical hypothesis. Sampling should be done in order to confirm potential sources, but routine sampling of air to detect bioaerosols is not recommended.
Existing Guidelines and Standards
Different international organizations such as the World Health Organization (WHO) and the International Council of Building Research (CIBC), private organizations such as ASHRAE and countries like the United States and Canada, among others, are establishing exposure guidelines and standards. For its part, the European Union (EU) through the European Parliament, has presented a resolution on the quality of air in indoor spaces. This resolution establishes the need for the European Commission to propose, as soon as possible, specific directives that include:
- a list of substances to be proscribed or regulated, both in the construction and in the maintenance of buildings
- quality standards that are applicable to the different types of indoor environments
- prescriptions for the consideration, construction, management and maintenance of air-conditioning and ventilation installations
- minimum standards for the maintenance of buildings that are open to the public.
Many chemical compounds have odours and irritating qualities at concentrations that, according to current knowledge, are not dangerous to the occupants of a building but that can be perceived by—and therefore annoy—a large number of people. The reference values in use today tend to cover this possibility.
Given the fact that the use of occupational hygiene standards is not recommended for the control of indoor air unless a correction is factored in, in many cases it is better to consult the reference values used as guidelines or standards for the quality of ambient air. The US Environmental Protection Agency (EPA) has set standards for ambient air intended to protect, with an adequate margin of safety, the health of the population in general (primary standards) and even its welfare (secondary standards) against any adverse effects that may be predicted due to a given pollutant. These reference values are, therefore, useful as a general guide to establish an acceptable standard of air quality for a given indoor space, and some standards like ASHRAE-92 use them as quality criteria for the renewal of air in a closed building. Table 1 shows the reference values for sulphur dioxide, carbon monoxide, nitrogen dioxide, ozone, lead and particulate matter.
Table 1. Standards of air quality established by the US Environmental Protection Agency
|
Average concentration |
|||
|
Pollutant |
μg/m3 |
ppm |
Time frame for exposures |
|
Sulphur dioxide |
80a |
0.03 |
1 year (arithmetic mean) |
|
365a |
0.14 |
24 hoursc |
|
|
1,300b |
0.5 |
3 hoursc |
|
|
Particulate matter |
150a,b |
— |
24 hoursd |
|
50a,b |
— |
1 yeard (arithmetic mean) |
|
|
Carbon monoxide |
10,000a |
9.0 |
8 hoursc |
|
40,000a |
35.0 |
1 hourc |
|
|
Ozone |
235a,b |
0.12 |
1 hour |
|
Nitrogen dioxide |
100a,b |
0.053 |
1 year (arithmetic mean) |
|
Lead |
1.5a,b |
— |
3 months |
a Primary standard. b Secondary standard. c Maximum value that should not be exceeded more than once a year. d Measured as particles of diameter ≤10 μm. Source: US Environmental Protection Agency. National Primary and Secondary Ambient Air Quality Standards. Code of Federal Regulations, Title 40, Part 50 (July 1990).
For its part, WHO has established guidelines intended to provide a baseline to protect public health from adverse effects due to air pollution and to eliminate or reduce to a minimum those air pollutants that are known or suspected of being dangerous for human health and welfare (WHO 1987). These guidelines do not make distinctions as to the type of exposure they are dealing with, and hence they cover exposures due to outdoor air as well as exposures that may occur in indoor spaces. Tables 2 and 3 show the values proposed by WHO (1987) for non-carcinogenic substances, as well as the differences between those that cause health effects and those that cause sensory discomfort.
Table 2. WHO guideline values for some substances in air based on known effects on human health other than cancer or odour annoyance.a
|
Pollutant |
Guideline value (time- |
Duration of exposure |
|
Organic compounds |
||
|
Carbon disulphide |
100 μg/m3 |
24 hours |
|
1,2-Dichloroethane |
0.7 μg/m3 |
24 hours |
|
Formaldehyde |
100 μg/m3 |
30 minutes |
|
Methylene chloride |
3 μg/m3 |
24 hours |
|
Styrene |
800 μg/m3 |
24 hours |
|
Tetrachloroethylene |
5 μg/m3 |
24 hours |
|
Toluene |
8 μg/m3 |
24 hours |
|
Trichloroethylene |
1 μg/m3 |
24 hours |
|
Inorganic compounds |
||
|
Cadmium |
1-5 ng/m3 |
1 year (rural areas) |
|
Carbon monoxide |
100 μg/m3 c |
15 minutes |
|
Hydrogen sulphide |
150 μg/m3 |
24 hours |
|
Lead |
0.5-1.0 μg/m3 |
1 year |
|
Manganese |
1 μg/m3 |
1 hour |
|
Mercury |
1 μg/m3 b |
1 hour |
|
Nitrogen dioxide |
400 μg/m3 |
1 hour |
|
Ozone |
150-200 μg/m3 |
1 hour |
|
Sulphur dioxide |
500 μg/m3 |
10 minutes |
|
Vanadium |
1 μg/m3 |
24 hours |
a Information in this table should be used in conjunction with the rationales provided in the original publication.
b This value refers to indoor air only.
c Exposure to this concentration should not exceed the time indicated and should not be repeated within 8 hours. Source: WHO 1987.
Table 3. WHO guideline values for some non-carcinogenic substances in air, based on sensory effects or annoyance reactions for an average of 30 minutes
|
Pollutant |
Odour threshold |
||
|
Detection |
Recognition |
Guideline value |
|
|
Carbon |
|
|
|
|
Hydrogen |
|
|
|
|
Styrene |
70 μg/m3 |
210-280 μg/m3 |
70 μg/m3 |
|
Tetracholoro- |
|
|
|
|
Toluene |
1 mg/m3 |
10 mg/m3 |
1 mg/m3 |
b In the manufacture of viscose it is accompanied by other odorous substances such as hydrogen sulphide and carbonyl sulphide. Source: WHO 1987.
For carcinogenic substances, the EPA has established the concept of units of risk. These units represent a factor used to calculate the increase in the probability that a human subject will contract cancer due to a lifetime’s exposure to a carcinogenic substance in air at a concentration of 1 μg/m3. This concept is applicable to substances that can be present in indoor air, such as metals like arsenic, chrome VI and nickel; organic compounds like benzene, acrylonitrile and polycyclic aromatic hydrocarbons; or particulate matter, including asbestos.
In the concrete case of radon, Table 20 shows the reference values and the recommendations of different organizations. Thus the EPA recommends a series of gradual interventions when the levels in indoor air rise above 4 pCi/l (150 Bq/m3), establishing the time frames for the reduction of those levels. The EU, based on a report submitted in 1987 by a task force of the International Commission on Radiological Protection (ICRP), recommends an average yearly concentration of radon gas, making a distinction between existing buildings and new construction. For its part, WHO makes its recommendations keeping in mind exposure to radon’s decay products, expressed as a concentration of equilibrium equivalent of radon (EER) and taking into account an increase in the risk of contracting cancer between 0.7 x 10-4 and 2.1 x 10-4 for a lifetime exposure of 1 Bq/m3 EER.
Table 4. Reference values for radon according to three organizations
|
Organization |
Concentration |
Recommendation |
|
Environmental |
4-20 pCi/l |
Reduce the level in years |
|
European Union |
>400 Bq/m3 a,b >400 Bq/m3 a |
Reduce the level Reduce the level |
|
World Health |
>100 Bq/m3 EERc |
Reduce the level |
a Average annual concentration of radon gas.
b Equivalent to a dose of 20 mSv/year.
c Annual average.
Finally, it must be remembered that reference values are established, in general, based on the known effects that individual substances have on health. While this may often represent arduous work in the case of assaying indoor air, it does not take into account the possible synergistic effects of certain substances. These include, for example, volatile organic compounds (VOCs). Some authors have suggested the possibility of defining total levels of concentrations of volatile organic compounds (TVOCs) at which the occupants of a building may begin to react. One of the main difficulties is that, from the point of view of analysis, the definition of TVOCs has not yet been resolved to everyone’s satisfaction.
In practice, the future establishment of reference values in the relatively new field of indoor air quality will be influenced by the development of policies on the environment. This will depend on the advancements of knowledge of the effects of pollutants and on improvements in the analytical techniques that can help us to determine these values.
More...
Control of Indoor Environments: General Principles
People in urban settings spend between 80 and 90% of their time in indoor spaces while carrying out sedentary activities, both during work and during leisure time. (See figure 1).
Figure 1. Urban dwellers spend 80 to 90% of their time indoors
This fact led to the creation within these indoor spaces of environments that were more comfortable and homogeneous than those found outdoors with their changing climatic conditions. To make this possible, the air within these spaces had to be conditioned, being warmed during the cold season and cooled during the hot season.
For air conditioning to be efficient and cost-effective it was necessary to control the air coming into the buildings from the outside, which could not be expected to have the desired thermal characteristics. The result was increasingly airtight buildings and more stringent control of the amount of ambient air that was used to renew stagnant indoor air.
The energy crisis at the beginning of the 1970s—and the resulting need to save energy—represented another state of affairs often responsible for drastic reductions in the volume of ambient air used for renewal and ventilation. What was commonly done then was to recycle the air inside a building many times over. This was done, of course, with the aim of reducing the cost of air-conditioning. But something else began to happen: the number of complaints, discomfort and/or health problems of the occupants of these buildings increased considerably. This, in turn, increased the social and financial costs due to absenteeism and led specialists to study the origin of complaints that, until then, were thought to be independent of pollution.
It is not a complicated matter to explain what led to the appearance of complaints: buildings are built more and more hermetically, the volume of air supplied for ventilation is reduced, more materials and products are used to insulate buildings thermally, the number of chemical products and synthetic materials used multiplies and diversifies and individual control of the environment is gradually lost. The result is an indoor environment that is increasingly contaminated.
The occupants of buildings with degraded environments then react, for the most part, by expressing complaints about aspects of their environment and by presenting clinical symptoms. The symptoms most commonly heard of are the following sort: irritation of mucous membranes (eyes, nose and throat), headaches, shortness of breath, higher incidence of colds, allergies and so on.
When the time comes to define the possible causes that trigger these complaints, the apparent simplicity of the task gives way in fact to a very complex situation as one attempts to establish the relation of cause and effect. In this case one must look at all the factors (whether environmental or of other origins) that may be implicated in the complaints or the health problems that have appeared.
The conclusion—after many years of studying this problem—is that these problems have multiple origins. The exceptions are those cases where the relationship of cause and effect has been clearly established, as in the case of the outbreak of Legionnaires’ disease, for example, or the problems of irritation or of increased sensitivity due to exposure to formaldehyde.
The phenomenon is given the name of sick building syndrome, and is defined as those symptoms affecting the occupants of a building where complaints due to malaise are more frequent than might be reasonably expected.
Table 1 shows some examples of pollutants and the most common sources of emissions that can be associated with a drop in the quality of indoor air.
In addition to indoor air quality, which is affected by chemical and biological pollutants, sick building syndrome is attributed to many other factors. Some are physical, such as heat, noise and illumination; some are psychosocial, chief among them the way work is organized, labour relations, the pace of work and the workload.
Table 1. The most common indoor pollutants and their sources
|
Site |
Sources of emission |
Pollutant |
|
Outdoors |
Fixed sources |
|
|
Industrial sites, energy production |
Sulphur dioxide, nitrogen oxides, ozone, particulate matter, carbon monoxide, organic compounds |
|
|
Motor vehicles |
Carbon monoxide, lead, nitrogen oxides |
|
|
Soil |
Radon, microorganisms |
|
|
Indoors |
Construction materials |
|
|
Stone, concrete |
Radon |
|
|
Wood composites, veneer |
Formaldehyde, organic compounds |
|
|
Insulation |
Formaldehyde, fiberglass |
|
|
Fire retardants |
Asbestos |
|
|
Paint |
Organic compounds, lead |
|
|
Equipment and installations |
||
|
Heating systems, kitchens |
Carbon monoxide and dioxide, nitrogen oxides, organic compounds, particulate matter |
|
|
Photocopiers |
Ozone |
|
|
Ventilation systems |
Fibres, microorganisms |
|
|
Occupants |
||
|
Metabolic activity |
Carbon dioxide, water vapour, odours |
|
|
Biological activity |
Microorganisms |
|
|
Human activity |
||
|
Smoking |
Carbon monoxide, other compounds, particulate matter |
|
|
Air fresheners |
Fluorocarbons, odours |
|
|
Cleaning |
Organic compounds, odours |
|
|
Leisure, artistic activities |
Organic compounds, odours |
Indoor air plays a very important role in sick building syndrome, and controlling its quality can therefore help, in most cases, to rectify or help improve conditions that lead to the appearance of the syndrome. It should be remembered, however, that air quality is not the only factor that should be considered in evaluating indoor environments.
Measures for the Control of Indoor Environments
Experience shows that most of the problems that occur in indoor environments are the result of decisions made during the design and construction of a building. Although these problems can be solved later by taking corrective measures, it should be pointed out that preventing and correcting deficiencies during the design of the building is more effective and cost-efficient.
The great variety of possible sources of pollution determines the multiplicity of corrective actions that can be taken to bring them under control. The design of a building may involve professionals from various fields, such as architects, engineers, interior designers and others. It is therefore important at this stage to keep in mind the different factors that can contribute to eliminate or minimize the possible future problems that may arise because of poor air quality. The factors that should be considered are
- selection of the site
- architectural design
- selection of materials
- ventilation and air conditioning systems used to control the quality of indoor air.
Selecting a building site
Air pollution may originate at sources that are close to or far from the chosen site. This type of pollution includes, for the most part, organic and inorganic gases that result from combustion—whether from motor vehicles, industrial plants, or electrical plants near the site—and airborne particulate matter of various origins.
Pollution found in the soil includes gaseous compounds from buried organic matter and radon. These contaminants can penetrate into the building through cracks in the building materials that are in contact with the soil or by migration through semi-permeable materials.
When the construction of a building is in the planning stages, the different possible sites should be evaluated. The best site should be chosen, taking these facts and information into consideration:
- Data that show the levels of environmental pollution in the area, to avoid distant sources of pollution.
- Analysis of adjacent or nearby sources of pollution, taking into account such factors as the amount of vehicular traffic and possible sources of industrial, commercial or agricultural pollution.
- The levels of pollution in soil and water, including volatile or semivolatile organic compounds, radon gas and other radioactive compounds that result from the disintegration of radon. This information is useful if a decision must be made to change the site or to take measures to mitigate the presence of these contaminants within the future building. Among the measures that can be taken are the effective sealing of the channels of penetration or the design of general ventilation systems that will insure a positive pressure within the future building.
- Information on the climate and predominant wind direction in the area, as well as daily and seasonal variations. These conditions are important in order to decide the proper orientation of the building.
On the other hand, local sources of pollution must be controlled using various specific techniques, such as draining or cleaning the soil, depressurizing the soil or using architectural or scenic baffles.
Architectural design
The integrity of a building has been, for centuries, a fundamental injunction at the time of planning and designing a new building. To this end consideration has been given, today as in the past, to the capacities of materials to withstand degradation by humidity, temperature changes, air movement, radiation, the attack of chemical and biological agents or natural disasters.
The fact that the above-mentioned factors should be considered when undertaking any architectural project is not an issue in the current context: in addition, the project must implement the right decisions with regard to the integrity and well-being of the occupants. During this phase of the project decisions must be made about such concerns as the design of interior spaces, the selection of materials, the location of activities that could be potential sources of pollution, the openings of the building to the outside, the windows and the ventilation system.
Building openings
Effective measures of control during the design of the building consist of planning the location and orientation of these openings with an eye to minimizing the amount of contamination that can enter the building from previously detected sources of pollution. The following considerations should be kept in mind:
- Openings should be far from sources of pollution and not in the predominant direction of the wind. When openings are close to sources of smoke or exhaust, the ventilation system should be planned to produce positive air pressure in that area in order to avoid the re-entry of vented air, as shown in figure 2.
- Special attention should be given to guarantee drainage and to prevent seepage where the building comes in contact with the soil, into the foundation, in areas that are tiled, where the drainage system and conduits are located, and other sites.
- Access to loading docks and garages should be built far from the normal air intake sites of the building as well as from the main entrances.
Figure 2. Penetration of pollution from the outside
Windows
During recent years there has been a reversal of the trend seen in the 1970s and the 1980s, and now there is a tendency to include working windows in new architectural projects. This confers several advantages. One of them is the ability to provide supplementary ventilation in those areas (few in number, it is hoped) that need it, assuming that the ventilation system has sensors in those areas to prevent imbalances. It should be kept in mind that the ability to open a window does not always guarantee that fresh air will enter a building; if the ventilation system is pressurized, opening a window will not provide extra ventilation. Other advantages are of a definitely psychosocial character, allowing the occupants a certain degree of individual control over their surroundings and direct and visual access to the outdoors.
Protection against humidity
The principal means of control consist of reducing humidity in the foundations of the building, where micro-organisms, especially fungi, can frequently spread and develop.
Dehumidifying the area and pressurizing the soil can prevent the appearance of biological agents and can also prevent the penetration of chemical pollutants that may be present in the soil.
Sealing and controlling the enclosed areas of the building most susceptible to humidity in the air is another measure that should be considered, since humidity can damage the materials used to clad the building, with the result that these materials may then become a source of microbiological contamination.
Planning of indoor spaces
It is important to know during the planning stages the use to which the building will be put or the activities that will be carried out within it. It is important above all to know which activities may be a source of contamination; this knowledge can then be used to limit and control these potential sources of pollution. Some examples of activities that may be sources of contamination within a building are the preparation of food, printing and graphic arts, smoking and the use of photocopying machines.
The location of these activities in specific locales, separate and insulated from other activities, should be decided in such a way that occupants of the building are affected as little as possible.
It is advisable that these processes be provided with a localized extraction system and/or general ventilation systems with special characteristics. The first of these measures is intended to control contaminants at the source of emission. The second, applicable when there are numerous sources, when they are dispersed within a given space, or when the pollutant is extremely dangerous, should comply with the following requirements: it should be capable of providing volumes of new air which are adequate given the established standards for the activity in question, it should not reuse any of the air by mixing it with the general flow of ventilation in the building and it should include supplementary forced-air extraction where needed. In such cases the flow of air in these locales should be carefully planned, to avoid transferring pollutants between contiguous spaces—by creating, for example, negative pressure in a given space.
Sometimes control is achieved by eliminating or reducing the presence of pollutants in the air by filtration or by cleaning the air chemically. In using these control techniques, the physical and chemical characteristics of the pollutants should be kept in mind. Filtration systems, for instance, are adequate for the removal of particulate matter from the air—so long as the efficiency of the filter is matched to the size of the particles that are being filtered—but allow gases and vapours to pass through.
The elimination of the source of pollution is the most effective way to control pollution in indoor spaces. A good example that illustrates the point are the restrictions and prohibitions against smoking in the workplace. Where smoking is permitted, it is generally restricted to special areas that are equipped with special ventilation systems.
Selection of materials
In trying to prevent possible pollution problems within a building, attention should be given to the characteristics of the materials used for construction and decoration, to the furnishings, the normal work activities that will be performed, the way the building will be cleaned and disinfected and the way insects and other pests will be controlled. It is also possible to reduce the levels of volatile organic compounds (VOCs), for example, by considering only materials and furniture that have known rates of emission for these compounds and selecting those with the lowest levels.
Today, even though some laboratories and institutions have carried out studies on emissions of this kind, the information available on the rates of emission of contaminants for construction materials is scarce; this scarcity is moreover aggravated by the vast number of products available and the variability they exhibit over time.
In spite of this difficulty, some producers have begun to study their products and to include, usually at the request of the consumer or the construction professional, information on the research that has been done. Products are more and more frequently labelled environmentally safe, non-toxic and so on.
There are still many problems to overcome, however. Examples of these problems include the high cost of the necessary analyses both in time and money; the lack of standards for the methods used to assay the samples; the complicated interpretation of results obtained due to lack of knowledge of the health effects of some contaminants; and the lack of agreement among researchers on whether materials with high levels of emission that emit for a short period of time are preferable to materials with low levels of emission that emit over longer periods of time.
But the fact is that in coming years the market for construction and decoration materials will become more competitive and will come under more legislative pressure. This will result in the elimination of some products or their substitution with other products that have lower rates of emission. Measures of this sort are already being taken with the adhesives used in the production of moquette fabric for upholstery and are further exemplified by the elimination of dangerous compounds such as mercury and pentachlorophenol in the production of paint.
Until more is known and legislative regulation in this field matures, decisions as to the selection of the most appropriate materials and products to use or install in new buildings will be left to the professionals. Outlined here are some considerations that can help them arrive at a decision:
- Information should be available on the chemical composition of the product and the emission rates of any pollutants, as well as any information regarding the health, safety and comfort of occupants exposed to them. This information should be provided by the manufacturer of the product.
- Products should be selected which have the lowest rates of emission possible of any contaminants, giving special attention to the presence of carcinogenic and teratogenic compounds, irritants, systemic toxins, odoriferous compounds and so on. Adhesives or materials that present large emission or absorption surfaces, such as porous materials, textiles, uncoated fibres and the like, should be specified and their use restricted.
- Preventive procedures should be implemented for the handling and installation of these materials and products. During and after the installation of these materials the space should be exhaustively ventilated and the bake out process (see below) should be used to cure certain products. The recommended hygienic measures should also be applied.
- One of the procedures recommended to minimize exposure to emissions of new materials during the installation and finishing stages, as well as during the initial occupation of the building, is to ventilate the building for 24 hours with 100 per cent outside air. The elimination of organic compounds by the use of this technique prevents the retention of these compounds in porous materials. These porous materials may act as reservoirs and later sources of pollution as they release the stored compounds into the environment.
- Incrementing ventilation to the maximum possible level before reoccupying a building after it has been closed for a period—during the first hours of the day—and after weekends or vacation shut-downs is also a convenient measure that can be implemented.
- A special procedure, known as bake out, has been used in some buildings to “cure” new materials. The bake out procedure consists in elevating the temperature of a building for 48 hours or more, keeping air flow at a minimum. The high temperatures favour the emission of volatile organic compounds. The building is then ventilated and its pollution load is thereby reduced. The results obtained so far show that this procedure can be effective in some situations.
Ventilation systems and the control of indoor climates
In enclosed spaces, ventilation is one of the most important methods for the control of air quality. There are so many sources of pollution in these spaces, and the characteristics of these pollutants are so varied, that it is almost impossible to manage them completely in the design stage. The pollution generated by the very occupants of the building—by the activities they engage in and the products they use for personal hygiene—are a case in point; in general, these sources of contamination are beyond the control of the designer.
Ventilation is, therefore, the method of control normally used to dilute and eliminate contaminants from polluted indoor spaces; it may be carried out with clean outdoor air or recycled air that is conveniently purified.
Many different points need to be considered in designing a ventilation system if it is to serve as an adequate pollution control method. Among them are the quality of outside air that will be used; the special requirements of certain pollutants or of their generating source; the preventive maintenance of the ventilation system itself, which should also be considered a possible source of contamination; and the distribution of air inside the building.
Table 2 summarizes the main points that should be considered in the design of a ventilation system for the maintenance of quality indoor environments.
In a typical ventilation/air conditioning system, air that has been taken from outside and that has been mixed with a variable portion of recycled air passes through different air conditioning systems, is usually filtered, is heated or cooled according to the season and is humidified or dehumidified as needed.
Table 2. Basic requirements for a ventilation system by dilution
|
System component |
Requirement |
|
Dilution by outside air |
A minimum volume of air by occupant per hour should be guaranteed. |
|
The aim should be to renew the volume of inside air a minimum number of times per hour. |
|
|
The volume of outside air supplied should be increased based on the intensity of the sources of pollution. |
|
|
Direct extraction to the outside should be guaranteed for spaces where pollution-generating activities will take place. |
|
|
Air intake locations |
Placing air intakes near plumes of known sources of pollution should be avoided. |
|
One should avoid areas near stagnant water and the aerosols that emanate from refrigeration towers. |
|
|
The entry of any animals should be prevented and birds should be prevented from perching or nesting near intakes. |
|
|
Location of air extraction |
Extraction vents should be placed as far as possible from air intake locations and the height of the discharge vent should be increased. |
|
Orientation of discharge vents should be in the opposite direction from air intake hoods. |
|
|
Filtration and cleaning |
Mechanical and electrical filters for particulate matter should be used. |
|
One should install a system for the chemical elimination of pollutants. |
|
|
Microbiological control |
Placing any porous materials in direct contact with air currents, including those in the distribution conduits, should be avoided. |
|
One should avoid the collection of stagnant water where condensation is formed in air-conditioning units. |
|
|
A preventive maintenance programme should be established and the periodic cleaning of humidifiers and refrigeration towers should be scheduled. |
|
|
Air distribution |
One should eliminate and prevent the formation of any dead zones (where there is no ventilation) and the stratification of air. |
|
It is preferable to mix the air where the occupants breathe it. |
|
|
Adequate pressures should be maintained in all locales based on the activities that are performed in them. |
|
|
Air propulsion and extraction systems should be controlled to maintain equilibrium between them. |
Once treated, air is distributed by conduits to every area of the building and is delivered through dispersion gratings. It then mixes throughout the occupied spaces exchanging heat and renewing the indoor atmosphere before it is at last drawn away from each locale by return ducts.
The amount of outside air that should be used to dilute and to eliminate pollutants is the subject of much study and controversy. In recent years there have been changes in the recommended levels of outside air and in the published ventilation standards, in most cases involving increases in the volumes of outside air used. In spite of this, it has been noted that these recommendations are insufficient to control effectively all the sources of pollution. This is because the established standards are based on occupancy and disregard other important sources of pollution, such as the materials employed in construction, the furnishings and the quality of the air taken from the outside.
Therefore, the amount of ventilation required should be based on three fundamental considerations: the quality of air that you wish to obtain, the quality of outside air available and the total load of pollution in the space that will be ventilated. This is the starting point of the studies that have been carried out by professor PO Fanger and his team (Fanger 1988, 1989). These studies are geared to establishing new ventilation standards that meet air quality requirements and that provide an acceptable level of comfort as perceived by the occupants.
One of the factors that affects the quality of air in inside spaces is the quality of outside air available. The characteristics of exterior sources of pollution, like vehicular traffic and industrial or agricultural activities, put their control beyond the reach of the designers, the owners and the occupants of the building. It is in cases of this sort that the environmental authorities must assume the responsibility for establishing environmental protection guidelines and of making sure that they are adhered to. There are, however, many control measures that can be applied and that are useful in the reduction and the elimination of airborne pollution.
As was mentioned above, special care should be given to the location and orientation of air intake and exhaust ducts, in order to avoid drawing pollution back in from the building itself or from its installations (refrigeration towers, kitchen and bathroom vents, etc.), as well as from buildings in the immediate vicinity.
When outside air or recycled air is found to be polluted, the recommended control measures consist of filtering it and cleaning it. The most effective method of removing particulate matter is with electrostatic precipitators and mechanical retention filters. The latter will be most effective the more precisely they are calibrated to the size of the particles to be eliminated.
The use of systems capable of eliminating gases and vapours through chemical absorption and/or adsorption is a technique rarely used in nonindustrial situations; however, it is common to find systems that mask the pollution problem, especially smells for example, by the use of air fresheners.
Other techniques to clean and improve the quality of air consist of using ionizers and ozonizers. Prudence would be the best policy on the use of these systems to achieve improvements in air quality until their real properties and their possible negative health effects are clearly known.
Once air has been treated and cooled or heated it is delivered to indoor spaces. Whether the distribution of air is acceptable or not will depend, in great measure, on the selection, the number and the placement of diffusion grates.
Given the differences of opinion on the effectiveness of the different procedures that should be followed to mix air, some designers have begun to use, in some situations, air distribution systems that deliver air at floor level or on the walls as an alternative to diffusion grates on the ceiling. In any case, the location of the return registers should be carefully planned to avoid short-circuiting the entry and exit of air, which would prevent it from mixing completely as shown in figure 3.
Figure 3. Example of how air distribution can be shortcircuited in indoor spaces
Depending on how compartmentalized workspaces are, air distribution may present a variety of different problems. For example, in open workspaces where diffusion grates are on the ceiling, air in the room may not mix completely. This problem tends to be compounded when the type of ventilation system used can supply variable volumes of air. The distribution conduits of these systems are equipped with terminals that modify the amount of air supplied to the conduits based on the data received from area thermostats.
A difficulty can develop when air flows at a reduced rate through a significant number of these terminals—a situation that arises when the thermostats of different areas reach the desired temperature—and the power to the fans that push the air is automatically reduced. The result is that the total flow of air through the system is less, in some cases much less, or even that the immission of new outside air is interrupted altogether. Placing sensors that control the flow of outside air at the intake of the system can insure that a minimum flow of new air is maintained at all times.
Another problem that regularly emerges is that air flow is blocked due to the placement of partial or total partitions in the workspace. There are many ways to correct this situation. One way is to leave an open space at the lower end of the panels that divide the cubicles. Other ways include the installation of supplementary fans and the placement of the diffusion grilles on the floor. The use of supplementary induction fan coils aid in mixing the air and allow individualized control of the thermal conditions of the given space. Without detracting from the importance of air quality per se and the means to control it, it should be kept in mind that a comfortable indoor environment is attained by the equilibrium of the different elements that affect it. Taking any action—even positive action—affecting one of the elements without regard to the rest may affect the equilibrium among them, leading to new complaints from the occupants of the building. Tables 3 and 4 show how some of these actions, intended to improve the quality of indoor air, lead to the failure of other elements in the equation, so that adjusting the working environment may have repercussions on the quality of indoor air.
Table 3. Indoor air quality control measures and their effects on indoor environments
|
Action |
Effect |
|
Thermal environment |
|
|
Increase in volume of fresh air |
Increase in draughts |
|
Reduction of relative humidity to check microbiological agents |
Insufficient relative humidity |
|
Acoustic environment |
|
|
Intermittent supplying of outside air to conserve |
Intermittent noise exposure |
|
Visual environment |
|
|
Reduction in the use of fluorescent lights to reduce |
Reduction in the effectiveness of the illumination |
|
Psychosocial environment |
|
|
Open offices |
Loss of intimacy and of a defined workspace |
Table 4. Adjustments of the working environment and their effects on indoor air quality
|
Action |
Effect |
|
Thermal environment |
|
|
Basing the supply of outside air on thermal |
Insufficient volumes of fresh air |
|
The use of humidifiers |
Potential microbiological hazard |
|
Acoustic environment |
|
|
Increase in the use of insulating materials |
Possible release of pollutants |
|
Visual environment |
|
|
Systems based solely on artificial illumination |
Dissatisfaction, plant mortality, growth of microbiological agents |
|
Psychosocial environment |
|
|
Using equipment in the workspace, such as photocopiers and printer |
Increase in the level of pollution |
Insuring the quality of the overall environment of a building when it is in the design stages depends, to a great extent, on its management, but above all on a positive attitude towards the occupants of that building. The occupants are the best sensors the owners of the building can rely on in order to gauge the proper functioning of the installations intended to provide a quality indoor environment.
Control systems based on a “Big Brother” approach, making all the decisions regulating interior environments such as lighting, temperature, ventilation, and so on, tend to have a negative effect on the psychological and sociological well-being of the occupants. Occupants then see their capacity to create environmental conditions that meet their needs diminished or blocked. In addition, control systems of this type are sometimes incapable of changing to meet the different environmental requirements that may arise due to changes in the activities performed in a given space, the number of people working in it or changes in the way space is allocated.
The solution could consist of installing a system of centralized control for the indoor environment, with localized controls regulated by the occupants. This idea, very commonly used in the realm of the visual environment where general illumination is supplemented by more localized illumination, should be expanded to other concerns: general and localized heating and air-conditioning, general and localized supplies of fresh air and so on.
To sum up, it can be said that in each instance a portion of the environmental conditions should be optimized by means of a centralized control based on safety, health and economic considerations, while the different local environmental conditions should be optimized by the users of the space. Different users will have different needs and will react differently to given conditions. A compromise of this sort between the different parts will doubtless lead to greater satisfaction, well-being and productivity.
Indoor Air: Methods for Control and Cleaning
The quality of air inside a building is due to a series of factors that include the quality of outside air, the design of the ventilation/airconditioning system, the way that the system works and is maintained and the sources of indoor pollution. In general terms, the level of concentration of any contaminant in an indoor space will be determined by the balance between the generation of the pollutant and the rate of its elimination.
As for the generation of contaminants, the sources of pollution may also be external or internal. The external sources include atmospheric pollution due to industrial combustion processes, vehicular traffic, power plants and so on; pollution emitted near the intake shafts where air is drawn into the building, such as that from refrigeration towers or the exhaust vents of other buildings; and emanations from contaminated soil such as radon gas, leaks from gasoline tanks or pesticides.
Among the sources of internal pollution, it is worth mentioning those associated with the ventilation and air-conditioning systems themselves (chiefly the microbiological contamination of any segment of such systems), the materials used to build and decorate the building, and the occupants of the building. Specific sources of indoor pollution are tobacco smoke, laboratories, photocopiers, photographic labs and printing presses, gyms, beauty parlours, kitchens and cafeterias, bathrooms, parking garages and boiler rooms. All these sources should have a general ventilation system and air extracted from these areas should not be recycled through the building. When the situation warrants it, these areas should also have a localized ventilation system that operates by extraction.
Evaluating the quality of indoor air comprises, among other tasks, the measurement and evaluation of contaminants that may be present in the building. Several indicators are used to ascertain the quality of air inside a building. They include the concentrations of carbon monoxide and carbon dioxide, total volatile organic compounds (TVOC), total suspended particles (TSP) and the rate of ventilation. Various criteria or recommended target values exist for the evaluation of some of the substances found in interior spaces. These are listed in different standards or guidelines, such as the guidelines for the quality of interior air promulgated by the World Health Organization (WHO), or the standards of the American Society of Heating, Refrigerating and Air Conditioning Engineers (ASHRAE).
For many of these substances, however, there are no defined standards. For now the recommended course of action is to apply the values and standards for industrial environments provided by the American Conference of Governmental Industrial Hygienists (ACGIH 1992). Safety or correction factors are then applied on the order of one-half, one-tenth or one-hundredth of the values specified.
The methods of control of indoor air can be divided in two main groups: control of the source of pollution, or control of the environment with ventilation and air cleaning strategies.
Control of the Source of Pollution
The source of pollution can be controlled by various means, including the following:
- Elimination. Eliminating the source of pollution is the ideal method for the control of indoor air quality. This measure is permanent and requires no future maintenance operations. It is applied when the source of pollution is known, as in the case of tobacco smoke, and it requires no substitution for polluting agents.
- Substitution. In some cases, substitution of the product that is the source of contamination is the measure that should be used. Changing the kind of products used (for cleaning, decoration, etc.) with others that provide the same service but are less toxic or present less risk to the people who use them is sometimes possible.
- Isolation or spatial confinement. These measures are designed to reduce exposure by limiting access to the source. The method consists in interposing barriers (partial or total) or containments around the source of pollution to minimize emissions to the surrounding air and to limit the access of people to the area near the source of pollution. These spaces should be equipped with supplementary ventilation systems that can extract air and provide a directed flow of air where needed. Examples of this approach are closed ovens, boiler rooms and photocopying rooms.
- Sealing the source. This method consists of using materials that emit minimal levels of pollution or that emit none at all. This system has been suggested as a way to inhibit the dispersal of loose asbestos fibres from old insulation, as well as to inhibit the emission of formaldehyde from walls treated with resins. In buildings contaminated with radon gas, this technique is used to seal cinder blocks and crevices in basement walls: polymers are used that prevent the immission of radon from the soil. Basement walls may also be treated with epoxy paint and a polymeric sealant of polyethylene or polyamide to prevent contamination that may seep in through walls or from the soil.
- Ventilation by localized extraction. Localized ventilation systems are based on the capture of the pollutant at, or as close as possible to, the source. The capture is accomplished by a bell designed to trap the pollutant in a current of air. The air then flows by conduits with the help of a fan to be purified. If the extracted air cannot be purified or filtered, then it should be vented outside and should not be recycled back into the building.
Control of the Environment
The indoor environments of nonindustrial buildings usually have many sources of pollution and, in addition, they tend to be scattered. The system most commonly employed to correct or prevent pollution problems indoors, therefore, is ventilation, either general or by dilution. This method consists of moving and directing the flow of air to capture, contain and transport pollutants from their source to the ventilation system. In addition, general ventilation also permits the control of the thermal characteristics of the indoor environment by air conditioning and recirculating air (see “Aims and principles of general and dilution ventilation”, elsewhere in this chapter).
In order to dilute internal pollution, increasing the volume of outside air is advisable only when the system is of the proper size and does not cause a lack of ventilation in other parts of the system or when the added volume does not prevent proper air-conditioning. For a ventilation system to be as effective as possible, localized extractors should be installed at the sources of pollution; air mixed with pollution should not be recycled; occupants should be placed near air diffusion vents and sources of pollution near extraction vents; pollutants should be expelled by the shortest possible route; and spaces that have localized sources of pollution should be kept at negative pressure relative to outside atmospheric pressure.
Most ventilation deficiencies seem to be linked to an inadequate amount of outside air. An improper distribution of ventilated air, however, can also result in poor air quality problems. In rooms with very high ceilings, for instance, where warm (less dense) air is supplied from above, air temperature may become stratified and ventilation will then fail to dilute the pollution present in the room. The placement and location of air diffusion vents and air return vents relative to the occupants and the sources of contamination is a consideration that requires special attention when the ventilation system is being designed.
Air Cleaning Techniques
Air cleaning methods should be precisely designed and selected for specific, very concrete types of pollutants. Once installed, regular maintenance will prevent the system from becoming a new source of contamination. The following are descriptions of six methods used to eliminate pollutants from air.
Filtration of particles
Filtration is a useful method to eliminate liquids or solids in suspension, but it should be borne in mind that it does not eliminate gases or vapours. Filters may capture particles by obstruction, impact, interception, diffusion and electrostatic attraction. Filtration of an indoor air conditioning system is necessary for many reasons. One is to prevent the accumulation of dirt that may cause a diminution of its heating or cooling efficiency. The system may also be corroded by certain particles (sulphuric acid and chlorides). Filtration is also necessary to prevent a loss of equilibrium in the ventilation system due to deposits on the fan blades and false information being fed to the controls because of clogged sensors.
Indoor air filtration systems benefit from placing at least two filters in series. The first, a pre-filter or primary filter, retains only the larger particles. This filter should be changed often and will lengthen the life of the next filter. The secondary filter is more efficient than the first, and can filter out fungal spores, synthetic fibres and in general finer dust than that collected by the primary filter. These filters should be fine enough to eliminate irritants and toxic particles.
A filter is selected based on its effectiveness, its capacity to accumulate dust, its loss of charge and the required level of air purity. A filter’s effectiveness is measured according to ASHRAE 52-76 and Eurovent 4/5 standards (ASHRAE 1992; CEN 1979). Their capacity for retention measures the mass of dust retained multiplied by the volume of air filtered and is used to characterize filters that retain only large particles (low and medium efficiency filters). To measure its retention capacity, a synthetic aerosol dust of known concentration and granulometry is forced through a filter. the portion retained in the filter is calculated by gravimetry.
The efficiency of a filter is expressed by multiplying the number of particles retained by the volume of air filtered. This value is the one used to characterize filters that also retain finer particles. To calculate the efficiency of a filter, a current of atmospheric aerosol is forced through it containing an aerosol of particles with a diameter between 0.5 and 1 μm. The amount of captured particles is measured with an opacitimeter, which measures the opacity caused by the sediment.
The DOP is a value used to characterize very high-efficiency particulate air (HEPA) filters. The DOP of a filter is calculated with an aerosol made by vapourizing and condensing dioctylphthalate, which produces particles 0.3 μm in diameter. This method is based on the light-scattering property of drops of dioctylphthalate: if we put the filter through this test the intensity of scattered light is proportional to the surface concentration of this material and the penetration of the filter can be measured by the relative intensity of scattered light before and after filtering the aerosol. For a filter to earn the HEPA designation it must be better than 99.97 per cent efficient on the basis of this test.
Although there is a direct relationship between them, the results of the three methods are not directly comparable. The efficiency of all filters diminishes as they clog up, and they can then become a source of odours and contamination. The useful life of a high efficiency filter can be greatly extended by using one or several filters of a lower rating in front of the high efficiency filter. Table 1 shows the initial, final and mean yields of different filters according to criteria established by ASHRAE 52-76 for particles 0.3 μm in diameter.
Table 1. The effectiveness of filters (according to ASHRAE standard 52-76) for particles of 3 mm diameter
|
Filter description |
ASHRAE 52-76 |
Efficiency (%) |
|||
|
Dust spot (%) |
Arrestance (%) |
Initial |
Final |
Median |
|
|
Medium |
25–30 |
92 |
1 |
25 |
15 |
|
Medium |
40–45 |
96 |
5 |
55 |
34 |
|
High |
60–65 |
97 |
19 |
70 |
50 |
|
High |
80–85 |
98 |
50 |
86 |
68 |
|
High |
90–95 |
99 |
75 |
99 |
87 |
|
95% HEPA |
— |
— |
95 |
99.5 |
99.1 |
|
99.97% HEPA |
— |
— |
99.97 |
99.7 |
99.97 |
Electrostatic precipitation
This method proves useful for controlling particulate matter. Equipment of this sort works by ionizing particles and then eliminating them from the air current as they are attracted to and captured by a collecting electrode. Ionization occurs when the contaminated effluent passes through the electrical field generated by a strong voltage applied between the collecting and the discharge electrodes. The voltage is obtained by a direct current generator. The collecting electrode has a large surface and is usually positively charged, while the discharge electrode consists of a negatively charged cable.
The most important factors that affect the ionization of particles are the condition of the effluent, its discharge and the characteristics of the particles (size, concentration, resistance, etc.). The effectiveness of capture increases with humidity, and the size and density of the particles, and decreases with the increased viscosity of the effluent.
The main advantage of these devices is that they are highly effective at collecting solids and liquids, even when particle size is very fine. In addition, these systems may be used for heavy volumes and high temperatures. The loss of pressure is minimal. The drawbacks of these systems are their high initial cost, their large space requirements and the safety risks they pose given the very high voltages involved, especially when they are used for industrial applications.
Electrostatic precipitators are used in a full range, from industrial settings to reduce the emission of particles to domestic settings to improve the quality of indoor air. The latter are smaller devices that operate at voltages in the range of 10,000 to 15,000 volts. They ordinarily have systems with automatic voltage regulators which ensure that enough tension is always applied to produce ionization without causing a discharge between both electrodes.
Generation of negative ions
This method is used to eliminate particles suspended in air and, in the opinion of some authors, to create healthier environments. The efficacy of this method as a way to reduce discomfort or illness is still being studied.
Gas adsorption
This method is used to eliminate polluting gases and vapours like formaldehyde, sulphur dioxide, ozone, nitrogen oxides and organic vapours. Adsorption is a physical phenomena by which gas molecules are trapped by an adsorbent solid. The adsorbent consists of a porous solid with a very large surface area. To clean this kind of pollutant from the air, it is made to flow through a cartridge full of the adsorbent. Activated carbon is the most widely used; it traps a wide range of inorganic gases and organic compounds. Aliphatic, chlorinated and aromatic hydrocarbons, ketones, alcohols and esters are some examples.
Silica gel is also an inorganic adsorbent, and is used to trap more polar compounds such as amines and water. There are also other, organic adsorbents made up of porous polymers. It is important to keep in mind that all adsorbent solids trap only a certain amount of pollutant and then, once saturated, need to be regenerated or replaced. Another method of capture through adsorbent solids is to use a mixture of active alumina and carbon impregnated with specific reactants. Some metallic oxides, for instance, capture mercury vapour, hydrogen sulphide and ethylene. It must be borne in mind that carbon dioxide is not retained by adsorption.
Gas absorption
Eliminating gases and fumes by absorption involves a system that fixes molecules by passing them through an absorbent solution with which they react chemically. This is a very selective method and it uses reagents specific to the pollutant that needs to be captured.
The reagent is generally dissolved in water. It also must be replaced or regenerated before it is used up. Because this system is based on transferring the pollutant from the gaseous phase to the liquid phase, the reagent’s physical and chemical properties are very important. Its solubility and reactivity are especially important; other aspects that play an important part in this transfer from gaseous to liquid phase are pH, temperature and the area of contact between gas and liquid. Where the pollutant is highly soluble, it is sufficient to bubble it through the solution to fix it to the reagent. Where the pollutant is not as readily soluble the system that must be employed must ensure a greater area of contact between gas and liquid. Some examples of absorbents and the contaminants for which they are especially suited are given in table 2.
Table 2. Reagents used as absorbents for various contaminants
|
Absorbent |
Contaminant |
|
Diethylhydroxamine |
Hydrogen sulphide |
|
Potassium permangenate |
Odiferous gases |
|
Hydrochloric and sulphuric acids |
Amines |
|
Sodium sulphide |
Aldehydes |
|
Sodium hydroxide |
Formaldehyde |
Ozonization
This method of improving the quality of indoor air is based on the use of ozone gas. Ozone is generated from oxygen gas by ultraviolet radiation or electric discharge, and employed to eliminate contaminants dispersed in air. The great oxidizing power of this gas makes it suitable for use as an antimicrobial agent, a deodorant and a disinfectant and it can help to eliminate noxious gases and fumes. It is also employed to purify spaces with high concentrations of carbon monoxide. In industrial settings it is used to treat the air in kitchens, cafeterias, food and fish processing plants, chemical plants, residual sewage treatment plants, rubber plants, refrigeration plants and so on. In office spaces it is used with air conditioning installations to improve the quality of indoor air.
Ozone is a bluish gas with a characteristic penetrating smell. At high concentrations it is toxic and even fatal to man. Ozone is formed by the action of ultraviolet radiation or an electric discharge on oxygen. The intentional, accidental and natural production of ozone should be differentiated. Ozone is an extremely toxic and irritating gas both at short-term and long-term exposure. Because of the way it reacts in the body, no levels are known for which there are no biological effects. These data are discussed more fully in the chemicals section of this Encyclopaedia.
Processes that employ ozone should be carried out in enclosed spaces or have a localized extraction system to capture any release of gas at the source. Ozone cylinders should be stored in refrigerated areas, away from any reducing agents, inflammable materials or products that may catalyze its breakdown. It should be kept in mind that if ozonizers function at negative pressures, and have automatic shut-off devices in case of failure, the possibility of leaks is minimized.
Electrical equipment for processes that employ ozone should be perfectly insulated and maintenance on them should be done by experienced personnel. When using ozonizers, conduits and accessory equipment should have devices that shut ozonizers down immediately when a leak is detected; in case of a loss of efficiency in the ventilation, dehumidifying or refrigeration functions; when there occurs an excess of pressure or a vacuum (depending on the system); or when the output of the system is either excessive or insufficient.
When ozonizers are installed, they should be provided with ozone specific detectors. The sense of smell cannot be trusted because it can become saturated. Ozone leaks can be detected with reactive strips of potassium iodide that turn blue, but this is not a specific method because the test is positive for most oxidants. It is better to monitor for leaks on a continuing basis using electrochemical cells, ultraviolet photometry or chemiluminesence, with the chosen detection device connected directly to an alarm system that acts when certain concentrations are reached.
Aims and Principles of General and Dilution Ventilation
When pollutants generated at a worksite are to be controlled by ventilating the entire locale we speak of general ventilation. The use of general ventilation implies accepting the fact that the pollutant will be distributed to some degree through the entire space of the worksite, and could therefore affect workers who are far from the source of contamination. General ventilation is, therefore, a strategy that is the opposite of localized extraction. Localized extraction seeks to eliminate the pollutant by intercepting it as closely as possible to the source (see “Indoor air: methods for control and cleaning”, elsewhere in this chapter).
One of the basic objectives of any general ventilation system is the control of body odours. This can be achieved by supplying no less than 0.45 cubic metres per minute, m3/min, of new air per occupant. When smoking is frequent or the work is physically strenuous, the rate of ventilation required is greater, and may surpass 0.9 m3/min per person.
If the only environmental problems that the ventilation system must overcome are the ones just described, it is a good idea to keep in mind that every space has a certain level of “natural” air renewal by means of so-called “infiltration,” which occurs through doors and windows, even when they are closed, and through other sites of wall penetration. Air-conditioning manuals usually provide ample information in this regard, but it can be said that as a minimum the level of ventilation due to infiltration falls between 0.25 and 0.5 renewals per hour. An industrial site will commonly experience between 0.5 and 3 renewals of air per hour.
When used to control chemical pollutants, general ventilation must be limited to only those situations where the amounts of pollutants generated are not very high, where their toxicity is relatively moderate and where workers do not carry out their tasks in the immediate vicinity of the source of contamination. If these injunctions are not respected, it will be difficult to obtain acceptance for adequate control of the work environment because such high renewal rates must be used that the high air speeds will likely create discomfort, and because high renewal rates are expensive to maintain. It is therefore unusual to recommend the use of general ventilation for the control of chemical substances except in the case of solvents which have admissible concentrations of more than 100 parts per million.
When, on the other hand, the goal of general ventilation is to maintain the thermal characteristics of the work environment with a view to legally acceptable limits or technical recommendations such as the International Organization for Standardization (ISO) guidelines, this method has fewer limitations. General ventilation is therefore used more often to control the thermal environment than to limit chemical contamination, but its usefulness as a complement of localized extraction techniques should be clearly recognized.
While for many years the phrases general ventilation and ventilation by dilution were considered synonymous, today that is no longer the case because of a new general ventilation strategy: ventilation by displacement. Even though ventilation by dilution and ventilation by displacement fit within the definition of general ventilation we have outlined above, they both differ widely in the strategy they employ to control contamination.
Ventilation by dilution has the goal of mixing the air that is introduced mechanically as completely as possible with all the air that is already within the space, so that the concentration of a given pollutant will be as uniform as possible throughout (or so that the temperature will be as uniform as possible, if thermal control is the goal desired). To achieve this uniform mixture air is injected from the ceiling as streams at a relatively high speed, and these streams generate a strong circulation of air. The result is a high degree of mixing of the new air with the air already present inside the space.
Ventilation by displacement, in its ideal conceptualization, consists of injecting air into a space in such a way that new air displaces the air previously there without mixing with it. Ventilation by displacement is achieved by injecting new air into a space at a low speed and close to the floor, and extracting air near the ceiling. Using ventilation by displacement to control the thermal environment has the advantage that it profits from the natural movement of air generated by density variations that are themselves due to temperature differences. Even though ventilation by displacement is already widely used in industrial situations, the scientific literature on the subject is still quite limited, and the evaluation of its effectiveness is therefore still difficult.
Ventilation by Dilution
The design of a system of ventilation by dilution is based on the hypothesis that the concentration of the pollutant is the same throughout the space in question. This is the model that chemical engineers often refer to as a stirred tank.
If you assume that the air that is injected into the space is free of the pollutant and that at the initial time the concentration within the space is zero, you will need to know two facts in order to calculate the required rate of ventilation: the amount of the pollutant that is generated in the space and the level of environmental concentration that is sought (which hypothetically would be the same throughout).
Under these conditions, the corresponding calculations yield the following equation:
![]()
where
c(t) = the concentration of the contaminant in the space at time t
a = the amount of the pollutant generated (mass per unit of time)
Q = the rate at which new air is supplied (volume per unit of time)
V = the volume of the space in question.
The above equation shows that the concentration will tend to a steady state at the value a/Q, and that it will do so faster the smaller the value of Q/V, frequently referred to as “the number of renewals per unit of time”. Although occasionally the index of the quality of ventilation is regarded as practically equivalent to that value, the above equation clearly shows that its influence is limited to controlling the speed of stabilization of the environmental conditions, but not the level of concentration at which such a steady state will occur. That will depend only on the amount of the pollutant that is generated (a), and on the rate of ventilation (Q).
When the air of a given space is contaminated but no new amounts of the pollutant are generated, the speed of diminution of the concentration over a period of time is given by the following expression:
![]()
where Q and V have the meaning described above, t1 and t2 are, respectively, the initial and the final times and c1 and c2 are the initial and final concentrations.
Expressions can be found for calculations in instances where the initial concentration is not zero (Constance 1983; ACGIH 1992), where the air injected into the space is not totally devoid of the pollutant (because to reduce heating costs in the winter part of the air is recycled, for example), or where the amounts of the pollutant generated vary as a function of time.
If we disregard the transition stage and assume that the steady state has been achieved, the equation indicates that the rate of ventilation is equivalent to a/clim, where clim is the value of the concentration that must be maintained in the given space. This value will be established by regulations or, as an ancillary norm, by technical recommendations such as the threshold limit values (TLV) of the American Conference of Governmental Industrial Hygienists (ACGIH), which recommends that the rate of ventilation be calculated by the formula
![]()
where a and clim have the meaning already described and K is a safety factor. A value of K between 1 and 10 must be selected as a function of the efficacy of the air mixture in the given space, of the toxicity of the solvent (the smaller clim is, the greater the value of K will be), and of any other circumstance deemed relevant by the industrial hygienist. The ACGIH, among others, cites the duration of the process, the cycle of operations and the usual location of the workers with respect to the sources of emission of the pollutant, the number of these sources and their location in the given space, the seasonal changes in the amount of natural ventilation and the anticipated reduction in the functional efficacy of the ventilation equipment as other determining criteria.
In any case, the use of the above formula requires a reasonably exact knowledge of the values of a and K that should be used, and we therefore provide some suggestions in this regard.
The amount of pollutant generated may quite frequently be estimated by the amount of certain materials consumed in the process that generates the pollutant. So, in the case of a solvent, the amount used will be a good indication of the maximum amount that can be found in the environment.
As indicated above, the value of K should be determined as a function of the efficacy of the air mixture in the given space. This value will, therefore, be smaller in direct proportion to how good the estimation is of finding the same concentration of the pollutant at any point within the given space. This, in turn, will depend on how air is distributed within the space being ventilated.
According to these criteria, minimum values of K should be used when air is injected into the space in a distributed fashion (by using a plenum, for example), and when the injection and extraction of air are at opposite ends of the given space. On the other hand, higher values for K should be used when air is supplied intermittently and air is extracted at points close to the intake of new air (figure 1).
Figure 1. Schematic of air circulation in room with two supply openings
It should be noted that when air is injected into a given space—especially if it is done at a high speed—the stream of air created will exert a considerable pull on the air surrounding it. This air then mixes with the stream and slows it down, creating measurable turbulence as well. As a consequence, this process results in intense mixing of the air already in the space and the new air that is injected, generating internal air currents. Predicting these currents, even generally, requires a large dose of experience (figure 2).
Figure 2. Suggested K factors for inlet and exhaust locations
In order to avoid problems that result from workers’ being subjected to streams of air at relatively high speeds, air is commonly injected by way of diffusing grates designed in such a way that they facilitate the rapid mixing of new air with the air already present in the space. In this way, the areas where air moves at high speeds are kept as small as possible.
The stream effect just described is not produced near points where air escapes or is extracted through doors, windows, extraction vents or other openings. Air reaches extraction grates from all directions, so even at a relatively short distance from them, air movement is not easily perceived as an air current.
In any case, in dealing with air distribution, it is important to keep in mind the convenience of placing workstations, to the extent possible, in such a way that new air reaches the workers before it reaches the sources of contamination.
When in the given space there are important sources of heat, the movement of air will largely be conditioned by the convection currents that are due to density differences between denser, cold air and lighter, warm air. In spaces of this kind, the designer of air distribution must not fail to keep in mind the existence of these heat sources, or the movement of air may turn out to be very different from the one predicted.
The presence of chemical contamination, on the other hand, does not alter in a measurable way the density of air. While in a pure state the pollutants may have a density that is very different from that of air (usually much greater), given the real, existing concentrations in the workplace, the mix of air and pollutant does not have a density significantly different than the density of pure air.
Furthermore, it should be pointed out that one of the most common mistakes made in applying this type of ventilation is supplying the space only with air extractors, without any forethought given to adequate intakes of air. In these cases, the effectiveness of the extraction ventilators is diminished and, therefore, the actual rates of air extraction are much less than planned. The result is greater ambient concentrations of the pollutant in the given space than those initially calculated.
To avoid this problem some thought should be given to how air will be introduced into the space. The recommended course of action is to use immission ventilators as well as extraction ventilators. Normally, the rate of extraction should be greater than the rate of immission in order to allow for infiltration through windows and other openings. In addition, it is advisable to keep the space under slightly negative pressure to prevent the contamination generated from drifting into areas that are not contaminated.
Ventilation by Displacement
As mentioned above, with ventilation by displacement one seeks to minimize the mixing of new air and the air previously found in the given space, and tries to adjust the system to the model known as plug flow. This is usually accomplished by introducing air at slow speeds and at low elevations in the given space and extracting it near the ceiling; this has two advantages over ventilation by dilution.
In the first place, it makes lower rates of air renewal possible, because pollution concentrates near the ceiling of the space, where there are no workers to breathe it. The average concentration in the given space will then be higher than the clim value we have referred to before, but that does not imply a higher risk for the workers because in the occupied zone of the given space the concentration of the pollutant will be the same or lower than a clim.
In addition, when the goal of ventilation is the control of the thermal environment, ventilation by displacement makes it possible to introduce warmer air into the given space than would be required by a system of ventilation by dilution. This is because the warm air that is extracted is at a temperature several degrees higher than the temperature in the occupied zone of the space.
The fundamental principles of ventilation by displacement were developed by Sandberg, who in the early 1980s developed a general theory for the analysis of situations where there were nonuniform concentrations of pollutants in enclosed spaces. This allowed us to overcome the theoretical limitations of ventilation by dilution (which presupposes a uniform concentration throughout the given space) and opened the way for practical applications (Sandberg 1981).
Even though ventilation by displacement is widely used in some countries, particularly in Scandinavia, very few studies have been published in which the efficacy of different methods are compared in actual installations. This is no doubt because of the practical difficulties of installing two different ventilation systems in a real factory, and because the experimental analysis of these types of systems require the use of tracers. Tracing is done by adding a tracer gas to the air ventilation current and then measuring the concentrations of the gas at different points within the space and in the extracted air. This sort of examination makes it possible to infer how air is distributed within the space and to then compare the efficacy of different ventilation systems.
The few studies available that have been carried out in actual existing installations are not conclusive, except as regards the fact that systems that employ ventilation by displacement provide better air renewal. In these studies, however, reservations are often expressed about the results in so far as they have not been confirmed by measurements of the ambient level of contamination at the worksites.
Ventilation Criteria for Nonindustrial Buildings
One of the chief functions of a building in which nonindustrial activities are carried out (offices, schools, dwellings, etc.) is to provide the occupants with a healthy and comfortable environment in which to work. The quality of this environment depends, to a large degree, on whether the ventilation and climatization systems of the building are adequately designed and maintained and function properly.
These systems must therefore provide acceptable thermal conditions (temperature and humidity) and an acceptable quality of indoor air. In other words, they should aim for a suitable mix of outside air with indoor air and should employ filtration and cleaning systems capable of eliminating pollutants found in the indoor environment.
The idea that clean outdoor air is necessary for well-being in indoor spaces has been expressed since the eighteenth century. Benjamin Franklin recognized that air in a room is healthier if it is provided with natural ventilation by opening the windows. The idea that providing great quantities of outside air could help reduce the risk of contagion for illnesses like tuberculosis gained currency in the nineteenth century.
Studies carried out during the 1930s showed that, in order to dilute human biological effluvia to concentrations that would not cause discomfort due to odours, the volume of new outside air required for a room is between 17 and 30 cubic metres per hour per occupant.
In standard No. 62 set in 1973, the American Society of Heating, Refrigerating and Air Conditioning Engineers (ASHRAE) recommends a minimum flow of 34 cubic metres of outside air per hour per occupant to control odours. An absolute minimum of 8.5 m3/hr/occupant is recommended to prevent carbon dioxide from surpassing 2,500 ppm, which is half of the exposure limit set for industrial settings.
This same organization, in standard No. 90, set in 1975—in the middle of an energy crisis—adopted the aforementioned absolute minimum leaving aside, temporarily, the need for greater ventilation flows to dilute pollutants such as tobacco smoke, biological effluvia and so forth.
In its standard No. 62 (1981) ASHRAE rectified this omission and established its recommendation as 34 m3/hr/occupant for areas where smoking is permitted and 8.5 m3/hr/occupant in areas where smoking is forbidden.
The last standard published by ASHRAE, also No. 62 (1989), established a minimum of 25.5 m3/hr/occupant for occupied indoor spaces independently of whether smoking is permitted or not. It also recommends increasing this value when the air brought into the building is not mixed adequately in the breathing zone or if there are unusual sources of pollution present in the building.
In 1992, the Commission of European Communities published its Guidelines for Ventilation Requirements in Buildings. In contrast with existing recommendations for ventilation standards, this guide does not specify volumes of ventilation flow that should be provided for a given space; instead, it provides recommendations that are calculated as a function of the desired quality of indoor air.
Existing ventilation standards prescribe set volumes of ventilation flow that should be supplied per occupant. The tendencies evidenced in the new guidelines show that volume calculations alone do not guarantee a good quality of indoor air for every setting. This is the case for three fundamental reasons.
First, they assume that occupants are the only sources of contamination. Recent studies show that other sources of pollution, in addition to the occupants, should be taken into consideration as possible sources of pollution. Examples include furniture, upholstery and the ventilation system itself. The second reason is that these standards recommend the same amount of outside air regardless of the quality of air that is being conveyed into the building. And the third reason is that they do not clearly define the quality of indoor air required for the given space. Therefore, it is proposed that future ventilation standards should be based on the following three premises: the selection of a defined category of air quality for the space to be ventilated, the total load of pollutants in the occupied space and the quality of outside air available.
The Perceived Quality of Air
The quality of indoor air can be defined as the degree to which the demands and requirements of the human being are met. Basically, the occupants of a space demand two things of the air they breathe: to perceive the air they breathe as fresh and not foul, stale or irritating; and to know that the adverse health effects that may result from breathing that air are negligible.
It is common to think that the degree of quality of the air in a space depends more on the components of that air than on the impact of that air on the occupants. It may thus seem easy to evaluate the quality of the air, assuming that by knowing its composition its quality can be ascertained. This method of evaluating air quality works well in industrial settings, where we find chemical compounds that are implicated in or derived from the production process and where measuring devices and reference criteria to assess the concentrations exist. This method does not, however, work in nonindustrial settings. Nonindustrial settings are places where thousands of chemical substances can be found, but at very low concentrations, sometimes a thousand times lower than the recommended exposure limits; evaluating these substances one by one would result in a false assessment of the quality of that air, and the air would likely be judged to be of a high quality. But there is a missing aspect that remains to be considered, and that is the lack of knowledge that exists about the combined effect of those thousands of substances on human beings, and that may be the reason why that air is perceived as being foul, stale or irritating.
The conclusion that has been reached is that traditional methods used for industrial hygiene are not well-adapted to define the degree of quality that will be perceived by the human beings that breathe the air being evaluated. The alternative to chemical analysis is to use people as measuring devices to quantify air pollution, employing panels of judges to make the evaluations.
Human beings perceive the quality of air by two senses: the olfactory sense, situated in the nasal cavity and sensitive to hundreds of thousands of odorous substances, and the chemical sense, situated in the mucous membranes of the nose and eyes, and sensitive to a similar number of irritating substances present in air. It is the combined response of these two senses that determines how air is perceived and that allows the subject to judge whether its quality is acceptable.
The olf unit
One olf (from Latin = olfactus) is the emission rate of air pollutants (bioeffluents) from a standard person. One standard person is an average adult who works in an office or in a similar nonindustrial workplace, sedentary and in thermal comfort with a hygienic standard equipment to 0.7 bath/day. Pollution from a human being was chosen to define the term olf for two reasons: the first is that biological effluvia emitted by a person are well-known, and the second is that there was much data on the dissatisfaction caused by such biological effluvia.
Any other source of contamination can be expressed as the number of standard persons (olfs) needed to cause the same amount of dissatisfaction as the source of contamination that is being evaluated.
Figure 1 depicts a curve that defines an olf. This curve shows how contamination produced by a standard person (1 olf) is perceived at different rates of ventilation, and allows the calculation of the rate of dissatisfied individuals—in other words, those that will perceive the quality of air to be unacceptable just after they have entered the room. The curve is based on different European studies in which 168 people judged the quality of air polluted by over a thousand people, both men and women, considered to be standard. Similar studies conducted in North America and Japan show a high degree of correlation with the European data.
Figure 1. Olf definition curve
The decipol unit
The concentration of pollution in air depends on the source of contamination and its dilution as a result of ventilation. Perceived air pollution is defined as the concentration of human biological effluvia that would cause the same discomfort or dissatisfaction as the concentration of polluted air that is being evaluated. One decipol (from the Latin pollutio) is the contamination caused by a standard person (1 olf) when the rate of ventilation is 10 litres per second of noncontaminated air, so that we may write
1 decipol = 0.1 olf/(litre/second)
Figure 2, derived from the same data as the previous figure, shows the relation between the perceived quality of air, expressed as a percentage of dissatisfied individuals and in decipols.
Figure 2. Relation between the perceived quality of air expressed as a percentage of dissatisfied individuals and in decipols
To determine the rate of ventilation required from the point of view of comfort, selecting the degree of air quality desired in the given space is essential. Three categories or levels of quality are proposed in Table 1, and they are derived from Figures 1 and 2. Each level corresponds to a certain percentage of dissatisfied people. The selection of one or another level will depend, most of all, on what the space will be used for and on economic considerations.
Table 1. Levels of quality of indoor air
|
Perceived air quality |
|||
|
Category |
Percentage of dissatisfied |
Decipols |
Rate of ventilation required1 |
|
A |
10 |
0.6 |
16 |
|
B |
20 |
1.4 |
7 |
|
C |
30 |
2.5 |
4 |
1 Assuming that outside air is clean and the efficiency of the ventilation system is equal to one.
Source: CEC 1992.
As noted above, the data are the result of experiments carried out with panels of judges, but it is important to keep in mind that some of the substances found in air that can be dangerous (carcinogenic compounds, micro-organisms and radioactive substances, for example) are not recognized by the senses, and that the sensory effects of other contaminants bear no quantitative relationship to their toxicity.
Sources of Contamination
As was indicated earlier, one of the shortcomings of today’s ventilation standards is that they take into account only the occupants as the sources of contamination, whereas it is recognized that future standards should take all the possible sources of pollution into account. Aside from the occupants and their activities, including the possibility that they might smoke, there are other sources of pollution that contribute significantly to air pollution. Examples include furniture, upholstery and carpeting, construction materials, products used for decoration, cleaning products and the ventilation system itself.
What determines the load of pollution of air in a given space is the combination of all these sources of contamination. This load can be expressed as chemical contamination or as sensory contamination expressed in olfs. The latter integrates the effect of several chemical substances as they are perceived by human beings.
The chemical load
Contamination that emanates from a given material can be expressed as the rate of emission of each chemical substance. The total load of chemical pollution is calculated by adding all the sources, and is expressed in micrograms per second (μg/s).
In reality, it may be difficult to calculate the load of pollution because often little data are available on the rates of emission for many commonly used materials.
Sensory load
The load of pollution perceived by the senses is caused by those sources of contamination that have an impact on the perceived quality of air. The given value of this sensory load can be calculated by adding all the olfs of different sources of contamination that exist in a given space. As in the previous case, there is still not much information available on the olfs per square metre (olfs/m2) of many materials. For that reason it turns out to be more practical to estimate the sensory load of the entire building, including the occupants, the furnishings and the ventilation system.
Table 2 shows the pollution load in olfs by the occupants of the building as they carry out different types of activities, as a proportion of those who smoke and don’t smoke, and the production of various compounds like carbon dioxide (CO2), carbon monoxide (CO) and water vapour. Table 3 shows some examples of the typical occupancy rates in different kinds of spaces. And last, table 4 reflects the results of the sensory load—measured in olfs per square metre—found in different buildings.
Table 2. Contamination due to the occupants of a building
|
Sensory load olf/occupant |
CO2 |
CO3 |
Water vapour4 |
|
|
Sedentary, 1-1.2 met1 |
||||
|
0% smokers |
2 |
19 |
50 |
|
|
20% smokers2 |
2 |
19 |
11x10-3 |
50 |
|
40% smokers2 |
3 |
19 |
21x10-3 |
50 |
|
100% smokers2 |
6 |
19 |
53x10-3 |
50 |
|
Physical exertion |
||||
|
Low, 3 met |
4 |
50 |
200 |
|
|
Medium, 6 met |
10 |
100 |
430 |
|
|
High (athletic), |
20 |
170 |
750 |
|
|
Children |
||||
|
Child care centre |
1.2 |
18 |
90 |
|
|
School |
1.3 |
19 |
50 |
|
1 1 met is the metabolic rate of a sedentary person at rest (1 met = 58 W/m2 of skin surface).
2 Average consumption of 1.2 cigarettes/hour per smoker. Average rate of emission, 44 ml of CO per cigarette.
3 From tobacco smoke.
4 Applicable to people close to thermal neutrality.
Source: CEC 1992.
Table 3. Examples of the degree of occupancy of different buildings
|
Building |
Occupants/m2 |
|
Offices |
0.07 |
|
Conference rooms |
0.5 |
|
Theatres, other large gathering places |
1.5 |
|
Schools (classrooms) |
0.5 |
|
Child-care centres |
0.5 |
|
Dwellings |
0.05 |
Source: CEC 1992.
Table 4. Contamination due to the building
|
Sensory load—olf/m2 |
||
|
Average |
Interval |
|
|
Offices1 |
0.3 |
0.02–0.95 |
|
Schools (classrooms)2 |
0.3 |
0.12–0.54 |
|
Child care facilities3 |
0.4 |
0.20–0.74 |
|
Theatres4 |
0.5 |
0.13–1.32 |
|
Low-pollution buildings5 |
0.05–0.1 |
|
1 Data obtained in 24 mechanically ventilated offices.
2 Data obtained in 6 mechanically ventilated schools.
3 Data obtained in 9 mechanically ventilated child-care centres.
4 Data obtained in 5 mechanically ventilated theatres.
5 Goal that should be reached by new buildings.
Source: CEC 1992.
Quality of Outside Air
Another premise, one that rounds out the inputs needed for creation of ventilation standards for the future, is the quality of available outside air. Recommended exposure values for certain substances, both from inside and outside spaces, appear in the publication Air Quality Guidelines for Europe by the WHO (1987).
Table 5 shows the levels of perceived outside air quality, as well as the concentrations of several typical chemical pollutants found out of doors.
Table 5. Quality levels of outside air
|
Perceived |
Environmental pollutants2 |
||||
|
Decipol |
CO2 (mg/m3) |
CO (mg/m3) |
NO2 (mg/m3) |
SO2 (mg/m3) |
|
|
By the sea, in the mountains |
0 |
680 |
0-0.2 |
2 |
1 |
|
City, high quality |
0.1 |
700 |
1-2 |
5-20 |
5-20 |
|
City, low quality |
>0.5 |
700-800 |
4-6 |
50-80 |
50-100 |
1 The values of perceived air quality are daily average values.
2 The values of pollutants correspond to average yearly concentrations.
Source: CEC 1992.
It should be kept in mind that in many cases the quality of outside air can be worse than the levels indicated in the table or in the guidelines of the WHO. In such cases air needs to be cleaned before it is conveyed into occupied spaces.
Efficiency of Ventilation Systems
Another important factor that will affect the calculation of the ventilation requirements for a given space is the efficiency of ventilation (Ev), which is defined as the relation between the concentration of pollutants in extracted air (Ce) and the concentration in the breathing zone (Cb).
Ev = Ce/Cb
The efficiency of ventilation depends on the distribution of air and the location of the sources of pollution in the given space. If air and the contaminants are mixed completely, the efficiency of ventilation is equal to one; if the quality of air in the breathing zone is better than that of extracted air, then the efficiency is greater than one and the desired quality of air can be attained with lower rates of ventilation. On the other hand, greater rates of ventilation will be needed if the efficiency of ventilation is less than one, or to put it differently, if the quality of air in the breathing zone is inferior to the quality of extracted air.
In calculating the efficiency of ventilation it is useful to divide spaces into two zones, one into which the air is delivered, the other comprising the rest of the room. For ventilation systems that work by the mixing principle, the zone where air is delivered is generally found above the breathing zone, and the best conditions are reached when mixing is so thorough that both zones become one. For ventilation systems that work by the displacement principle, air is supplied in the zone occupied by people and the extraction zone is usually found overhead; here the best conditions are reached when mixing between both zones is minimal.
The efficiency of ventilation, therefore, is a function of the location and characteristics of the elements that supply and extract air and the location and characteristics of the sources of contamination. In addition, it is also a function of the temperature and of the volumes of air supplied. It is possible to calculate the efficiency of a ventilation system by numerical simulation or by taking measurements. When data are not available the values in figure 3 can be used for different ventilation systems. These reference values take into consideration the impact of air distribution but not the location of sources of pollution, assuming instead that they are uniformly distributed throughout the ventilated space.
Figure 3. Effectiveness of ventilation in breathing zone according to different ventilation principles
Calculating Ventilation Requirements
Figure 4 shows the equations used to calculate ventilation requirements from the point of view of comfort as well as that of protecting health.
Figure 4. Equations for calculating ventilation requirements
Ventilation requirements for comfort
The first steps in the calculation of comfort requirements is to decide the level of quality of indoor air that one wishes to obtain for the ventilated space (see Table 1), and to estimate the quality of outside air available (see Table 5).
The next step consists in estimating the sensory load, using Tables 8, 9, and 10 to select the loads according to the occupants and their activities, the type of building, and the level of occupancy by square metre of surface. The total value is obtained by adding all the data.
Depending on the operating principle of the ventilation system and using Figure 9, it is possible to estimate the efficiency of ventilation. Applying equation (1) in Figure 9 will yield a value for the required amount of ventilation.
Ventilation requirements for health protection
A procedure similar to the one described above, but using equation (2) in Figure 3, will provide a value for the stream of ventilation required to prevent health problems. To calculate this value it is necessary to identify a substance or group of critical chemical substances which one proposes to control and to estimate their concentrations in air; it is also necessary to allow for different evaluation criteria, taking into account the effects of the contaminant and the sensitivity of the occupants that you wish to protect—children or the elderly, for example.
Unfortunately, it is still difficult to estimate the ventilation requirements for health protection owing to the lack of information on some of the variables that enter into the calculations, such as the rates of emission of the contaminants (G), the evaluation criteria for indoor spaces (Cv) and others.
Studies carried out in the field show that spaces where ventilation is required to achieve comfortable conditions the concentrations of chemical substances is low. Nevertheless, those spaces may contain sources of pollution that are dangerous. The best policy in these cases is to eliminate, to substitute or to control the sources of pollution instead of diluting the contaminants by general ventilation.
" DISCLAIMER: The ILO does not take responsibility for content presented on this web portal that is presented in any language other than English, which is the language used for the initial production and peer-review of original content. Certain statistics have not been updated since the production of the 4th edition of the Encyclopaedia (1998)."